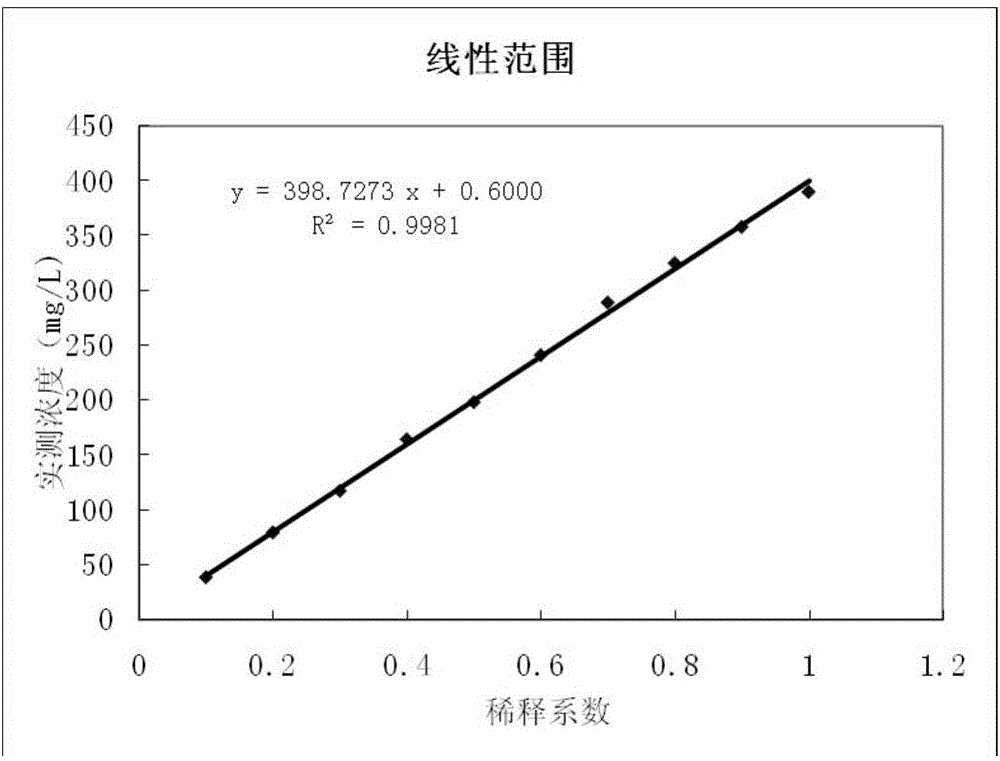
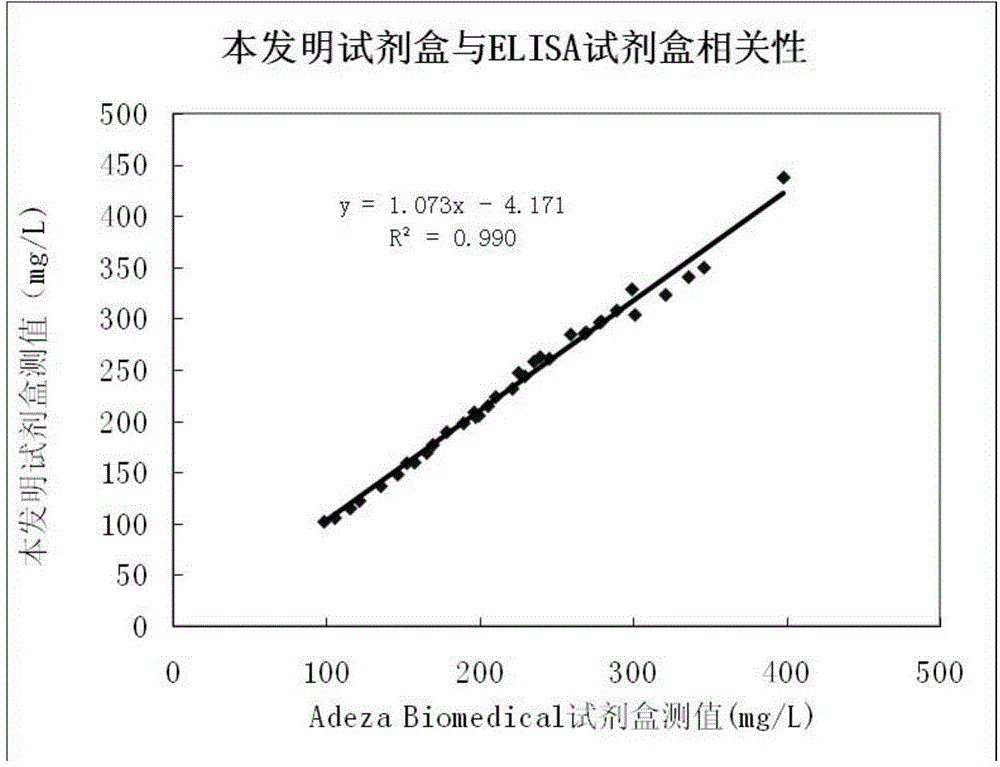
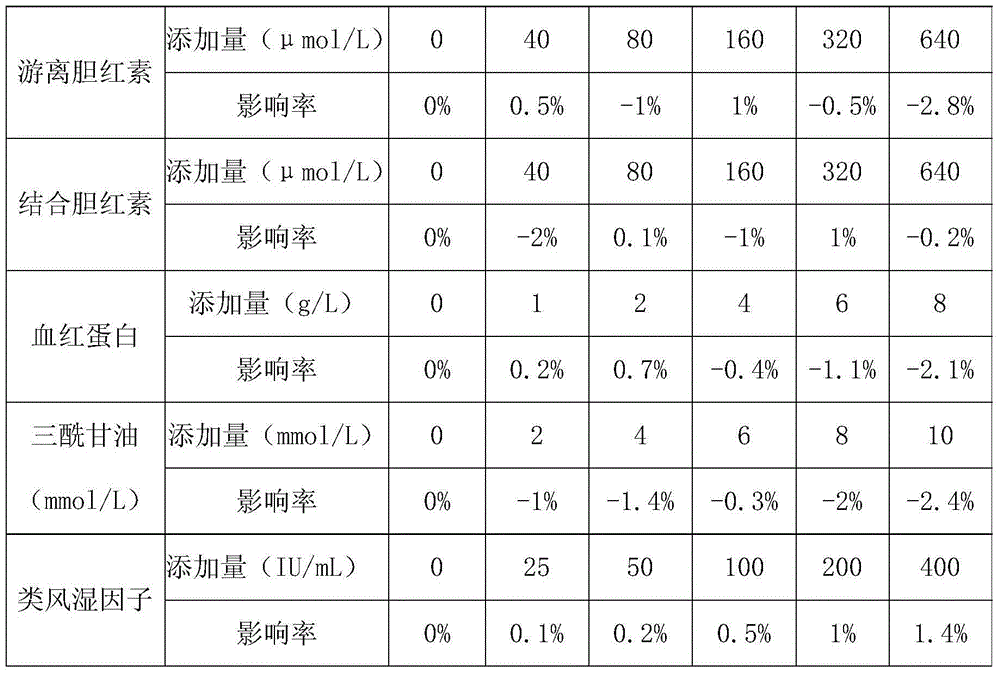
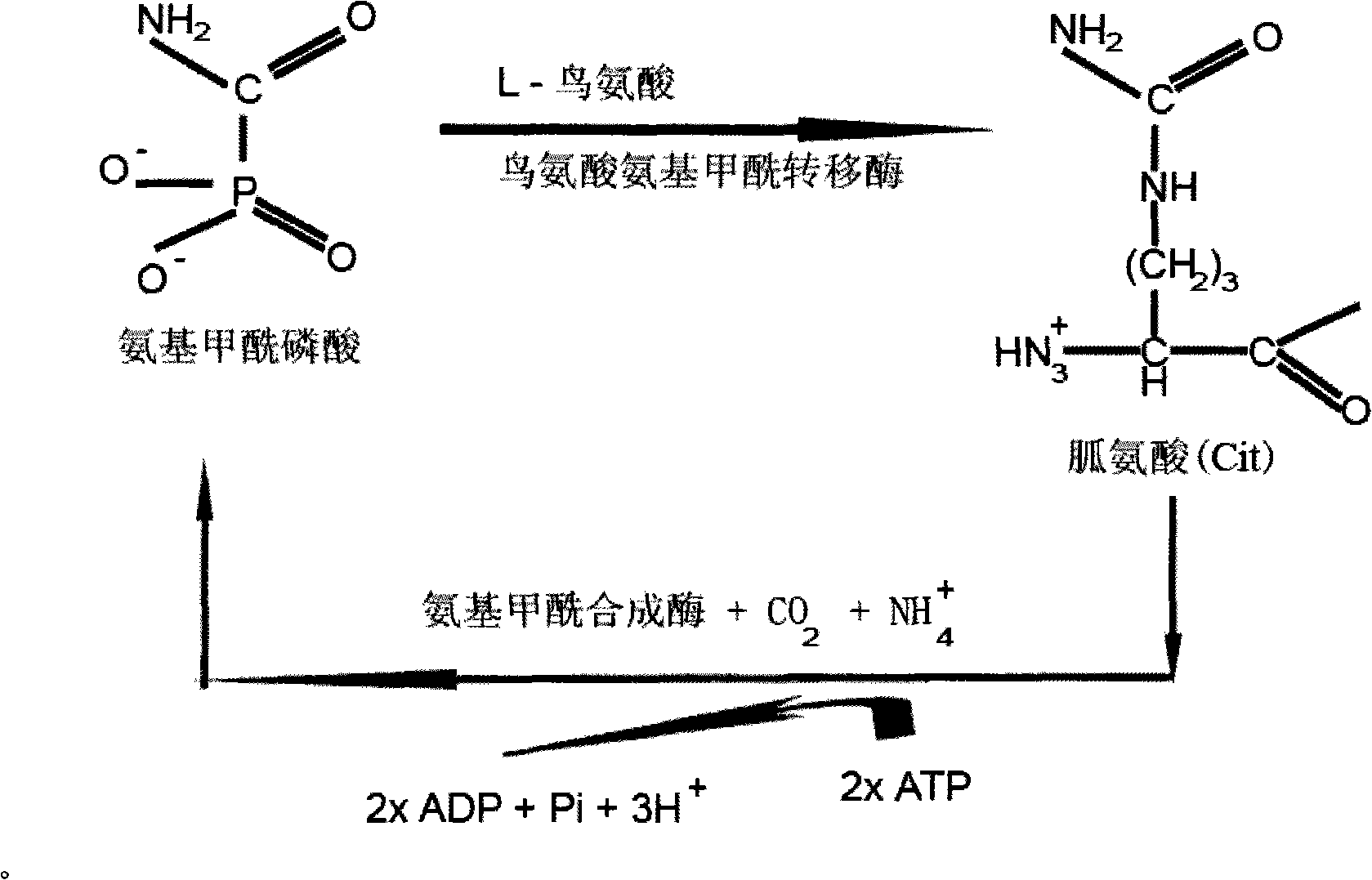
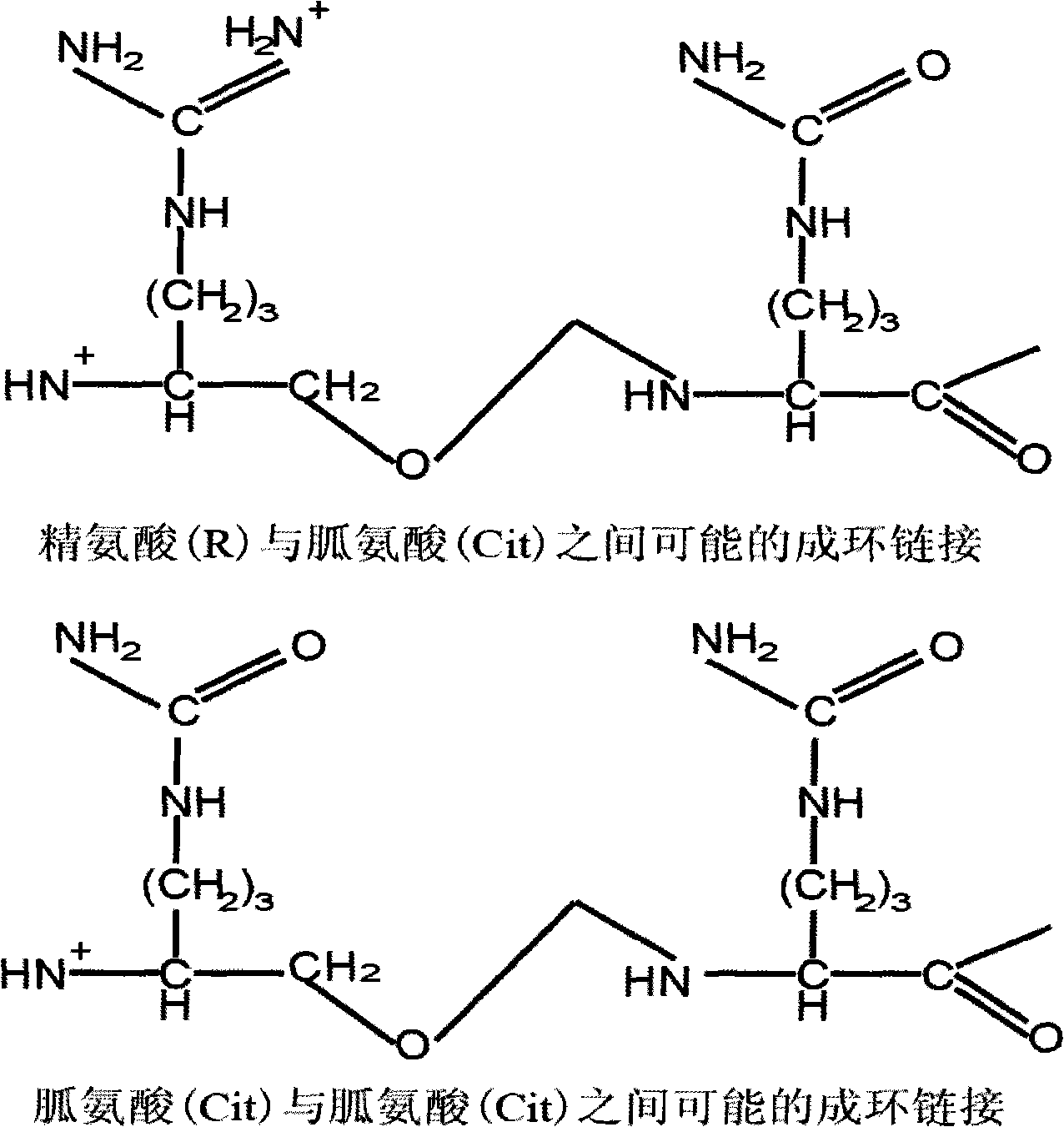
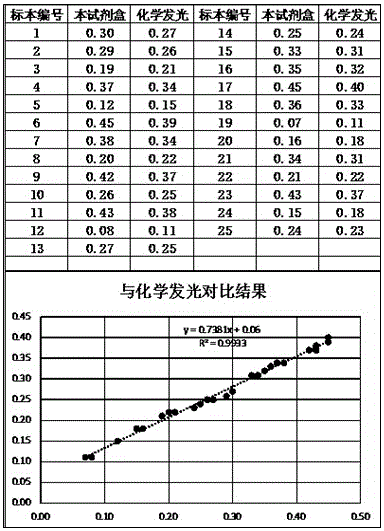
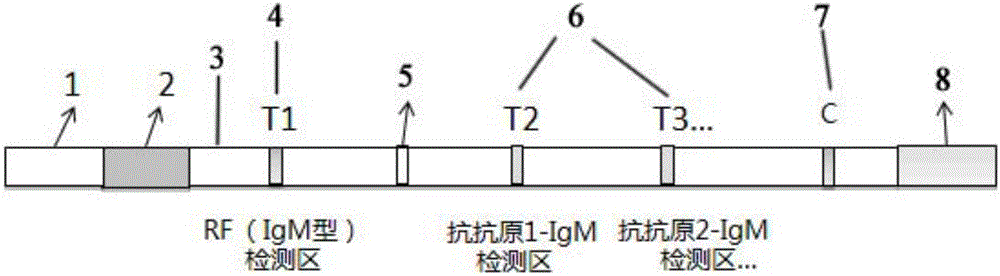
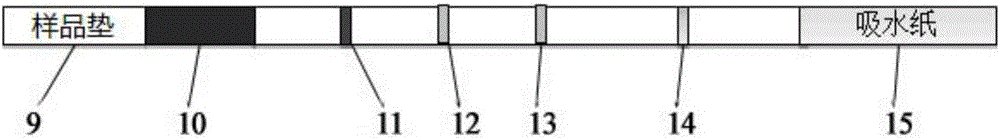
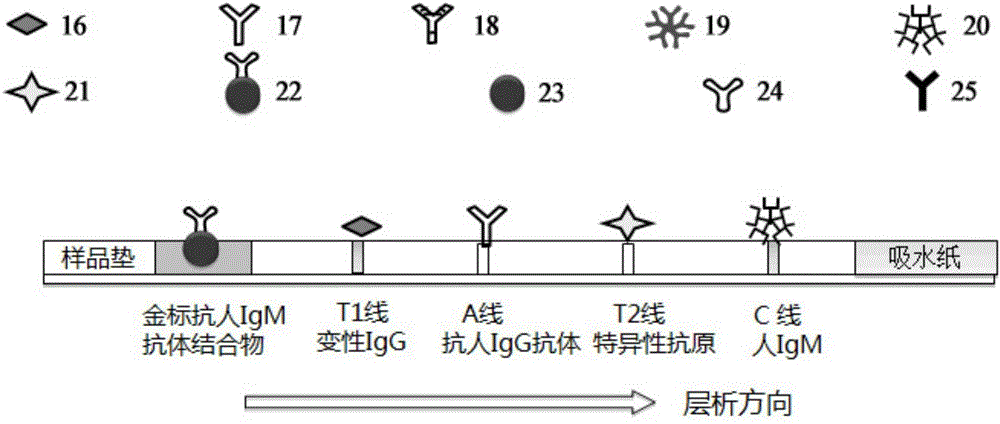
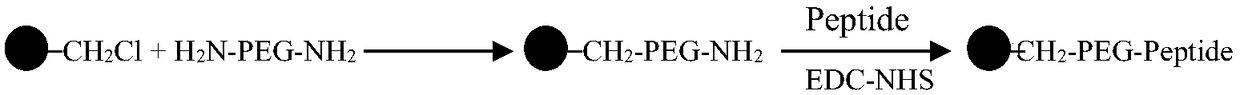Patents
Literature
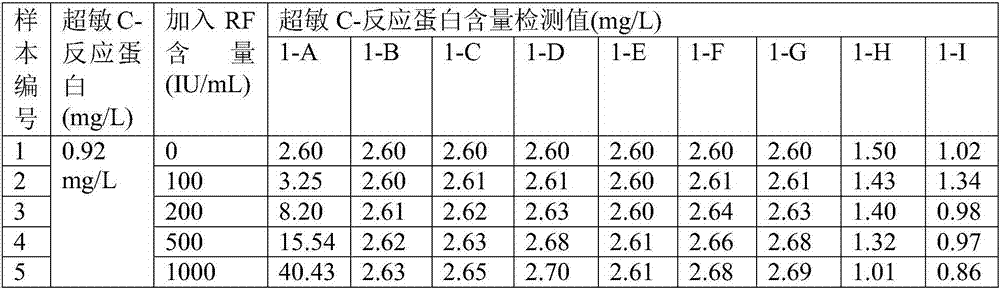
91 results about "Rheumatoid factor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
<ul><li>Normal results are shown in absence of rheumatoid factor in blood.</li><li>Positive results suggest that RF is present in the blood.</li></ul>
Traditional Chinese medicine composition for treating chronic hepatitis and preparation method thereof
InactiveCN101757561AAchieve standardizationAchieve scaleAnthropod material medical ingredientsDigestive systemMedicinal herbsImmune complex deposition
The invention discloses a new traditional Chinese medicine composition for treating chronic hepatitis and a preparation method thereof. The traditional Chinese medicine composition mainly comprises the following Chinese medicinal herbs of dried orange peel, rhizoma cyperi, pericarpium citri reticulatae viride, radix scrophulariae, root of rehmannia, folium isatidis, root of kudzu vine, houttuynia cordata, indigo naturalis, peach kernel, red flower, radix paeoniae alba, Tuckahoe, rhizoma alismatis, oriental wormwood, desmodium, polygonum cuspidatum, root of red-rooted salvia, radix bupleuri, angelica sinensis, herba lycopi, earthworm, goldthread, felwort and root bark of the peony tree. The traditional Chinese medicine composition can be prepared into a common oral preparation according to a conventional traditional Chinese medicine preparation method. The invention can remarkably improve the symptoms of mild acratia, inappetence, abdominal distension, pain over the liver and the like of chronic persisting hepatitis, and can improve the symptoms of asthenia, poor appetite, abdominal distension, semiliquid, pain over the liver, poor complexion, poorer health, manpower reduction, hepatomegaly accompanied with haphalgesia and rap pain, and splenomegaly of the chronic active hepatitis and the symptoms of jaundice, spider angioma, liver palms, acne and the like caused by the chronic hepatitis. The invention can also improve the symptoms of long-term obvious dysfunction of liver, ALT continuous increase or repeated fluctuation, albumin reduction, globulin increase, gamma globulin or IgG increase, time extension of protrombin time, capability of positive reaction in self antibody and rheumatoid factors, capability of circulating immune complex increase, capability of addiments C3 and C4 reduction and the like, and has accurate remarkable clinical treatment effect and rapid effect taking.
Owner:TAIYI HEPU BEIJING RES INST OF TCM
Fibronectin detection kit
InactiveCN104483475AReduce dosageImprove anti-interference abilityMaterial analysis by observing effect on chemical indicatorBiological testingAntibody fragmentsLatex particle
The invention discloses a fibronectin detection kit which comprises a reagent R1 and a reagent R2, wherein the reagent R1 adopts a buffer solution containing an anti-human rheumatoid factor antibody and a coagulant, and the reagent R2 adopts a buffer solution containing latex particles for marking an anti-human fibronectin antibody; the anti-human rheumatoid factor antibody is an intact antibody or an antibody fragment containing a functional part, and is a rabbit anti-human polyclonal antibody, a goat anti-human polyclonal antibody or a monoclonal antibody. The fibronectin detection kit can meet requirements for high sensitivity and automatic and fast batch detection simultaneously, the anti-interference capacity is high, the stability is high, the dosage of the antibody is small, and the cost is reduced.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
Blood-purifying adsorbing agent for cleaning antibody
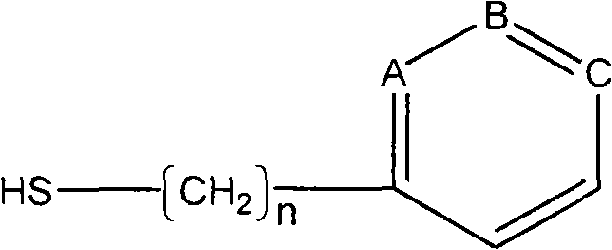
InactiveCN101279242AHigh adsorption selectivityImprove stabilityOther blood circulation devicesOther chemical processesImmune complex depositionSorbent
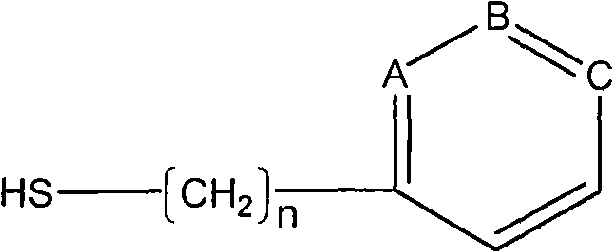
A blood purification sorbent for antibody removal belongs to the technical field of biomedicine, which consists of the two parts of solid-phase carrier material and a petunidin fixed on the carrier by chemical coupling. The molecular structure of the petunidin is shown as above, wherein, one atom among A, B and C is N, the others are C; n is 0-2. The blood purification sorbent can adsorb the antibody component in plasma and autoantibodies such as rheumatoid factor, antinuclear antibody, etc. in heavy load, has limited nonspecific adsorption to other plasma components such as seralbumin, etc., and also has low preparation cost and stable physicochemical property. The material can be used as adsorption filler of a blood purification device for removing the autoantibody and immune complex in the plasma.
Owner:DALIAN UNIV OF TECH
Latex reinforced immunoturbidimetric reagent for inhibiting rheumatoid factor interference
The present invention discloses a latex reinforced immunoturbidimetric reagent for inhibiting rheumatoid factor interference, wherein the latex reinforced immunoturbidimetric reagent comprises a reagent R1 and a reagent R2, the reagent R2 comprises latex micro-particles, and the PH value of the reagent R1 is 5.5-6.0. According to the present invention, the latex reinforced immunoturbidimetric reagent has characteristics of simple composition and good versatility, and with the latex reinforced immunoturbidimetric reagent, the interference of most of the rheumatoid factors can be effectively eliminated, and the specificity and the accuracy of the detection result can be improved.
Owner:NINGBO RUI BIO TECH
Heart-type fatty acid binding protein content detection kit and preparation method thereof
ActiveCN103123319AImprove interference effectStrong interference abilityColor/spectral properties measurementsPreservativeHeart-type fatty acid binding protein
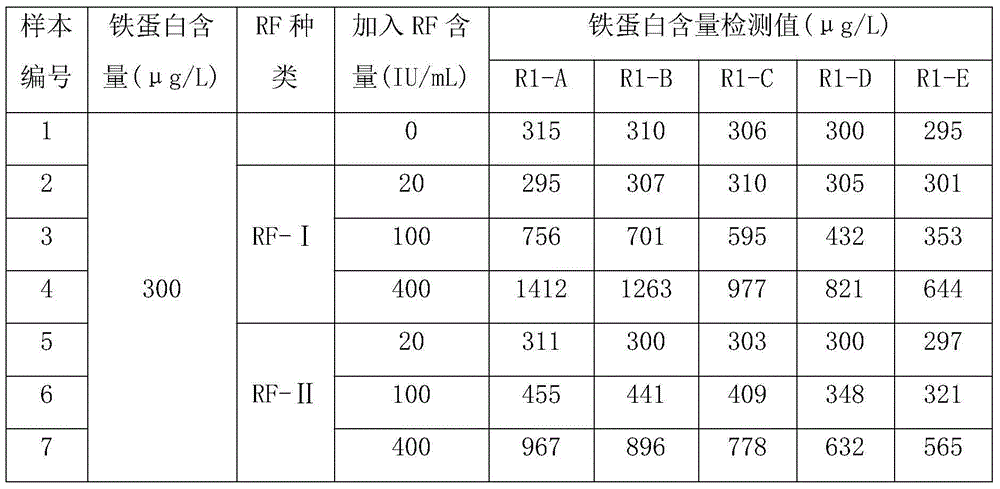
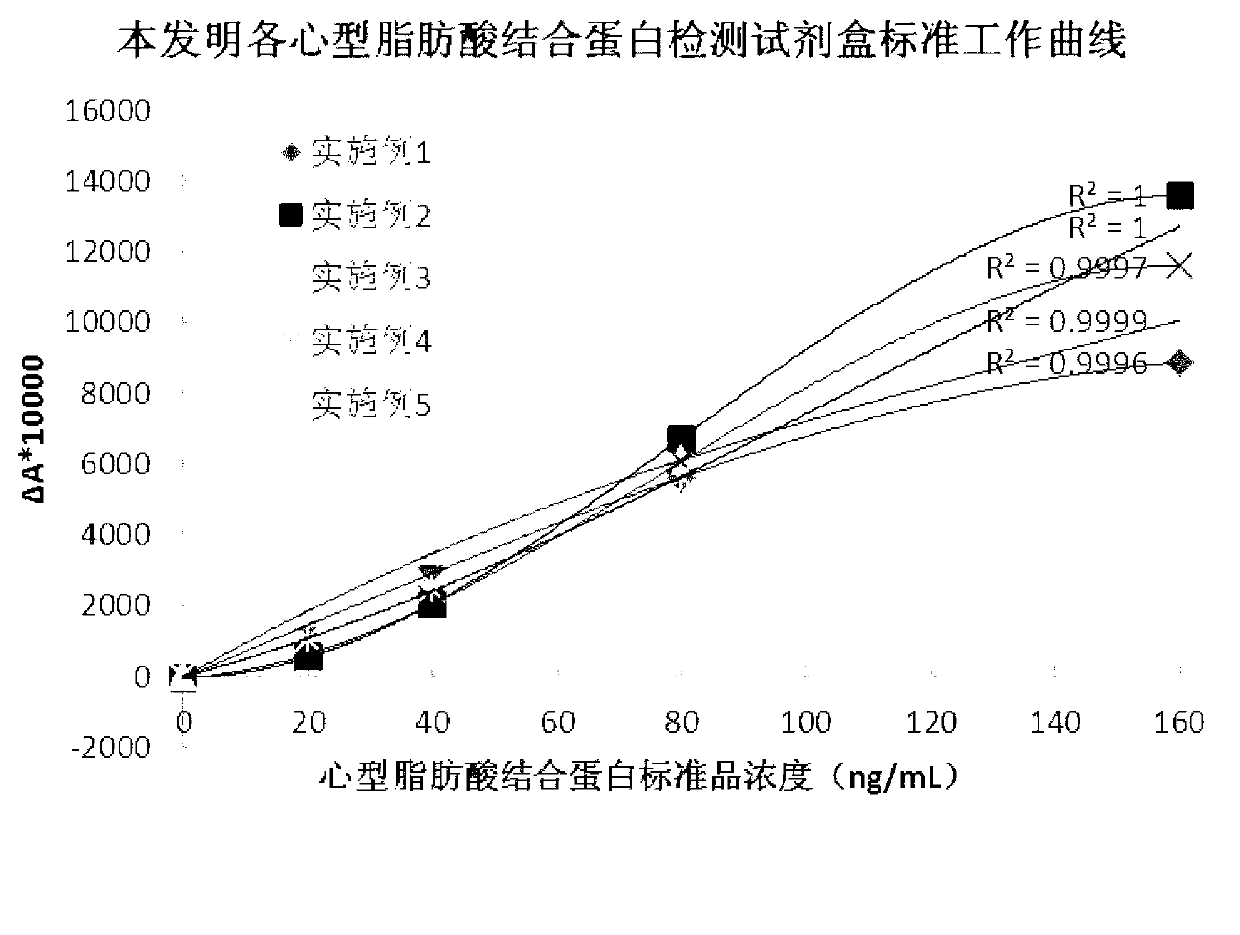
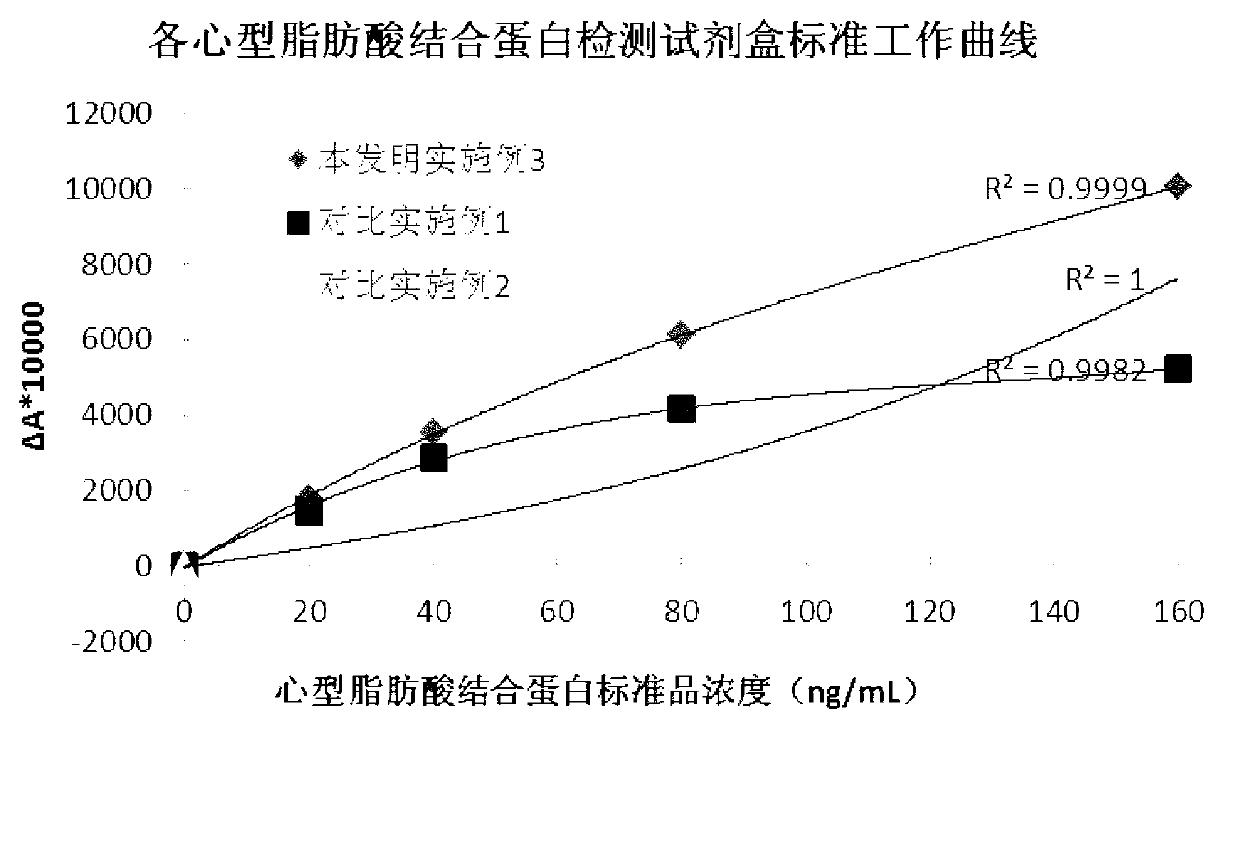
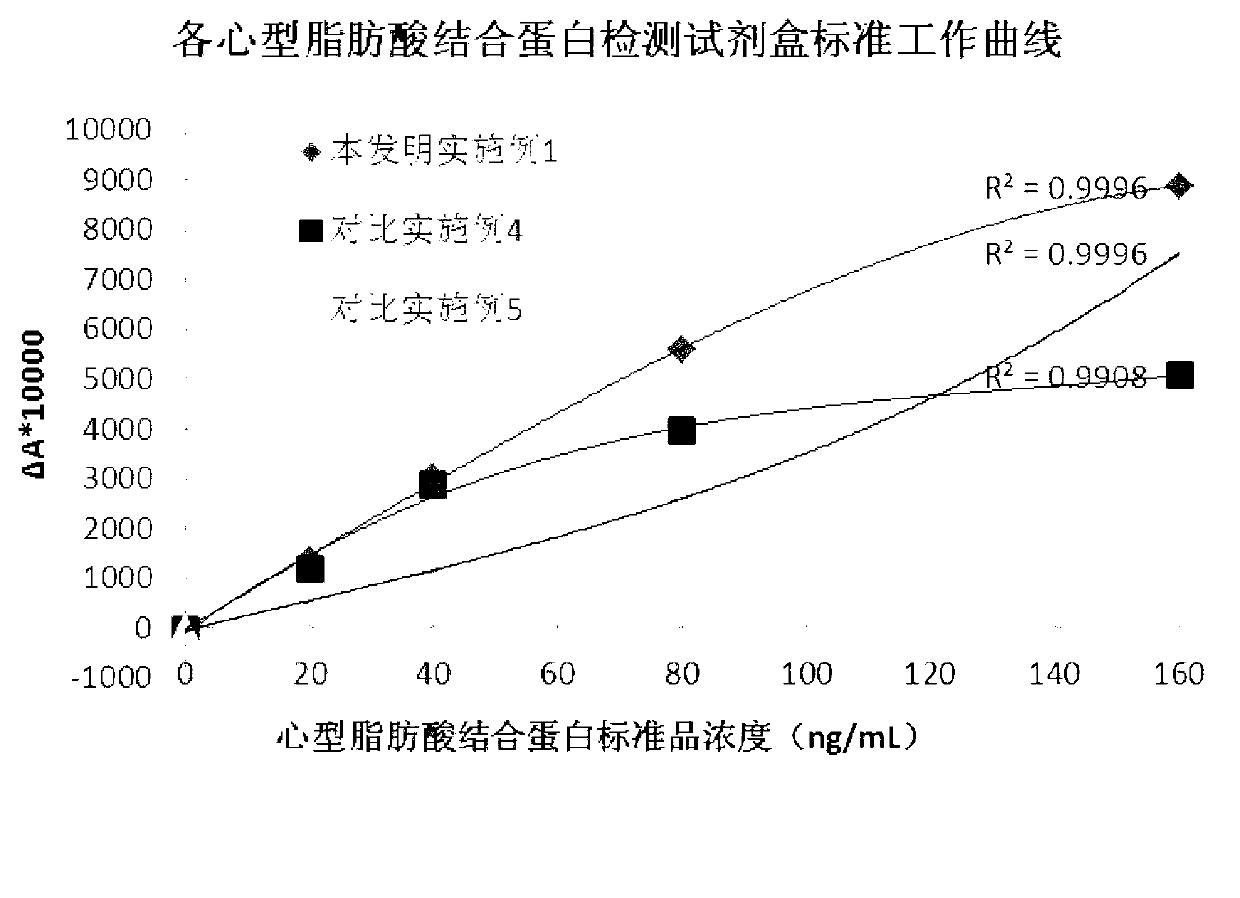
The invention discloses a heart-type fatty acid binding protein content detection kit and a preparation method thereof. The kit disclosed by the invention is formed by a reagent I and a reagent II which are mutually independent; the reagent I comprises the following components of: a biological buffering agent, a surfactant, a coagulation accelerator, a preservative, a stabilizing agent, a blocking agent, a chelating agent and water; the reagent II comprises the following components of: latex grains covered by a heart-type fatty acid binding protein antibody, a biological buffering agent, a chelating agent, a surfactant, a preservative, a suspending aid, a sealing agent, a stabilizing agent and water; and the grain diameters of the latex grains covered by the heart-type fatty acid binding protein antibody are within 90-200nm. The detection kit disclosed by the invention has a series of advantages of good stability, strong specificity, high sensitivity, strong anti-interference capability on rheumatoid factors, good linear range, simplicity and convenience for detection and operation and the like, and is good for large-scale popularization and application.
Owner:WUHAN LIFE ORIGIN BIOTECH LTD
Lipoprotein (a) detection kit
The invention discloses a lipoprotein (a) detection kit which is characterized by consisting of a reagent 1, a reagent 2 and a working calibration solution. The reagent 1 consists of a buffer solution, bull serum albumin (BSA), a surfactant, goat IgG and NaN3; the reagent 2 consists of a buffer solution, a lipoprotein (a) monoclonal antibody latex microsphere, BSA, the surfactant, the goat IgG, a stabilizer and NaN3. The lipoprotein (a) detection kit can be applied to a fully automatic biochemical analyzer, the detection result is high in accuracy, and the lipoprotein (a) has high comparability with absolute concentration of lipoprotein (a) of different crowds and different sizes. Moreover, the lipoprotein (a) detection kit does not have a cross reaction with plasminogen, is not interfered by rheumatoid factors and is high in reagent stability, convenient to use and convenient for large-scale popularization, only a monoclonal antibody of the lipoprotein (a) is needed, and the preparation cost and time of the kit are saved.
Owner:BEIJING LEADMAN BIOCHEM
Modified allosteric type cyclic citrulline polypeptide, and fusion protein, antibody and reagent kit thereof
ActiveCN101407541ABuild specificityImmunoglobulins against animals/humansBiological testingAntigenAutoantibody
The invention discloses five categories of denaturizing allosteric cyclic citrulline polypeptide, fusion protein, antibodies and a kit thereof, which are formed by synthesizing related antigens that antibodies of rheumatoid arthritis patients can specifically recognize, independent designing amino acid sequences and structures with special derivations, and tests prove that the invention can combine with antibodies in RA blood serum and have better immunity reaction compared with the existing cyclic citrulline polypeptide detections, such as CCP and the like. The polypeptides carry out denaturizing and allosteric design and synthesis according to metabolism of arginine residues, and compared with projects of CCP, C-CRP and RF and the like, the five categories of denaturizing allosteric cyclic citrulline polypeptides are characterized by good specificity, high relevance ratio and high primitive diagnosis rate, etc.
Owner:上海精臻生物科技有限公司
Lipoprotein (a) determination kit and preparation method thereof
The invention discloses a lipoprotein (a) determination kit and a preparation method thereof. The kit comprises a reagent R1 and a reagent R2 which are liquid components and are mutually independent, the reagent R1 comprises a buffer solution, a stabilizer, an accelerator, an antiseptic, an anti-human rheumatoid factor antibody and purified water used as a solvent, and the reagent R2 comprises the buffer solution, a surfactant, the stabilizer, a suspending aid, the antiseptic, a latex coated anti-human lipoprotein (a) antibody and purified water used as a solvent. The preparation method comprises the following steps: preparing the reagents according to the component content; mixing a sample to be determined with the reagent R1 and the reagent R2 to fully react the sample to be determined with the reagent R1 and the reagent R2; determining the absorbance difference by using a fully-automatic biochemical analyzer after the reaction; and calculating the concentration of lipoprotein (a) in the sample according to the absorbance change value. The kit has high accuracy.
Owner:ANHUI IPROCOM BIOTECH CO LTD
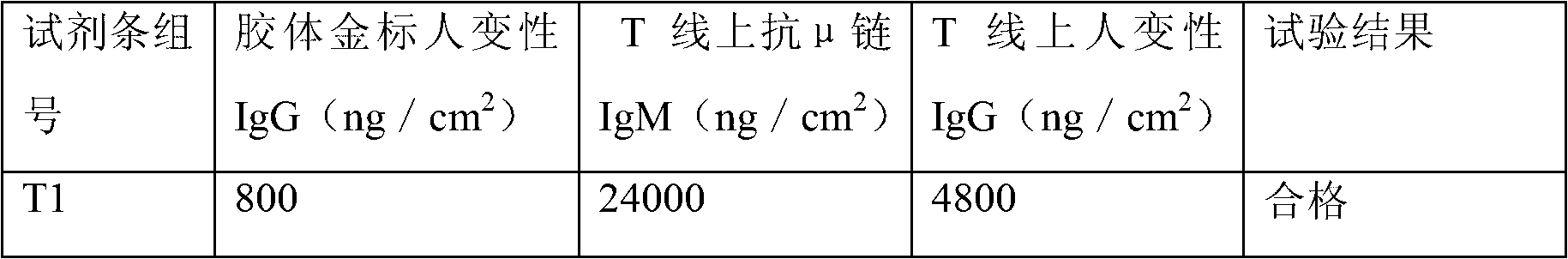
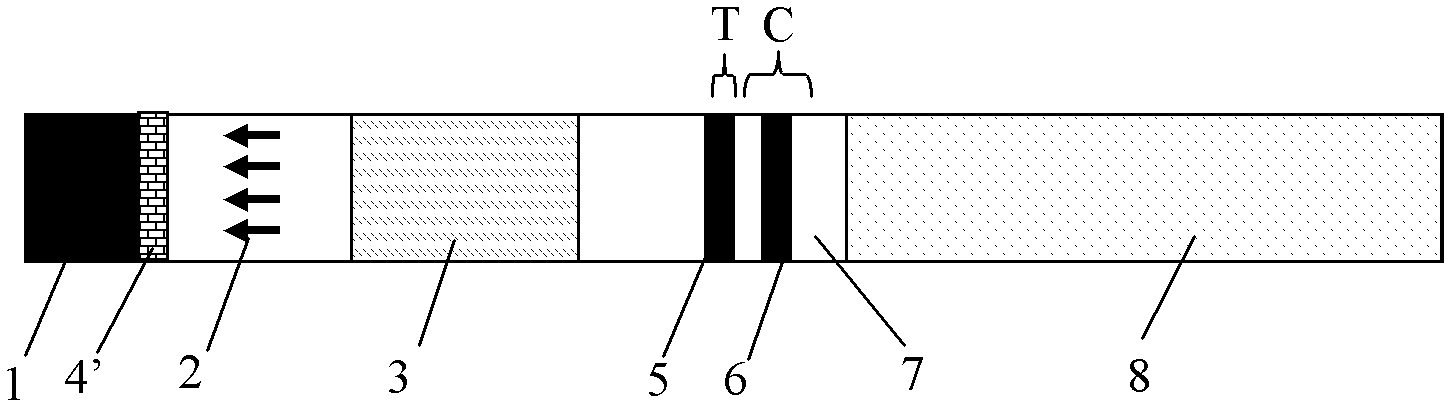
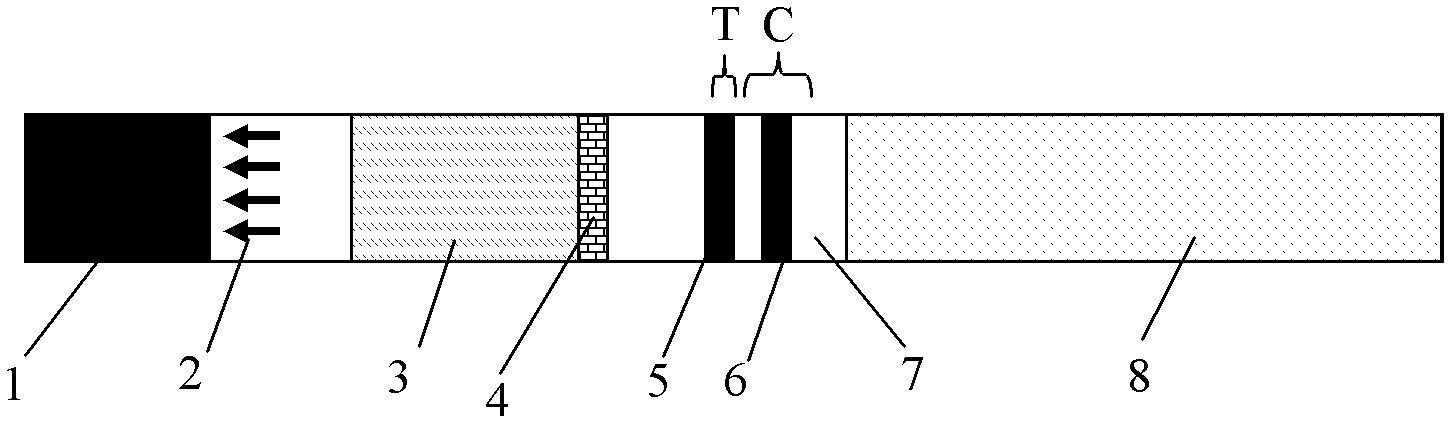
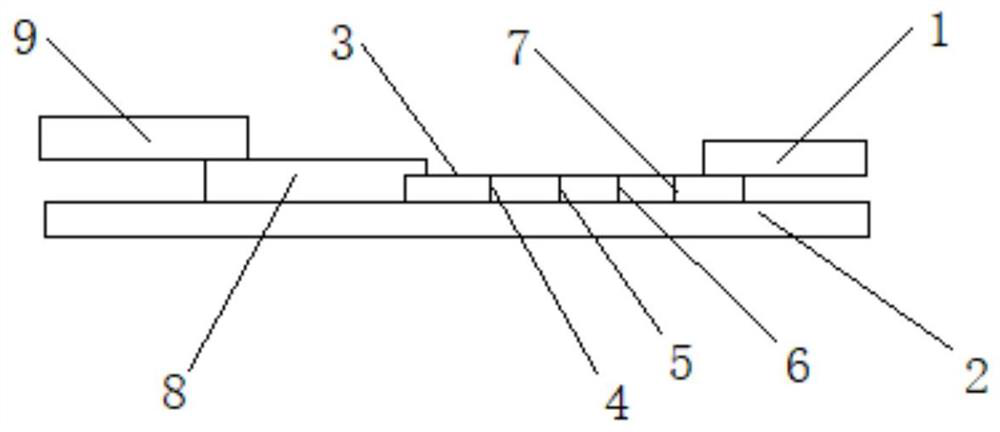
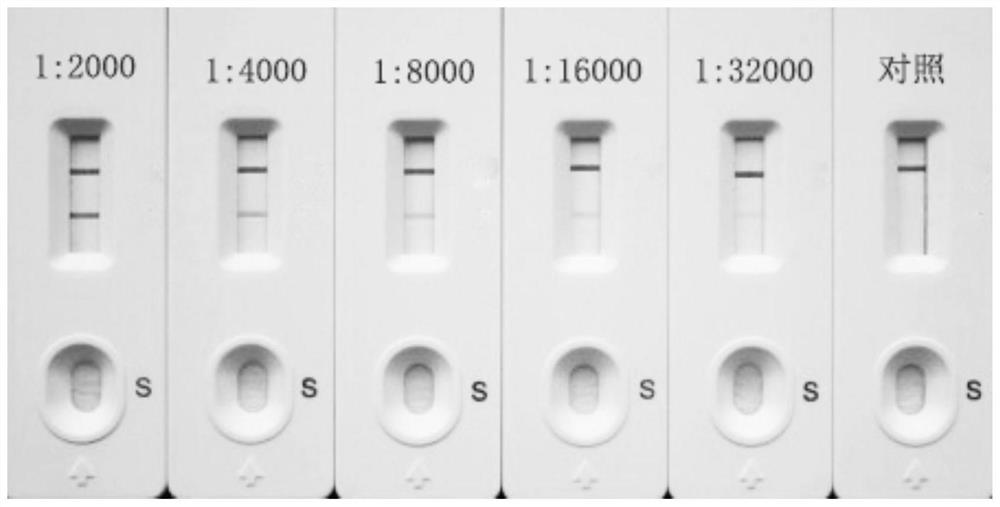
Colloidal gold test strip for fast detecting rheumatoid factors
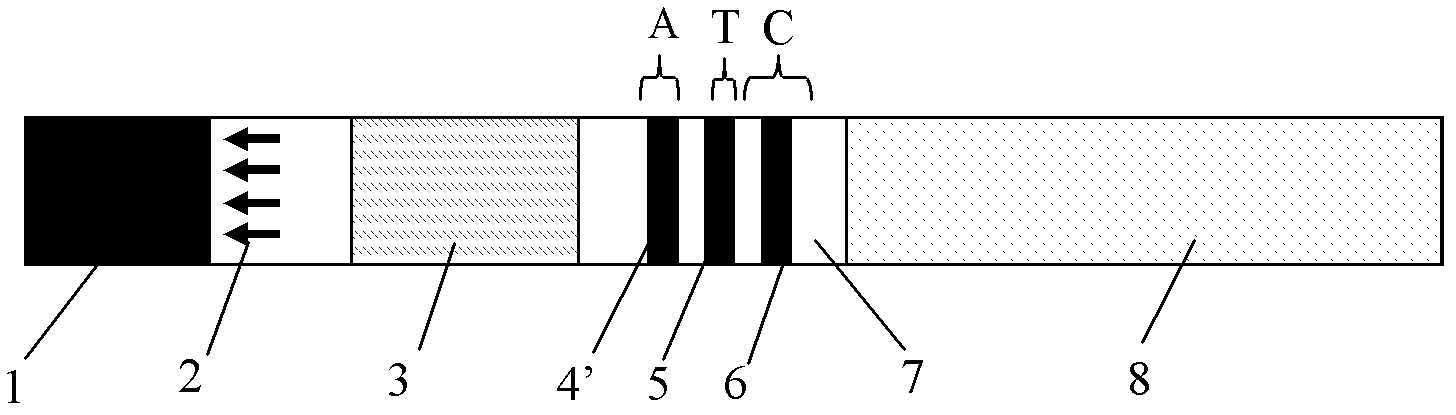
The invention discloses a colloidal gold test strip for fast detecting rheumatoid factors, belonging to medical test consumables. The colloidal gold test strip is characterized in that human denatured IgG with a colloidal gold label is enveloped on a gold label fiber pad; anti-mu chain IgM and human denatured IgG are enveloped on a T line, and the ratio of unit area envelop quantity of the human denatured IgG on the colloidal gold label, the anti-mu chain IgM and human denatured IgG on the T line on the gold label fiber pad is 0.133-0.889 to 5 to 1. The test strip uses an antibody capture method and a double antigen sandwich method principle during detection, has sensitivity and specificity, can fast detect the rheumatoid factors in human serum, plasma or whole blood by combining with an immune colloidal gold technique and has the advantages of simplicity, sensitivity and accuracy.
Owner:蓝十字生物药业(北京)有限公司
Rheumatoid factor detection reagent
The present invention discloses a rheumatoid factor detection reagent, which comprises a reagent R1 and a reagent R2, wherein the reagent R1 comprises a phosphate buffer solution, lauryl imidazolinium betaine, dioctadecyl dimethyl ammonium chloride, dodecyl trimethyl ammonium chloride, alkyl phenol ethoxylates (APEO), 1-hydroxyethylidene-1,1-diphosphonic acid, sodium azide and a stabilizer SHE-50, and the reagent R2 comprises a phosphate buffer solution, thermal polymerization I human IgG, latex microspheres, Kathon-CG, alkyl phenol ethoxylates (APEO) and sodium azide. According to the present invention, a variety of the reagents in the detection reagent of the present invention provide the synergy effect, and the detection reagent has characteristics of good accuracy, good stability, wide linear range, cheap price and easy use, and can completely meet the clinical needs.
Owner:郁东
IgM (immunoglobulin M) antibody detection test strip
The invention relates to an IgM (immunoglobulin M) antibody detection test strip, comprising an IgM antibody detection line, wherein a matter capable of absorbing human IgM and rheumatoid factors is coated in front of the IgM antibody detection line. Preferably, the matter is an anti-huamn IgG antibody, Protein G and human IgG aptamer; both the IgM antibody detection line and the matter are both on a nitrocellulose membrane; or the matter is on an absorption pad in front of the IgM antibody detection line, and the absorption pad can be arranged between the nitrocellulose membrane and a combined pad, and also can be arranged between the combined pad and a sample pad. The IgM antibody detection test strip provided by the invention can simply and quickly detect the IgM antibody, obviously improve the detection accuracy, provide a powerful support for the next accurate treatment and is suitable for large-scale promotion and application.
Owner:无锡博慧斯生物医药科技有限公司
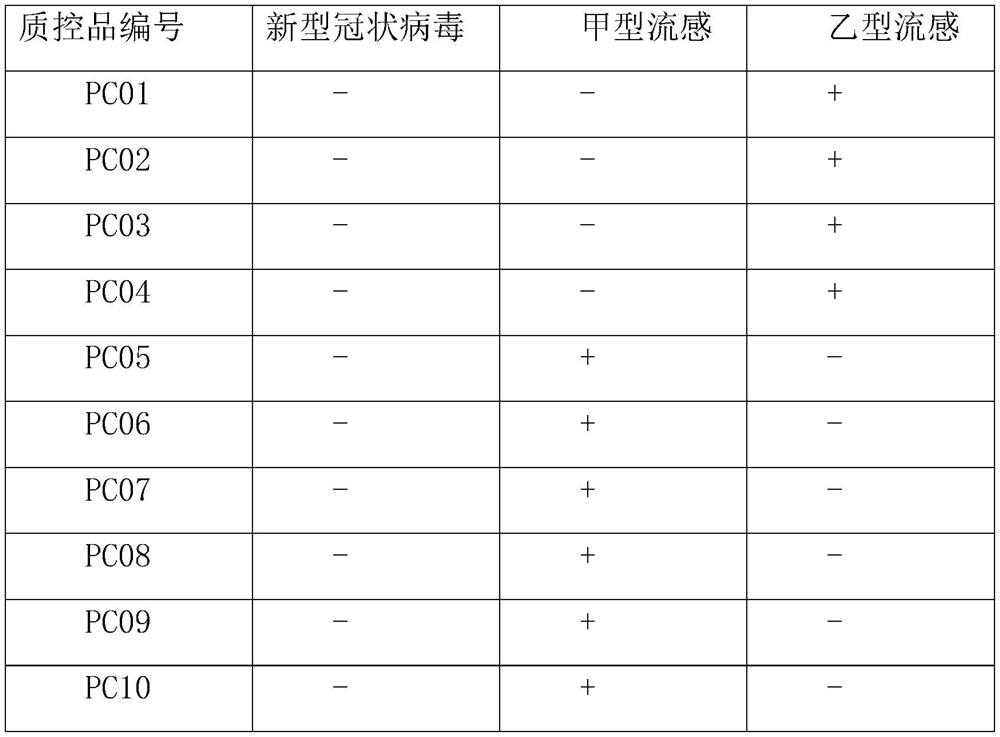
Novel coronavirus antigen and influenza virus antigen combined detection reagent strip and preparation method thereof
PendingCN112198312AReduce false positive interferenceHigh sensitivityBiological testingImmunoassaysReagent stripCoronavirus antibody
The invention discloses a novel coronavirus antigen and influenza virus antigen combined detection reagent strip which comprises a bottom plate. A sample pad, a gold-labeled pad, a nitrocellulose membrane and absorbent paper are sequentially pasted on the bottom plate, and the surface of the nitrocellulose membrane is sequentially coated with an anti-novel coronavirus antibody, an anti-influenza Avirus antibody and an anti-influenza B virus antibody. A rabbit anti-mouse IgG antibody coats the end close to the absorbent paper. A colloidal gold labeled anti-novel coronavirus antibody, an anti-influenza A virus antibody and an anti-influenza B virus antibody are sprayed on the gold-labeled pad. The binding protein A / G is marked on the surface of the colloidal gold, the protein A / G is specifically bound with the Fc end of the antibody, so that the ideal conformation of Fab end abduction is constructed, and meanwhile, the binding of the protein A / G with the Fc can reduce the false positiveinterference of rheumatoid factors on a detection structure. The reagent strip can simultaneously realize qualitative detection of the novel coronavirus antigen and the influenza antigen in one test,and is convenient to use, good in sensitivity, high in specificity and short in detection time.
Owner:南京佰抗生物科技有限公司
Preparation method of rheumatoid factor antigen, detection kit and preparation method of detection kit
The invention relates to a preparation method of a rheumatoid factor antigen, a detection kit and a preparation method of the detection kit. The preparation method of rheumatoid factor antigen comprises the following steps: uniformly mixing an IgG solution with a denaturing buffer at 4 DEG C; after IgG molecules and the denaturant are fully reacted, carrying out heat polymerization reaction in a constant-temperature water bath at 55-65 DEG C for 30-60 min to obtain multimeric modified IgG; filtering to remove impurities by using a dialysis buffer; diluting to the proper concentration with a stock buffer solution and preserving, and applying the prepared rheumatoid factor antigen to the detection kit. Compared with the prior art, the preparation method of rheumatoid factor antigen, detection kit and preparation method of detection kit can greatly improve the reagent performance of such products, and has a simple preparation method, the difference between the preparation batches is controllable, and large-batch industrial production can be performed.
Owner:SHANGHAI FOSUN LONG MARCH MEDICAL SCI CO LTD
Immunological measurement reagent for use in measurement of kl-6
ActiveCN102422159AQuick testEasy to testBiological testingImmunoassaysImmunologic assayAmount of substance
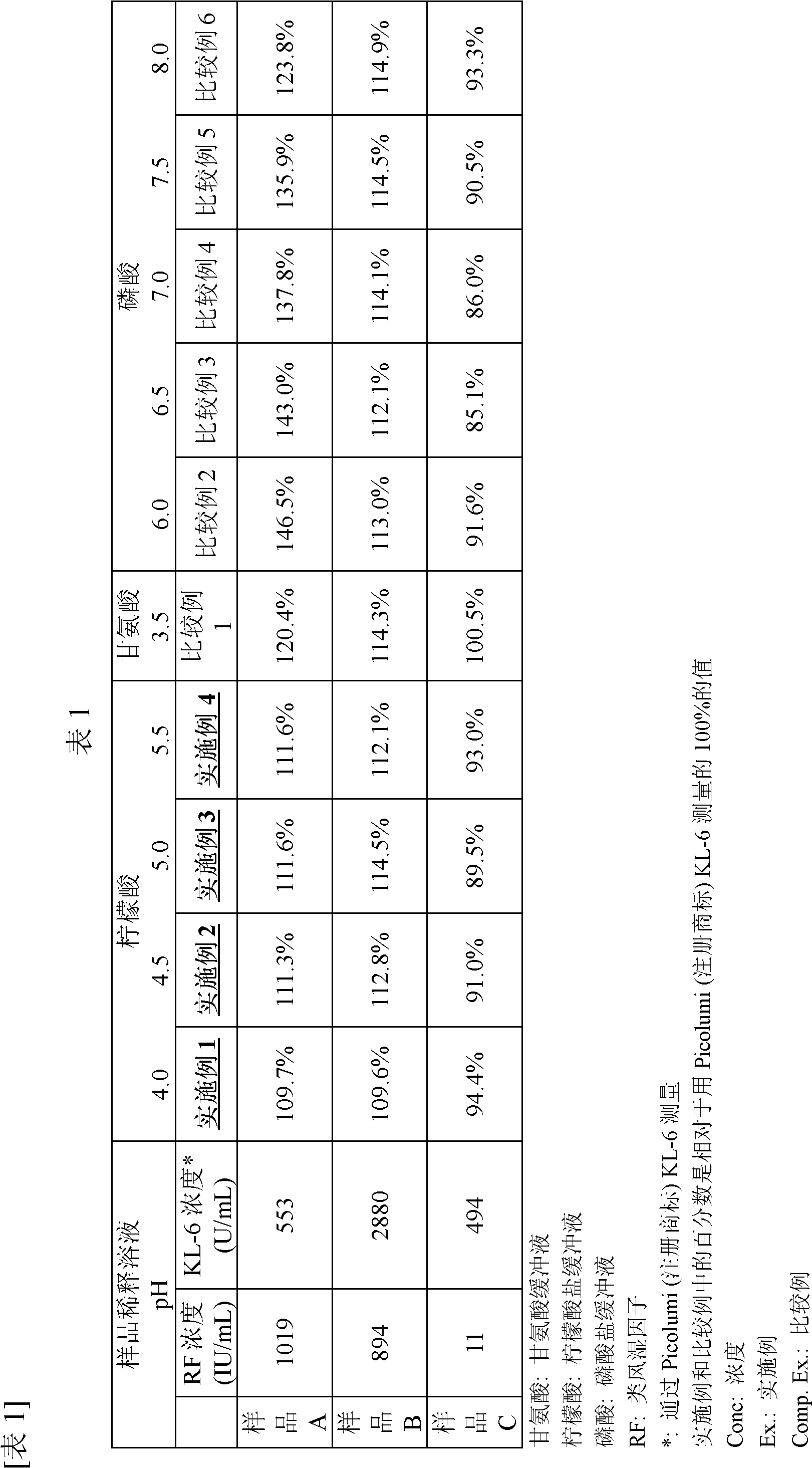
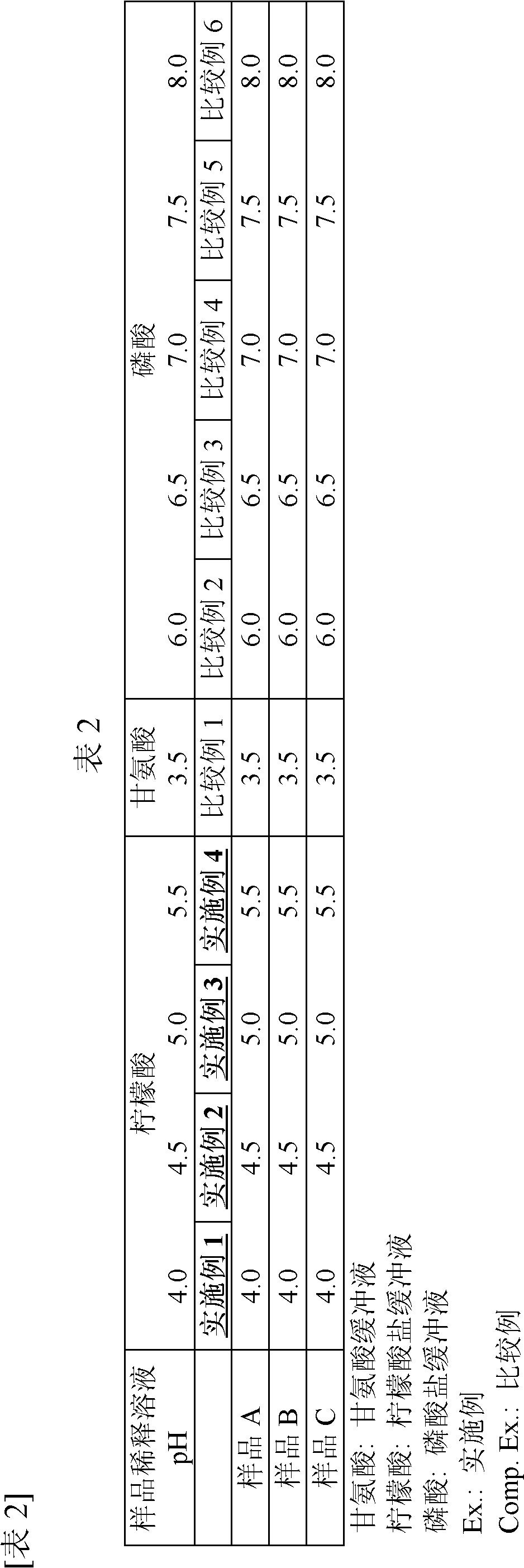
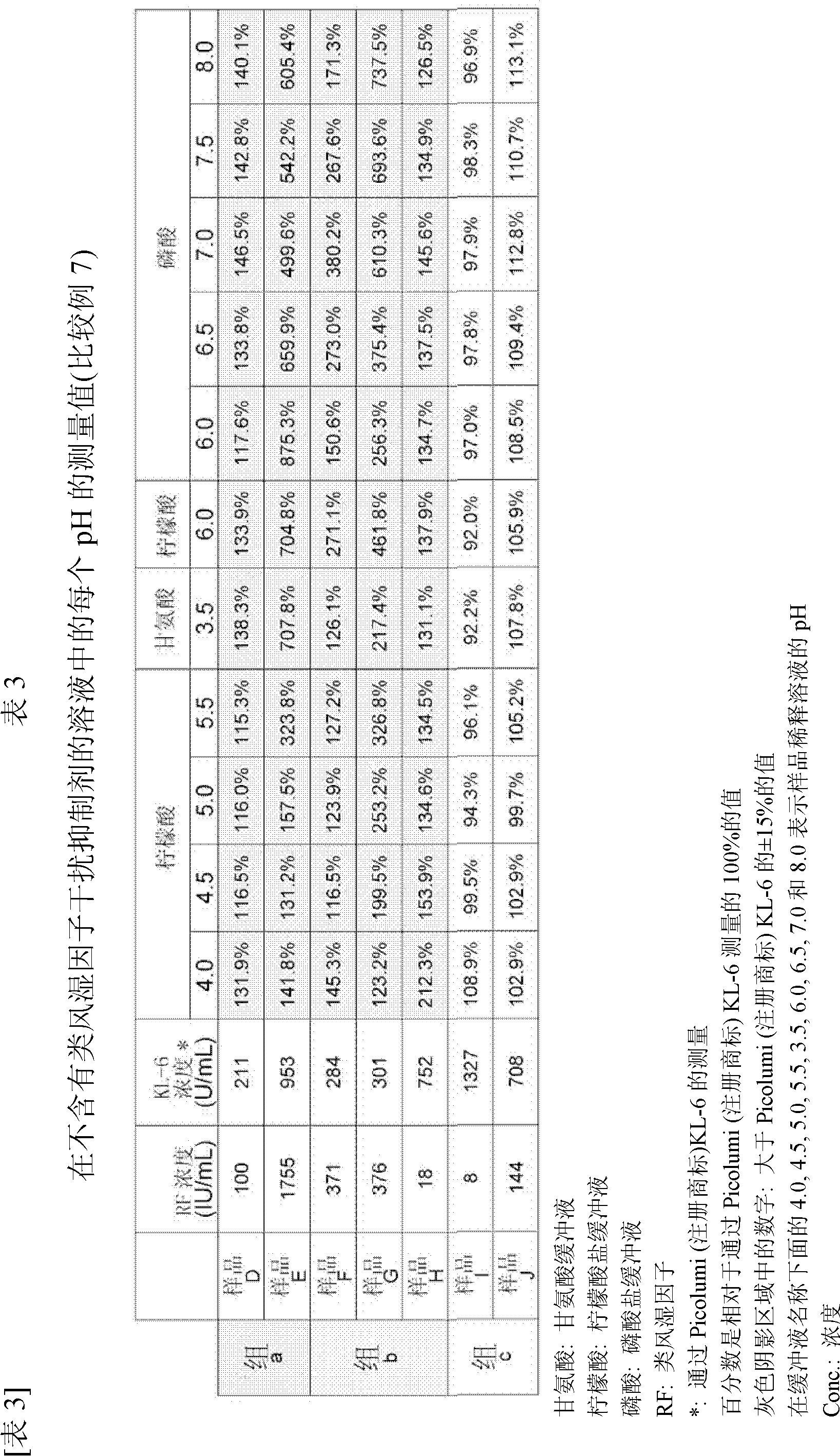
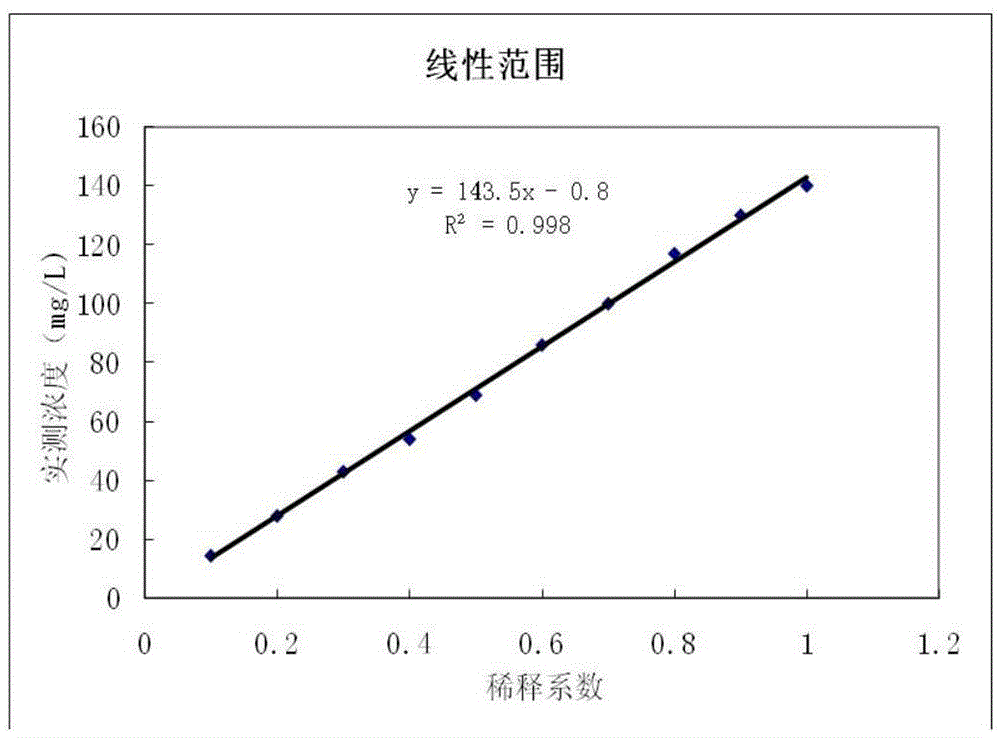
Disclosed are a measurement reagent and a measurement method both for accurately measuring KL-6, particularly a measurement reagent and a measurement method for accurately measuring KL-6 in a sample that contains a rheumatoid factor and / or a non-specific substance other than the rheumatoid factor. Specifically disclosed is an immunological measurement reagent which comprises a solution containing a rheumatoid factor interference inhibitor and having a pH value of 4.0 to 5.5 and a solution containing an insoluble carrier, wherein an anti-KL-6 antibody is supported on the insoluble carrier. The use of the immunological measurement reagent enables the accurate measurement of KL-6 in a sample that contains a rheumatoid factor and / or a non-specific substance other than the rheumatoid factor.
Owner:SEKISUI MEDICAL CO LTD
Kit capable of detecting alpha-1-microglobulin in urine and serum samples simultaneously
ActiveCN105467131AHigh sensitivityImprove anti-interference abilityBiological testingSerum igeSerum samples
A kit capable of detecting alpha-1-microglobulin in urine and serum samples simultaneously comprises a regent 1 and a reagent 2, and is characterized in that the reagent 1 is a buffer solution containing antibodies against human rheumatoid factors and a coagulation accelerator; the reagent 2 is a buffer solution containing large-particle-size polystyrene nano-particles coated with monoclonal antibodies against human alpha-1-microglobulin. The kit has the advantages of high sensitivity, wide linearity, better specificity and high anti-interference capacity and can be used for detecting human urine and the serum.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
Convenient procalcitonin detection kit
InactiveCN105467116AAvoid interferenceAvoid being copied and tampered withPreparing sample for investigationBiological testingCelluloseLap joint
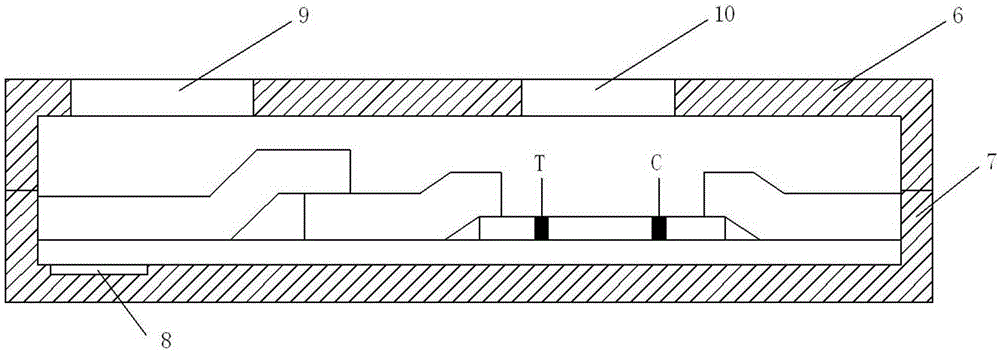
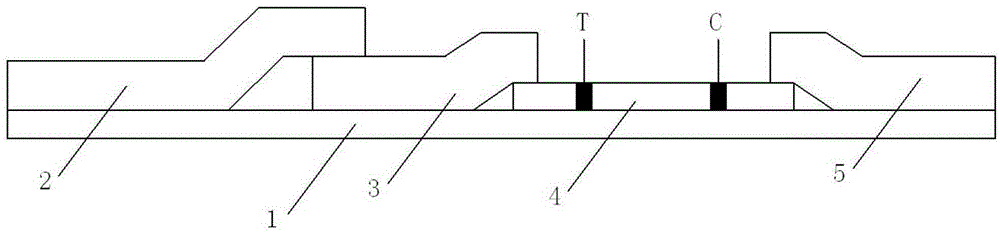
A convenient procalcitonin detection kit comprises a test strip, an upper clamping groove, a lower clamping groove and an RFID (radio frequency identification) chip, wherein the test strip comprises a plastic rubber slab; a sample pad, a first antibody bearing pad, a cellulose membrane and a water absorption pad are arranged sequentially on the plastic rubber slab; the first antibody bearing pad and the water absorption pad are in lap joint at two ends of the cellulose membrane; one end of the sample pad is in lap joint on the first antibody bearing pad; a detection band T for immobilizing a second C-reactive protein antibody and a quality control band C for immobilizing a goat-anti-mouse antibody are arranged on the cellulose membrane; the test strip is arranged in a cavity formed by splicing the upper clamping groove and the lower clamping groove; a sampling hole and a detection window are formed in the upper clamping groove; a sample diluent adopts a buffer containing rheumatoid factors and a heterophil antibody blocker; the RFID chip is arranged in the lower clamping groove close to the left end. The detection kit has the advantages that the antibody activity can be reflected accurately, an error detection result can be avoided, the kit is simple to use, and the like.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
Rheumatoid factor detection kit and detection method thereof
ActiveCN109406796AHigh sensitivityWide linear rangeDisease diagnosisBiological testingInorganic saltsPreservative
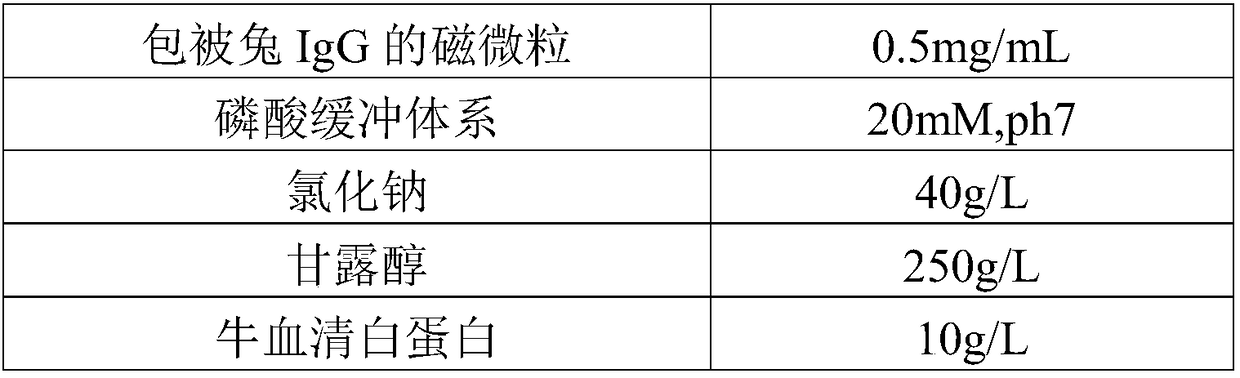
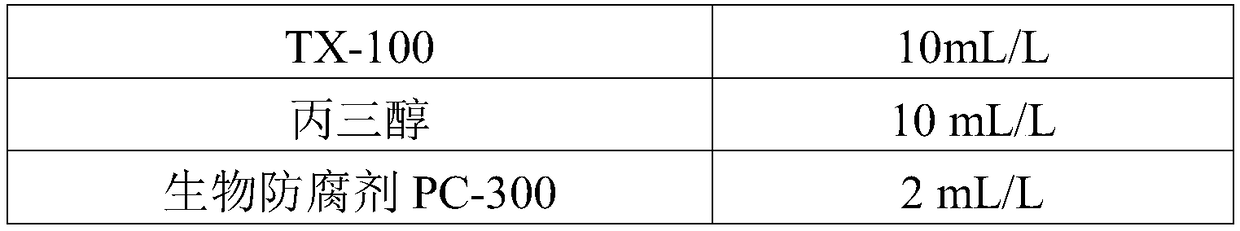
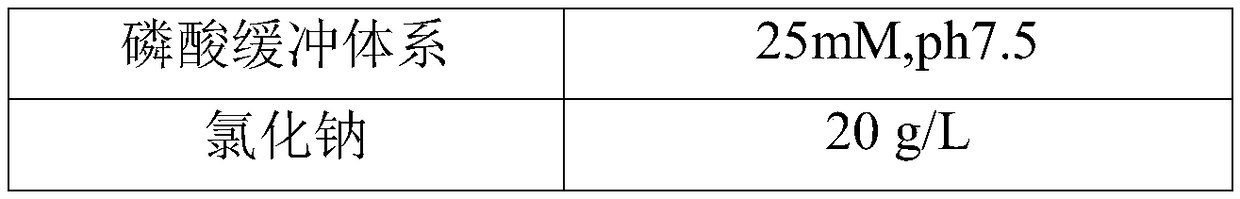
The invention discloses a rheumatoid factor detection kit and a preparation method thereof. The kit comprises a reagent R1, a reagent R2 and a reagent R3, wherein the reagent R1 contains magnetic particles coated with rabbit IgG; the reagent R2 contains markers of anti-human IgM / IgG / IgA antibodies or anti-human IgM antibodies or anti-human IgG antibodies or anti-human IgA antibodies; the reagent R3 is prepared by mixing a buffering system, inorganic salt, saccharides, proteins, a coloring agent, a nonionic surfactant and a preservative in a certain ratio. The rheumatoid factor detection kit ishigh in sensitivity, wide in linearity range, high in anti-interference capacity and capable of safely and quickly detecting rheumatoid factors.
Owner:SICHUAN MACCURA BIOTECH CO LTD
Preparation method for rheumatoid factor detection reagent
ActiveCN101644704AStrong specificityReduce manufacturing costBiological testingLatex particleFab Fragments
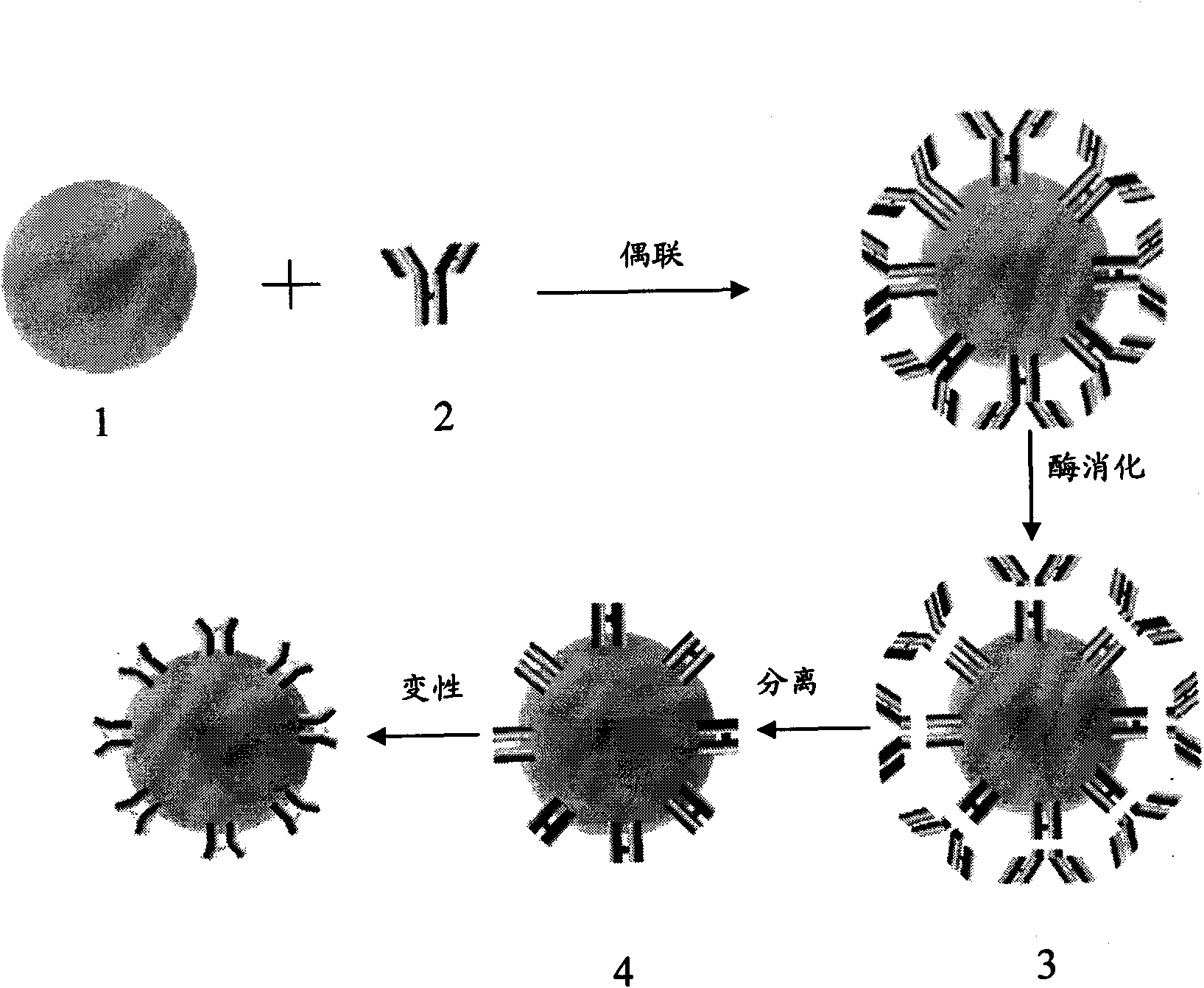
The invention provides a preparation method for rheumatoid factor detection reagent. The method comprises the following steps of: coupling IgG molecules and latex particles to obtain IgG sensitizing latex; digesting and decomposing obtained IgG sensitizing latex by enzyme to obtain mixture of Fab fragment and Fc fragment sensitizing latex; separating and purifying the Fab fragment and Fc fragmentsensitizing latex to obtain the Fc fragment sensitizing latex; causing the Fc fragment on the sensitizing latex to be denatured, and obtaining the denatured Fc fragment sensitizing latex which is prepared into rheumatoid factor detection reagent. The method not only can produce rheumatoid factor detection reagent with high specificity, but also has low production cost, simple technique and is convenient to large-scale industrialization production.
Owner:BEIJING LEADMAN BIOCHEM
Latex enhanced immunoturbidimetry kit capable of inhibiting rheumatoid factor interference
InactiveCN106950363AReduce the binding forceBinding is not affectedBiological testingAntigenLatex particle
The invention provides a latex enhanced immunoturbidimetry kit capable of inhibiting the rheumatoid factor interference. The kit comprises a reagent A and a reagent B. The reagent A is a solution capable of promoting the reactions between antigens and antibodies. The reagent B is a latex particle dispersion liquid containing sensitizing antibodies. The reagent A contains N-hydroxysuccinimide (1 to 40 g / L). The provided kit can effectively eliminate the interference of rheumatoid factor in a latex enhanced immunoturbidimetry sample on the immune-detection results; the accuracy and precision of measurement are both improved, and the kit has very good versatility.
Owner:SICHUAN MACCURA BIOTECH CO LTD
Blood purification material for removing rheumatoid factors, and preparation method thereof
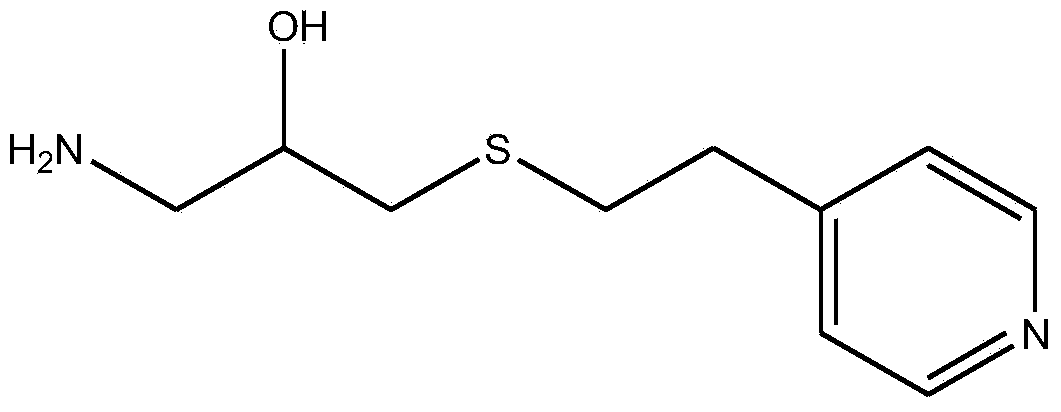
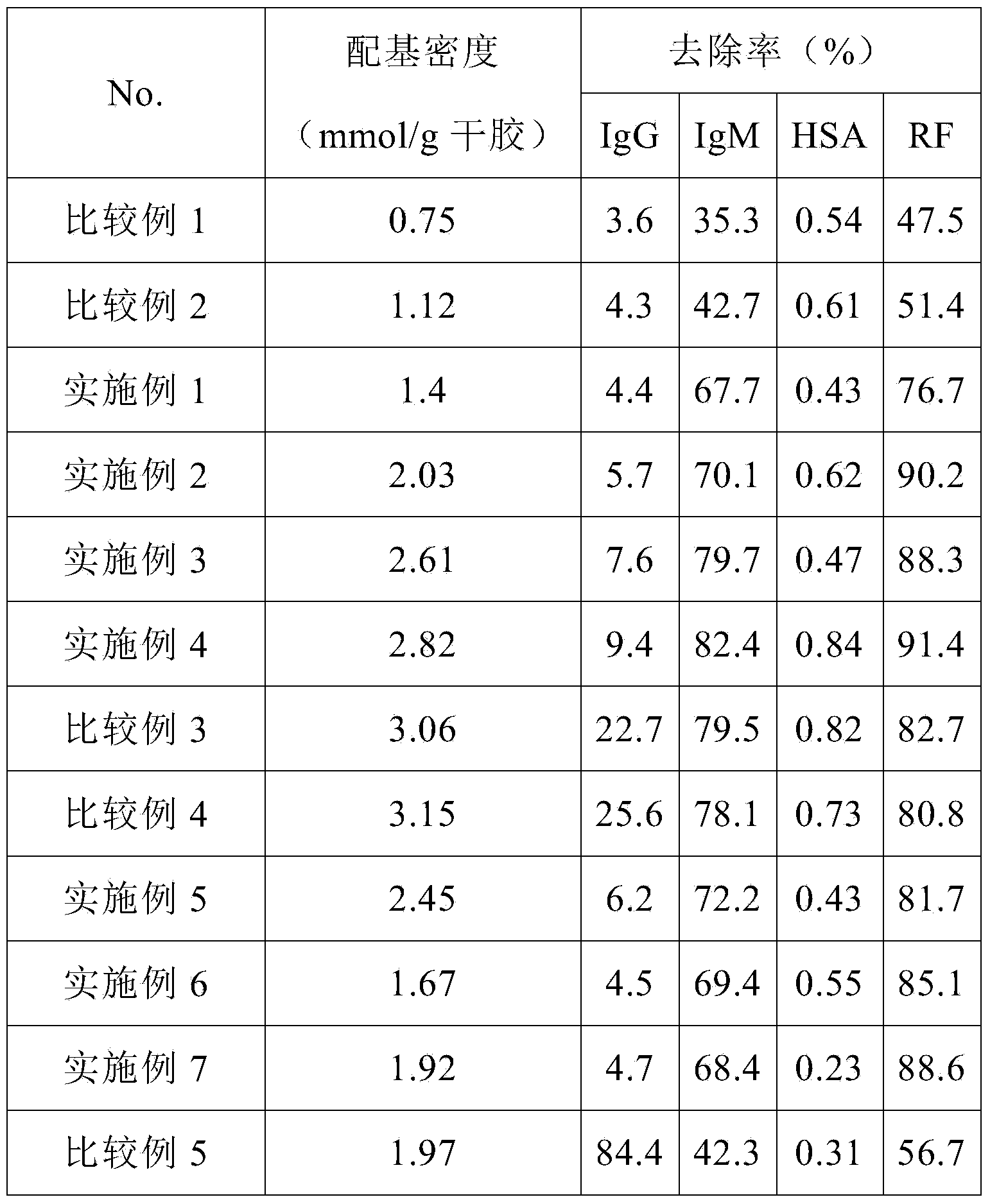
ActiveCN103933947AEasy to synthesizeReduce lossesIon-exchange process apparatusOther chemical processesVolumetric Mass DensityBlood plasma
The invention provides a blood purification material for removing rheumatoid factors, and a preparation method of the blood purification material, belonging to the technical field of biomedicine. The blood purification material comprises a solid phase carrier and a ligand fixed on the solid phase carrier by chemical coupling, wherein the solid phase carrier is a polysaccharide natural polymer material; the ligand is 1-amino-3-(2-(4-pyridyl)-ethyl sulfydryl)-2-propanol; the ligand fixed on the solid phase carrier by chemical coupling has the density of 1.4-2.8mmol / g dry solid phase carrier. The blood purification material is capable of selectively absorbing the rheumatoid factors in the blood, and is limited in nonspecific adsorption for blood plasma components such as human serum albumin, immunoglobulin G (Ig G) and the like; the blood purification material is low in preparation cost and stable in physicochemical property, and can be used as an absorption filler of a blood purification device for removing the rheumatoid factors in the blood of rheumatoid patients.
Owner:DALIAN UNIV OF TECH
Portable C reaction protein detection kit
InactiveCN105334318AAvoid interferenceAvoid copying and tamperingMaterial analysisCelluloseProtein detection
A portable C reaction protein detection kit comprises a test strip, an upper clamp slot, a lower clamp slot and a radio frequency identification chip; the test strip comprises a plastic cement plate, on which a sample pad, a first antibody bearing pad, a cellulose membrane and a water absorption pad are arranged in sequence; the first antibody bearing pad and the water absorption pad are respectively overlapped at two ends of the cellulose membrane; one end of the sample pad is overlapped on the first antibody bearing pad; the cellulose membrane is provided with a detection belt T for fixing a C reaction protein second antibody and a quality control belt for fixing a sheep anti-mouse antibody; the test strip is arranged in a cavity formed by splicing the upper clamp slot and the lower clamp slot; the upper clamp slot is provided with a sampling hole and a detection window; a sample diluent is a buffer solution containing a rheumatoid factor and heterophil antibody blocking agent; the lower clamp slot is provided with the radio frequency identification chip close to the left end. The kit has the advantages of being capable of accurately reflecting antibody activity, capable of avoiding wrong detection results, simple to use, and the like.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
Immunosorbent for rheumatoid factors for blood perfusion and preparation method thereof
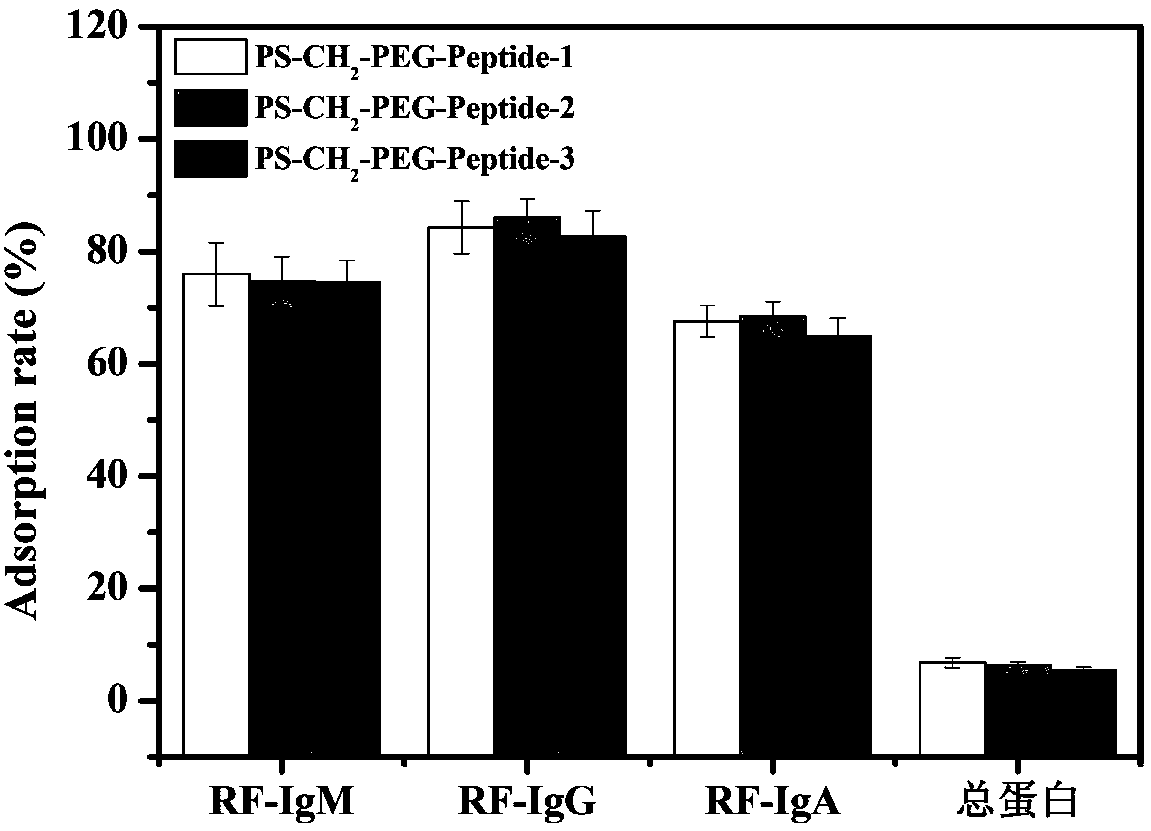
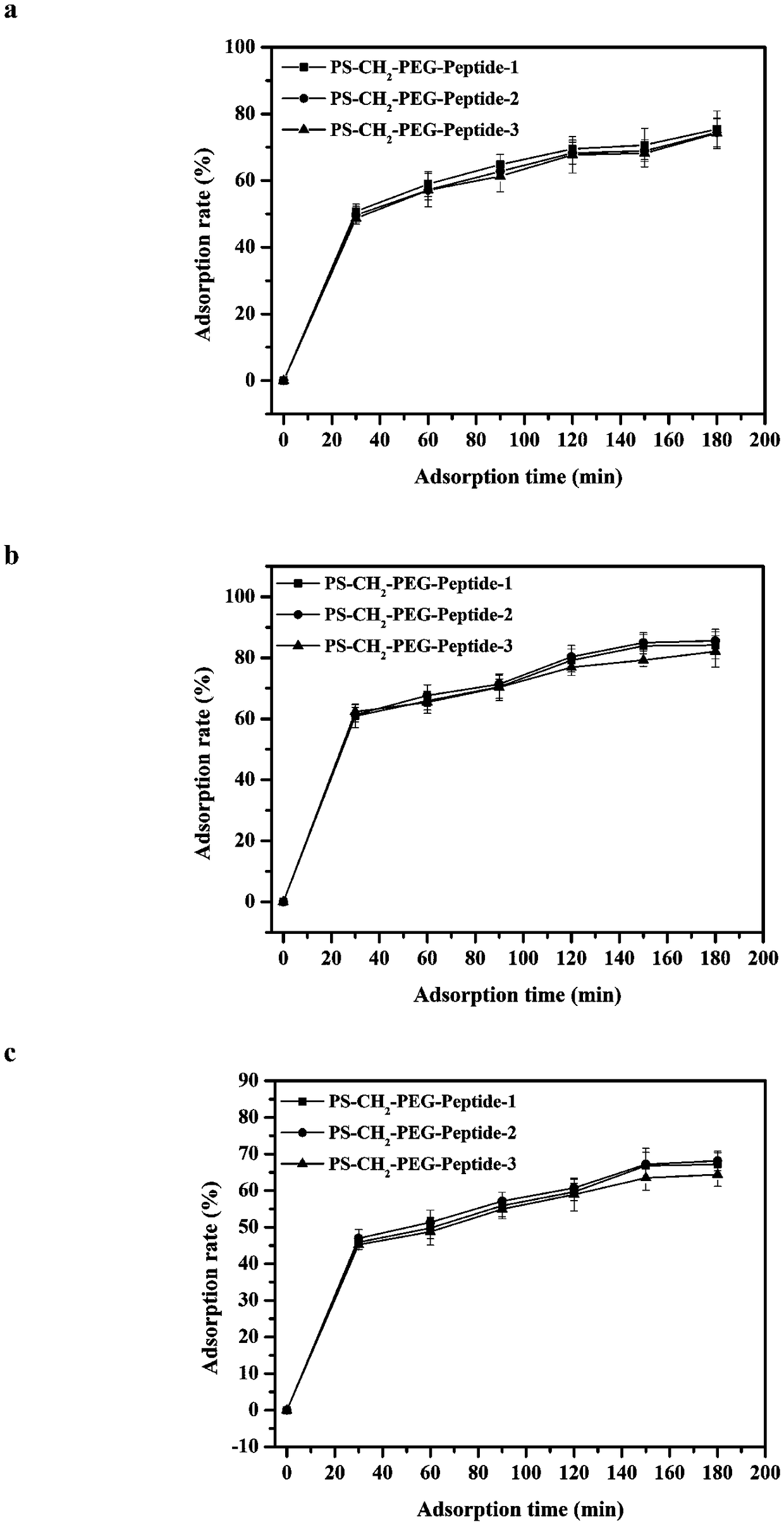
ActiveCN108586794AImprove adsorption capacityHigh adsorption selectivityOther chemical processesMicroballoon preparationSpecific adsorptionMicrosphere
The invention relates to an immunosorbent for rheumatoid factors for blood perfusion and a preparation method thereof. The immunosorbent uses chloromethylation macroporous structure styrene-divinylbenzene resin as carriers, uses micro-molecule polypeptides (consisting of 6 to 14 amino acids) as ligands, uses amino-functionalization polyethylene glycol (H2N-PEG-NH2) with different molecular weightas arms, so that carrier microspheres are coupled with the ligands through the arms. The preparation of the immunosorbent is simple; on the basis of maintaining the specific adsorption on the rheumatoid factors, the relevant side effects of potential immunogenicity and the like of the macromolecule (antigen or antibody) ligand immunosorbent is avoided; the cost is low; the immunosorbent is suitable for being used for the excessive rheumatoid factors in a body of the patient through blood or plasma perfusion.
Owner:NANKAI UNIV
Adrenocorticotropic hormone (ACTH) chemiluminescence detection kit
ActiveCN108548930AReduce signal backgroundIncrease signal strengthChemiluminescene/bioluminescenceBiological material analysisAlcoholMonoclonal antibody
The invention discloses an adrenocorticotropic hormone (ACTH) chemiluminescence detection kit, which comprises a reagent (1) and a reagent (2). The reagent (1) comprises a buffer solution, magnetic particles, a biotin labeled anti-ACTH monoclonal antibody, and polyhydric alcohol. The reagent (2) comprises a buffer solution, an acridinium ester labeled anti-ACTH monoclonal antibody and polyhydroxylsaccharides. The provided kit has the advantages of good stability, high sensitivity, good accuracy, simple and convenient operation, and low cost, and is not influenced by rheumatoid factors.
Owner:SICHUAN MACCURA BIOTECH CO LTD
Kit for determining immunoglobulin A and preparation method thereof
InactiveCN105911293AImprove accuracyEasy to operateColor/spectral properties measurementsBiological testingInorganic saltsEmulsion
The invention discloses a kit for determining immunoglobulin A and a preparation method thereof. The kit comprises double liquid components including a reagent R1 and a reagent R2 which are independent to each other, wherein the reagent R1 is composed of a buffering solution, inorganic salt ions, a coagulant, a preservative and an anti-human rheumatoid factor antibody; and the reagent R2 is composed of a buffering solution, a stabilizer, a preservative and an emulsion-coated anti-human immunoglobulin A antibody. The preparation method comprises the following steps: preparing the reagents according to the content of the components; mixing a sample to be detected with the reagent R1 and the reagent R2 to have a sufficient reaction; determining a reacted absorbance difference value by utilizing a full-automatic biochemical analyzer; and calculating the concentration of the immunoglobulin A in a sample according to an absorbance change value. The kit gas has the advantages of high detection accuracy and the like.
Owner:ANHUI IPROCOM BIOTECH CO LTD
IgG type rheumatoid factor enzyme immunity detection method
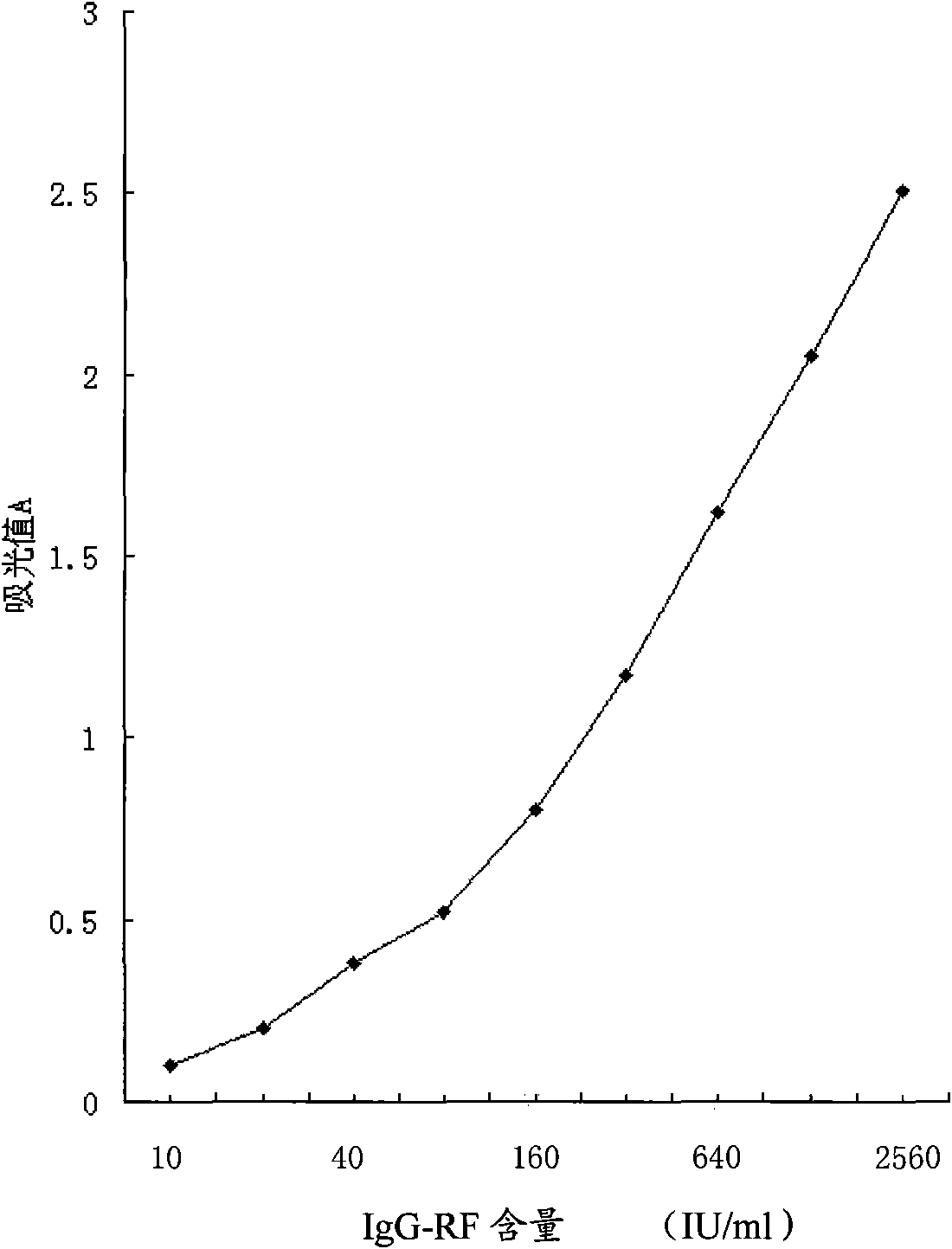
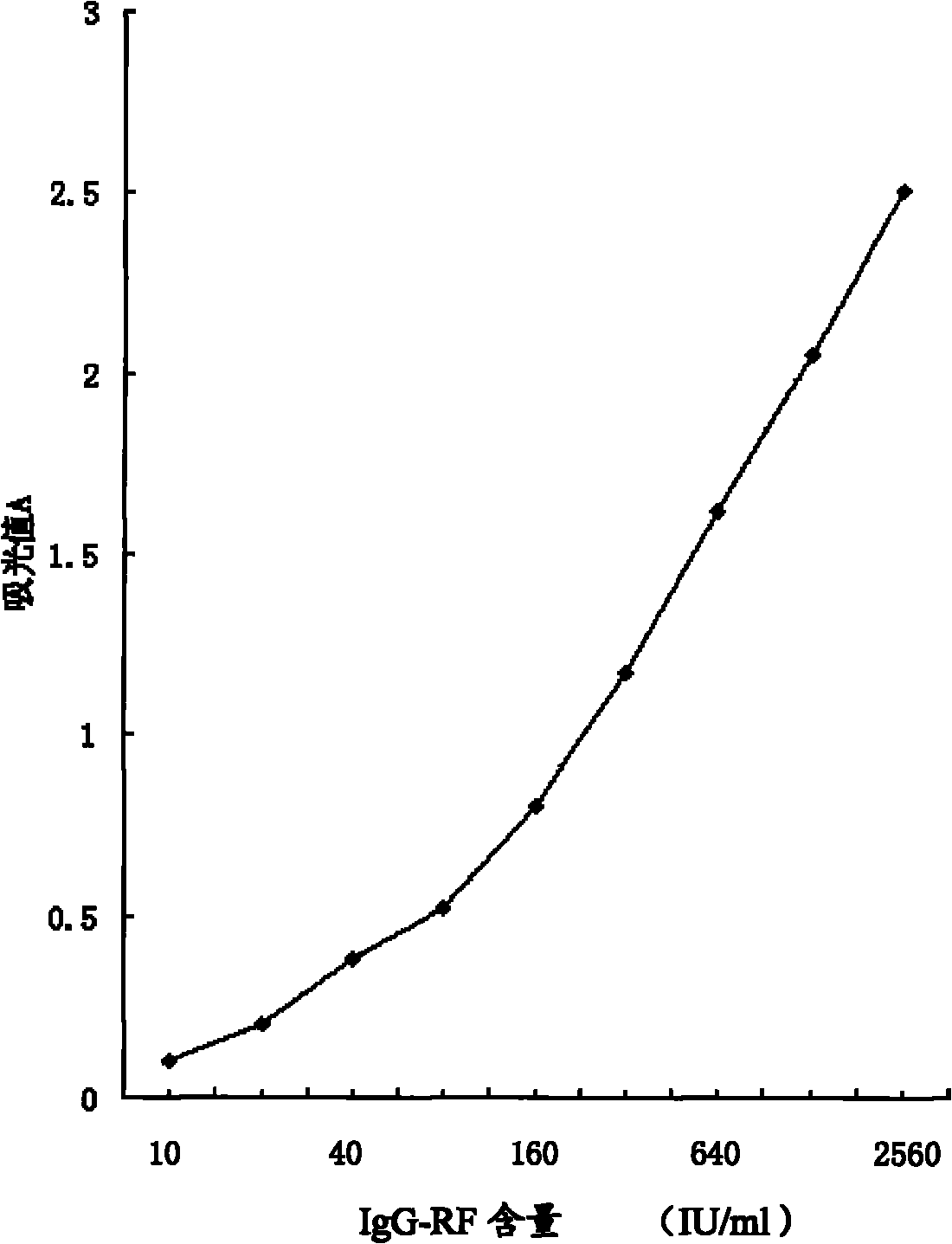
InactiveCN101871940AEliminate the effects of cross-reactivityImprove accuracyMaterial analysisImmuno detectionBiology
The invention discloses an IgG type rheumatoid factor enzyme immunity detection method. The method comprises the following steps of: (1) preparing reagents and specimens; (2) adding IgG-RF standard products, specimens and quality control products into coated plate micropores; (3) under the action of a certain temperature, pouring off liquid in the micropores, washing and beating to dry; (4) adding enzyme markers into all the micropores; (5) repeating the step (3); (6) adding substrates into all the micropores; (7) adding stopping solutions into all the micropores and uniformly shaking; (8) reading a light-sucking value A by using a microplate reader; and (9) calculating, wherein the coating plate micropores in the step (2) are IgG-Fc segment coated plate micropores, and the enzyme markers in the step (4) are anti-IgG-F(ab')2 segment antibodies. The IgG type rheumatoid factor enzyme immunity detection method eliminates the influence of a cross reaction, greatly improves the result accuracy, and obtains a good result proved by clinical applications.
Owner:上海市长宁区光华中西医结合医院
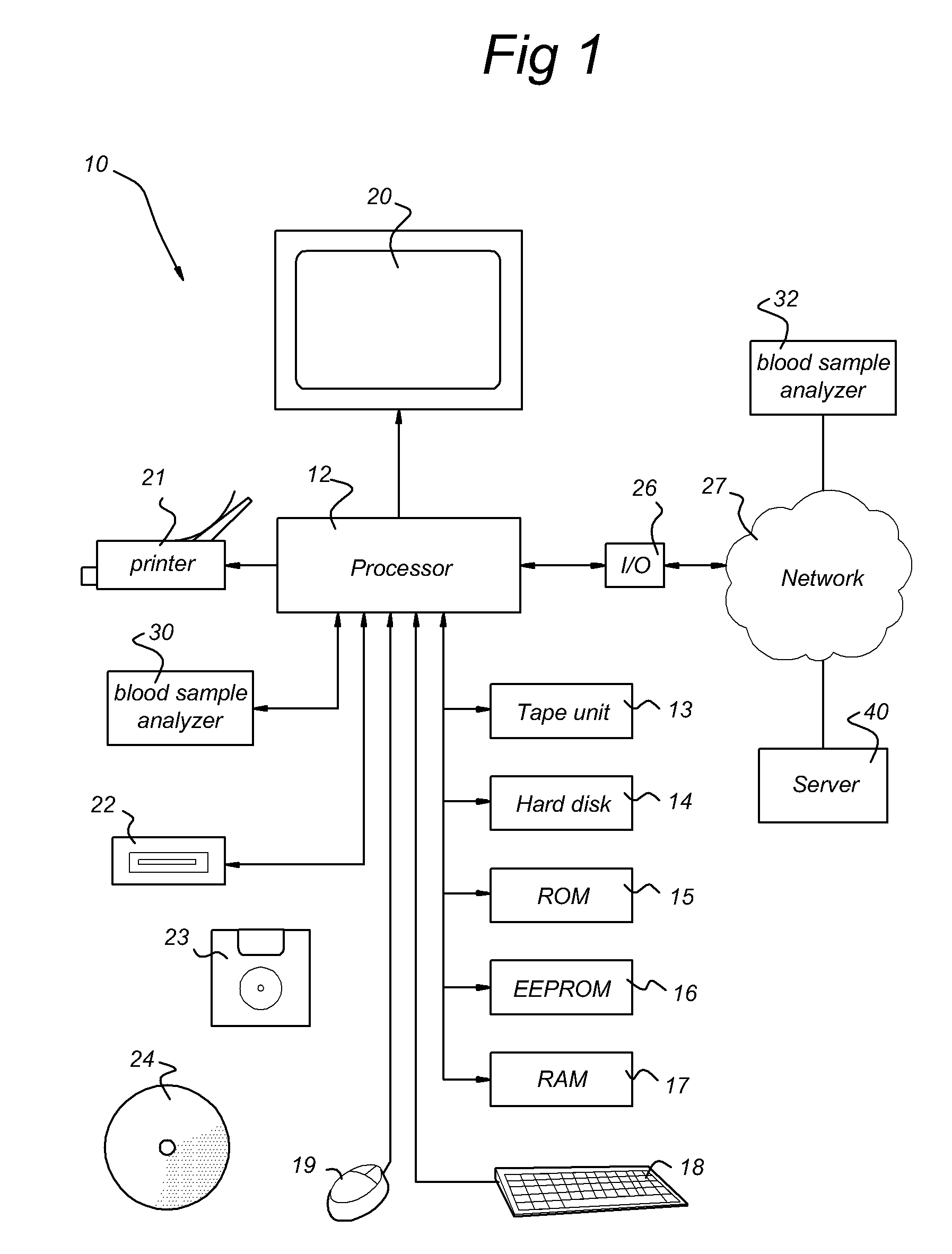
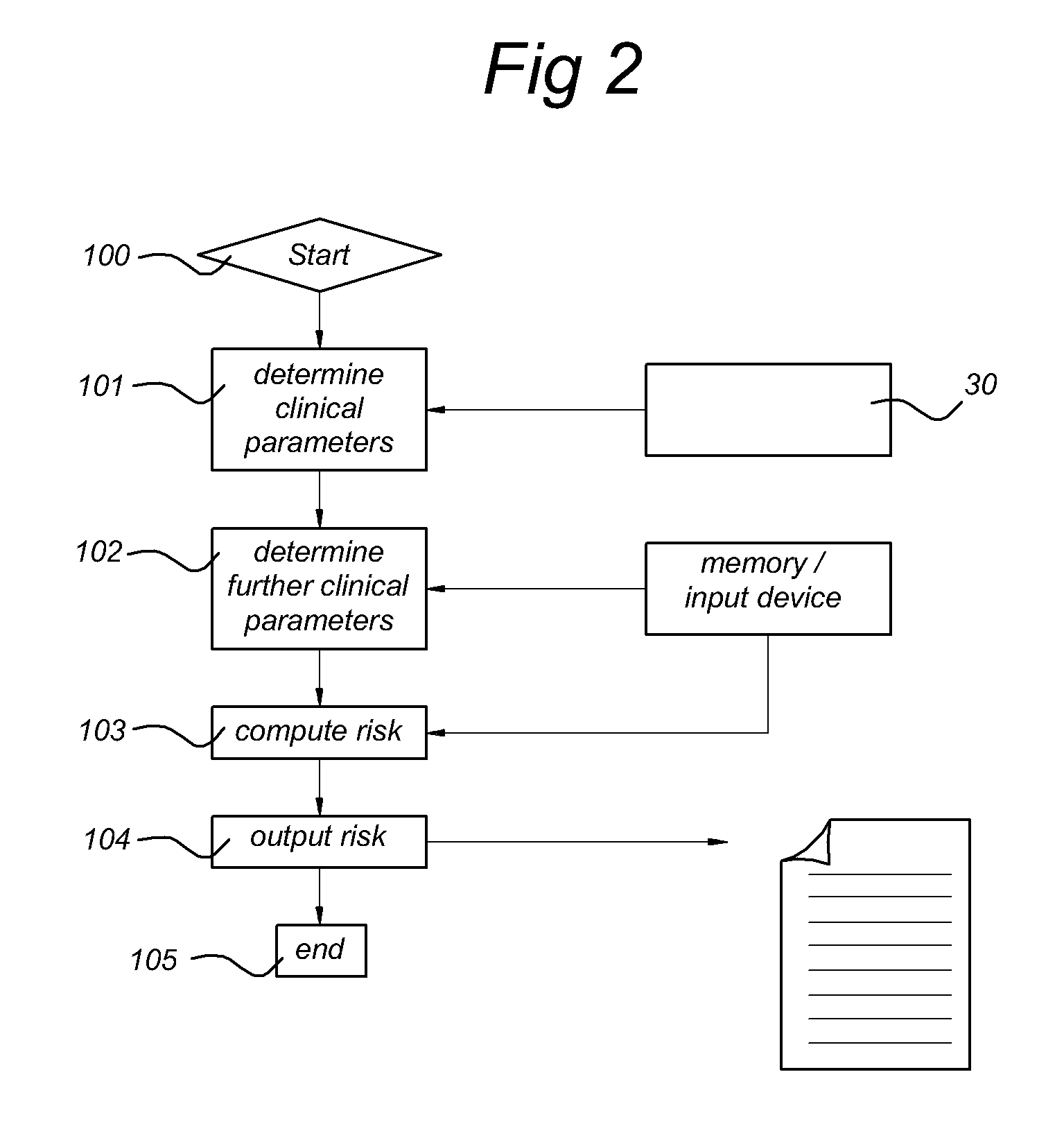
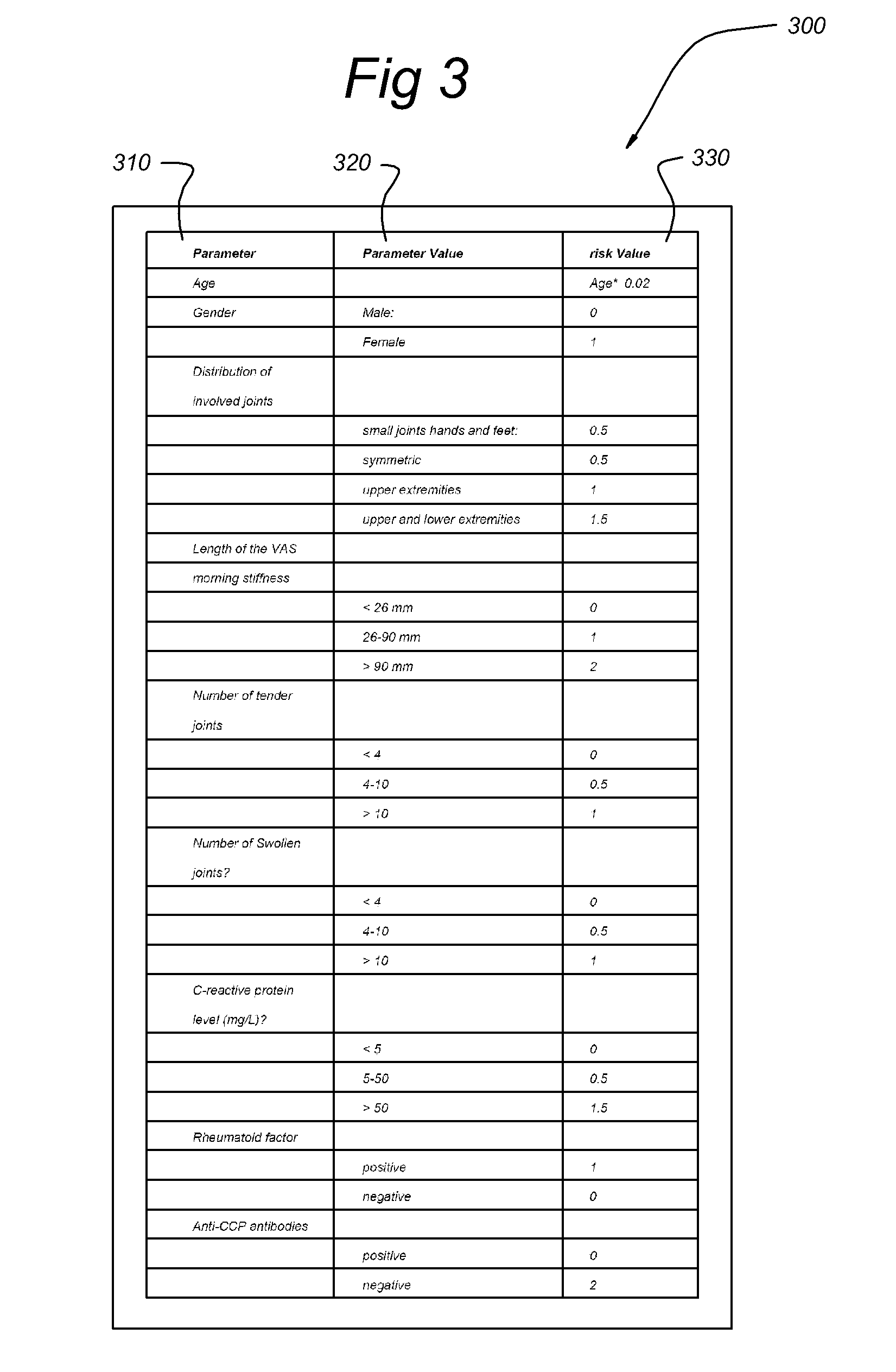
Systems and methods for predicting an individual's risk of developing rheumatoid arthritis
InactiveUS7702469B2Medical simulationHealth-index calculationAntiendomysial antibodiesUndifferentiated arthritis
Methods for predicting the likelihood of development of rheumatoid arthritis for individuals that present with recent-onset undifferentiated arthritis. The methods are based on the determination of a set of clinical parameter values and determining a predicted risk for developing rheumatoid arthritis by correlating the parameter values with predefined risk values associated with ranges of parameter values. Parameters values that are decisive for the risk for developing rheumatoid arthritis may include serum levels of C-reactive protein, Rheumatoid factors, anti-CCP antibodies, as well as age, gender, localization of the joint complaints, length of morning stiffness, and number of tender and / or swollen joints. The method may be performed by a computer. The invention further relates to a computer, a sample analyser and a computer program product for performing the method and a data carrier with the computer program product.
Owner:ACADEMISCH ZIEKENHUIS BIJ DE UNIV VAN AMSTERDAM ACADEMISCH MEDISCH CENT
A detection kit for rheumatoid factor detection through immunonephelometry, and a preparing method thereof
InactiveCN107356750AHigh precisionImprove accuracyDisease diagnosisAbsorption cellMonoclonal antibody
A detection kit for rheumatoid factor detection through immunonephelometry is disclosed. The kit includes a detection absorption cell, a detection absorption cell cover, a blank liquid, a calibrating substance and a quality control substance. A detection reagent R1 is sub-packaged in the detection absorption cell in advance. A detection reagent R2 is sub-packaged in the detection absorption cell cover in advance. The detection reagent R1 is a diluting liquid. The detection reagent R2 is a mixture of carboxylated polystyrene latex coupled to a rheumatoid factor monoclonal antibody and carboxylated polystyrene latex coupled to a rheumatoid factor polyclonal antibody in a mixing ratio of 3:2-5. The rheumatoid factor monoclonal and polyclonal antibodies and the carboxylated polystyrene latex are successfully coupled, and through this manner, the rheumatoid factor monoclonal and polyclonal antibodies are marked respectively, obtained large-grain-size latex and small-grain-size latex are mixed according to a specific ratio and then the mixture is used in match with the special-purpose diluting agent. Accuracy, precision, the detection speed and sensitivity of rheumatoid factor detection are increased.
Owner:江苏澳格姆生物科技有限公司
Kit for measuring procalcitonin
InactiveCN106018816AHigh detection sensitivityImprove detection accuracyBiological testingEmulsionRheumatism
The invention relates to the technical fields of medicine and biochemistry, in particular to a kit for measuring procalcitonin. The purpose of the present invention is to solve the problem of complex operation and low measurement accuracy in the detection process of fixed procalcitonin in the prior art. The double reagent used in the present invention firstly mixes the reagent R1 containing the anti-human rheumatism factor antibody with the sample , so that the rheumatoid factor antigen in the sample combines with the anti-human rheumatoid factor antibody in reagent R1 to form an antigen-antibody complex, so as to achieve the purpose of clearing the rheumatoid factor antigen; The reagent R2 is mixed with the above sample, and the procalcitonin antigen is specifically combined to form an insoluble polystyrene microsphere-antigen-polystyrene microsphere particle complex emulsion, which produces a certain turbidity, and at a certain wavelength The content of procalcitonin detected in the sample can be measured by performing turbidimetric determination. The invention has the advantages of high accuracy, no pollution, convenient operation and the like.
Owner:ANHUI IPROCOM BIOTECH CO LTD
Joint detection method for rheumatoid factors (IgM type) and other antigen-specific IgM antibodies
ActiveCN105974111AQuick checkLow priceDisease diagnosisBiological testingIgm antibodyPolyvinyl chloride
The invention provides a joint detection test strip for rheumatoid factors (IgM type) and other antigen-specific IgM antibodies. The test strip comprises a sample pad, a conjugate pad, a nitrocellulose membrane and an absorbent pad which are sequentially attached to a polyvinyl chloride rubber slab, wherein a first detection line, an adsorption line, one or more detection lines and a quality control line are sequentially and respectively coated on the nitrocellulose membrane; the first detection line is a denatured rat / human IgG; the adsorption line is an anti-human IgG antibody; the quality control line is human IgM; and the one or more detection lines are respectively selected from one or more specific antigens: a mycoplasma pneumoniae specific antigen, an influenza B specific antigen, a toxoplasma pathogen specific antigen, an autoimmune antigen and allergens. The invention also provides a kit comprising the test strip, a method for preparing the test strip, a detection method by using the test strip or kit, and application of the test strip or kit.
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
Kit for determining rheumatoid factors
InactiveCN105911285AHigh detection sensitivityEasy to detectDisease diagnosisColor/spectral properties measurementsPolyethylene glycolRheumatism
The invention relates to the technical field of medicine and biochemistry and particularly relates to a kit for determining rheumatoid factors, aiming at solving the problems in the prior art that a rheumatoid factor detection process is complicated to operate and the determination accuracy is low. Aiming at solving the technical problems above, the technical scheme provided by the invention is as follows: a kit for determining hyaluronic acid is provided, wherein the kit is composed of double liquid reagents R1 and R2, the reagent R1 is prepared from the following components: 30mmol / L-230mmol / L of a Tris buffering solution, 2.0mL / L-6.0mL / L of Tween-80, 5g / L-15g / L of polyethylene glycol-8000, 30mmol / L-50mmol / L of EDTA (Ethylene Diamine Tetraacetic Acid), 0.4g / L-1.0g / L of sodium azide and a solvent which is a purified water; the reagent R2 is prepared from the following components: 50mmol / L-150mmol / L of a Tris buffering solution, 5mmol / L-25mmol / L of glycerol, 0.4g / L-1.0g / L of sodium azide, 20g / L-60g / L of bovine serum albumin, 0.5%-4.0% of an emulsion-coated anti-human rheumatoid factor antibody and a solvent which is purified water. The kit for determining the rheumatoid factors has the advantages of high accuracy, convenience for operation and the like.
Owner:ANHUI IPROCOM BIOTECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com