Yeast-expressed Coxsackievirus A10 virus-like particles and applications thereof
A Coxsackie virus, A10 technology, applied in the field of biomedicine, can solve the problems of no vaccine and little research.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0117] Expression and purification of embodiment 1 VLP
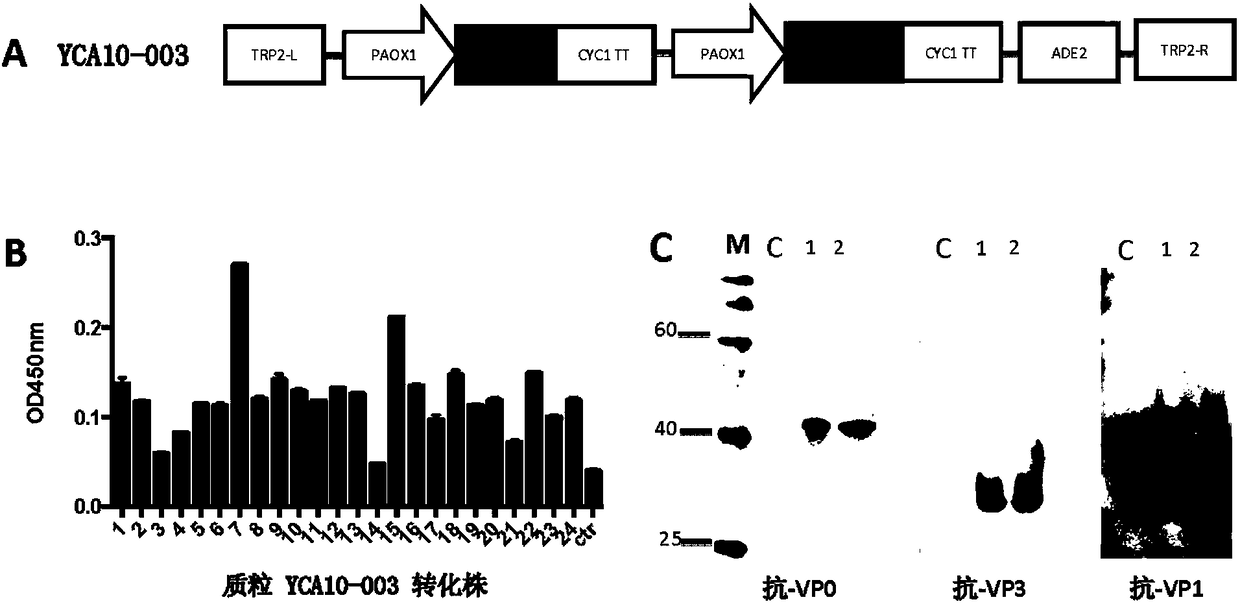
[0118] In order to express CA10-VLP, the 3CD and P1 genes were inserted into the pPink-HC vector together to construct plasmid YCA10-003 ( figure 1 A), and then use the plasmid to transform Pichia pastoris competent, small expression to obtain cell lysate centrifuged supernatant for ELISA and Western blotting analysis. Yeast clones transformed with empty vector pPink-HC were operated in parallel as negative controls. Compared with the control group, the yeast lysate supernatant transformed with plasmid YCA10-003 showed obvious antigen-antibody reactions ( figure 1 B). The two most responsive transformants were selected from the yeast clones for Western blotting analysis, and the experimental results are shown in the figure ( figure 1 C), using anti-CA10VP0, anti-CA10VP3, and anti-CA10VP1 as detection antibodies, corresponding bands appeared in the three yeast lysate samples, with sizes of 39kDa, 29kDa, and 37kDa, resp...
Embodiment 2
[0123] Example 2. Immunogenicity of CA10-VLP in mice
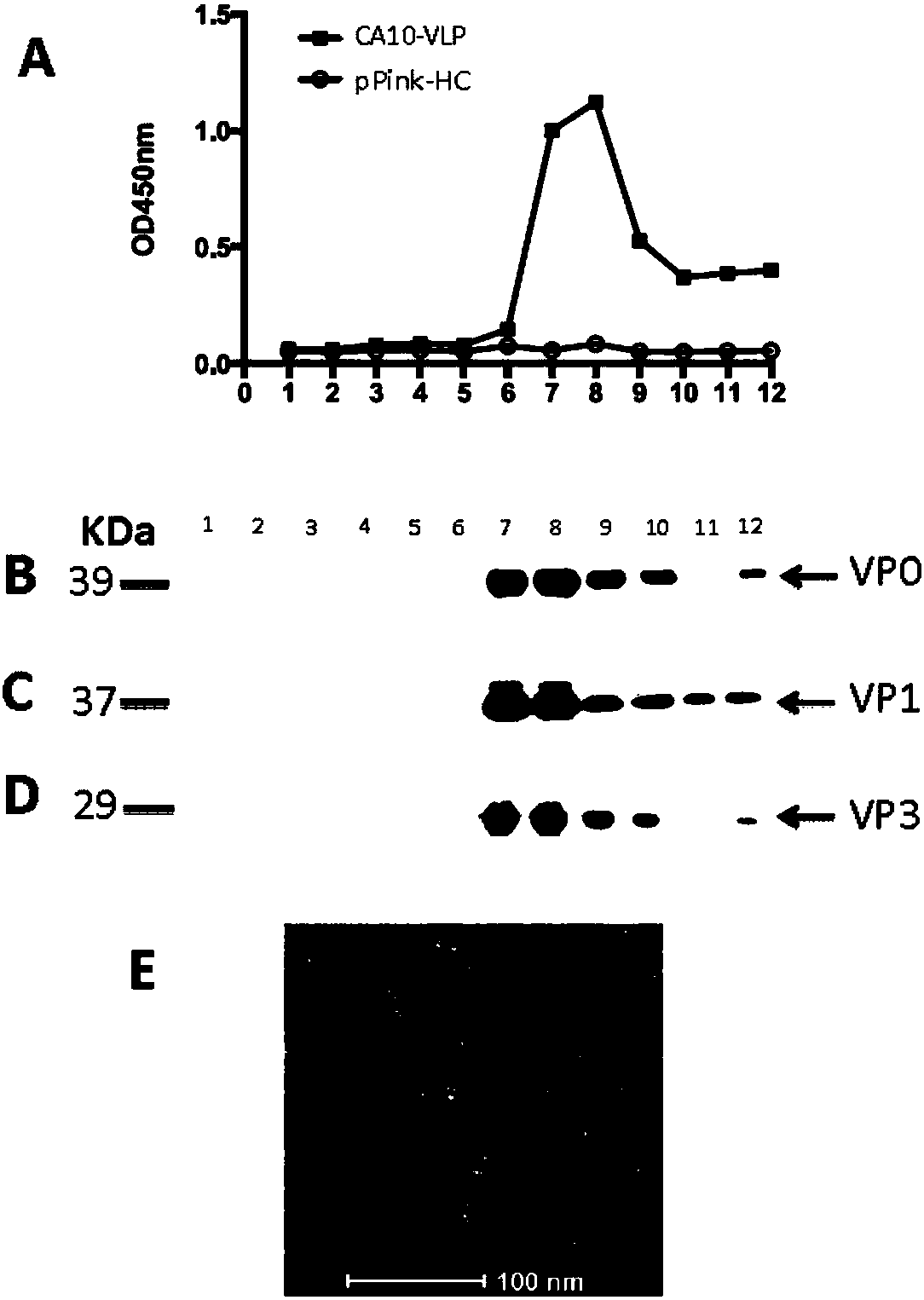
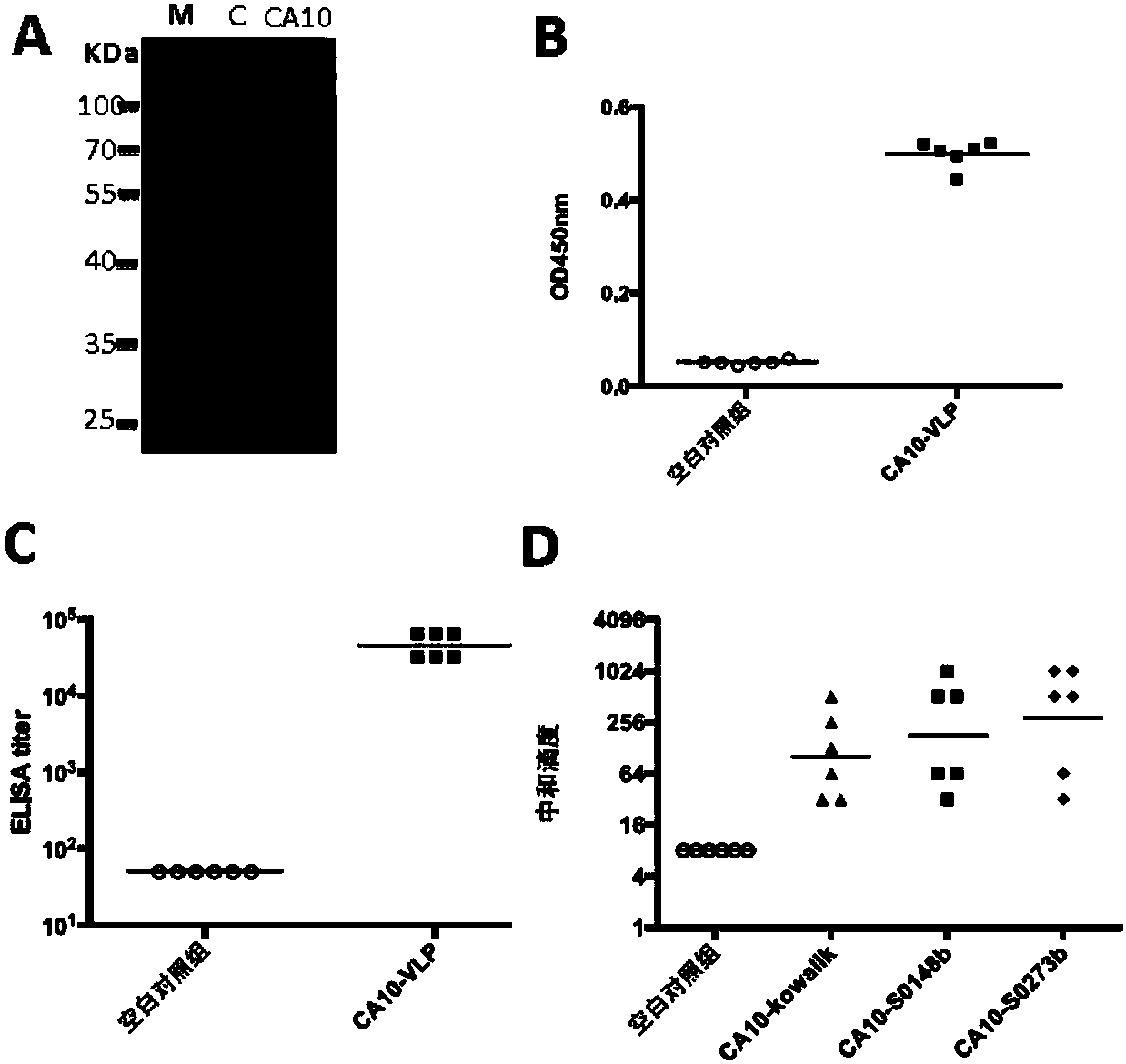
[0124] Before immunizing mice, CA10-VLP and control antigen were prepared and quantified ( image 3 A), followed by the addition of aluminum adjuvants, respectively. Two groups (6 / group) of ICR mice were injected intraperitoneally with CA10-VLP vaccine and control antigen at 0, 2, and 4 weeks, and blood was collected at 6 weeks for ELISA analysis and neutralization experiments. The results of ELISA with CA10-VLP coated plates showed that the mice in the vaccine group had obvious antigen-antibody responses ( image 3 B), and the geometric mean titer reached 45254.8 ( image 3 C); while the control group had no obvious response. In the virus microneutralization experiment, the serum of mice in the control group failed to show neutralization ability even at the lowest dilution (1:16); while the serum of mice in the vaccine group was resistant to homologous strain CA10 / S0273b and heterologous strains Strains CA10 / kowalik a...
Embodiment 3
[0125] Example 3.CA10-VLP vaccine antiserum has protective effect in vivo
[0126] Firstly, the in vivo protective effect of anti-VLP antibody was evaluated by virus challenge test. 6-day-old neonatal mice were intraperitoneally injected with 75 μl of anti-VLP serum or control serum, and 24 hours later, intraperitoneally injected with a lethal dose of CA10 / Kowalik or CA10 / S0148b virus, and then observed the clinical symptoms and death of virus-challenged mice . The results showed that mice injected with control serum gradually developed severe clinical symptoms after CA10 / Kowalik virus challenge, including slow movement, ataxia and paralysis, and all died within 9 days after challenge; The mice with VLP serum did not appear obvious clinical symptoms in the observation period of 15 days ( Figure 4 A-B). Similarly, after CA10 / S0148b virus challenge, all mice in the control group died within 7 days, and all mice injected with anti-VLP serum survived without obvious clinical s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com