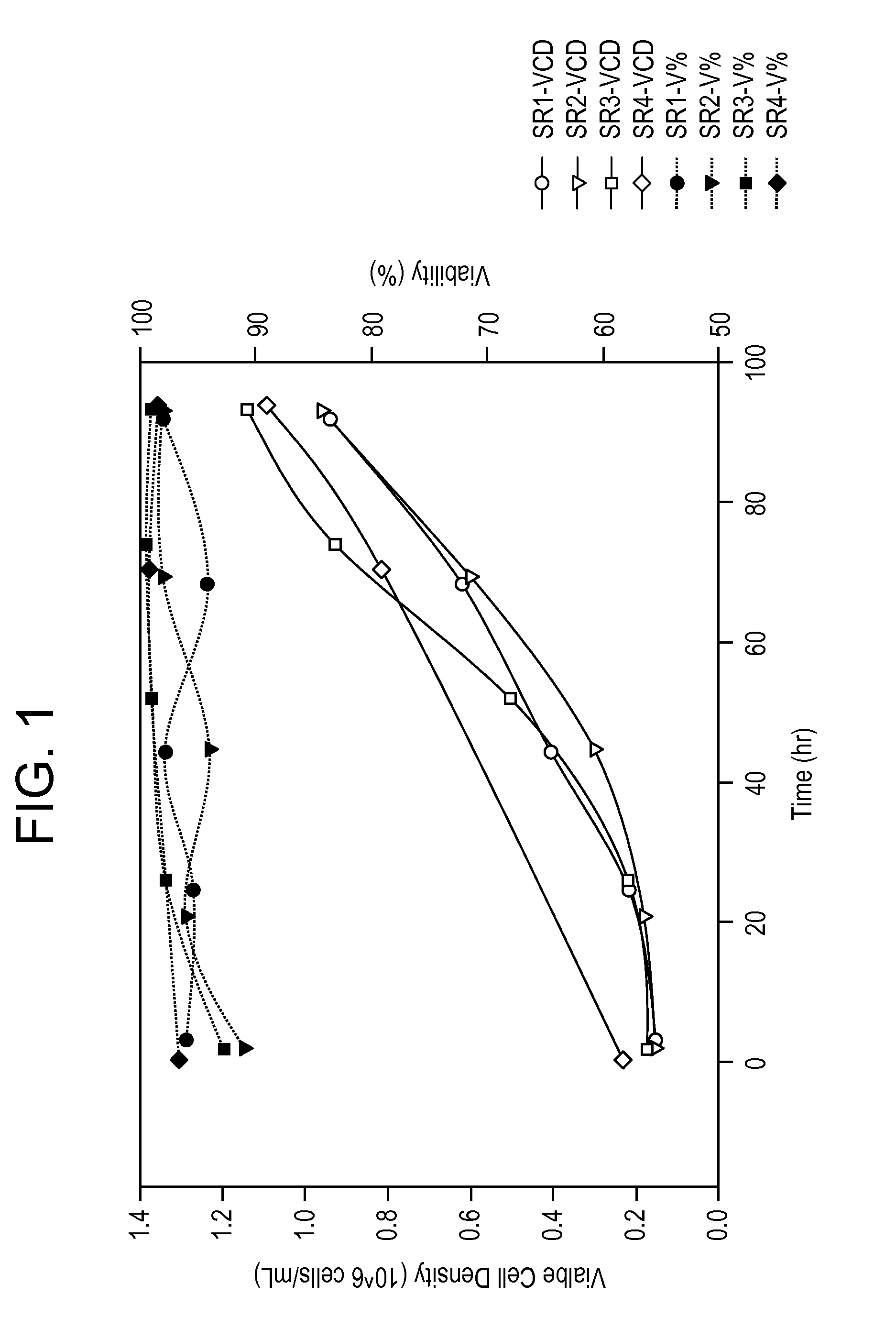
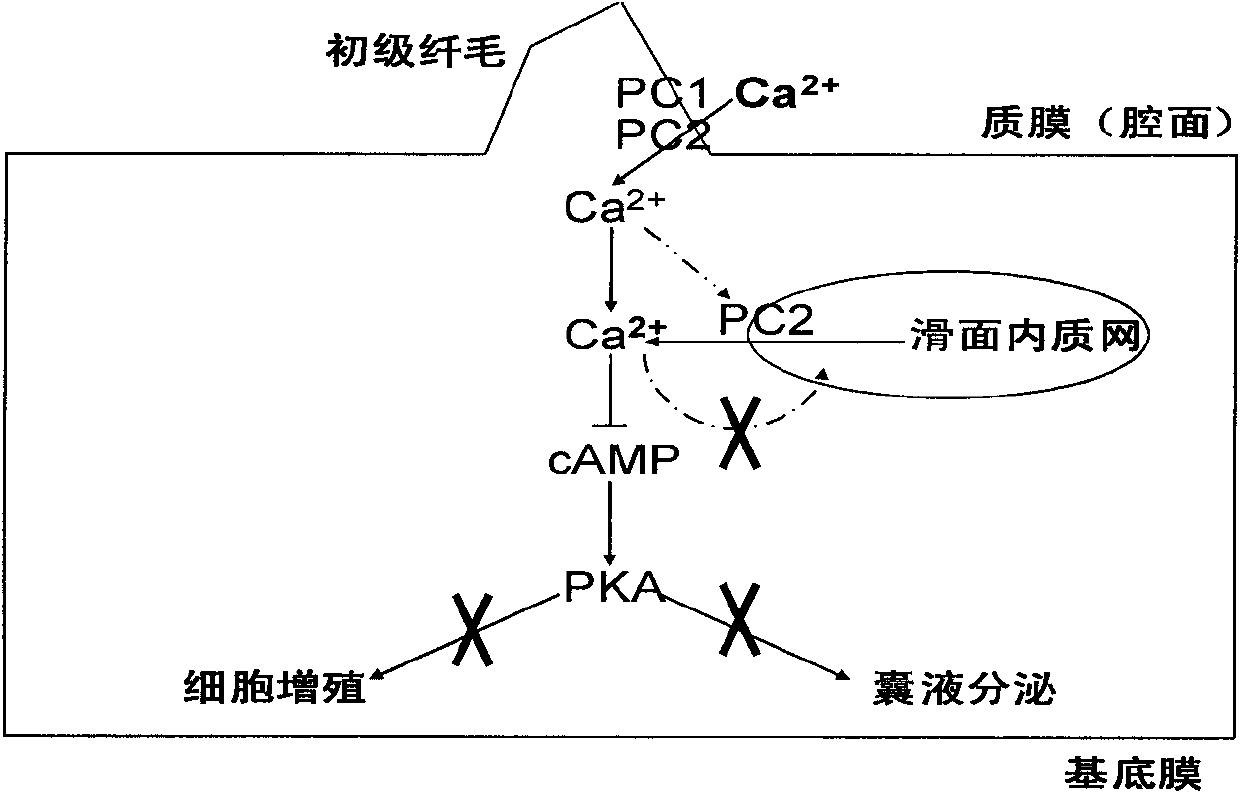
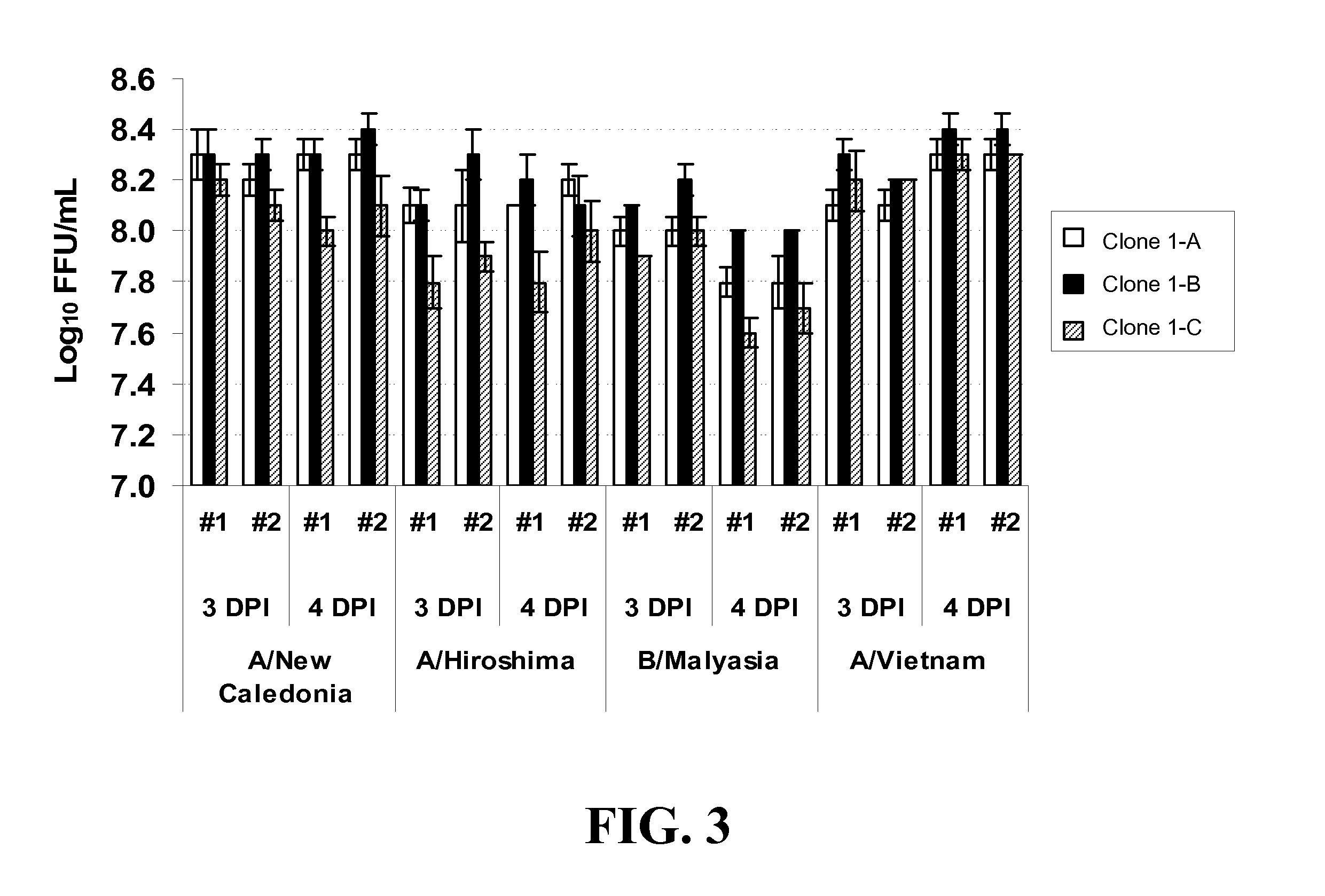
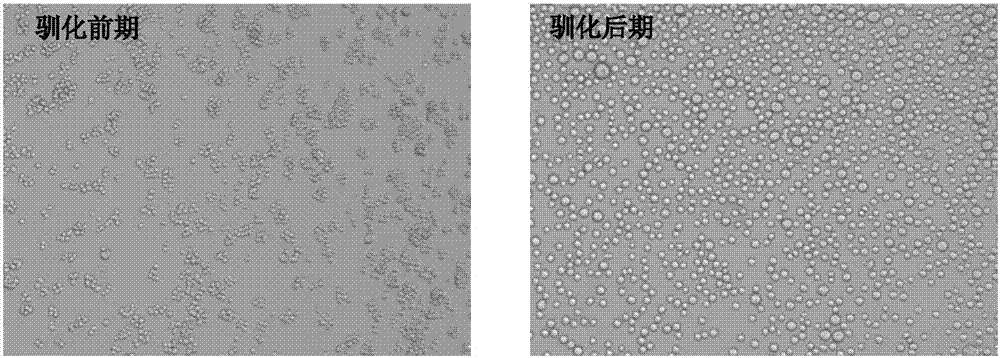
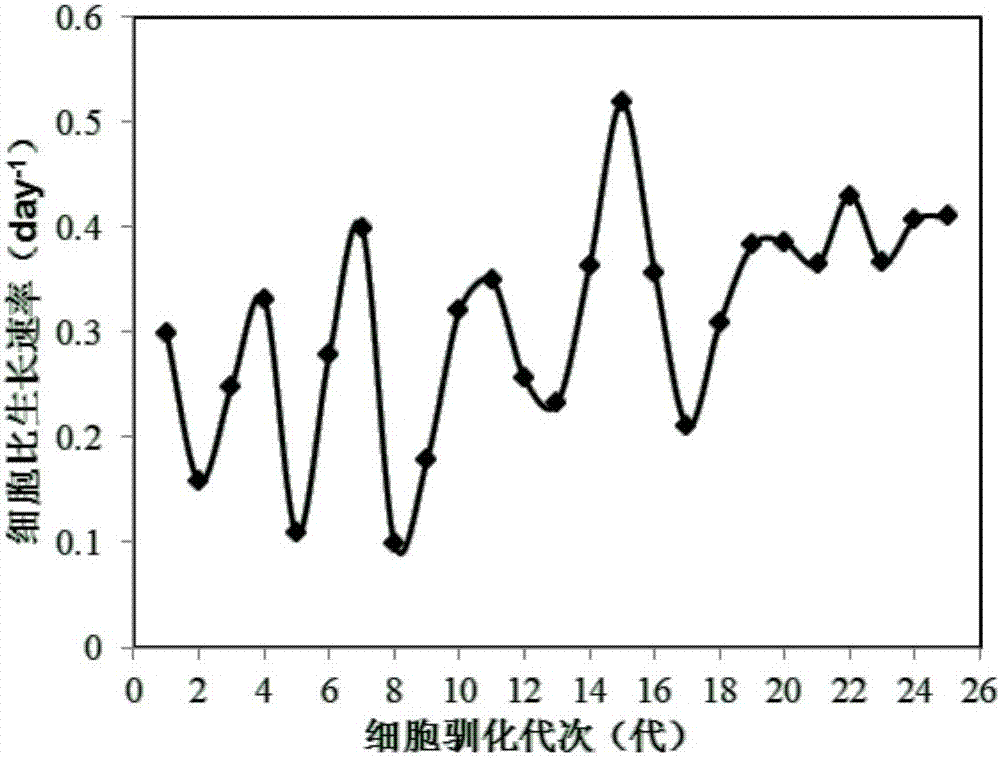
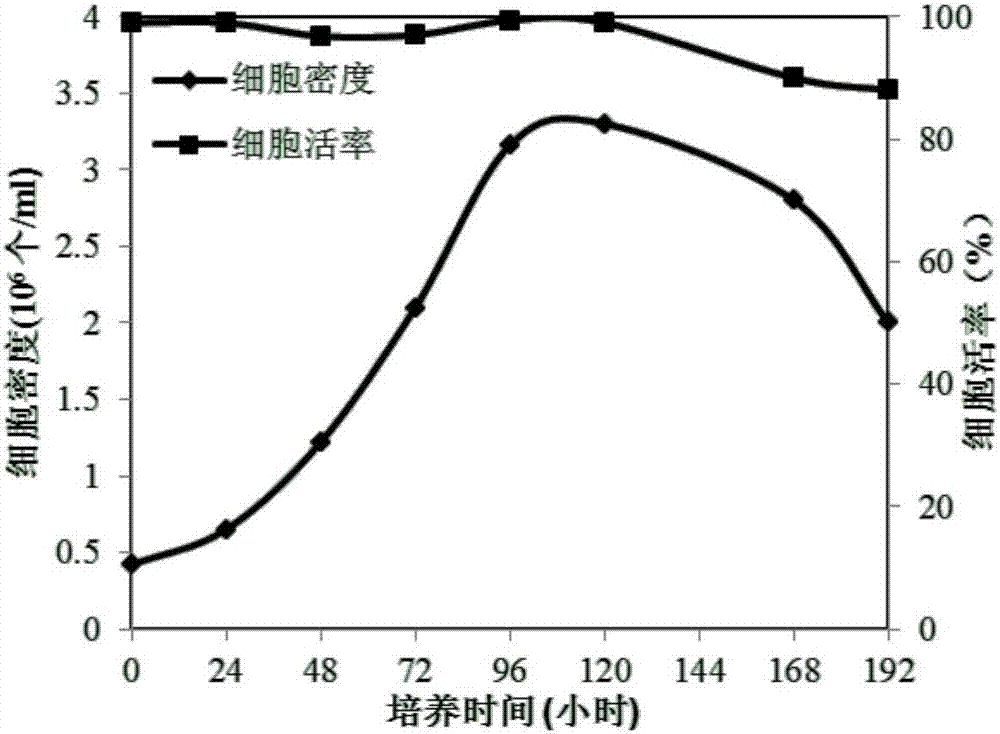
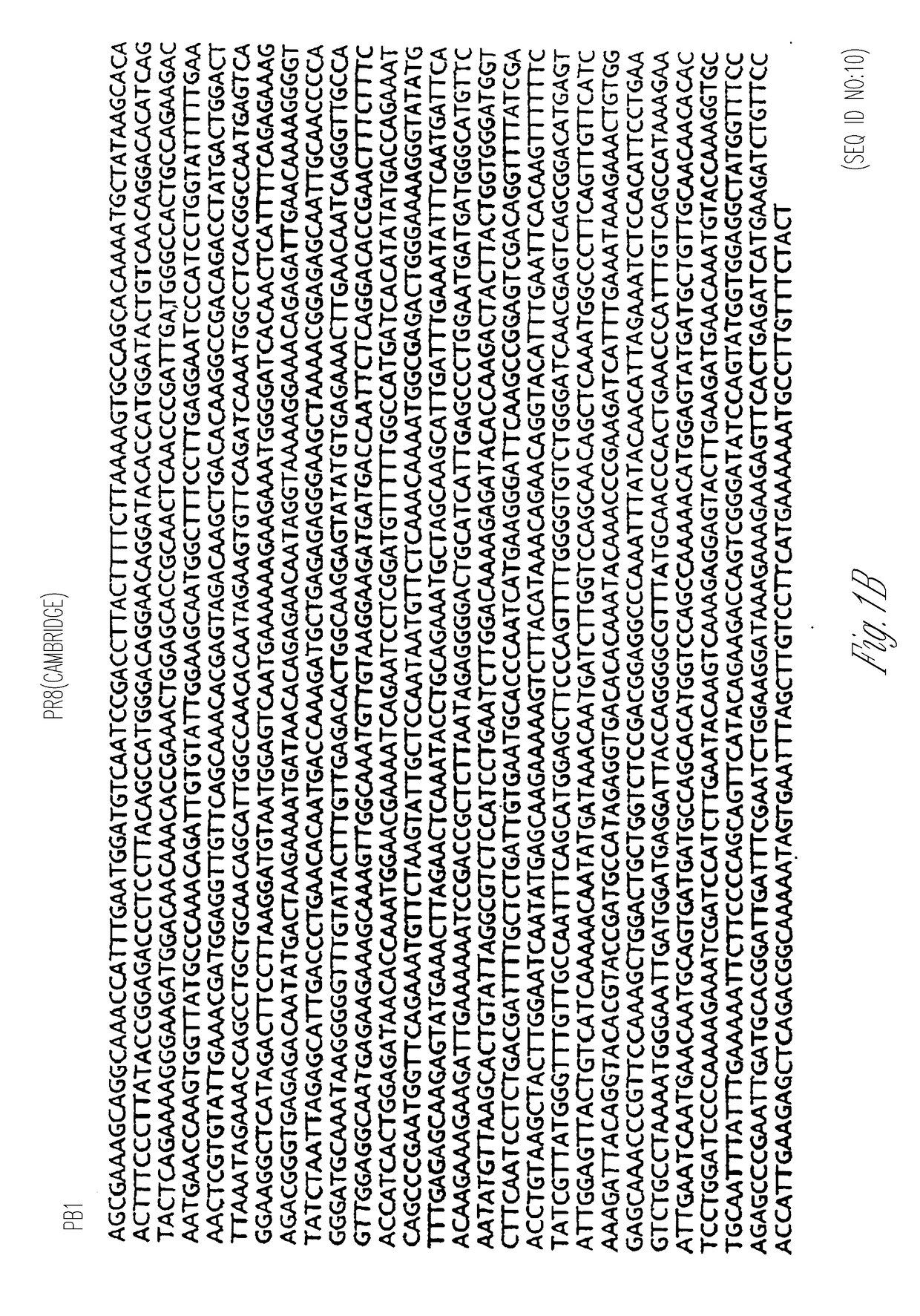
Patents
Literature
248 results about "Mdck cell" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Madin-Darby Canine Kidney (MDCK) cells are a model mammalian cell line used in biomedical research. MDCK cells are used for a wide variety of cell biology studies including cell polarity, cell-cell adhesions (termed adherens junctions), collective cell motility, as well as responses to growth factors.
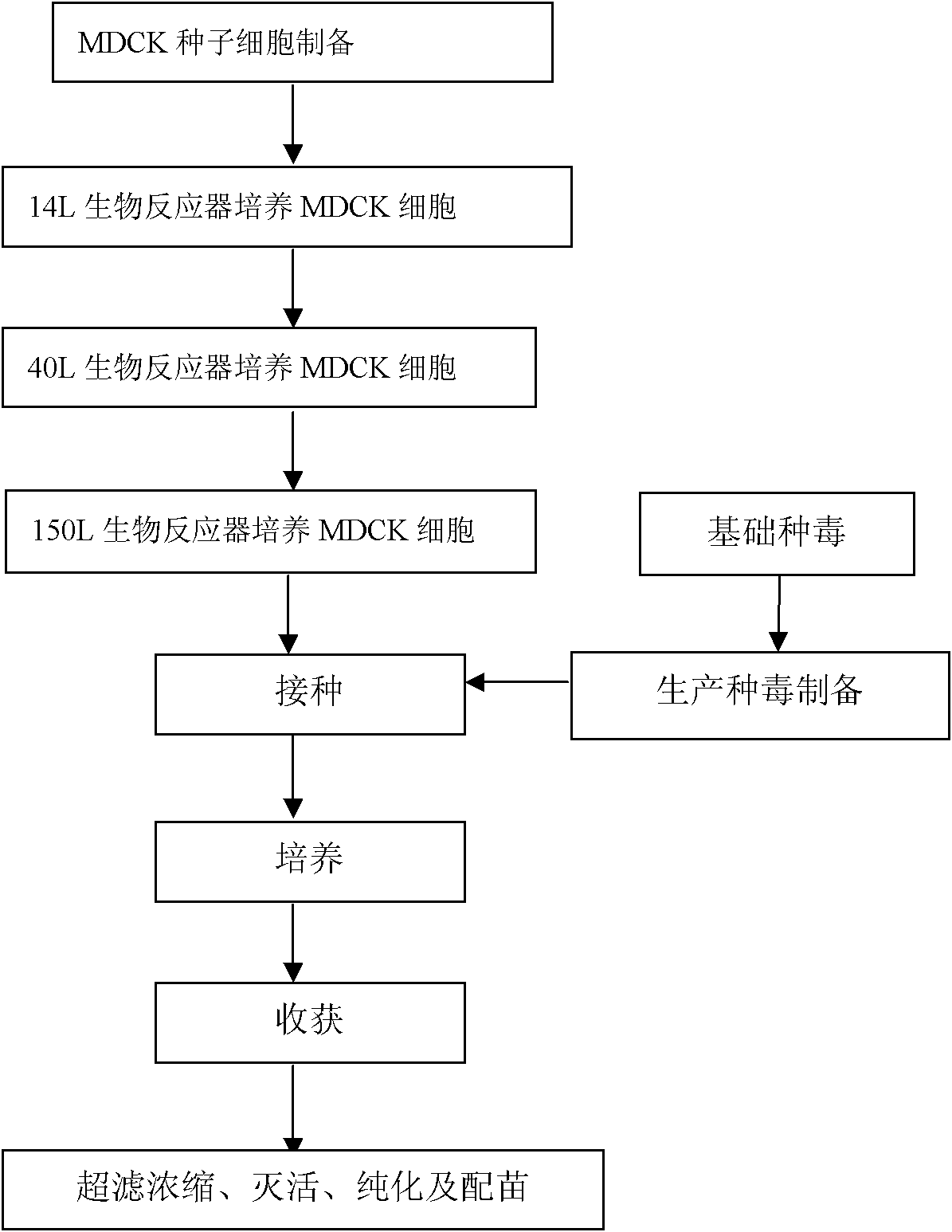
Serum-free medium for MDCK cell large-scale adherent culture and single-cell suspension culture
ActiveCN101760442ASupports adherent growthReduce the burden of separation and purification in the later stageVertebrate cellsArtificial cell constructsLipid formationSerum free media
The invention relates to the culture medium research and development technical field of modern biological technology and provides a serum-free medium for MDCK cell large-scale adherent culture and single-cell suspension culture, which comprises 21 amino acids, 6 vitamins, 8 salts, 8 lipids, 4 trace elements, 2 buffers, 1 protein hydrolysate, 1 acid-base indicator and 6 other additives. The serum-free medium can be prepared by the conventional preparation method, and an application method thereof is the conventional method. The serum-free medium has the beneficial effects that: the serum-free medium does not contain serum, has clear components, is beneficial for separating and purifying the product and improves the product quality; the serum-free medium supports long-term subculture of MDCK cells and does not require long-term and complex adaptation process; and the serum-free medium can well support the adherent growth and single-cell suspension growth of the MDCK cells, has clear components and easy preparation and utilization, and is suitable for mass production of biological products.
Owner:EAST CHINA UNIV OF SCI & TECH
Serum-free medium for Madin-Darby canine kidney (MDCK) cell full suspension culture
ActiveCN103555659AHigh densityClear ingredientsVertebrate cellsArtificial cell constructsCanine kidneyFlu immunization
The invention discloses a serum-free medium for Madin-Darby canine kidney (MDCK) cell full suspension culture. The serum-free medium for MDCK cell full suspension culture comprises an amino acid part, a vitamin part, an inorganic salt part and other additive parts. The serum-free medium has the beneficial effects that the serum-free medium for MDCK cell full suspension culture, provided by the invention, is high in cell culture density and clear in composition and does not contain animal serum, a downstream product is purified, the product quality is improved, and the serum-free medium is convenient to prepare and use and suitable for large-scale production of influenza vaccines and avian influenza vaccines.
Owner:无锡市赛尔百灵生物技术有限公司
Mdck-derived cell lines adapted to serum-free culture and suspension culture and method for preparing vaccine virus using the cells
InactiveUS20130183741A1Low and no tumorigenicityEasy to useSsRNA viruses negative-senseViral antigen ingredientsSerum igeSerum free
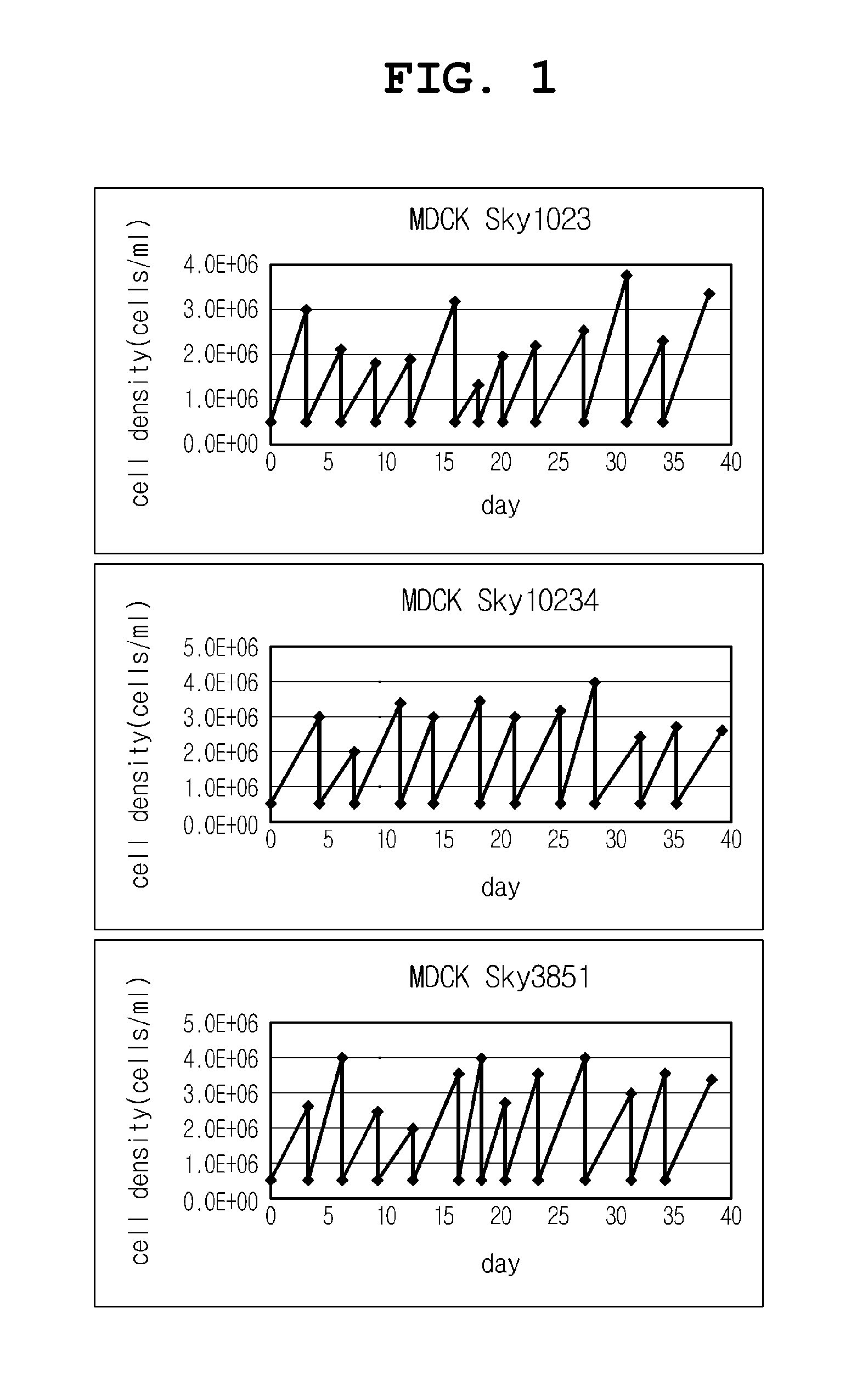
Disclosed is a Madin-Darby canine kidney (MDCK)-derived cell line. The MDCK-derived cell line is derived from MDCK cells deposited under accession number ATCC CCL-34. The MDCK-derived cell line can be prepared by serum-free culture and suspension culture. Preferably, the MDCK-derived cell line has low or no tumorigenicity. The MDCK-derived cell line is preferably selected from MDCK Sky1023, MDCK Sky10234 and MDCK Sky3851. Further disclosed are a culture method for growing the MDCK-derived cells and a method for producing a vaccine virus using the MDCK-derived cells.
Owner:SK CHEM CO LTD
Mdck cell lines supporting viral growth to high titers and bioreactor process using the same
The present invention relates to novel MDCK cells which can be to grow viruses, e.g., influenza viruses, in cell culture to higher titer than previously possible. The MDCK cells can be adapted to serum-free culture medium. The present invention further relates to cell culture compositions comprising the MDCK cells and cultivation methods for growing the MDCK cells. The present invention further relates to methods for producing influenza viruses in cell culture using the MDCK cells of the invention.
Owner:MEDIMMUNE LLC
Non-tumorigenic MDCK cell line for propagating viruses
The present invention provides novel MDCK-derived adherent non-tumorigenic cell lines that can be grown in the presence or absence of serum. The cell lines of the present invention are useful for the production of vaccine material (e.g., viruses). More specifically, the cell lines of the present invention are useful for the production of influenza viruses in general and ca / ts influenza viruses in particular. The invention further provides methods and media formulations for the adaptation and cultivation of MDCK cells such that they remain non-tumorigenic. Additionally, the present invention provides methods for the production of vaccine material (e.g., influenza virus) in the novel cell lines of the invention.
Owner:MEDIMMUNE LLC
Method of purifying influenza virus and removing MDCK cell DNA contaminants
The present invention relates to novel MDCK cells which can be to grow viruses, e.g., influenza viruses, in cell culture to higher titer than previously possible. The MDCK cells can be adapted to serum-free culture medium. The present invention further relates to cell culture compositions comprising the MDCK cells and cultivation methods for growing the MDCK cells. The present invention further relates to methods for producing influenza viruses in cell culture using the MDCK cells of the invention.
Owner:MEDIMMUNE LLC
Preparation method of avian influenza virus growing in serum-free full-suspended cultured MDCK cells and obtained avian influenza virus
ActiveCN106011083AStable characteristicsGet rid of dependenceVertebrate cellsArtificial cell constructsSerum freeF1 generation
The invention discloses a preparation method of an avian influenza virus suitable for growing in a serum-free full-suspended cultured MDCK cell line and the avian influenza virus obtained through the method. The preparation method comprises the following steps of 1 preparation of the MDCK cells to be inoculated; 2 virus seed preparation, wherein a chick embryo source avian influenza virus is prepared; 3 F1 generation virus domestication; 4 F2 generation virus domestication, wherein a supernatant sample retained at the time point when the blood clotting titer of the avian influenza virus obtained in the F1 generation is highest is taken, and the step 3 is repeated; 5 F3 generation virus domestication, wherein the F3 generation virus culturing temperature is 35 DEG C; 6 F4 generation-F10 generation virus domestication, wherein the step 5 is repeated, and the domesticated avian influenza virus is obtained. According to the preparation method, through a domestication method, the avian influenza virus is directly domesticated to completely adapt to be efficiently reproduced on the serum-free full-suspended cultured MDCK cells from the mode of being cultured by a chick embryo, the domestication efficiency is high, the avian influenza virus can be efficiently infected and copied in the MDCK cells, and the virus characteristic is stable.
Owner:ZHAOQING DAHUANONG BIOLOGIC PHARMA +2
Methods for cultivating cells, propagating and purifying viruses
InactiveUS20100098725A1Increase cell densityHigh viral titersSsRNA viruses negative-senseViral antigen ingredientsCell culture mediaCultured cell
The present invention provides novel serum-free cell culture medium and methods for cultivating MDCK cells. In particular, non-tumorigenic MDCK cells. The present invention also provides methods for producing influenza viruses (e.g., particularly cold-adapted, and / or temperature sensitive, and / or attenuated influenza viruses) that eliminate the need for a cell culture medium exchange step. The novel medium and methods are useful to grow influenza viruses, in cell culture to high titer. The present invention further provides purification methods for purifying influenza viruses with high overall recovery of live virus and result in levels of host cell DNA (HCD), host cell protein (HCP) and non-specific endonuclease (e.g., Benzonase), which are below the specifications required by regulatory agencies. The immunogenic compositions can be used to actively immunize subjects or to generate antibodies for a variety of uses, including passive immunization and diagnostic immunoassays.
Owner:MEDIMMUNE LLC
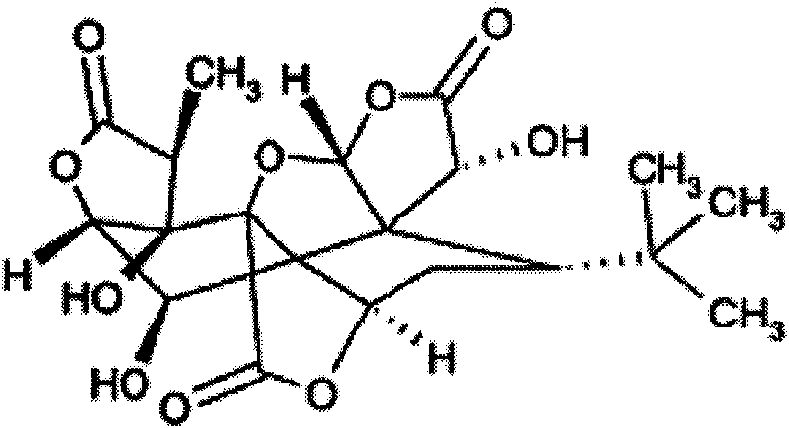
New application of ginkgolide B
InactiveCN102018702APromote apoptosisPromote growthOrganic active ingredientsUrinary disorderCanine kidneyCytotoxicity
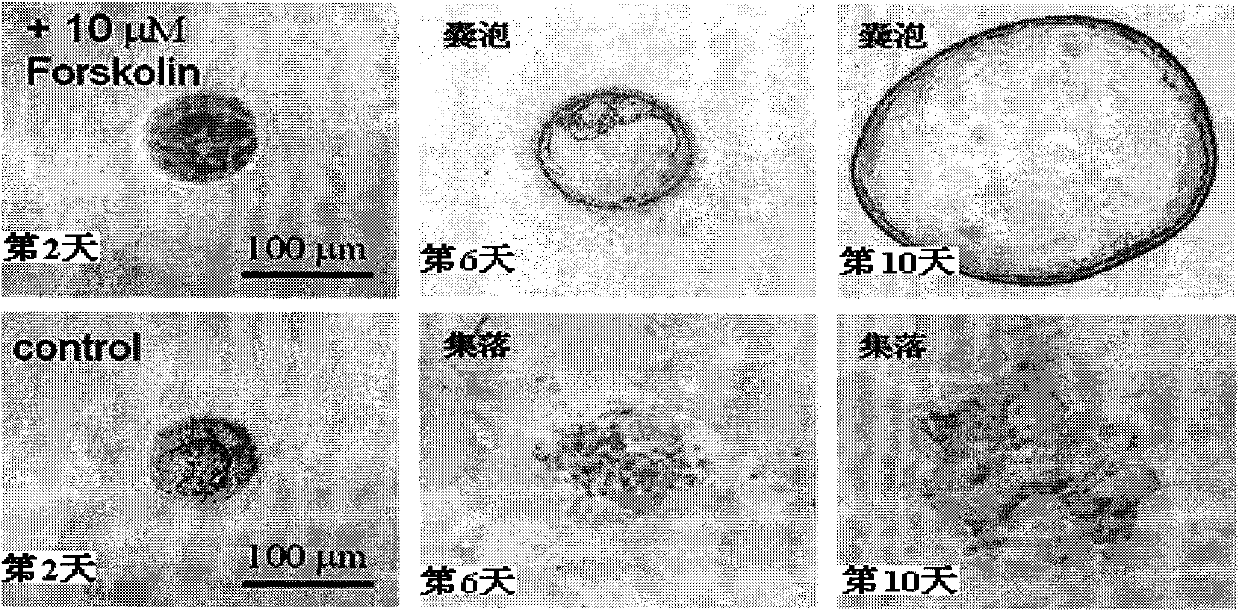
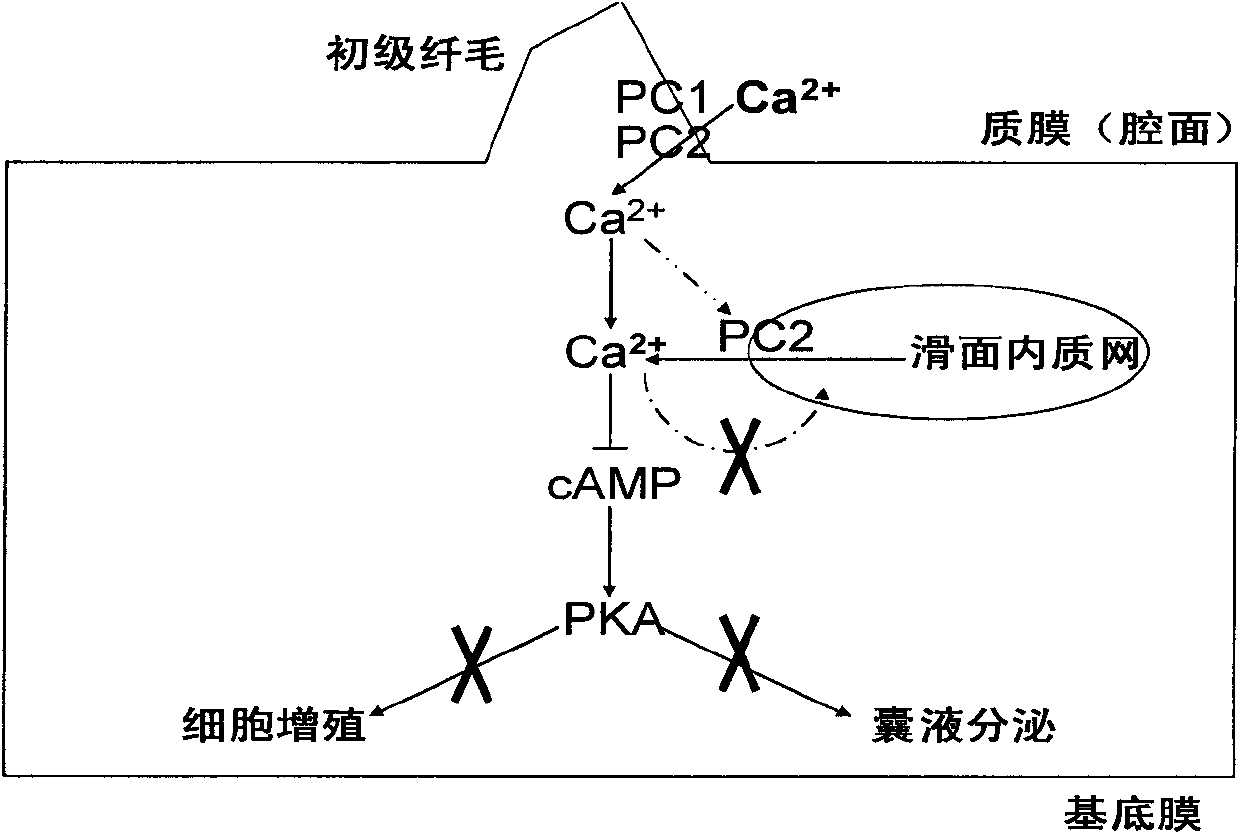
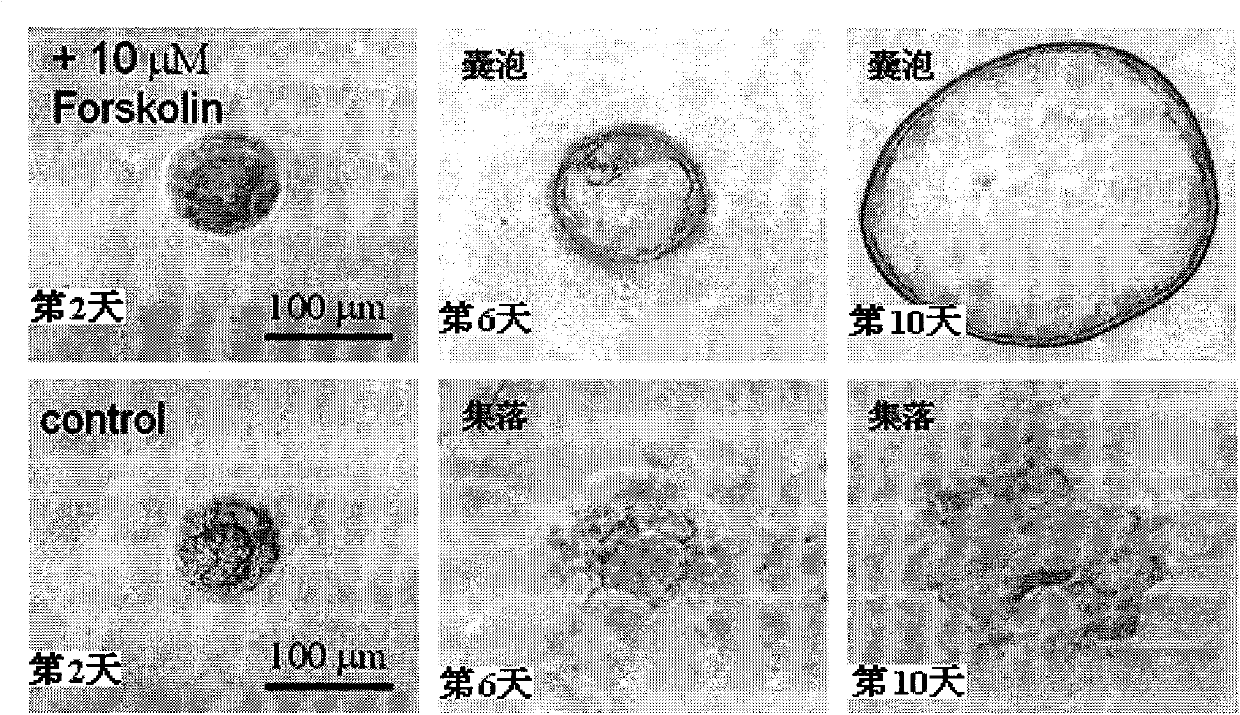
The invention discloses application of ginkgolide B to preparation of medicament for preventing and / or treating autosomal dominant polycystic kidney disease. A madin-darby canine kidney (MDCK) vesicle model is used for screening to find that the ginkgolide B inhibits formation and growth of vesicles. An experimental result shows that: the ginkgolide B has an obvious inhibiting effect on the formation and growth of MDCK vesicles and the effect of the ginkgolide B is in dose response relationship; the ginkgolide B has no cytotoxicity to MDCK cells, so that the vesicle inhibiting effect of the ginkgolide B is independent of the cytotoxicity; the ginkgolide B does not obviously induce MDCK cell apoptosis, so that the vesicle inhibiting effect of the ginkgolide B is independent of cell apoptosis promotion of the ginkgolide B; the ginkgolide B can promote the MDCK cells or vesicles to form tubular structures; and the effect is in dose response relationship; and the ginkgolide B has an inhibiting effect on the growth of the embryonic kidney vesicles. The ginkgolide B provides experimental data for development of a specific medicament for preventing and / or treating autosomal dominant polycystic kidney disease.
Owner:PEKING UNIV
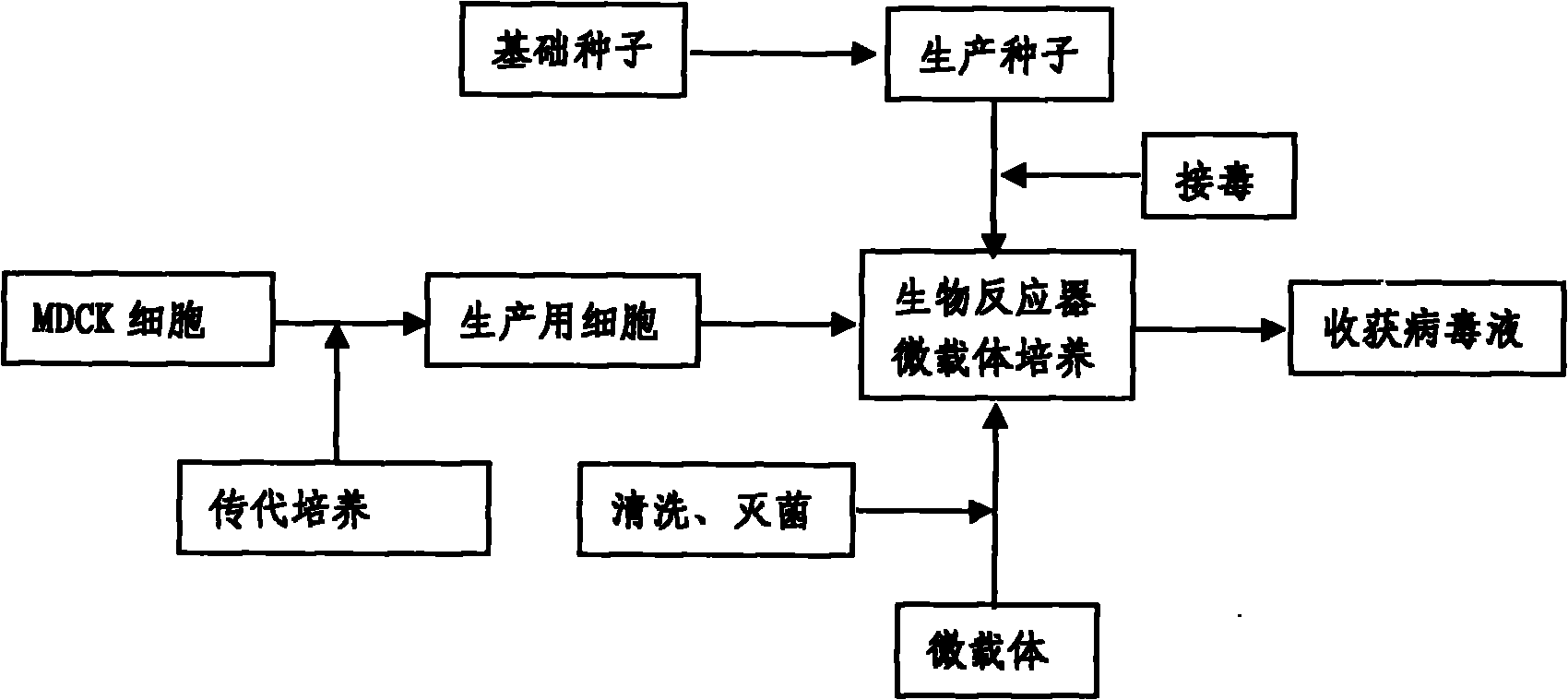
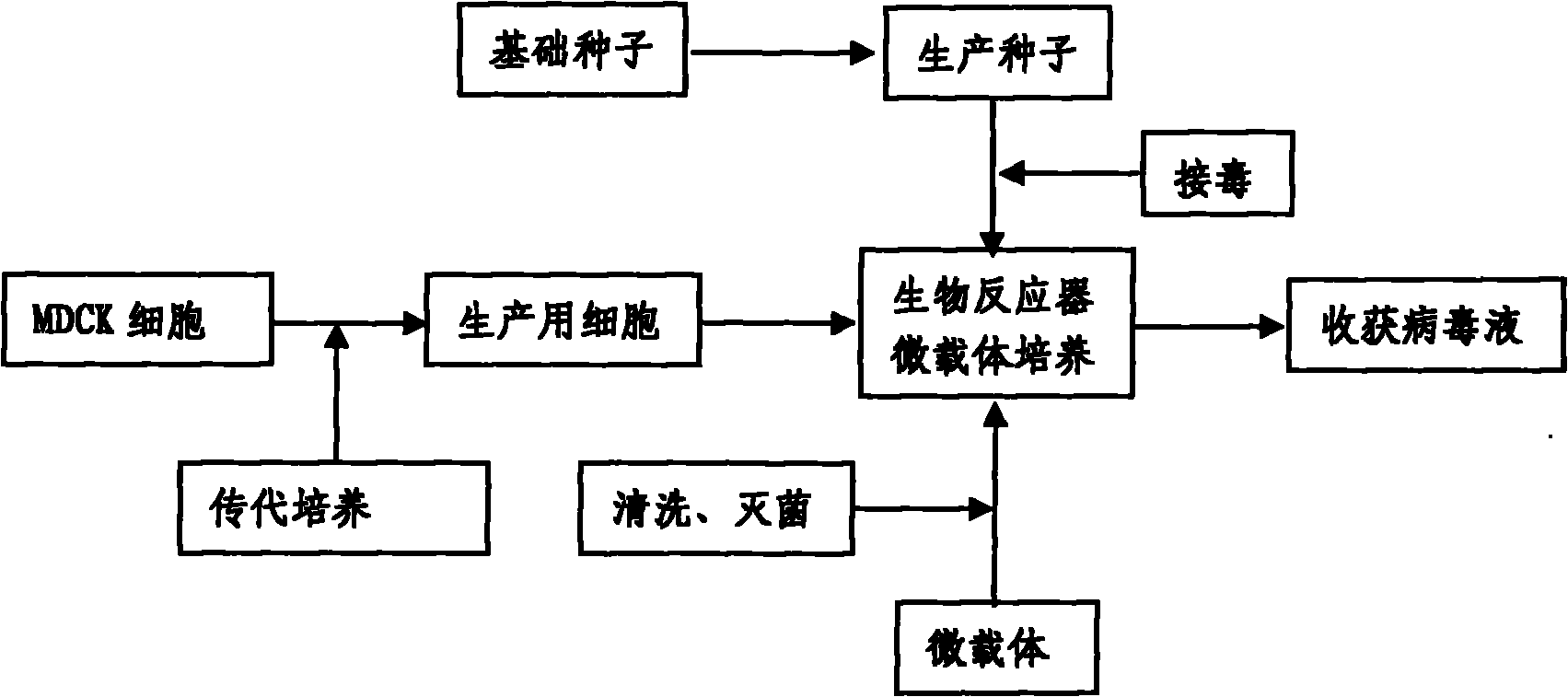
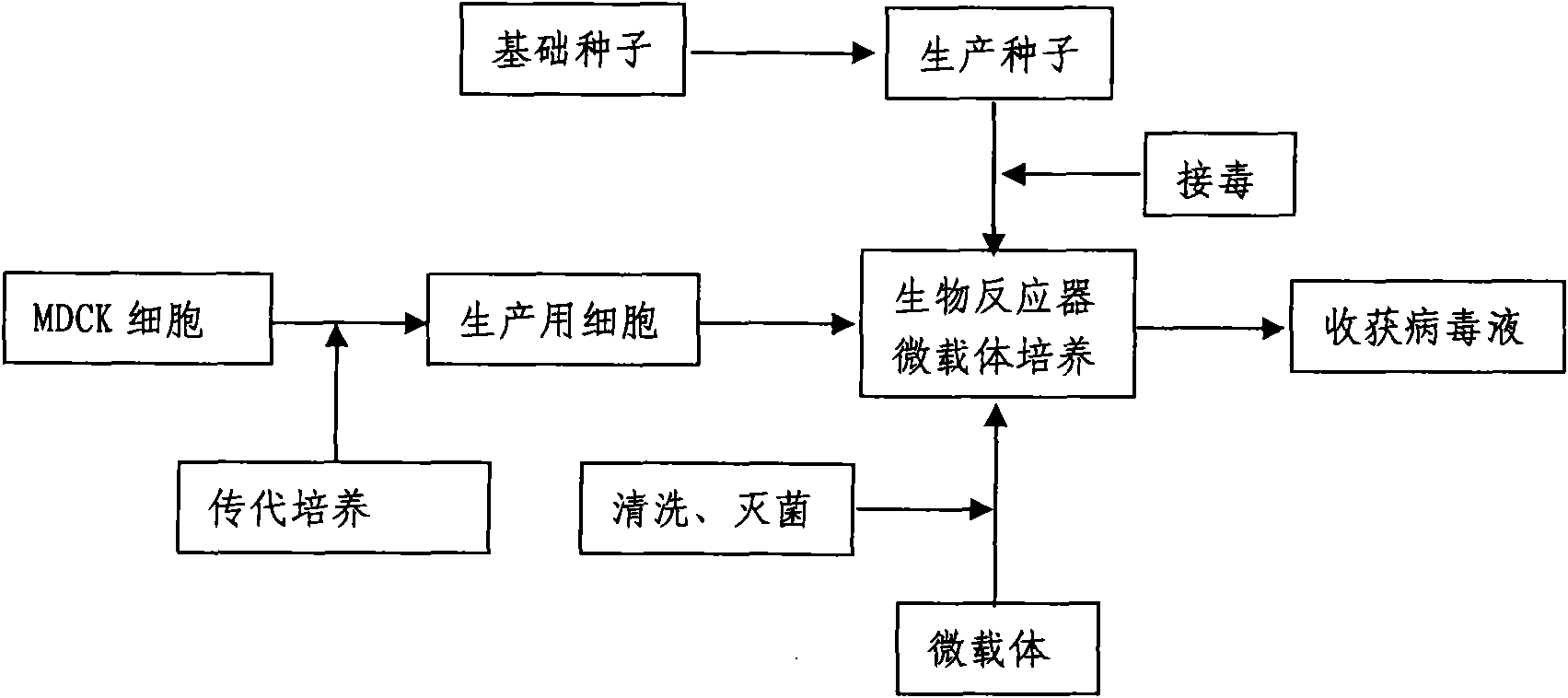
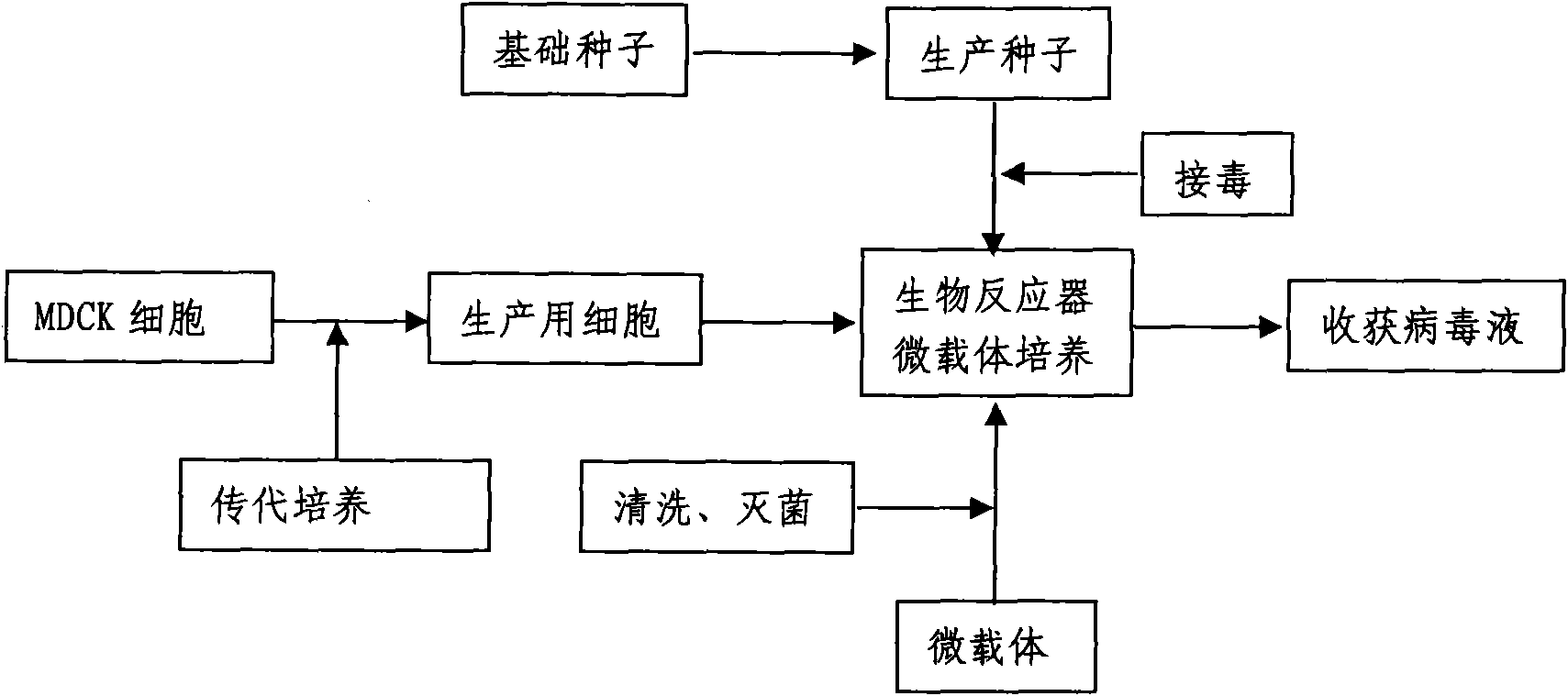
Method for industrially producing animal influenza vaccine by using bioreactor
InactiveCN102133398AImprove securityQuality improvementMicroorganism based processesAntiviralsAdjuvantInfluenza vaccine
The invention relates to a method for industrially producing animal influenza vaccine by using a bioreactor, comprising the following steps of: (1) adding Vero or MDCK cells in the sterilized bioreactor containing a micro-carrier and culturing in a serum-including culture medium; (2) replacing the culture medium including serum in the bioreactor by a serum-free culture medium which includes pancreatin; (3) inoculating animal influenza virus to culture continuously; (4) harvesting to obtain a cell-cultured influenza virus stock solution when the pathological change of the cells is up to 80%; and (5) hyper-filtering, concentrating and inactivating the influenza virus stock solution and then adding an adjuvant to obtain the influenza inactivated vaccine. The method provided by the invention has the advantages of controllable process parameters, high cell density, high virus titer, high production efficiency, low labor intensity and the like. The influenza vaccine produced by the invention has good security, stable and reliable quality, small side reaction and high immune effect and has a better immune protection function for the attack of the influenza virus.
Owner:WUHAN CHOPPER BIOLOGY
Method for preparing MDCK (Madin-Darby canine kidney) cell line adaptive to serum-free full-suspension culture and MDCK cell line
ActiveCN105861422AShort cycleLess generationsCulture processMicroorganism based processesCanine kidneySerum free
The invention discloses a method for preparing an MDCK (Madin-Darby canine kidney) cell line adaptive to serum-free full-suspension culture and the MDCK cell line. The method includes steps of 1), discarding culture media for cultivating MDCK cells, cleaning cell layers by the aid of pancreatin solution, then discarding the pancreatin solution, adding pancreatin solution into the MDCK cells again, digesting the MDCK cells, stopping digesting the MDCK cells after the cells are rounded, centrifuging cell suspension and then discarding supernatant to obtain cell clusters; 2), re-suspending the cell clusters obtained at the step 1) by the aid of serum-free culture media to obtain cell re-suspension; 3), arranging the cell re-suspension obtained at the step 2) in a shaking table, cultivating the cell re-suspension in culture tanks, diluting the cell re-suspension by the aid of the serum-free culture media and carrying out passage on the cell re-suspension to obtain the MDCK cell line. The method and the MDCK cell line have the advantages that the MDCK cell line is high in cell density and activity and uniform in size, individually grows in a dispersion manner, is plump in form and is suitable for serum-free suspension culture by the aid of bioreactors.
Owner:ZHAOQING DAHUANONG BIOLOGIC PHARMA +2
Serum-free culture medium used for full-suspension culture of MDCK (Madin Darby Canine Kidney) cells
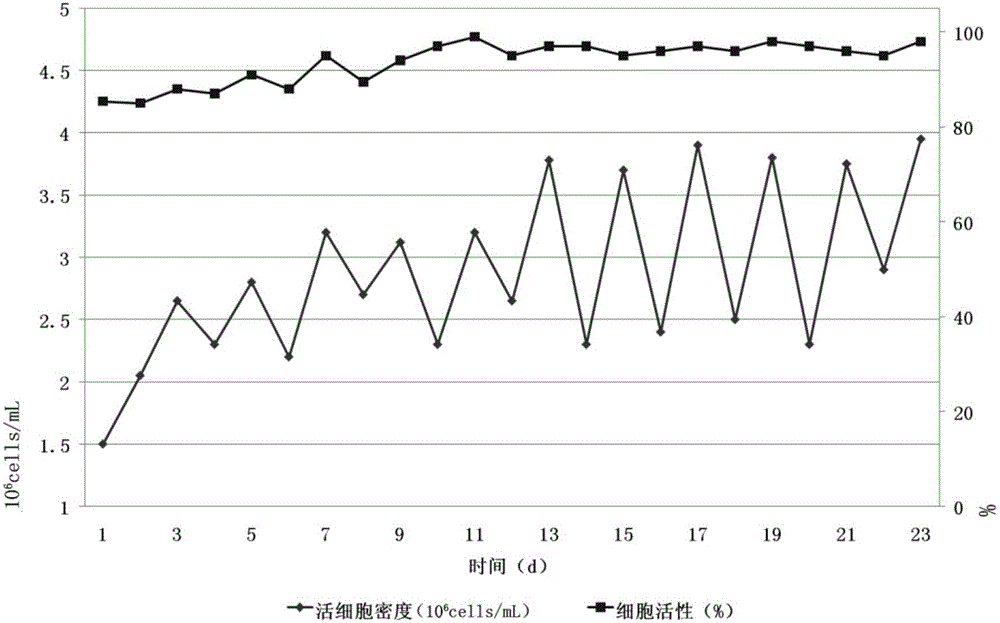
The invention provides a serum-free culture medium used for full-suspension culture of MDCK (Madin Darby Canine Kidney) cells. The culture medium is prepared from a DMEM / F12 culture medium containing 5g / L of NaCl and the following components: sodium stannite, recombinant human epidermal growth factors, hydrocortisone, recombinant full-chain insulin, prostaglandin E1, human transferrin, thyroxine (T3), vitamin E, cholesterol, ethanol amine, beta-mercaptoethanol, Tween-80, Hypep1510, recombinant human serum albumin ACF and mannitol. With the adoption of the culture medium provided by the invention, the full-suspension culture of the MDCK cells can be carried out very well under the condition that serum is not added; after the MDCK cells are continuously cultured for 20 generations, the average proliferation concentration of the MDCK cells is 2.308*10<6> / mL, the average cell viability is 97.4 percent and the average doubling time is 34.48 hours, so that the serum-free culture medium provides technical supports for developing influenza vaccines of cell matrixes of mammals in China.
Owner:马忠仁 +2
Method for propagating influenza virus
InactiveUS20090181446A1Increase productionReduce the amount of solutionSsRNA viruses negative-senseArtificial cell constructsTrypsin inhibitorCanis lupus familiaris
A method for producing influenza virus on large scale is provided. A method for propagating influenza virus which comprises, after removing or decreasing a trypsin inhibitor secreted into culture of MDCK cells (cell line derived from dog kidney) by washing with a culture medium or a buffer, inoculating influenza virus into said cells and culturing said influenza virus-inoculated cells in a culture medium supplemented with trypsin.
Owner:JURIDICAL FOUND THE CHEMO SERO THERAPEUTIC RES INST
Production method for influenza virus vaccines
InactiveCN101818131ASolve yourselfSolve pollutionAntiviralsViruses/bacteriophagesEquine influenza vaccineBioreactor
The invention discloses a production method for vaccines of avian influenza virus and other influenza virus such as swine influenza, dog influenza and equine influenza, which comprises the following steps of: (1) transfer of culture and cultivation of vaccine-made cells; (2) reproduction of vaccine-made virus seeds; (3) microcarrier suspension culture of MDCK cells in a bioreactor; (4) reproduction of vaccine-made virus liquid; and (5) harvest of the virus liquid. The production method has the advantages of reducing the production cost greatly and improving the yield and quality of the vaccines obviously, along with short production period, no restriction to raw material supply, small occupied area, easy and quick expansion of production scale, light environmental pollution, easy processing, high automaticity, low employment and easy realization of balanced and stable quality.
Owner:成都史纪生物制药有限公司
High titer recombinant influenza viruses with enhanced replication in mdck or vero cells or eggs
ActiveUS20170354730A1Improve scalabilitySignificantly higher viral titersSsRNA viruses negative-senseAntiviralsDiseaseEgg cell
The invention provides a composition useful to prepare high titer influenza viruses, e.g., in the absence of helper virus, which includes internal genes from an influenza virus vaccine strain or isolate, e.g., one that is safe in humans, for instance, one that does not result in significant disease, that confer enhanced growth in cells in culture, such as MDCK cells, or in eggs.
Owner:WISCONSIN ALUMNI RES FOUND
Establishment of MDCK cell line stably expressing beta-galactoside alpha-2, 3-sialytransferase I(ST3GAL I)
The invention provides an MDCK-ST3GAL I cell line, concretely an MDCK cell line stably expressing beta-galactoside alpha-2, 3-sialytransferase I(ST3GAL I) with the preservation number of CGMCC No. 2620; the invention further provides a method for establishing the MDCK-ST3GAL I cell line and a method for improving the growth titre of avian influenza virus and the forming capability of plaque. The invention further provides an application of the MDCK-ST3GAL I cell line in researching avian influenza virus 2, 3 linked receptor tendency; and the invention is used for applications in monitoring the avian influenza virus receptor associative property variant strain, screening medicines resisting avian influenza virus, measuring the neutralizing antibody and mass producing cell culture vaccine.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Mdck cells lines supporting viral growth to high titers and bioreactor process using the same
The present invention relates to novel MDCK cells which can be to grow viruses, e.g., influenza viruses, in cell culture to higher titer than previously possible. The MDCK cells can be adapted to serum-free culture medium. The present invention further relates to cell culture compositions comprising the MDCK cells and cultivation methods for growing the MDCK cells. The present invention further relates to methods for producing influenza viruses in cell culture using the MDCK cells of the invention.
Owner:MEDIMMUNE LLC
Serum-free whole suspension MDCK cell strain and application thereof to production of influenza viruses
ActiveCN107460156AAchieve serum-free cultureOvercoming anoikisSsRNA viruses negative-senseCulture processSerum freeInfluenza virus vaccine
The invention discloses a serum-free whole suspension cultured MDCK cell strain domesticated by a one-step adaptation method and application thereof to preparation of influenza virus vaccines, and belongs to the technical field of veterinary biology. Adherent cells are domesticated into a single dispersed whole suspension MDCK cell line named MDCK-S within two months by the one-step adaptation method, and the strain preservation number is CGMCC No. 12256. The invention further provides a method of culturing influenza viruses using the cell line. The method for producing the H1N1 and H3N2 subtype swine influenza viruses through MDCK cell suspension culture can replace traditional chicken embryo production to significantly reduce the production cost and improve downstream purification efficiency, and can rapidly and steadily increase the production scale.
Owner:兆丰华生物科技(南京)有限公司北京生物医药科技中心 +2
Influenza virus replication for vaccine development
ActiveUS20170067029A1Increase productionImprove scalabilitySsRNA viruses negative-senseVirus peptidesInfluenza virus vaccineViral Vaccine
The invention provides a composition useful to prepare high titer influenza viruses, e.g., in the absence of helper virus, which includes internal genes from an influenza virus vaccine strain or isolate, e.g., one that is safe in humans, for instance, one that does not result in significant disease, that confer enhanced growth in cells in culture, such as MDCK cells, or in eggs.
Owner:WISCONSIN ALUMNI RES FOUND
Method for producing influenza virus vaccine
ActiveCN101955915ASolve pollutionGuaranteed to be pureAntiviralsViruses/bacteriophagesCanine kidneyEquine influenza vaccine
The invention discloses a method for producing a vaccine for avian influenza viruses and other influenza viruses such as a swine influenza virus, a canine influenza virus and an equine influenza virus. The method comprises the following steps of: subculturing cells for preparing the vaccine; (2) reproducing cytotoxic varieties; (3) performing microcarrier suspension culture on darby canine kidney (MDCK) cells in a bioreactor; (4) reproducing venom for preparing the vaccine; and (5) harvesting virus liquid. The method has the advantages of great reduction in production cost, short production period, no restriction to raw material supply, small occupied area, easy and quick expansion in production scale, low and readily treated environmental pollution, high degree of automation, few labors, easy equilibrium and stabilization in quality and obvious improvement on the yield and the quality of the vaccines.
Owner:成都史纪生物制药有限公司
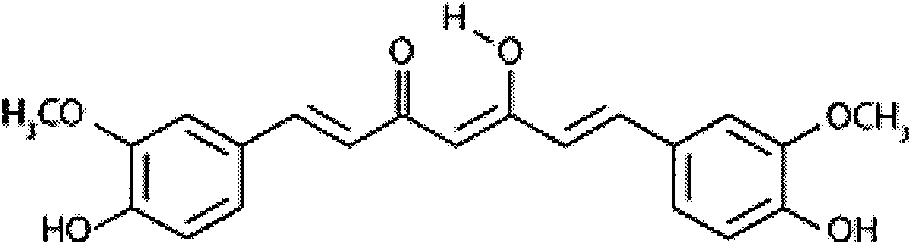
New application of curcumin
InactiveCN102018689ADoes not induce apoptosisPromote apoptosisKetone active ingredientsUrinary disorderCanine kidneyCytotoxicity
The invention discloses application of curcumin to preparation of medicaments for preventing and / or treating autosomal dominant polycystic kidney disease. A madin-darby canine kidney (MDCK) vesicle model is used for screening to obtain the curcumin which inhibits formation and growth of vesicles. An experimental result shows that: the curcumin has an obvious inhibiting effect on the formation and growth of MDCK vesicles and the effect of the curcumin is in dose response relationship; the curcumin has no cytotoxicity to MDCK cells, so that the vesicle inhibiting effect of the curcumin is independent of the cytotoxicity; the curcumin does not obviously induce MDCK cell apoptosis, so that the vesicle inhibiting effect of the curcumin is independent of cell apoptosis promotion of the curcumin; the curcumin can promote the MDCK cells or vesicles to form tubular structures; and the effect is in dose response relationship; and the curcumin has an inhibiting effect on the growth of the embryonic kidney vesicles. The curcumin is expected to be developed into a specific medicament for preventing and / or treating autosomal dominant polycystic kidney disease.
Owner:PEKING UNIV
Canine distemper virus (CDV) sensitive cell line and establishment method and application thereof
InactiveCN102807970AMicrobiological testing/measurementMicroorganism based processesMadin Darby canine kidney cellCanine distemper virus CDV
The invention discloses a canine distemper virus (CDV) sensitive cell line and an establishment method and application thereof, and belongs to the technical field of biology. According to the hundestaupe virus sensitive cell line, lentivirus four-plasmid packaging system is adopted to obtain a strain of stable cell line madin-darby canine kidney-cell isolate (MDCK-CSL) which expresses canine source signal lymphocyte activating molecules (SLAM), wherein the conservation number of strains is CGMCC No. 5881. The comparison of the propagation conditions of a CDV standard strain on the MDCK-CSL and MDCK cells indicates that after the CDV-Snyder Hill standard strain infects the MDCK-CSL cell line for 24 hours, cytopathy of the fusion, rounding contraction, obvious death and the like of cells can be observed; and the MDCK cells infected for 6 days continuously grow slowly, and the cytopathy does not occur. The CDV sensitive cell line MDCK-CSL provides a technical platform for the separation of CDV wild strains and the complete study of biological properties of the CDV wild strains, and also establishes a foundation for the prevention and control of canine distemper.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Method for cultivating recombinant avian influenza subtype virus through full-suspension cell
InactiveCN108220221AStable toxicityImprove performance and stabilitySsRNA viruses negative-senseArtificial cell constructsInfluenza virus vaccineAvian influenza virus
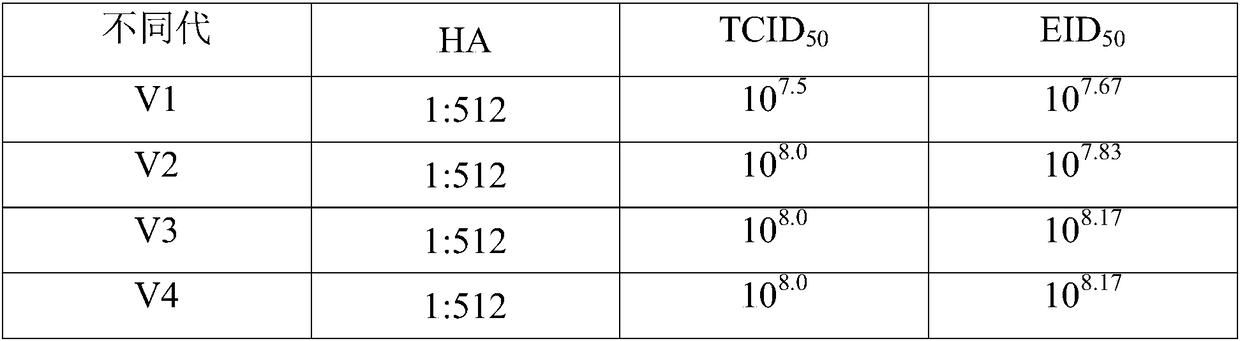
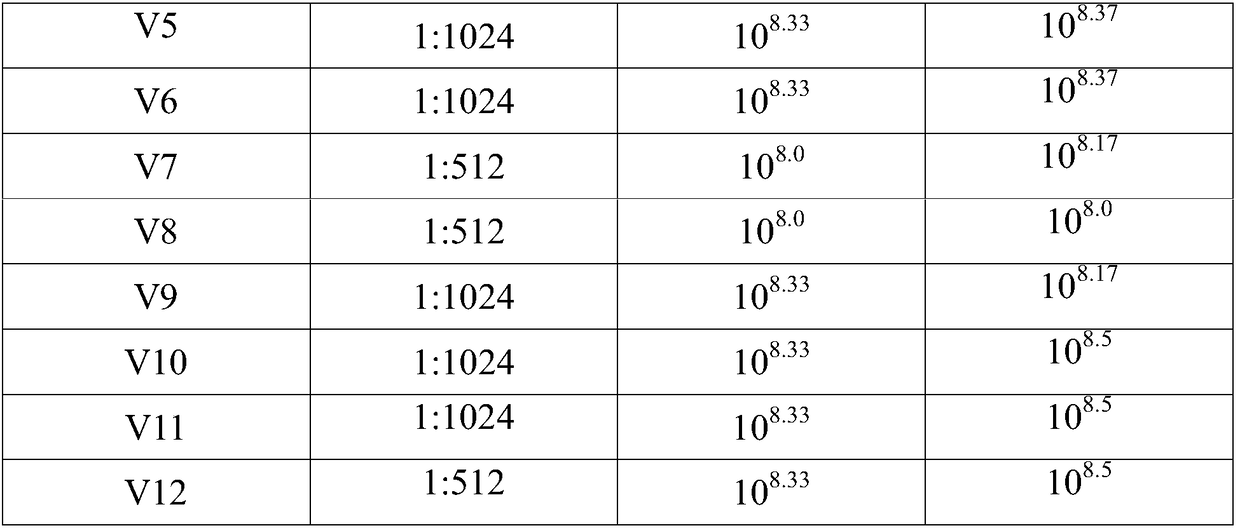
The invention relates to the preparation field of the avian influenza virus vaccine, and especially relates to a method for cultivating recombinant avian influenza subtype virus through full-suspension cell. The method comprises the following steps: inoculating the recombinant avian influenza subtype virus chick embryo virus into MDCK monolayer cell to acclimate and culture, inoculating the harvested culture, repeating the cultivating until the proliferation speed of the recombinant avian influenza subtype virus is stable, wherein the virus content is larger than or equal to 10<7.5>EID[50]; and then inoculating the suspension cell; acquiring virus liquid while cultivating until the cytopathy achieves 75% or above, namely obtaining the recombinant avian influenza subtype virus. The chick embryo virus is firstly inoculated into the MDCK monolayer cell to acclimate and cultivate, and then is inoculated to the suspension MDCK cell, thereby effectively increasing the performance stability of the suspension virus obtained through the production, wherein the obtained suspension virus HA is larger than or equal to 1 to 1024, each 0.1ml virus content is larger than or equal to 10<8.0>EID[50], and each 1ml virus content is larger than or equal to 10<8.0>TCID[50].
Owner:吉林冠界生物技术有限公司 +1
Serum-free medium for full-suspension culture of MDCK cells (madin-darby canine kidney cells) and preparation method of serum-free medium
ActiveCN106119186ALow costClear ingredientsCulture processCell culture mediaMadin Darby canine kidney cellSerum free media
The invention discloses a serum-free medium for full-suspension culture of MDCK cells (madin-darby canine kidney cells) and a preparation method of the serum-free medium. The serum-free medium for full-suspension culture of the MDCK cells consists of a basic metabolism nutrient, nucleotide, vitamins, inorganic salt, a shear force protective agent, a cell clustering resisting agent, a pH buffer agent, a pH indicator, an influenza virus proliferation accelerant and other additives; and the preparation method of the serum-free medium for full-suspension culture of the MDCK cells comprises the following steps: 1) preparing mixed liquid: dissolving and mixing the raw materials; and 2) regulating pH: regulating pH of the mixed liquid to 6.3-6.7, and setting a constant volume, so that the serum-free medium for full-suspension culture of the MDCK cells is obtained. The medium supports the high-density full-suspension culture of the MDCK single cells and is capable of greatly shortening a time of domesticating the MDCK cells from adherent cells into the serum-free full-suspension cells; and the medium is applicable to the large-scale production of biological products, in particular veterinary biological products.
Owner:ZHAOQING DAHUANONG BIOLOGIC PHARMA +2
Cryopreservation method for protecting cell junction
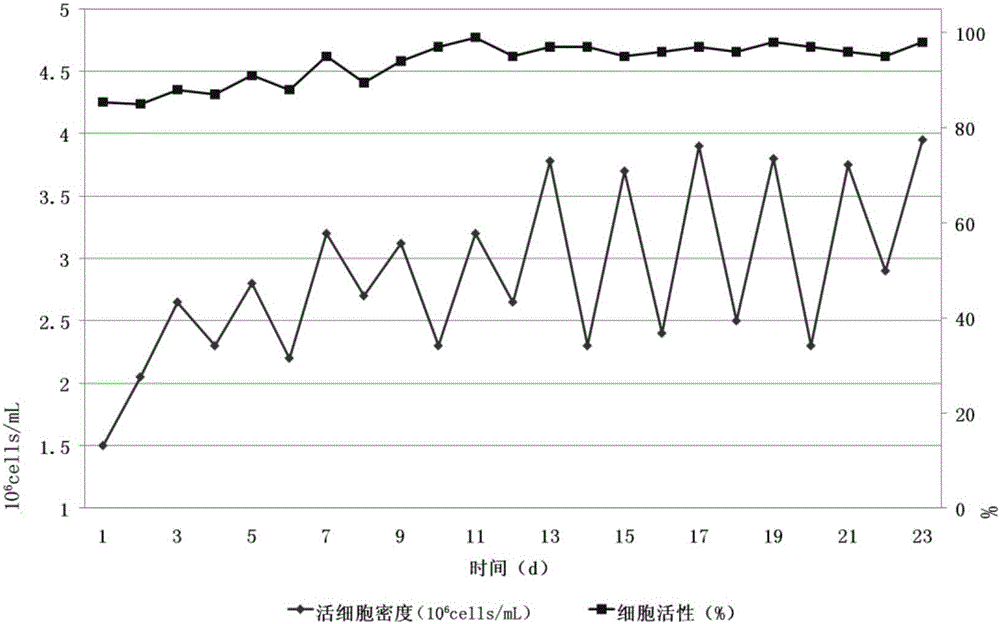
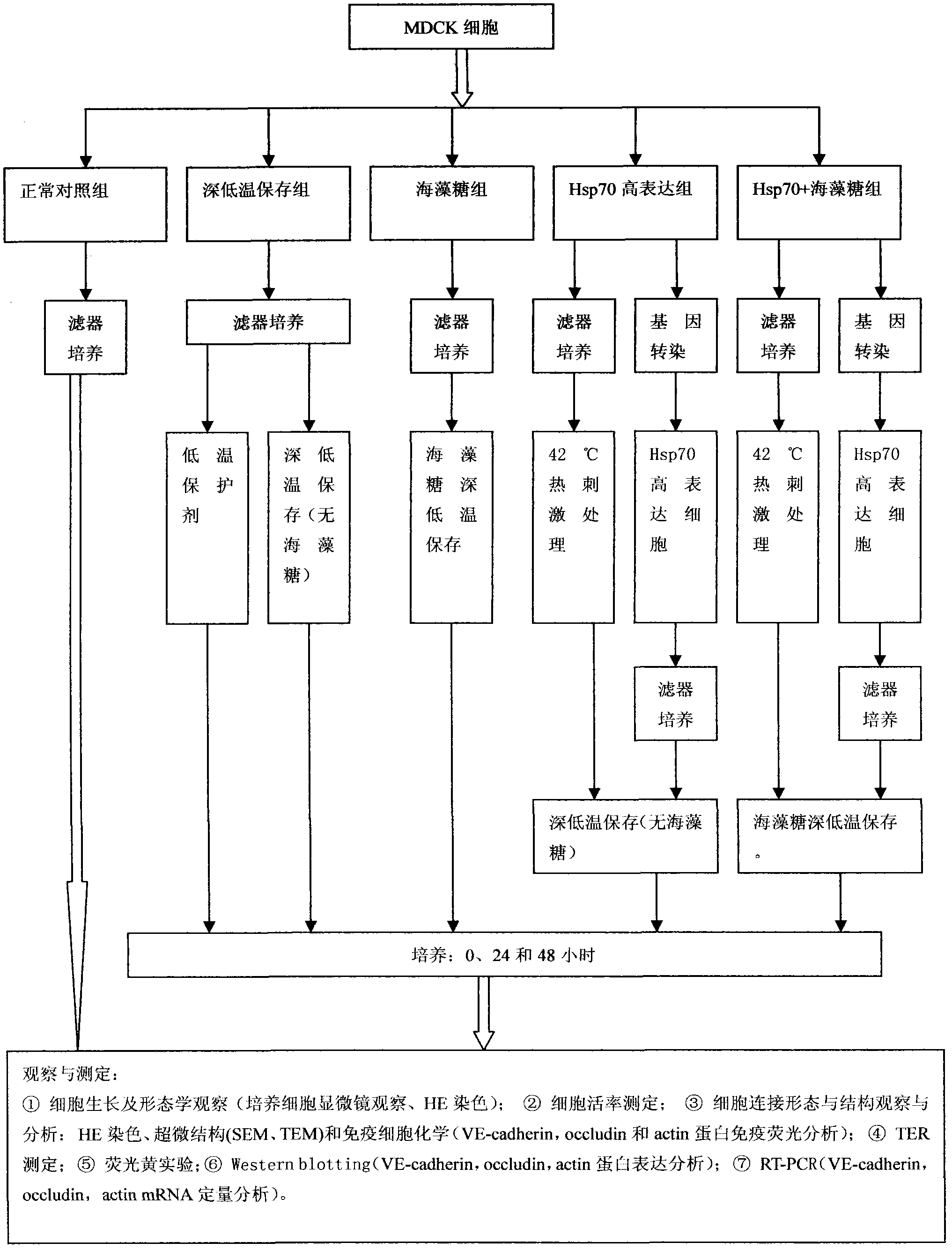
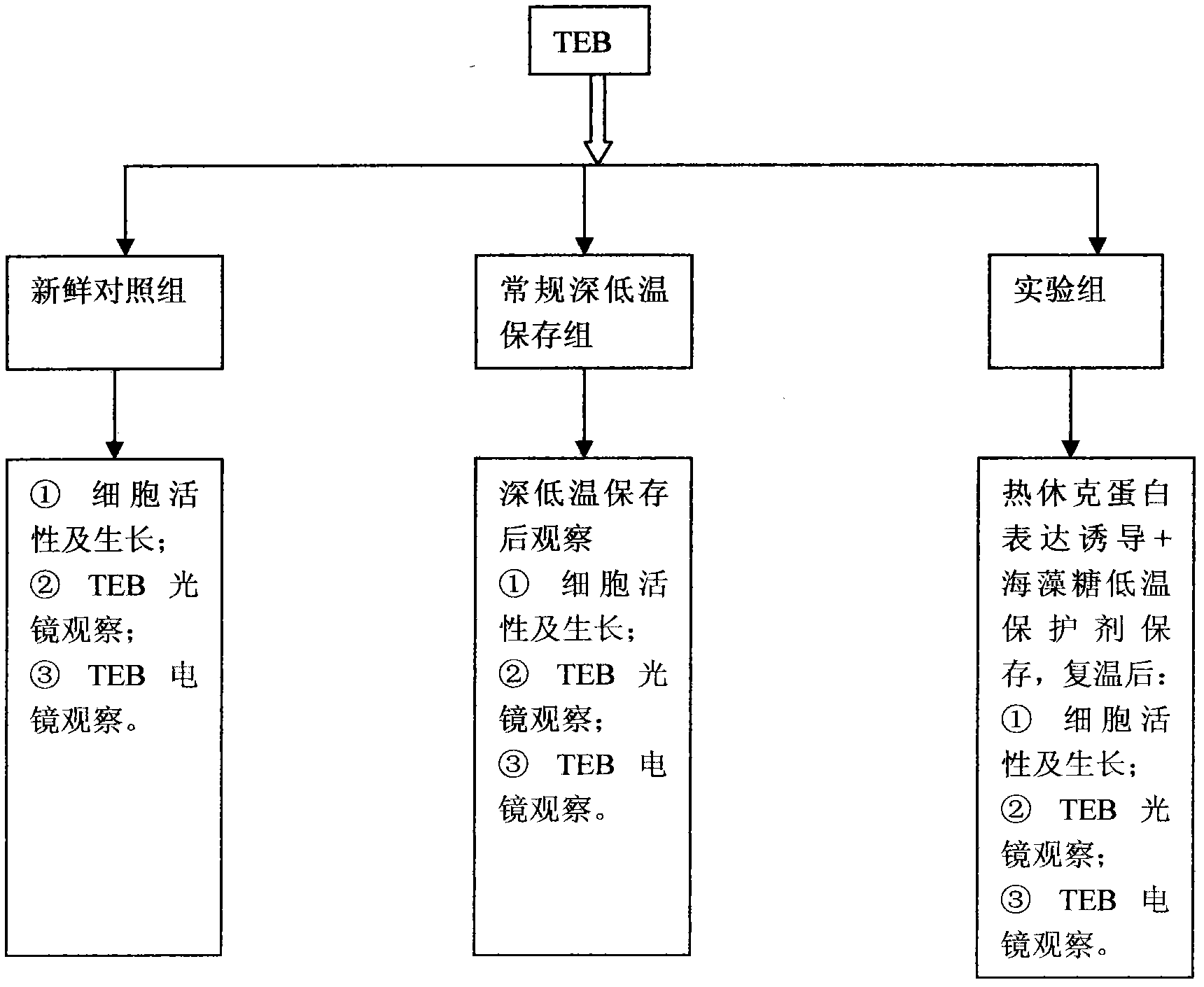
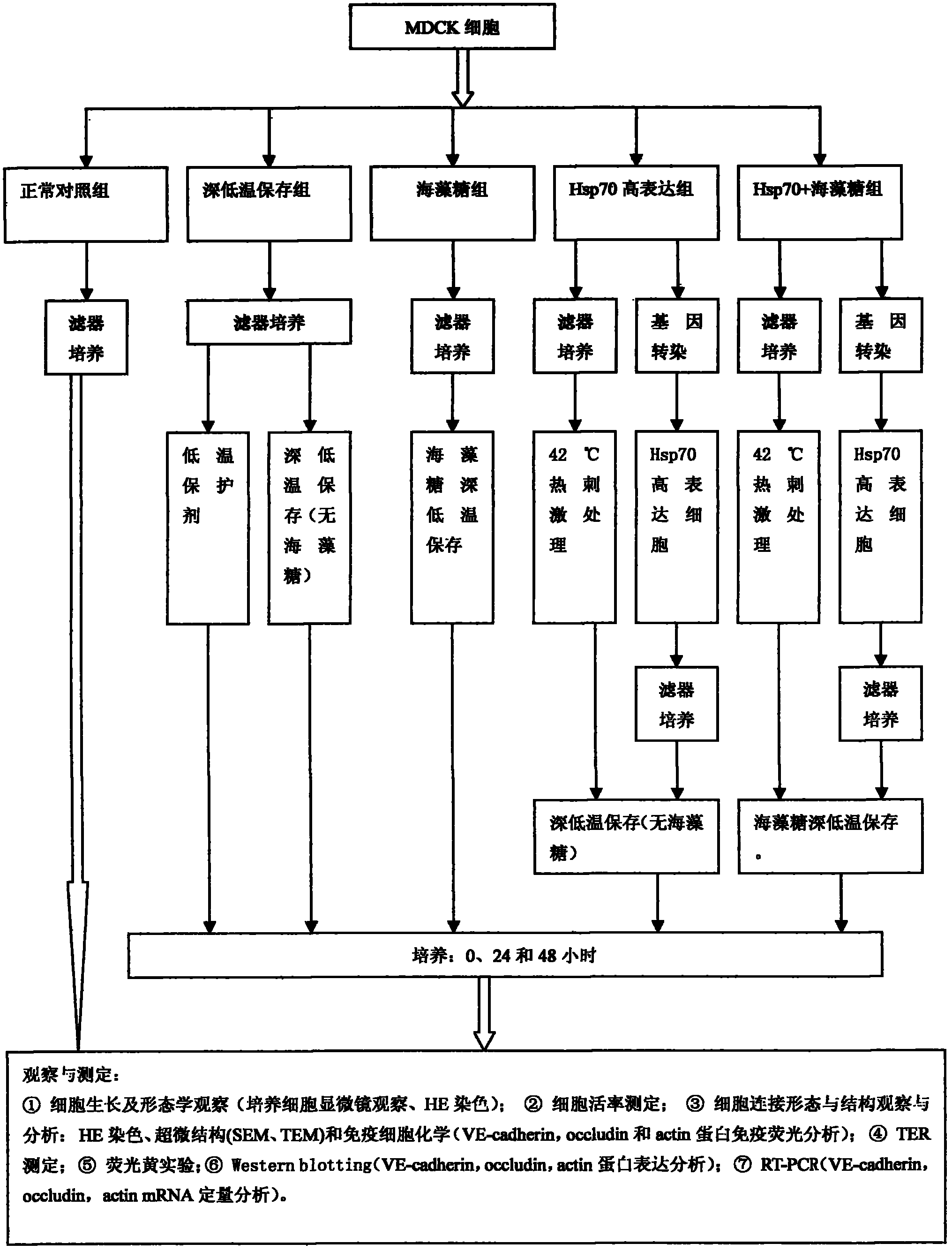
InactiveCN102487938AImprove toleranceFree from damageDead animal preservationCanine kidneyCultured cell
The invention discloses a cryopreservation method for protecting cell junction. The method comprises the following steps of: a) culturing Madin Darby canine kidney (MDCK) cells; b) dividing the MDCK cells in the step a) into a normal control group, a cryopreservation group, a trehalose group, an Hsp70 high expression group and an Hsp70+ trehalose group; c) performing filter culture on the normal control group, the cryopreservation group and the trehalose group respectively, performing filter culture and gene transfection on the Hsp70 high expression group, and performing filter culture and gene transfection on the Hsp70+ trehalose group; d) performing cryopreservation on the normal control group, the cryopreservation group, the trehalose group, the Hsp70 high expression group and the Hsp70+ trehalose group; e) growing cells and performing morphologic observation (the cultured cells are observed by a microscope and are subjected to HE staining); f) testing the survival rate of the cells; g) observing and analyzing the cell junction morphology and structure; and h) performing a fluorescein experiment. The method is simple, and convenient to use; and the structure and function of the cell junction after tissues and organs are subjected to cryopreservation can be kept complete, and the damage of the cell junction can be effectively avoided.
Owner:HOSPITAL ATTACHED TO QINGDAO UNIV
Urea transporter inhibitors, and preparation method and application thereof
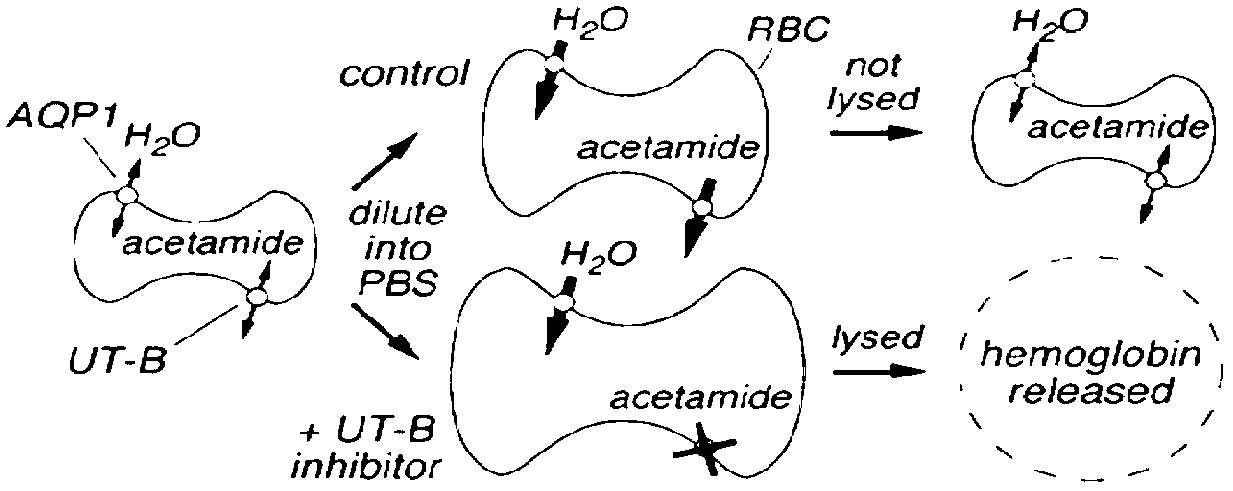
InactiveCN102757447AGood selective diuretic effectIncrease urine outputOrganic chemistryBlood disorderErythrocyte membraneCanine kidney
The invention discloses a series of urea transporter inhibitors of which the structural formula is disclosed in the specification. An erythrocyte model is used for screening to obtain compounds for inhibiting urea transporters. The experimental result indicates that the compounds (such as Youti) can inhibit the permeation of urea transporter UT-B mediated erythrocyte membranes for urea, and the action forms a dosage dependency relationship; Youti within effective dose range has no cytotoxic action on the MDCK (Madin-Darby Canine Kidney) cells, which indicates that the action of Youti for inhibiting cell permeable urea is irrelevant to cytotoxicity; the inhibiting action of Youti on the urea transporter UT-B gradually increases; the inhibiting action of Youti on the urea transporter UT-B is reversible; and the in-vivo test result proves that Youti can obviously increase the uresis amount of a rat, lower the concentration of urea in the urine of the rat, and lower the osmotic pressure, which indicates that Youti has selective diuresis action on urea in vivo.
Owner:PEKING UNIV
Stable cell line MDCK for amplifying recombinant canine adenovirus (CAV2) and construction method of stable cell line
InactiveCN108342362ASolve problems that are difficult to expressImprove stabilityGenetically modified cellsVirus peptidesLentivirusTiter
The invention provides a stable cell line for expressing canine adenovirus genes E1A and E1B and a construction method and application of the stable cell line. The two genes E1A and E1B in an E1 region are constructed in a lentivirus carrier, then an MDCK cell stably expresses the genes E1A and E1B respectively, and the MDCK-E1A-E1B stable cell line is constructed. The E1 gene which is long in segment and not easy to construct is split into the genes E1A and E1B which are short in segment and easy to construct, so that the success rate of constructing stable cells is increased; the genes E1A and E1B carry fluorescent proteins with different colors so that the stability of the stable cells can be monitored in real time. Experiments prove that the MDCK-E1A-E1B stable cell line is higher in titer of the amplified canine adenovirus than that of an MDCK-E1 stable cell line, and a good foundation is laid for application of the canine adenovirus in the fields of virus live carrier vaccines, gene therapy, cancer treatment and the like.
Owner:BRAINVTA (WUHAN) CO LTD
Method for producing inactivated vaccine of H9N2 subtype of avian influenza virus and product of inactivated vaccine
InactiveCN104338127AHigh titerAvoid biosafety issuesAntiviralsAntibody medical ingredientsCell factoryCytotoxicity
The invention discloses a method for producing an inactivated vaccine of H9N2 subtype of avian influenza virus and a product of the inactivated vaccine, and belongs to the field of production and application of inactivated vaccines of H9N2 subtype of avian influenza virus. The method comprises the following steps: (1) culturing a MDCK cell by utilizing a cell factory; (2) inoculating H9N2 subtype of avian influenza virus, and proliferating virus in the cell factory, wherein the microbial preservation number of the H9N2 subtype of avian influenza virus is CGMCC No.9325; and (3) harvesting the virus, and preparing the inactivated vaccine. Immune protection tests prove that the protective rate of the H9N2 subtype of avian influenza virus produced by the method is 100 percent, and the antibody level of a cytotoxicity immunity group at each point in time is higher than that of a germ toxicity immunity group, and rises relatively high. Virus liquid produced by the method can be used for preparing medicines or reagents for preventing or treating H9N2 subtype of avian influenza virus.
Owner:JIANGSU ACAD OF AGRI SCI
Influenza virus replication for vaccine development
ActiveUS9890363B2Increase productionImprove scalabilitySsRNA viruses negative-senseVirus peptidesDiseaseEgg cell
The invention provides a composition useful to prepare high titer influenza viruses, e.g., in the absence of helper virus, which includes internal genes from an influenza virus vaccine strain or isolate, e.g., one that is safe in humans, for instance, one that does not result in significant disease, that confer enhanced growth in cells in culture, such as MDCK cells, or in eggs.
Owner:WISCONSIN ALUMNI RES FOUND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com