Mdck-derived cell lines adapted to serum-free culture and suspension culture and method for preparing vaccine virus using the cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
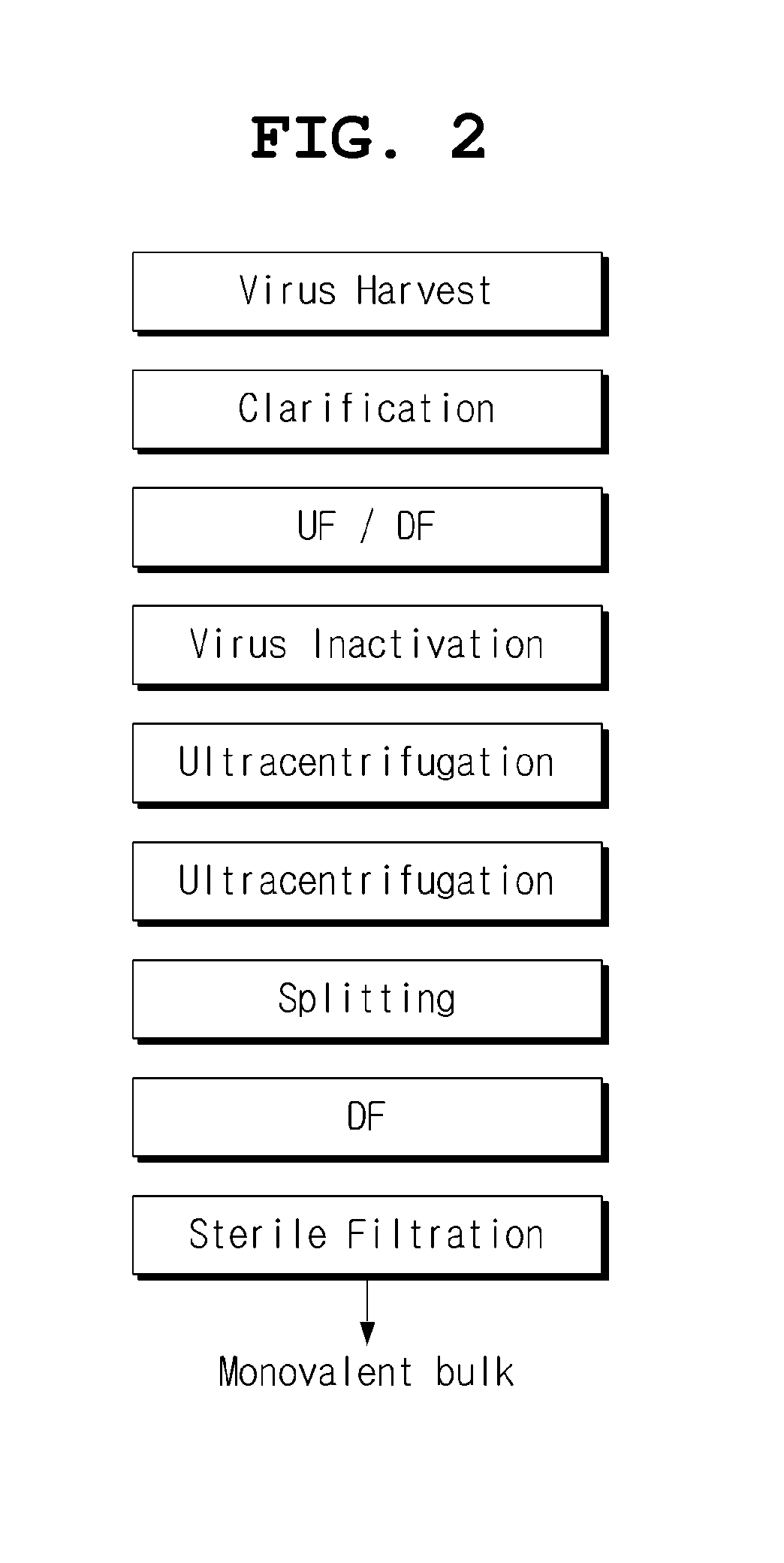
Preparation of MDCK-Derived Cell Lines by Serum-Free Culture and Suspension Culture
[0026]CCL-34, an MDCK cell line, was furnished from ATCC. CCL-34 was cultured in an EMEM medium supplemented with 10% serum in a T-25 flask at 37° C. and 5% CO2. After the cells were expanded, they were cultured in a medium consisting of the EMEM medium and a serum-free medium (50%). It was confirmed whether growth of the cells was normal or not during culture. When growth of the cells was confirmed to be normal, the grown cells were cultured in a medium containing a serum-free medium (75%). This procedure was repeated to obtain a cell line adapted to a serum-free medium (100%). EX-CELL MDCK (Sigma), UltraMDCK (Lonza) and VP-SFM (Invitrogen) may be used as the media for serum-free culture.
[0027]The cell line adapted to the serum-free medium was sufficiently expanded in a T-flask. Thereafter, the expanded cell line was adapted to suspension culture with stirring at a rate of 40-80 rpm in a spinner flas...
example 2
Evaluation of Proliferation Potentials of the Cell Lines
[0028]The MDCK-derived cell lines prepared by culture in the serum-free media were cultured under the conditions indicated in Table 1. The proliferation potentials of the MDCK-derived cell lines were evaluated. The MDCK cell line (ATCC CCL-34) as a control was grown in a medium supplemented with 10% serum.
TABLE 1MDCK-derived cell linesControl (ATCC CCL-34)CultureEX-CELL MDCK (Sigma),EMEM (Lonza)mediaUltraMDCK (Lonza),VP-SFM (Invitrogen)AdditivesL-Glutamine (Lonza) L-Glutamine (Lonza) 2% v / v,2% v / vFetal calf serum (Lonza) 10% v / vCulture37° C., 5% CO2, moist37° C., 5% CO2, moistconditionsCultureT-75 flask (15 ml)T-75 flask (15 ml)volume
[0029]The cell concentrations were about 1.0×105 cells / ml at the beginning of culture. When the cell concentrations reached about 1×106 cells / ml or 3-4 days after the culture, the cells were subcultured. The cell concentrations at the beginning of subculture were adjusted to 1×105 cells / ml.
[0030]Ea...
example 3
Evaluation of Proliferation Profiles and Subculture Stability of the Cell Lines
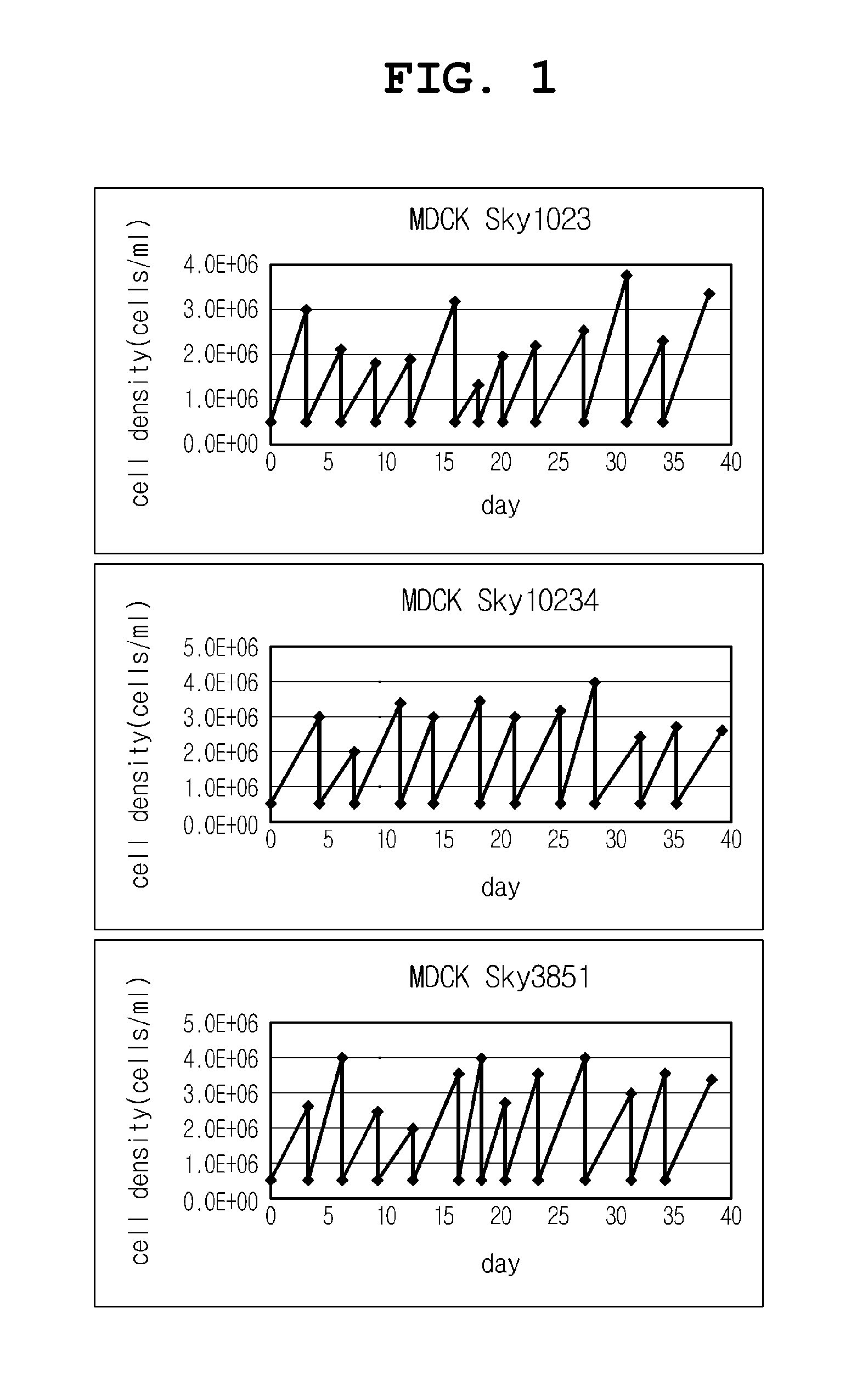
[0031]After the three kinds of cell lines adapted to serum-free culture and suspension culture were continuously cultured in respective spinner flasks, their proliferation profiles and subculture stability were evaluated. The cell concentrations at the beginning of culture were adjusted to about 4×105 cells / ml. About 3-4 days after the culture, the cell concentrations reached about 2×106 cells / ml or more. The culture was conducted under the following conditions. The results are shown in FIG. 1.
[0032]Initial cell concentration: 4×105 cells / ml
[0033]Culture scale: 50 ml spinner flask
[0034]Spinner rotational rate: 60 rpm
[0035]Culture conditions: 37° C., 5% CO2, moist
[0036]Subculture condition: 3-4 days after culture
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Acidity | aaaaa | aaaaa |
| Angular velocity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com