Serum-free whole suspension MDCK cell strain and application thereof to production of influenza viruses
An influenza virus, full-suspension technology, applied in the direction of viruses, animal cells, viruses/phages, etc., can solve the problems of reducing the immune effect of vaccines, greatly affecting the quality of viruses, and increasing the pressure of purification, so as to achieve cell density and reduce cultivation volume, to maximize the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1 Preparation of serum-free full suspension MDCK cell line
[0036] S1. Passage of adherent MDCK cells:
[0037] Take a T75 square flask to culture MDCK cells covered with a monolayer, digest the cells with 0.25% trypsin, blow and disperse the cells with DMEM / F12 (10% FBS) cell growth medium, add 20ml of cell growth medium, and place the MDCK cells in 37°C, 5% CO 2 Cultivate in a carbon dioxide incubator for 48-72h, and carry out amplified culture when a monolayer of cells is formed. The above cell growth medium is DMEM / F12 culture medium with 10% Jinyuankang fetal bovine serum.
[0038] S2. One-step adaptation method to adapt adherent cells to full suspension cells cultured without serum:
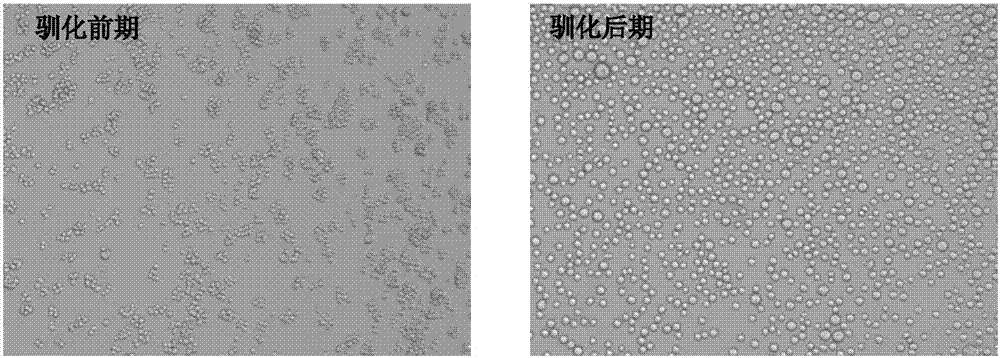
[0039] S21. Cell domestication process:
[0040] The MDCK cells expanded in step S1 were digested with 0.25% trypsin and inoculated into serum-free medium, placed on a shaker for shaking culture, and the initial inoculation density was 0.5×10 6 -2.0×10 6 Cells / ml, e...
Embodiment 2
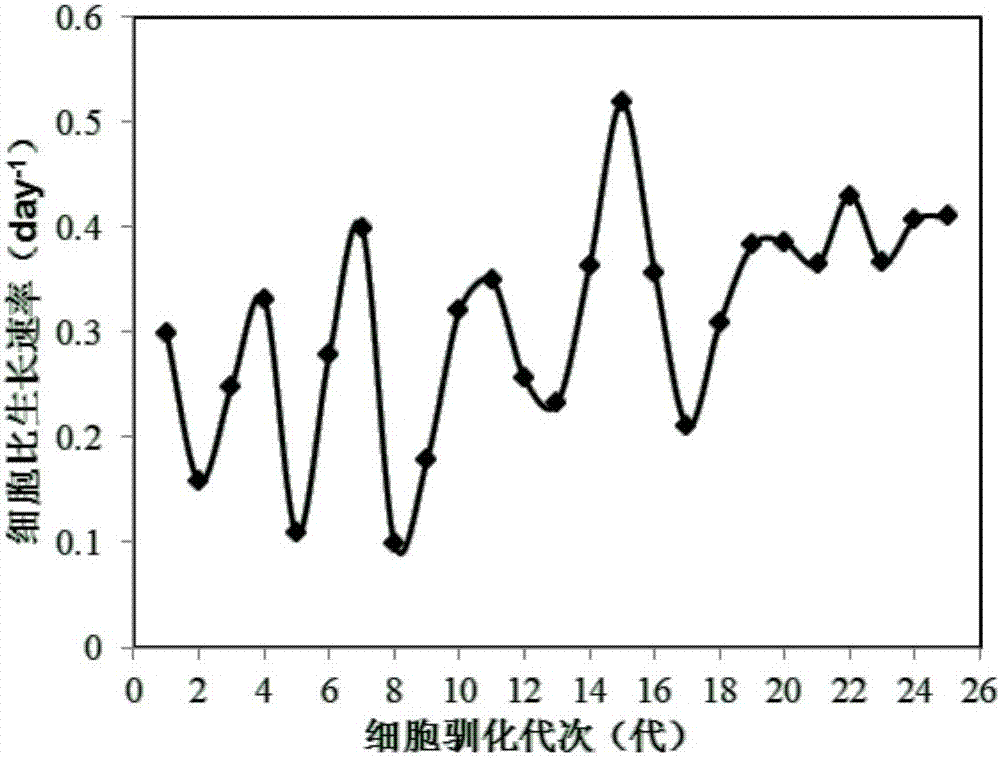
[0048] The clone screening of embodiment 2MDCK-S cell line
[0049] The MDCK-S cell line in Example 1 was screened for 3 rounds of clones by the limiting dilution method, and 3 cell lines with plump morphology, bright edges, faster growth rate and higher cell viability were obtained. In order to investigate the difference of these 3 clonal cell lines, the 3 lines of cells were divided into 0.5×10 6 Cells / ml were inoculated in 125ml shake flasks with a culture volume of 30 ml, placed at 37°C, 5% CO 2 Culture in a carbon dioxide incubator, take out 1ml of cell solution at 48h, count the cells after staining with trypan blue and calculate the cell viability. The cell density and viability of the three cell lines are shown in Table 1.
[0050] Table 1 Growth of three MDCK-S cell lines
[0051]
[0052] The MDCK-S3 cells with the fastest growth and highest activity were preserved on March 28, 2016 in the General Microbiology Center of the China Committee for the Collection of...
Embodiment 3
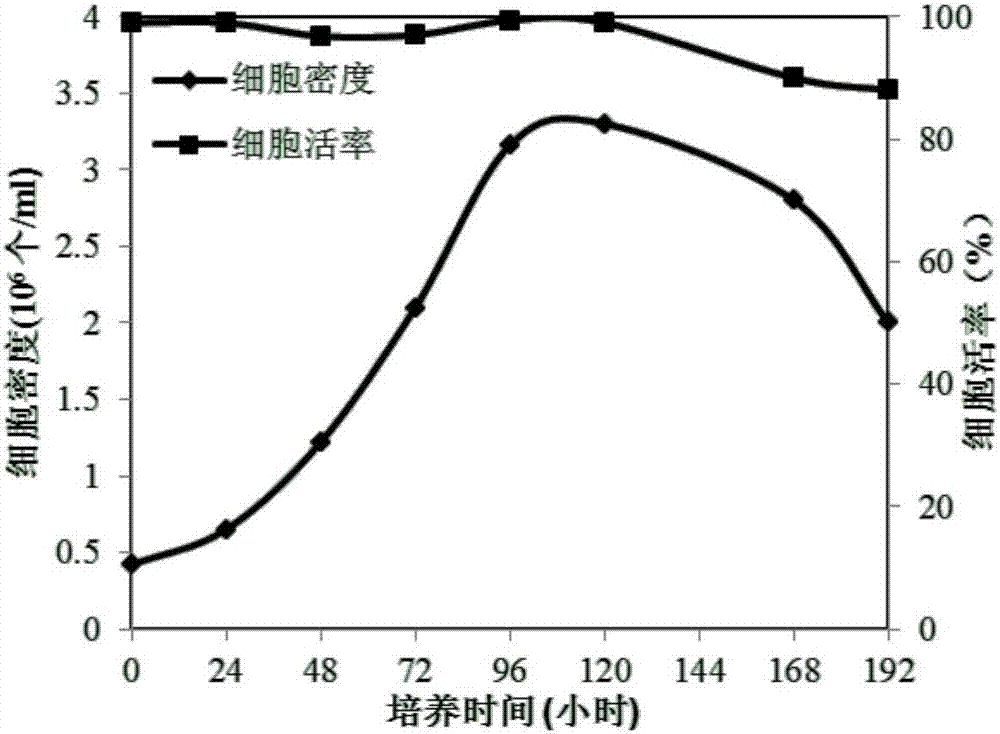
[0053] Example 3 Production of H1N1 Subtype Swine Influenza Virus by Suspension Culture MDCK-S Cells
[0055] Take out the frozen MDCK-S cells from the liquid nitrogen tank, put them in a 37°C water bath to thaw quickly, add an appropriate amount of medium to the thawed cells and centrifuge to remove dimethyl sulfoxide, and then resuspend the cells with fresh medium and cultured in shake flasks. The revived cells went through an adaptation period of 2-3 passages, and the experiment was carried out when the cell growth state was stable.
[0056] Propagation of S2.H1N1 subtype swine influenza virus cytotoxic species:
[0057] MDCK-S cells were divided into 0.3×10 6 -1×10 6 The cell density of each / ml was inoculated in serum-free medium for suspension culture, when the cell density reached 2×10 6 -3×10 6 Individuals / ml, inoculate the H1N1 subtype swine influenza virus produced by chicken embryos at a ratio of 0.1-0.01% (v / v), cultivate the harvest...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com