Medical implants and fibrosis-inducing agents
a technology of fibrosis and implants, applied in the field of pharmaceutical compositions, methods and devices, can solve the problems of inability to effectively attach the device to the surrounding tissue, inability to provide a good substrate for host tissue attachment and ingrowth, and devices that have a tendency to migrate within the vessel or tissue, etc., to achieve the effect of facilitating the “anchoring” of the device/implant, promoting adhesion or fibrosis in the surrounding tissue, and enhancing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
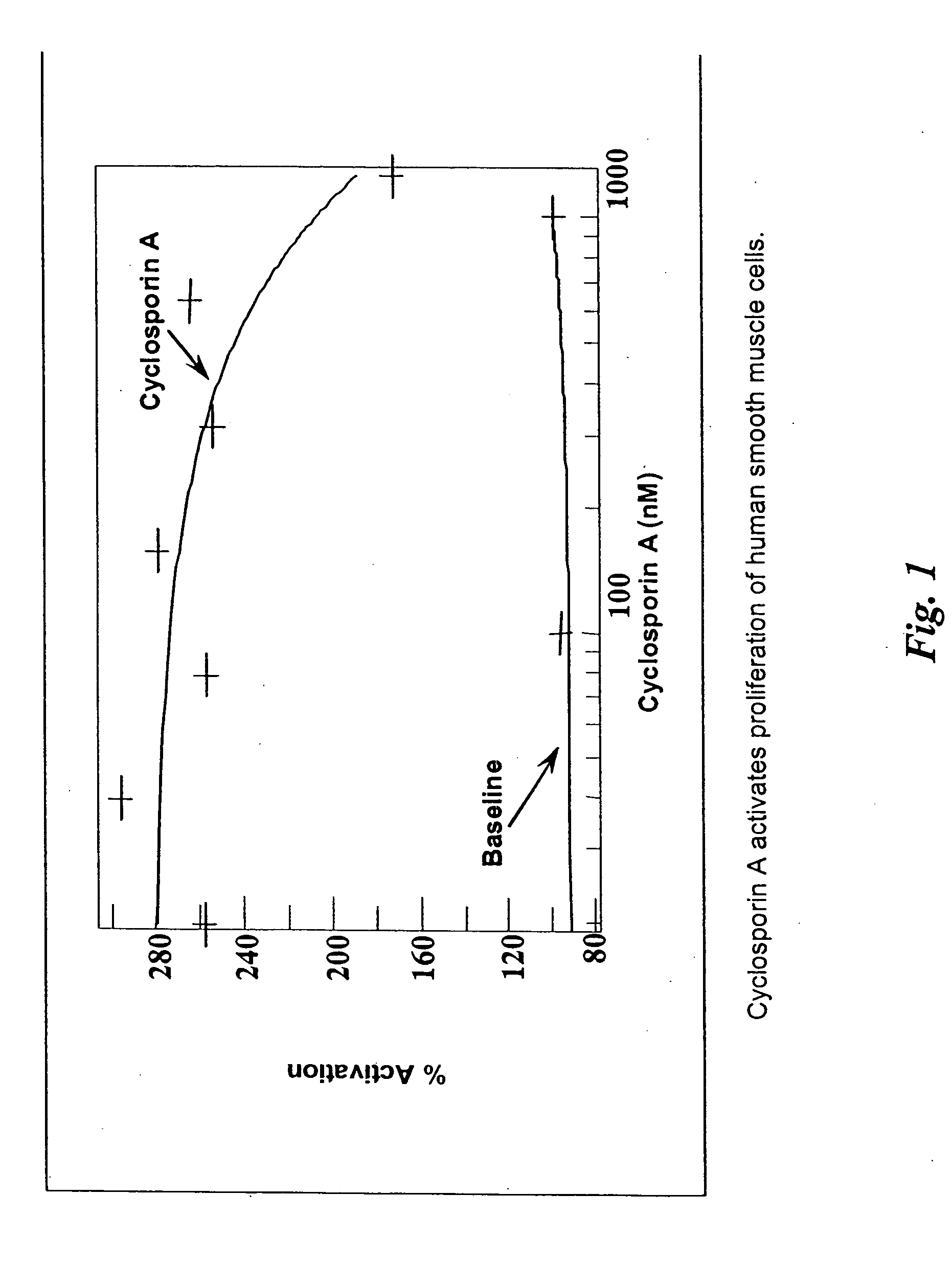
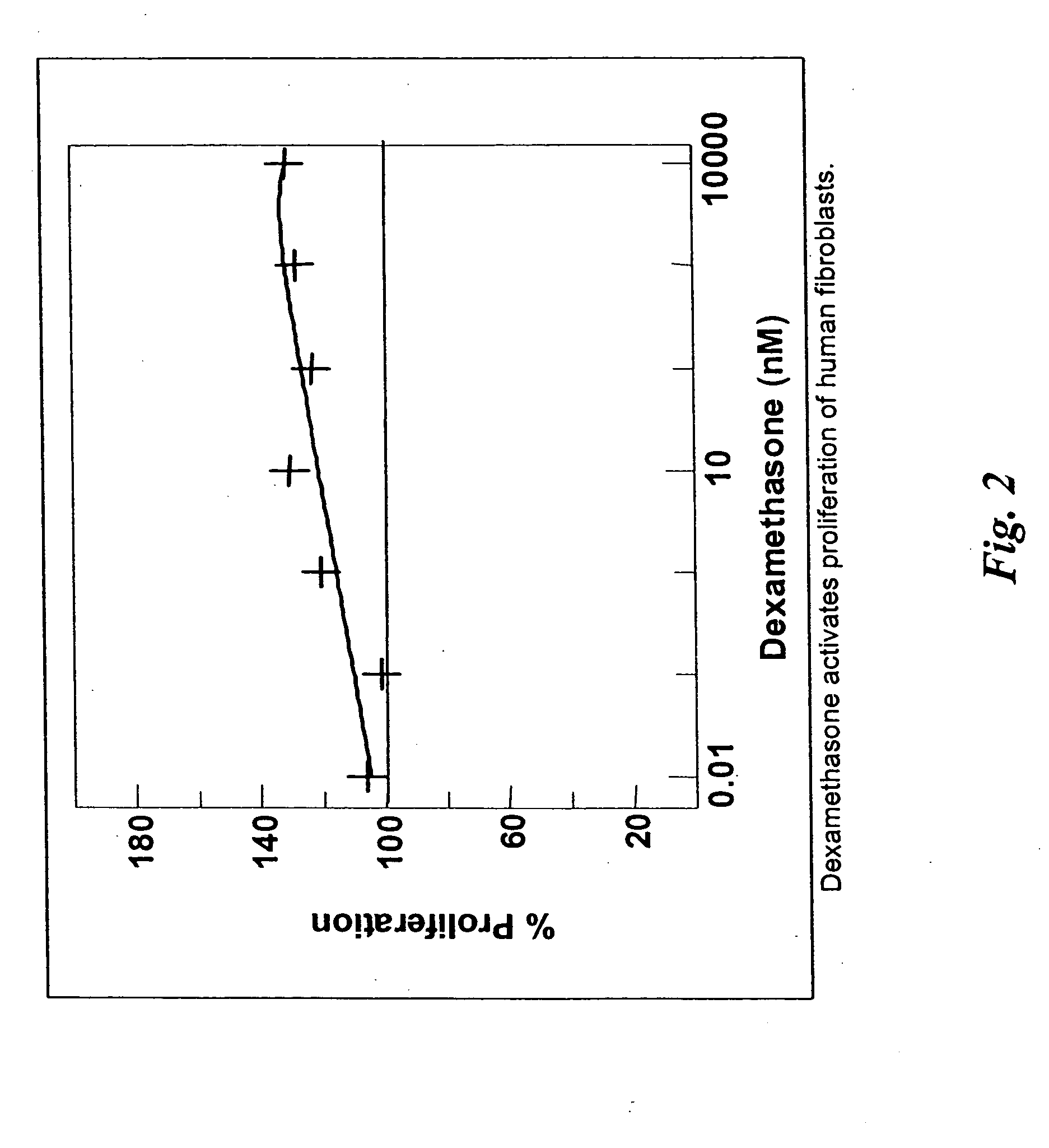
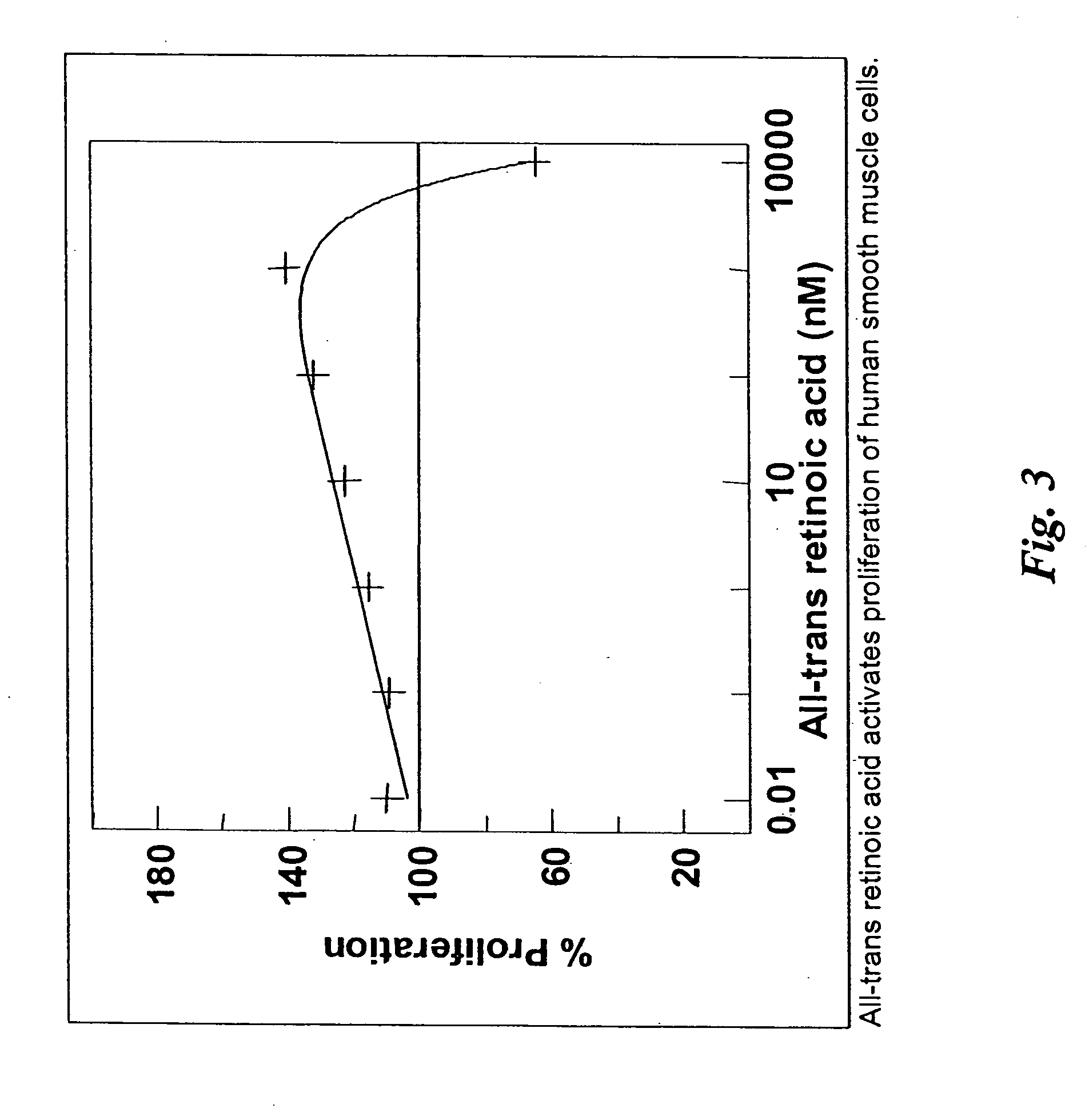
[0036] The present invention discloses pharmaceutical agents which promote one or more aspects of the production of fibrous (scar) tissue or tissue regeneration. Furthermore, compositions and methods are described for coating medical devices and implants with drug-delivery compositions such that the pharmaceutical agent is delivered in therapeutic levels over a period sufficient for fibrosis and healing to occur. The present invention also describes various compositions and methods for enhancing the production of scar tissue adjacent to or on the surface of the implant are described. Numerous specific implants and devices are described that are capable of producing superior clinical results as a result of being coated with agents that promote scarring and healing, as well as other related advantages.
Definitions
[0037] Prior to setting forth, the invention, it may be helpful to an understanding thereof to first set forth definitions of certain terms that is used hereinafter.
[0038]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com