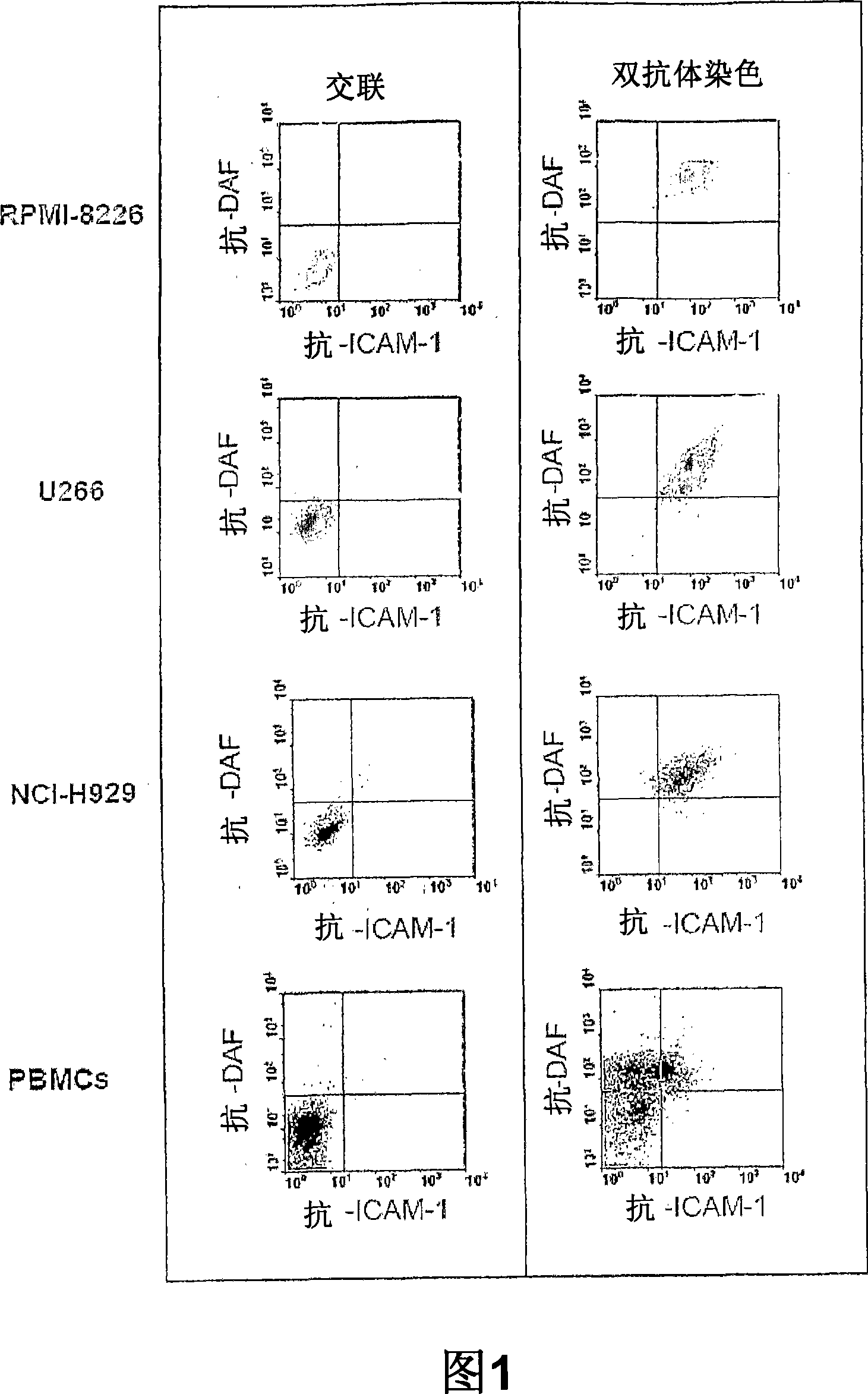
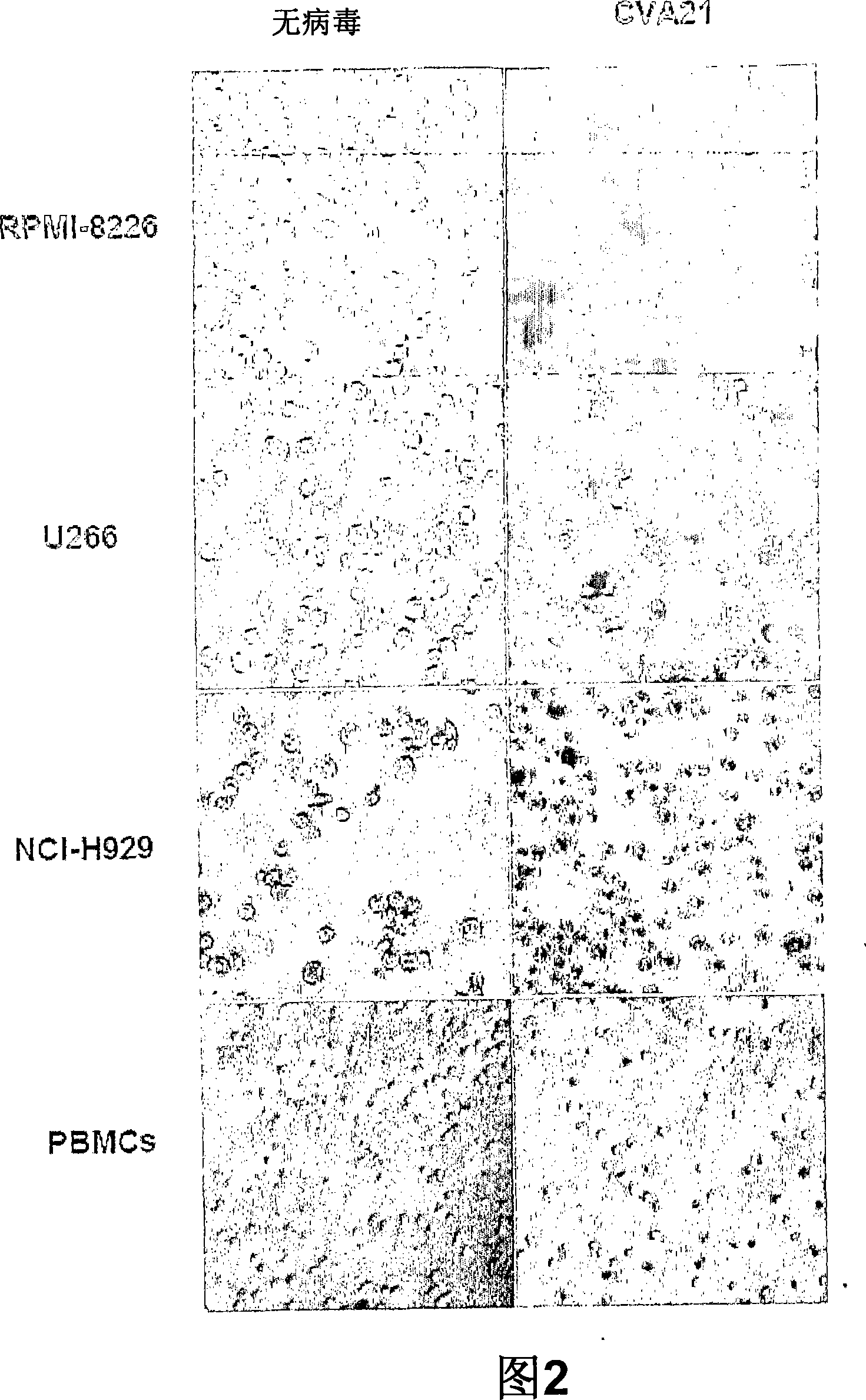
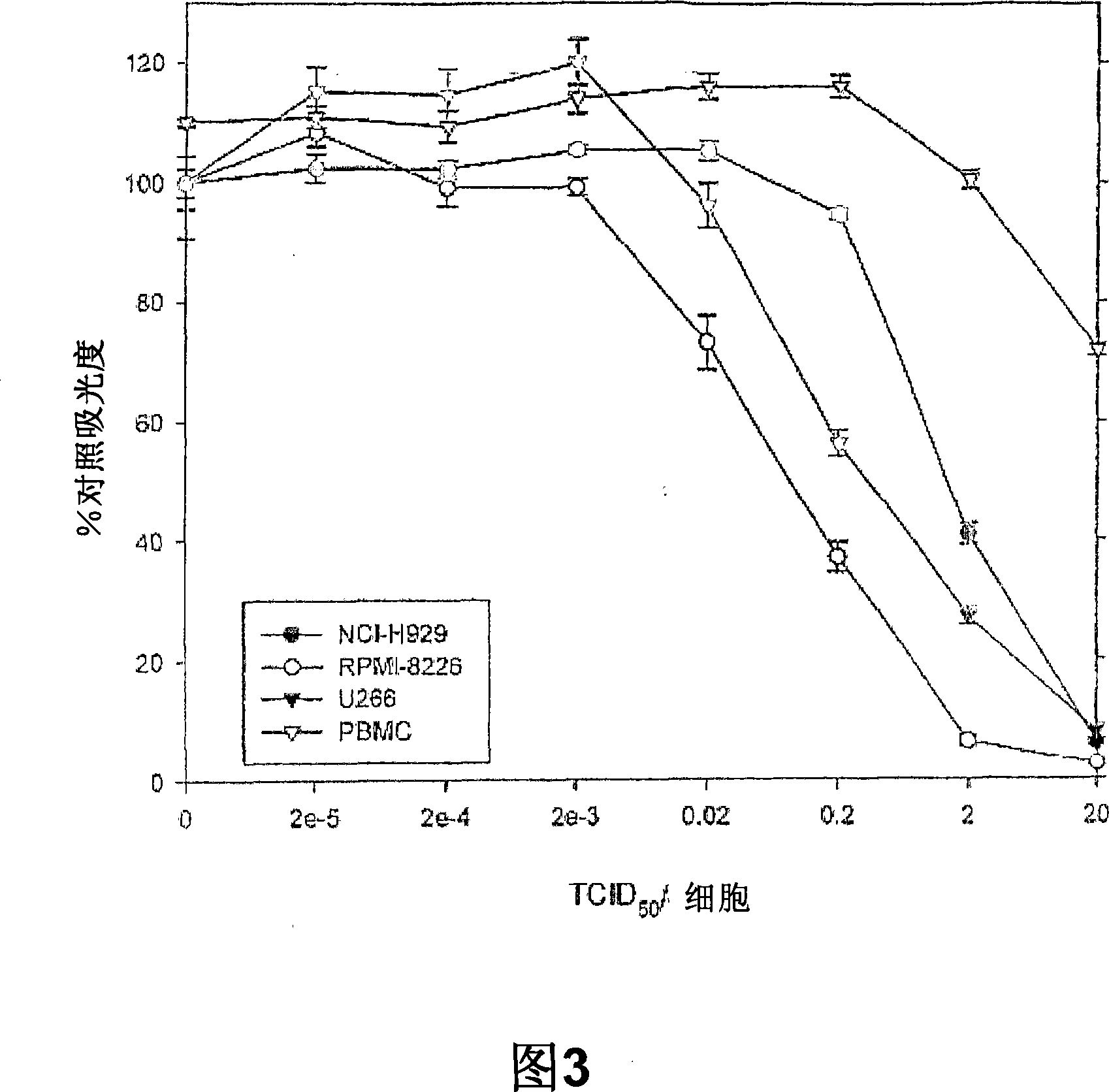
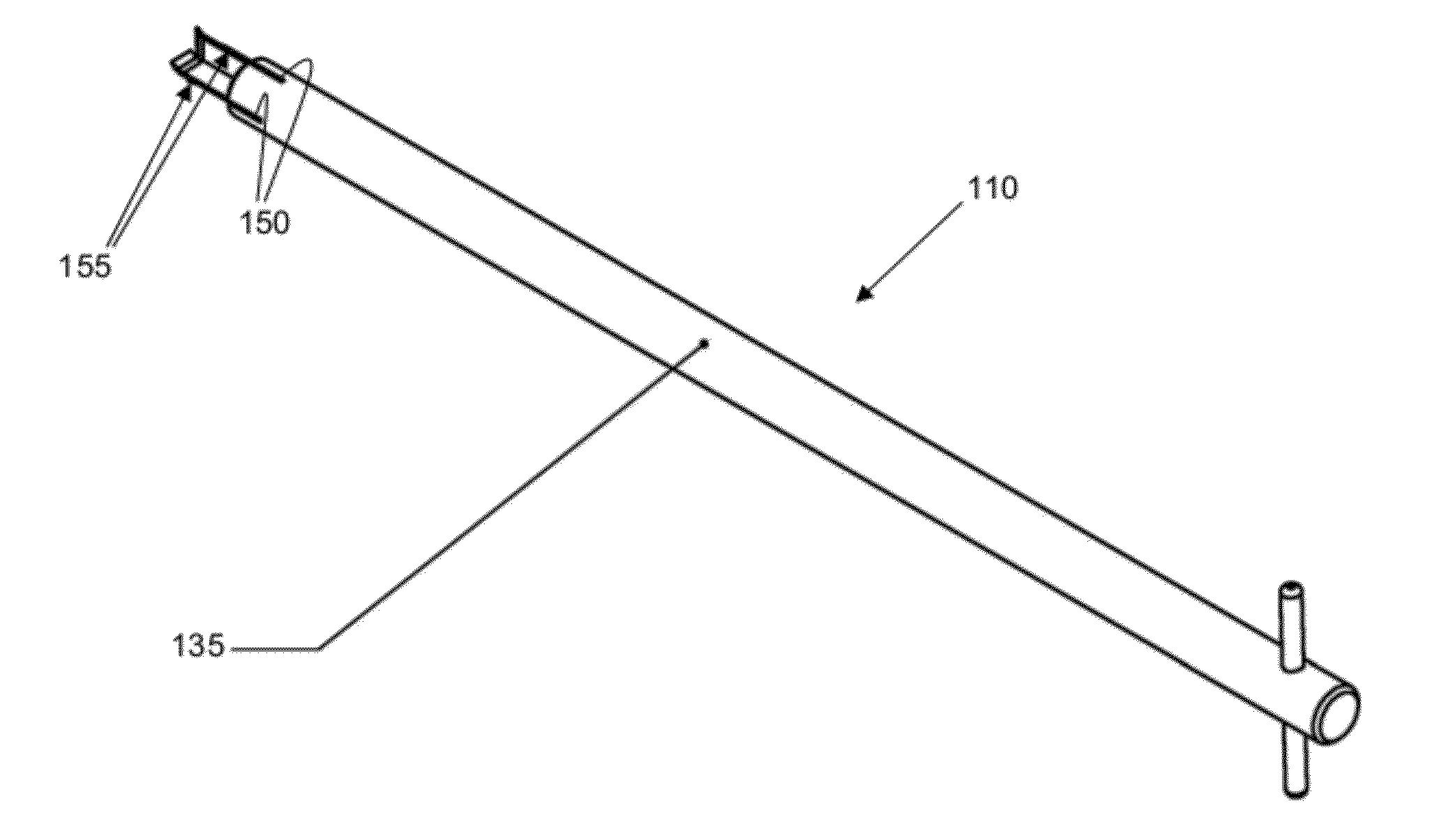
Patents
Literature
53 results about "Autogenous graft" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Collagen-calcium phosphate composites have been in use for over a decade and have demonstrated clinical usefulness equivalent to autogenous graft in reconstruction of fractures and bone defects.
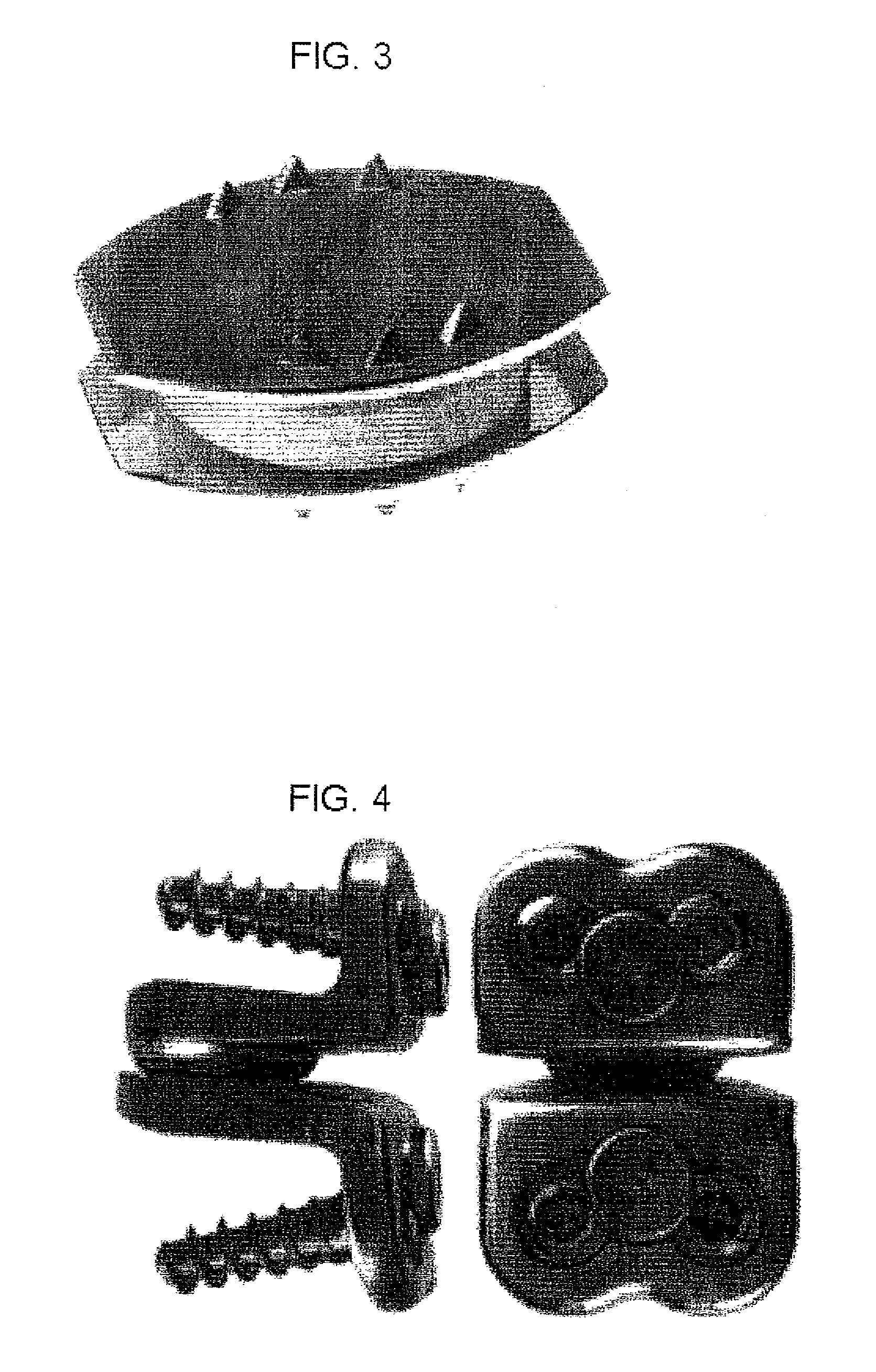
Intervertebral cage designs
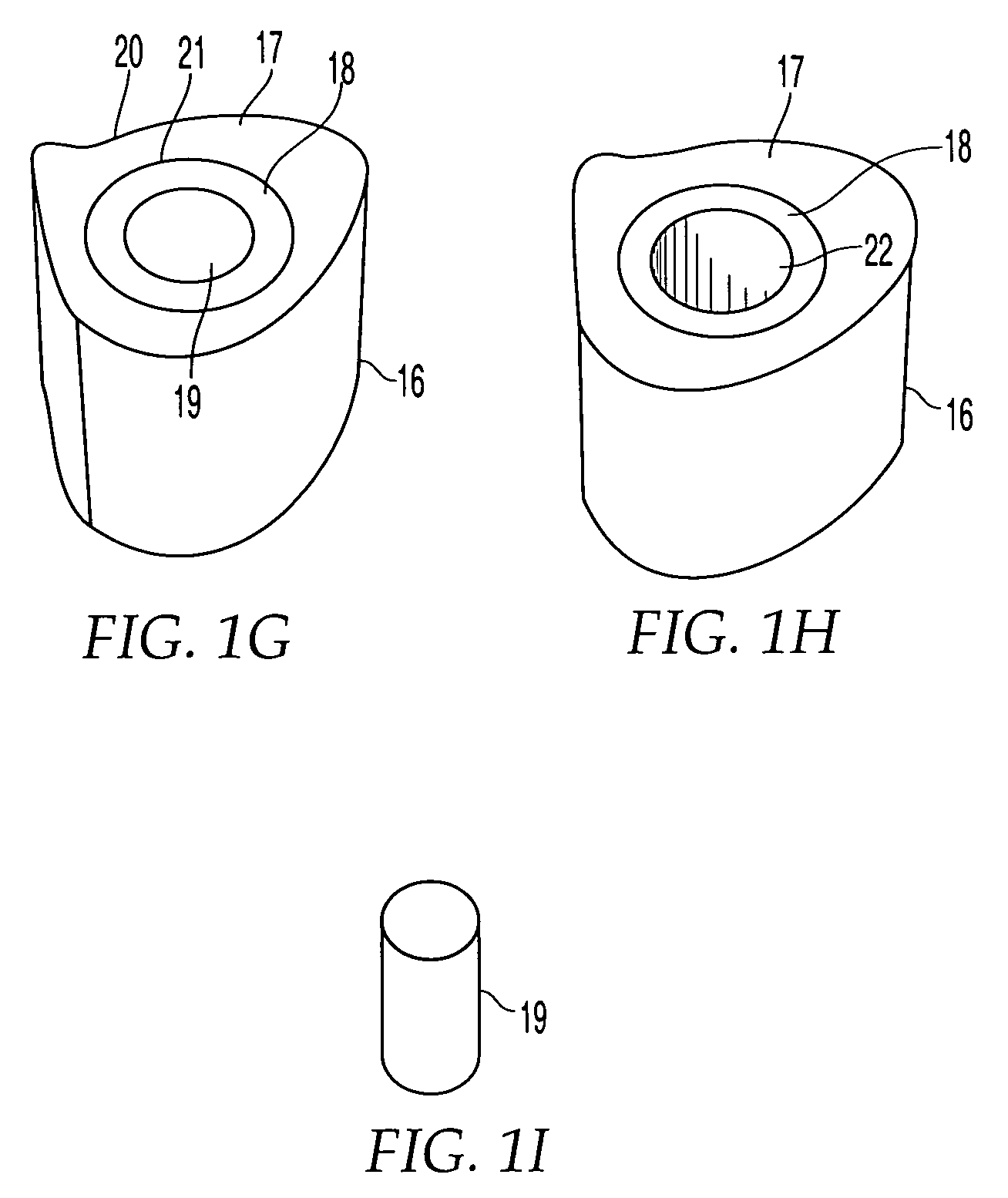
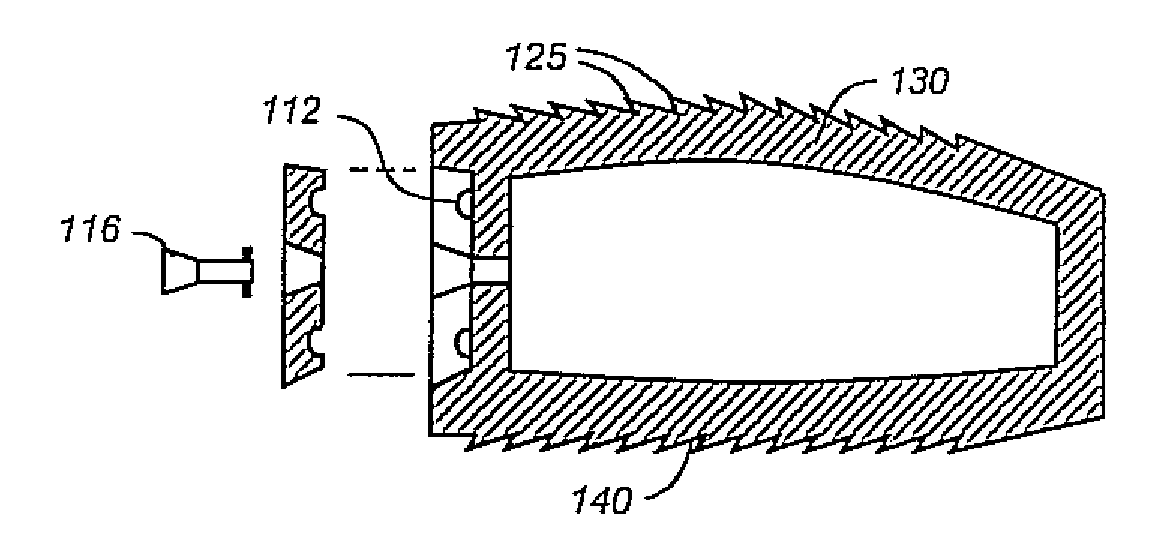
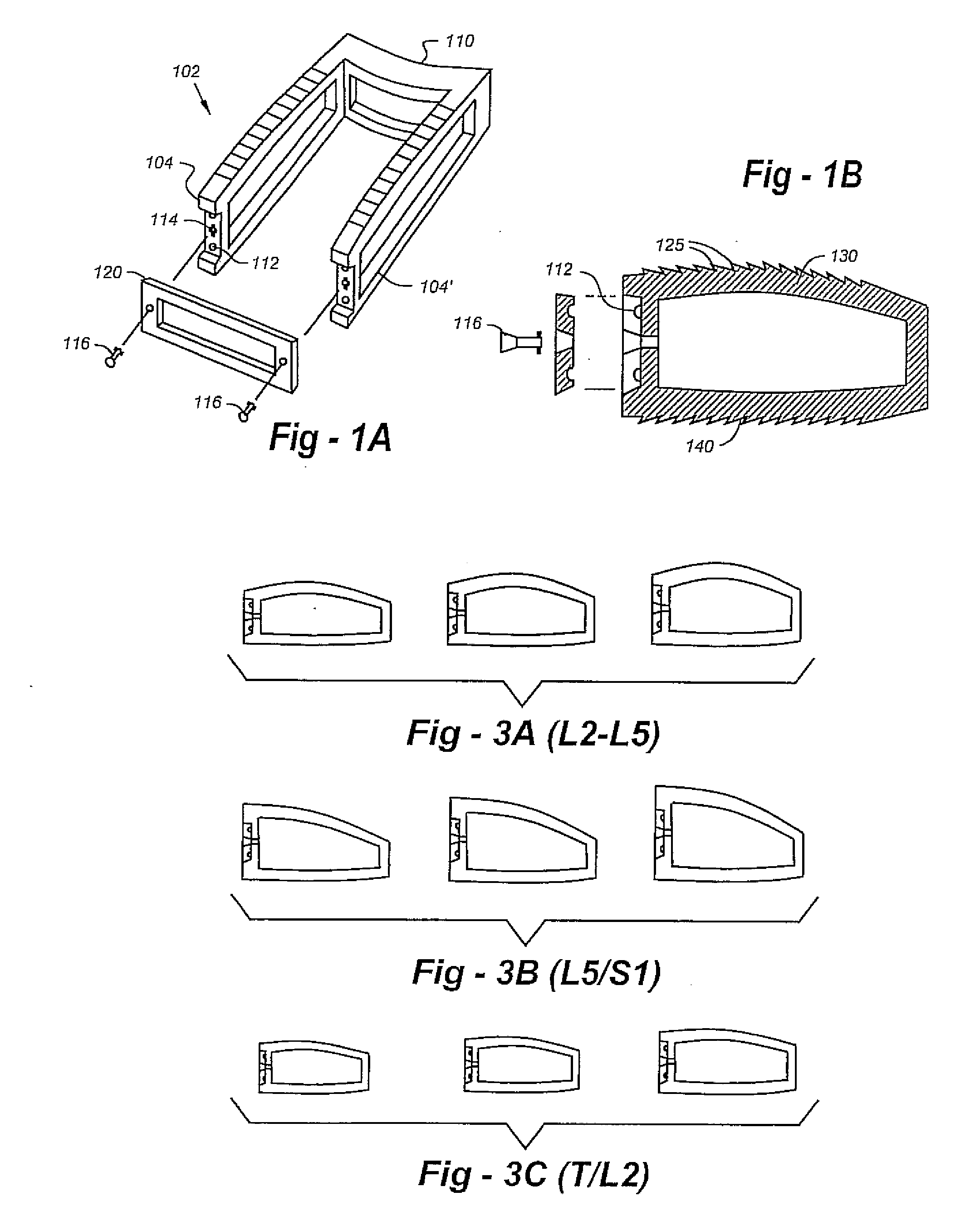
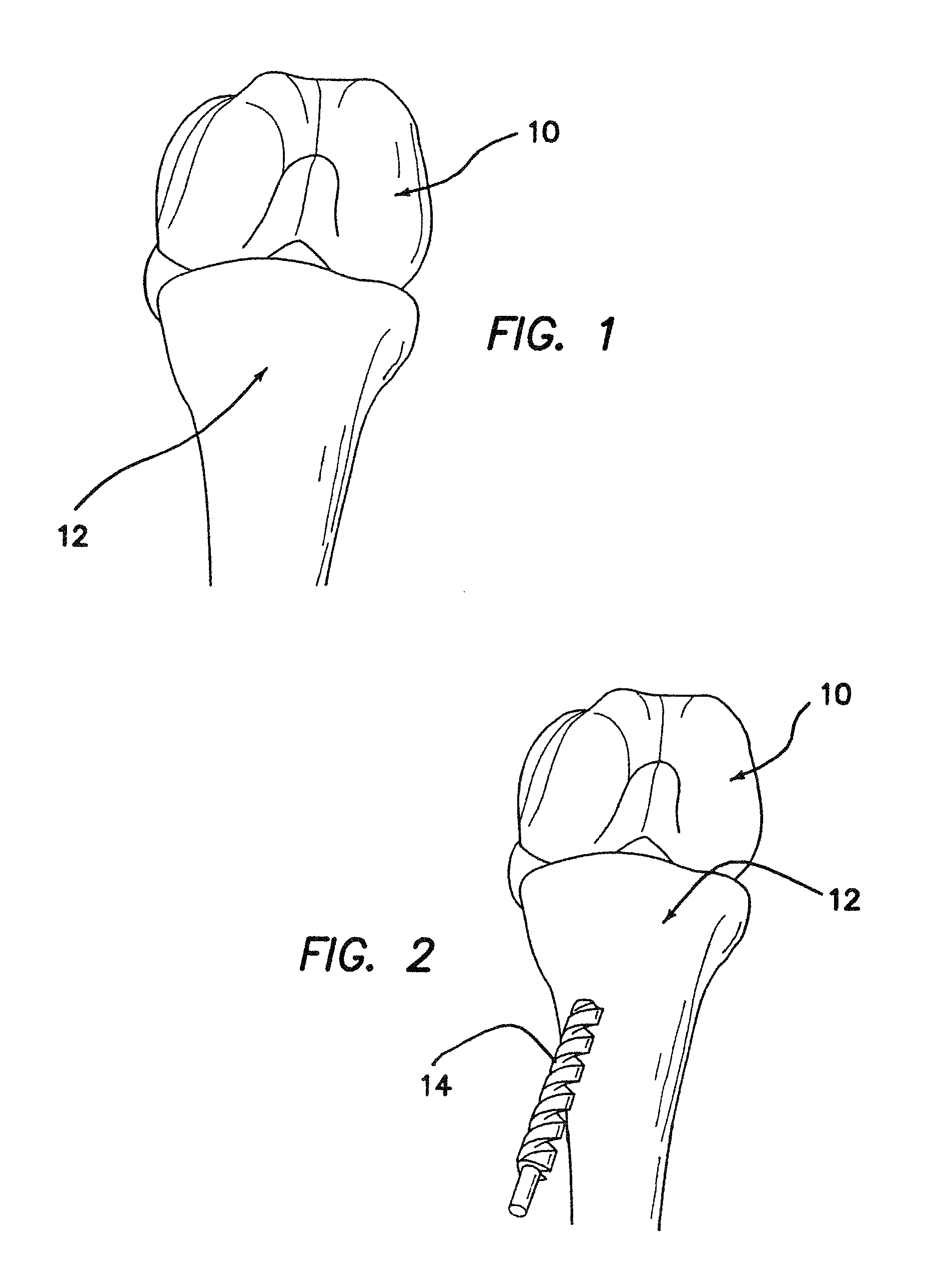
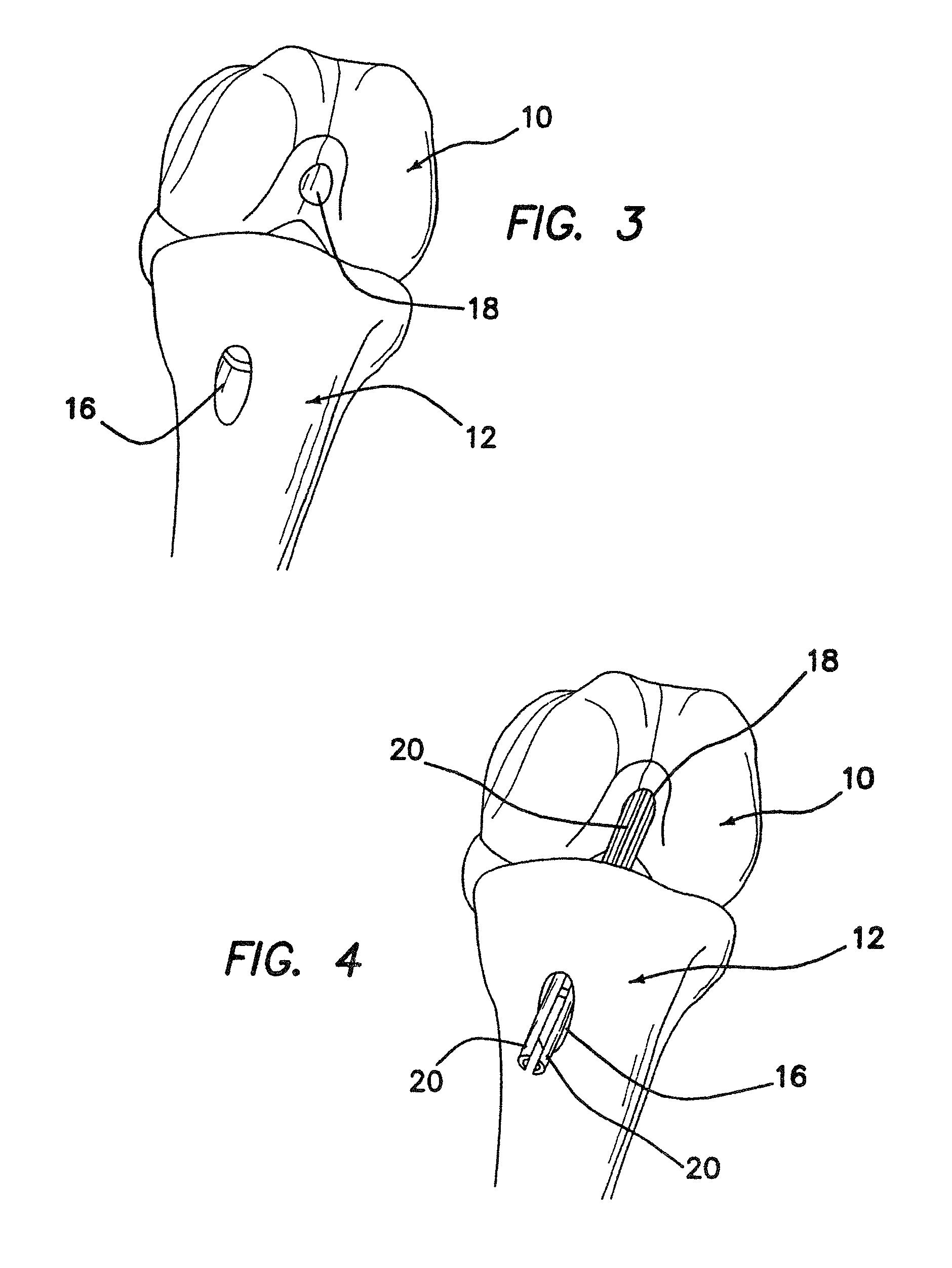
ActiveUS7232463B2Without excessive obscurationEasy and great accessDiagnosticsBone implantAutogenous graftCustom-fit
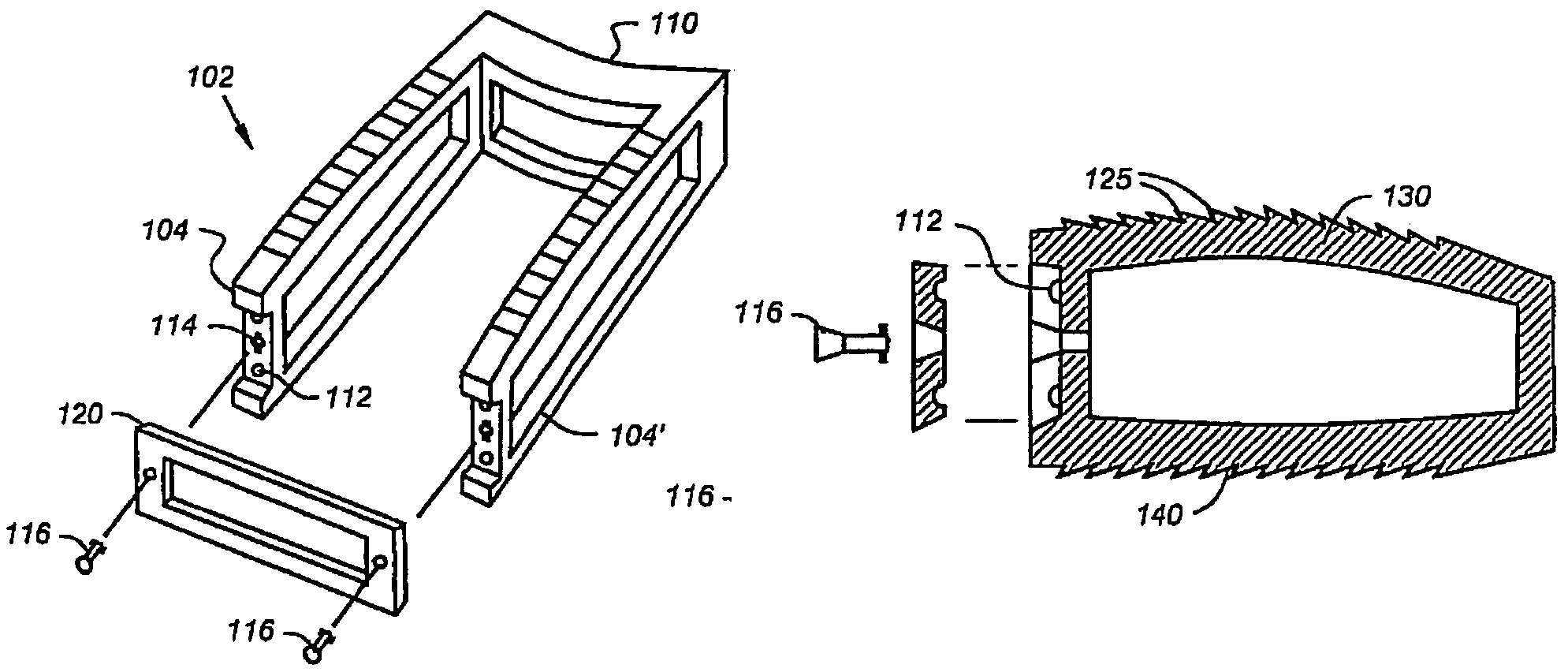
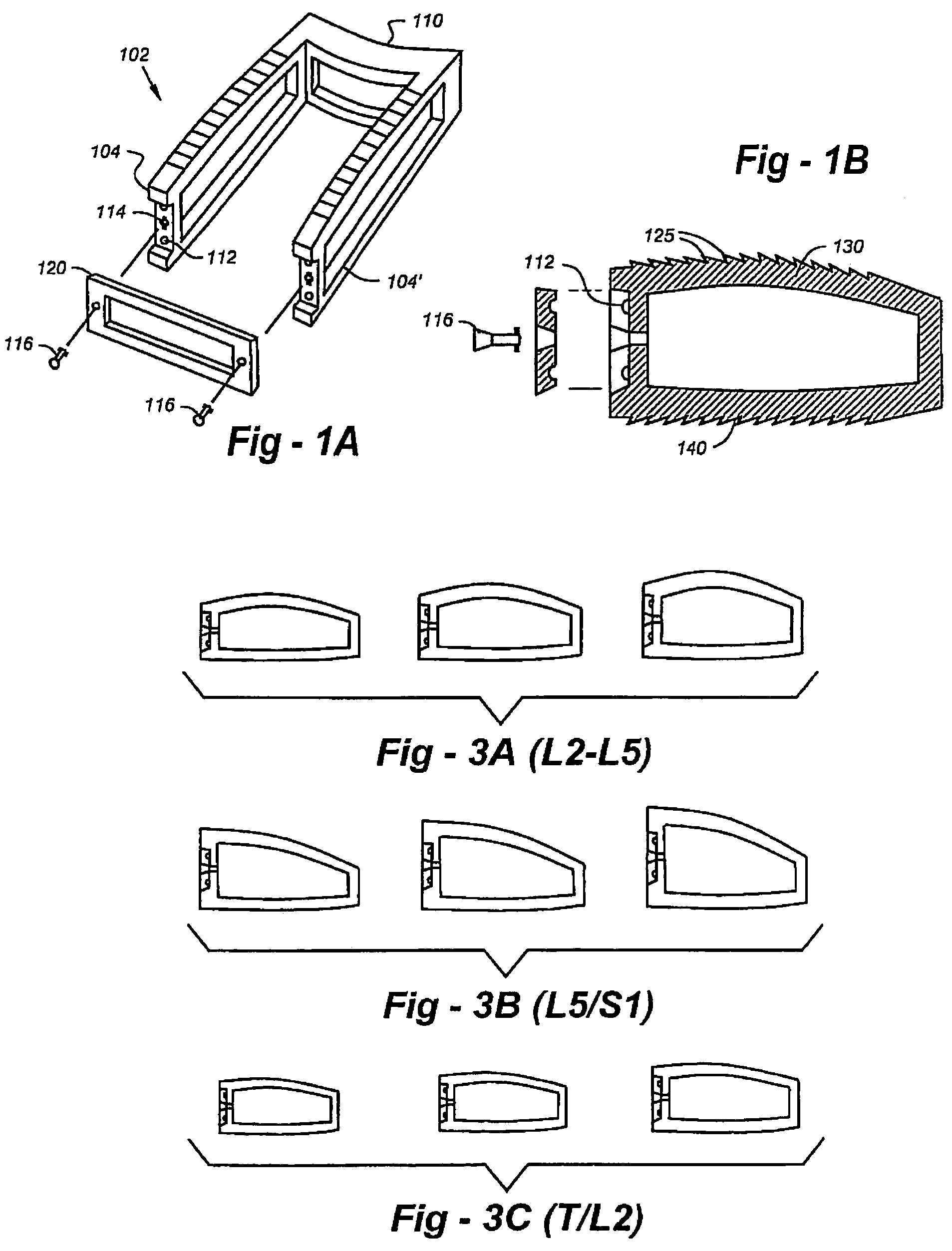
Cage systems that improve upon the prior art in various ways. In the preferred embodiments, devices are radiolucent, with markers, thereby allowing visualization of placement without excessive obscuration. Devices according to the invention eliminate multiple steps, instruments and trays, while being capable of a custom fit. The devices according to the invention permit easier and greater access to end plate surface area, and can be used with autografts, allografts, and biologics.
Owner:AMEDICA A DELAWARE
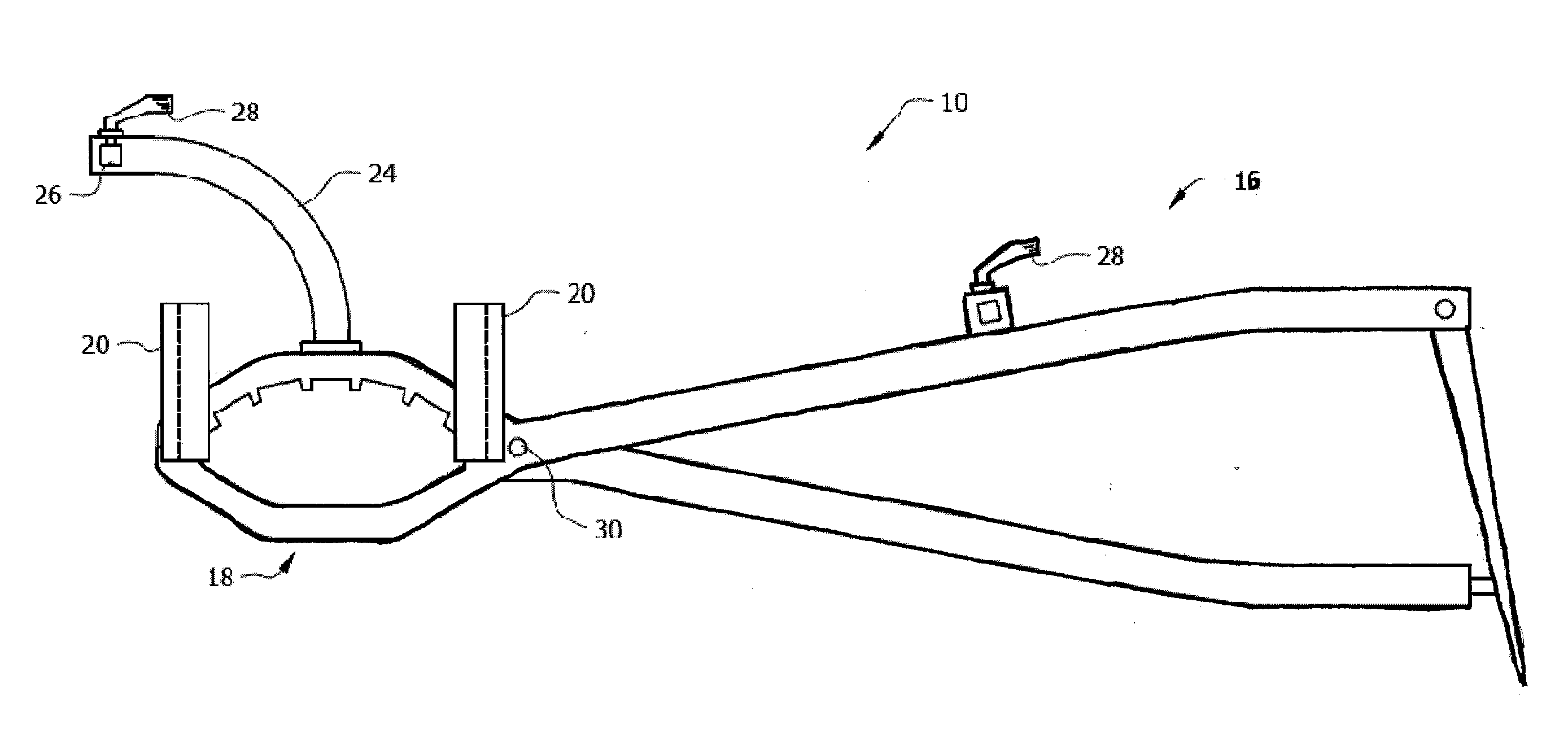
Devices, systems and methods for material fixation
A material fixation system is particularly adapted to improve the tendon-to-bone fixation of hamstring autografts, as well as other soft tissue ACL reconstruction techniques. The system is easy to use, requires no additional accessories, uses only a single drill hole, and can be implanted by one person. Additionally, it replicates the native ACL by compressing the tendons against the aperture of the tibial tunnel, which leads to a shorter graft and increased graft stiffness. It is adapted to accommodate single or double tendon bundle autografts or allografts. It also provides pull out strength measured to be greater than 1000 N.
Owner:CAYENNE MEDICAL INC
Bone implants with central chambers
InactiveUS7087082B2Easy to implantRestore disc height and natural curvature of spineJoint implantsSpinal implantsBone tissueBone splinters
A bone fusion implant for repair or replacement of bone includes a hollow body formed from at least two bone fragments which are configured and dimensioned for mutual engagement and which are coupled together. The hollow body may be formed of autograft, allograft, or xenograft bone tissue, and may include a core formed of at least one of bone material and bone inducing substances, with the core being disposed in the hollow body.
Owner:SYNTHES USA
Intervertebral cage designs
InactiveUS20090030519A1Without excessive obscurationEasy and great accessDiagnosticsBone implantAutogenous graftCustom-fit
Cage systems that improve upon the prior art in various ways. In the preferred embodiments, devices are radiolucent, with markers, thereby allowing visualization of placement without excessive obscuration. Devices according to the invention eliminate multiple steps, instruments and trays, while being capable of a custom fit. The devices according to the invention permit easier and greater access to end plate surface area, and can be used with autografts, allografts, and biologics.
Owner:US SPINE INC
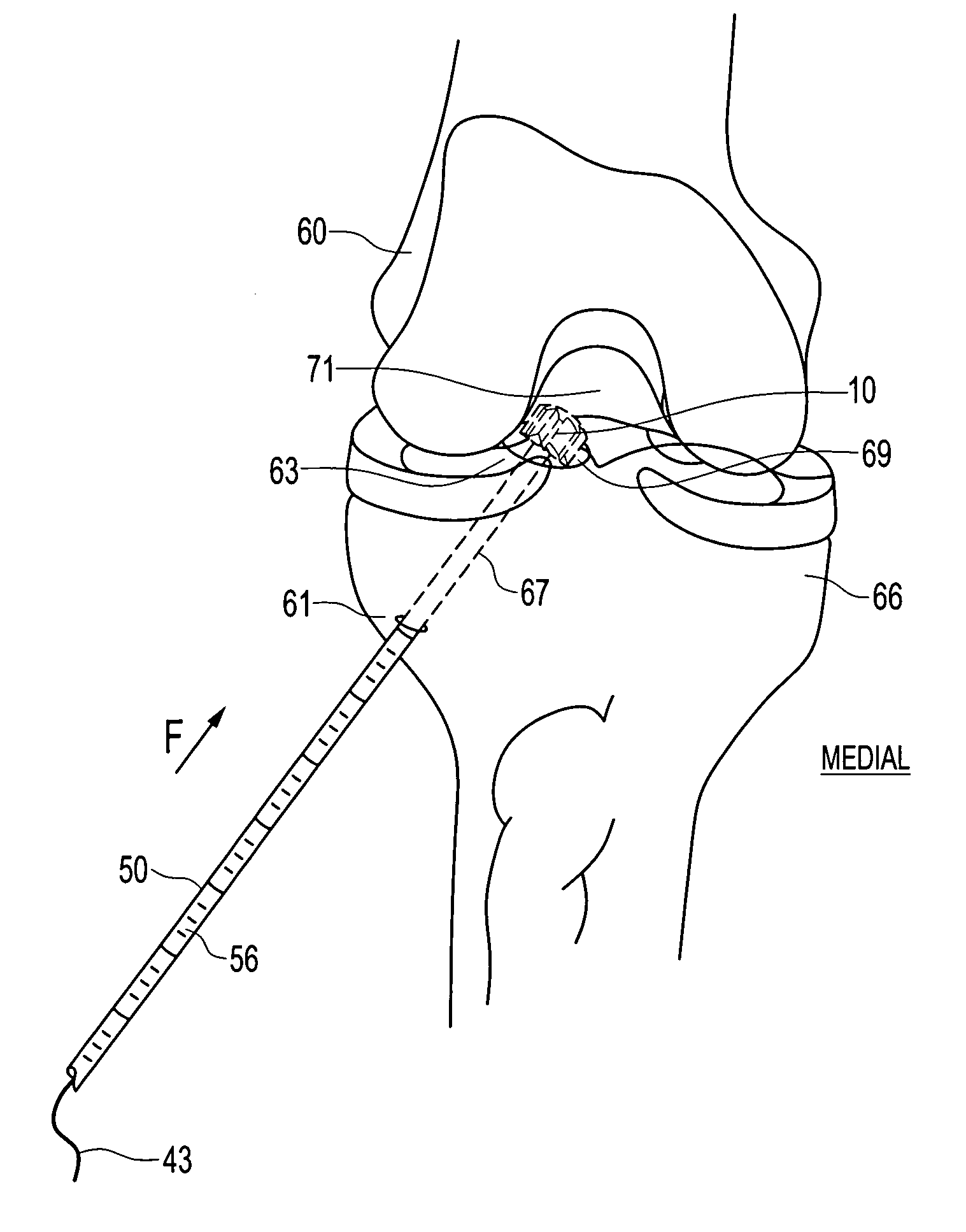
Retrodrill technique for insertion of autograft, allograft or synthetic osteochondral implants
Owner:ARTHREX
Devices, systems and methods for material fixation
A material fixation system is particularly adapted to improve the tendon-to-bone fixation of hamstring autografts, as well as other soft tissue ACL reconstruction techniques. The system is easy to use, requires no additional accessories, uses only a single drill hole, and can be implanted by one person. Additionally, it replicates the native ACL by compressing the tendons against the aperture of the tibial tunnel, which leads to a shorter graft and increased graft stiffness. It is adapted to accommodate single or double tendon bundle autografts or allografts. It also provides pull out strength measured to be greater than 1000 N.
Owner:CAYENNE MEDICAL INC
Devices for performing fusion surgery using a split thickness technique to provide vascularized autograft
A technique for providing vascularized autograft to a fusion site in the posteriolateral aspect of a lumbar fusion mass by using a split-thickness laminoplasty technique, is disclosed in co-pending U.S. Provisional Application 60 / 379,371 filed on May 10, 2002 and U.S. patent application Ser. No. ______ filed concurrently with the present application. Devices used to accomplish osteotomies of the laminae, facet joints, and transverse processes are disclosed. The devices include a shaft that can guide the devices, a method of deploying a wire bone saw for dividing these structures through a coronal plane, leaving the periosteum, musculature, and hence blood supply intact. This novel and non-obvious technique will allow for the application of vascularized autograft to the fusion bed in posterior or posteriolateral fusion of the spine.
Owner:TRUSPINE USA
Expandable interbody (lateral, posterior, anterior) multi-access cage for spinal surgery
An interbody cage can be utilized in a multi-access approach. Such a device can be inserted in an MIS exposure and then can be expanded insitu. Such a multi access device can expand in width to cover a larger area for fusion to occur. Such device includes a unique feature that allows for the graft material to stay in place upon deployment. Such a cage device can be configured with four graft boxes that can be filled with allograft or autograft material to allow for fusion to occur. Along with the graft boxes, such a cage can also be configured with a unique feature in the posterior piece of the device that has small cut outs or ports to allow for moldable allograft material to be injected through the inserter device.
Owner:MEDEVICE IP HLDG
Methods for collecting and processing autografts, processed autografts, kits for collecting and transporting autografts, and tools for preparing autografts
ActiveUS20110160857A1Easy to operateBioreactor/fermenter combinationsBiological substance pretreatmentsAnatomyAutogenous graft
The present invention is directed to methods for collecting and processing autografts, processed autografts, kits for collecting and transporting autografts, and tools for preparing autografts. It is also directed to autologous bone grafts, and methods of preparing them.
Owner:LIFENET HEALTH
Dynamic spinal implants incorporating cartilage bearing graft material
A dynamic spinal implant utilizes cartilage bearing graft material in dynamic disc replacement and / or facet arthroplasty. Methods and apparatus for dynamic spinal implants incorporate bulk articular graft tissues derived from donor joint sources in human (allograft or autograft) or non-human (xenograft) tissue. The donor joint is preferably prepared as a biological dynamic spinal implant with articular cartilage as a bearing interface between adjacent bone surfaces that naturally articulate with respect to one another.
Owner:BIOMET MFG CORP
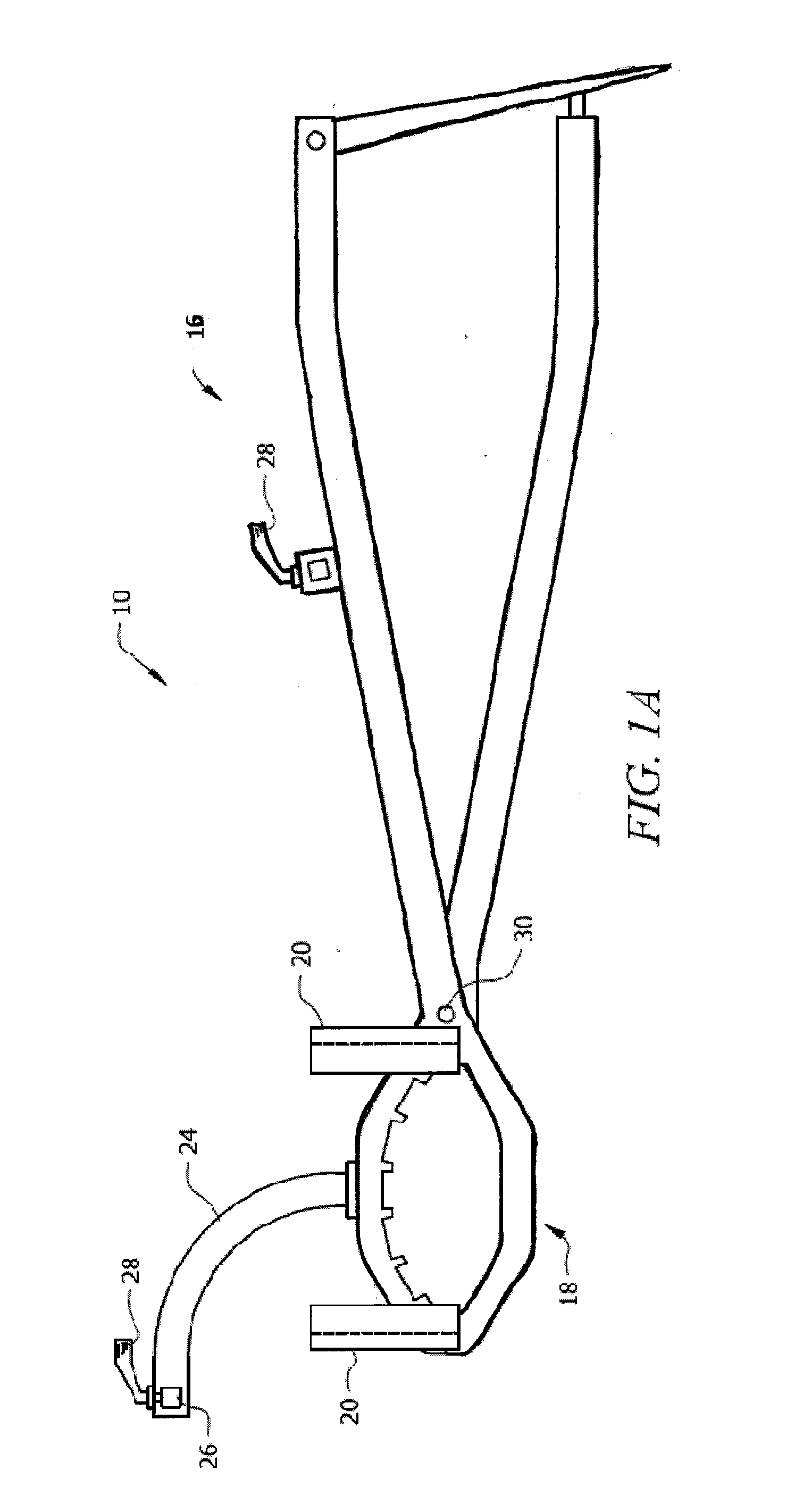
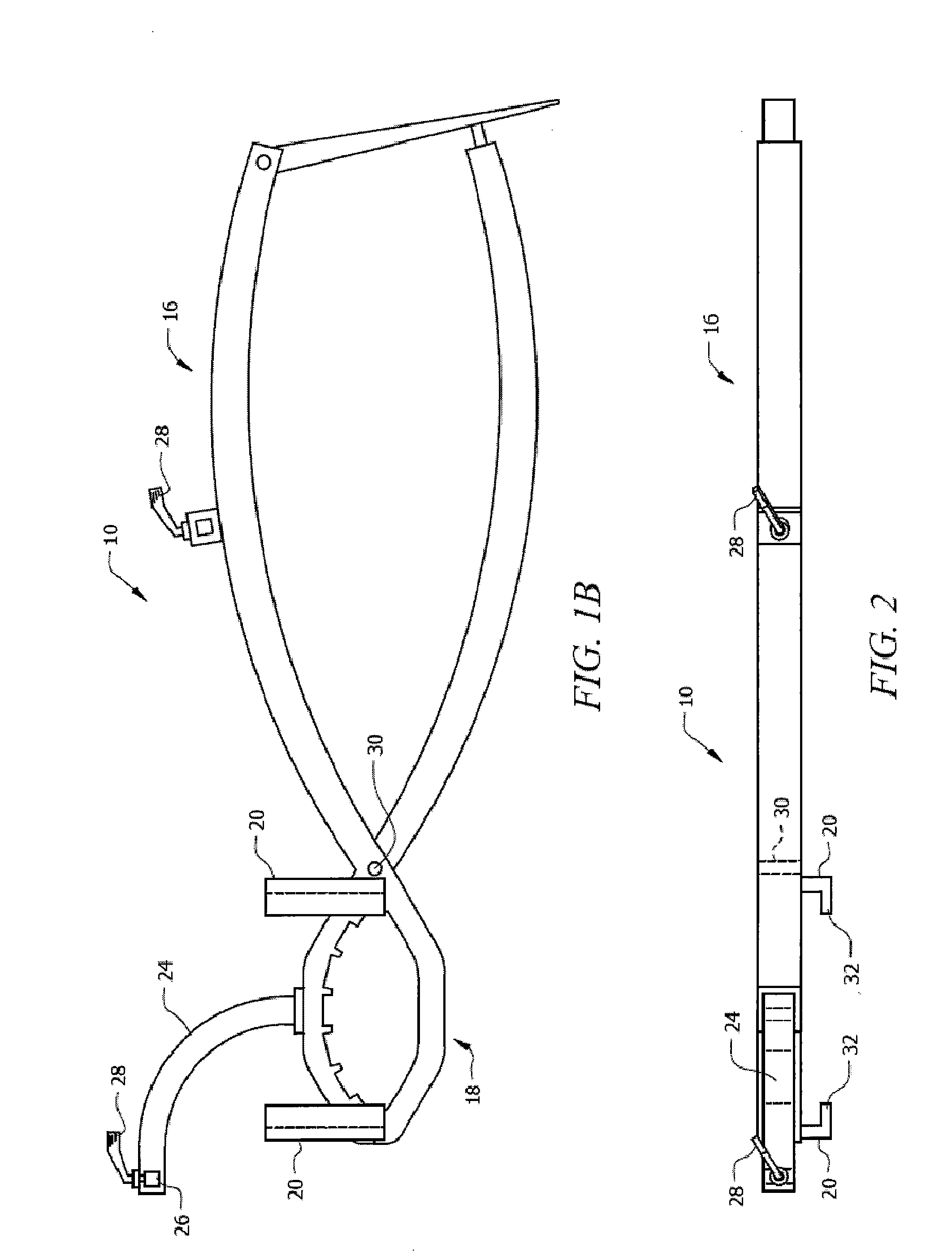
Apparatus for Osteotomy and Graft Preparation
InactiveUS20120130384A1Promote resultsPrecise resectionJoint implantsNon-surgical orthopedic devicesOrthopedic ProceduresPlastic surgery
Surgical tools that increase surgical accuracy for orthopedic procedures involving long bone osteotomy with or without supplementation with allograft or autograft. Two clamps on the operative side are rigidly interconnected to one another with an adjustable rod that is locked into place, preserving length and rotation of the bone. A graft preparation device is used on the operative table. It enables alignment of the graft to preserve the desired mechanical and anatomic axes and to provide rigid fixation of the graft for cutting. Adjustable jigs ensure exact cuts.
Owner:UNIV OF SOUTH FLORIDA
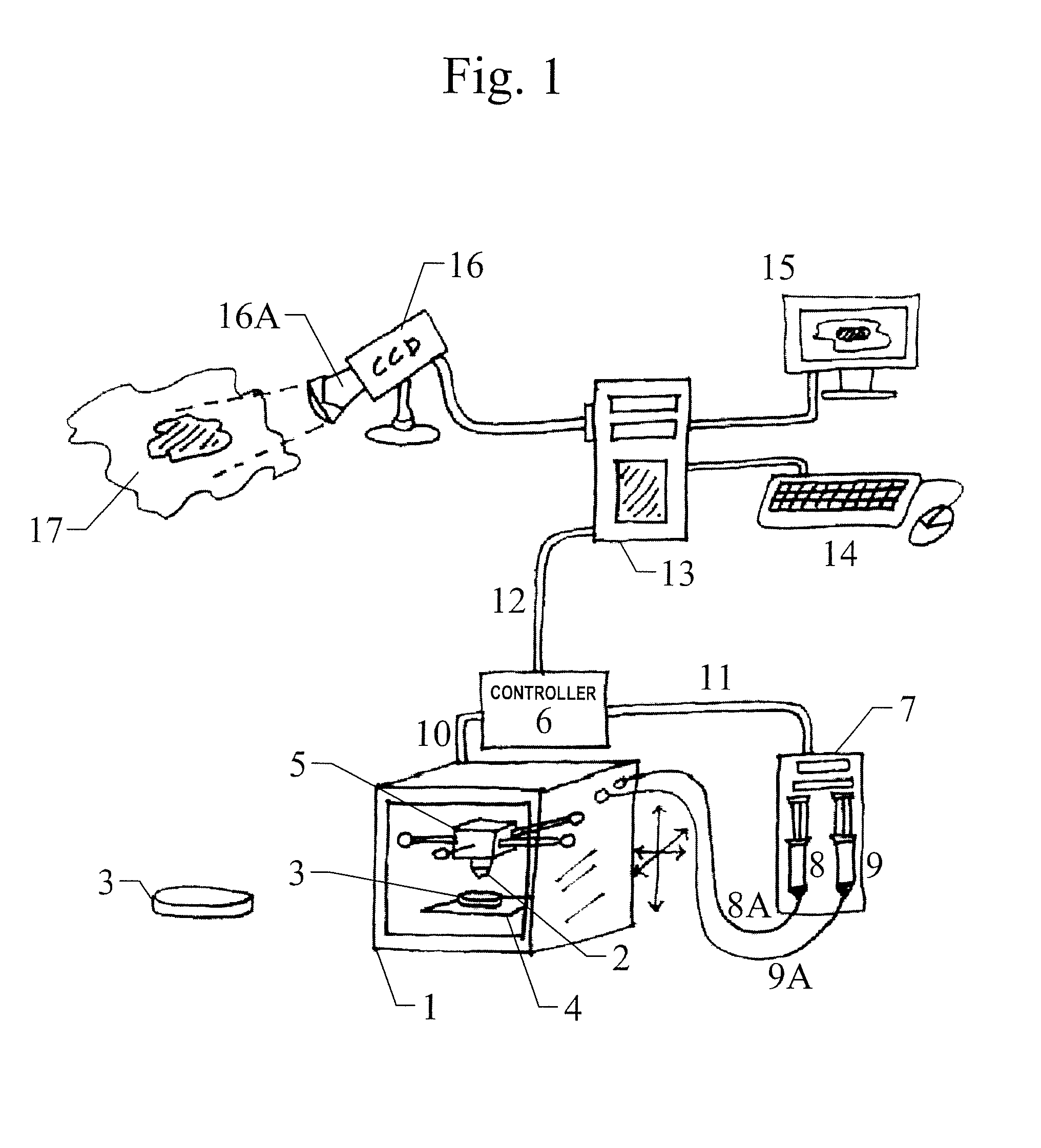
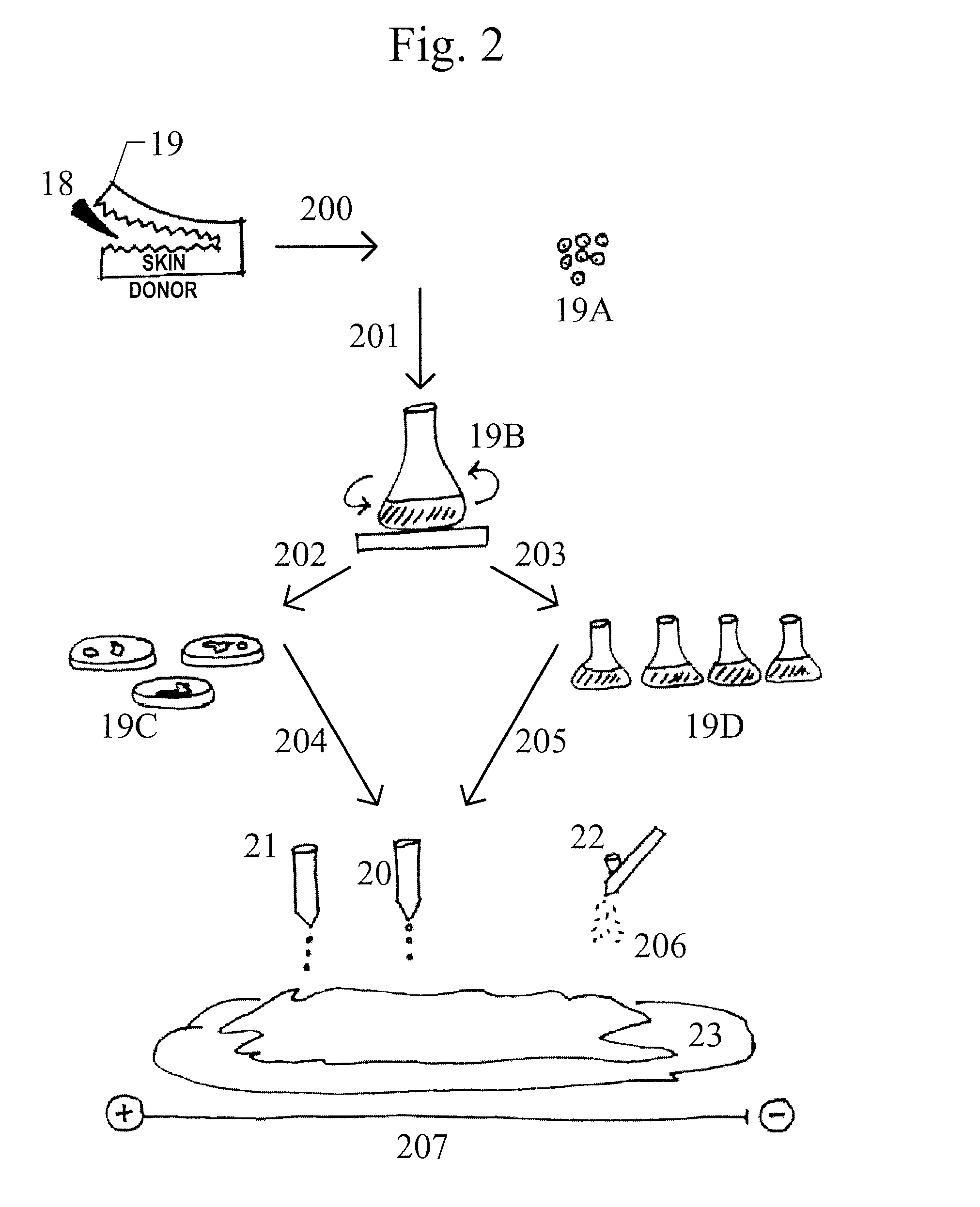
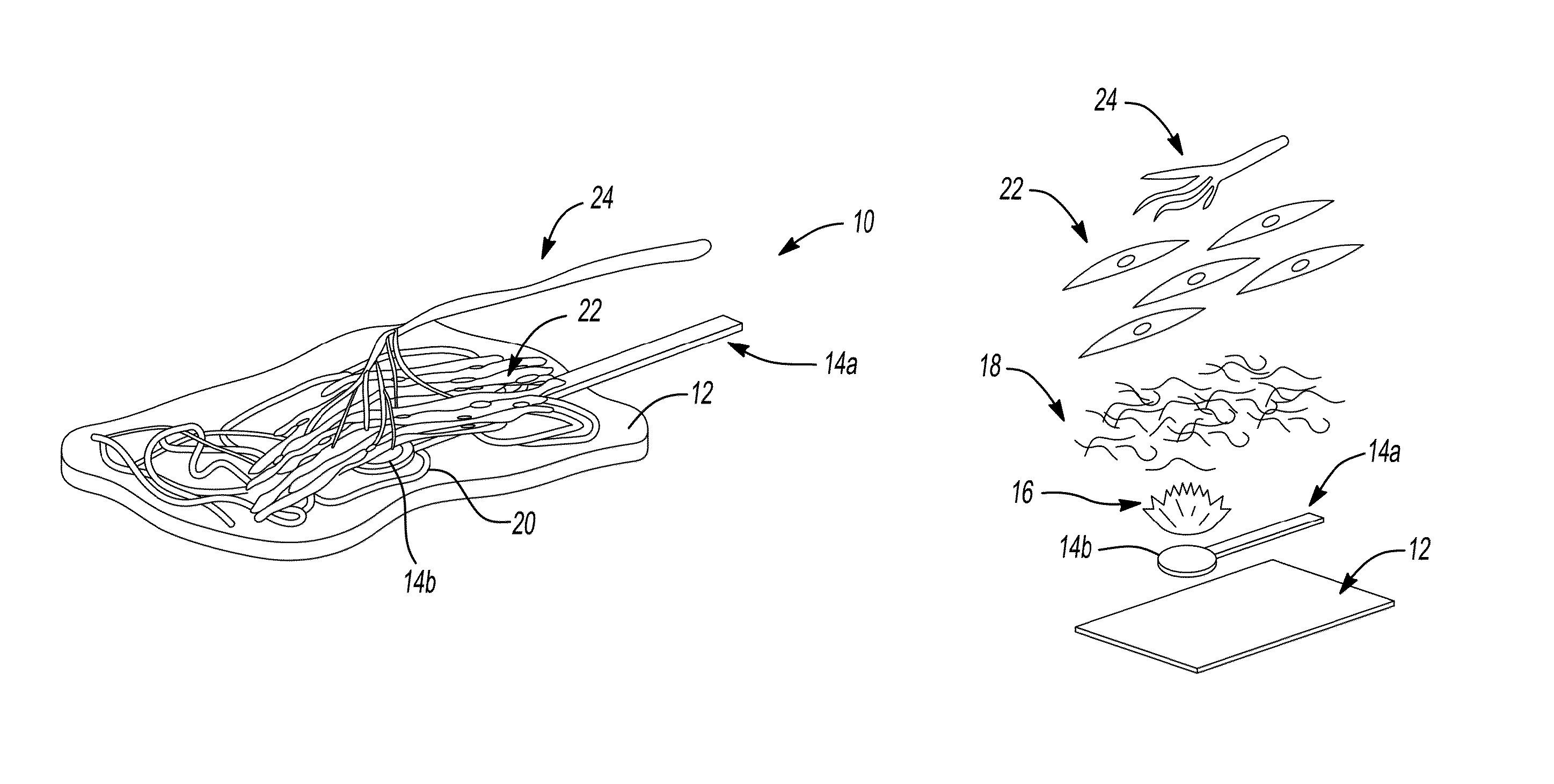
Skin printing and auto-grafting
Owner:DERMAGENESIS
Peripheral nerve interface devices for treatment and prevention of neuromas
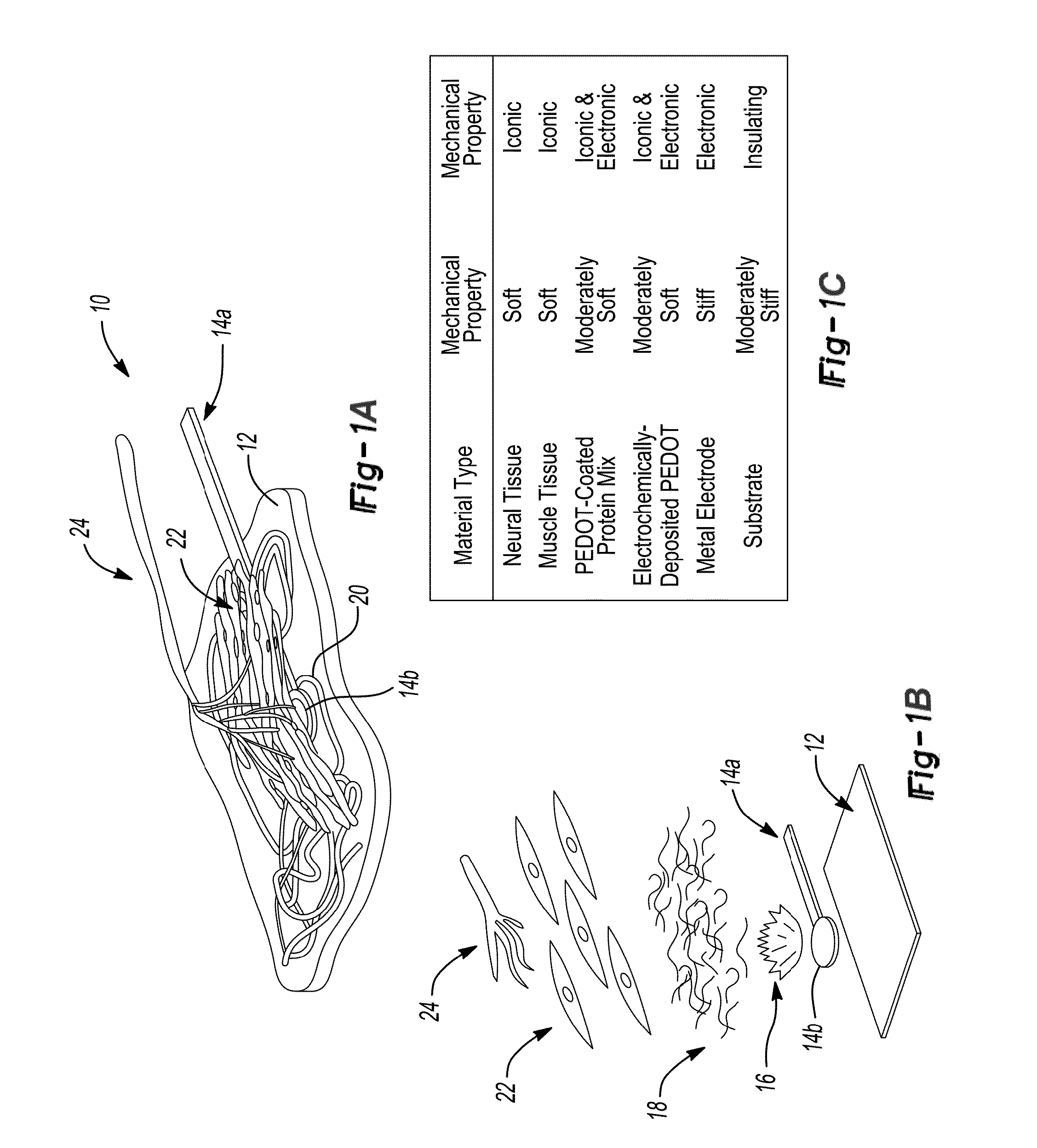
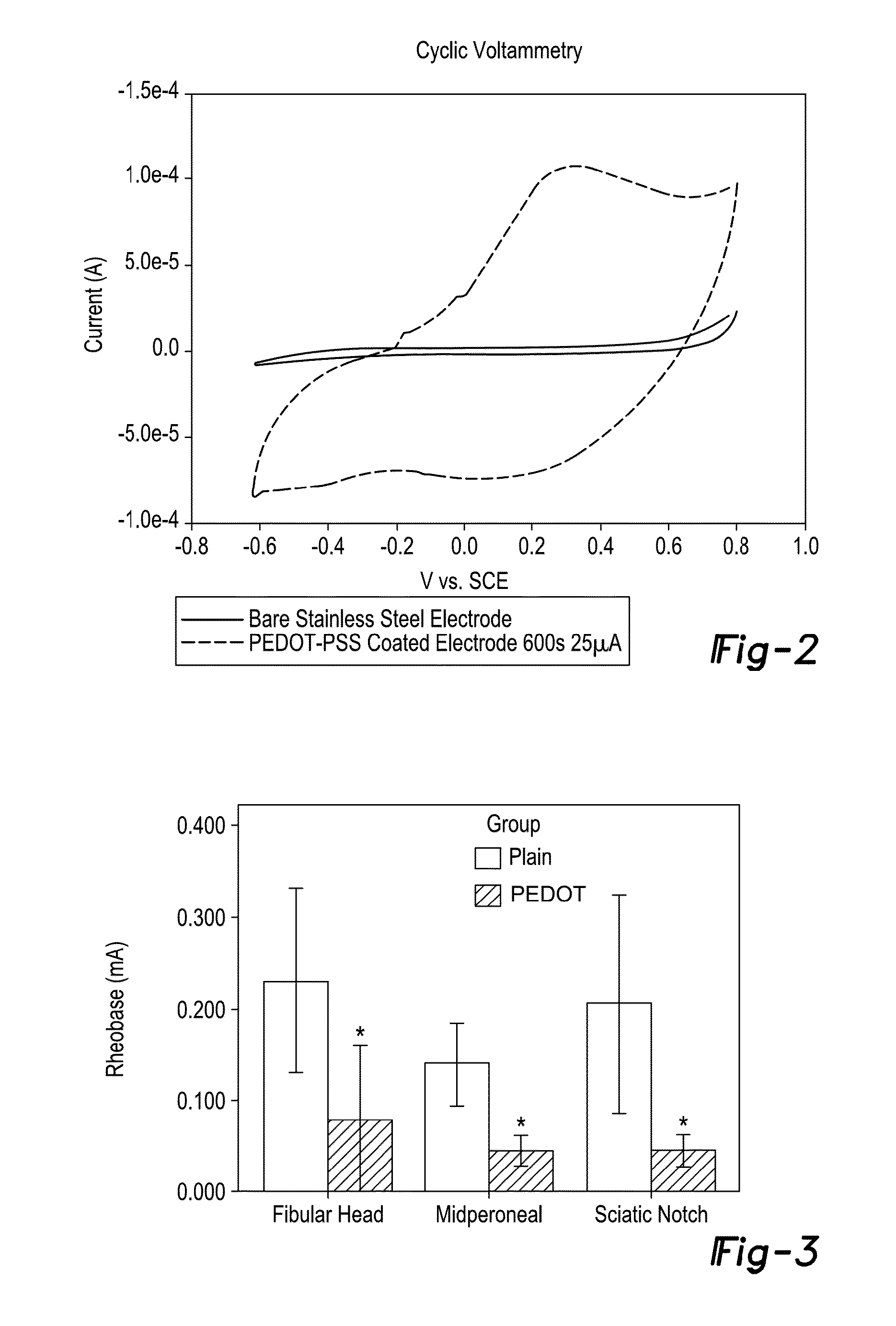
ActiveUS9352146B2Treat and minimize and prevent neuroma formationIncrease contactSpinal electrodesLigamentsConductive polymerAutogenous graft
The present disclosure provides nerve interface devices, such as passive or active nerve caps or regenerative peripheral nerve interface devices (RPNI), for a subject in need thereof. The nerve interface devices include nerve interface cap devices capable of treating, minimizing, or preventing formation of neuromas in severed or damaged nerve endings. Such a nerve interface device includes a housing that may be formed of a scaffold, such as a biotic material or hydrogel, an autograft, and optionally an electrode and / or conducting polymer. The autograft may be free muscle or free skin tissue, which is attached to the nerve ending to permit reinnervation. The present disclosure also provides methods for treating, minimizing, or preventing neuroma formation in a subject having a severed or damaged nerve, especially a peripheral nerve.
Owner:RGT UNIV OF MICHIGAN +1
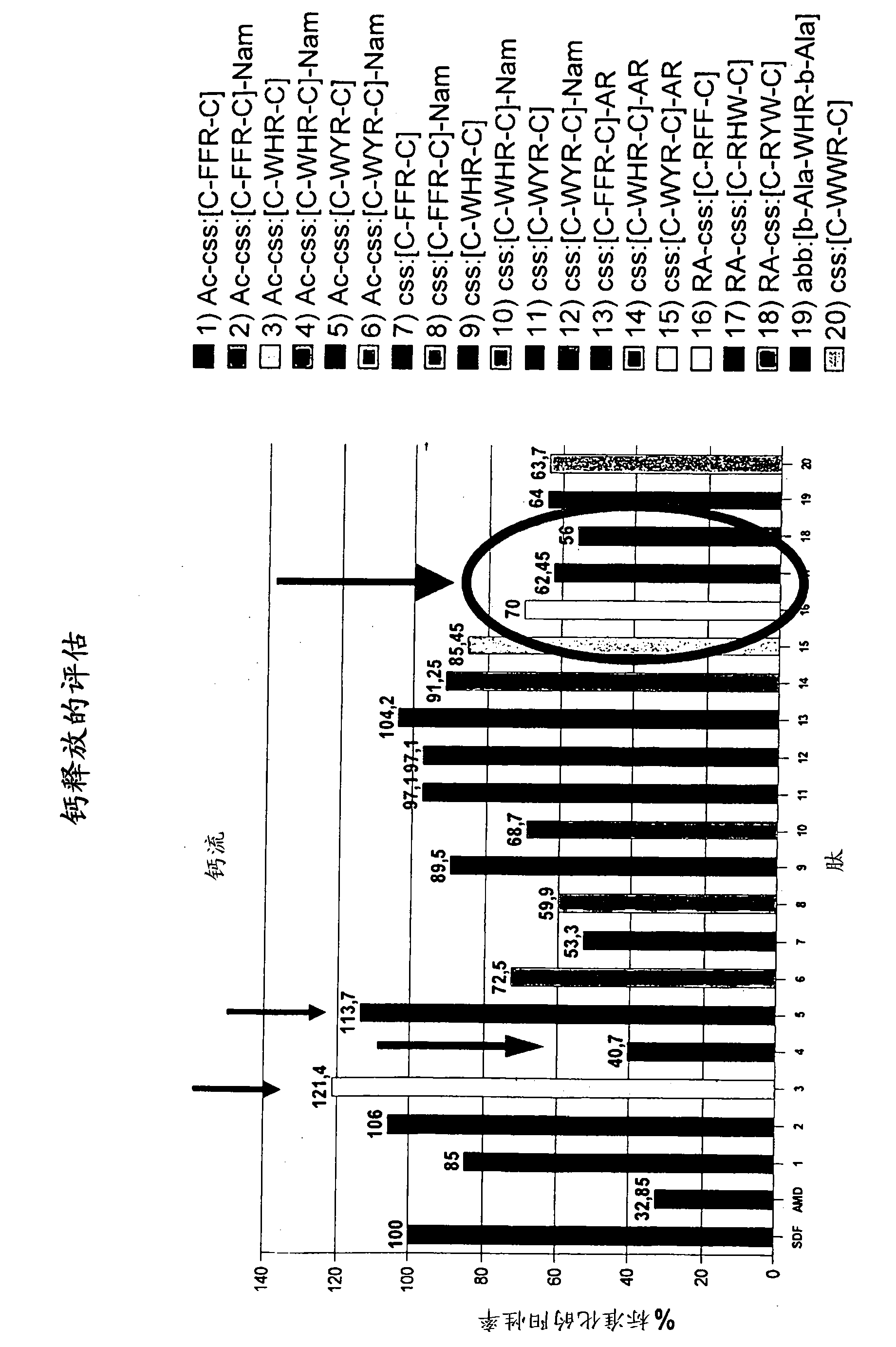
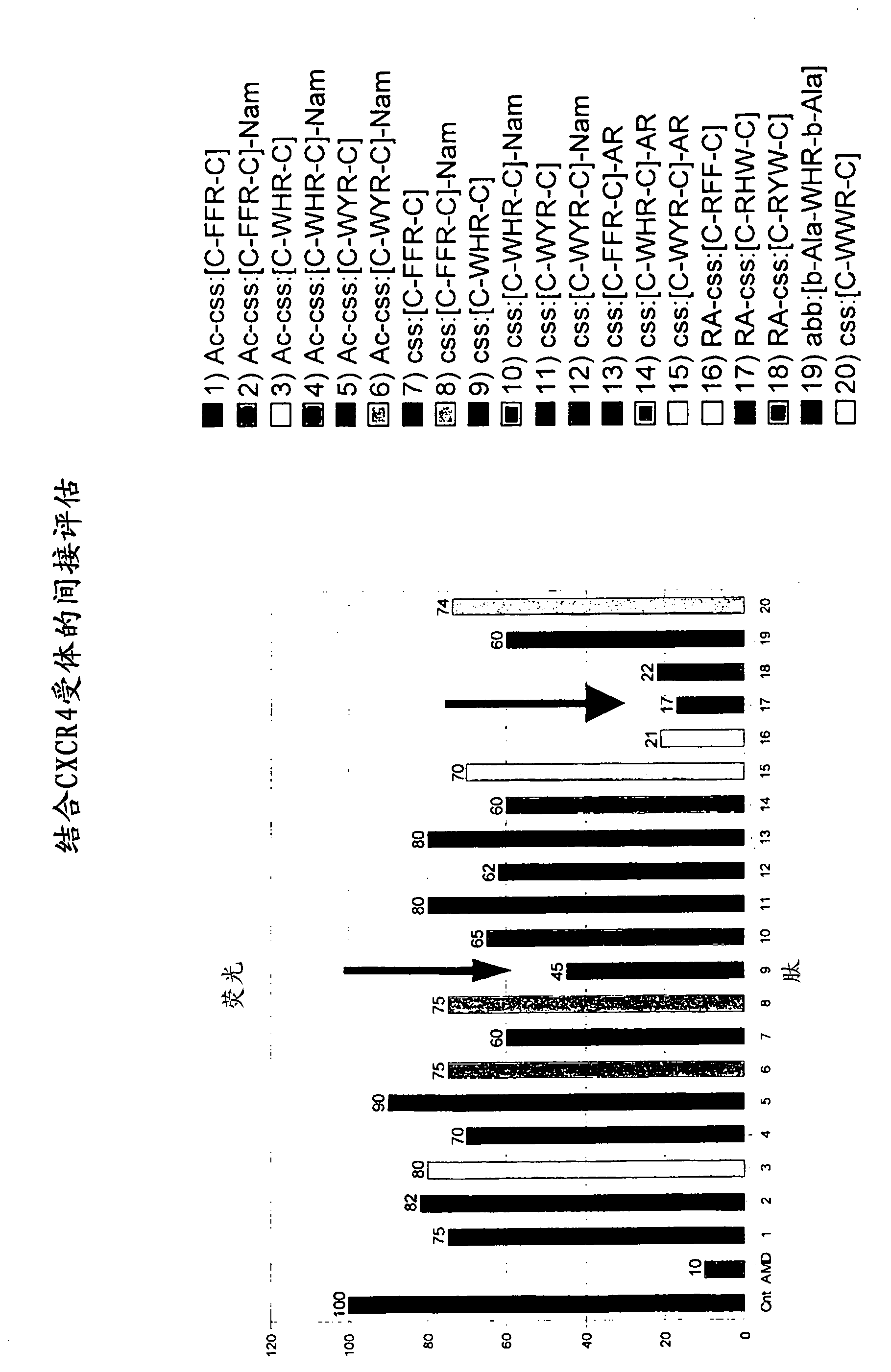
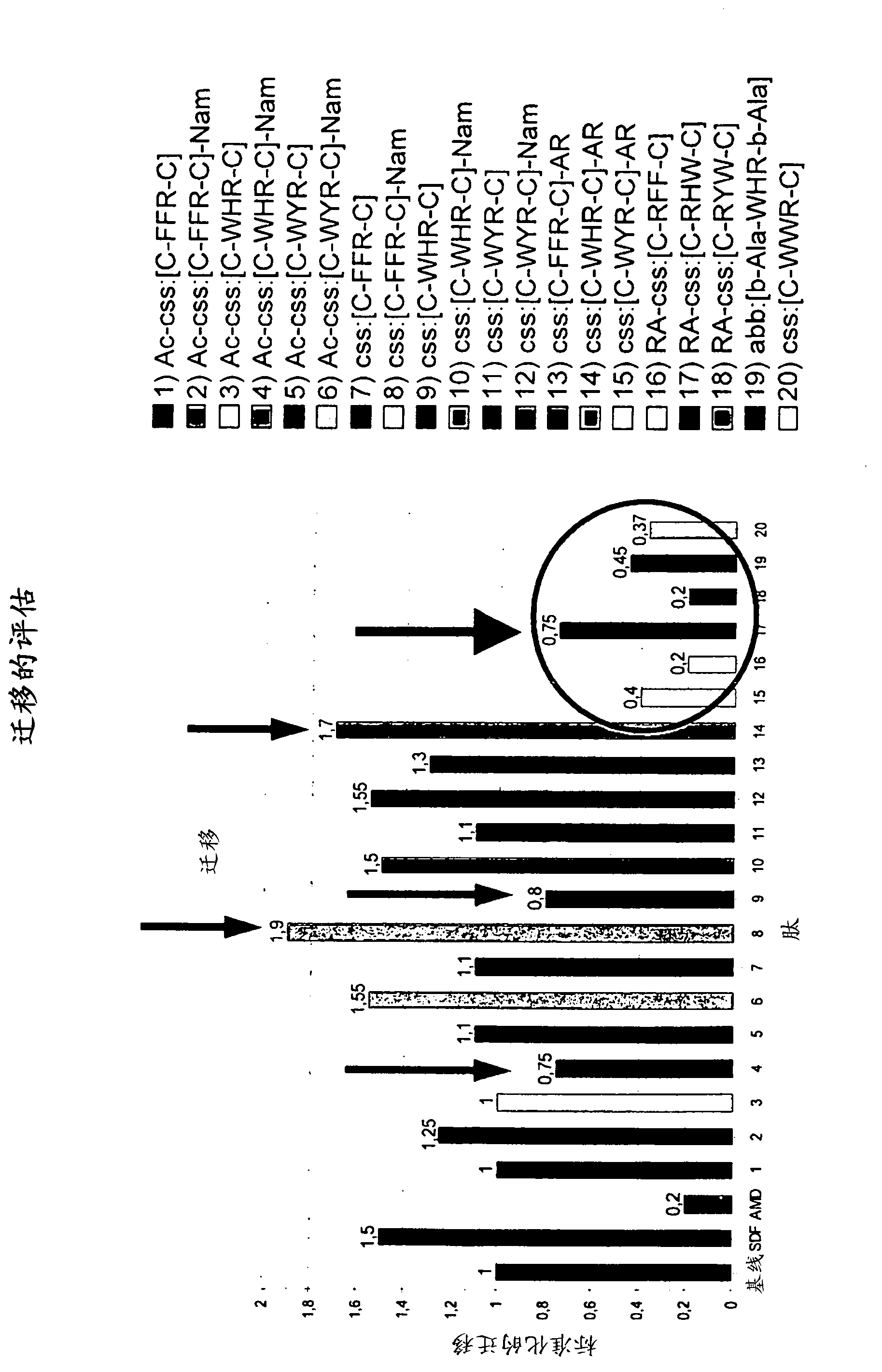
Cyclic peptides binding CXCR4 receptor and relative medical and diagnostic uses
The present invention relates to the identification of new peptides and peptidomimetics which bind the CXCR4 receptor, capable of forming complexes with receptors for chemokines, in particular with CXCR4. The invention also relates to the use of these peptides for the treatment, prevention and diagnosis of diseases which involve chemokine receptors (i.e. neoplasias, metastases, HIV-I virus infections), and also the mobilization of stem cells of hemopoietic precursors in the case of autologous transplants. Finally, the invention comprises pharmaceutical compositions and diagnostic kits comprising said peptides.
Owner:CONSIGLIO NAT DELLE RICERCHE +1
Bone preserving total hip arthroplasty using autograft
ActiveUS20050203524A1Easy to prepareDiagnosticsJoint implantsTotal hip arthroplastyFilling materials
This invention is generally directed to hip surgery (total hip arthroplasty or THA). The invention is more specifically directed to primary THA using autograft in the form of the patient's native and existing cancellous bone as a packing material for the intramedullary canal. This provides for many advantages, including, in the case where the patient later needs a revision surgery, the doctor has more bone to work with and there is less risk of complications.
Owner:MICROPORT ORTHOPEDICS HLDG INC
Methods for collecting and processing autografts, processed autografts, kits for collecting and transporting autografts, and tools for preparing autografts
Owner:LIFENET HEALTH
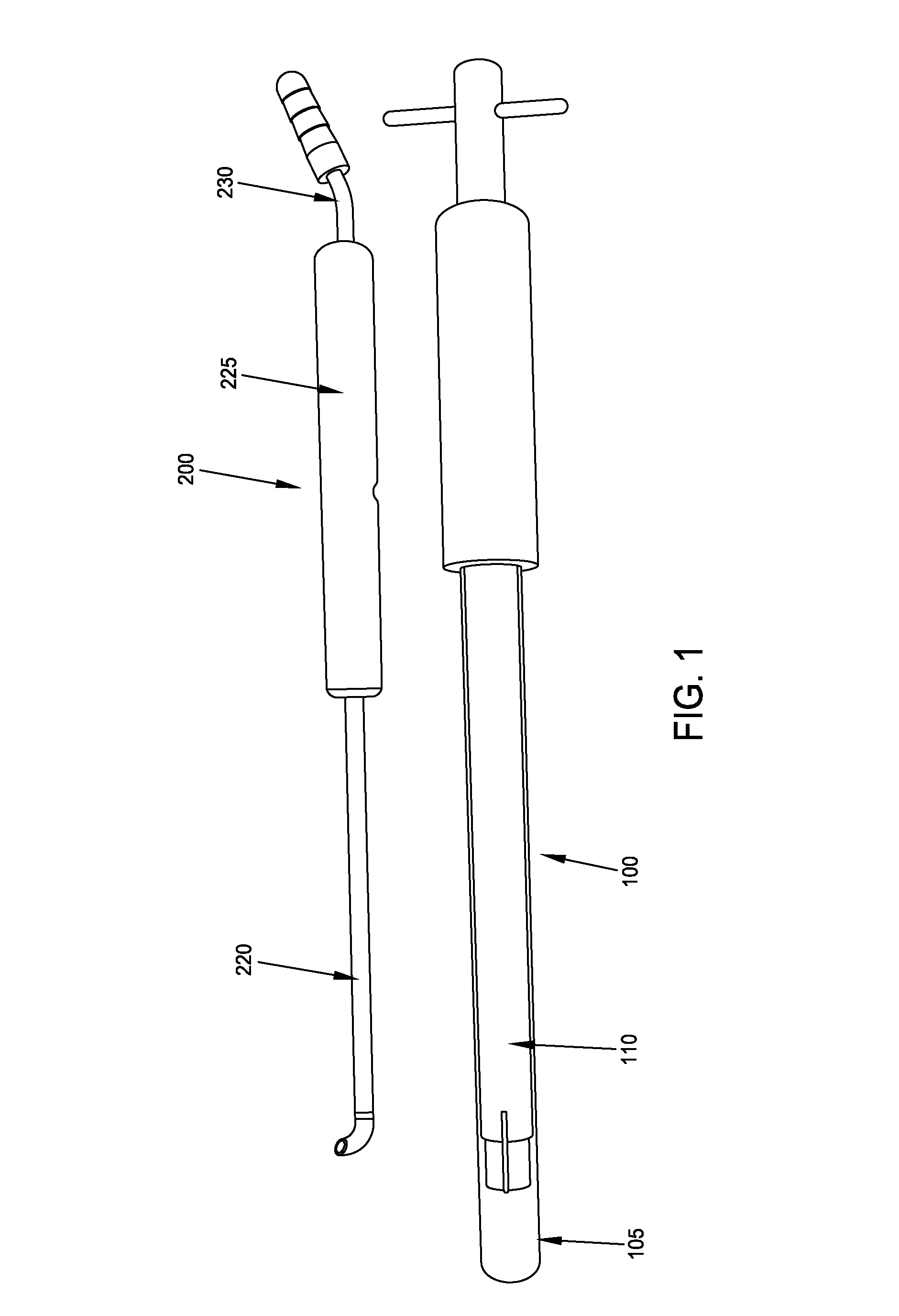
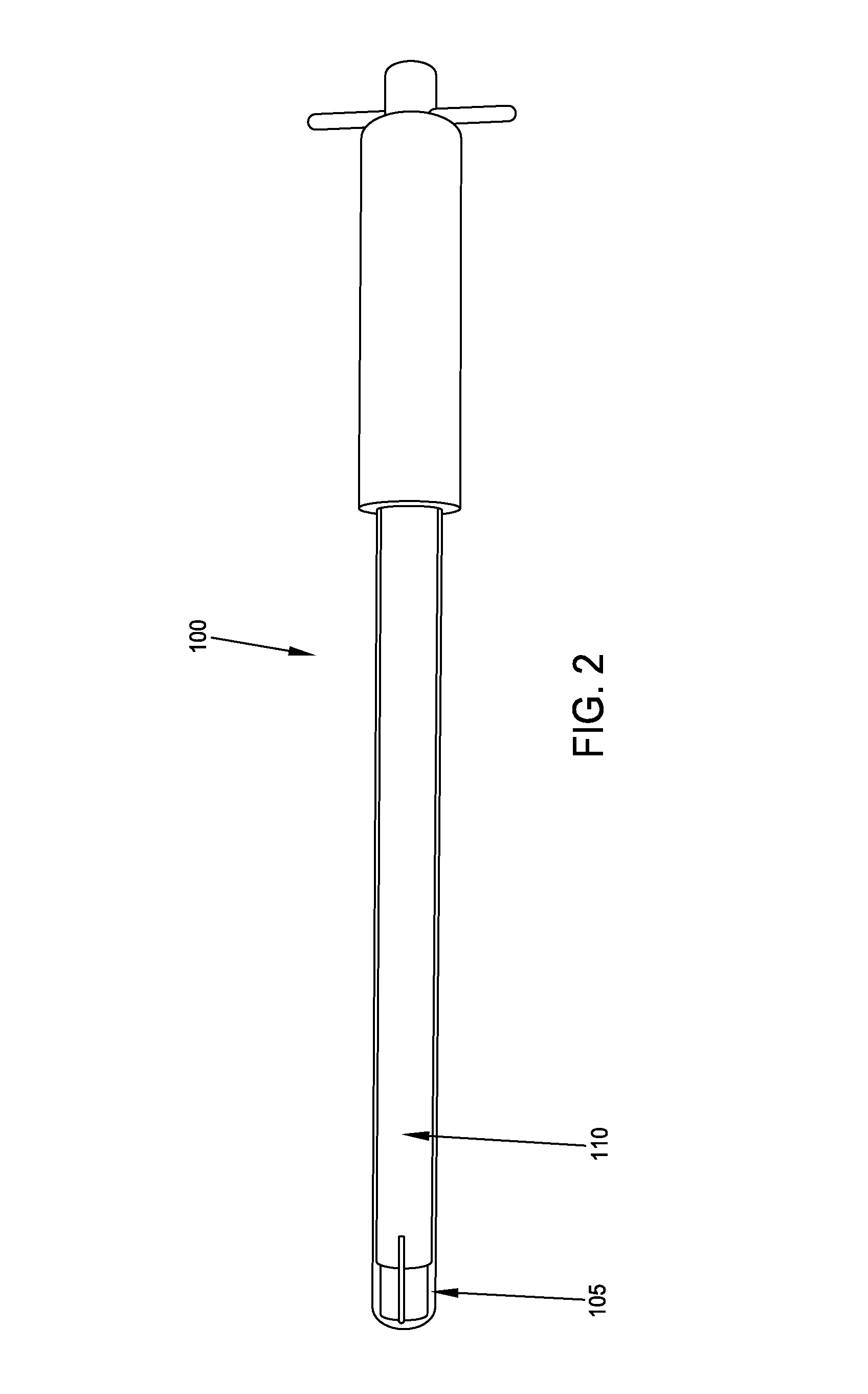
Implant placement trephine, prepackaged and sized implant / trephine kit, and methods of use
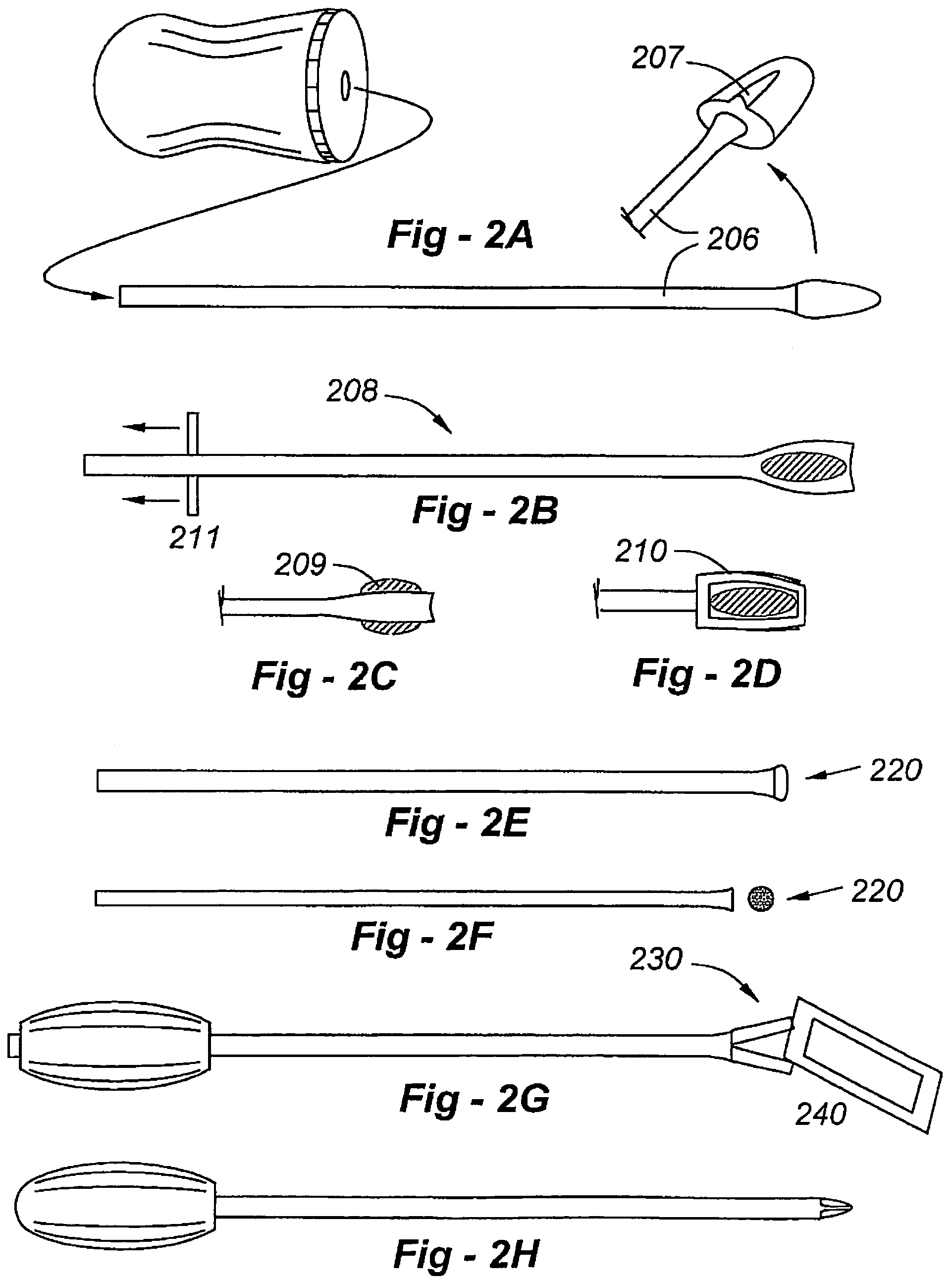
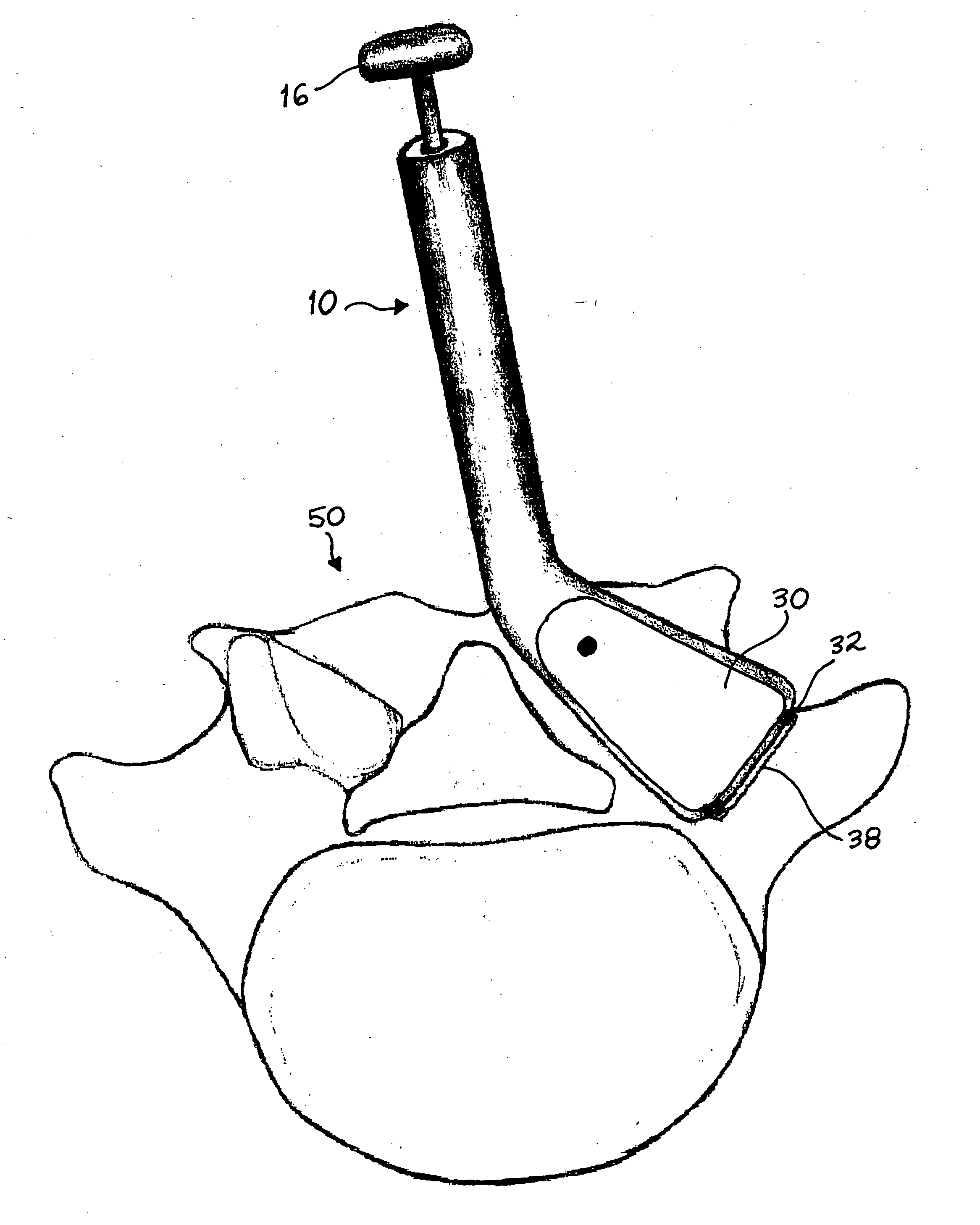
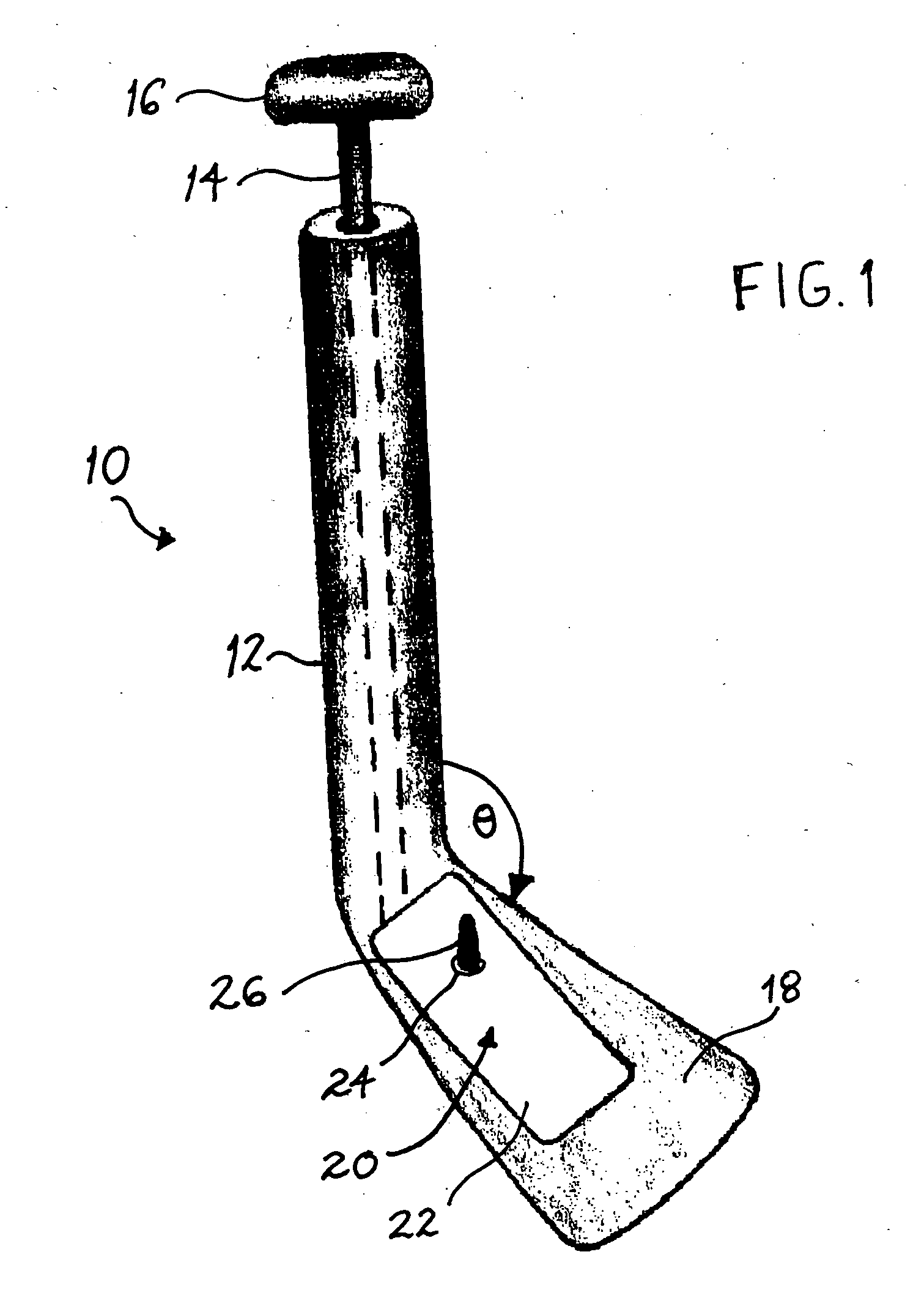
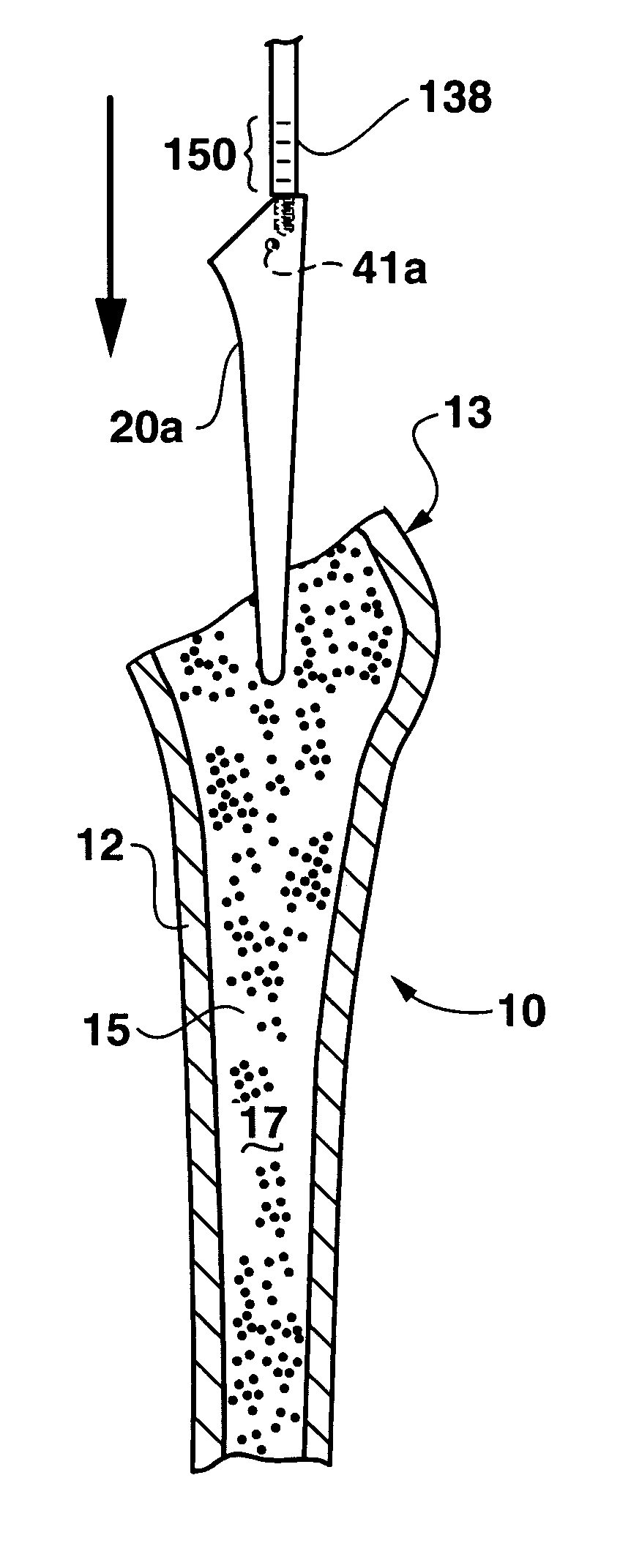
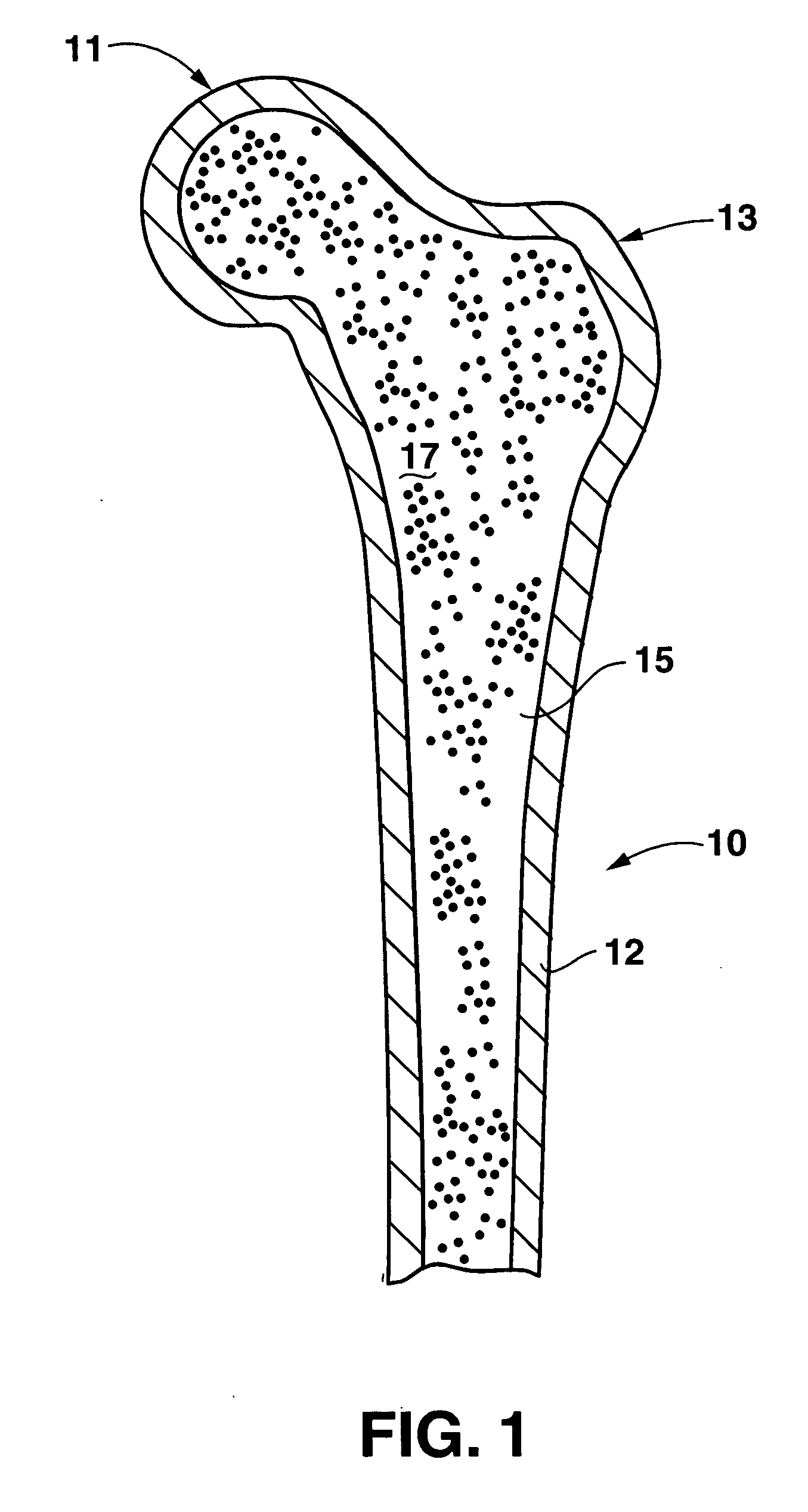
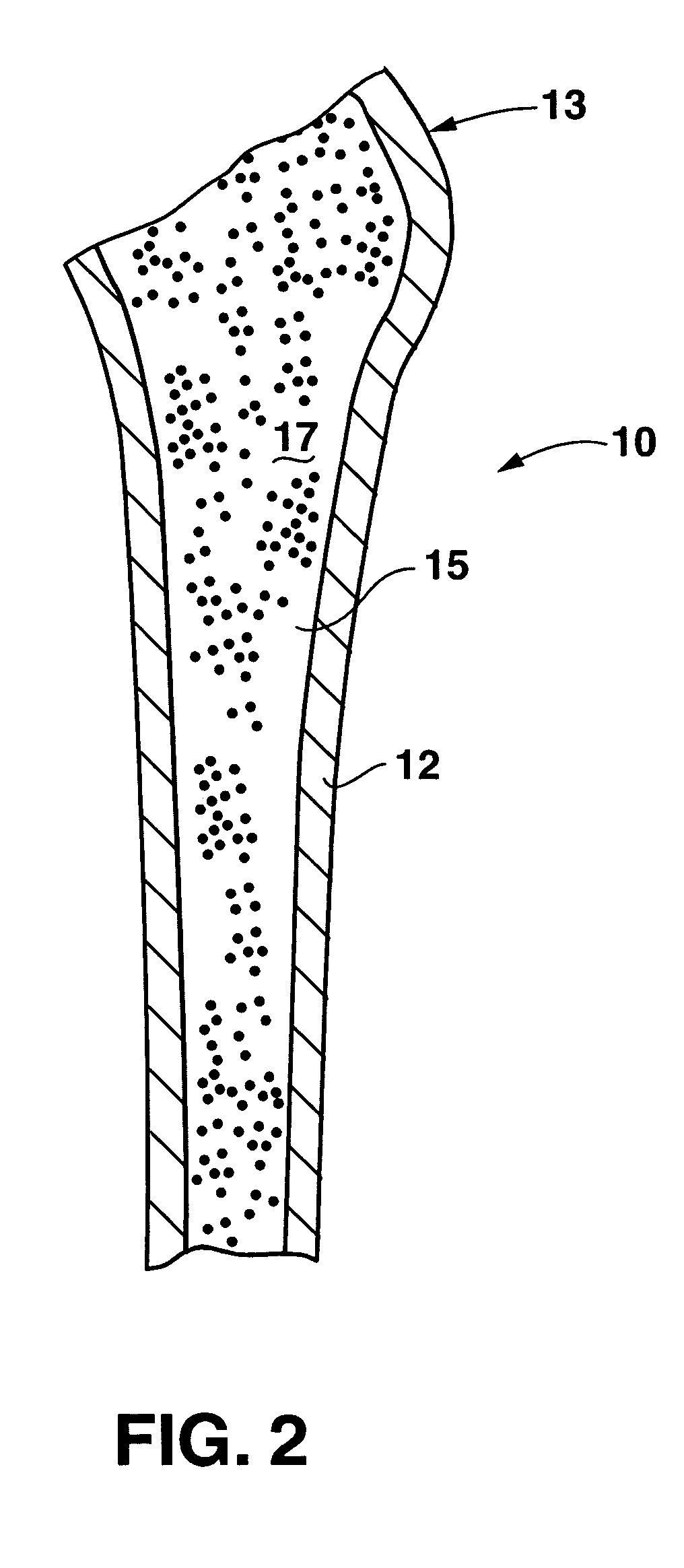
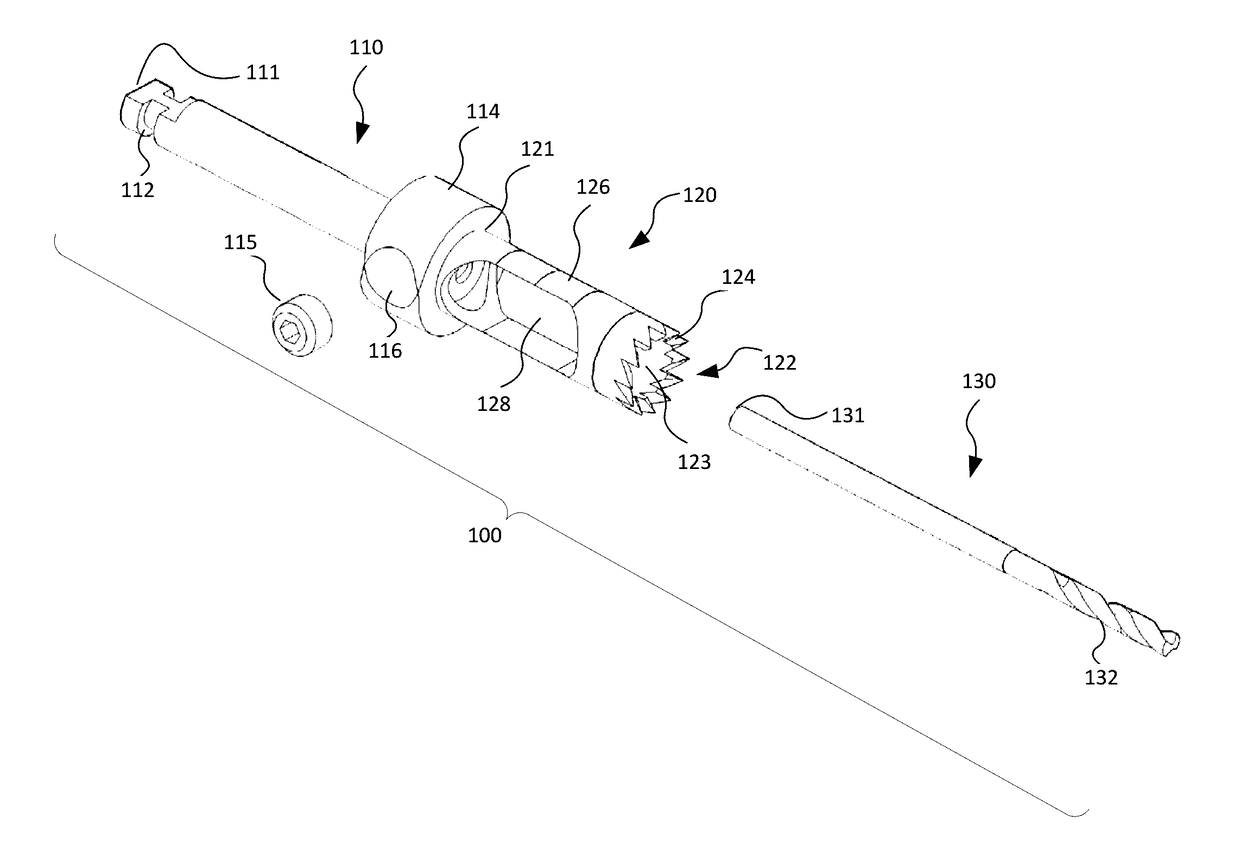
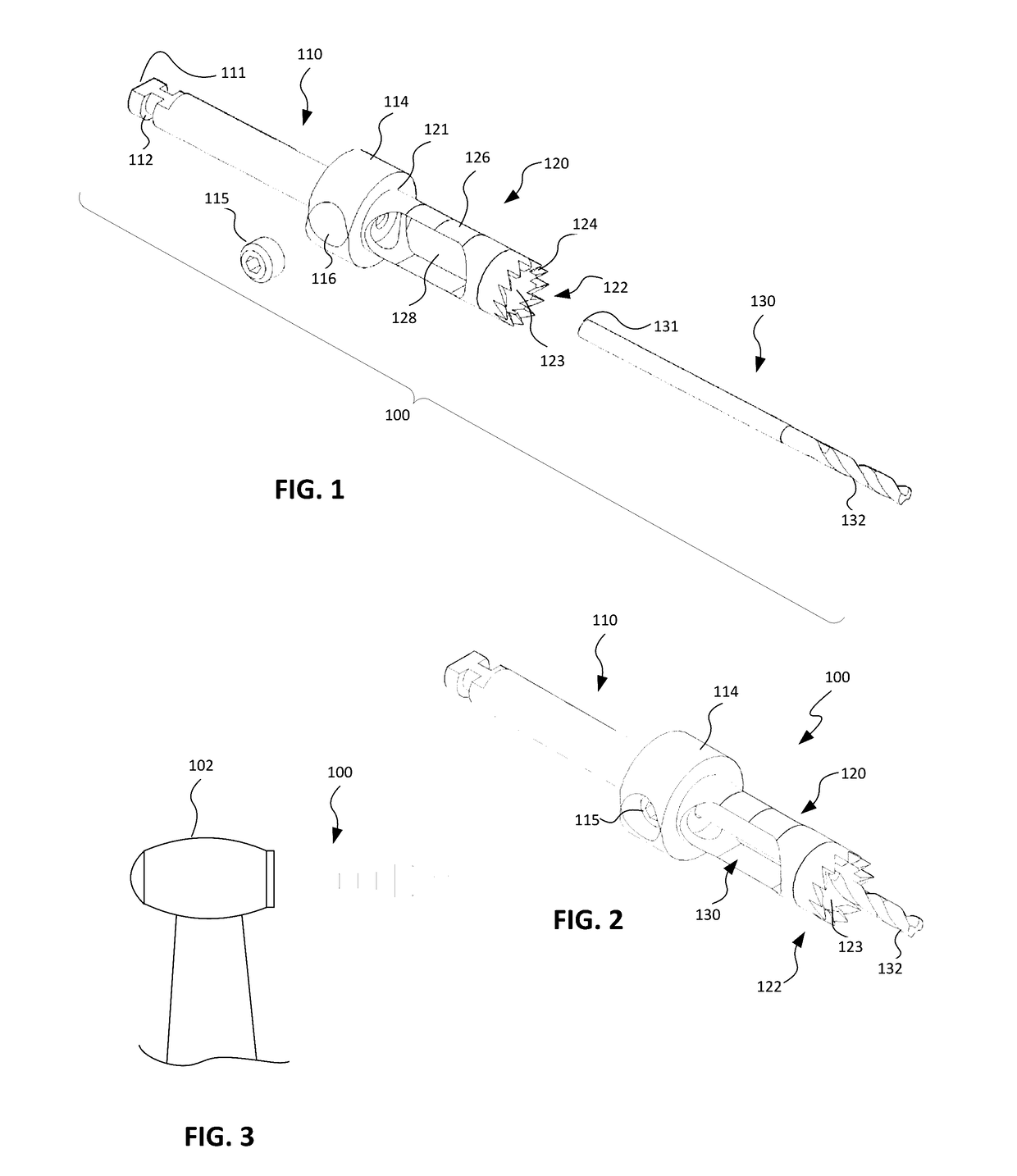
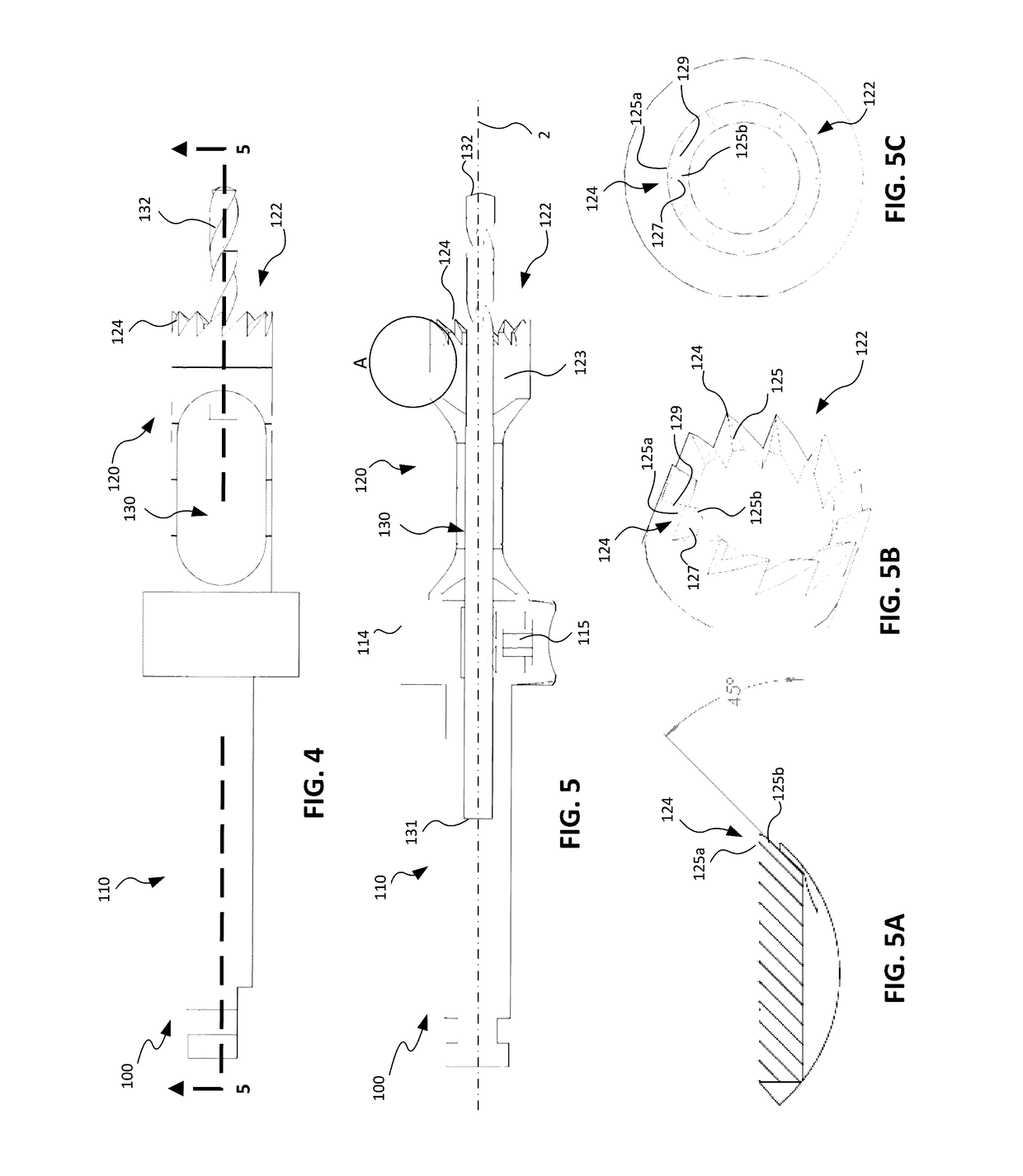
An implant placement trephine may be used to drill into bone and also to bore a core of bone for purposes of forming an implant channel for implant placement. The trephine generally includes a trephine body with a cutting blade at one end and a spindle extending through the trephine body with a pilot drill at one end extending beyond the cutting blade. The spindle may be adjustably attached to the trephine body to allow the pilot drill to be adjusted relative to the trephine cutting blade. The trephine may be prepackaged with an implant, such as a dental implant, having a corresponding size. The implant placement trephine may be used for different osteotomy applications, for example, to form a channel for dental implants or orthopedic retaining stabilizers. The trephine may also facilitate harvesting bone from the core of bone removed for an autograft at the implant site.
Owner:NELORUSSI CORP
Method and apparatus for restoring articular cartilage
The present invention comprises the provision and use of new and improved arthroscopic instrumentation for (i) harvesting a tissue biopsy from a non-critical section of a joint, and (ii) sizing and seating an autologous graft at an implant site.
Owner:PROCHON BIOTECH
Acellular tendon or ligament collagenous fiber material and preparation method thereof
ActiveCN101831724AEasy to separateLow antigenicityMonocomponent protein artificial filamentFiber bundleBiomechanics
The invention relates to the field of biological fiber materials. The conventional tendon and ligament substituent mainly comprises autograft, allograft or heteroplastic graft and synthetic material graft which all have unavoidable defects. The invention aims to provide a natural biological material with excellent biomechanical properties and biocompatibility, namely an acellular tendon / ligament collagenous fiber material, and a preparation method thereof. In the preparation method, a mechanical structural unit and a cell attachment structure which tendon / ligament collagenous fibers or fiber bundles originally have are reserved; the tendon / ligament fibers are processed by adopting a low-temperature drying method, and the separated fibers reserve certain mechanical strength; and the collagenous fibers or fiber bundles are selected by a physical loosening and carding method and then acellular treatment is performed. The tendon / ligament acellular collagenous fiber / fiber bundle material of the invention has wide source, large quantity and low price and not only can be used for ligament and tendon reconstruction and rehabilitation, but also can be widely applied to other biomedical fields because the tendon / ligament acellular collagenous fiber / fiber bundle material has properties of the fiber material.
Owner:陈雄生 +2
Supplemented matrices for the repair of bone fractures
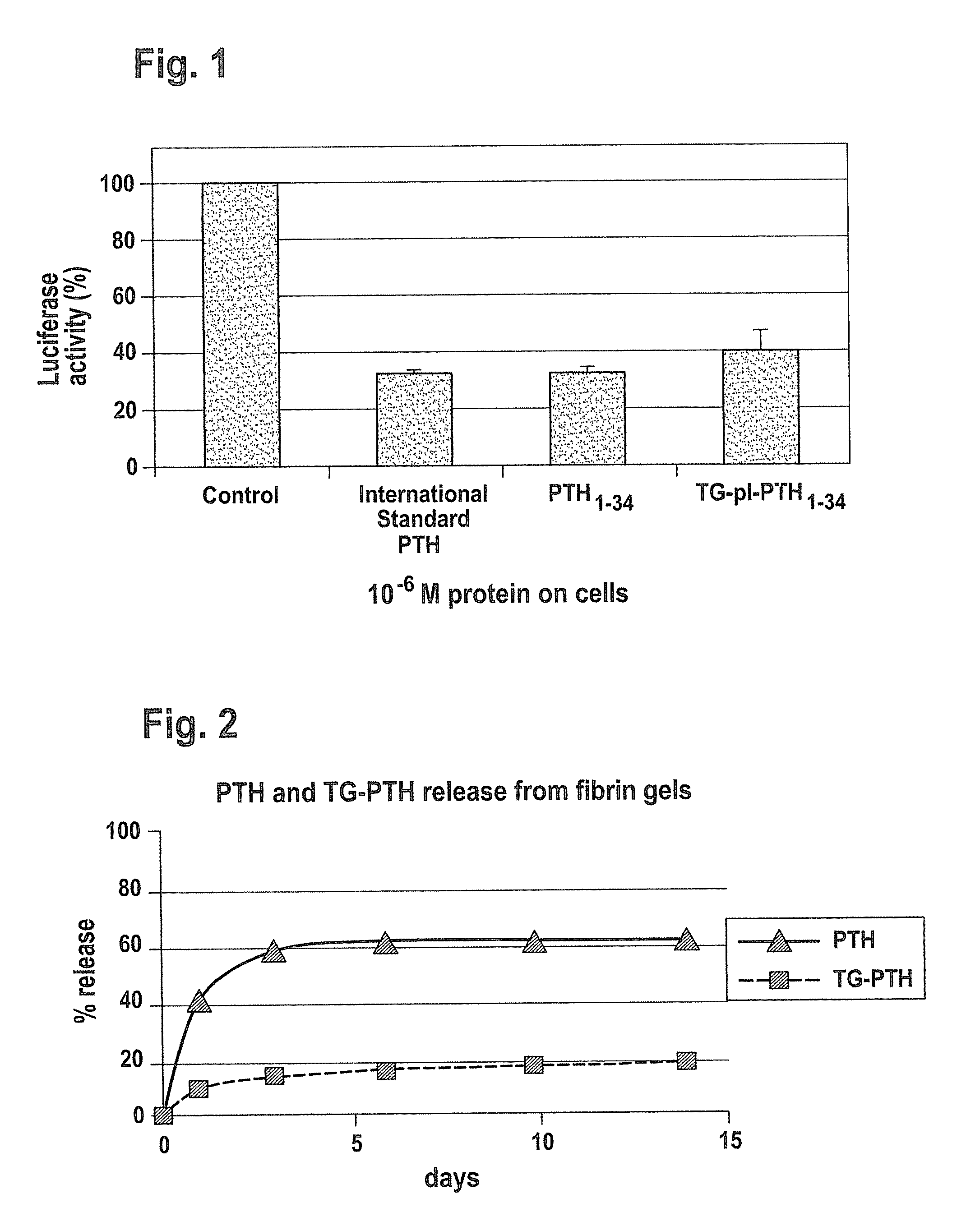
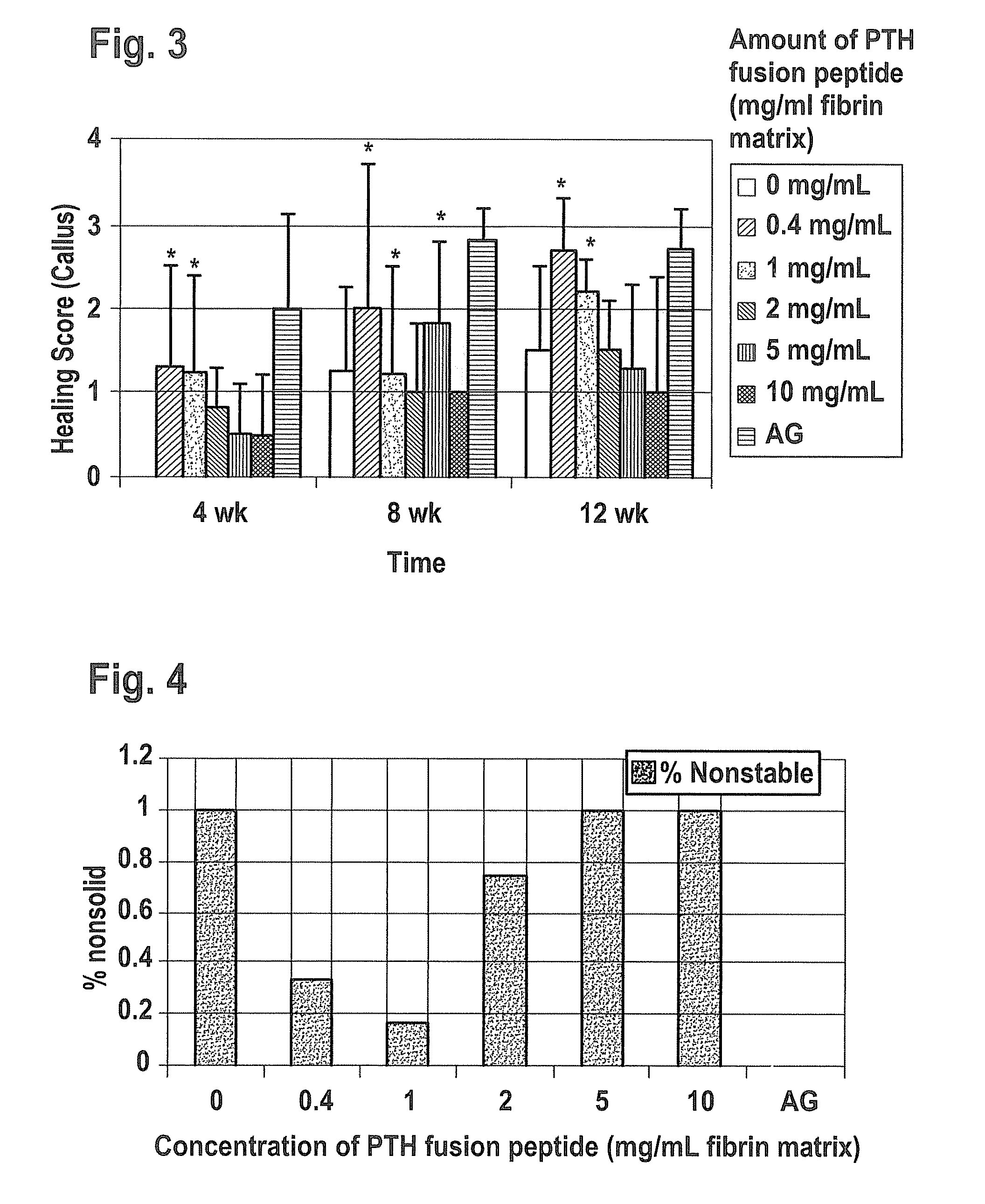
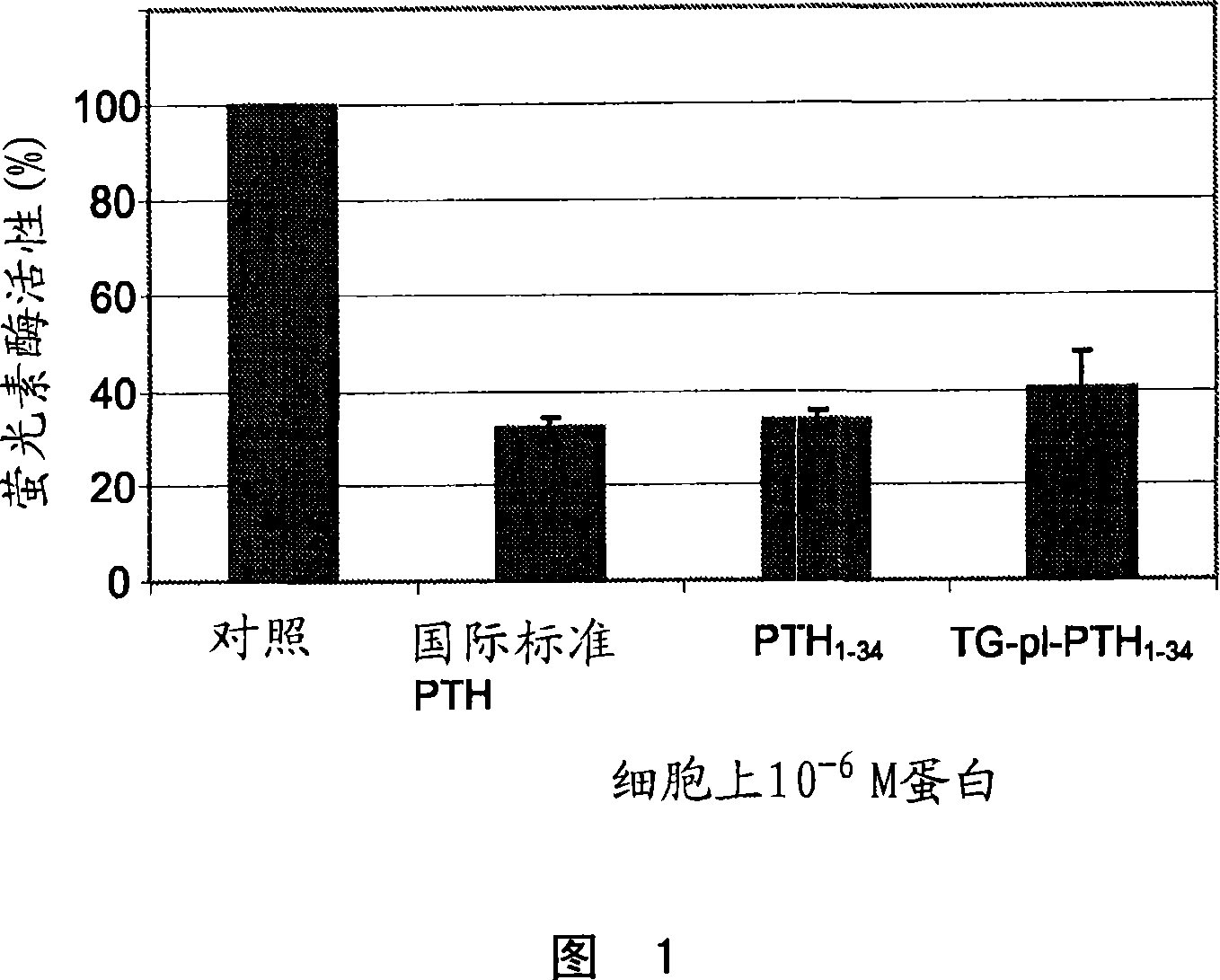
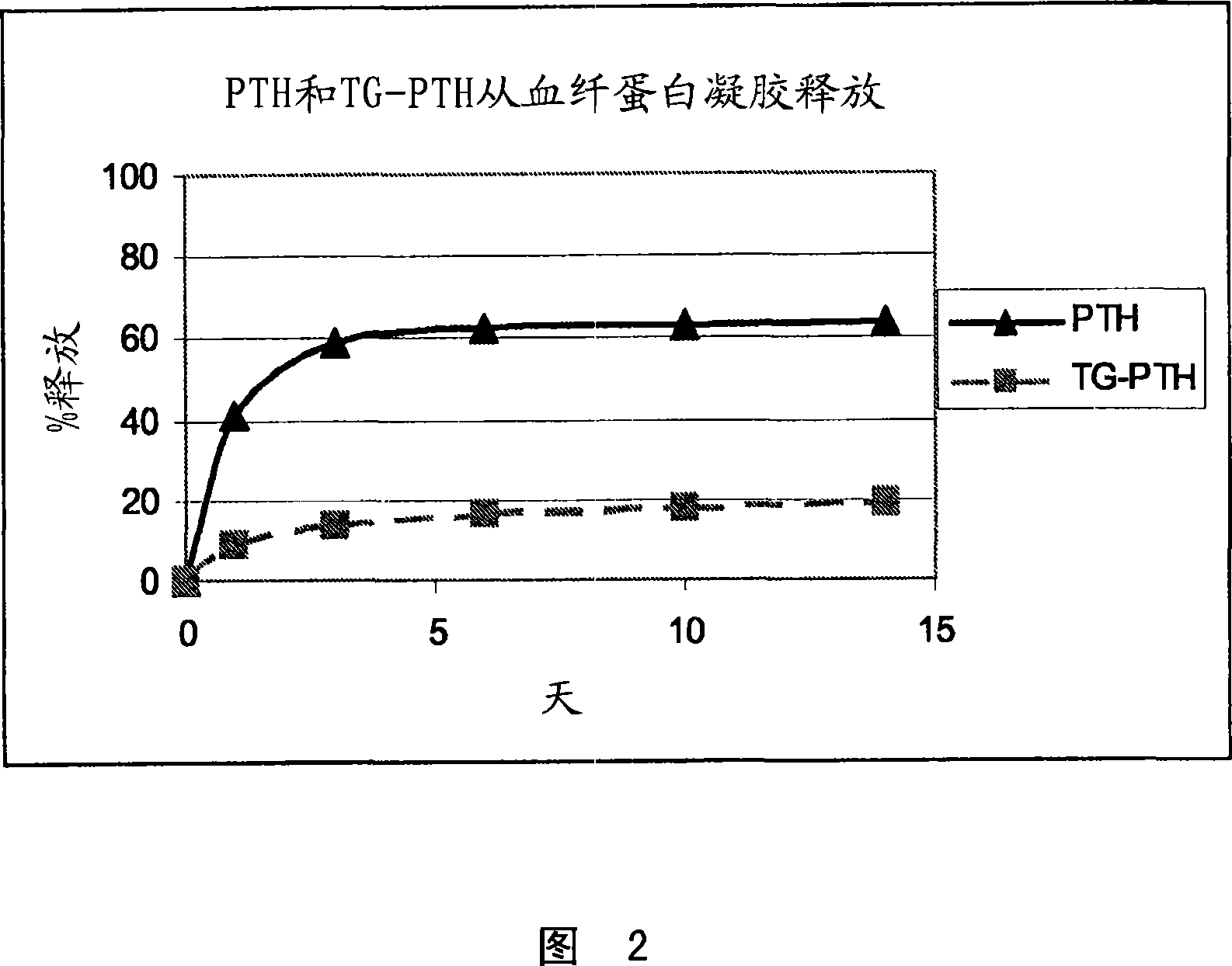
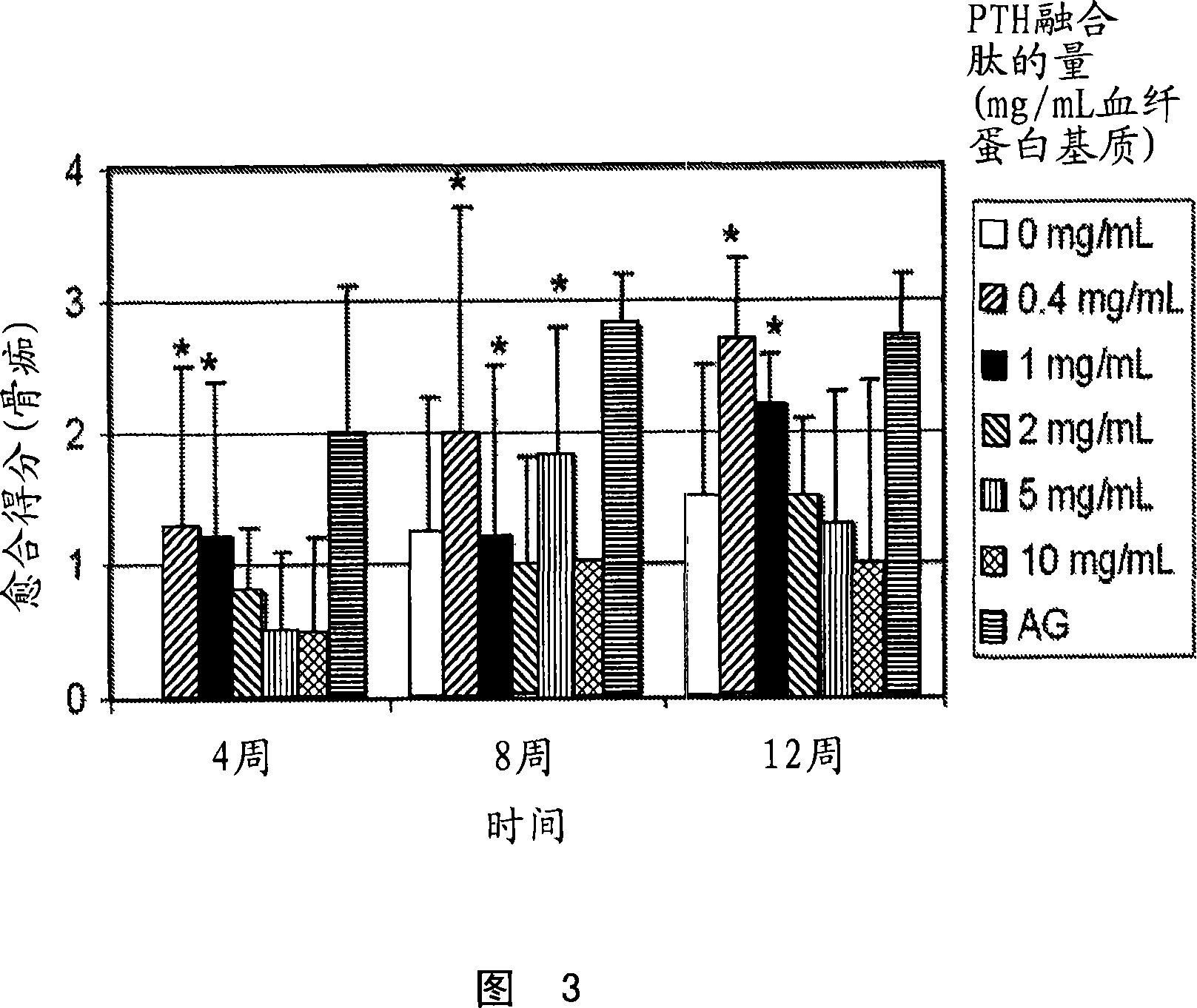
Supplemented matrices comprising a PTH releasably incorporated therein, optionally containing a granular material, which are used to heal bone fractures, particularly bone fractures with a risk of becoming delayed unions or non-unions, are described herein. The PTH is incorporated either through covalent linkage to the matrix or through non-covalent interaction with the matrix and / or the granules. These supplemented matrices decrease the time of healing compared to autograft and or trigger healing of bone fractures which otherwise would not heal. The matrices are biocompatible, preferably biodegradable, and can be formed in vitro or in vivo, at the time of implantation. The PTH may be a part of a fusion peptide. PTH can be incorporated into the matrices with full retention of its bioactivity. PTH can be releasably incorporated in the matrix.
Owner:KUROS BIOSURGERY AG
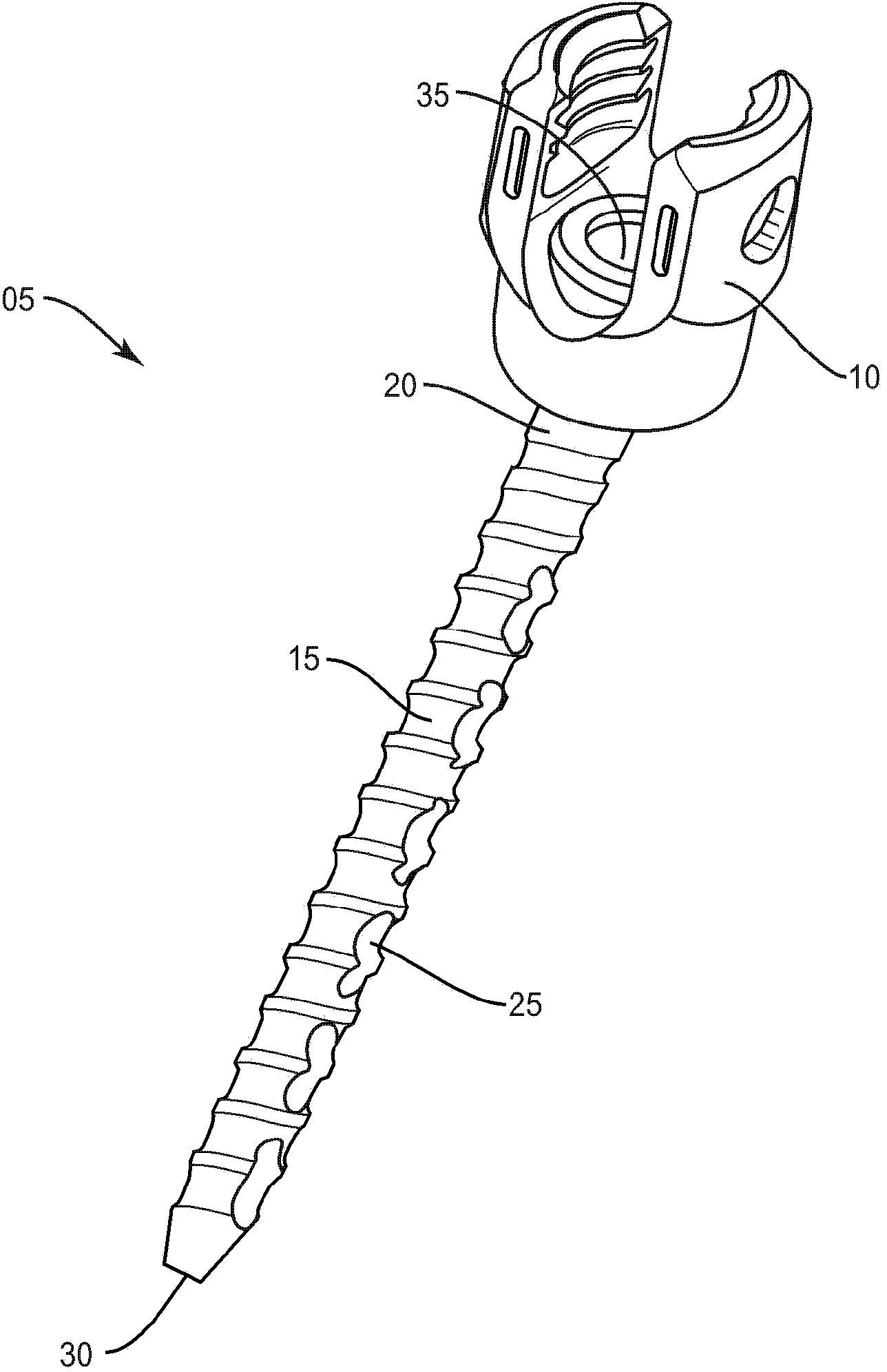
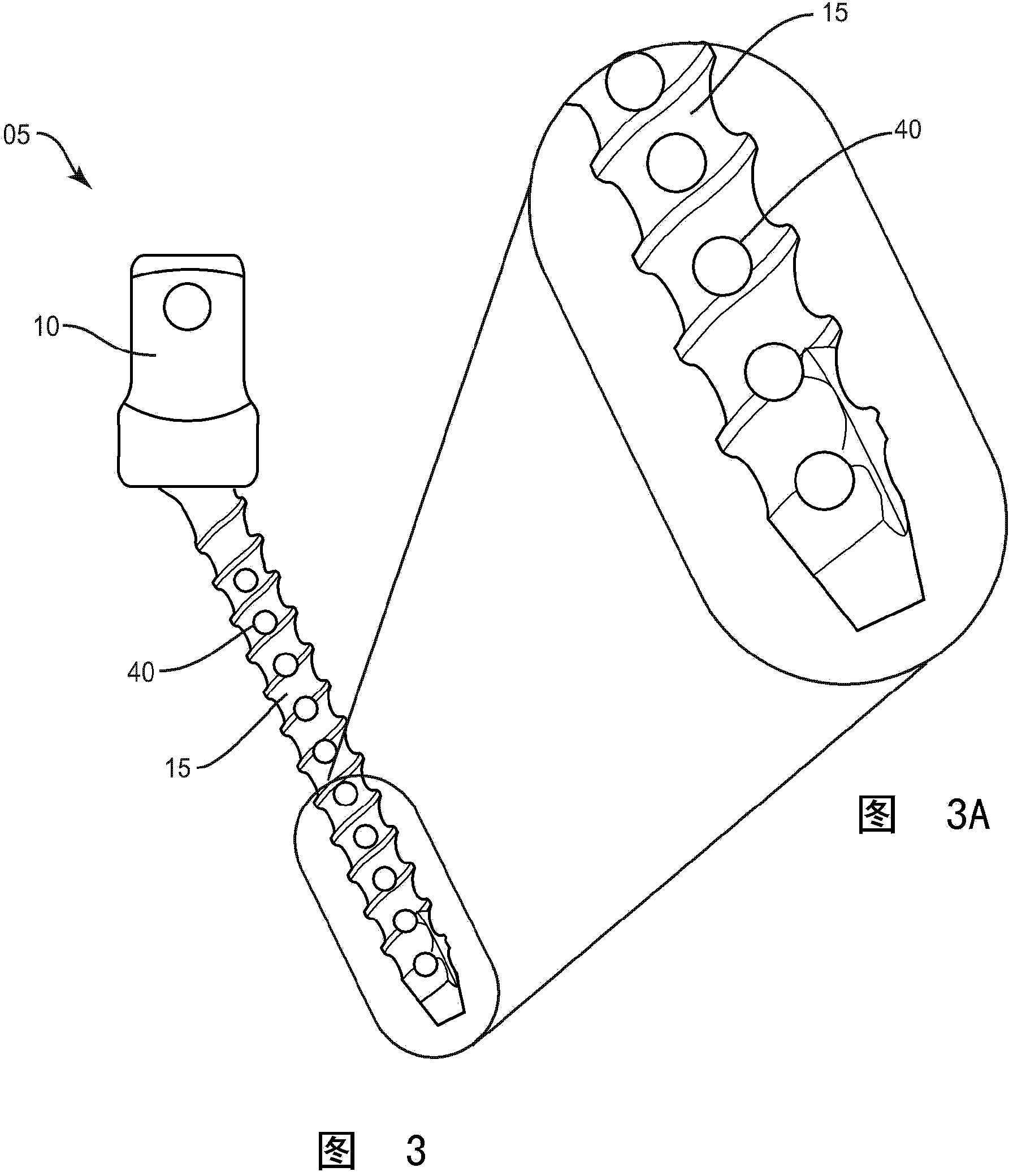
Bone fastener and methods of use
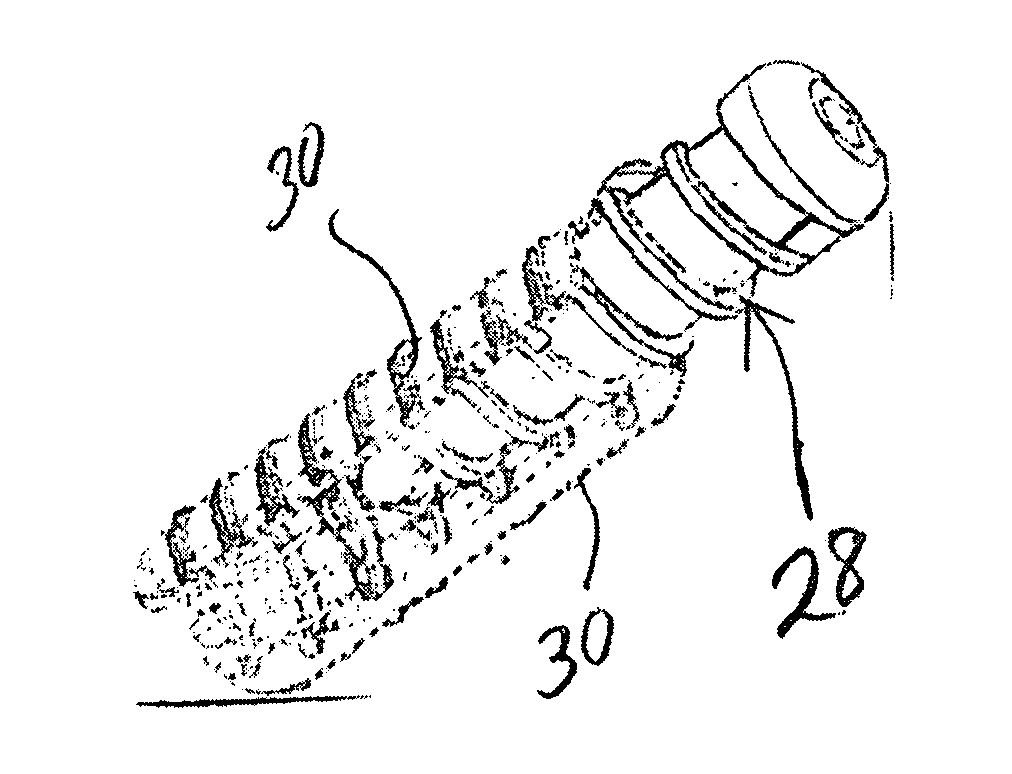
A bone fastener includes a head, an elongated shank defining a longitudinal axis having a proximal end and a distal end. The proximal end is connected to the head and the elongated shank has an inner surface defining a longitudinal cavity disposed along the longitudinal axis and at least one transverse cavity positioned in the elongated shank. The inner surface defining the transverse cavity and the elongated cavity as well as the outer surface of the elongated shank has bone conductive material disposed thereon so as to promote bone growth into the transverse cavities, elongated cavity and on the surface of the elongated shank in order to secure the bone fastener to bone. The distal end of the bone fastener can be configured to have cutting features that cut into bone and self-pack the elongated cavity with autograft material as it is inserted into bone.
Owner:WARSAW ORTHOPEDIC INC
Devices, methods, and systems for the treatment and/or monitoring of damaged tissue
Disclosed are methods, devices, and systems for treatment of an abnormal wound healing response. The methods, devices, and systems can be used to treat solid ulcerated tissue, such as diabetic foot ulcers (DFUs), arthritic tissue, muscle soreness, joint pain, varicose veins, obesity, and peripheral artery disease. Additionally, the methods, devices, and systems can promote the healing of xenograft, allograft, autograft, or engineered tissue following reconstruction surgery. The methods, devices, and systems include the ability to determine an optimal set of pulse parameters that are specific to wound healing. In embodiments, the methods, devices, and systems include components for guiding the user on electrode placement based on anatomical or electrical measurements, delivering a custom series of electric pulses, applying heat, and using feedback from physiologic measurements to control the device.
Owner:ADLORE INC
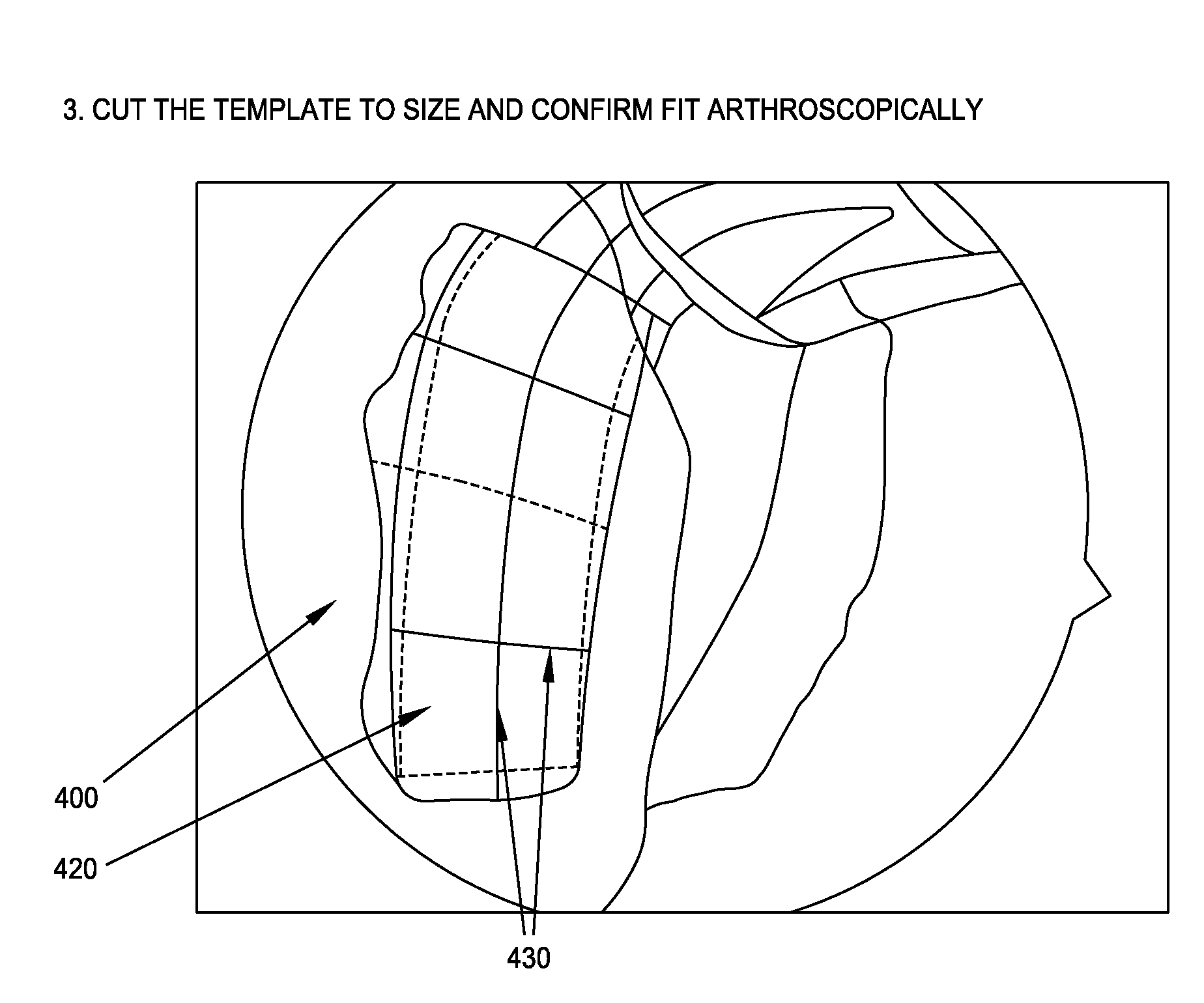
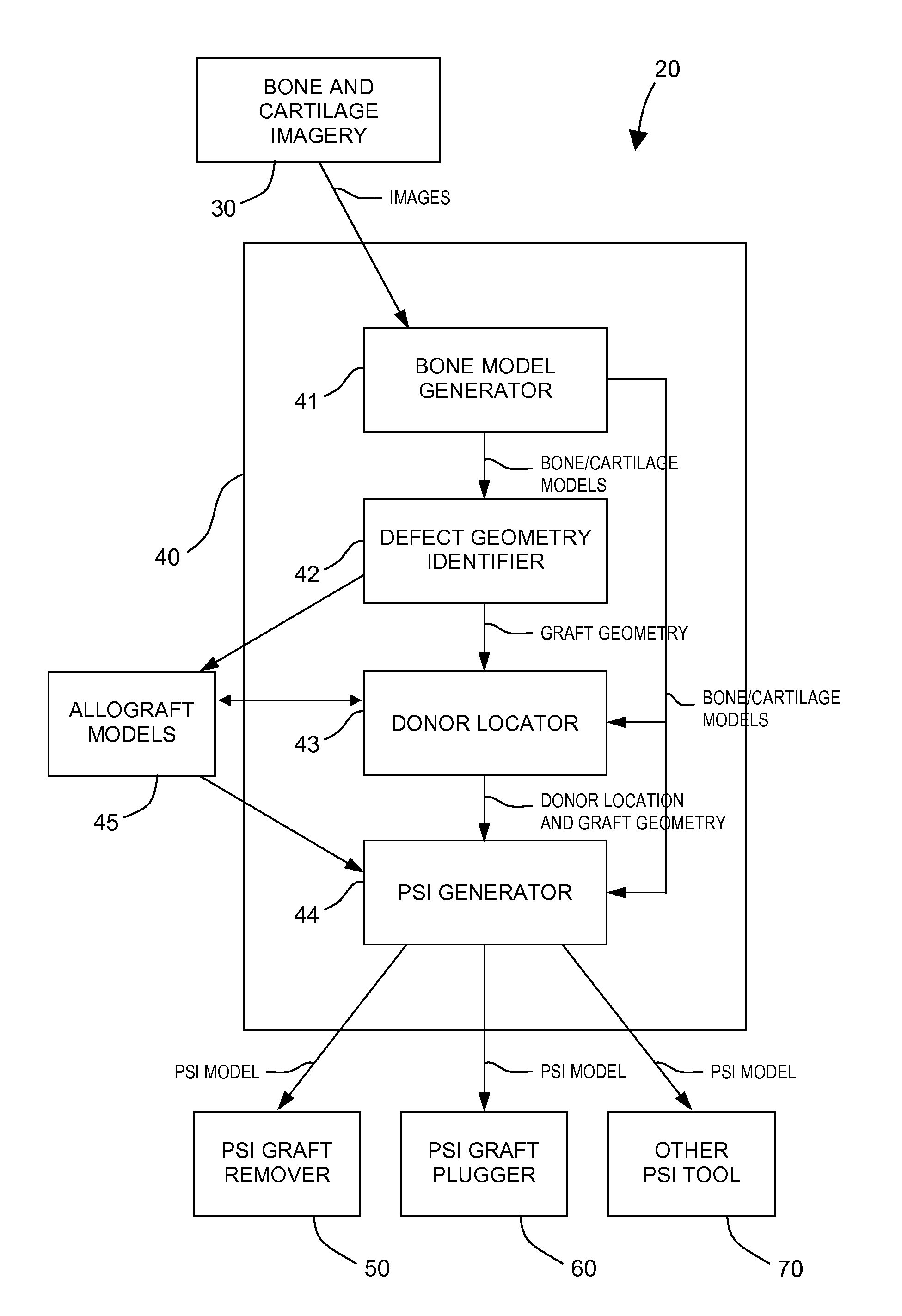
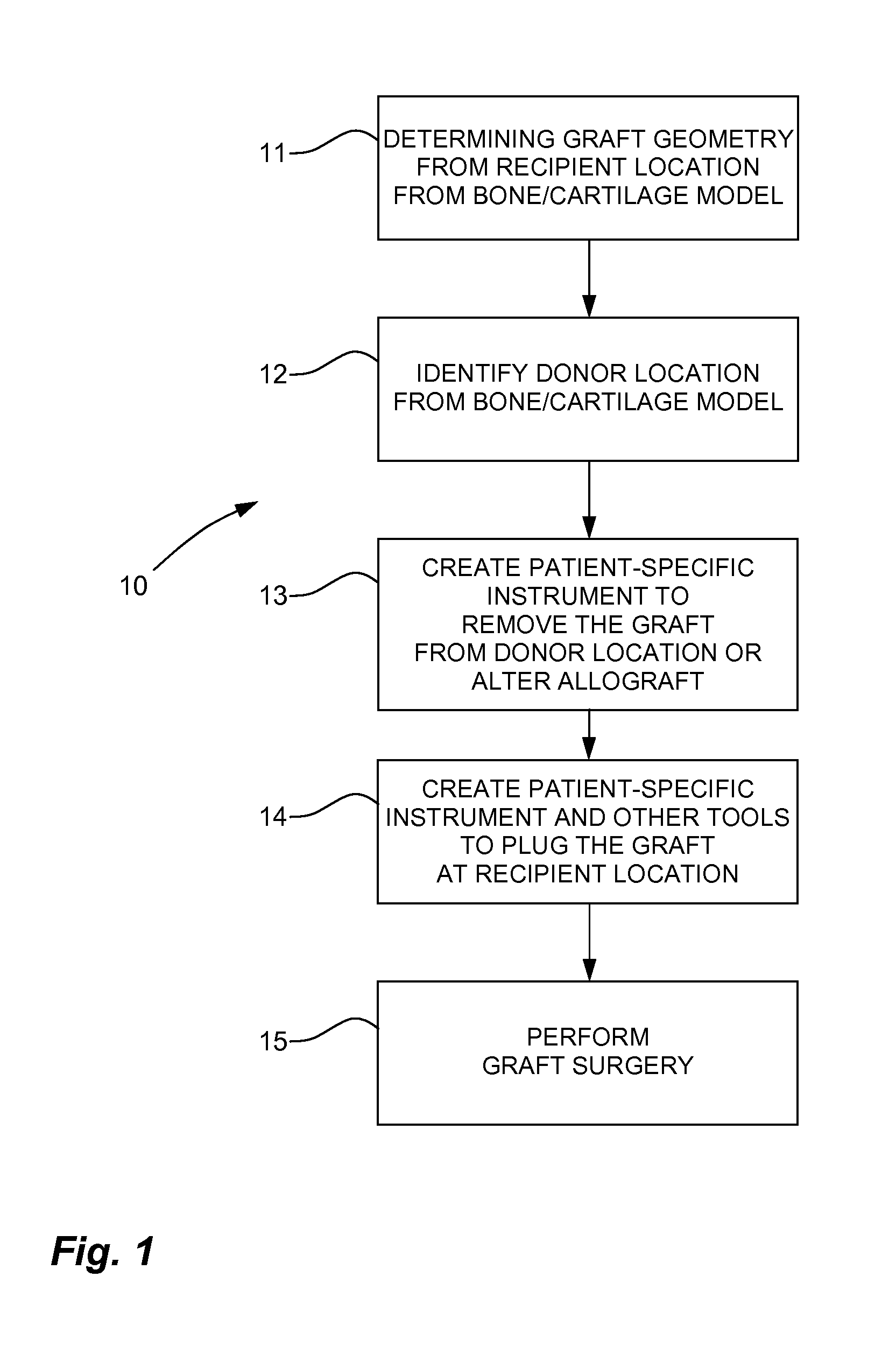
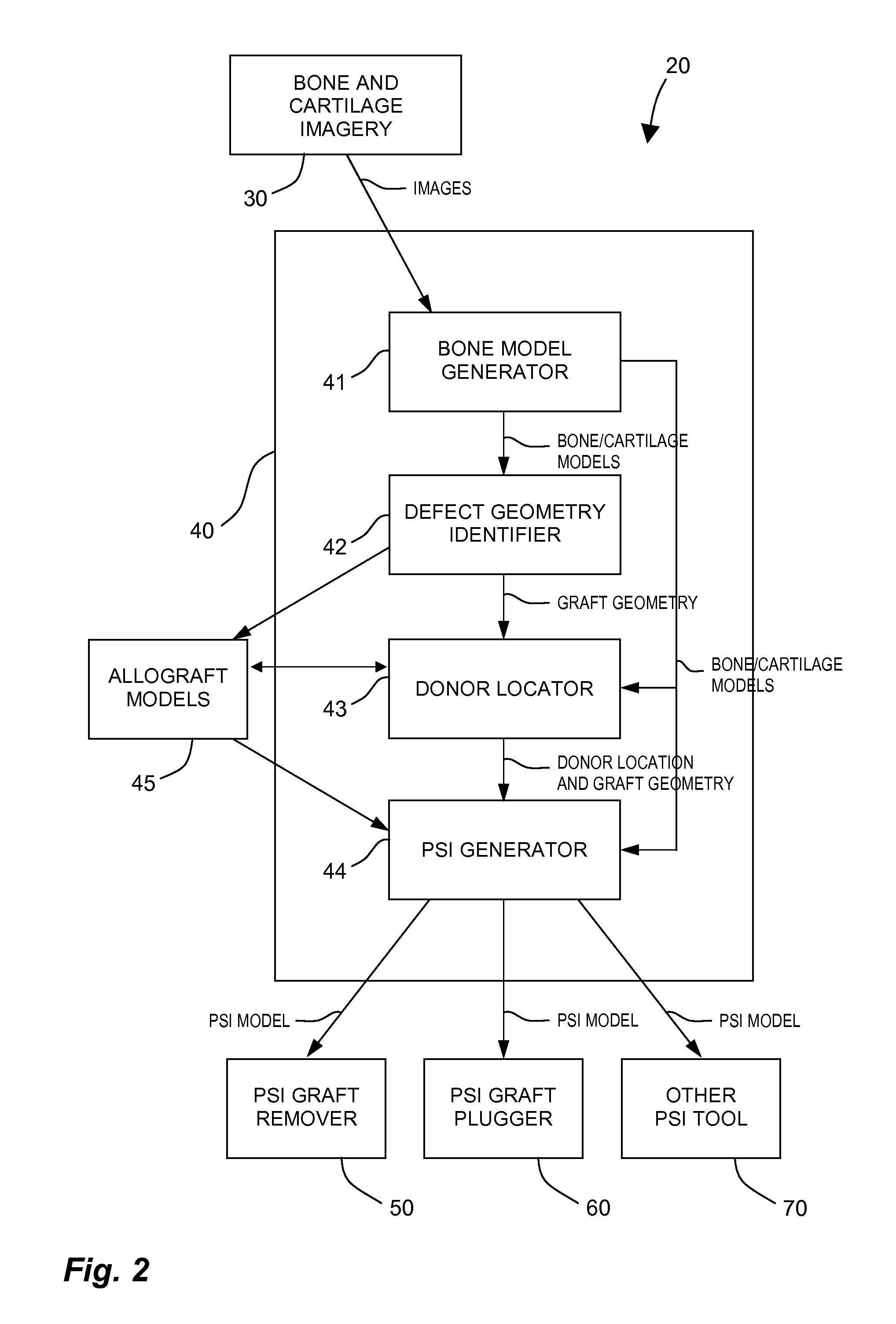
Method and system for creating patient-specific instrumentation for chondral graft transfer
A system for creating a patient-specific instrument model for chondral graft plugging comprises a bone model generator for producing a bone / cartilage model of an articular region of a bone from images thereof. A defect geometry identifier identifies a graft geometry from a defect region of said bone / cartilage model. A donor locator locates an autograft at a donor site or identifies an allograft from a database, using said bone / cartilage model and the graft geometry. A patient-specific instrument model generator creates a model of a graft-plugging patient-specific instrument from said bone / cartilage model, and the graft geometry, the graft-plugging patient-specific instrument model comprising a bone / cartilage interface surface shaped as a function of the bone / cartilage model for the at least one graft-plugging patient-specific instrument to be selectively positioned on the bone / cartilage to pose the autograft or the allograft at the defect region.
Owner:ZIMMER INC
Methods and compositions for treatment of hematologic cancers
The present invention relates to oncolytic picornaviruses and methods and compositions for treating subjects having hematologic cancers. These include methods and compositions for treatment of myeloma, using disclosed Picornavirus such as Coxsackievirus, in methods of direct or indirect administration to subjects and ex vivo purging of malignant cells within auto grafts prior to transplantation.
Owner:MERCK & CO INC
Method and apparatus for restoring articular cartilage
The present invention comprises the provision and use of new and improved arthroscopic instrumentation for (i) harvesting a tissue biopsy from a non-critical section of a joint, and (ii) sizing and seating an autologous graft at an implant site.
Owner:PROCHON BIOTECH
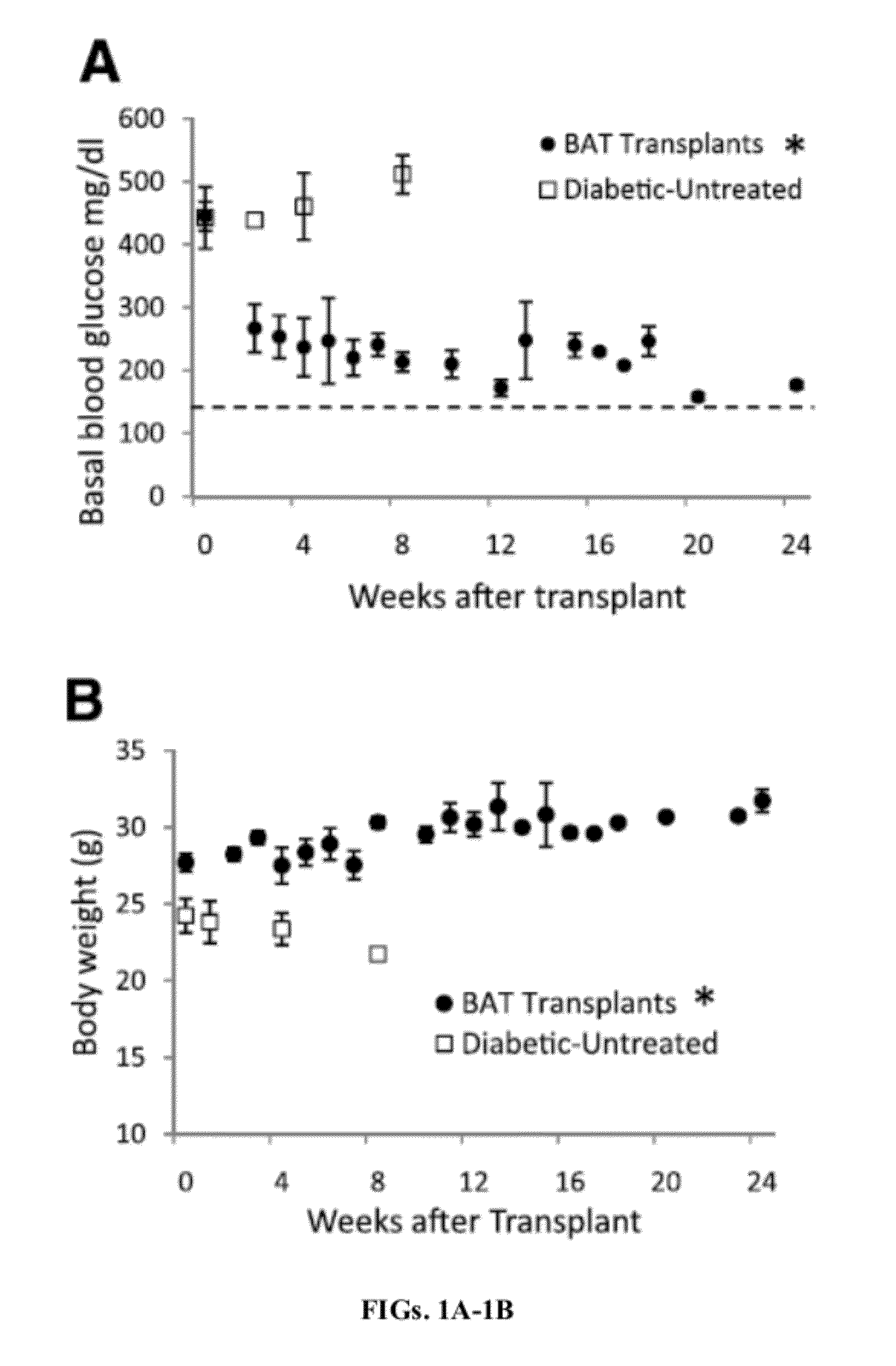
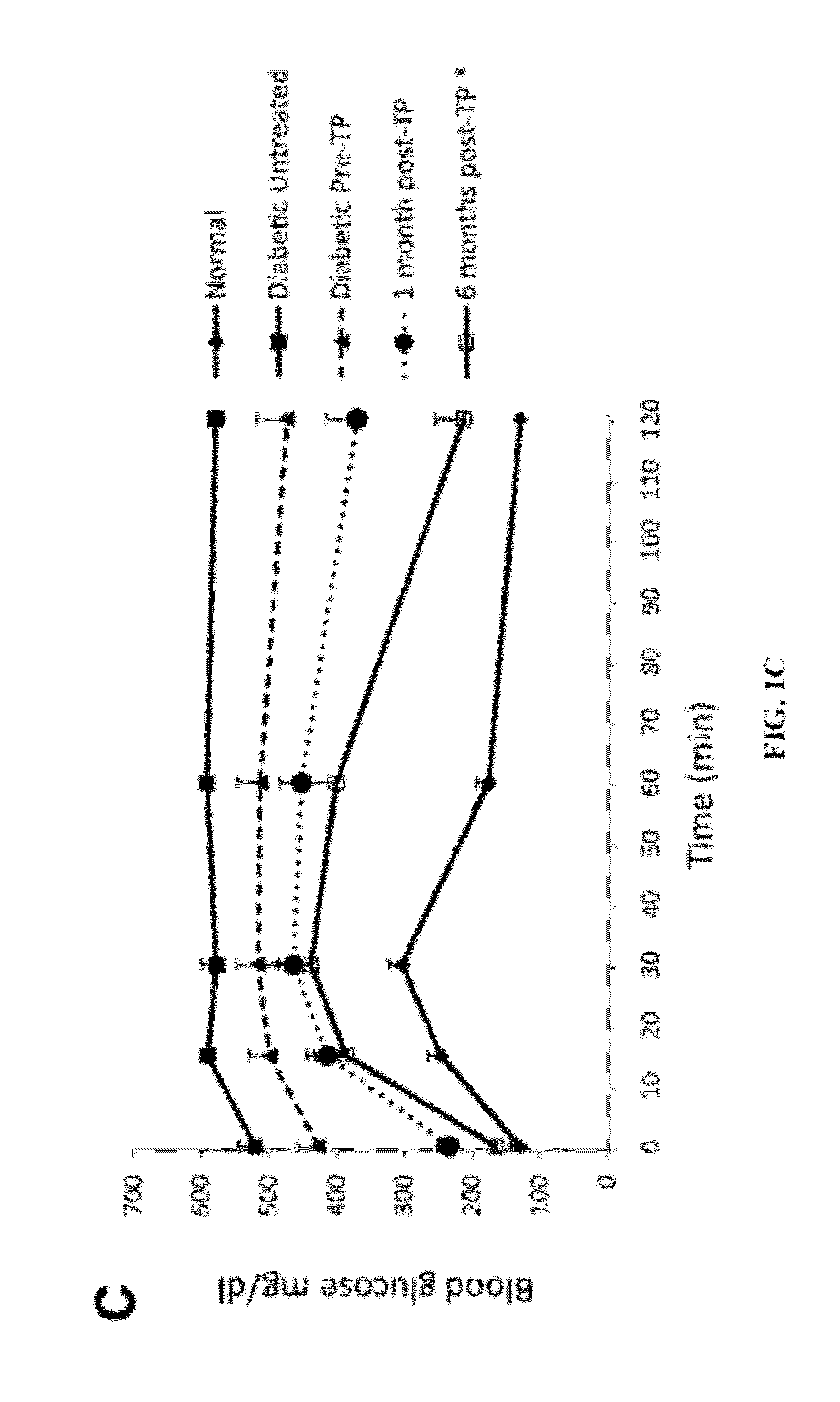
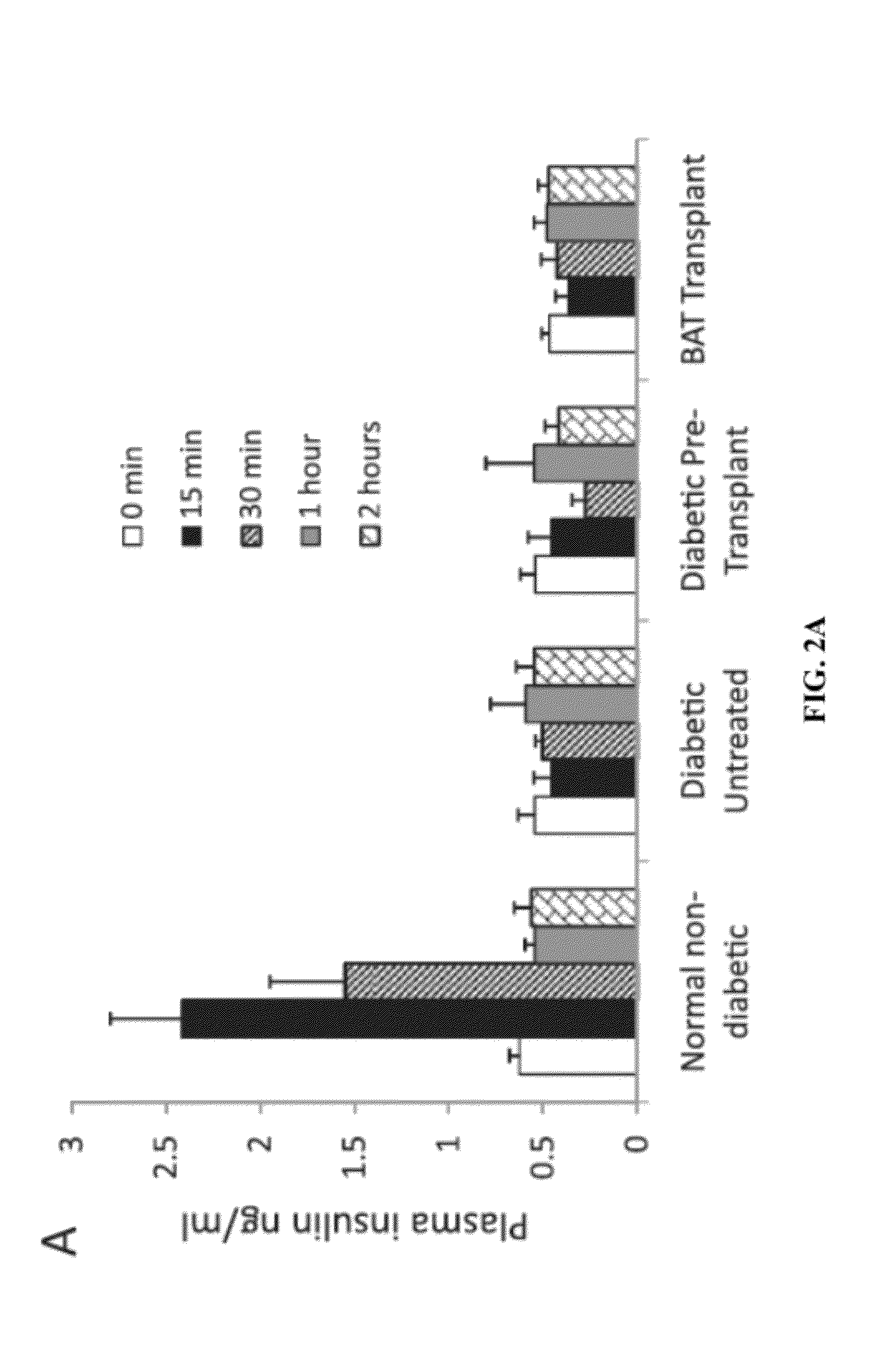
Methods For Treating Type Diabetes With Brown Adipose Transplants
InactiveUS20120225131A1Restoring normoglycemiaReducing and suppressing and attenuating and inhibitingMetabolism disorderMammal material medical ingredientsFat graftingBrown adipose tissue
Owner:VANDERBILT UNIV
System and method for spine ligament reconstruction
InactiveUS20160270903A1Readily conformsSpine mobilitySuture equipmentsInternal osteosythesisSpinal ligamentsProsthesis
A simple and flexible non-synthetic ligament which easily conforms to a patient's anatomy and can be used independently or in combination with an intervertebral graft, implant or prosthesis. A single length of allograft or autograft is provided, per each side of the vertebral column, to replace and recreate the spinal ligament spanning adjacent vertebrae. The single length allograft or autograft is secured to spine pedicles with fixation devices such as bone screws like interference screws and / or anchors such as SwiveLock® or PushLock® anchors.
Owner:ARTHREX
Apparatus for osteotomy and graft preparation
InactiveUS8920426B2Promote resultsAdequate healing of patient boneJoint implantsNon-surgical orthopedic devicesOrthopedic ProceduresPlastic surgery
Surgical tools that increase surgical accuracy for orthopedic procedures involving long bone osteotomy with or without supplementation with allograft or autograft. Two clamps on the operative side are rigidly interconnected to one another with an adjustable rod that is locked into place, preserving length and rotation of the bone. A graft preparation device is used on the operative table. It enables alignment of the graft to preserve the desired mechanical and anatomic axes and to provide rigid fixation of the graft for cutting. Adjustable jigs ensure exact cuts.
Owner:UNIV OF SOUTH FLORIDA
Bacterial antigen induced bone morphogenesis
InactiveUS20060040878A1Induce bone growthPromote bone growthBacterial antigen ingredientsGenetic material ingredientsBacteroidesBone growth
Bone growth following a spinal fusion procedure is enhanced by packing the fusion site with a mixture of a bone material such as allograft or autograft, and an antigen produced from bacteria or parasitic organisms. A composition for inducing bone morphogenesis also is disclosed.
Owner:SUDDABY LOUBERT
Supplemented matrices for the repair of bone fractures
Supplemented matrices comprising a PTH releasably incorporated therein, optionally containing a granular material, are described herein. The PTH is incorporated either through covalent linkage to the matrix or through non-covalent interaction with the matrix and / or the granules. These supplemented matrices decrease the time of healing compared to autograft and or trigger healing of bone fractures which otherwise would not heal. The matrices are biocompatible and biodegradable and can be formed in vitro or in vivo, at the time of implantation. The PTH may be a part of a fusion peptide. PTH can be incorporated into the matrices with full retention of its bio-activit y. PTH can be releasably incorporated, using techniques that provide control over how and when and to what degree the PTH is released using the matrix as a controlled release vehicle to heal bone fractures.
Owner:BAXTER INT INC +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com