Patents
Literature
229 results about "Implant placement" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
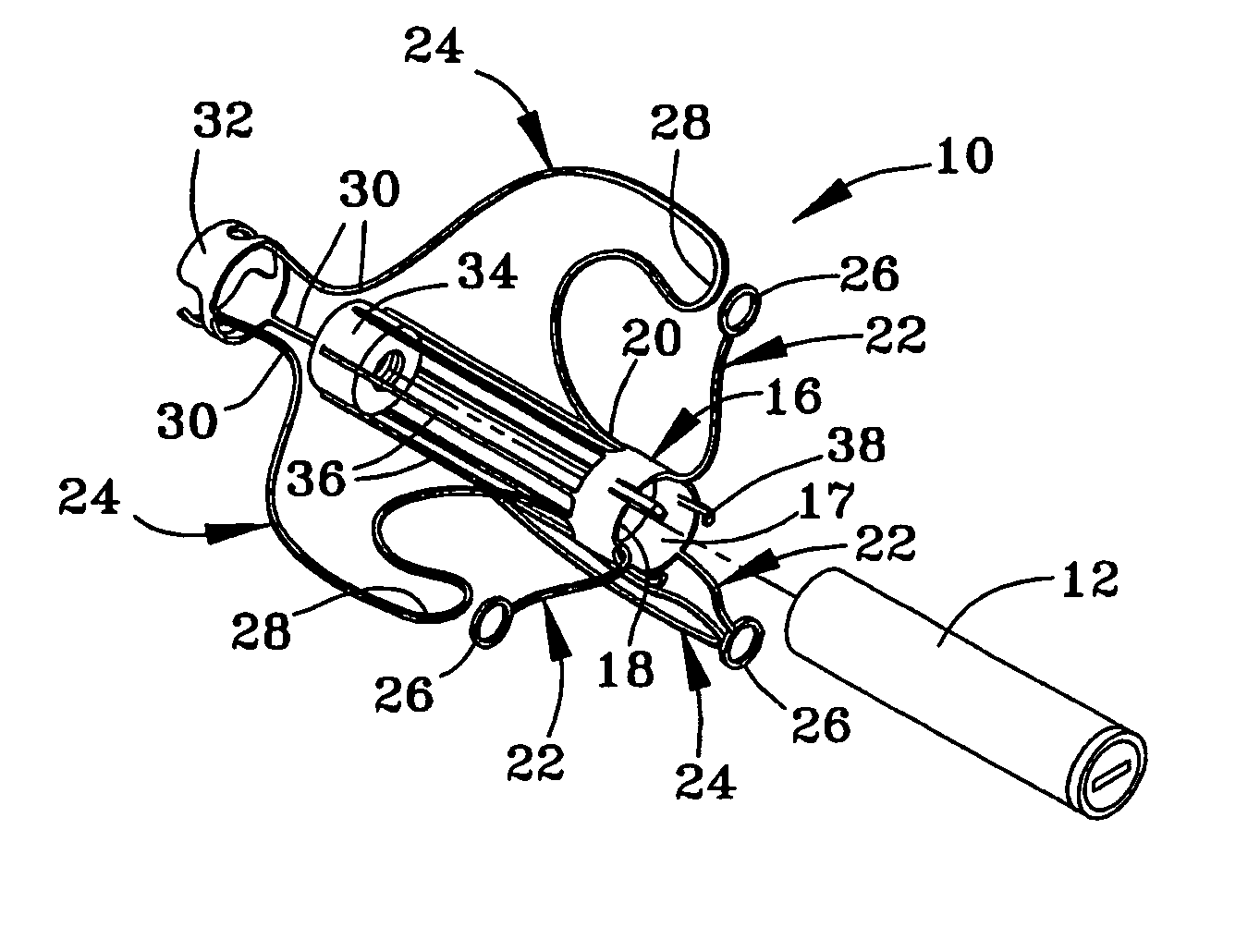
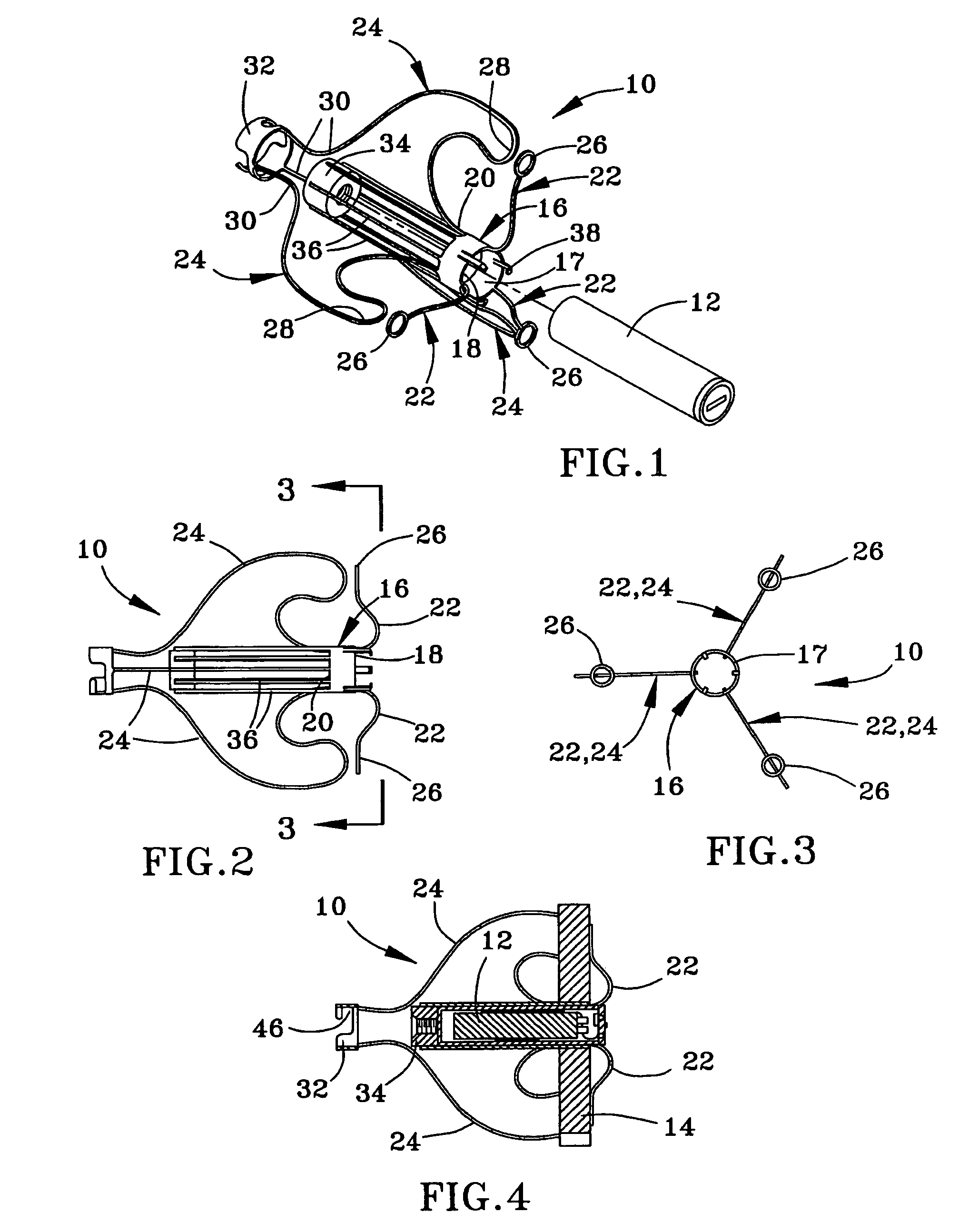
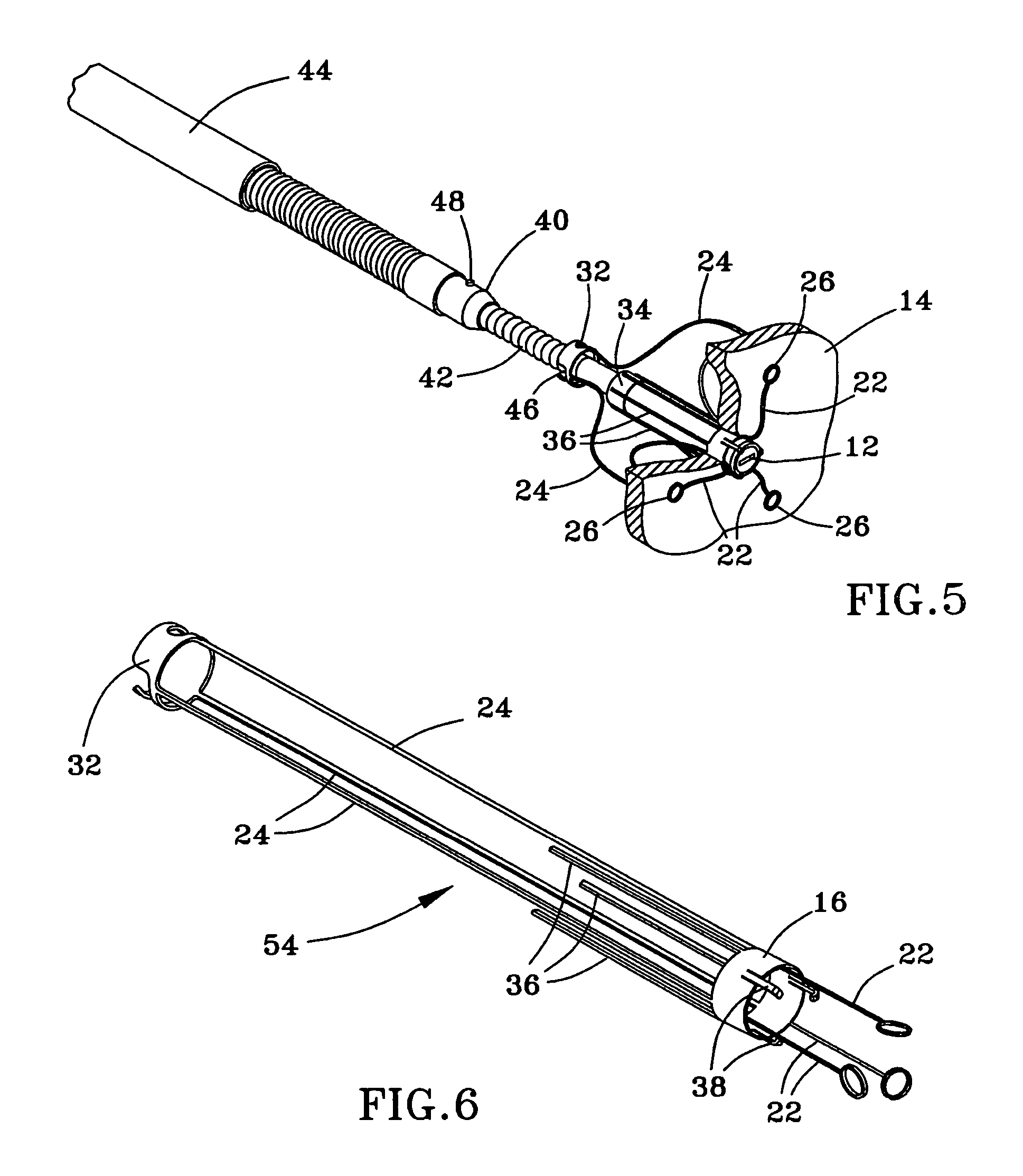
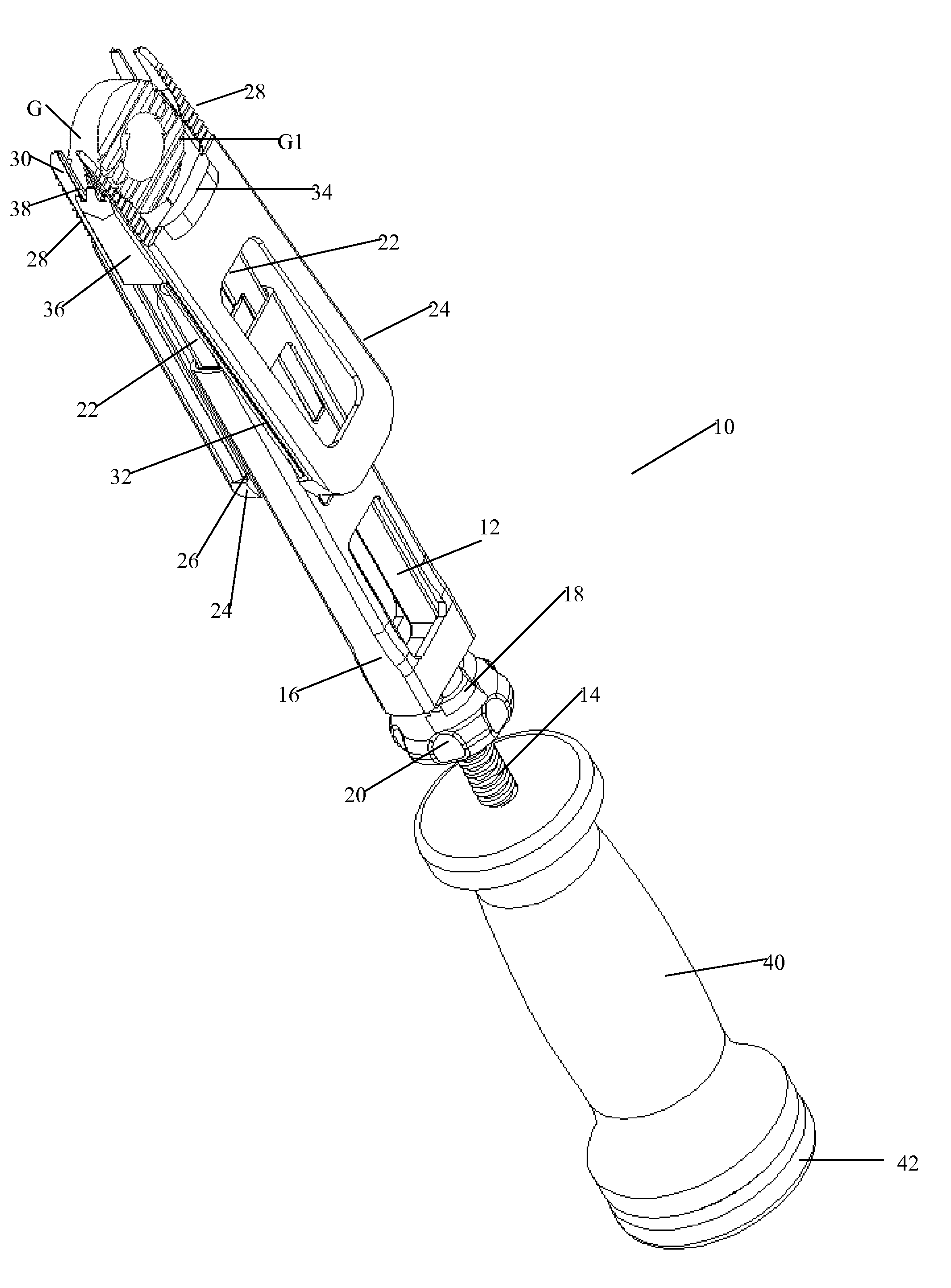
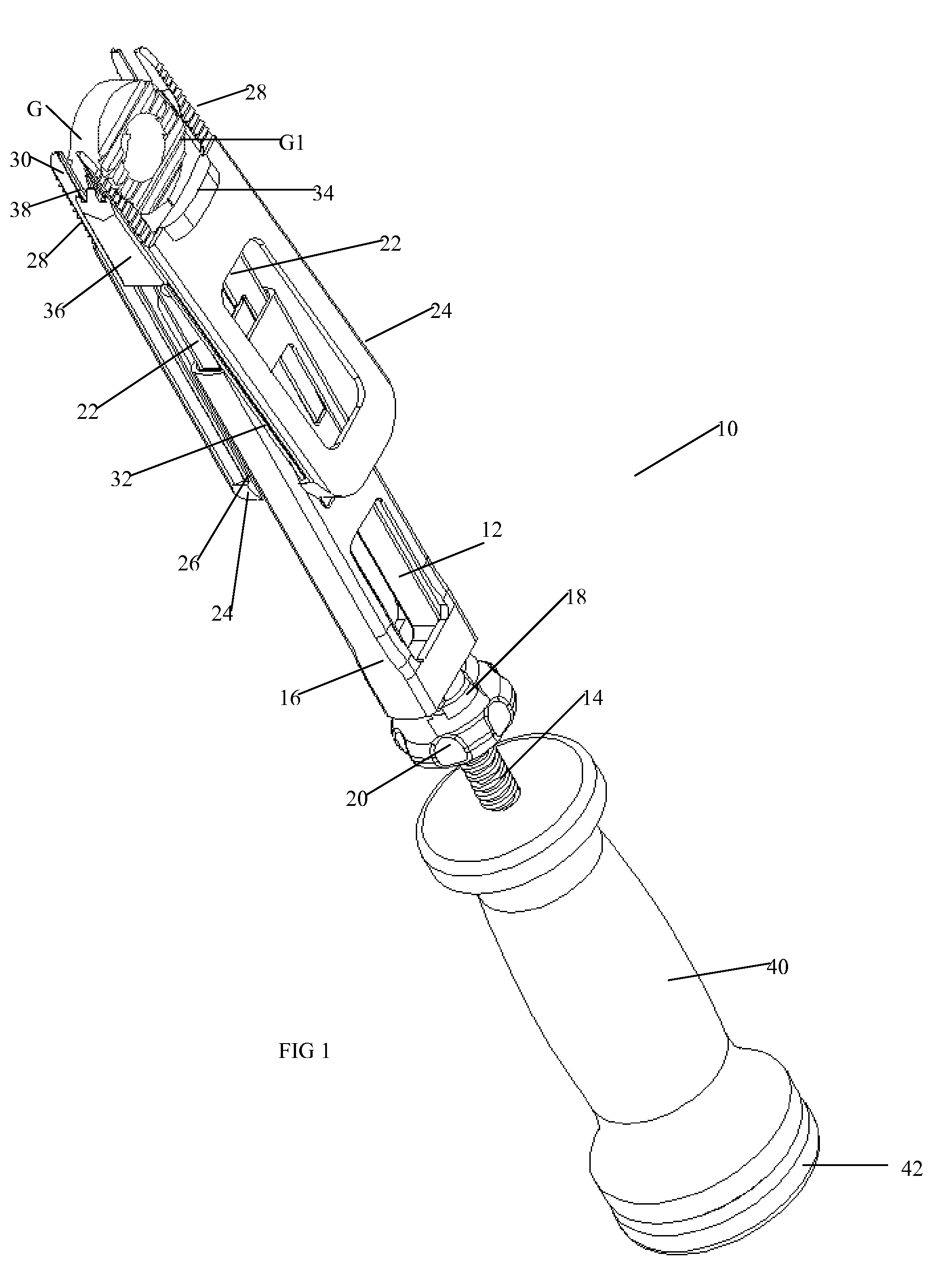
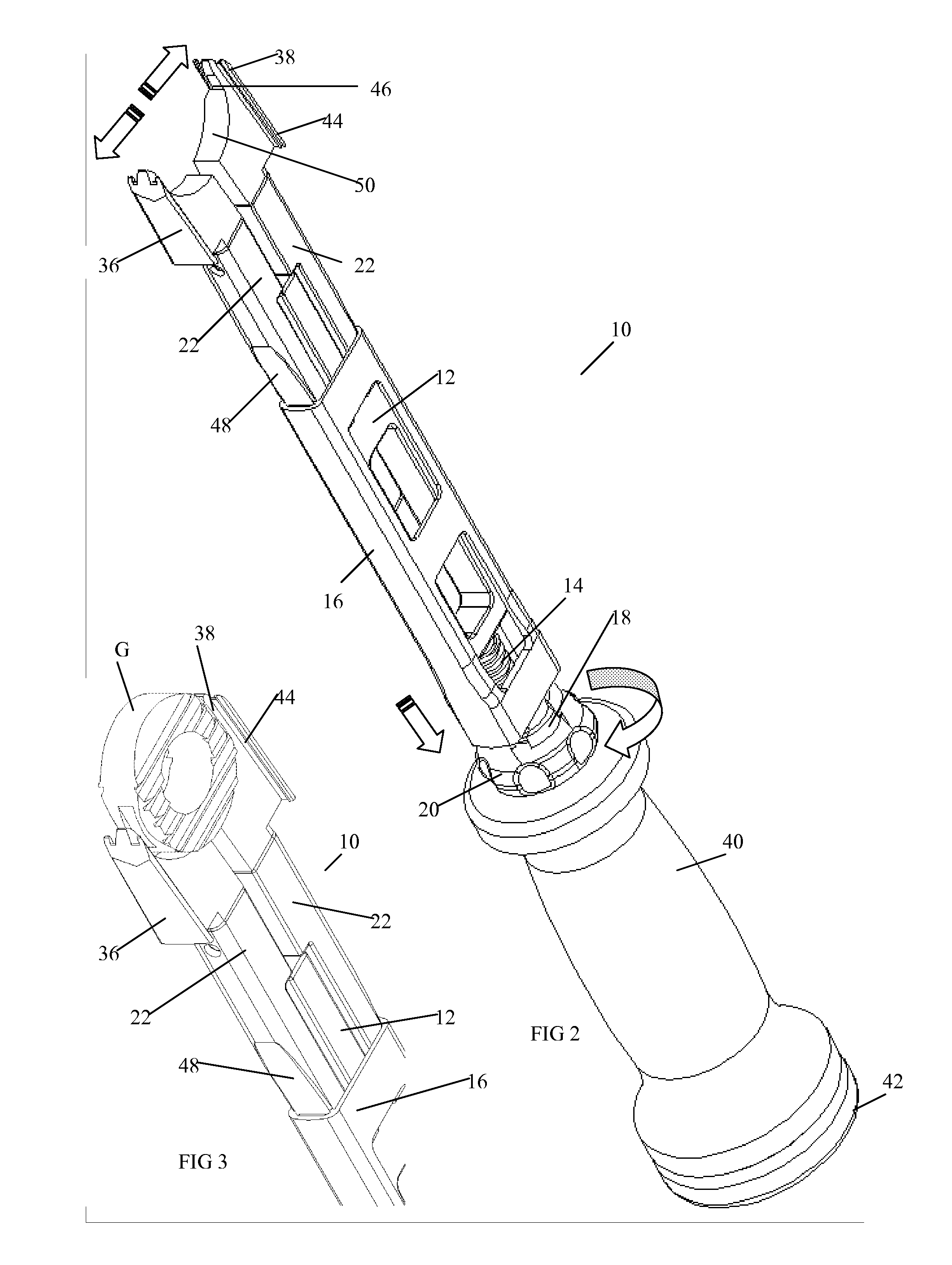
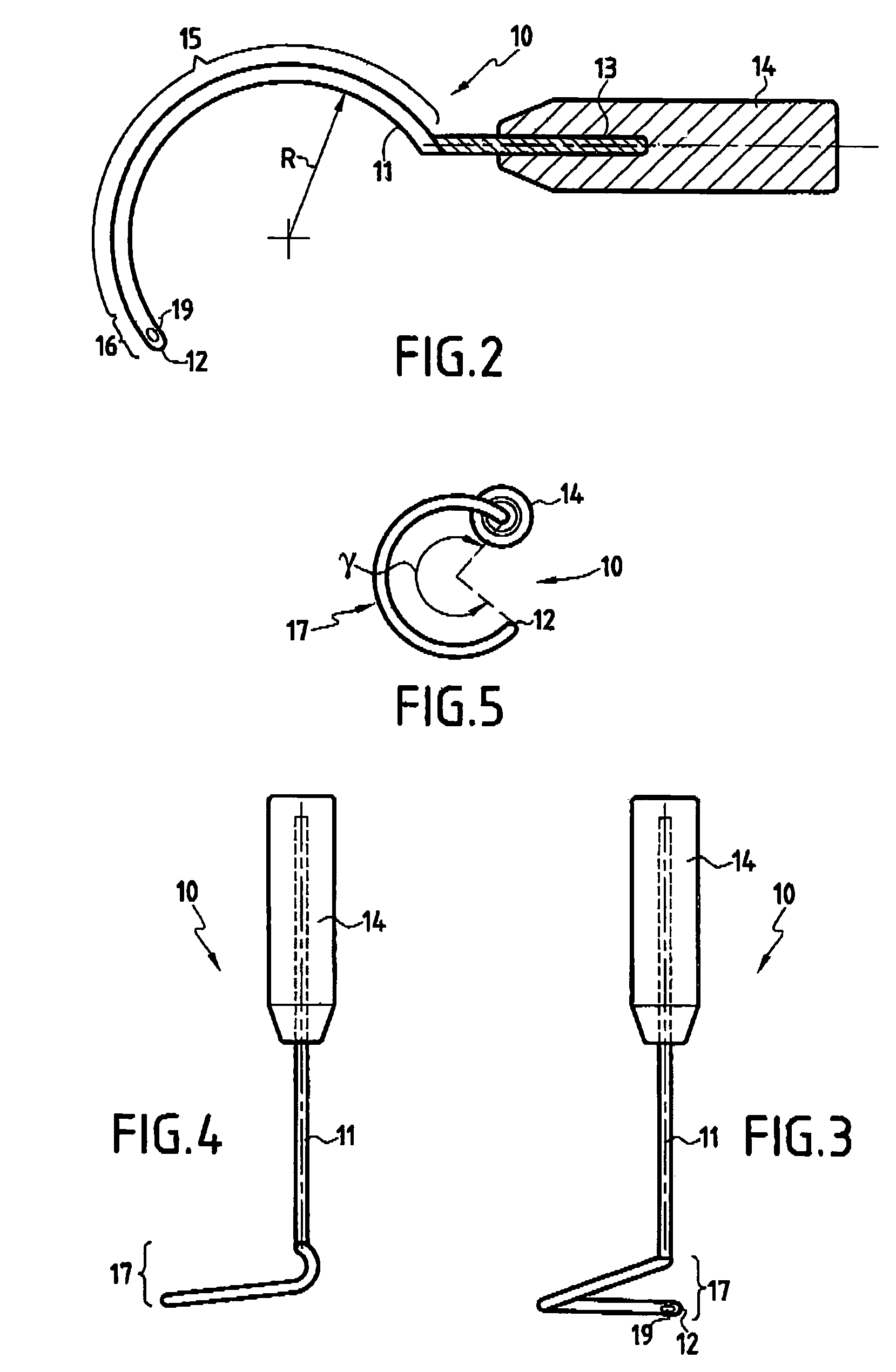
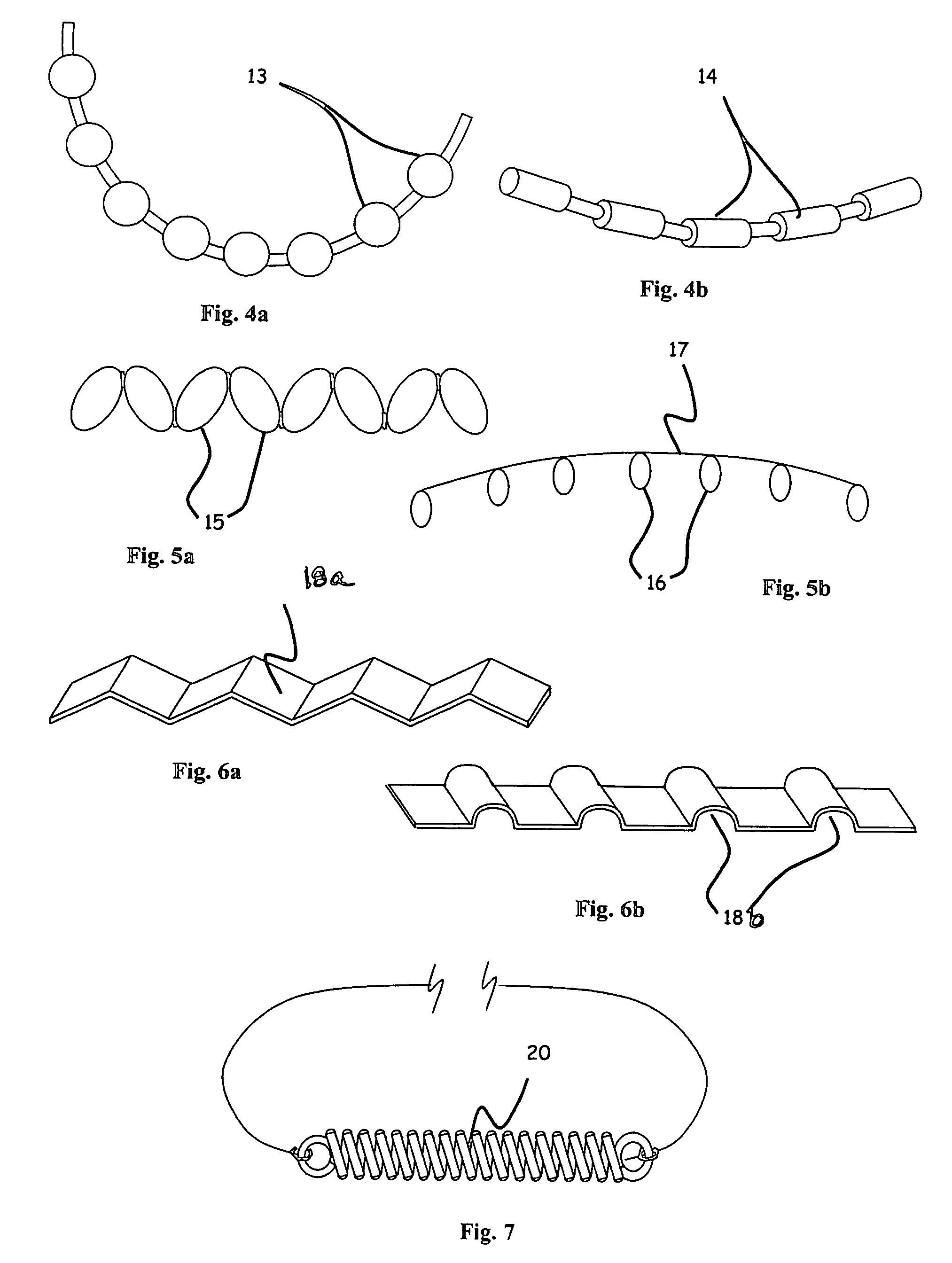
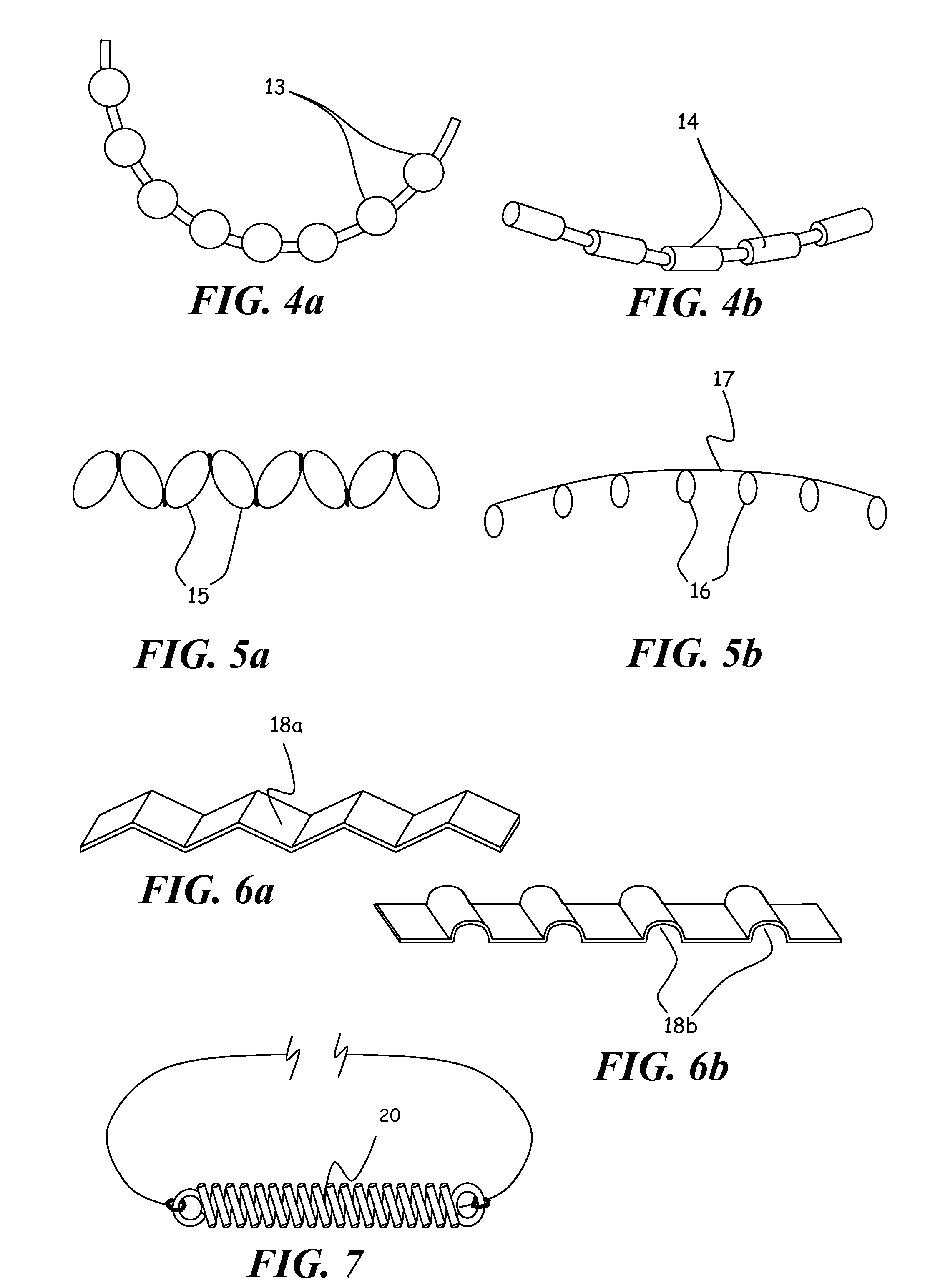
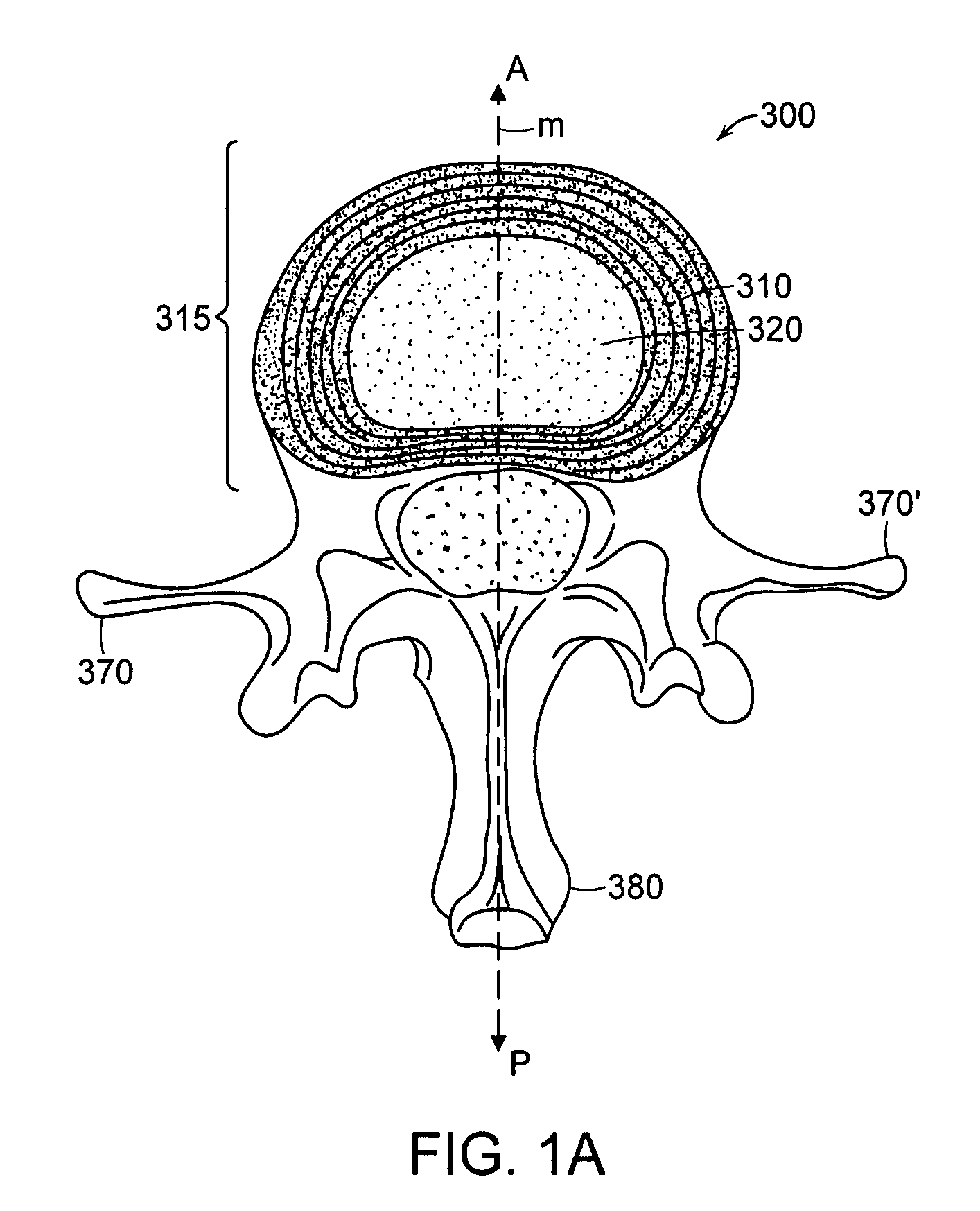
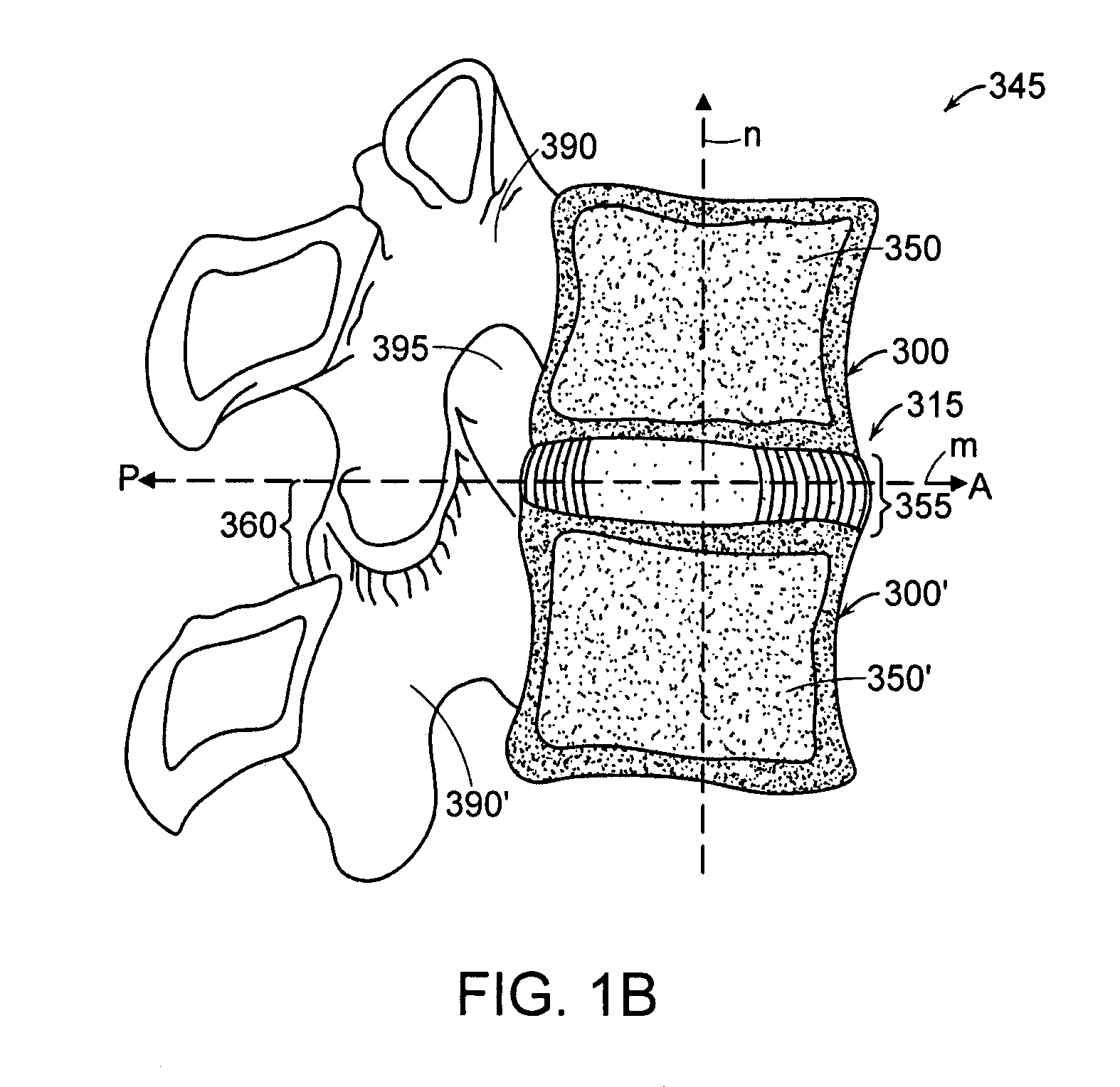
Instrument and method for the insertion and alignment of an intervertebral implant
ActiveUS20060084986A1The method is simple and reliablePrecise alignmentInternal osteosythesisJoint implantsDistractionCoronal plane
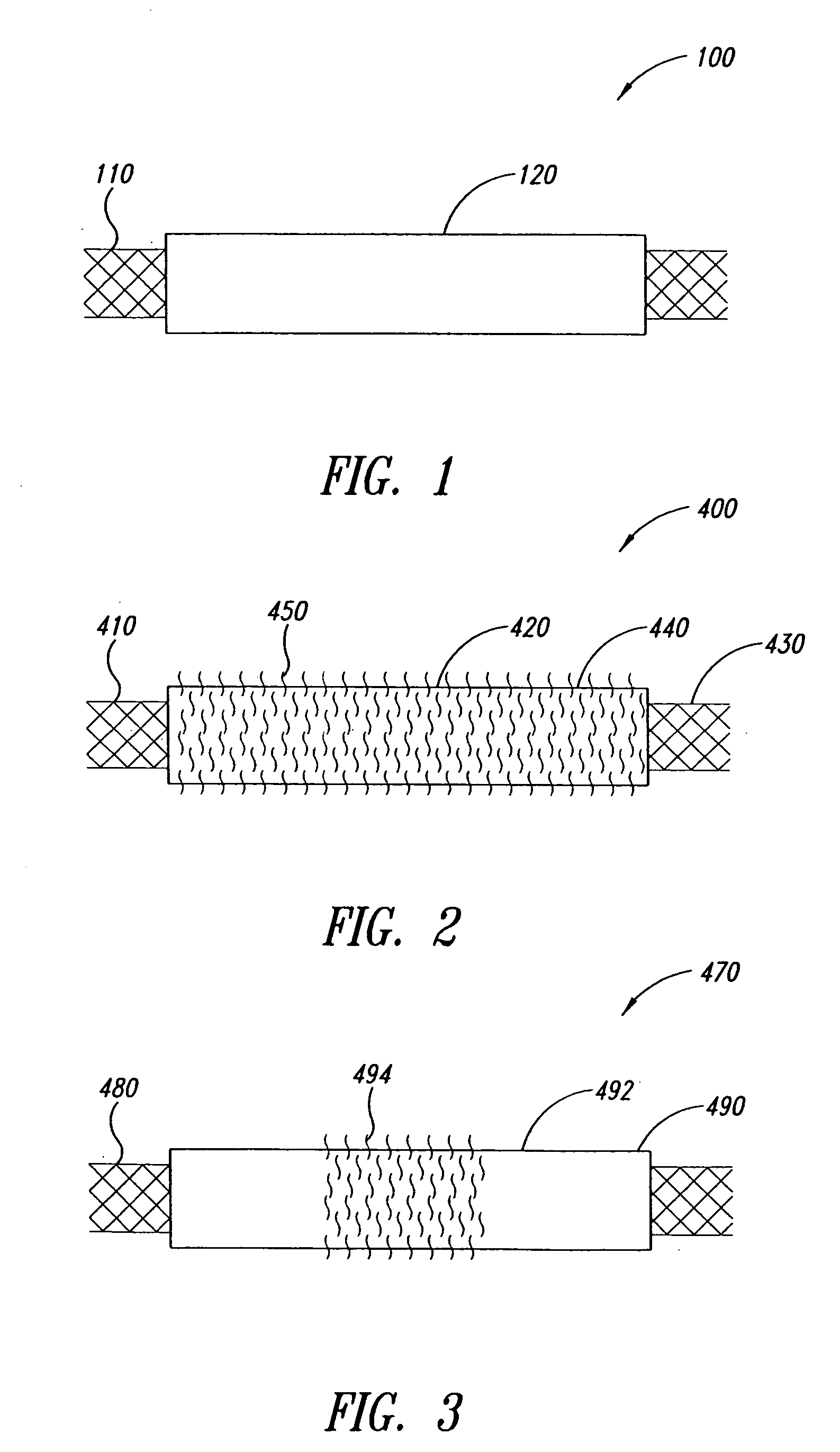
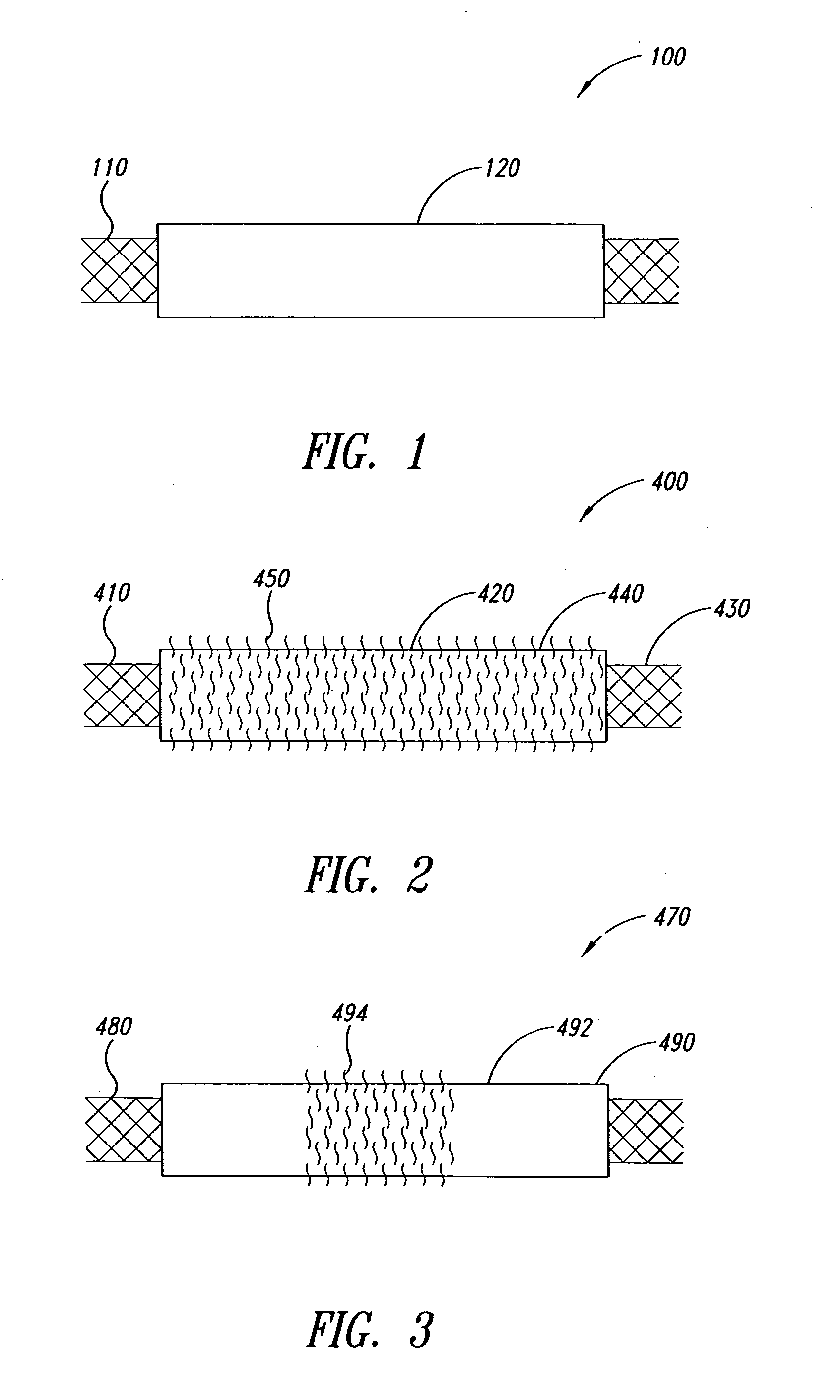
The present invention includes pin guides and methods for placing pins in adjacent vertebrae. The present invention also includes methods for placing pins in adjacent vertebrae using the pin guides described herein. The present invention also includes an intervertebral implant insertion and alignment instrument, a distraction instrument, an intervertebral implant insertion guide, and methods for inserting an implant into an intervertebral space. Despite existing tools and techniques, present positioning of implants in intervertebral spaces and pins in adjacent vertebrae often depend on a surgeon's skill, experience and technique. Practice of the present invention can aide in the placement of an implant into an intervertebral space and placement of pins in adjacent vertebrae, e.g., midline to the coronal plane spine and / or parallel to vertebral endplates that abut the intervertebral space.
Owner:DEPUY SPINE INC (US)
Intravascular devices and fibrosis-inducing agents
InactiveUS20050149173A1Reducing perigraft leakageFacilitate “anchoring”StentsPeptide/protein ingredientsFibrosisCoil embolization
Intravascular devices (e.g., stents, stent grafts, covered stents, aneurysm coils, embolic agents and drug delivery catheters and balloons) are used in combination with fibrosing agents in order to induce fibrosis that may otherwise not occur when the implant is placed within an animal or to promote fibrosis betweent the devices and the host tissues. Compositions and methods are described for use in the treatment of aneurysms and unstable arterial (vulnerable) plaque.
Owner:ANGIOTECH INT AG (CH)
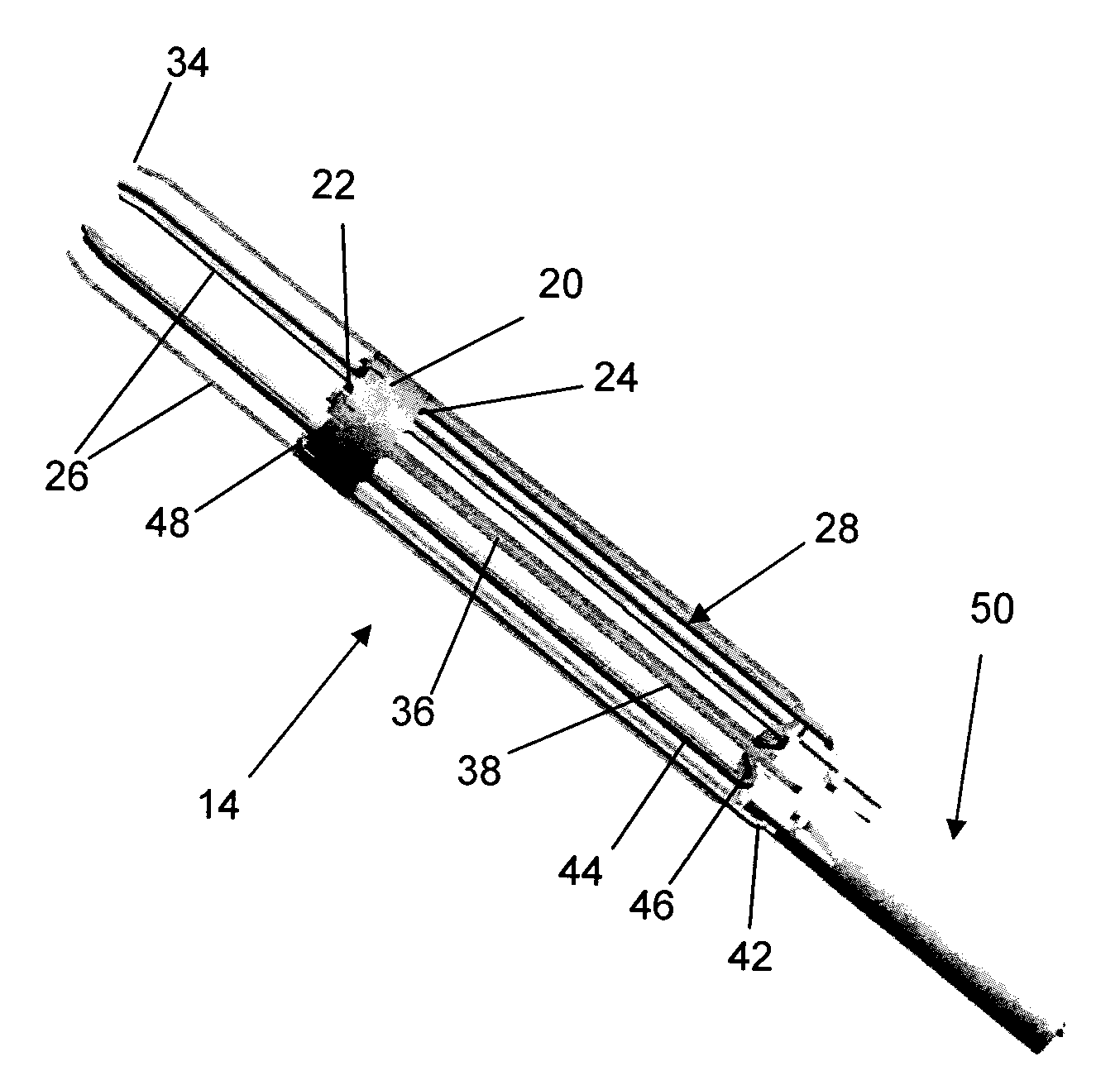
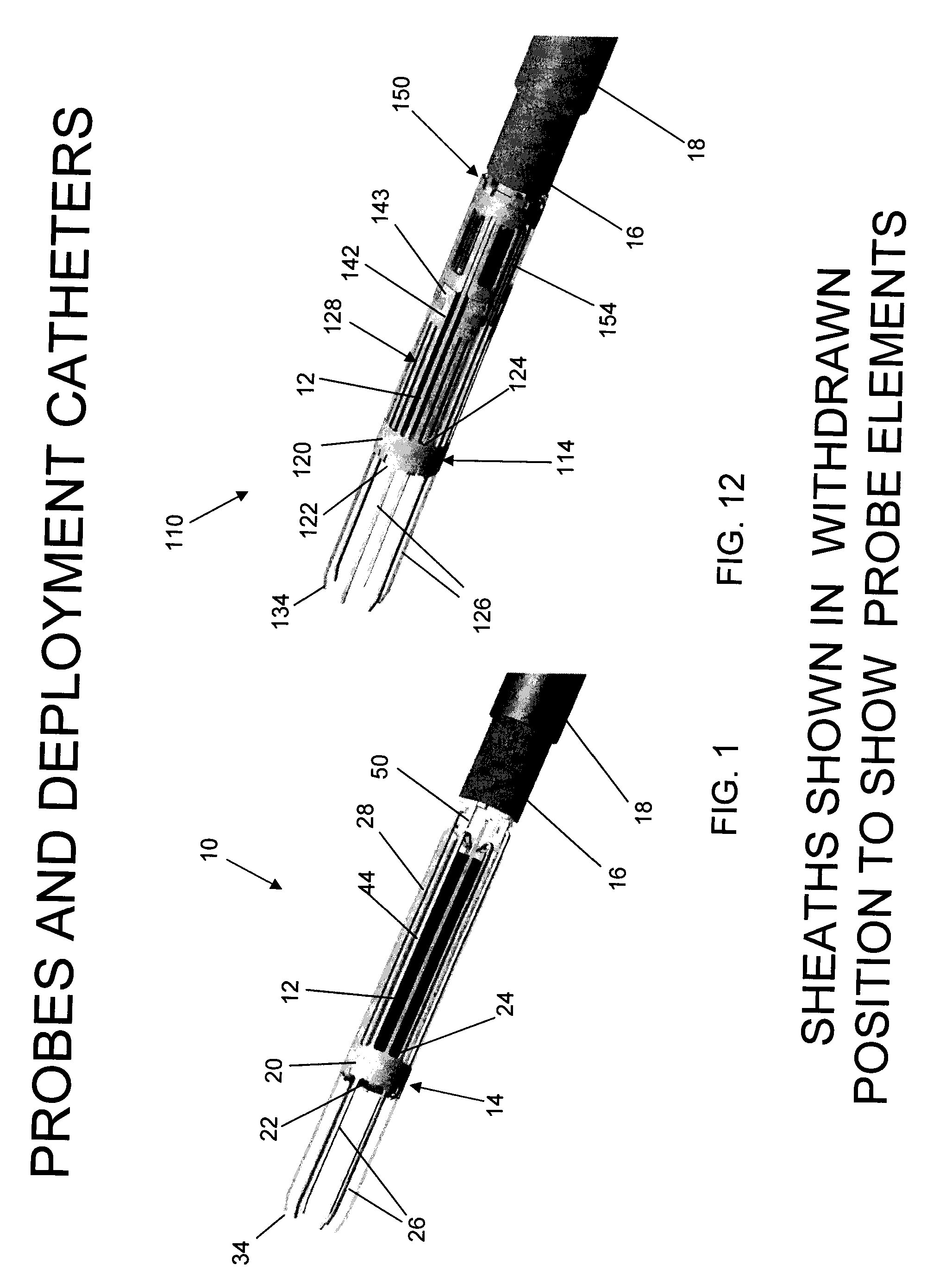
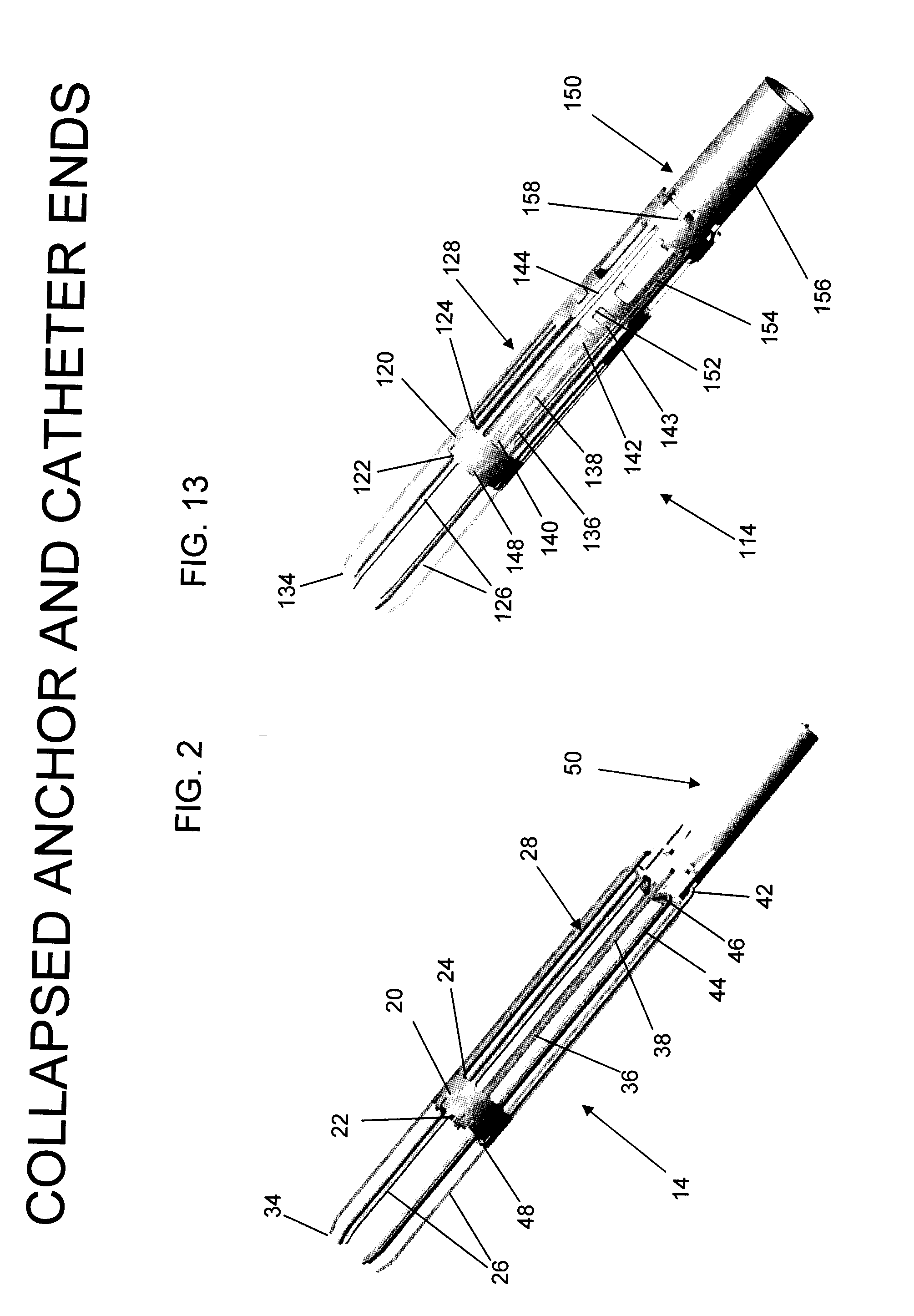
Method and anchor for medical implant placement, and method of anchor manufacture
ActiveUS20050065589A1Transvascular endocardial electrodesCatheterDistal portionMonitoring physiological parameters
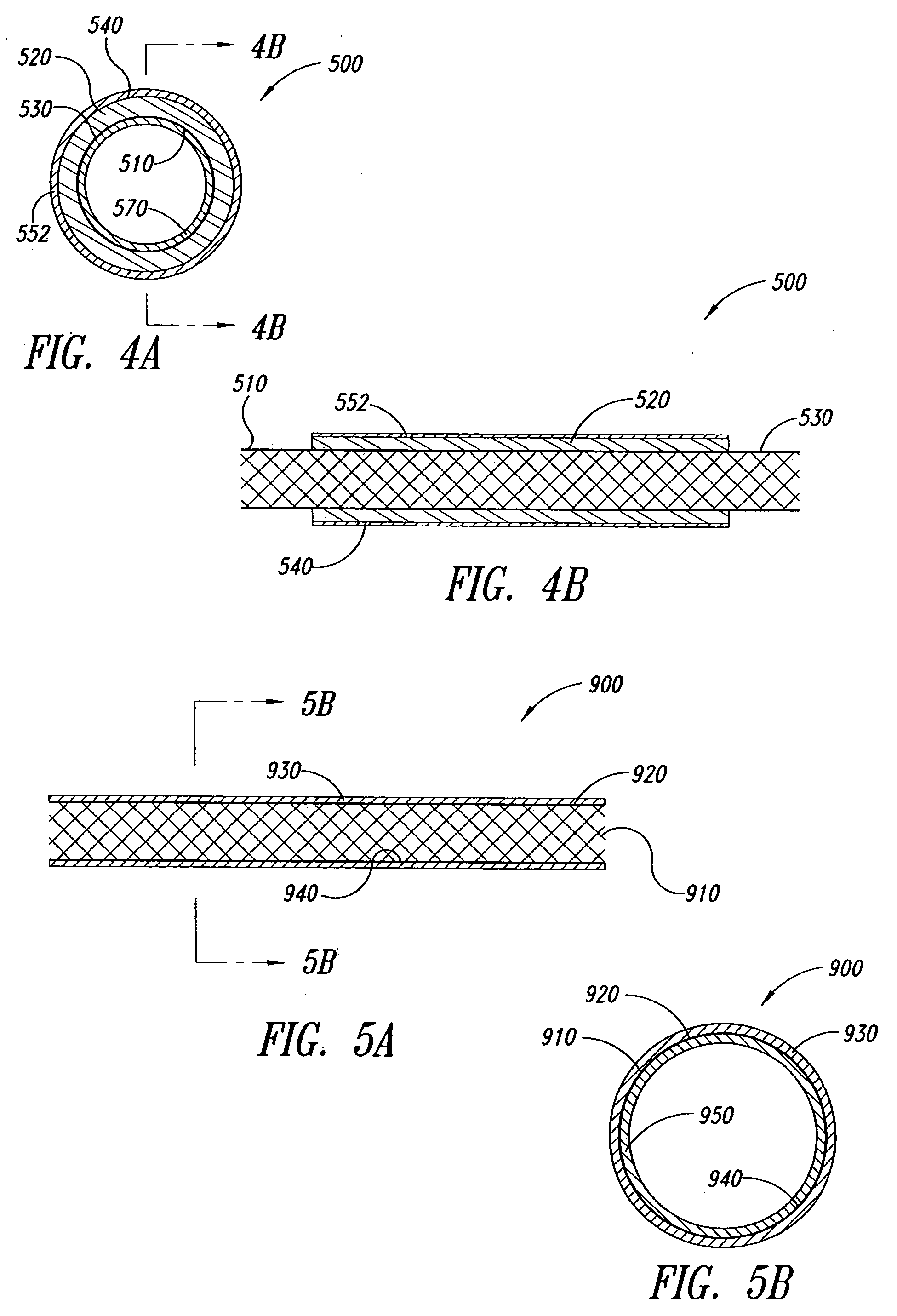
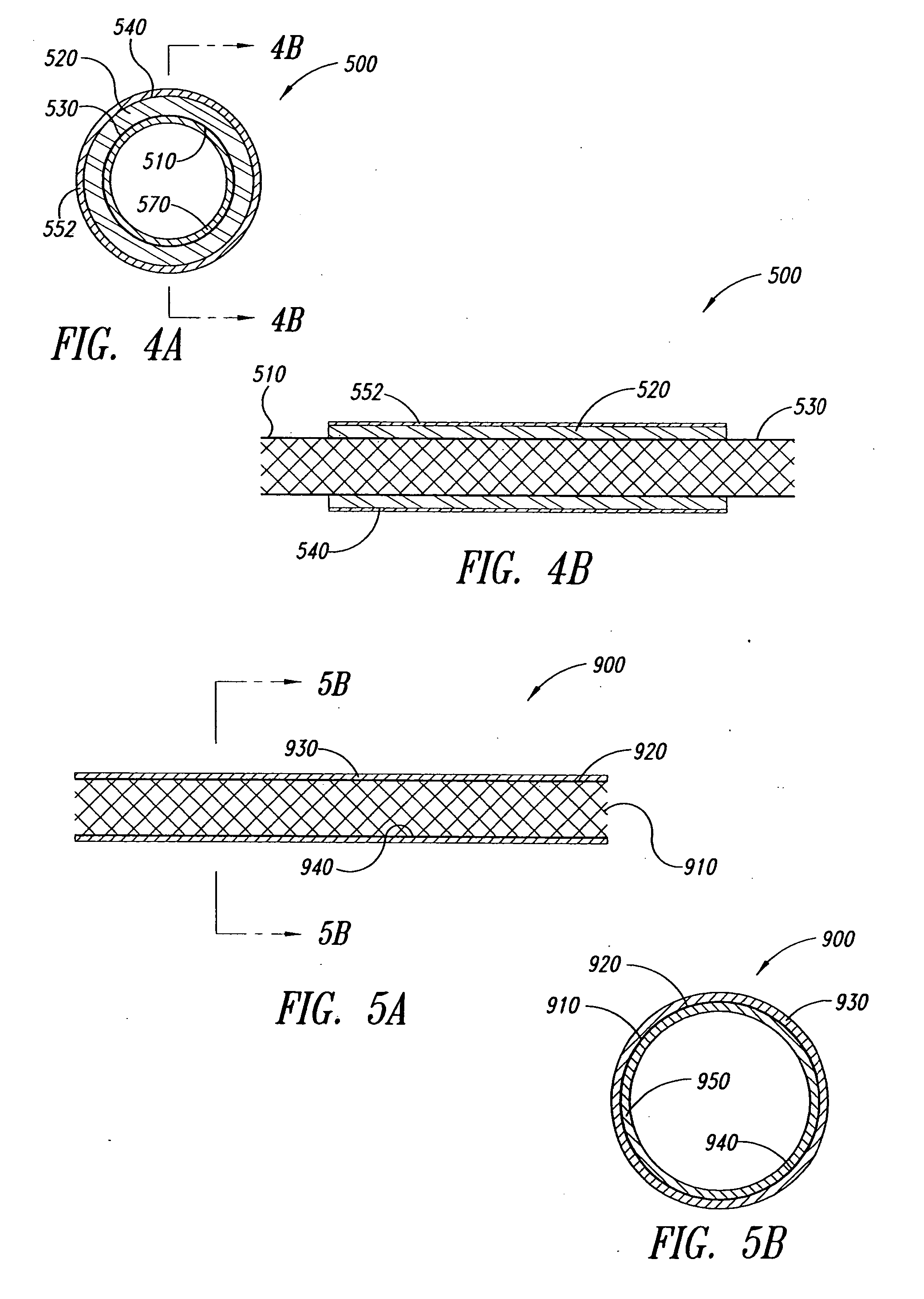
An anchor and procedure for placing a medical implant, such as for monitoring physiological parameters. The anchor includes a central body in which a medical implant can be received. arms and members extend radially from first and second ends, respectively, of the central body. Each member defines a leg extending toward distal portions of the arms to provide a clamping action. The anchor and its implant are placed by coupling first and second guidewires to first and second portions of the anchor, placing an end of a delivery catheter in a wall where implantation is desired, inserting the anchor in the catheter with the guidewires to locate the anchor within the wall, deploying the arms of the anchor at one side of the wall followed by deployment of the members at the opposite side of the wall, and thereafter decoupling the guidewires from the anchor.
Owner:UIM PRESSURE IMPLANT INC
Apparatus and Methods for Inserting an Implant
ActiveUS20080275455A1Easy to disassembleCombined height reductionSpinal implantsOsteosynthesis devicesBiomedical engineeringVertebral body
A system and method for inserting an implant into a cavity is disclosed, which may include advancing an implant insertion instrument toward a pair of adjacent bodies, the implant insertion instrument having two opposed ramps, wherein each ramp has a distal tip and wherein the longitudinal axes of the opposed ramps are separated by an initial angle; inserting the distal tips of the opposed ramps between the adjacent bodies, thereby creating an initial interbody cavity between the adjacent bodies; expanding the interbody cavity while maintaining the initial angle between the longitudinal axes of the opposed ramps; placing the implant in a final location between the adjacent bodies; transferring a compressive force urging the adjacent bodies together from the opposed ramps to the implant; and extracting the implant insertion instrument from the interbody cavity.
Owner:K2M
Surgical articles for placing an implant about a tubular tissue structure and methods
ActiveUS7104949B2Avoid difficultyWithout any changeSuture equipmentsStentsLess invasive surgerySurgical department
A minimally invasive surgical instrument for placing an implantable article about a tubular tissue structure is disclosed. The surgical instrument is particularly useful for treating urological disorders such as incontinence. Surgical methods using the novel instrument are also described.
Owner:BOSTON SCI SCIMED INC
Intravascular devices and fibrosis-inducing agents
InactiveUS20050149175A1Reducing perigraft leakageFacilitate “anchoring”StentsPeptide/protein ingredientsFibrosisCoil embolization
Intravascular devices (e.g., stents, stent grafts, covered stents, aneurysm coils, embolic agents and drug delivery catheters and balloons) are used in combination with fibrosing agents in order to induce fibrosis that may otherwise not occur when the implant is placed within an animal or to promote fibrosis betweent the devices and the host tissues. Compositions and methods are described for use in the treatment of aneurysms and unstable arterial (vulnerable) plaque.
Owner:ANGIOTECH INT AG (CH)
Rigidly guided implant placement with control assist
ActiveUS20070055291A1Reduce human errorAccurate and precise alignmentCannulasDiagnosticsEngineeringInstrumentation
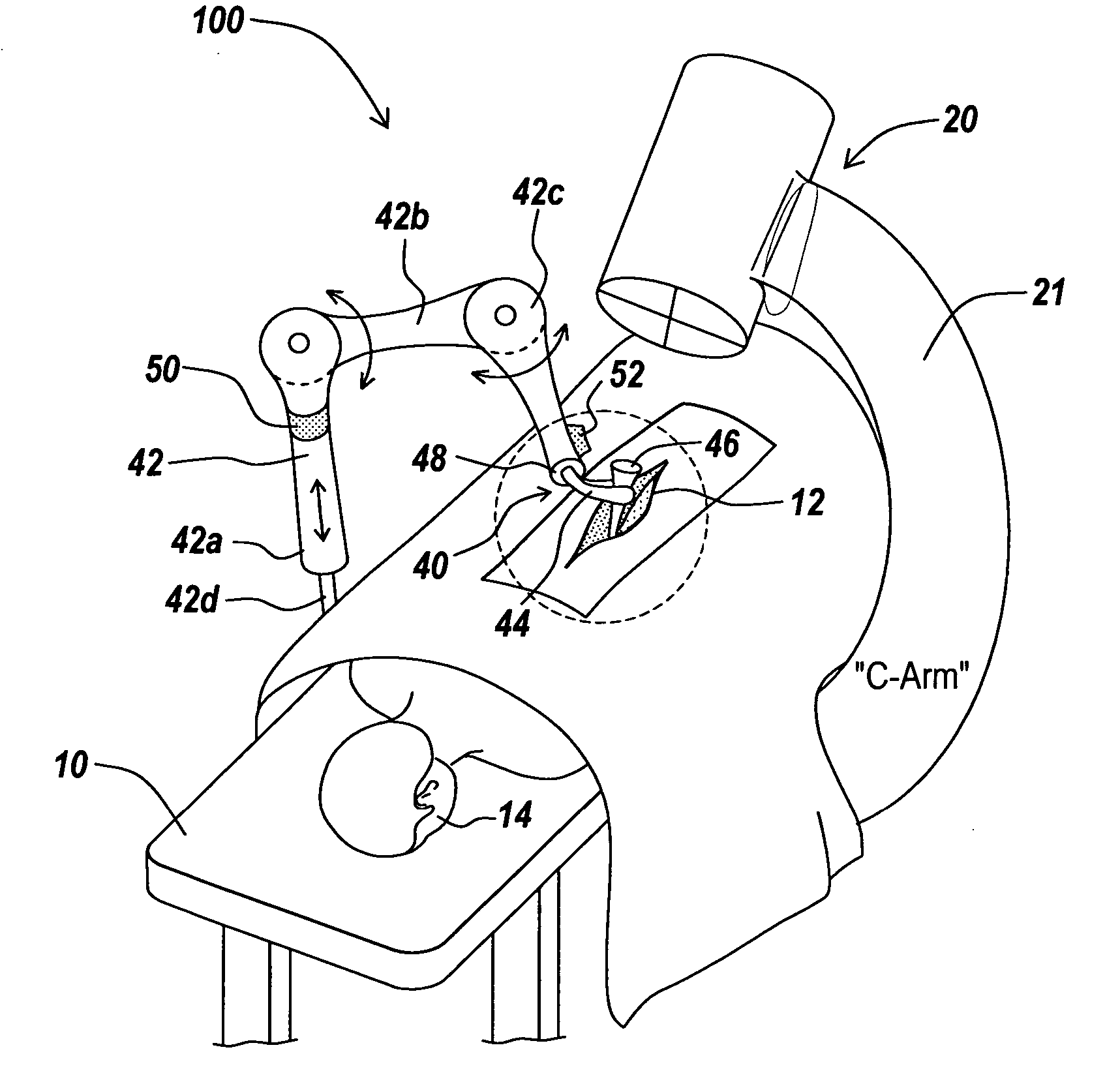
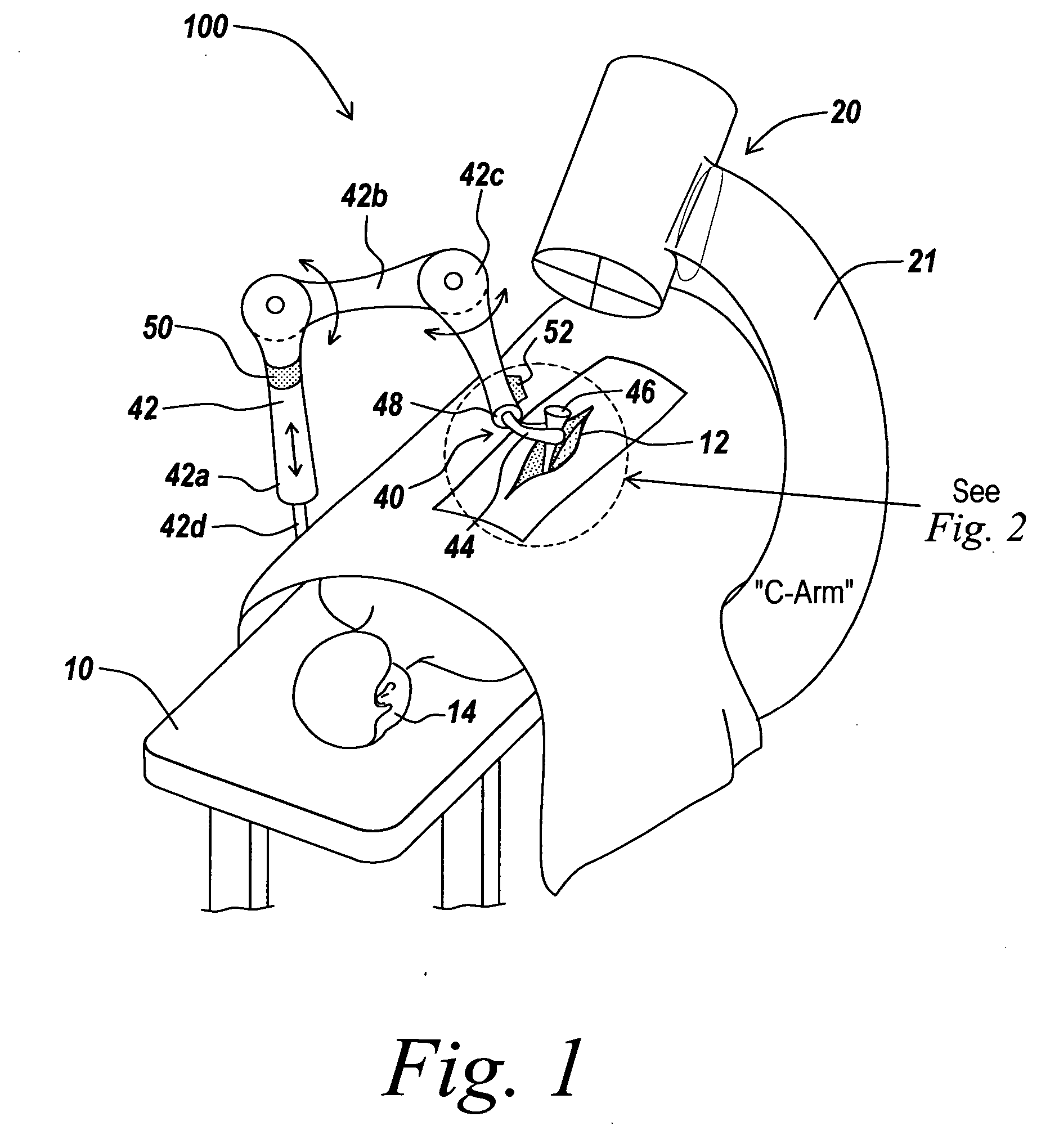
A system for guiding an implant to an optimal placement within a patient includes a trajectory guide for guiding instruments along a selected trajectory and a trajectory fixation device for fixing the trajectory guide in a selected position. The trajectory guide defines a path configured to align with the selected trajectory. A movable support mounts the trajectory guide and selectively moves the trajectory guide to align the trajectory guide with the selected trajectory prior to fixing the trajectory guide in the selected position. The trajectory is aligned coarsely by hand, then the trajectory is aligned using a fine adjustment system. After fixing the trajectory guide, instruments can be inserted along the trajectory through the path defined by the trajectory guide.
Owner:DEPUY SYNTHES PROD INC +1
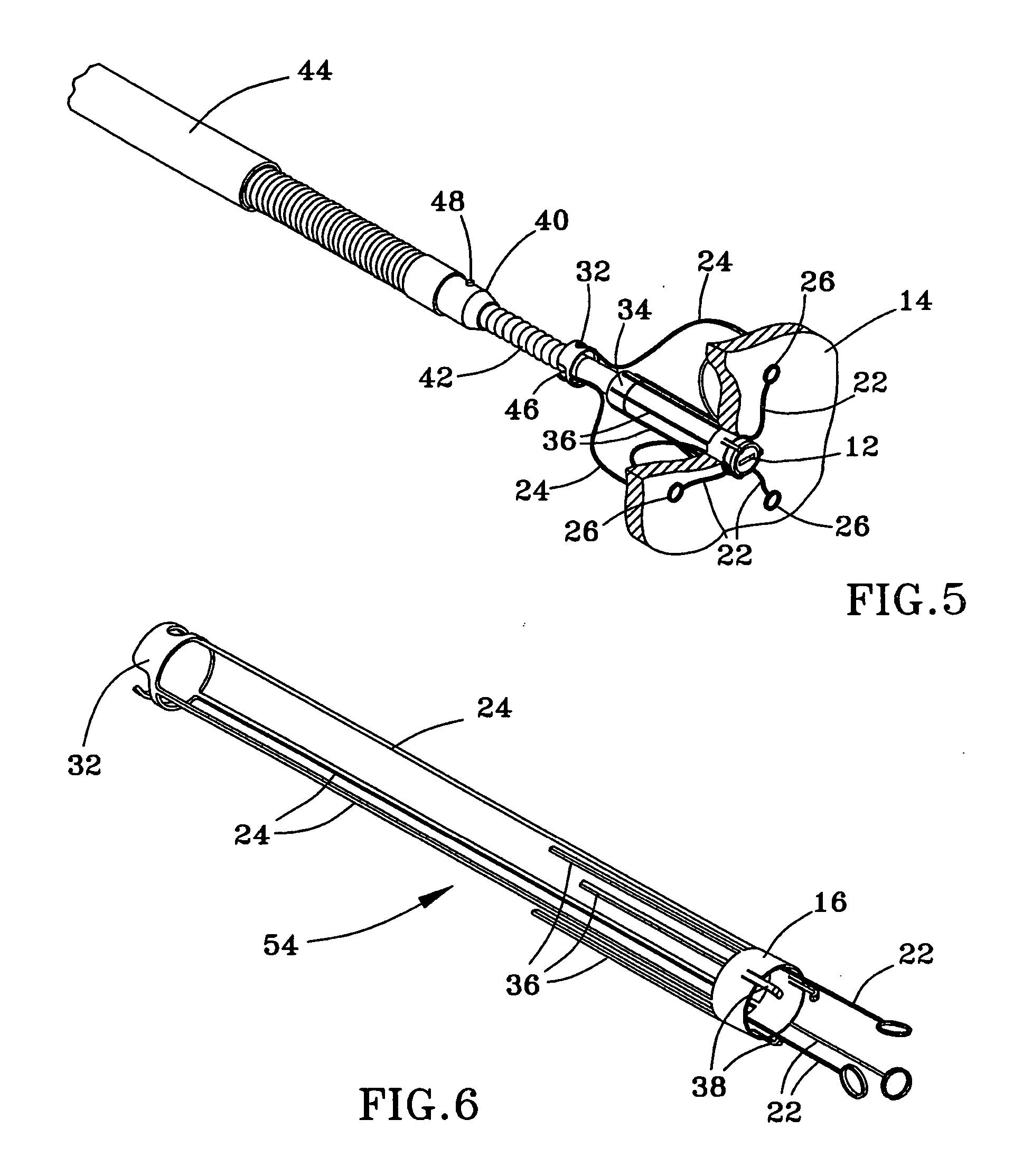
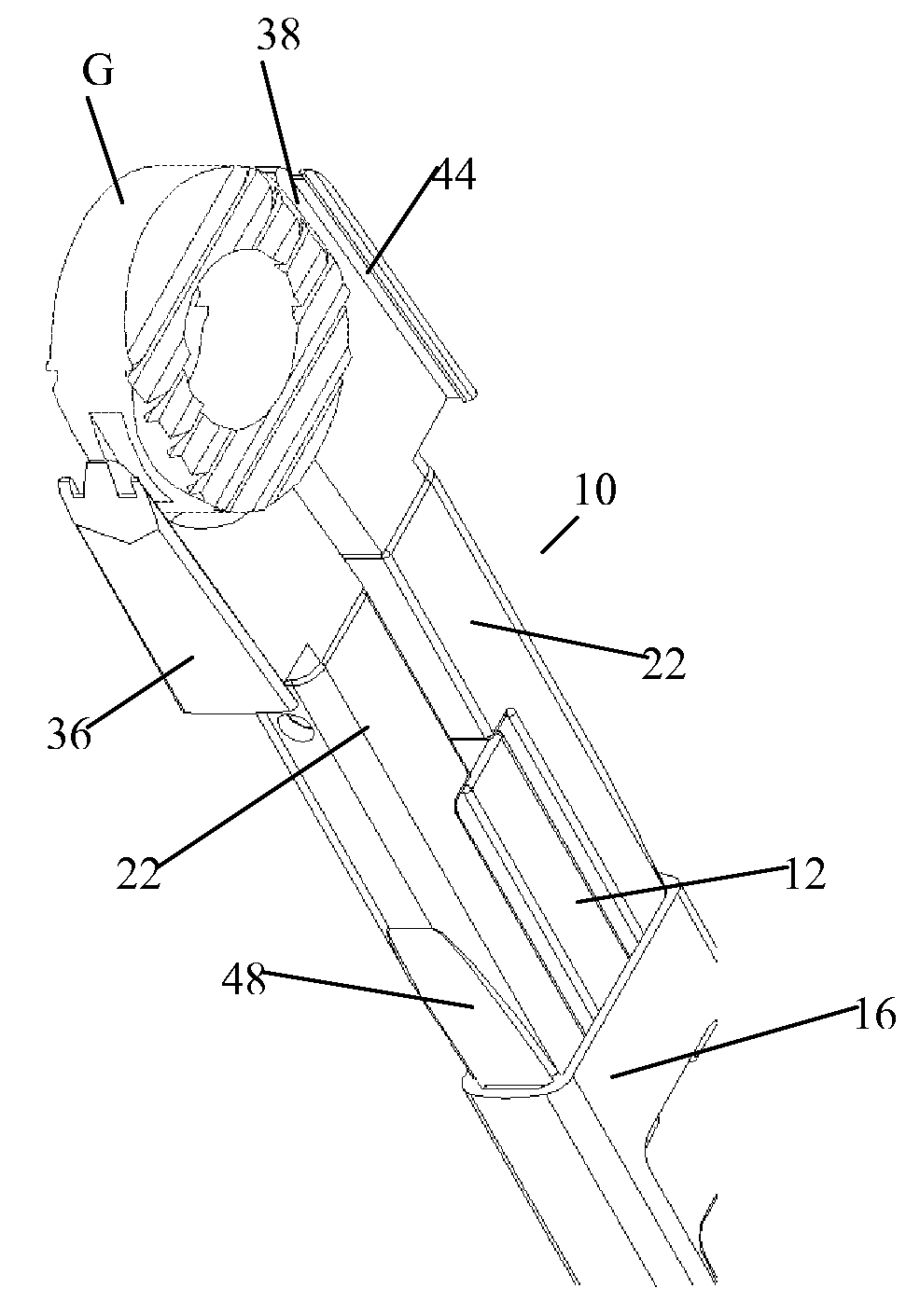
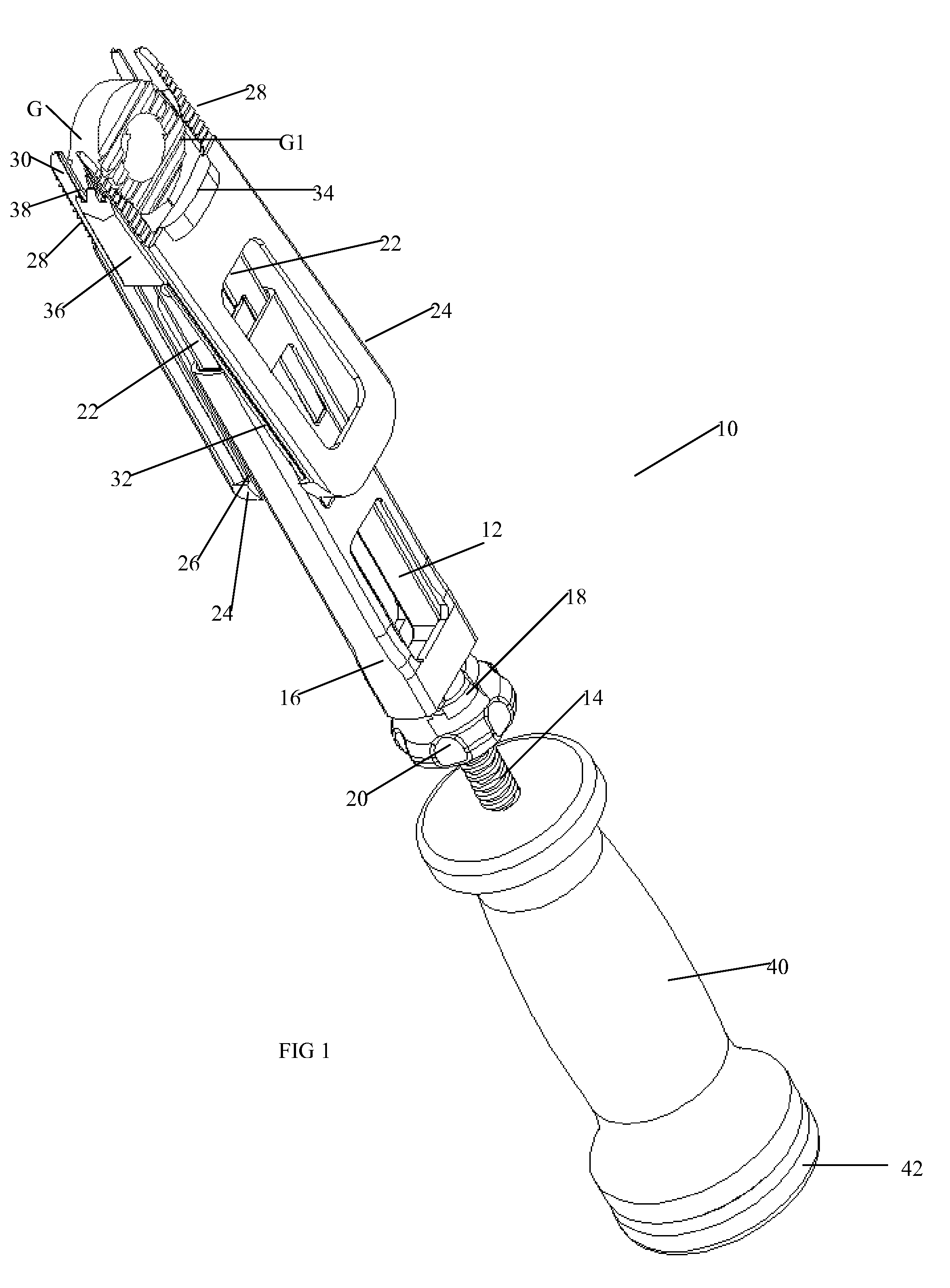
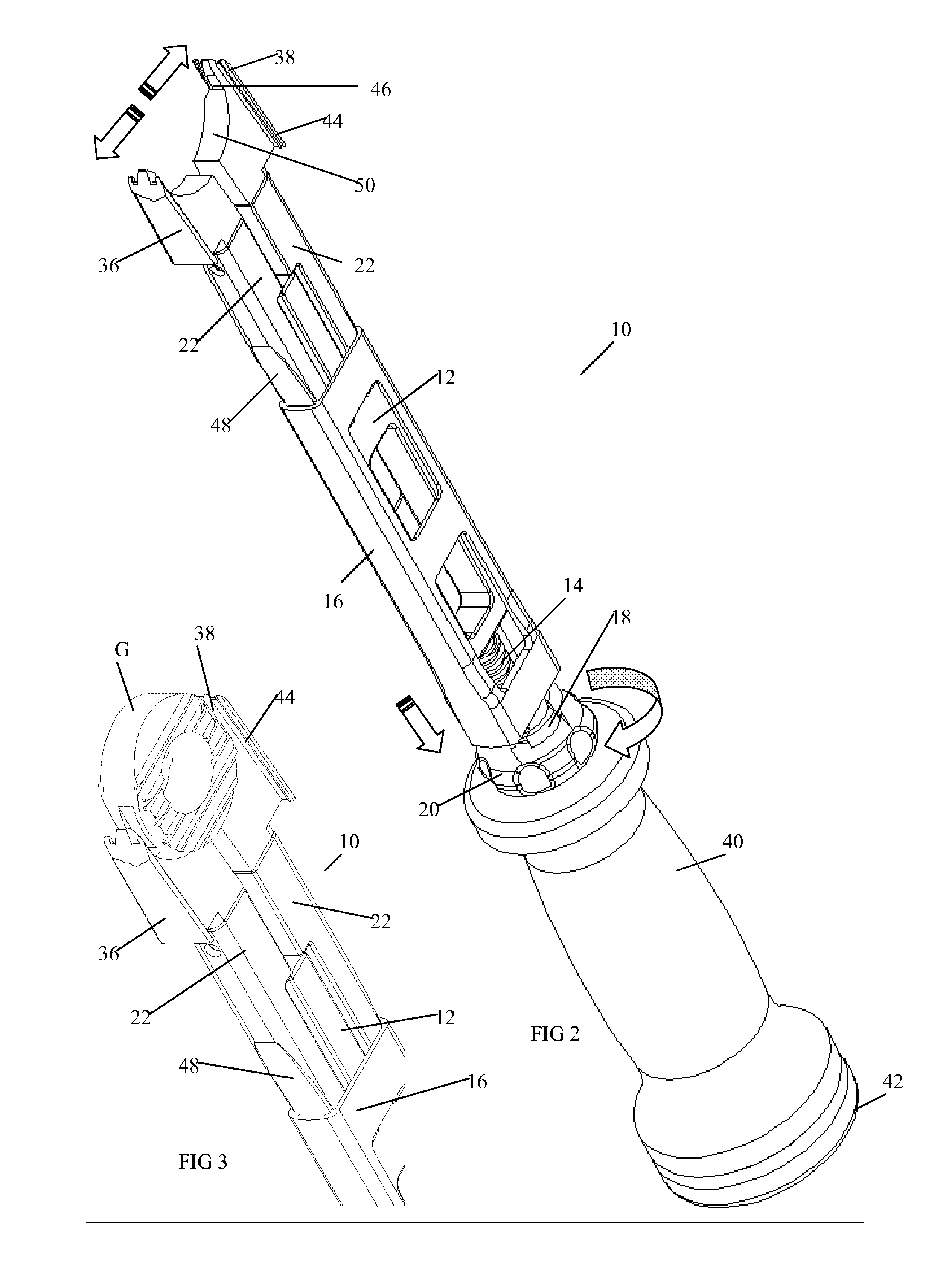
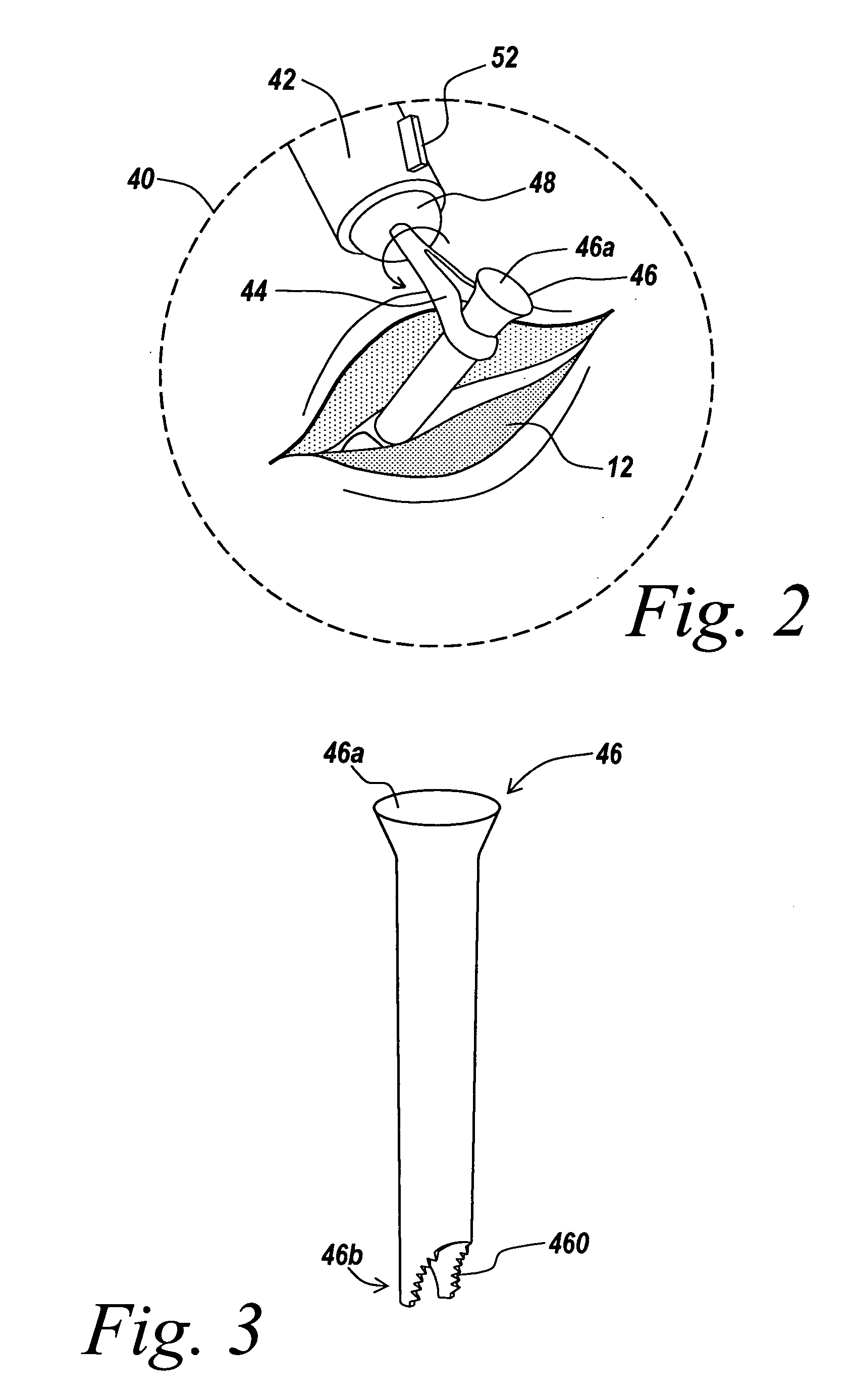
Vertebral implant and insertion tool
A vertebral implant and insertion tool for placing implants in the spine are disclosed. The implant has a tool engaging surface configured for intimate engagement with the insertion tool. The insertion tool configuration is particularly suited for being gripped at a plurality of angles. The insertion tool having a gripping end adapted for intimate engagement with an implant at a plurality of angles.
Owner:WARSAW ORTHOPEDIC INC
Soft tissue implants and anti-scarring agents
InactiveUS20050142162A1Guaranteed functionImprove clinical outcomesPeptide/protein ingredientsAntipyreticChinBiomedical engineering
Soft tissue implants (e.g., breast, pectoral, chin, facial, lip, and nasal implants) are used in combination with an anti-scarring agent in order to inhibit scarring that may otherwise occur when the implant is placed within an animal.
Owner:ANGIOTECH INT AG (CH)
Intravascular devices and fibrosis-inducing agents
InactiveUS20050154454A1Facilitate “anchoring”Good curative effectStentsPeptide/protein ingredientsFibrosisCoil embolization
Intravascular devices (e.g., stents, stent grafts, covered stents, aneurysm coils, embolic agents and drug delivery catheters and balloons) are used in combination with fibrosing agents in order to induce fibrosis that may otherwise not occur when the implant is placed within an animal or to promote fibrosis betweent the devices and the host tissues. Compositions and methods are described for use in the treatment of aneurysms and unstable arterial (vulnerable) plaque.
Owner:ANGIOTECH INT AG (CH)
Rigidly guided implant placement
A system for guiding an implant to an optimal placement within a patient includes a trajectory guide for guiding instruments along a selected trajectory and a trajectory fixation device for fixing the trajectory guide in a selected position. The trajectory guide defines a path configured to align with the selected trajectory. A movable support mounts the trajectory guide and selectively moves the trajectory guide to align the trajectory guide with the selected trajectory prior to fixing the trajectory guide in the selected position. After fixing the trajectory guide, instruments can be inserted along the trajectory through the path defined by the trajectory guide.
Owner:DEPUY SYNTHES PROD INC
Intravascular devices and fibrosis-inducing agents
InactiveUS20050154445A1Facilitate “anchoring”Good curative effectStentsPeptide/protein ingredientsFibrosisCoil embolization
Intravascular devices (e.g., stents, stent grafts, covered stents, aneurysm coils, embolic agents and drug delivery catheters and balloons) are used in combination with fibrosing agents in order to induce fibrosis that may otherwise not occur when the implant is placed within an animal or to promote fibrosis betweent the devices and the host tissues. Compositions and methods are described for use in the treatment of aneurysms and unstable arterial (vulnerable) plaque.
Owner:ANGIOTECH INT AG (CH)
Intravascular devices and fibrosis-inducing agents
InactiveUS20050154453A1Facilitate “anchoring”Good curative effectStentsPeptide/protein ingredientsFibrosisCoil embolization
Intravascular devices (e.g., stents, stent grafts, covered stents, aneurysm coils, embolic agents and drug delivery catheters and balloons) are used in combination with fibrosing agents in order to induce fibrosis that may otherwise not occur when the implant is placed within an animal or to promote fibrosis betweent the devices and the host tissues. Compositions and methods are described for use in the treatment of aneurysms and unstable arterial (vulnerable) plaque.
Owner:ANGIOTECH INT AG (CH)
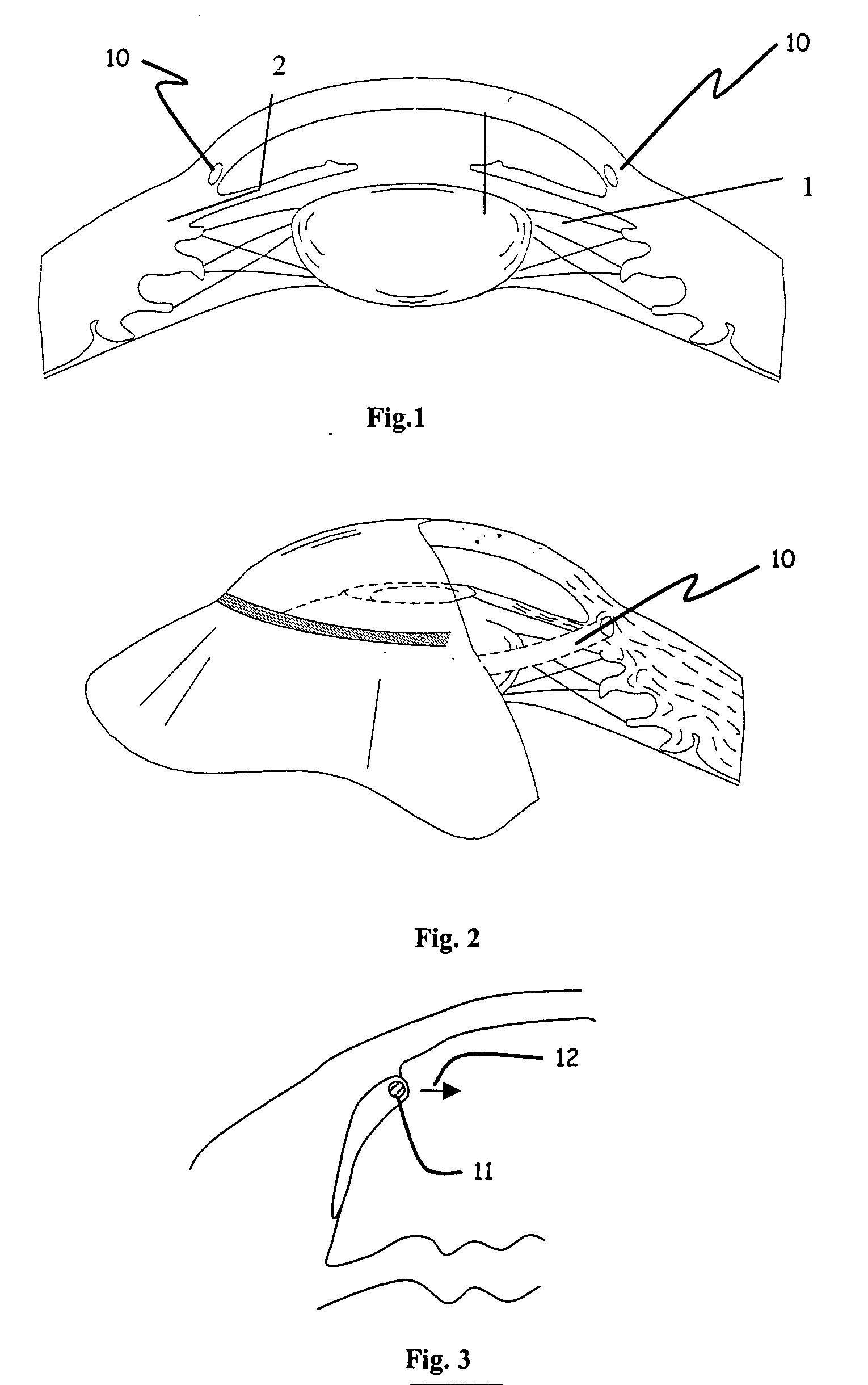
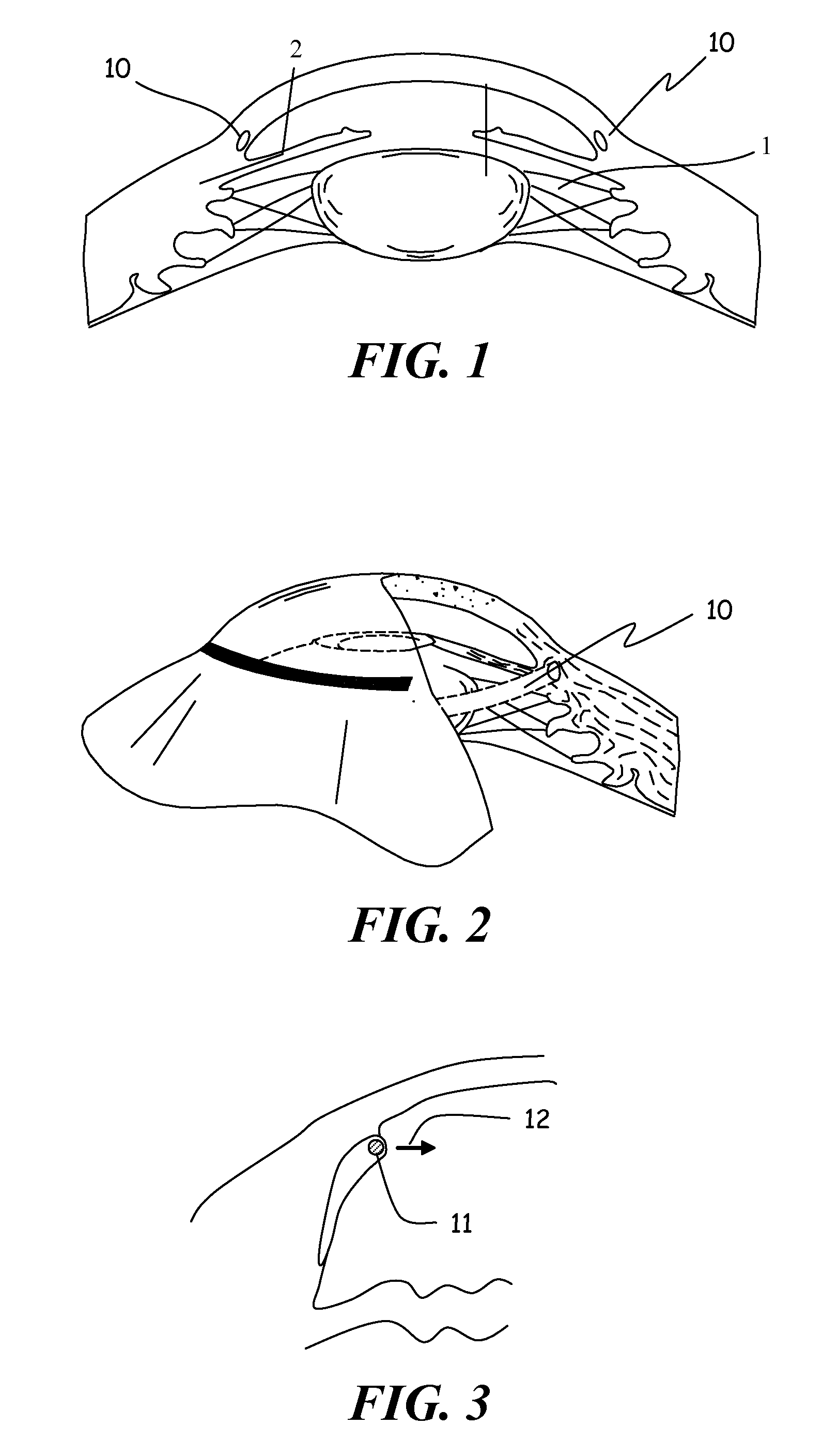
Ophthalmic implant for treatment of glaucoma
ActiveUS20060195187A1Increase outflowGood fluid permeabilityEye implantsEye surgeryOphthalmological implantSchlemm's canal
An implant is placed within Schlemm's canal of the eye and provides tension to the trabecular meshwork. The tension is continuous and increases the aqueous outflow without the necessity of administering cholinergic drugs to treat glaucoma.
Owner:NOVA EYE INC
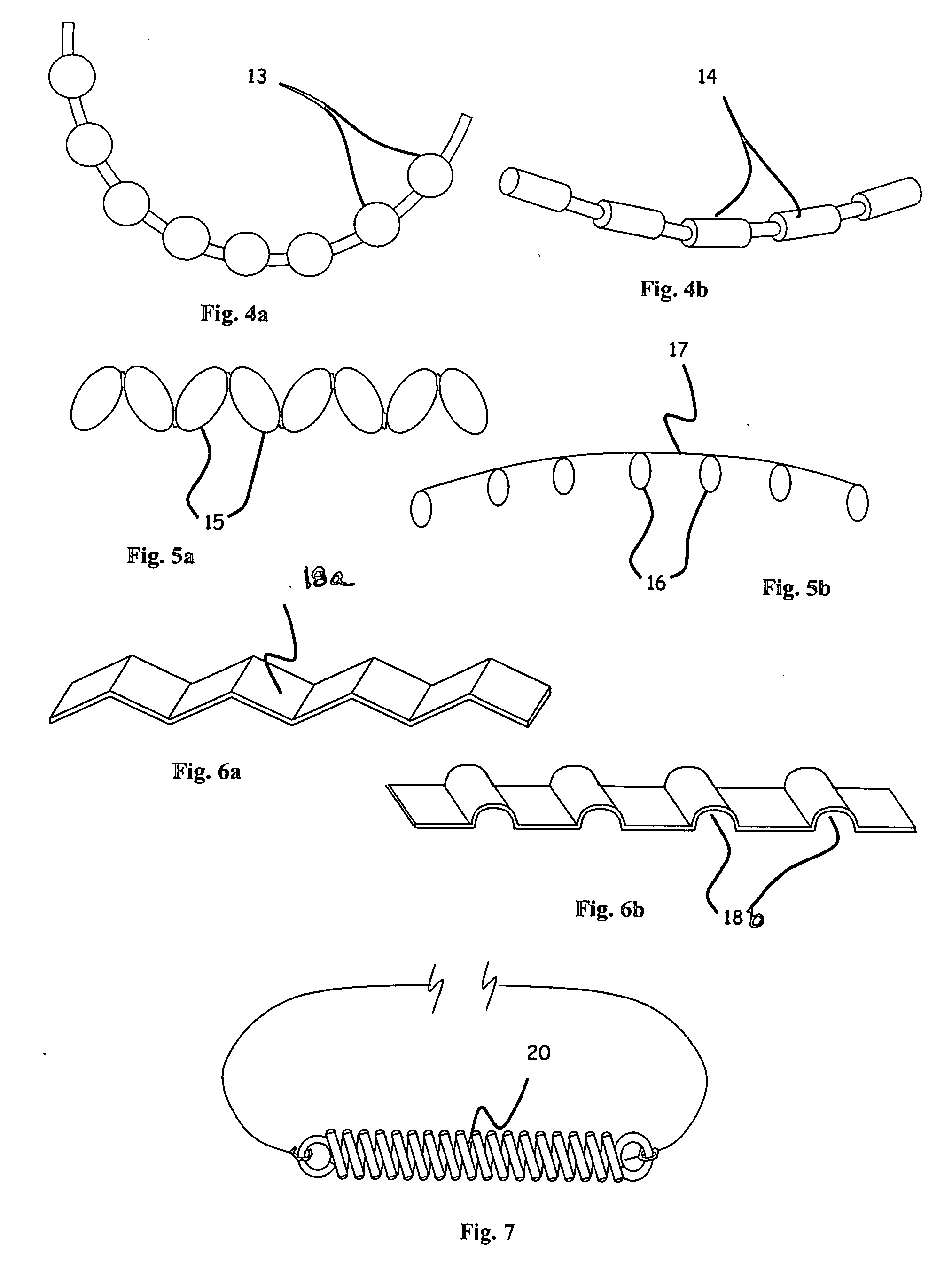
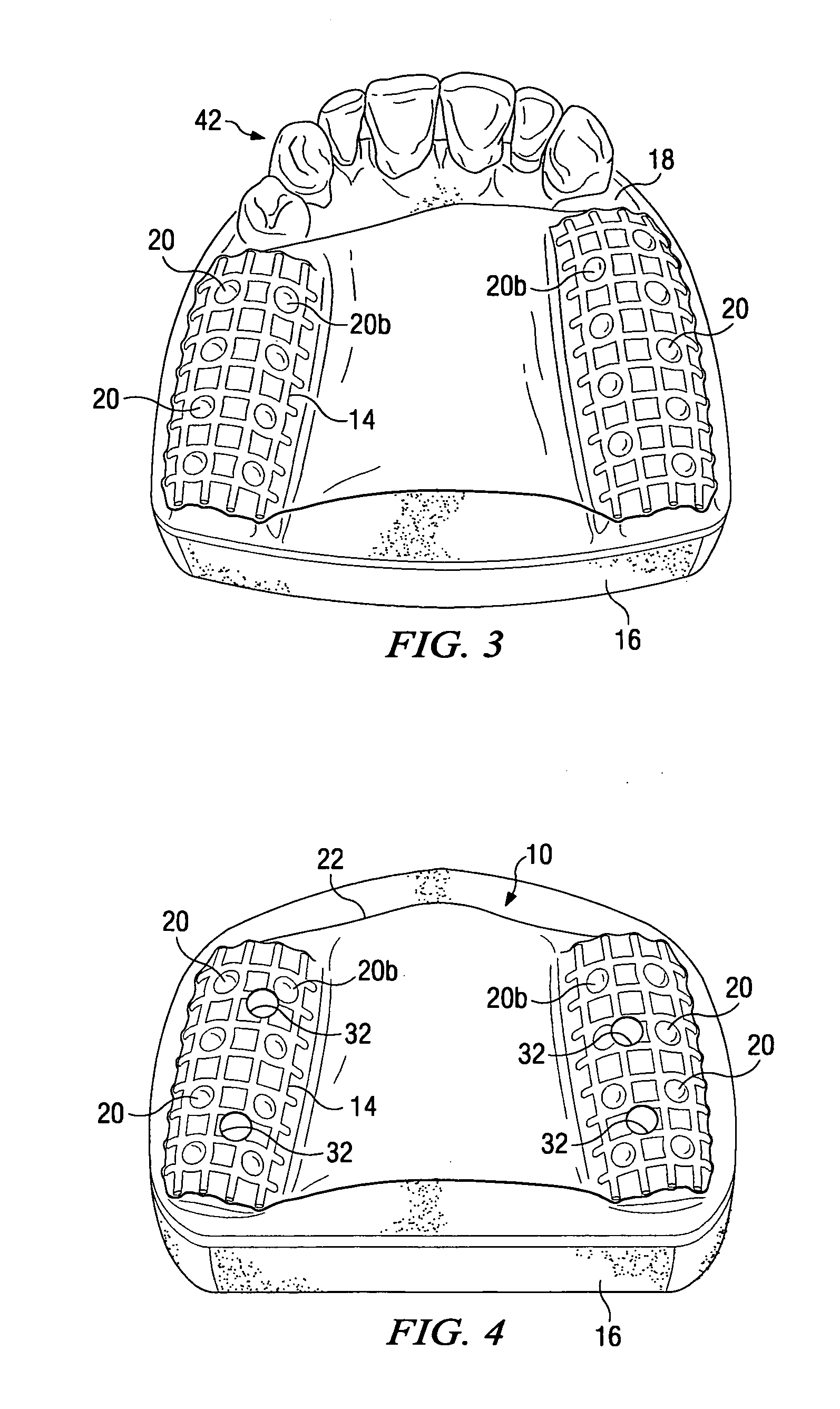
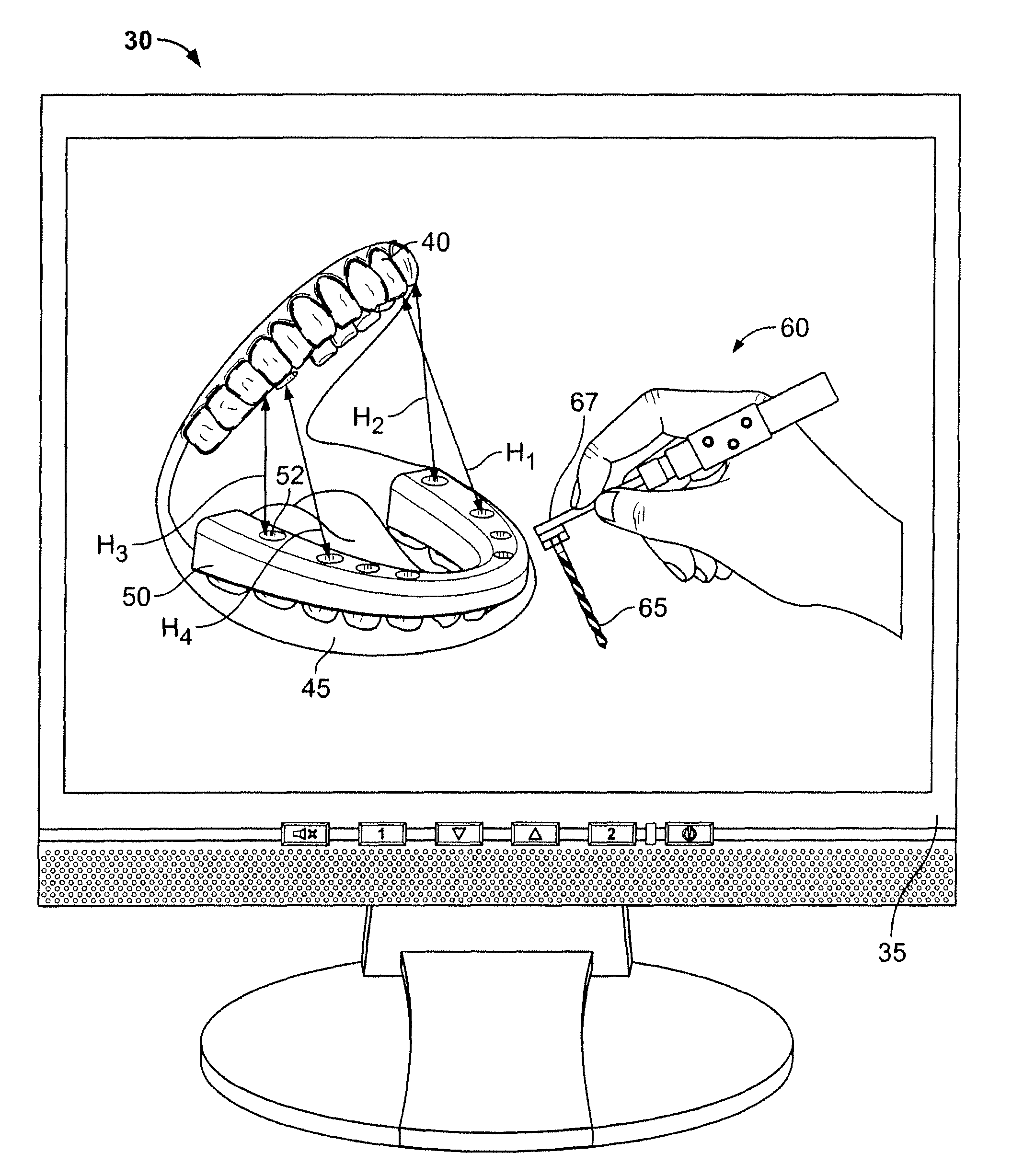
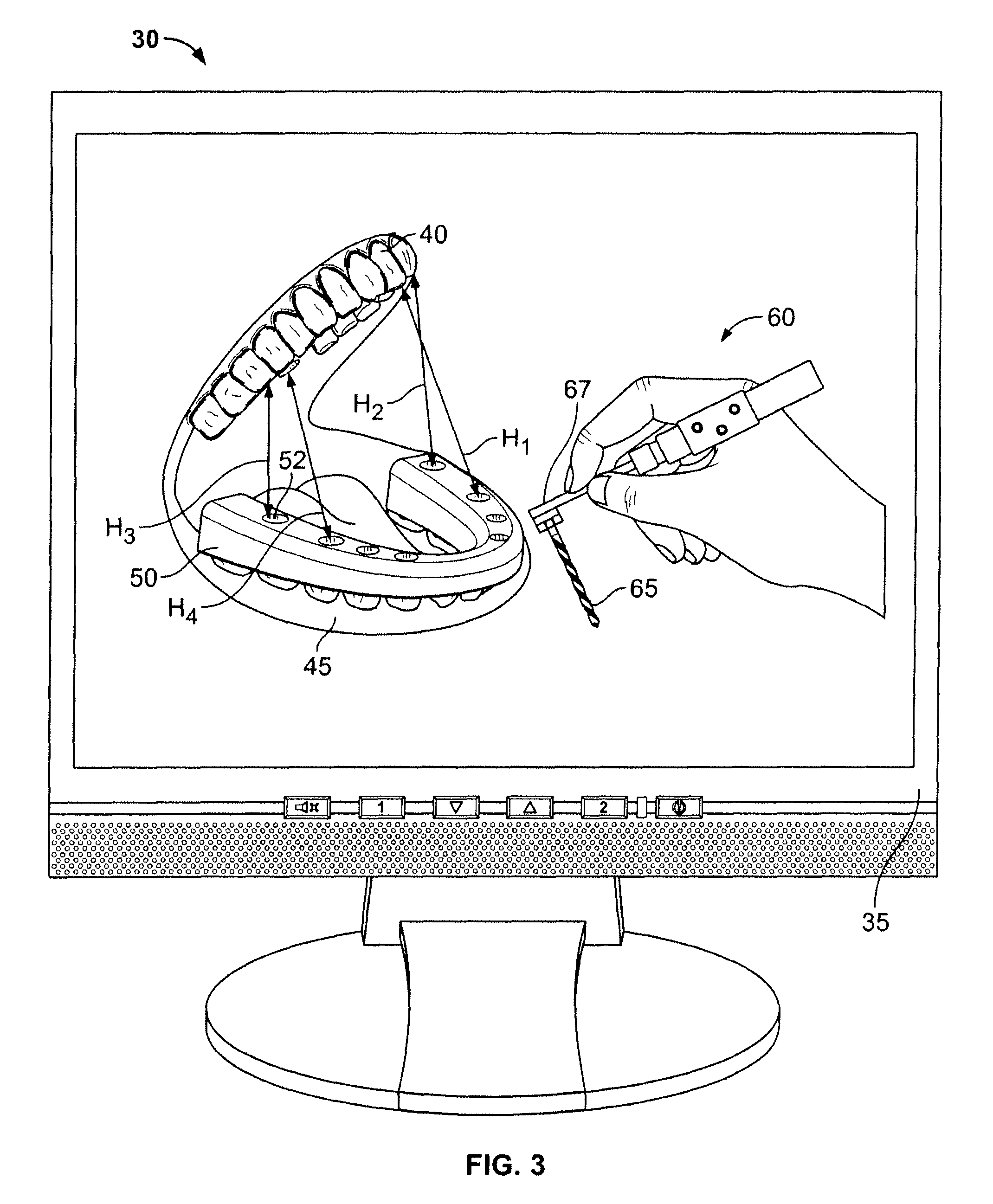
Dental implant placement locator and method of use
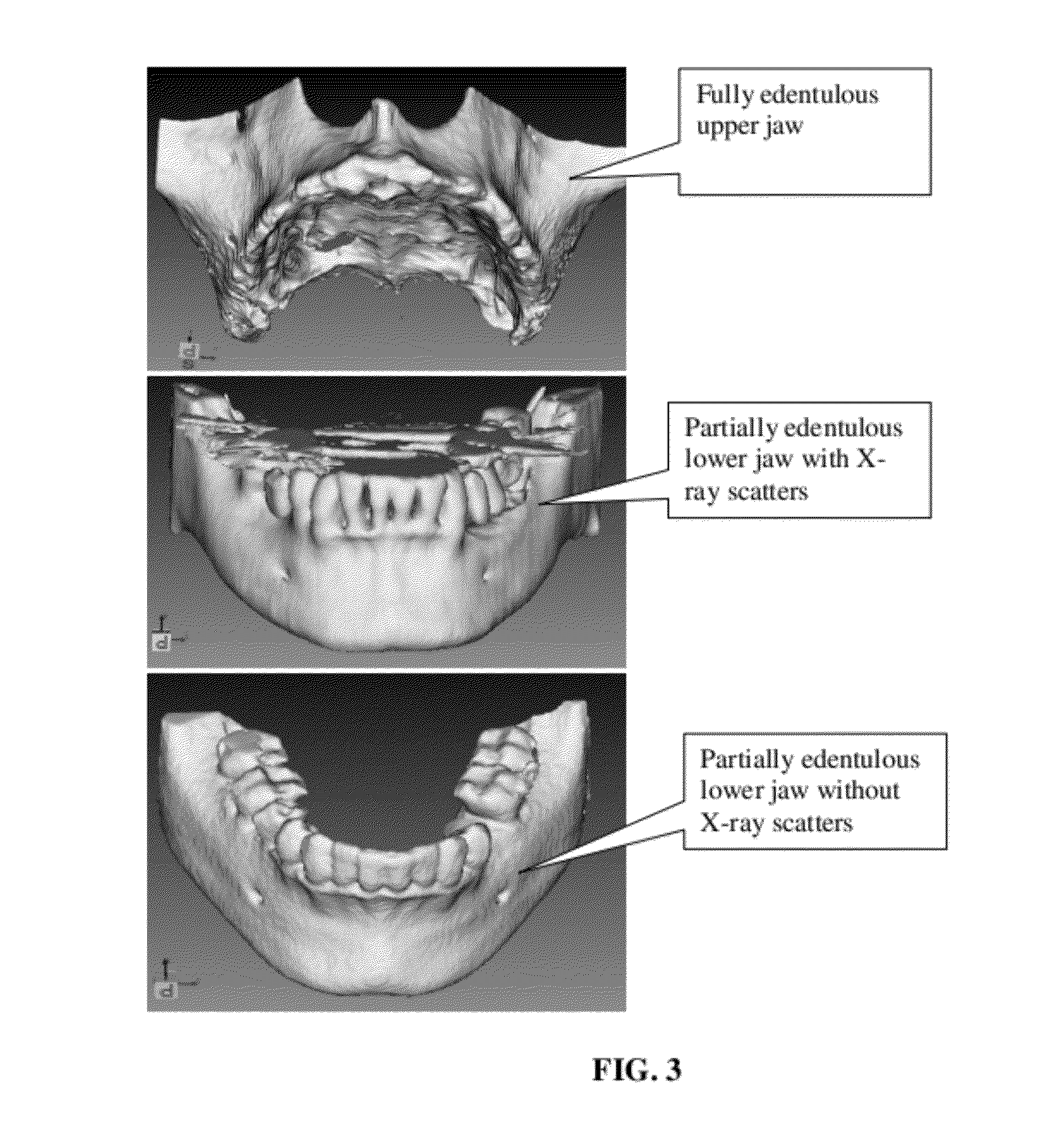
The present invention provides a device and method for facilitating the placement of dental implants by use of an implant placement locator and a sequentially sized drill orientation tube series. The implant placement locator disclosed herein comprises a visible radiolucent moldable grid, a set of radiopaque markers located at known intervals within the moldable grid, and a plastic sheeting encasing the visible radiolucent moldable grid and the radiopaque markers. The method of the present invention comprises obtaining a radiograph of a patient's mouth with the implant placement locator overlaying the patient's dental ridge and then transferring reference points as indicated by the location of the radiopaque markers relative to existing teeth and other oral structures to a dental stone model. In a preferred embodiment, drill bits are directed in the desired trajectory into the patient's available lower or upper jaw bone by use of drill orientation tubes in combination with the implant placement locator. A sequentially sized set of spacers is provided having sequentially sized inside diameters to direct varying diameter drills. Drill guide parallelism is thus provided by the invention.
Owner:SHELTON ROBERT
Delivery system, method, and anchor for medical implant placement
ActiveUS20070179583A1Transvascular endocardial electrodesSurgical needlesMonitoring physiological parametersBiomedical engineering
Owner:UIM PRESSURE IMPLANT INC
Anchor for medical implant placement and method of manufacture
ActiveUS7317951B2Transvascular endocardial electrodesCatheterDistal portionMonitoring physiological parameters
An anchor and procedure for placing a medical implant, such as for monitoring physiological parameters. The anchor includes a central body in which a medical implant can be received. Arms and members extend radially from first and second ends, respectively, of the central body. Each member defines a leg extending toward distal portions of the arms to provide a clamping action. The anchor and its implant are placed by coupling first and second guidewires to first and second portions of the anchor, placing an end of a delivery catheter in a wall where implantation is desired, inserting the anchor in the catheter with the guidewires to locate the anchor within the wall, deploying the arms of the anchor at one side of the wall followed by deployment of the members at the opposite side of the wall, and thereafter decoupling the guidewires from the anchor.
Owner:UIM PRESSURE IMPLANT INC
Apparatus and methods for inserting an implant
ActiveUS8062303B2Gently insert the intervertebral deviceForce is smallNon-surgical orthopedic devicesSpinal implantsBiomedical engineeringVertebral body
A system and method for inserting an implant into a cavity is disclosed, which may include advancing an implant insertion instrument toward a pair of adjacent bodies, the implant insertion instrument having two opposed ramps, wherein each ramp has a distal tip and wherein the longitudinal axes of the opposed ramps are separated by an initial angle; inserting the distal tips of the opposed ramps between the adjacent bodies, thereby creating an initial interbody cavity between the adjacent bodies; expanding the interbody cavity while maintaining the initial angle between the longitudinal axes of the opposed ramps; placing the implant in a final location between the adjacent bodies; transferring a compressive force urging the adjacent bodies together from the opposed ramps to the implant; and extracting the implant insertion instrument from the interbody cavity.
Owner:K2M
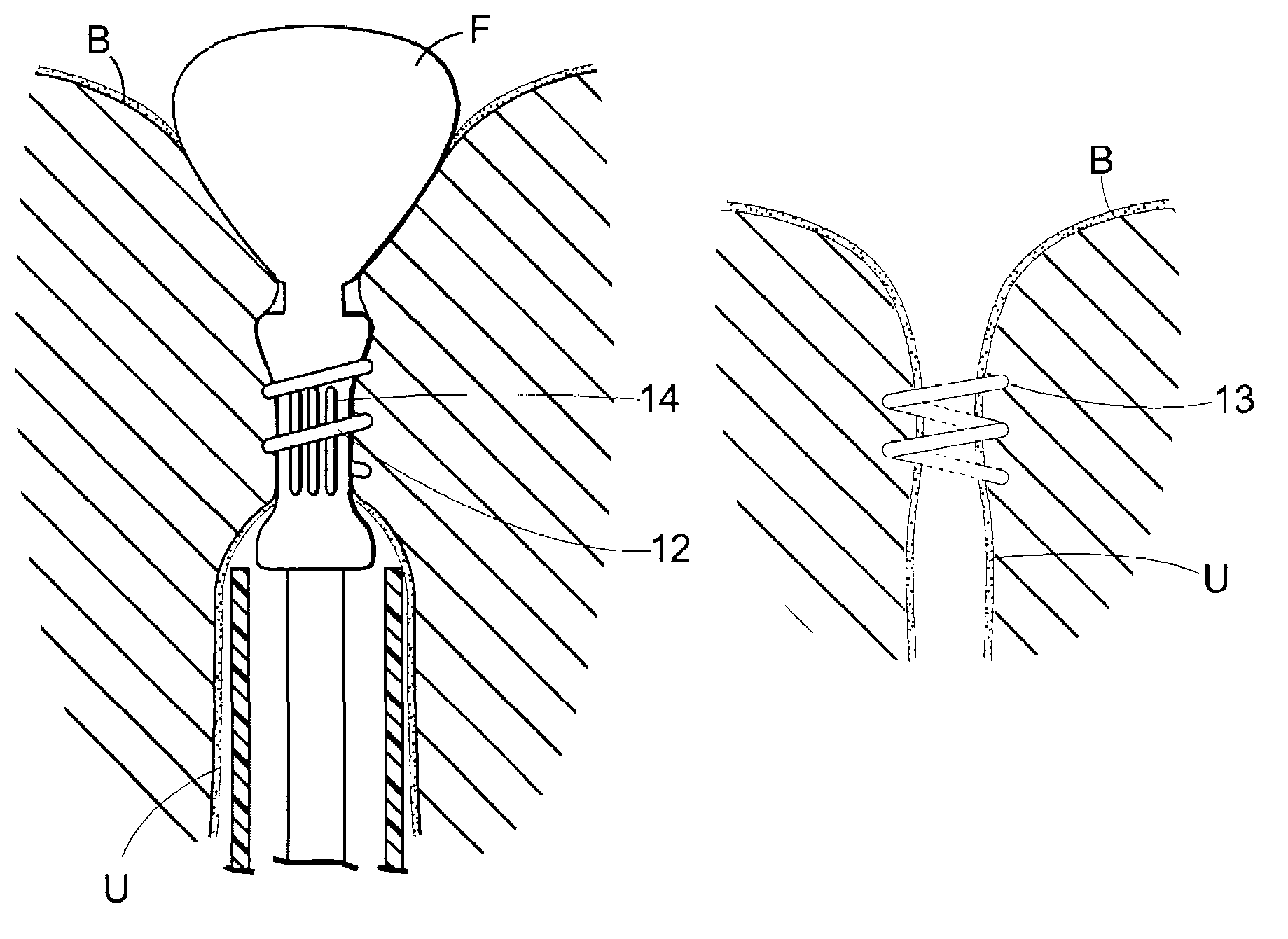
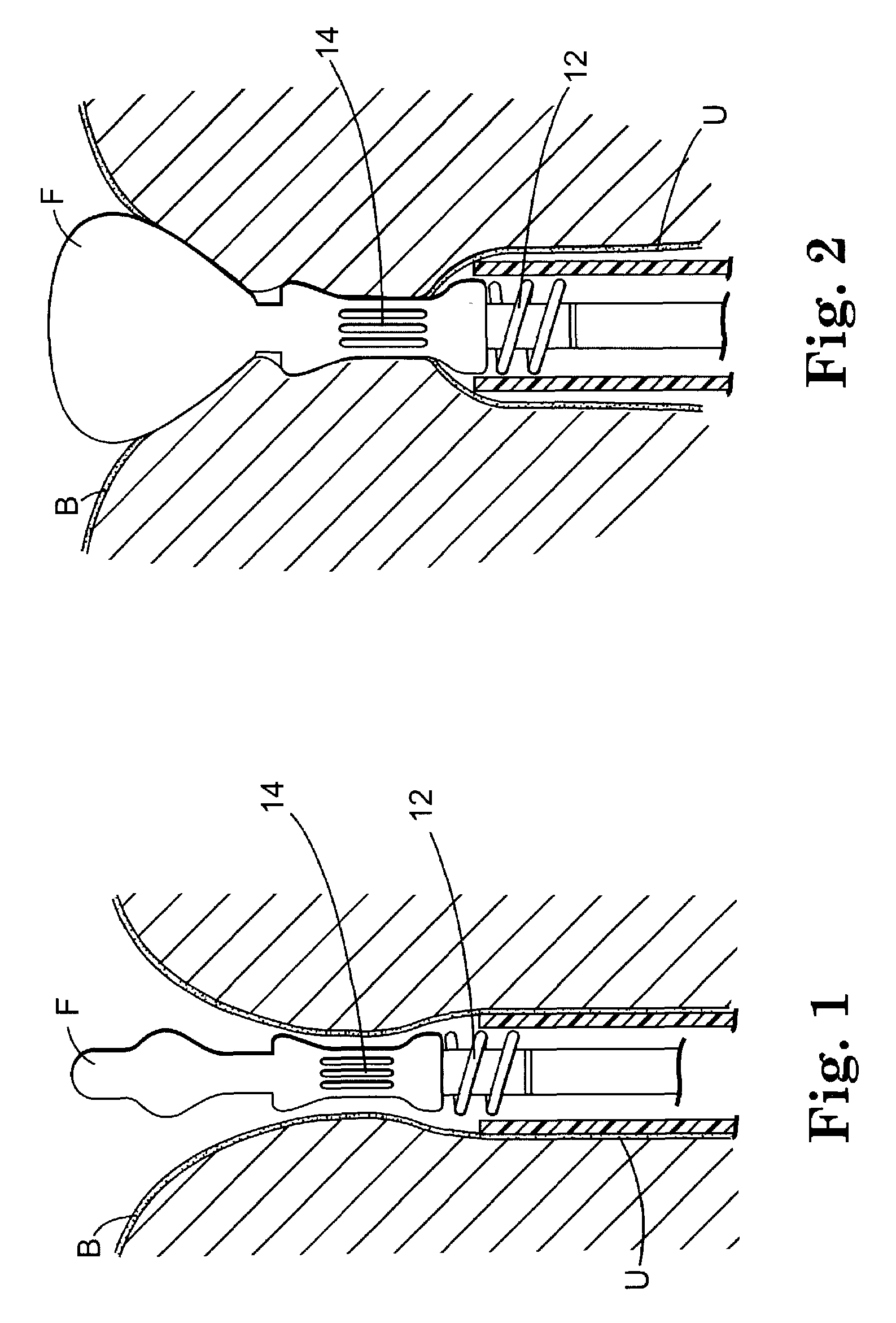
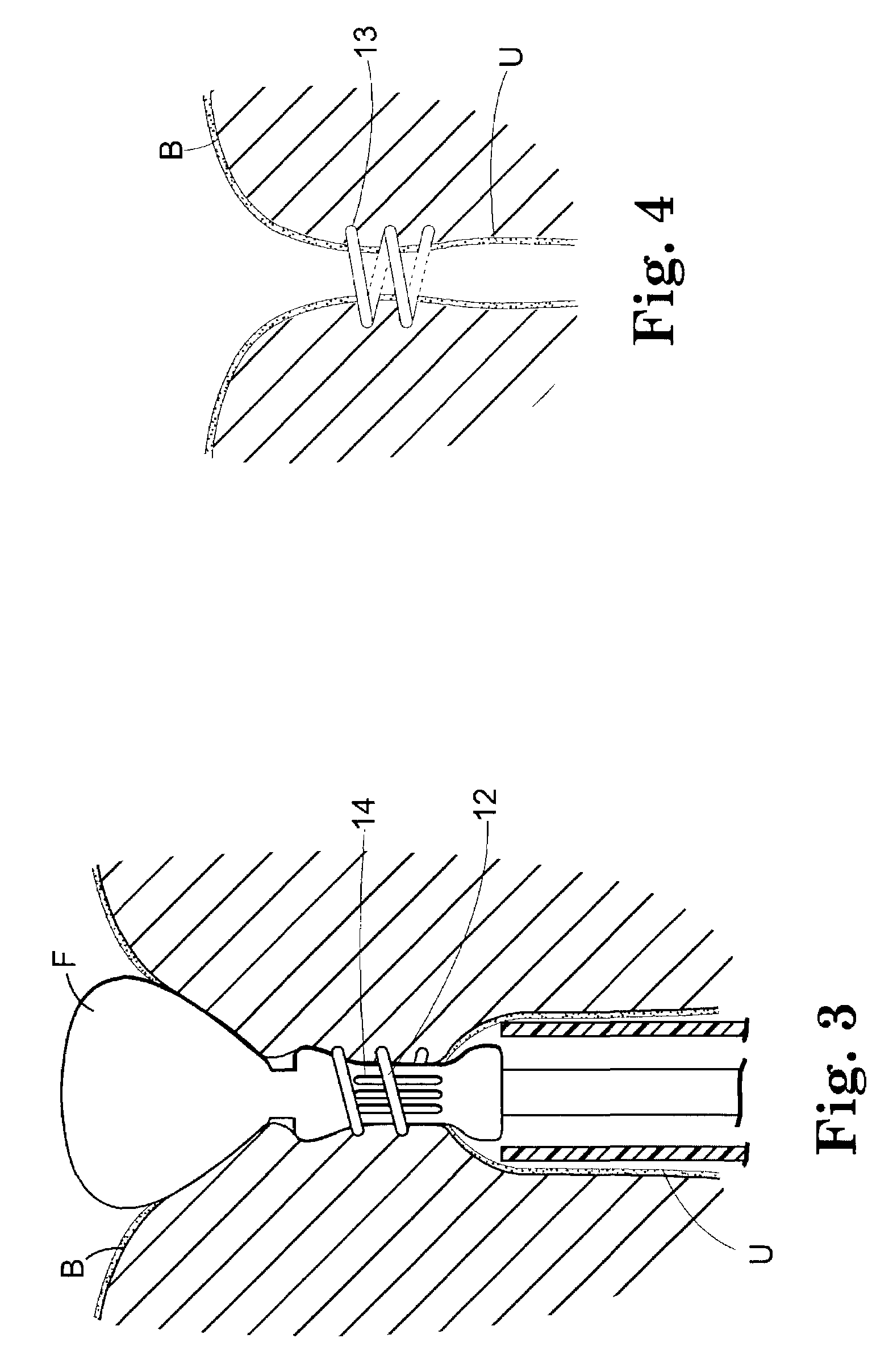
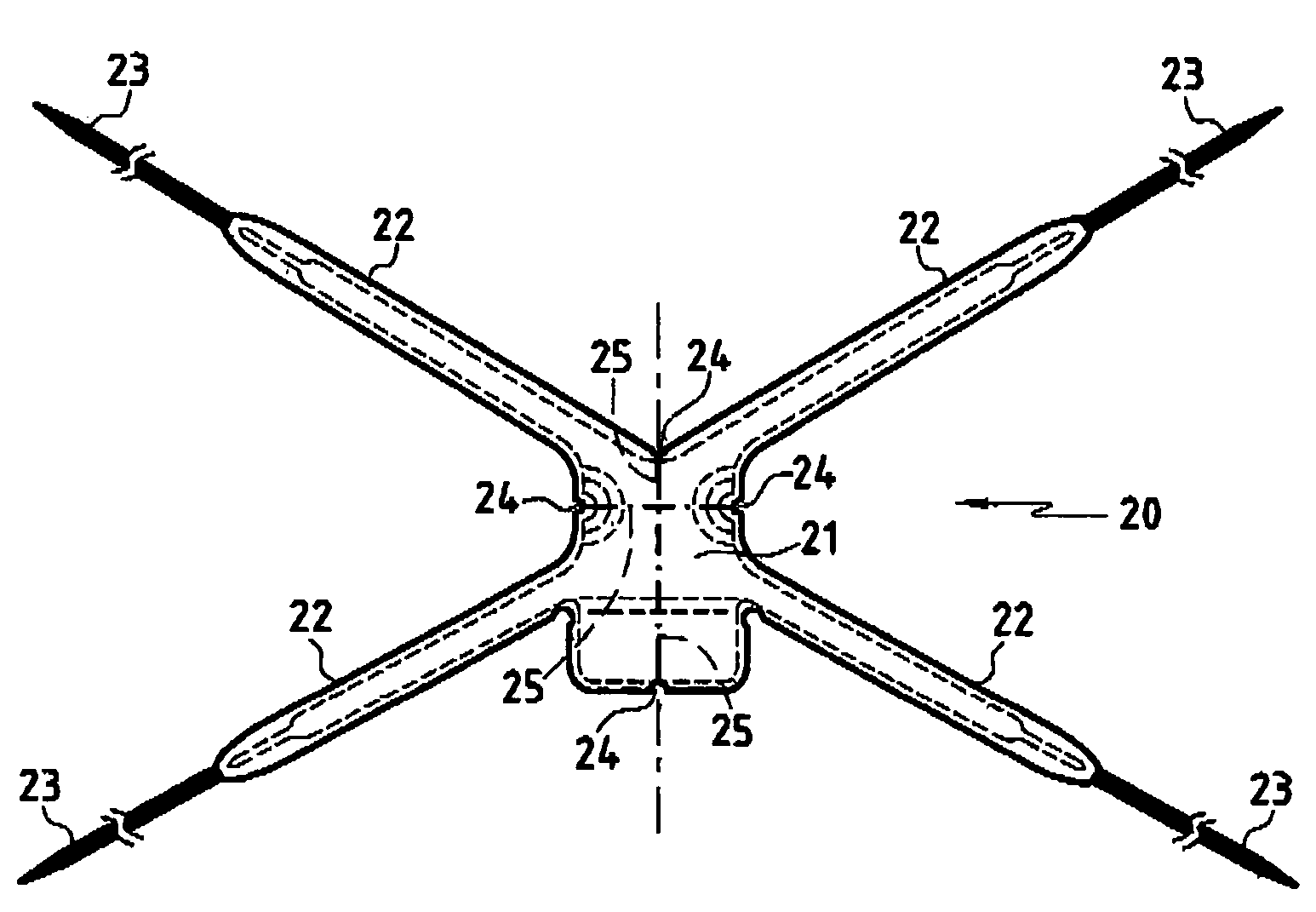
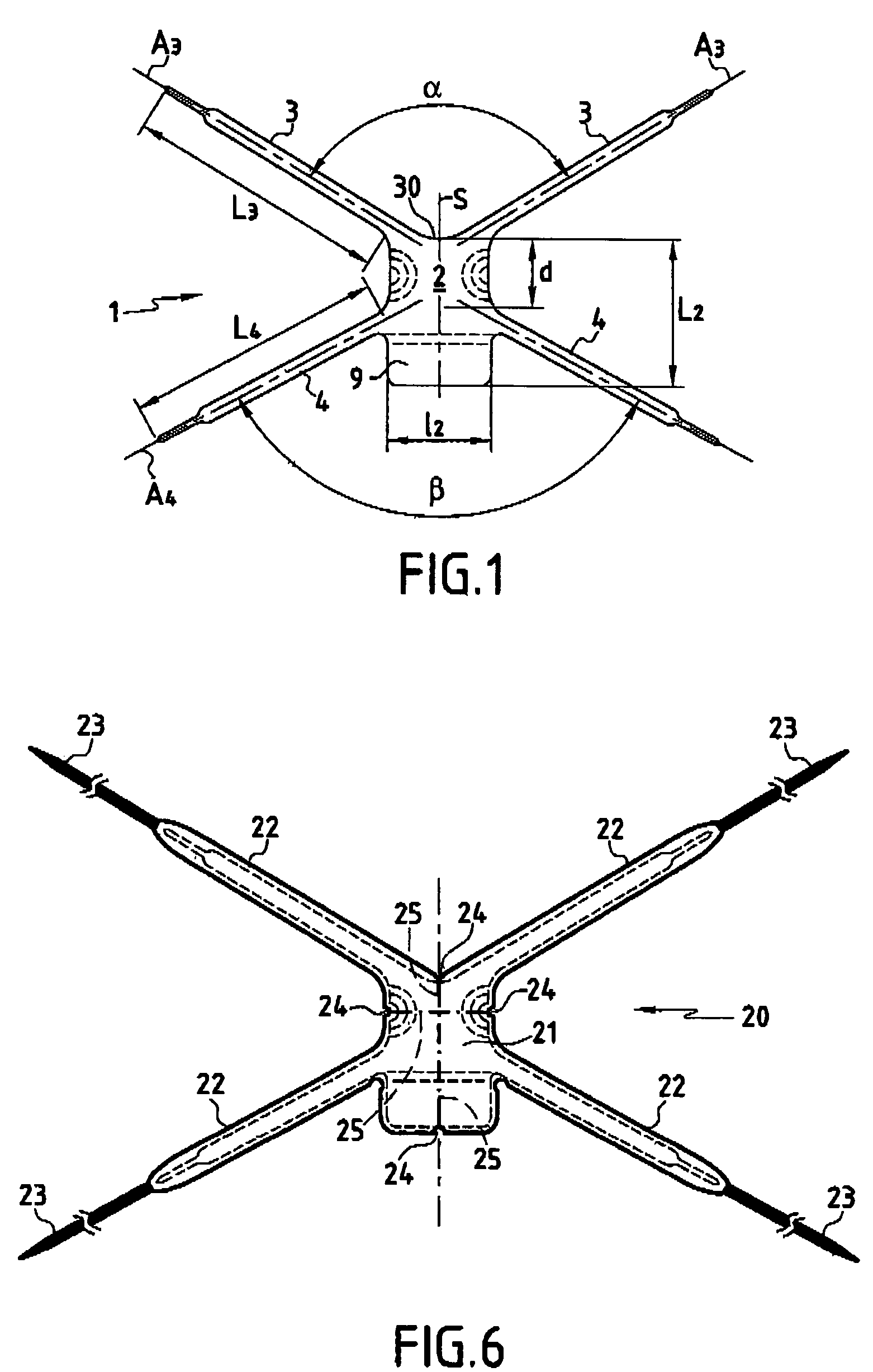
Implant for treating rectocele and a device for putting said implant into place
InactiveUS7588598B2Good stability of implantationMinimize traumaAnti-incontinence devicesSurgical needlesFiberPolyester
An implant for treating rectocele and / or prolapsus of the vaginal fornix is thin and flexible and includes a support body from which there extend at least two upper suspension stabilizers disposed on either side of a sagittal plane and two lower suspension stabilizers disposed on either side of the sagittal plane. The implant may be constructed from a suitable biocompatible material such as a woven synthetic material, or a knitted material of polypropylene or polyester fibers.
Owner:COLOPLAST AS
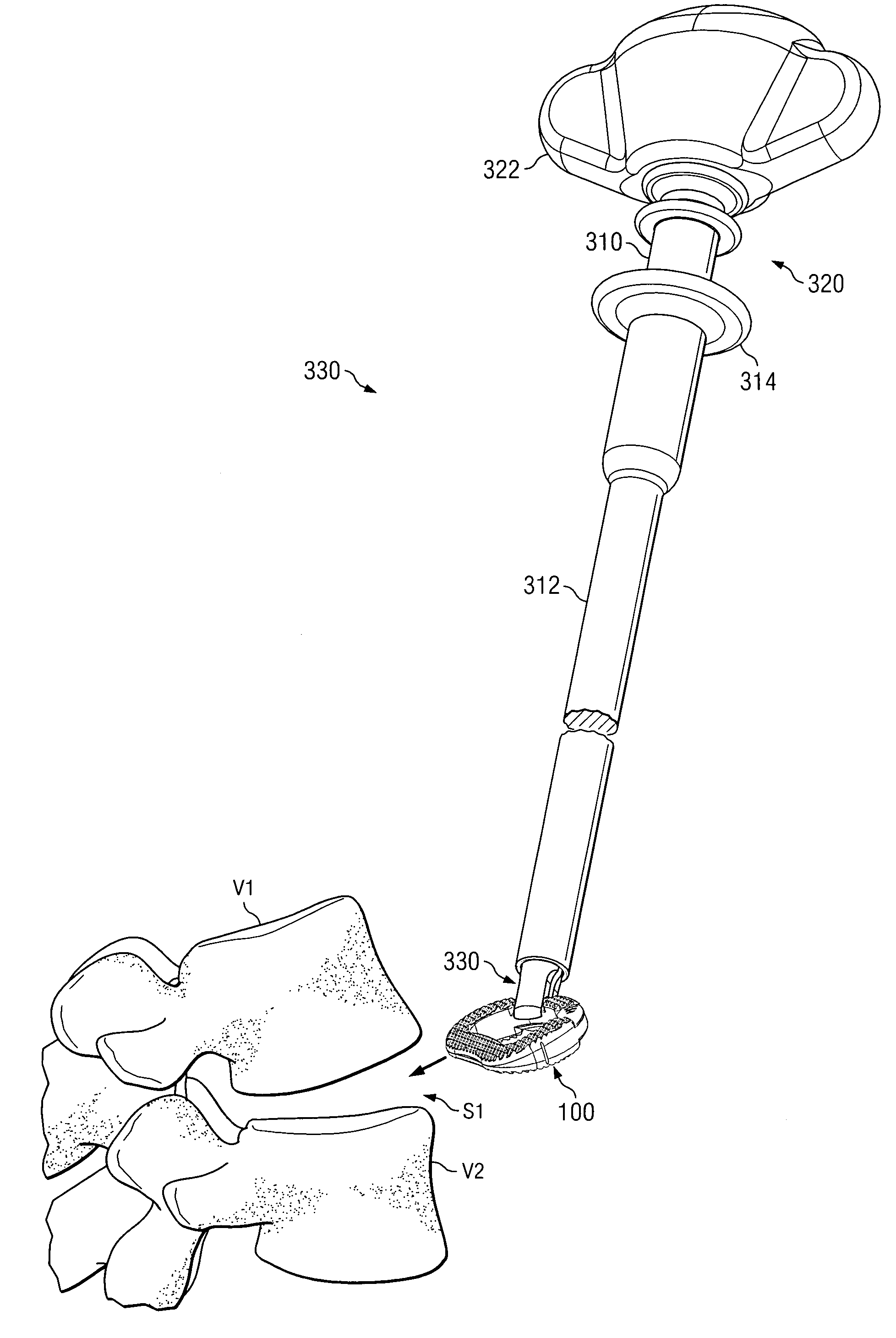
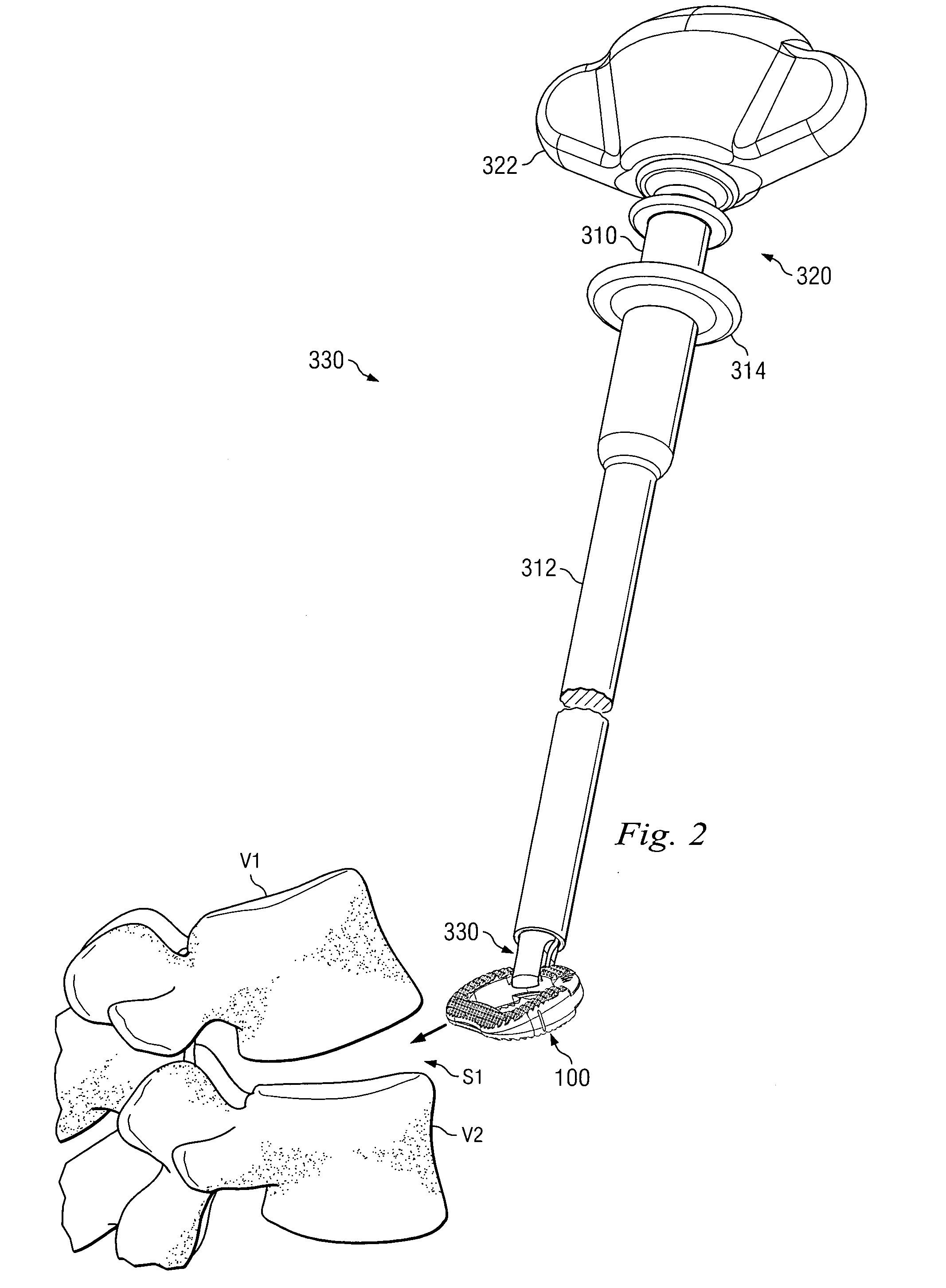
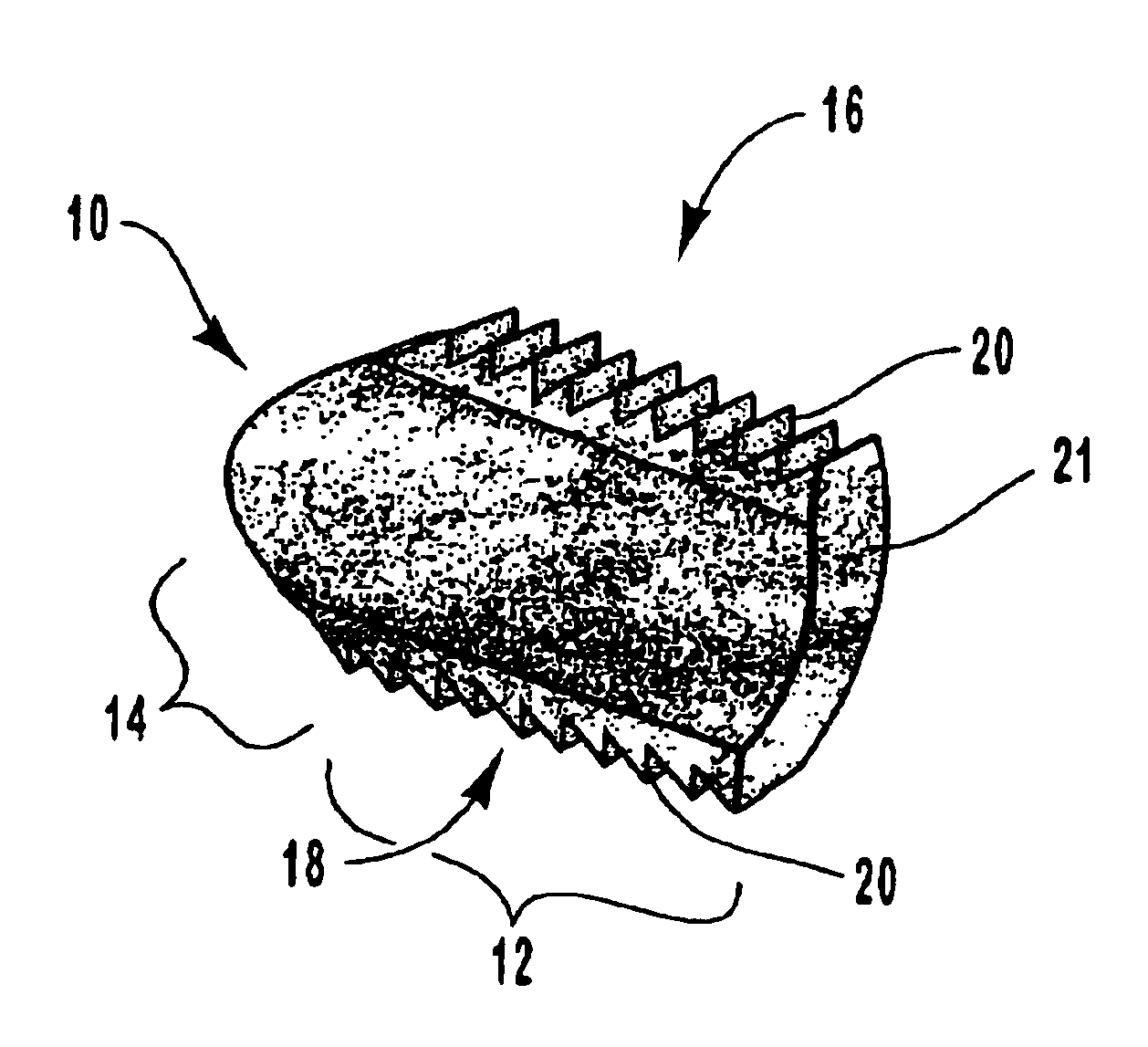
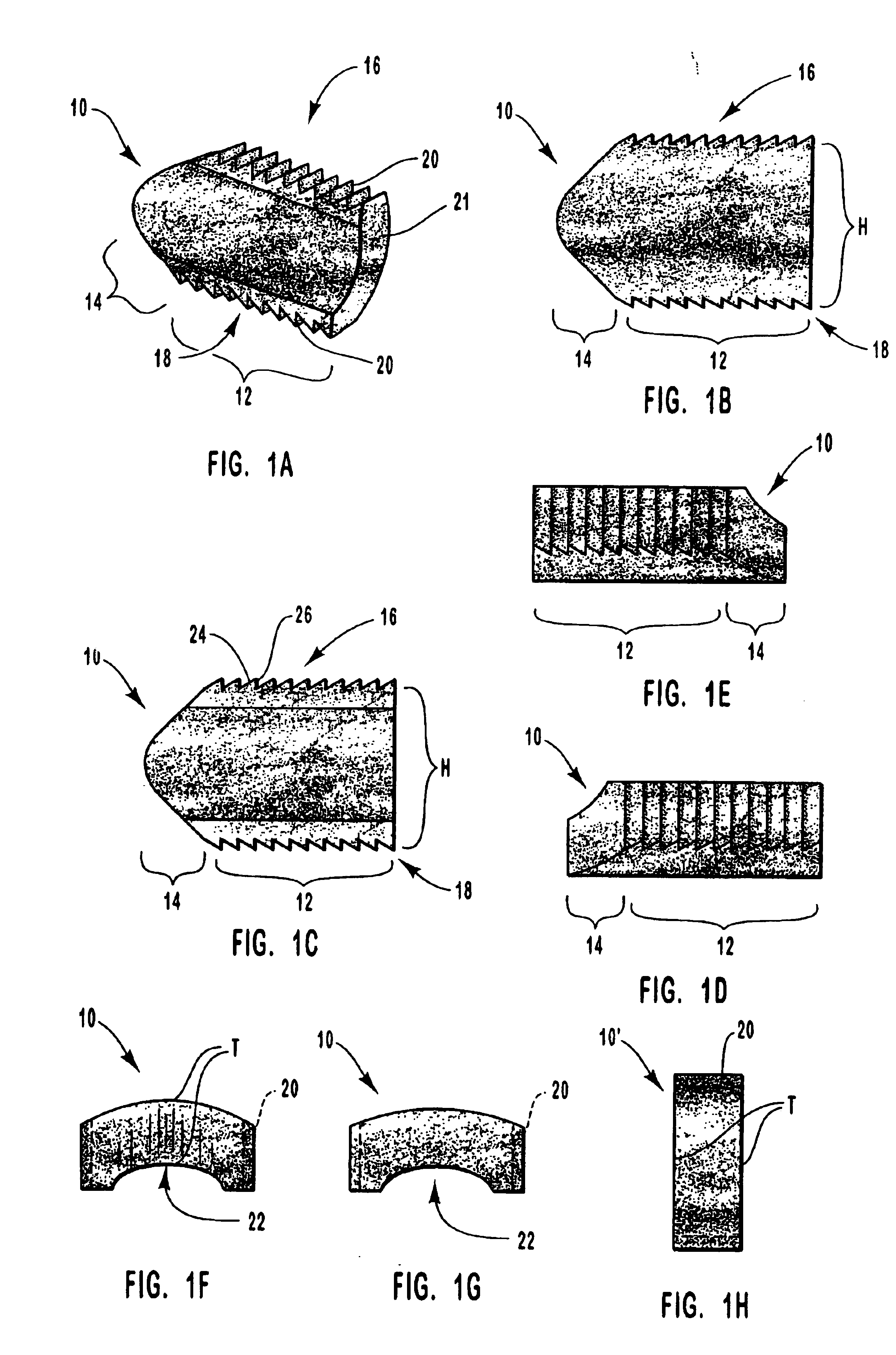
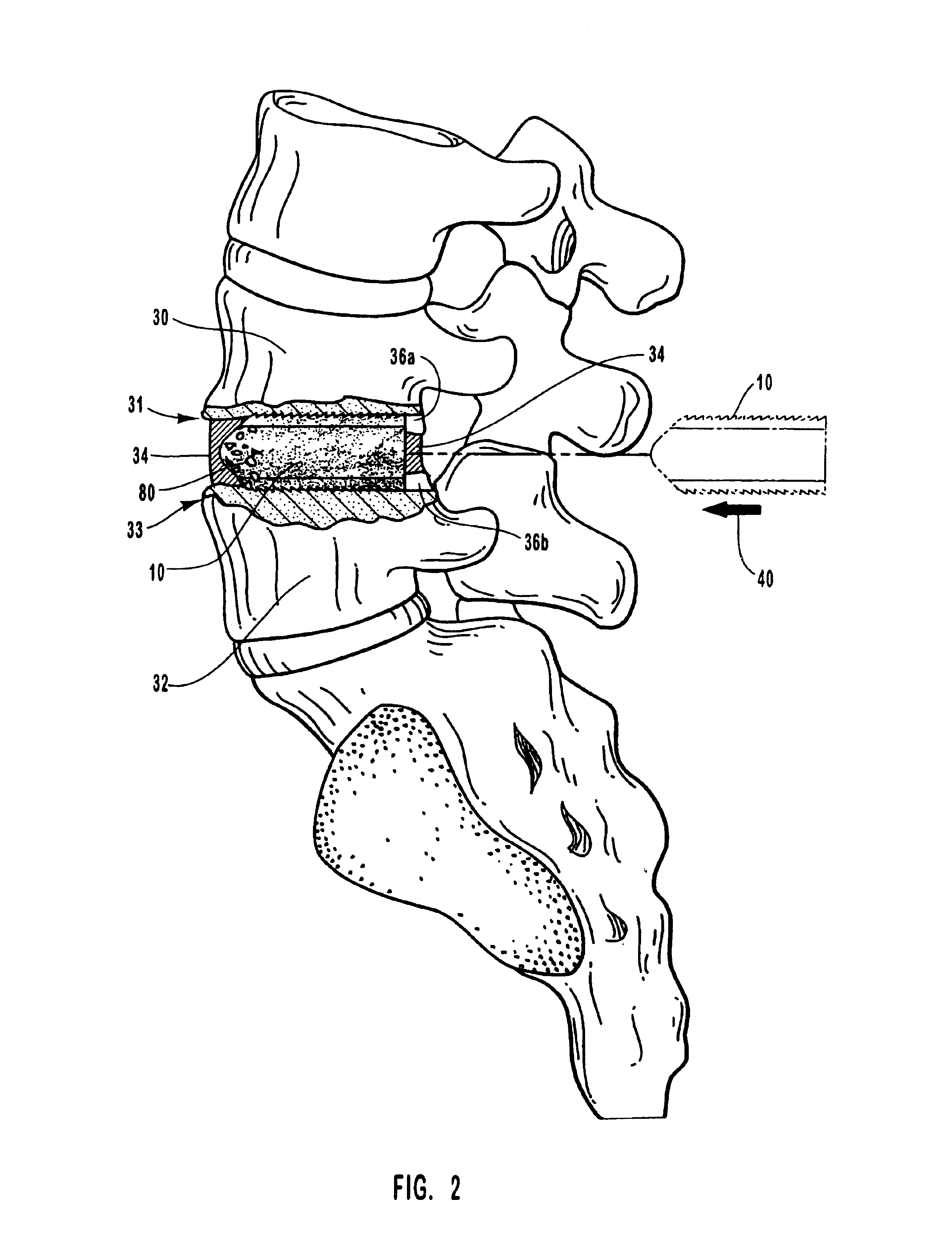
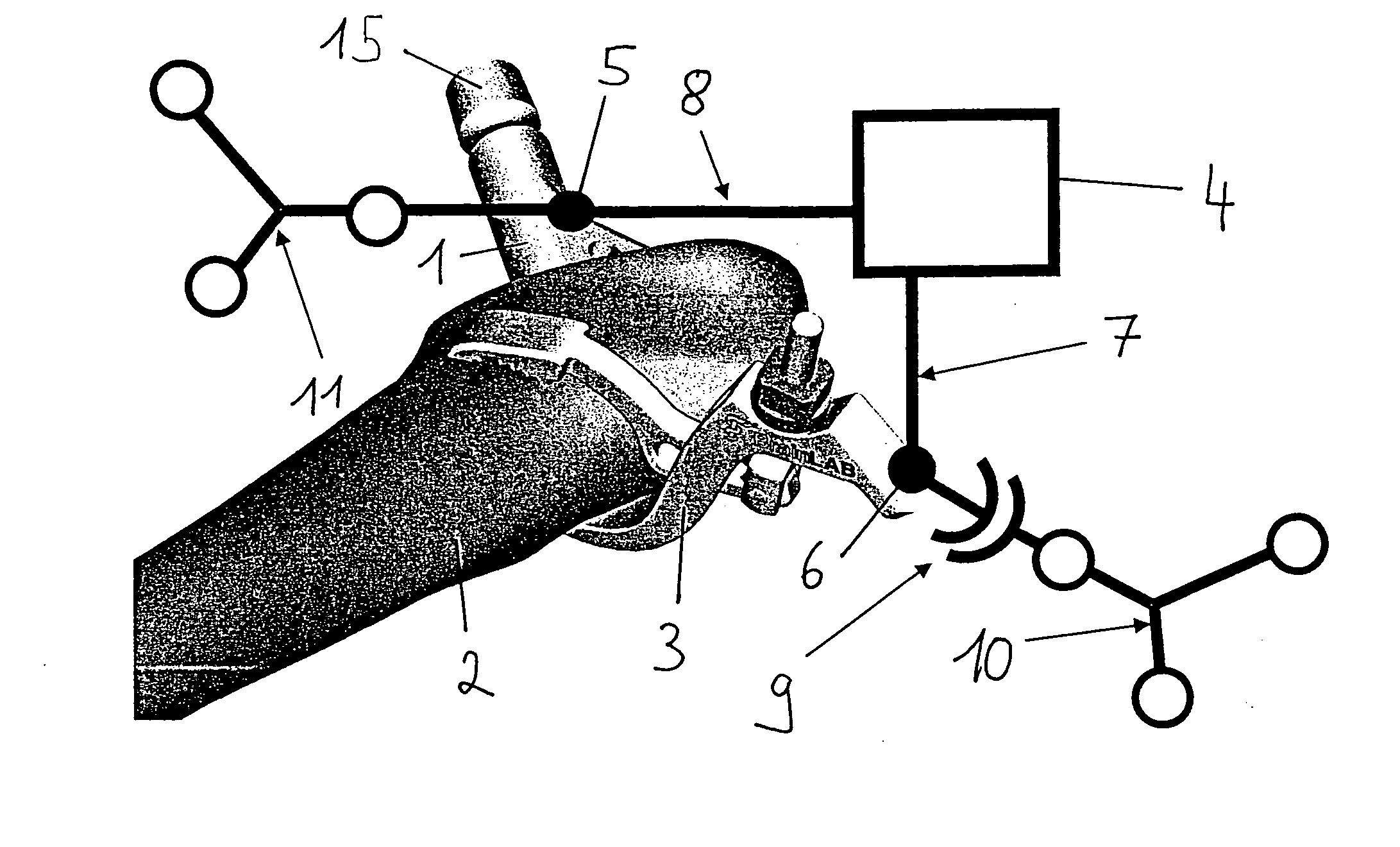
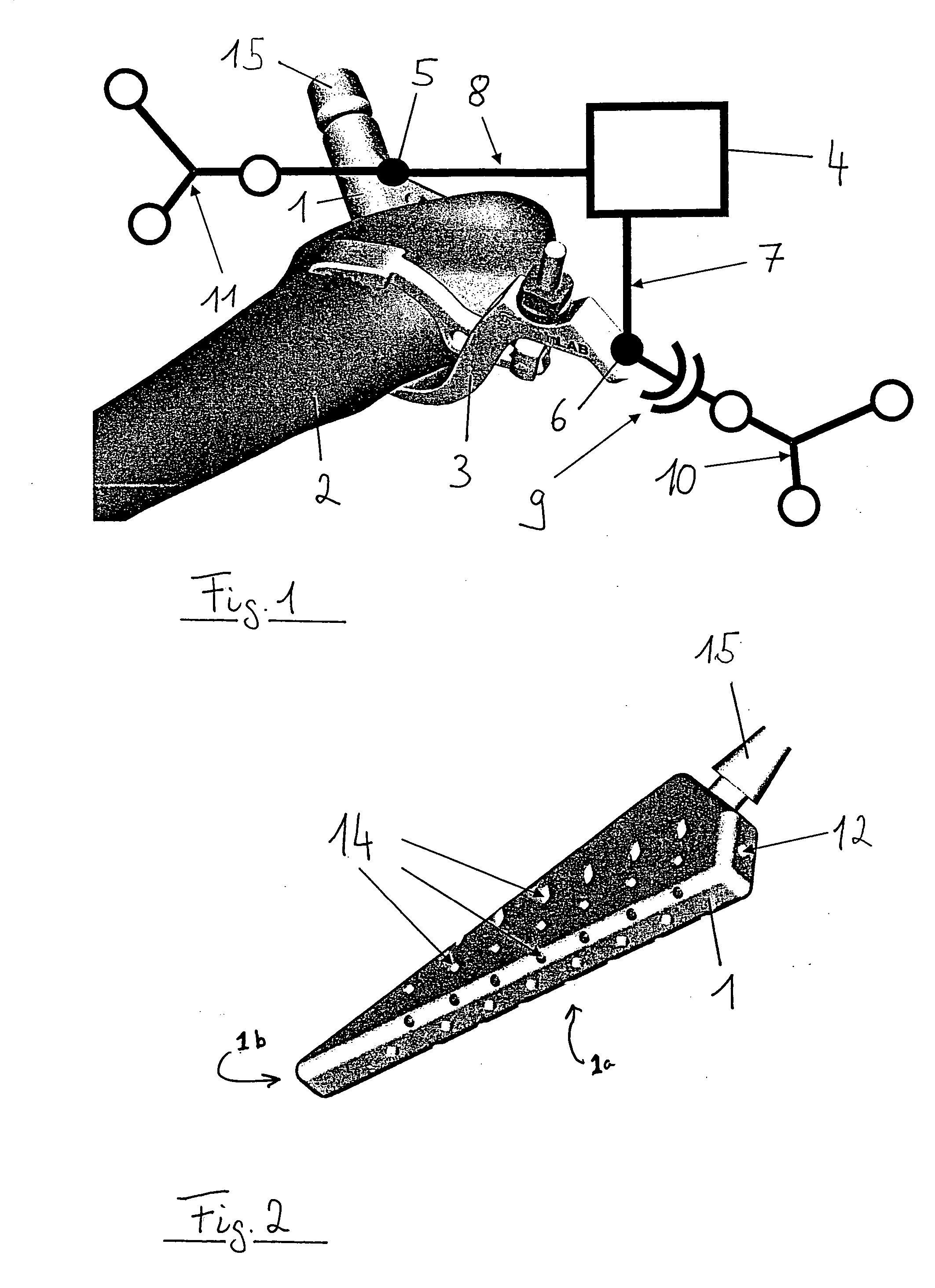
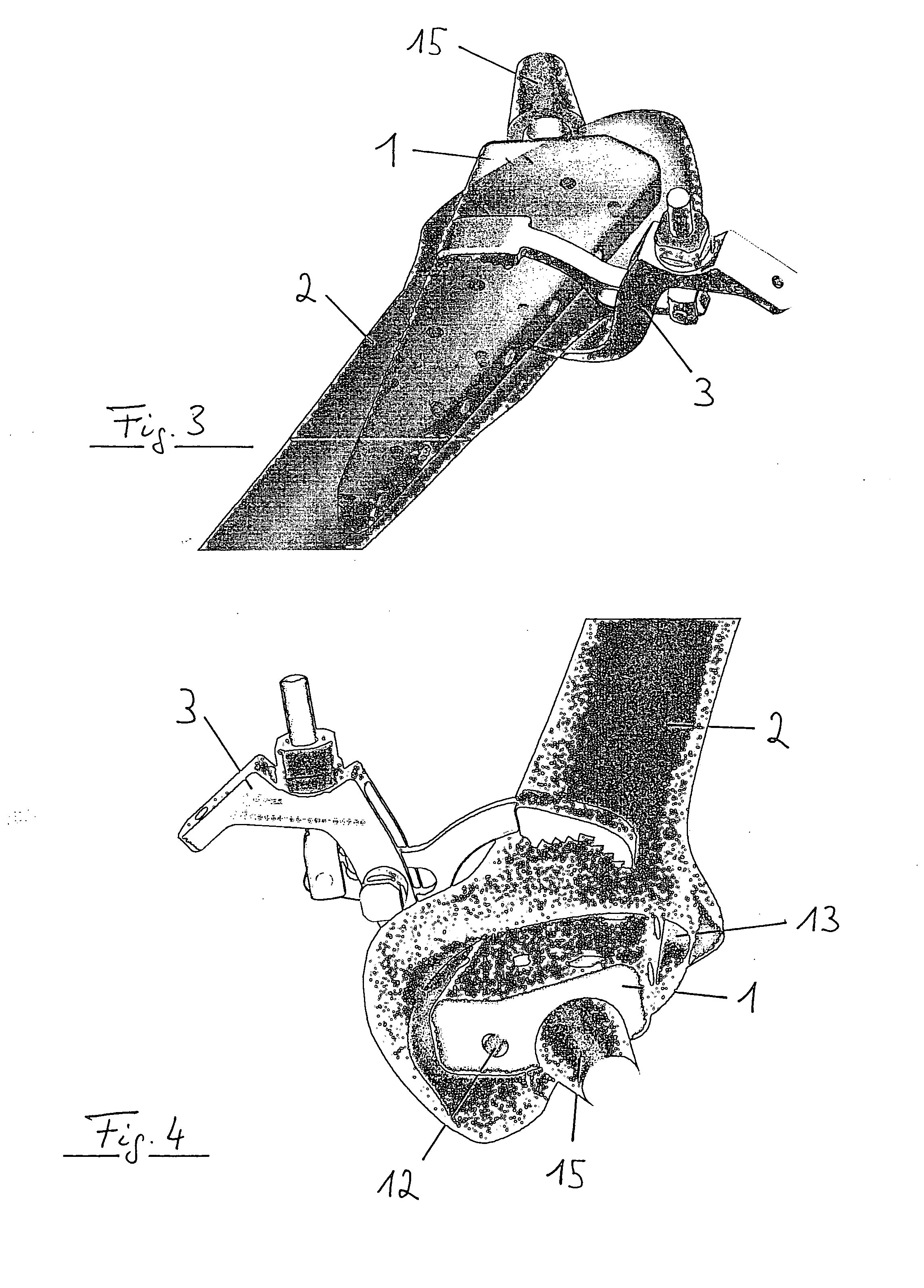
Spinal vertebral implant and methods of insertion
InactiveUS6843804B2Promote bone growthPrevent implantationInternal osteosythesisBone implantNoseIntervertebral space
A spinal vertebral implant includes a substantially rectangular shaped base section made from a solid piece of bone. A nose section extends integrally from the substantially rectangularly shaped base section and preferably has a generally tapering shape to foster entry between adjacent vertebrae. The nose section tapers distally and inwardly from the base section to form a generally pointed or rounded distal tip portion and comprises a solid piece of bone. Serrated sides assist the implant in gripping adjacent upper and lower vertebrae and in being maintained therebetween. The serrated sides are angled in a manner that encourages the implant to be placed between the vertebrae and locked therebetween upon such placement. First and second implants may be placed into respective left and right sides of an intervertebral space. A method for placing one or more implants between the adjacent vertebrae comprises forming a slot configured to receive an implant and inserting the implant into the slot. Each slot is preferably formed from an upper slot portion and a lower slot portion in the posterior portion of adjacent upper and lower vertebrae, respectively. Instruments for performing the method include osteotomes, impactors, and spacers.
Owner:BRYAN DONALD W
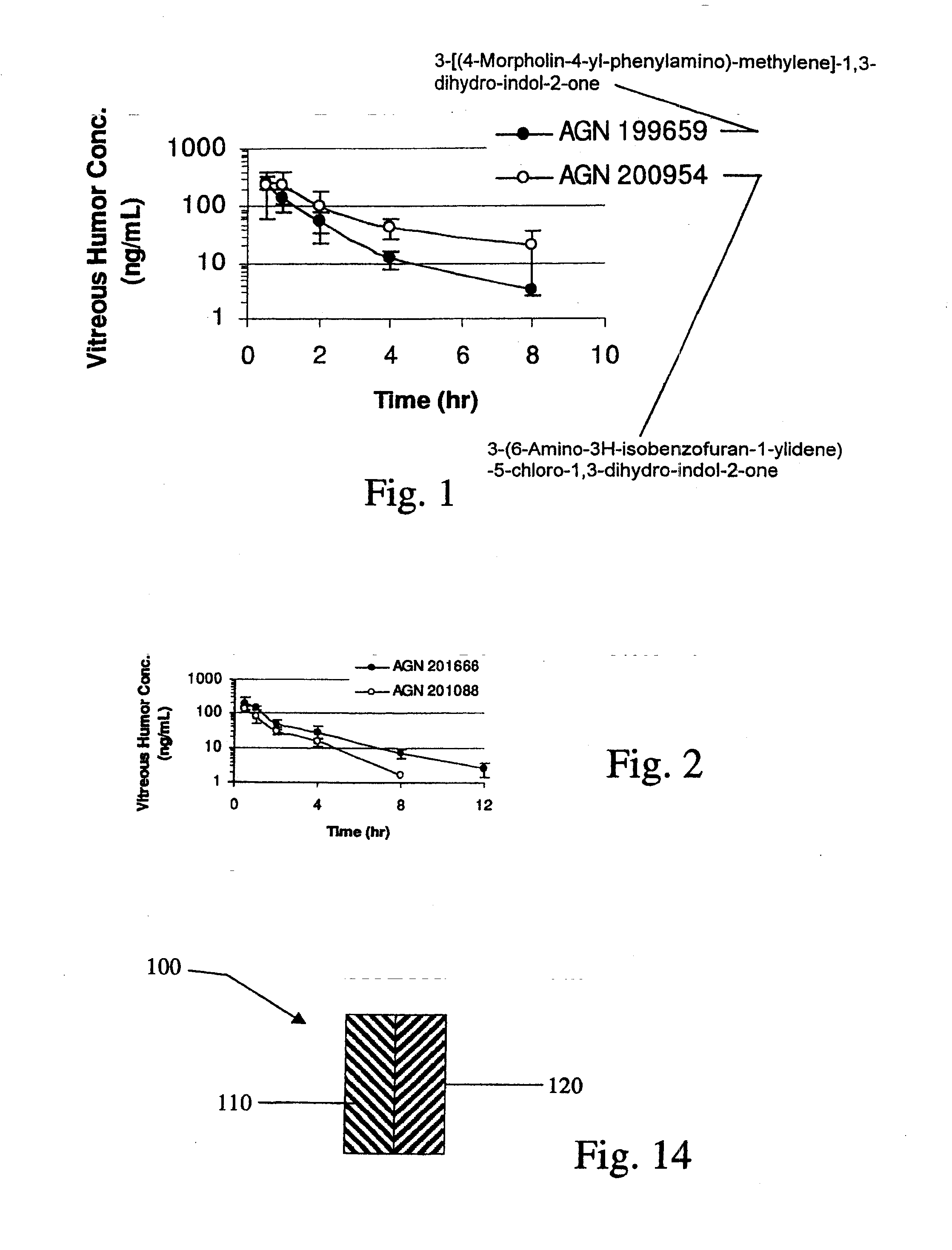
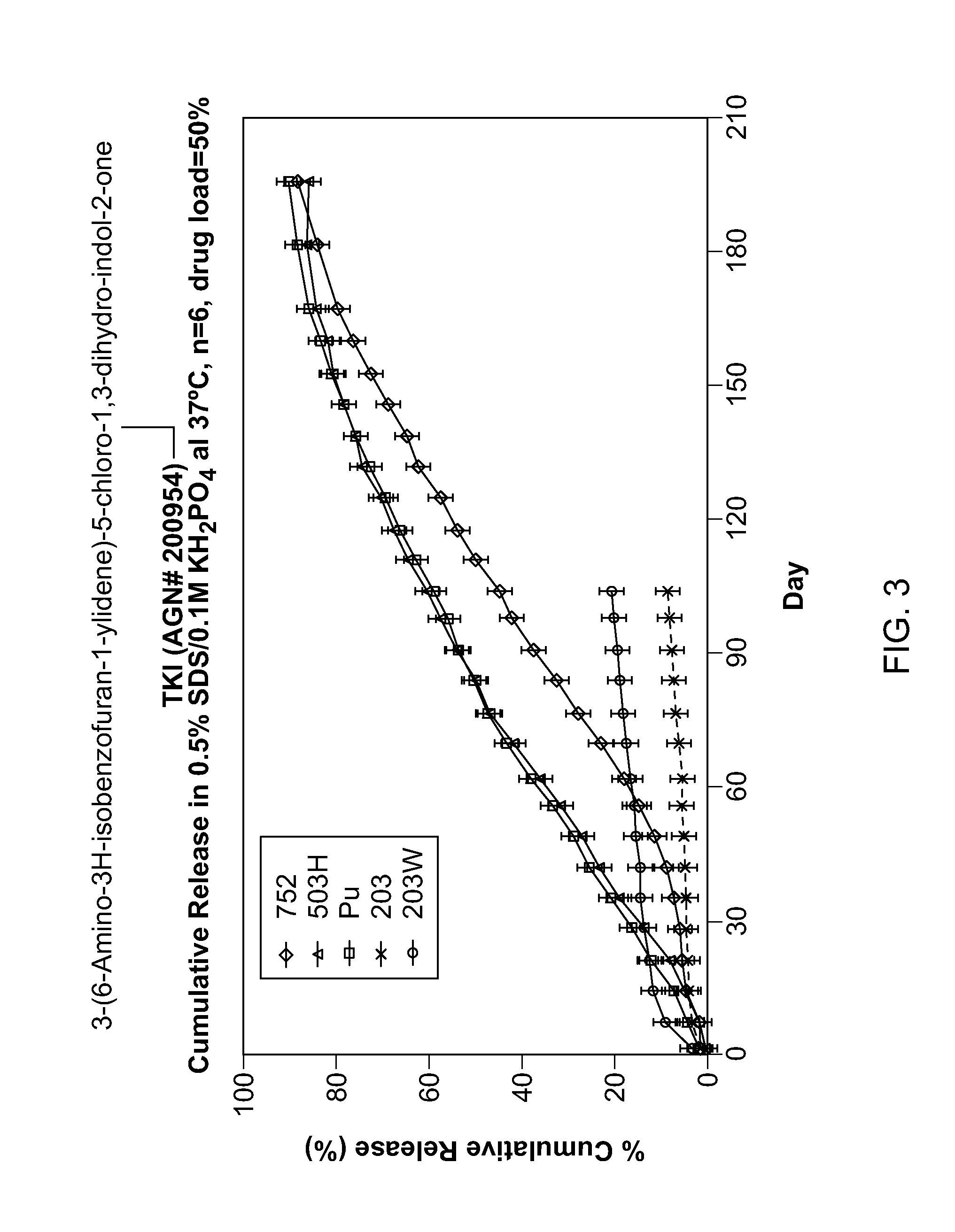
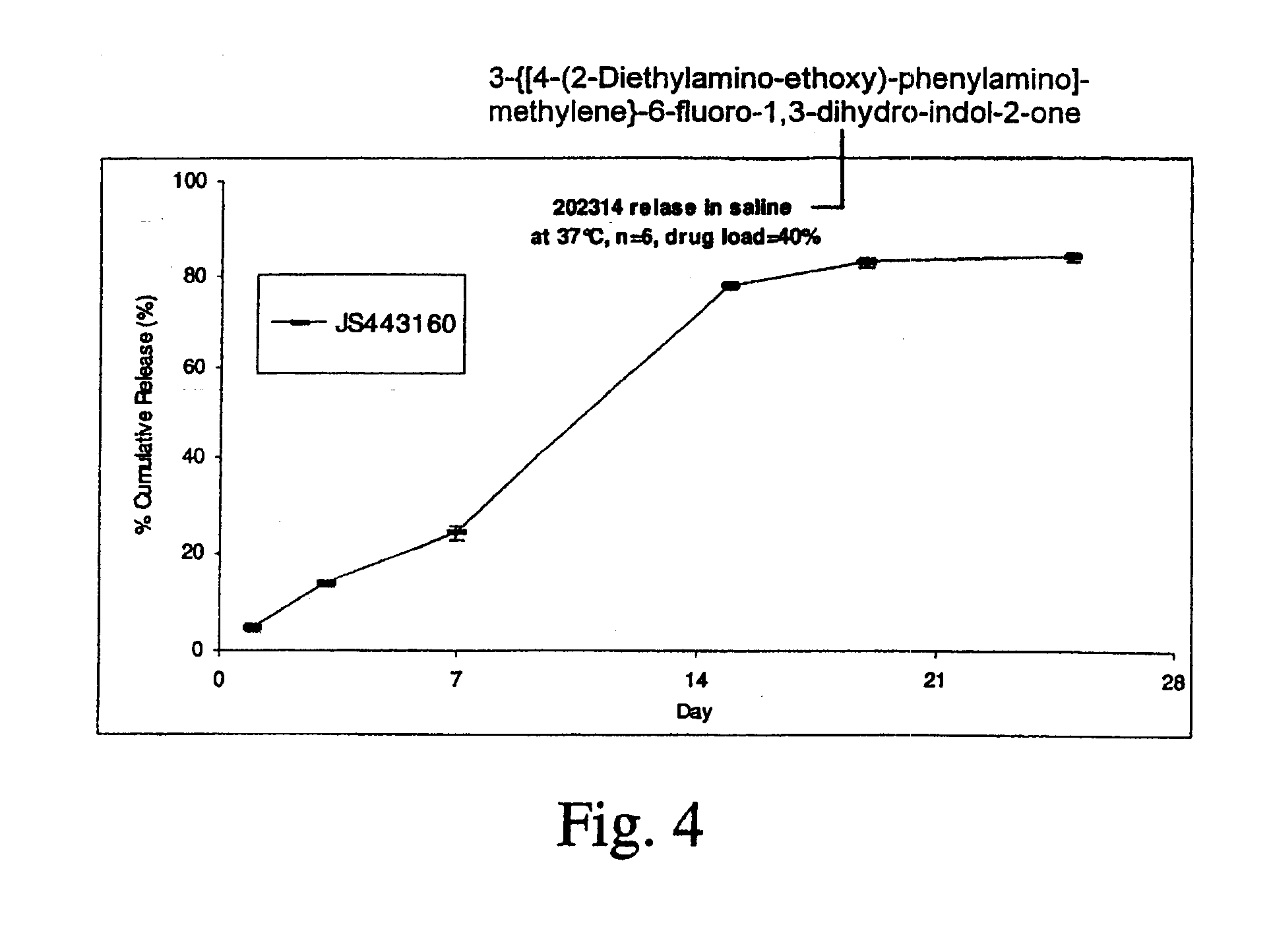
Biodegradable Intravitreal Tyrosine Kinase Implants
ActiveUS20140031408A1Reduce deliveryFacilitate obtaining successful treatment resultsBiocideSenses disorderOphthalmologyPolyvinyl alcohol
Biocompatible intraocular implants include a tyrosine kinase inhibitor and a biodegradable polymer that is effective to facilitate release of the tyrosine kinase inhibitor into the vitreous of an eye for an extended period of time. The therapeutic agents of the implants may be associated with a biodegradable polymer matrix, such as a matrix that is substantially free of a polyvinyl alcohol. The implants can be placed in an eye to treat or reduce the occurrence of one or more ocular conditions.
Owner:ALLERGAN INC
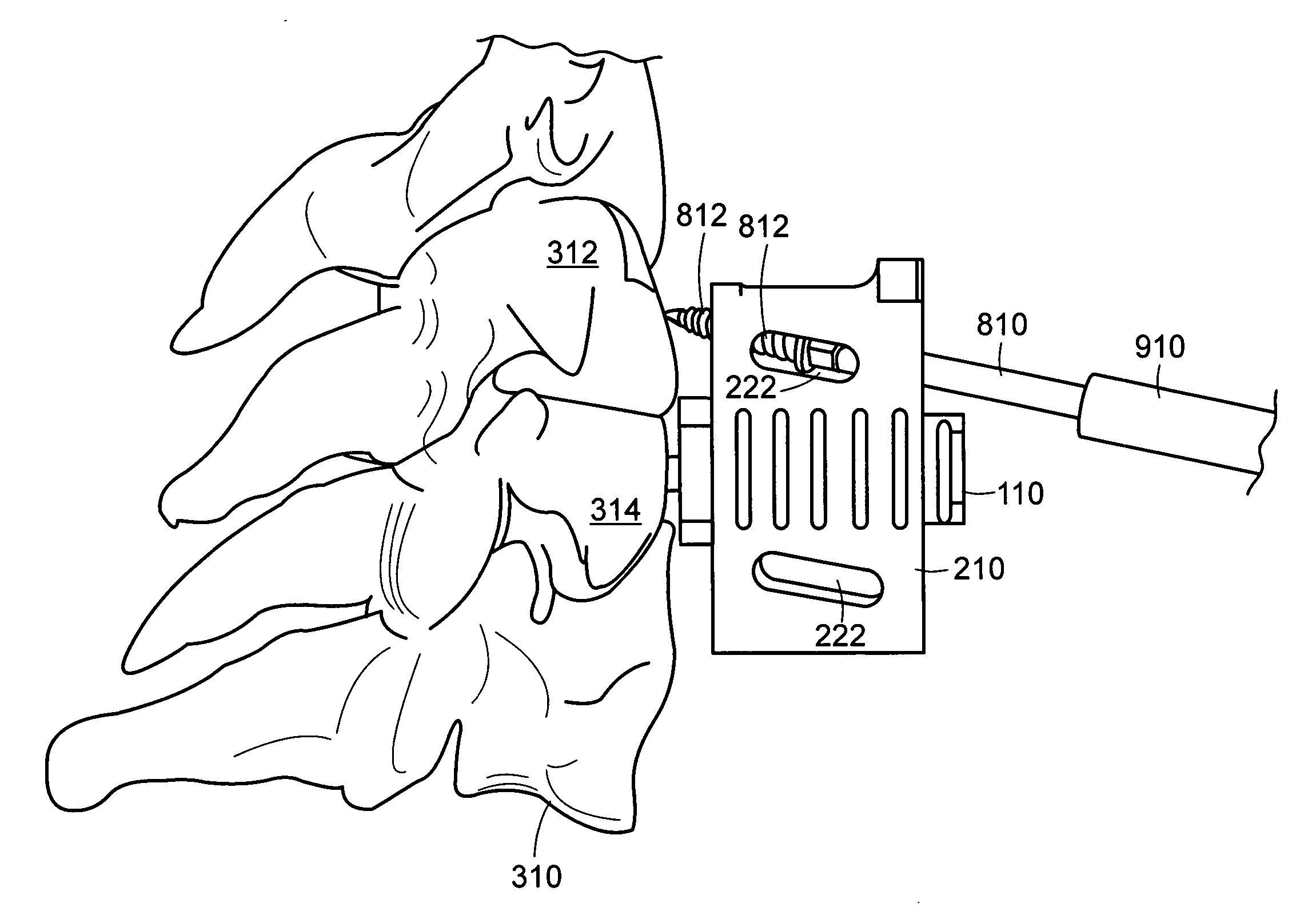
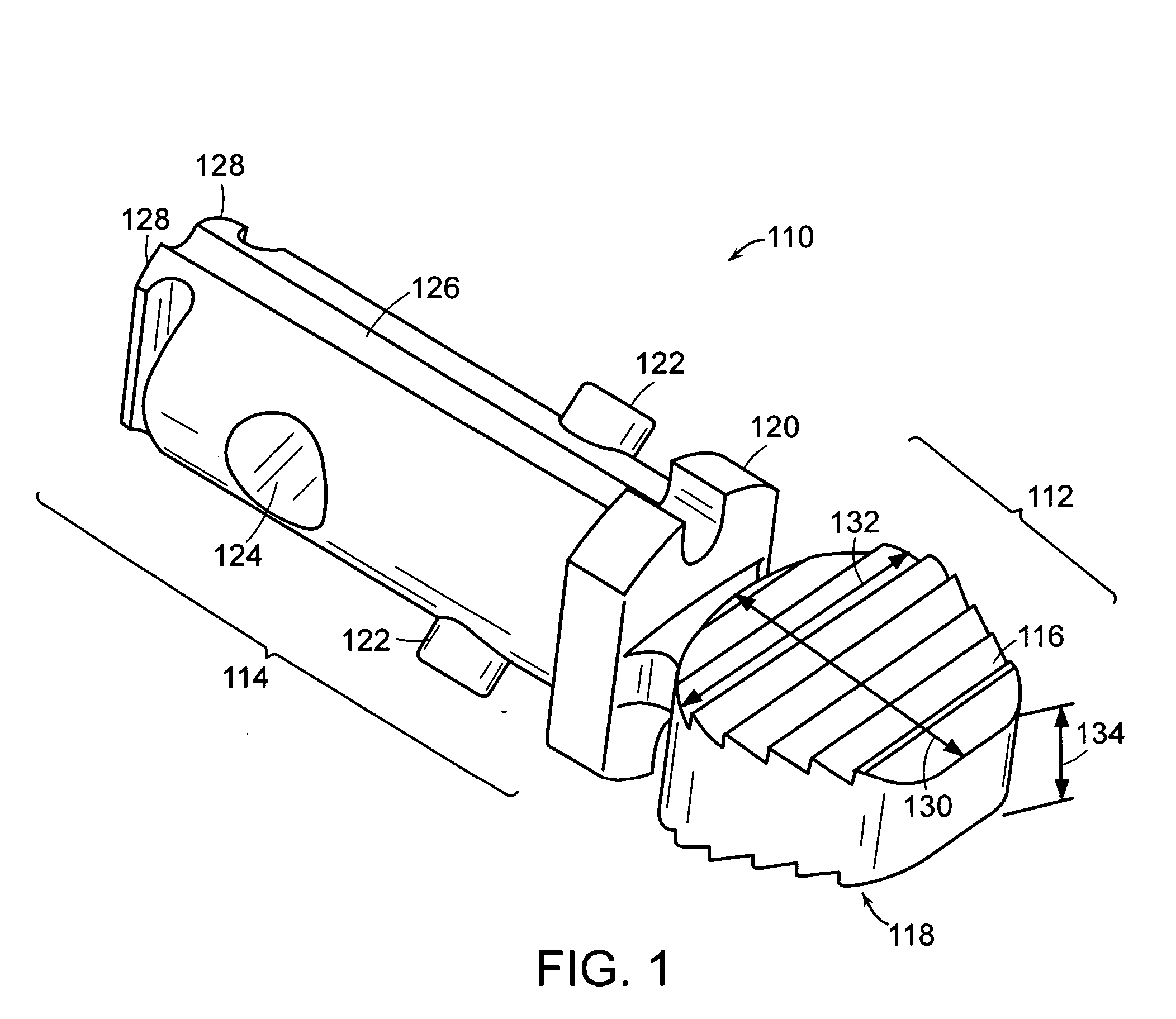
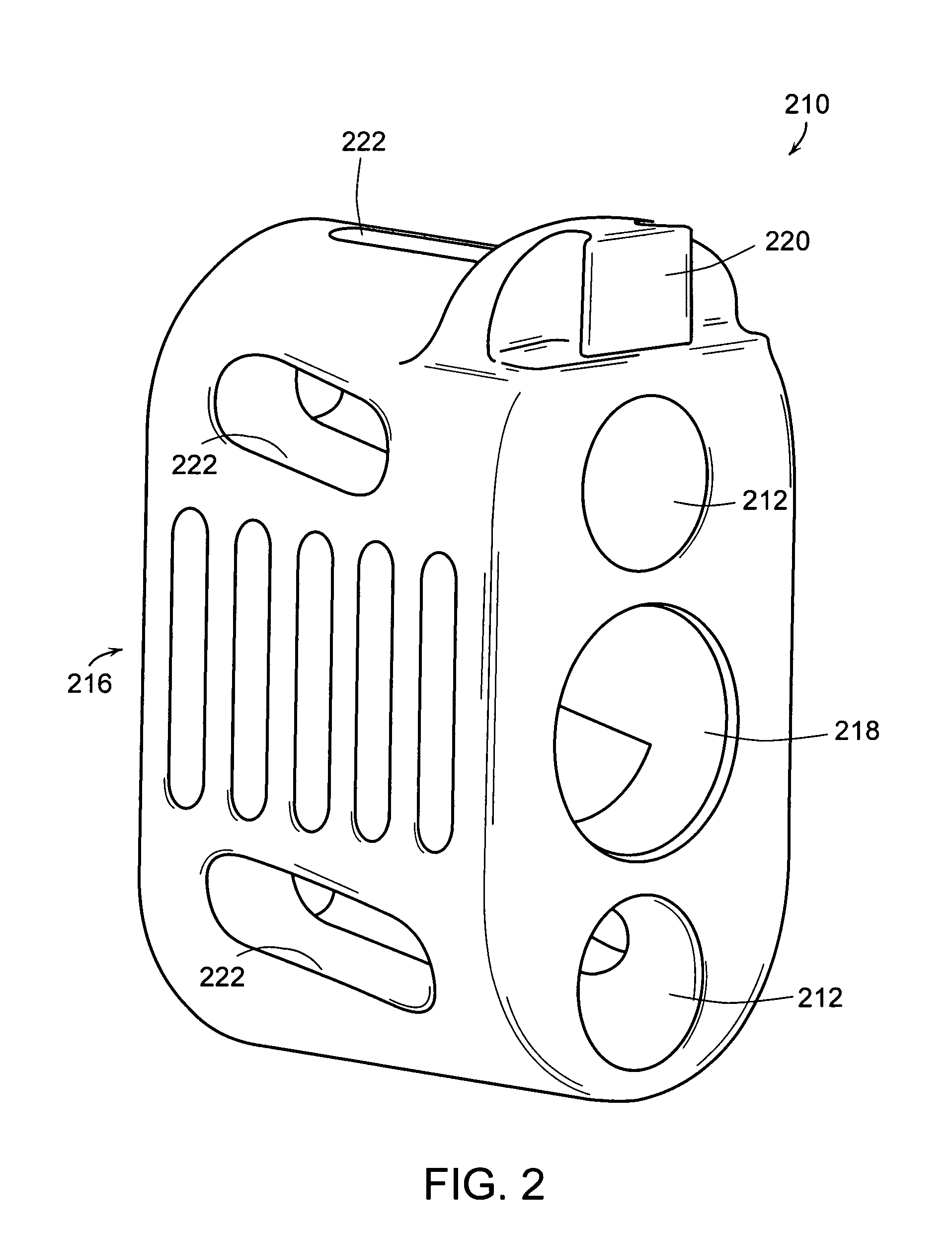
Geared spinal implant inserter-distractor
ActiveUS8114088B2Efficient and accurate deliveryGreat control and feedbackSpinal implantsOsteosynthesis devicesLinear motionPosterior approach
Embodiments of a geared spinal implant inserter-distractor disclosed herein provide a greater mechanical advantage in delivering an intervertebral implant via an anterior, anterior-lateral, or posterior approach. The geared spinal implant inserter-distractor comprises an inserter, a distractor structured to slidably receive the inserter with a collar and an intervertebral implant attached thereto, and a gear mechanism arranged to translate a surgeon's rotational motion into linear motion, allowing the surgeon to have a greater control and feedback when placing an implant within an intervertebral disc space. The gear arrangement comprises a pinion inside the distractor and a rack on the inserter. The pinion can be driven by a shaft connected to a knob or handle. With the gear mechanism, a surgeon can turn the knob or handle to drive the inserter in and out of the distractor in a quantifiable manner, which facilitates the desirable precision delivery of the intervertebral implant.
Owner:ZIMMER BIOMET SPINE INC
Medical implants and fibrosis-inducing agents
InactiveUS20050169959A1Good curative effectInduce adhesion or fibrosis in the surrounding tissueHeavy metal active ingredientsInternal osteosythesisHost tissueIncreased fibrosis
Owner:ANGIOTECH INT AG (CH)
Devices and methods for stabilizing a spinal region
InactiveUS20100114098A1Strengthening intervertebral spaceIncrease spacingInternal osteosythesisEar treatmentComputed tomographyOuter Cannula
Disclosed are apparatuses and methods for delivering implants through a posterior aspect of a vertebral body such as a pedicle and placing the implant or performing a procedure into the anterior aspect of the vertebral body. A representative apparatus includes an outer cannula, and advancer tube and a drill assembly. It is envisioned that at least one of the outer cannula, the advancer tube or the drill assembly can be viewed in vivo using for example, a CT scan or fluoroscope. The present invention is also directed to an apparatus for forming an arcuate channel in bone material. The apparatus includes and advancer tube and a drill assembly.
Owner:K2M
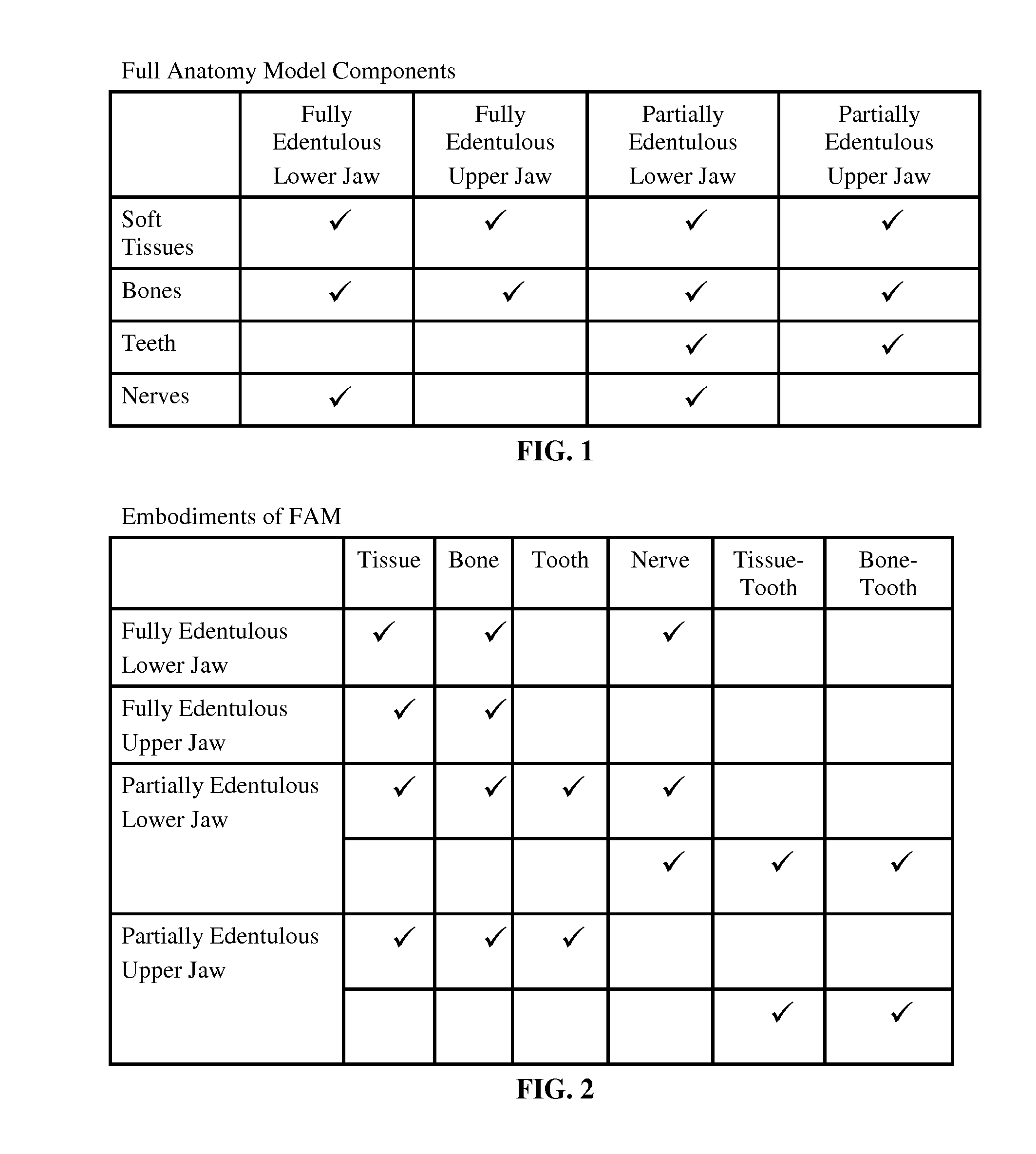
Method and system of anatomy modeling for dental implant treatment planning
InactiveUS20120100500A1Effective placementAdditive manufacturing apparatusAnalogue computers for chemical processesDICOMDifferentiator
This invention introduces an oral-dental anatomy modeling method and an implant treatment planning system based on a full anatomy model (FAM). Dental Implant Treatment Planning systems place implants on 2D slices of DICOM files, and sometimes on 3D models of bones and remaining teeth. Because the final look and feel of implants and restorations depend on how well they go along with the remaining teeth and soft tissues, treatment planning without tissue model presents safety and aesthetics risks. A FAM consists of models for bones, teeth, soft tissues and nerves. They are created from CT and optical scans, and assembled together with model registration techniques. The tissue model is the real differentiator. A treatment planning system uses FAM as a unique reference throughout the workflow. Its implant placement, restoration preview and surgical guide design are all based on FAM.
Owner:GUIDEMIA TECH
Ophthalmic implant for treatment of glaucoma
ActiveUS8034105B2Sufficient forceImprove permeabilityEye implantsEye surgeryOphthalmological implantSchlemm's canal
A method is provided for increasing the outflow of fluid through Schlemm's canal that is useful for treatment of glaucoma. The implant is placed in Schlemm's canal by use of a flexible delivery instrument attached to the implant. The instrument and implant are positioned within the canal, the implant is released and the distal and proximal ends of the implant are connected to apply sufficient axial tensioning force on the inner wall of the canal to increase fluid permeability. In another embodiment a delivery instrument attached to the implant is positioned in the canal securing one of the distal or proximal ends of the implant within the canal. The implant provides sufficient axial tensioning force on the inner wall of the canal to increase fluid permeability of the inner wall of the canal. The other of the distal or proximal ends may be secured to maintain the tensioning force on the inner wall of the canal.
Owner:NOVA EYE INC
Ophthalmic implant for treatment of glaucoma
InactiveUS20120010702A1Sufficient forceImprove permeabilityEye implantsEye surgeryOphthalmological implantSchlemm's canal
An implant is placed within Schlemm's canal of the eye and provides tension to the trabecular meshwork. The tension is continuous and increases the aqueous outflow without the necessity of administering cholinergic drugs to treat glaucoma.
Owner:NOVA EYE INC
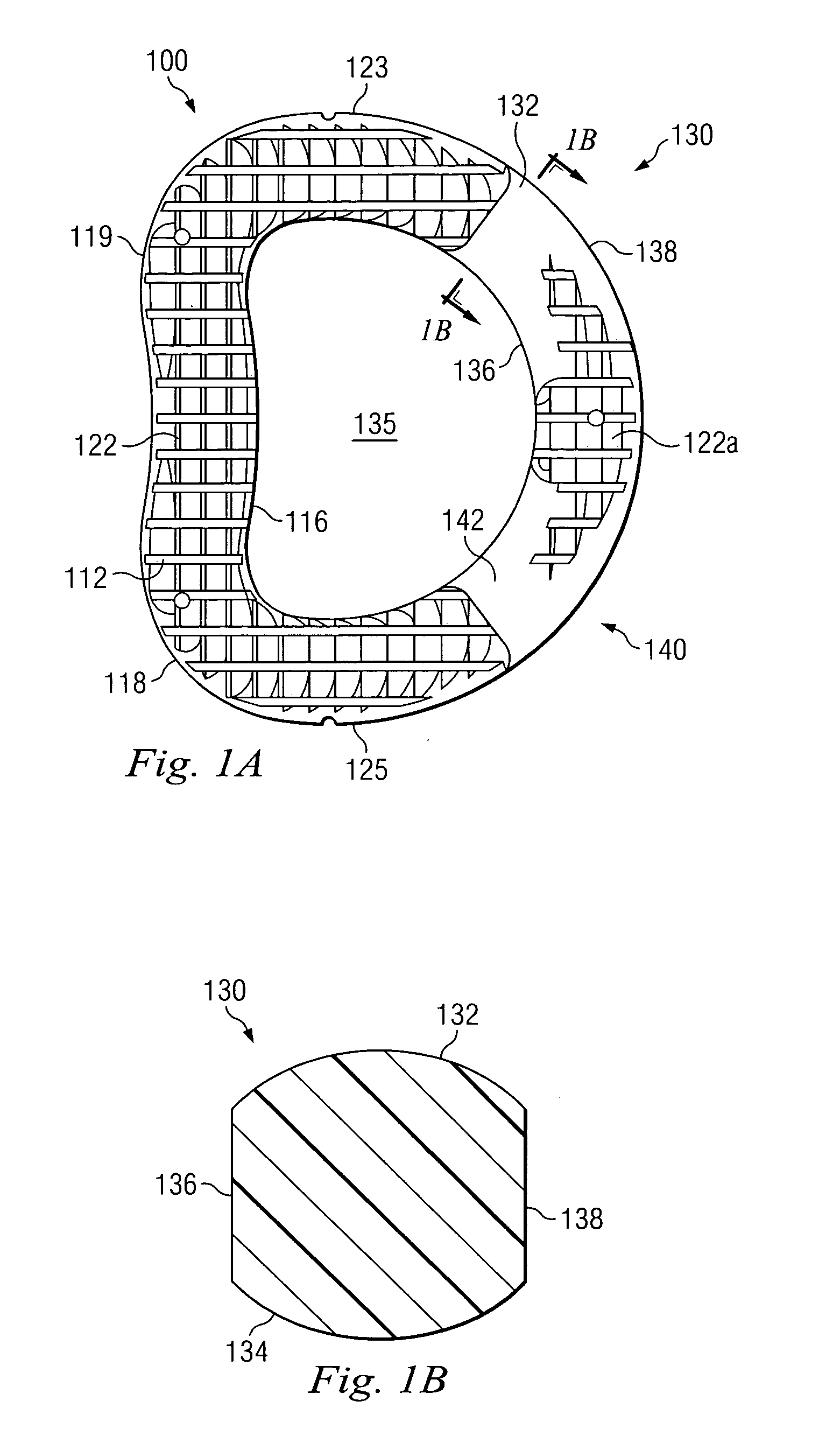
Positioning system with cannulated implant
InactiveUS20060015119A1Minimizes and eliminates large forcePrecise positioningDiagnosticsJoint implantsBiomedical engineeringImplant placement
A positioning system for placing an implant into a cavity of a bone, comprising a cannulated implant that is substantially smaller in the implanting area than the cavity and a positioning device that can hold the implant in a settable position in the cavity.
Owner:SMITH & NEPHEW INC +2
Devices and methods for stabilizing a spinal region
InactiveUS8579903B2Increase spacingInternal osteosythesisEar treatmentComputed tomographyOuter Cannula
Owner:K2M
Method for pre-operative visualization of instrumentation used with a surgical guide for dental implant placement
The invention relates to manufacturing a surgical guide to be placed in a patient's mouth. The patient's mouth is scanned to obtain surgical-region scan data at a region where an implant is to be located. The patient's mouth is also scanned in the opened position to acquire dental conditions opposite from the surgical region so as to obtain opposing-condition scan data. A virtual model is developed using the surgical-region scan data and the opposing-condition scan data. Using the virtual model, a surgical plan is developed that includes the location of the implant to be installed in the patient. A virtual surgical guide is also developed based on the surgical plan. The dimensions of instrumentation to be used with the surgical guide are checked to ensure they will fit within the mouth by use of the opposing-condition scan data. After checking, final surgical-guide manufacturing information is obtained for manufacturing the surgical guide.
Owner:BIOMET 3I LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com