Blood purification material for removing rheumatoid factors, and preparation method thereof
A technology for rheumatoid factor and blood purification, applied in the field of biomedicine, can solve the problems such as the ability of action is difficult to effectively reflect, and achieve the effects of high IgM adsorption selectivity, good stability and simple structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
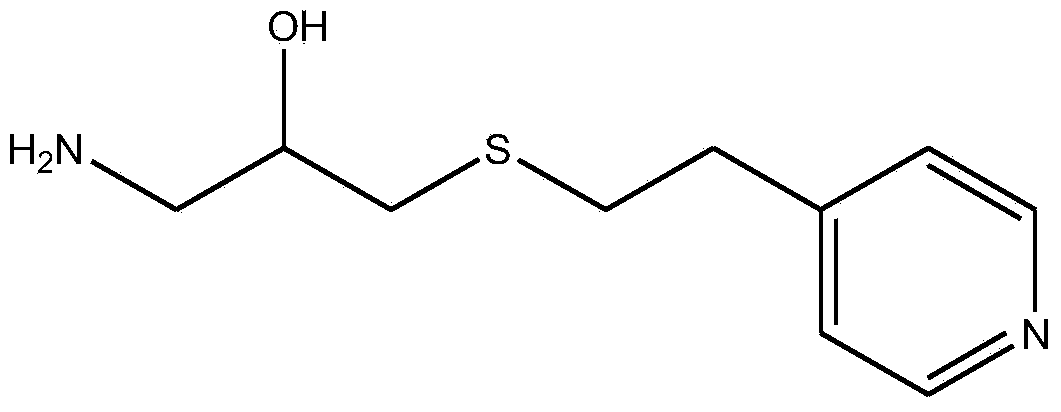
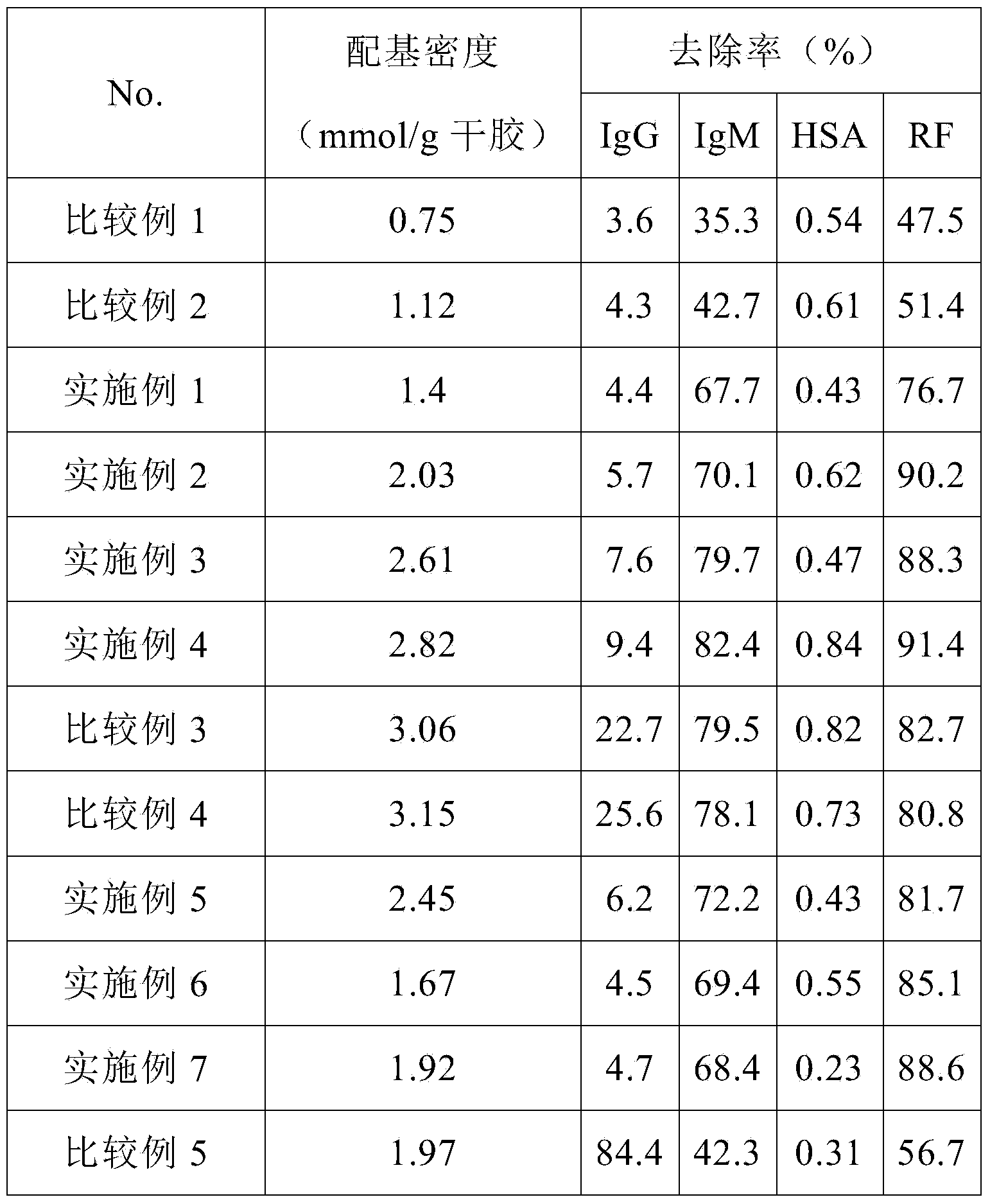
[0043]Example 1: Preparation of 1-amino-3-(2-(4-pyridyl)-ethylmercapto)-2-propanol cross-linked agarose blood purification material with a coupling density of 1.4 mmol / g dry gel ( activated by carbonyldiimidazole)
[0044] The specific steps are the same as in Example 1, the difference is that: the amount of CDI is increased to 0.9g / 10mL wet glue, and the activation reaction is carried out at 20°C for 2h; when the ligand is coupled, the reaction is carried out at 25°C for 2h; when the remaining imidazole activation group is hydrolyzed, NaOH solution at pH 10 was used. Elemental analysis showed that the ligand coupling density was 1.4mmol / g dry glue.
Embodiment 2
[0045] Example 2: Preparation of 1-amino-3-(2-(4-pyridyl)-ethylmercapto)-2-propanol cross-linked agarose blood purification material with a coupling density of 2.03 mmol / g dry gel ( activated by carbonyldiimidazole)
[0046] The specific steps are the same as in Example 1, the difference is that the amount of CDI is increased to 1.1g / 10mL wet glue, and the activation reaction is carried out at 25°C for 1h; when coupling ligands, 1-amino-3-(2-(4-pyridyl )-Ethylmercapto)-2-propanol is used in an amount of 10 times the volume, and the reaction is carried out at 25°C for 3 hours; when hydrolyzing the remaining imidazole activation groups, a NaOH solution with a pH of 10 is used. Elemental analysis showed that the ligand coupling density was 2.03mmol / g dry glue.
Embodiment 3
[0047] Example 3: Preparation of 1-amino-3-(2-(4-pyridyl)-ethylmercapto)-2-propanol cross-linked agarose blood purification material with a coupling density of 2.61 mmol / g dry gel ( activated by carbonyldiimidazole)
[0048] The specific steps are the same as in Example 1, the difference is that the amount of CDI is increased to 1.2g / 10mL wet glue, and the activation reaction is carried out at 25°C for 1h; when coupling ligands, 1-amino-3-(2-(4-pyridyl )-Ethylmercapto)-2-propanol is used in an amount of 10 times the volume, and the reaction is carried out at 25°C for 3 hours; when hydrolyzing the remaining imidazole activating groups, a NaOH solution with a pH of 13 is used. Elemental analysis showed that the ligand coupling density was 2.61mmol / g dry glue.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com