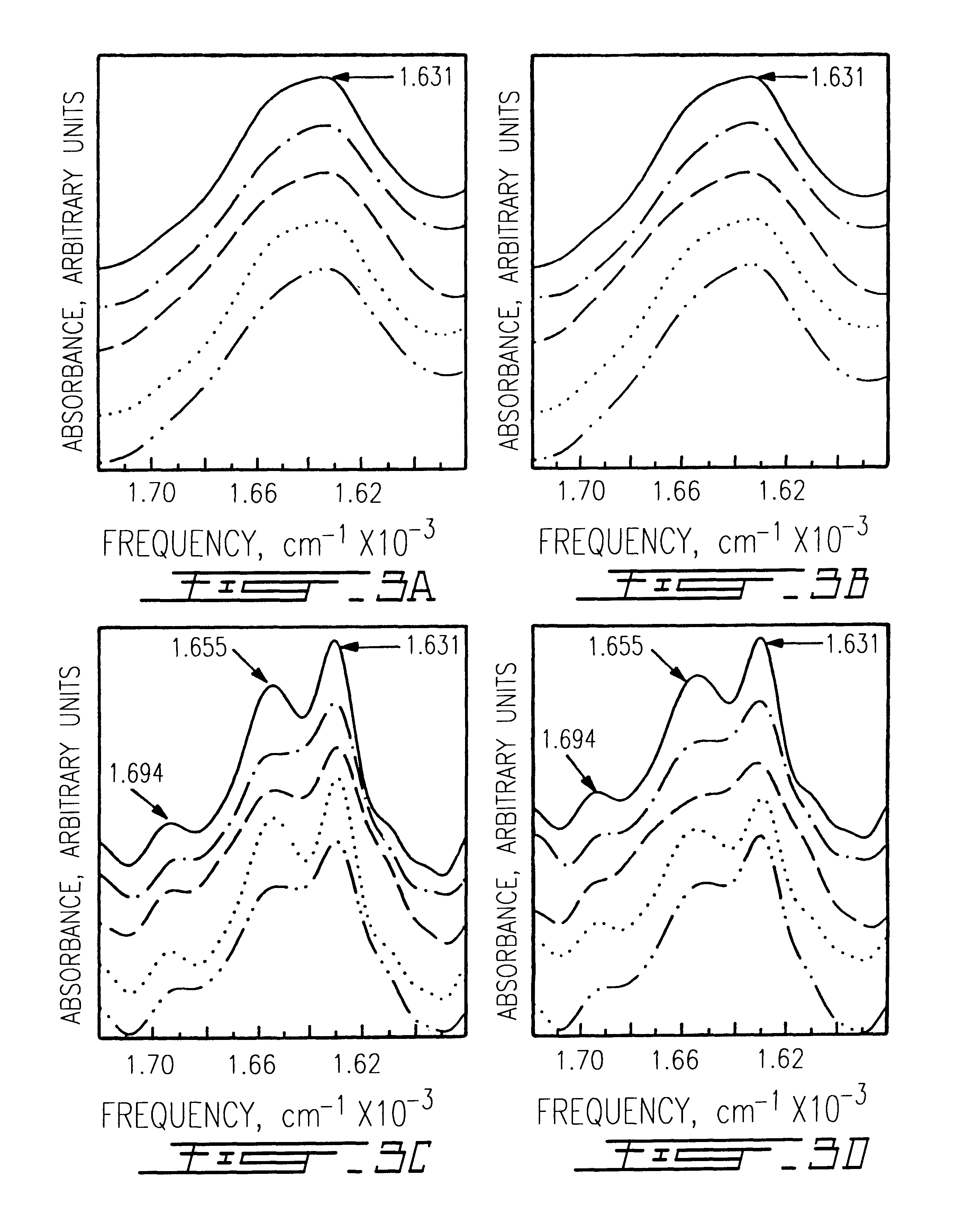
Patents
Literature
2690 results about "Physical chemical" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Physical Chemistry is the branch of chemistry dealing with the physical properties of chemical substances. That means describing and explaining how specific chemical substances look and behave in particular situations, e.g. under certain temperatures and pressures.
RFID device with changeable characteristics
ActiveUS20050012616A1Reduction in accessibilityChanging read characteristics of the deviceSolid-state devicesSubscribers indirect connectionCouplingEngineering
An RFID device includes a first, relatively permanent portion and a second alterable or inactivatable portion. Upon the occurrence of some predetermined event, the second portion and / or its coupling to the first portion is physically altered, inactivating it. The first portion may itself be an antennaless RFID device that may be read at short range, and the second portion may be an antenna that, when coupled to the first portion, substantially increases the range at which the first portion may be read. The second portion may be configured to be altered or inactivated by any of a variety of predetermined events, such as involving physical, chemical or electrical forces, performed either on the RFID device, or upon an object to which the RFID device is coupled.
Owner:AVERY DENNISON CORP
Apparatus and method for measuring biologic parameters
ActiveUS20070106172A1Precise positioningGood visual impactThermometer detailsUltrasonic/sonic/infrasonic diagnosticsInfraredVideo transmission
Support structures for positioning sensors on a physiologic tunnel for measuring physical, chemical and biological parameters of the body and to produce an action according to the measured value of the parameters. The support structure includes a sensor fitted on the support structures using a special geometry for acquiring continuous and undisturbed data on the physiology of the body. Signals are transmitted to a remote station by wireless transmission such as by electromagnetic waves, radio waves, infrared, sound and the like or by being reported locally by audio or visual transmission. The physical and chemical parameters include brain function, metabolic function, hydrodynamic function, hydration status, levels of chemical compounds in the blood, and the like. The support structure includes patches, clips, eyeglasses, head mounted gear and the like, containing passive or active sensors positioned at the end of the tunnel with sensing systems positioned on and accessing a physiologic tunnel.
Owner:BRAIN TUNNELGENIX TECH CORP
Boron-containing small molecules
ActiveUS20060234981A1Growth inhibitionAvoid infectionAntibacterial agentsBiocideFungal microorganismsTopical treatment
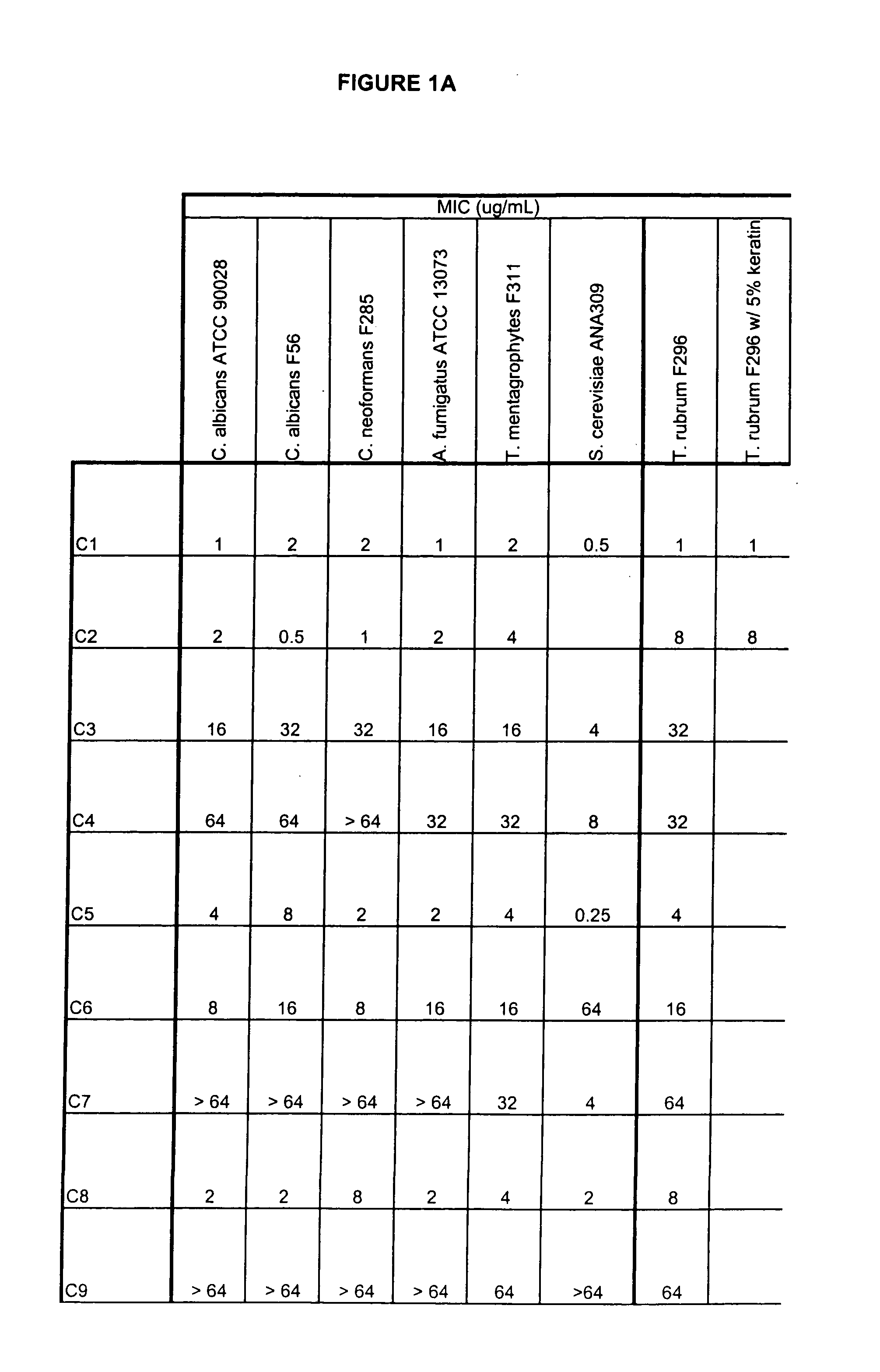
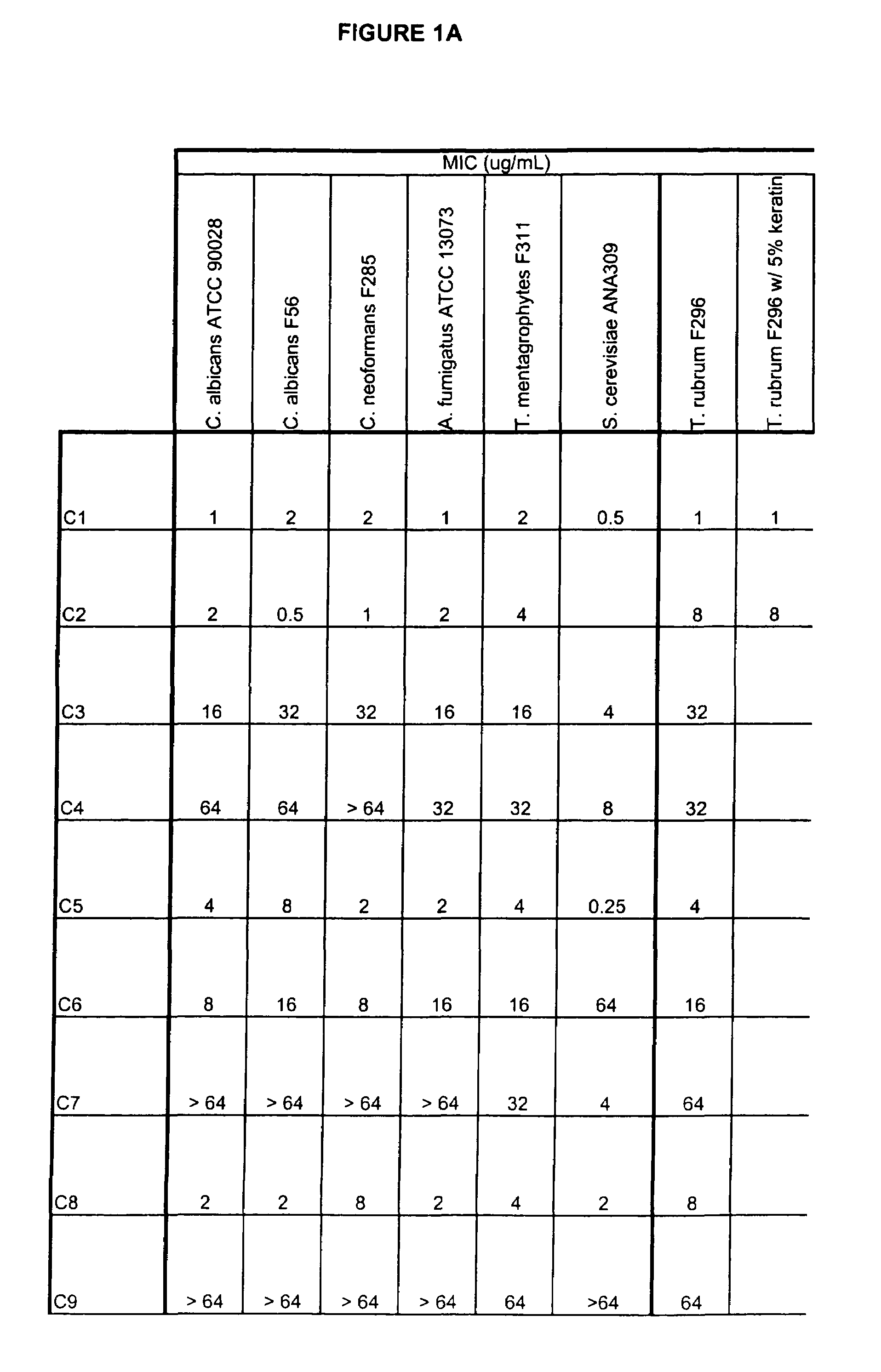
This invention relates to compounds useful for treating fungal infections, more specifically topical treatment of onychomycosis and / or cutaneous fungal infections. This invention is directed to compounds that are active against fungi and have properties that allow the compound, when placed in contact with a patient, to reach the particular part of the skin, nail, hair, claw or hoof infected by the fungus. In particular the present compounds have physiochemical properties that facilitate penetration of the nail plate.
Owner:ANACOR PHARMA INC
Modified Antibody Constant Region
ActiveUS20100298542A1Improving immunogenicityImprove propertiesAntipyreticAnalgesicsHigh concentrationHinge region
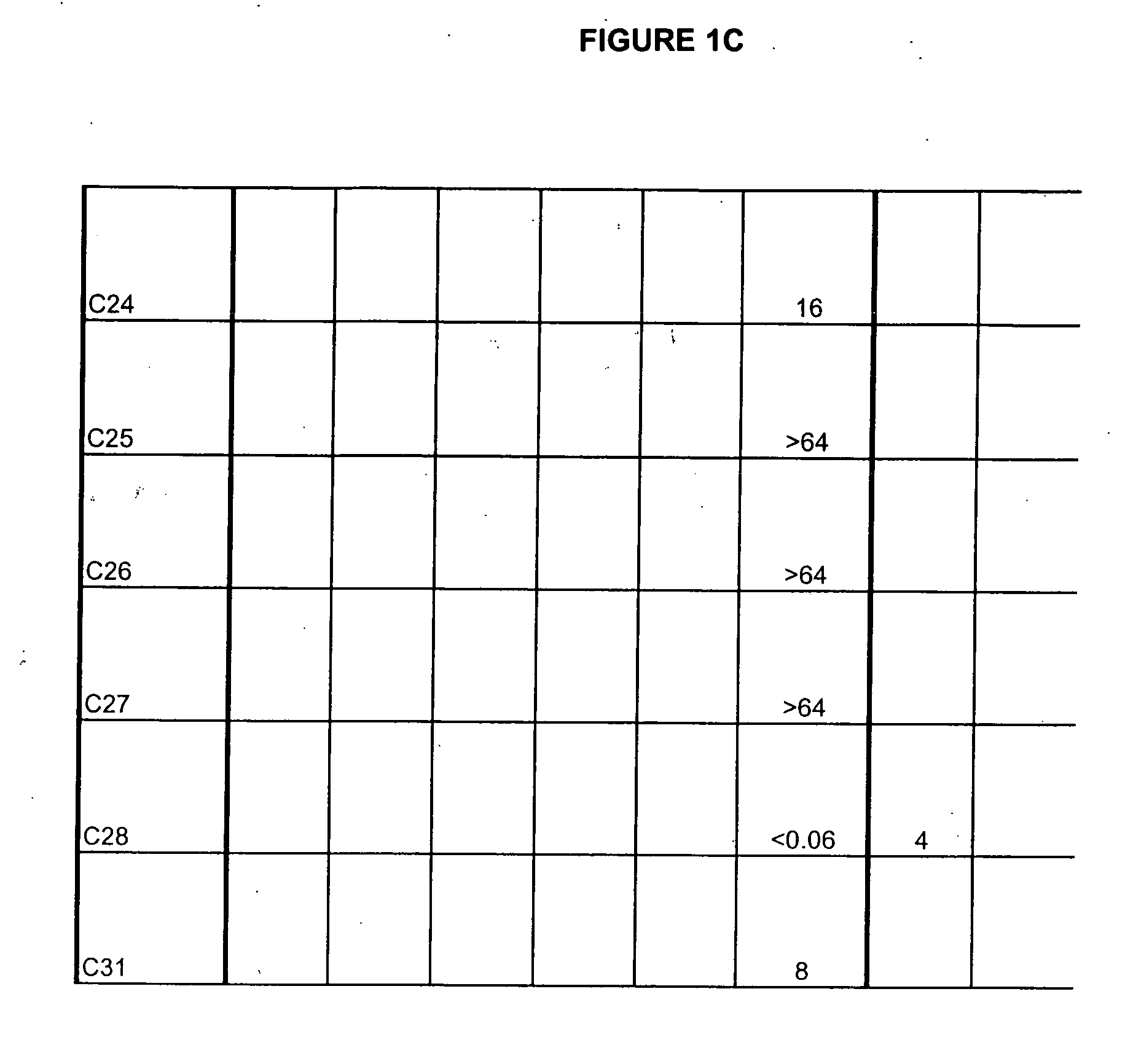
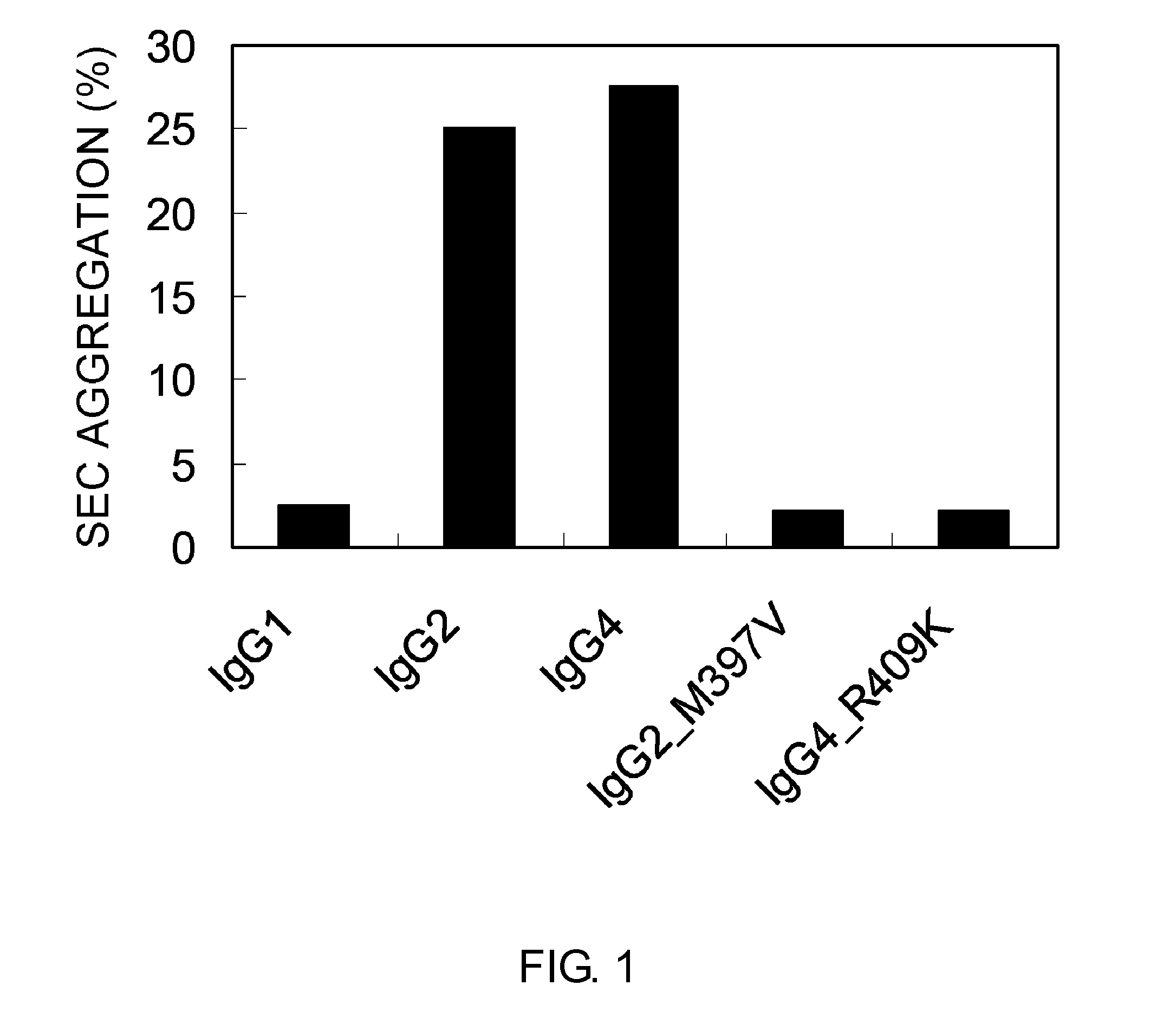
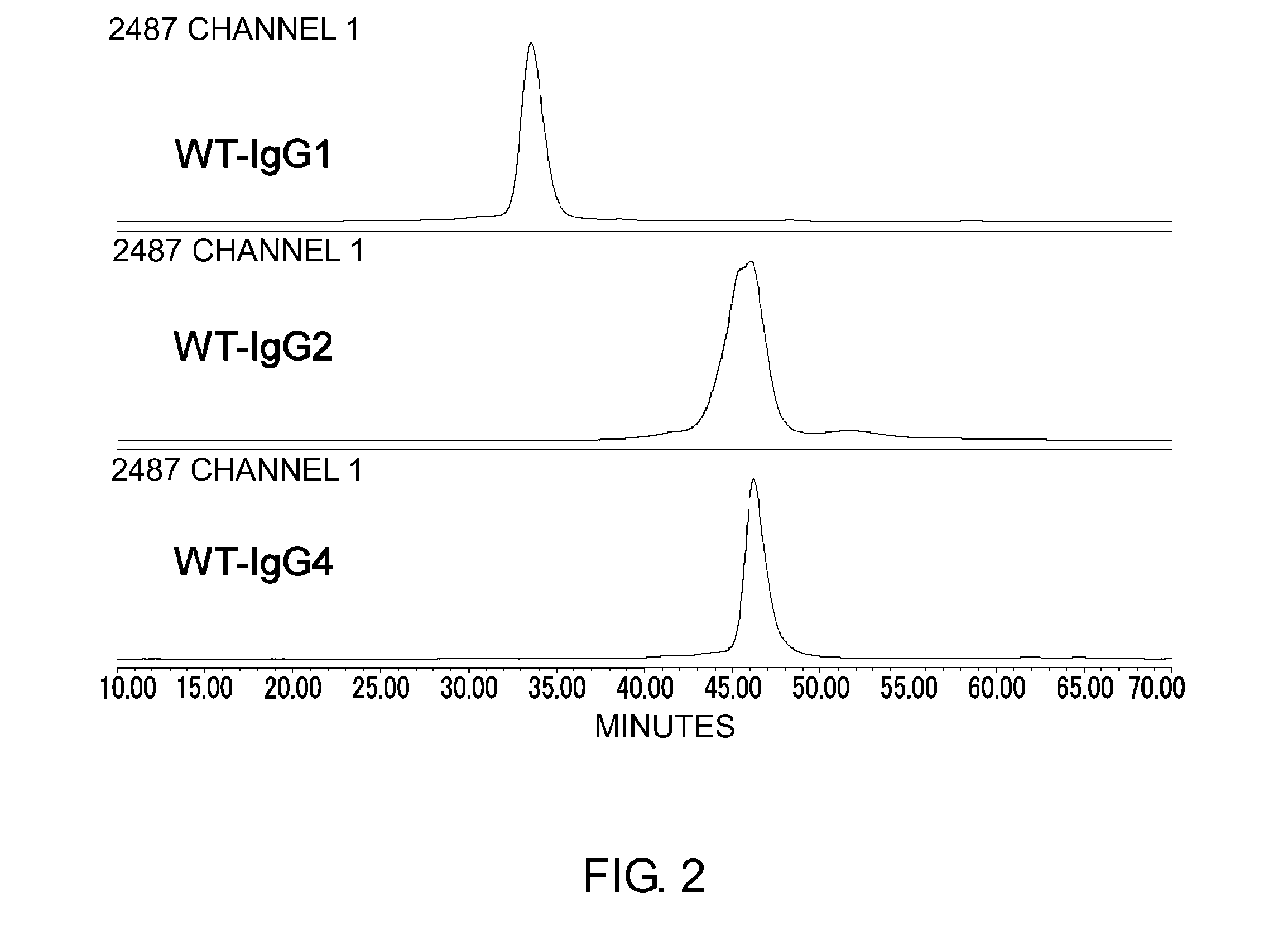
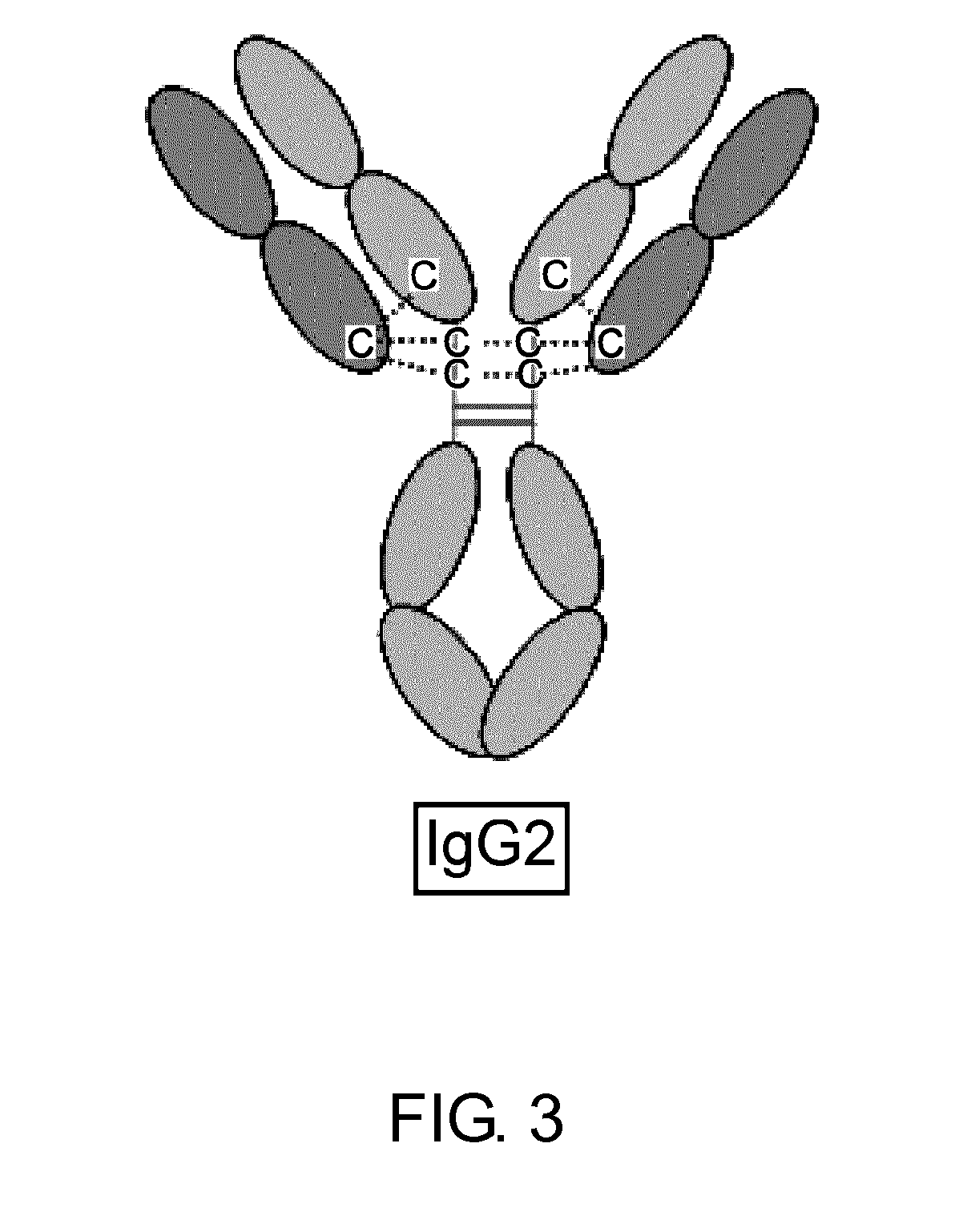
The present inventors succeeded in improving the antibody constant region to have increased stability under acid conditions, reduced heterogeneity originated from disulfide bonds in the hinge region, reduced heterogeneity originated from the H chain C terminus, and increased stability at high concentrations as well as in discovering novel constant region sequences having reduced Fcγ receptor-binding, while minimizing the generation of novel T-cell epitope peptides. As a result, the present inventors successfully discovered antibody constant regions with improved physicochemical properties (stability and homogeneity), immunogenicity, safety, and pharmacokinetics.
Owner:CHUGAI PHARMA CO LTD
Particles with modified physicochemical properties, their preparation and uses
InactiveUS6197349B1Easy to handleImprove bioavailabilityPowder deliveryOrganic active ingredientsActive agentDelivery system
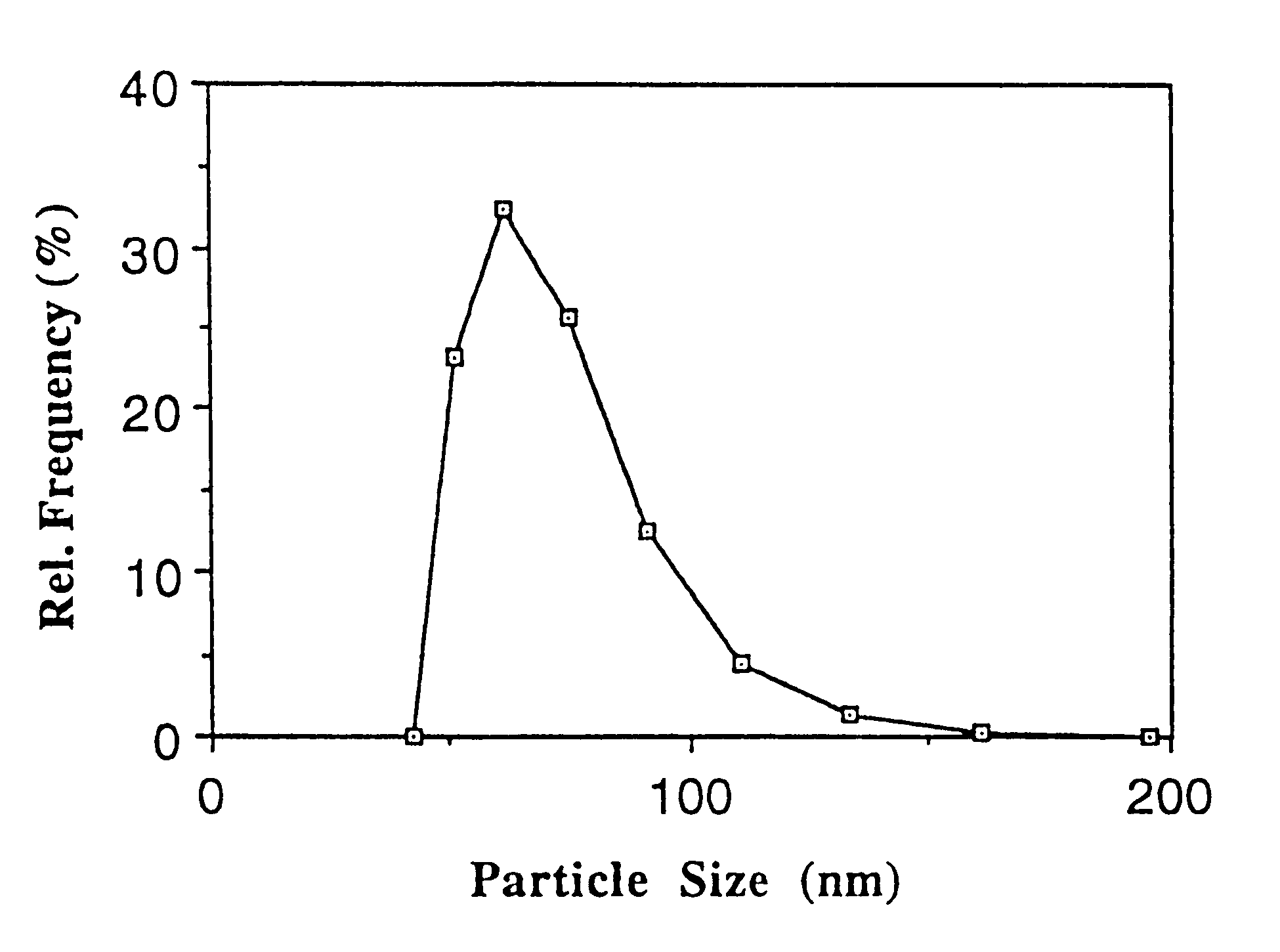
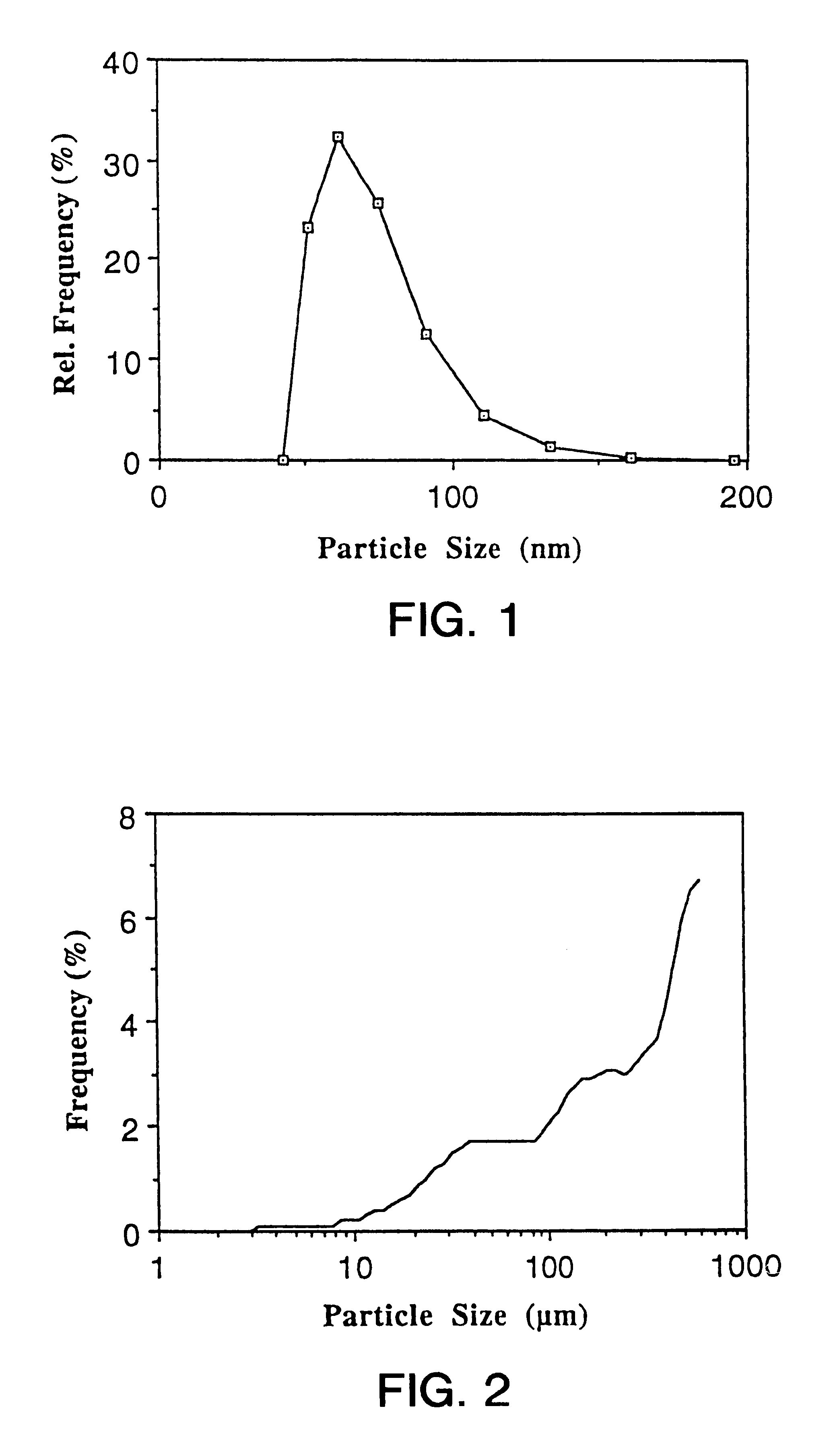
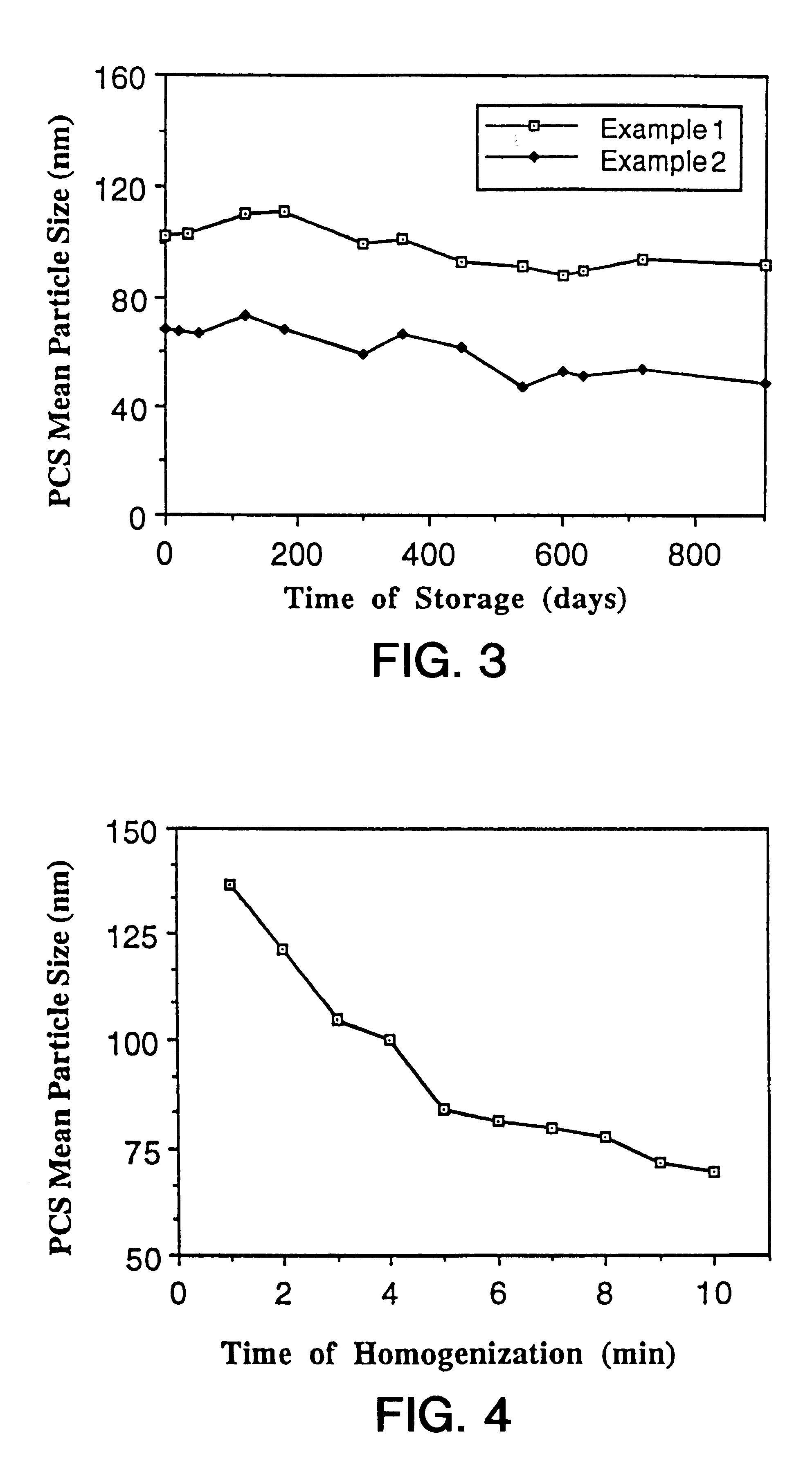
Particles comprising(a) a supercooled melt of a poorly water-soluble substance and(b) a stabilizing agent,which have a mean particle size of between 30 and 500 nm, and disperse compositions containing them, as administration forms and delivery systems for drugs, vaccines and other biologically active agents such as herbicides, pesticides, insecticides, fungicides, fertilizers, vitamins, nutrition additives and cosmetics.
Owner:MSE PHARMA
Boron-containing small molecules
This invention relates to compounds useful for treating fungal infections, more specifically topical treatment of onychomycosis and / or cutaneous fungal infections. This invention is directed to compounds that are active against fungi and have properties that allow the compound, when placed in contact with a patient, to reach the particular part of the skin, nail, hair, claw or hoof infected by the fungus. In particular the present compounds have physiochemical properties that facilitate penetration of the nail plate.
Owner:ANACOR PHARMA LLC
Compositions comprising lipophilic active compounds and method for their preparation
ActiveUS20090098200A1Quick releaseImprove bioavailabilityPowder deliveryBiocideHydrophilic polymersCompound (substance)
Compositions are provided comprising a lipophilic active compound, e.g., a human or veterinary drug or a nutraceutical, interwoven with a polymeric matrix formed by two or more polymers, wherein one of the polymers is an amphiphilic polymer and the other polymer is either an amphiphilic polymer with a different hydrophobic-hydrophilic balance or a hydrophilic polymer, and the active lipophilic compound has modified physicochemical properties. The composition forms colloidal nanodispersion upon contact with aqueous media.
Owner:SOLUBEST
Solid lipid particles, particles of bioactive agents and methods for the manufacture and use thereof
InactiveUS6207178B1Suppresses decrease in specific surface areaImprove bioavailabilityBiocideCosmetic preparationsLipid formationLipid particle
The present invention is in the area of administration forms and delivery systems for drugs, vaccines and other biologically active agents. More specifically the invention is related to the preparation of suspensions of colloidal solid lipid particles (SLPs) of predominantly anisometrical shape with the lipid matrix being in a stable polymorphic modification and of suspensions of micron and submicron particles of bioactive agents (PBAs); as well as to the use of such suspensions or the lyophilizates thereof as delivery systems primarily for the parenteral administration of preferably poorly water-soluble bioactive substances, particularly drugs, and to their use in cosmetic, food and agricultural products. SLPs and PBAs are prepared by the following emulsification process: (1) A solid lipid or bioactive agent or a mixture of solid lipids or bioactive agents is melted. (2) Stabilizers are added either to the lipid or bioactive agent and to the aqueous phase or to the aqueous phase only depending on their physicochemical characteristics. Stabilizers may also be added or exchanged after homogenization. (3) Drugs or other bioactive substances to be incorporated into the SLPs may be melted together with the lipids if the physicochemical characteristics of the substance permit or may be dissolved, solubilized or dispersed in the lipid melt before homogenization. (4) The aqueous phase is heated to the temperature of the melt before mixing and may contain for example stabilizers, isotonicity agents, buffering substances, cryoprotectants and / or preservatives. (5) The molten lipid compounds and the bioactive agents are emulsified in an aqueous phase preferably by high-pressure homogenization.
Owner:PHARMACIA AB
Boron-containing small molecules
This invention relates to compounds useful for treating fungal infections, more specifically topical treatment of onychomycosis and / or cutaneous fungal infections. This invention is directed to compounds that are active against fungi and have properties that allow the compound, when placed in contact with a patient, to reach the particular part of the skin, nail, hair, claw or hoof infected by the fungus. In particular the present compounds have physiochemical properties that facilitate penetration of the nail plate.
Owner:ANACOR PHARMA INC
Coating process for microfluidic sample arrays
InactiveUS20060105453A1Minimizes average amount of errorSerious errorSequential/parallel process reactionsLaboratory glasswaresEngineeringFixed position
A differentially coated device for conducting a plurality of nano-volume specified reactions, the device comprising a platen having at least one exterior surface modified to a specified physicochemical property, a plurality of nano-volume channels, each nano-volume channel having at least one interior surface in communication with the at least one exterior surface that is selectively coated with an optionally dissolvable coating agent physisorbed to at least one interior surface, wherein the optionally dissolvable coating agent comprises a coating agent and a first component for the plurality of specified reactions. Methods for preparing and using such devices are also provided, as well as a method of registering a location of a dispenser array in relation to a microfluidic array. A first one of the dispenser array and the microfluidic array is movable in relation to the frame, and the other of the first one of the dispenser array and the microfluidic array is fixed relative to the frame. Quantities related to a vector displacement from the alignment position to a fixed position on the one of the dispenser array and the microfluidic array is determined. The quantities thus determined are used to guide positioning of the dispenser array relative to the microfluidic array.
Owner:LIFE TECH CORP
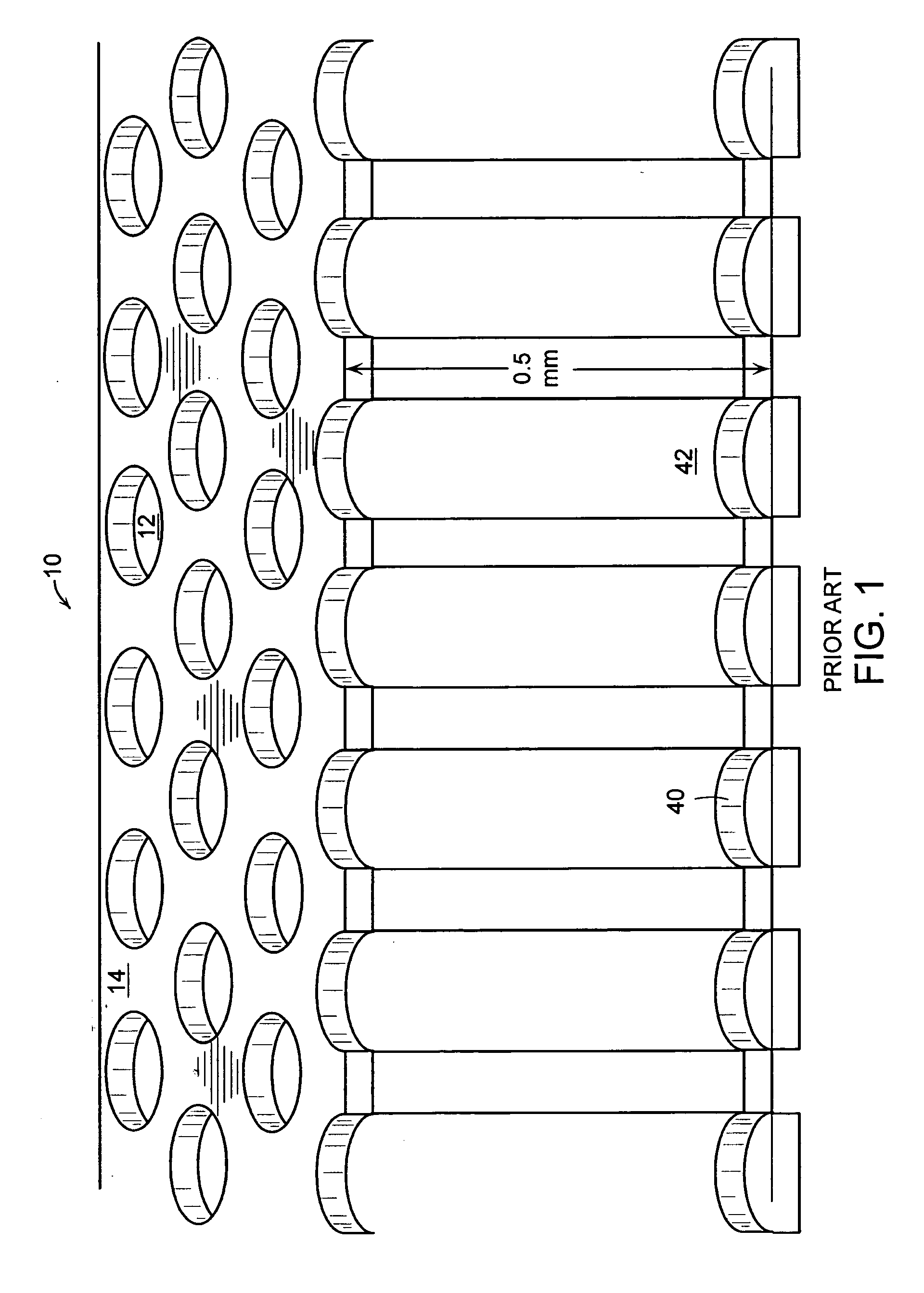
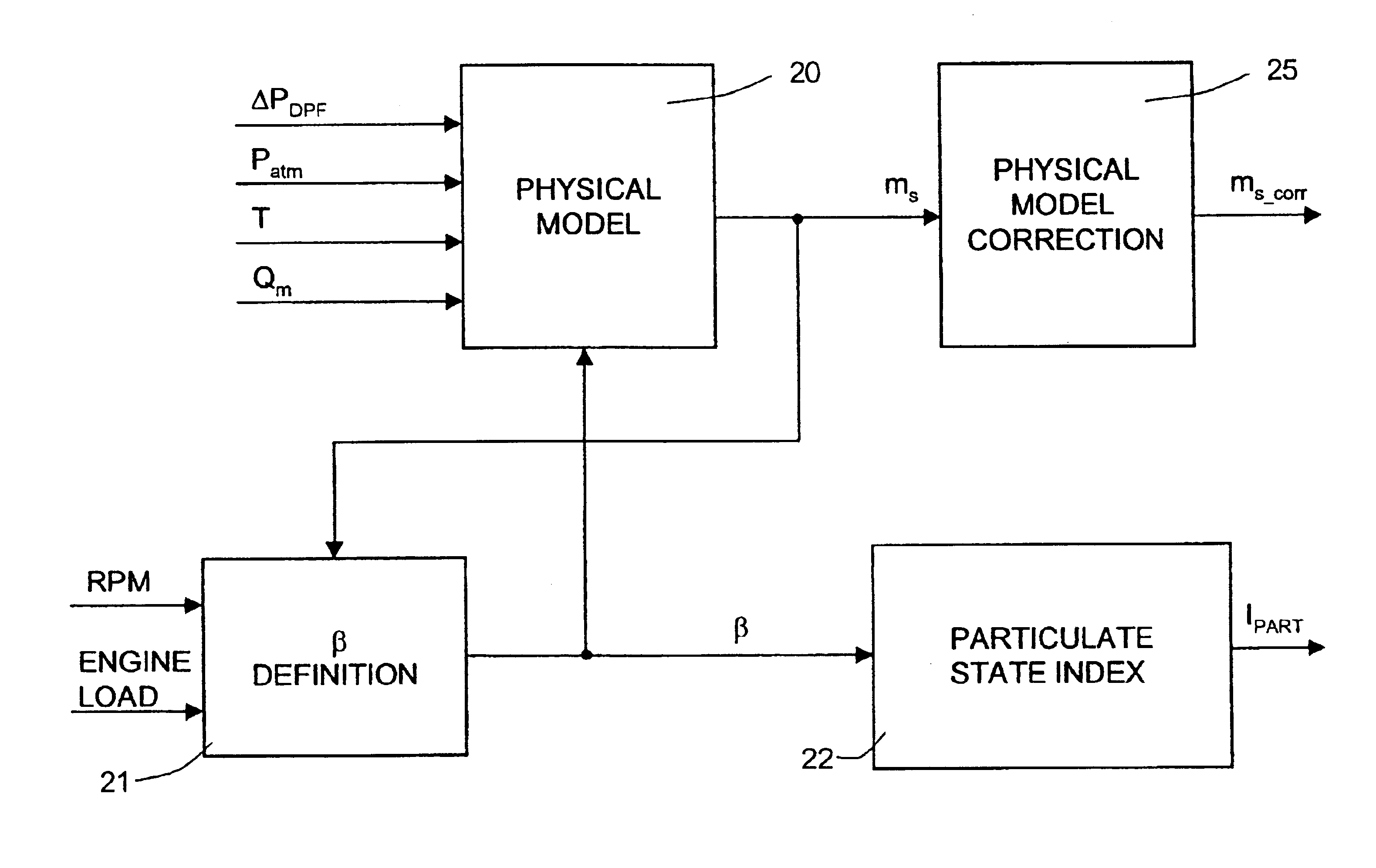
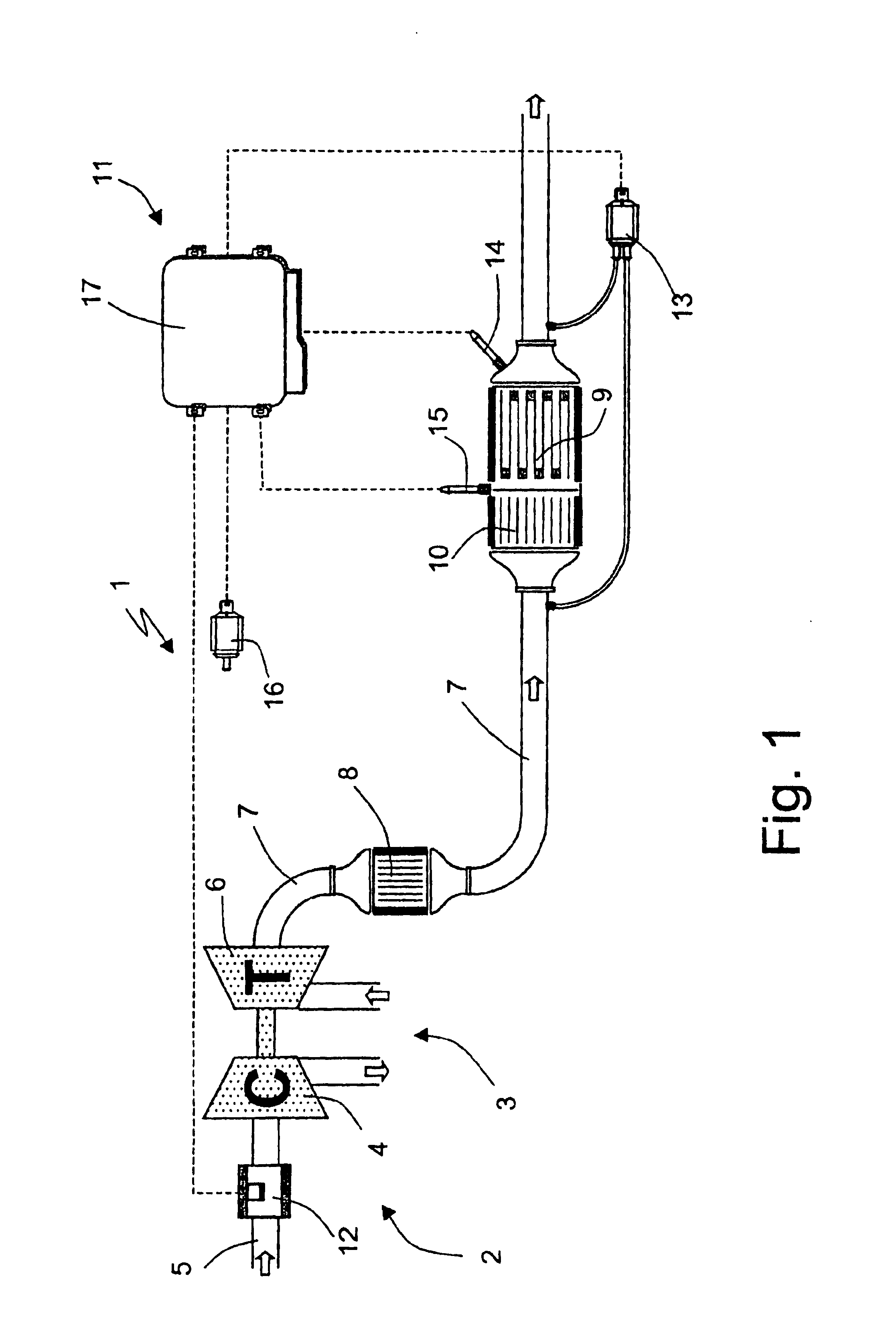
Method of determining the amount of particulate accumulated in a particulate filter
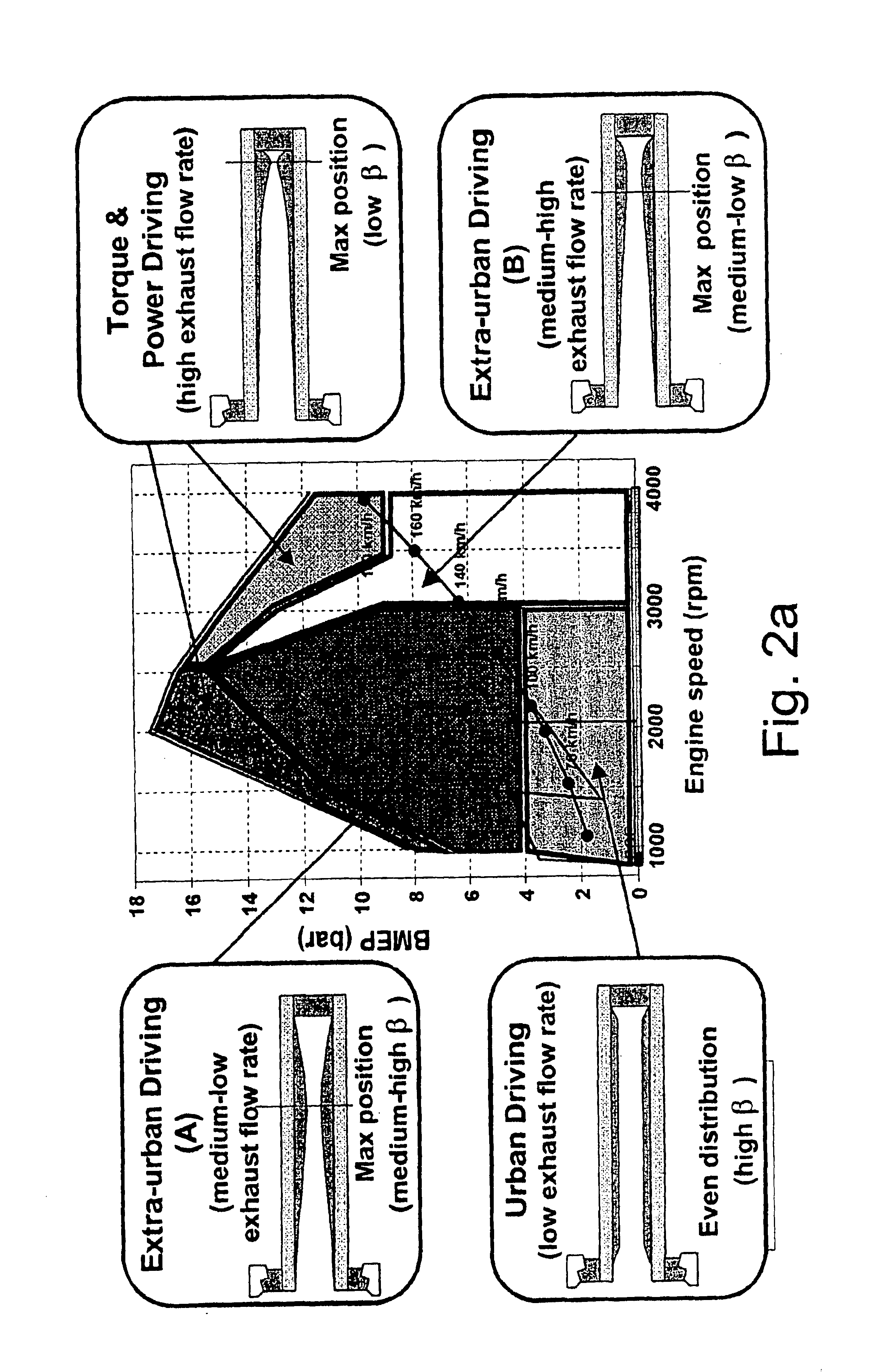
InactiveUS6941750B2Drawback can be obviatedElectrical controlInternal combustion piston enginesParticulatesEngineering
A method of determining the amount of particulate accumulated in a particulate filter, is based on determining possible variations in the spatial distribution and / or physical-chemical properties of the particulate as a function of engine operating conditions and past particulate accumulation in the particulate filter. A number of reference values defining a relationship between the amount of particulate accumulated in the particulate filter and the pressure drop across the particulate filter are mapped, each of the reference values relating to a respective steady engine operating condition in which particulate is accumulated in the particulate filter. In a given engine operating condition, an operating value of the parameter is then determined as a function of the reference value of the parameter relative to the same steady engine operating condition, and of past particulate accumulation in the particulate filter.
Owner:CENT RICERCHE FIAT SCPA
Sprayable compositions comprising a combination of pharmaceutical actives and an oily phase
InactiveUS20050281749A1Good acceptability and toleranceChemically stableBiocideAerosol deliveryDermatological disordersOil phase
Sprayable, anhydrous and physically / chemically stable dermatological / pharmaceutical compositions, well suited for the treatment of a variety of dermatological disorders, notably psoriasis, contain: a) a therapeutically effective amount of a solubilized corticoid, notably dissolved clobetasol propionate; b) a therapeutically effective amount of a solubilized vitamin D derivative, notably dissolved calcitriol; and c) an oily phase which comprises one or more oils; formulated into d), a sprayable and topically applicable, dermatologically / pharmaceutically acceptable vehicle therefor.
Owner:GALDERMA SA
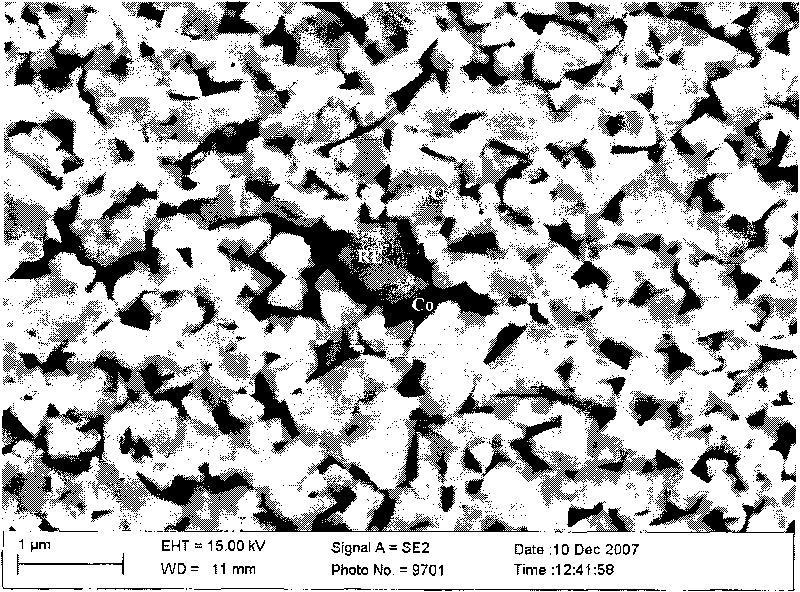
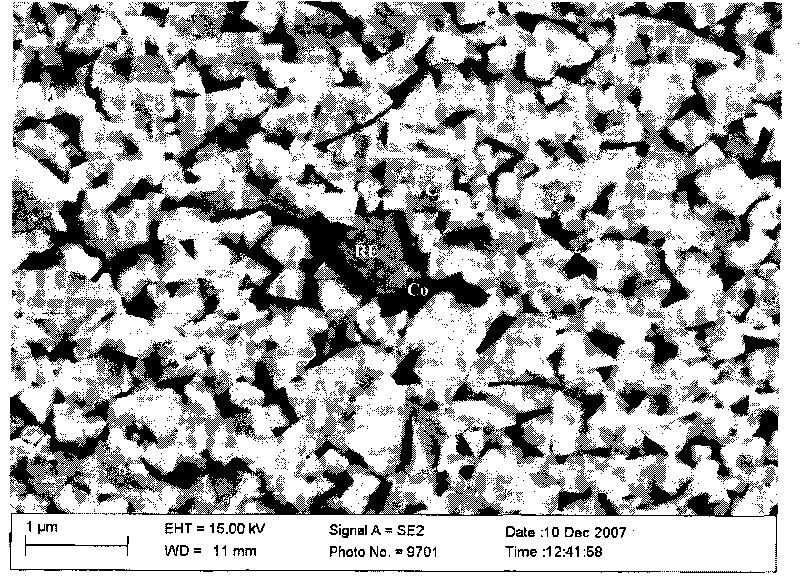
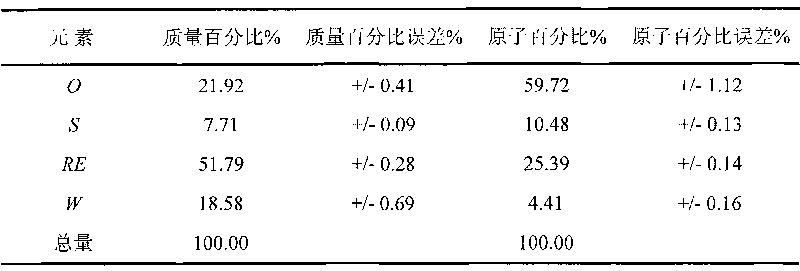
Superfine WC-Co cemented carbide containing rare-earth elements and preparation method thereof
ActiveCN101760685AChange processSteady improvement in overall performanceRare-earth elementChemical reaction
The invention discloses a superfine WC-Co cemented carbide containing rare-earth elements and a preparation method thereof, belonging to the technical field of cemented carbide. In the cemented carbide, the weight of WC rigid phase accounts for 85-94% of that of the cemented carbide, the weight of Co binder phase accounts for 5-14% of that of the cemented carbide, the weight of grain growth inhibitor accounts for 0.3-2.0% of that of the cemented carbide, and the weight of the thulium in the rare earth addition accounts for 0.2-1.2% of that of the Co binder phase. The method comprises the following steps: weighing various powder stocks, ball-milling, drying and pelletizing to form a compound; and suppressing and shaping the compound, sintering and cooling to obtain the cemented carbide. The adding mode of nano rare earth oxide or Co-RE composite powder can be implemented easily and conveniently, and the rare earth is diffusely and evenly distributed, thereby facilitating to perform physo-chemical reactions; and the cemented carbide has the advantages of low production cost, stable and enhanced performance and easy implementation, production and application.
Owner:GRIMAT ENG INST CO LTD
Method for water filtration
InactiveUS20090045135A1Great hydraulic resistancePromote circulationTreatment using aerobic processesTreatment involving filtrationNatural sourceSuspended particles
This is a method of filtration of a liquid comprising steps of sequential filtration of said liquid through at least one deep bed medium producing at least one first filtrate followed by at least one membrane medium filtration producing at least one second filtrate, wherein said membrane medium is at least periodically within said deep bed media Many types of deep bed and membrane media can be used. The domain of using contact clarification (direct filtration) can be expanded towards greater solids concentration. Operation and backwash, is simplified, continuous filtration becomes possible. Water can be water from natural source water, process water, wastewater, aqueous or non-aqueous suspensions, emulsions, solutions. Treatment can include mechanical interception of suspended particles, chemical, physical chemical, electrochemical, and biological processes. In water and wastewater processing, control over suspended solids, BOD, COD, nitrogen and phosphorus compounds, bacteria and viruses, heavy metals, color, and other constituents can be dramatically improved as compared to conventional processes. The method can be accommodated in new and modified existing treatment systems.
Owner:KHUDENKO ENG
Method for determining a treatment parameter on a haemofiltration device, and haemofiltration device for applying the method
ActiveUS6939471B2Change effectSolvent extractionSettling tanks feed/dischargeBlood filteringBlood filters
A method for determining a treatment parameter on a haemofiltration device, as well as a haemofiltration device for applying the method, based on the recognition that a determination method known from haemodialysis can, with little modification, be transferred to haemofiltration. To this effect a physical-chemical characteristic of the substitution fluid and of the fluid which is removed from the haemofilter by way of an ultrafiltrate outlet pipe, is determined. Furthermore, this characteristic is acquired before, after or during a change of this characteristic in the substitution fluid, and is evaluated. As a result, the ion dialysance and thus the urea clearance of a haemofiltration device, which can also be operated as a haemodiafiltration device, can be determined.
Owner:FRESENIUS MEDICAL CARE DEUTSCHLAND GMBH
Prion-free collagen and collagen-derived products and implants for multiple biomedical applications; methods of making thereof
InactiveUS6197935B1Preserve integrityPeptide/protein ingredientsImmunoglobulinsIn vivo biocompatibilityCollagen VI
The use of collagen as a biomedical implant raises safety issues towards viruses and prions. The physicochemical changes and the in vitro and in vivo biocompatibility of collagen treated with heat, and by formic acid (FA), trifluoroacetic acid (TFA), tetrafluoroethanol (TFE) and hexafluoroiso-propanol (HFIP) were investigated. FA and TFA resulted in extensive depurination of nucleic acids while HFIP and TFE did so to a lesser degree. The molecules of FA, and most importantly of TFA, remained within collagen. Although these two acids induced modification in the secondary structure of collagen, resistance to collagenase was not affected and, in vitro, cell growth was not impaired. Severe dehydrothermal treatment, for example 110° C. for 1-3 days under high vacuum, also succeeded in removing completely nucleic acids. Since this treatment also leads to slight cross-linking, it could be advantageously used to eliminate prion and to stabilize gelatin products. Finally, prolonged treatment with TFA provides a transparent collagen, which transparency is further enhanced by adding glycosaminoglycans or proteoglycans, particularly hyaluronic acid. All the above treatments could offer a safe and biocompatible collagen-derived material for diverse biomedical uses, by providing a virus or prion-free product.
Owner:UNIV LAVAL
Deposition of calcium-phosphate (CaP) and calcium-phosphate with bone morphogenic protein (CaP+BMP) coatings on metallic and polymeric surfaces
InactiveUS20080097618A1Reduce the amount of solutionHigh densityImpression capsBone implantPolymeric surfaceSolubility
The invention is a medical implantable device which is coated by the method according to the invention. The surface of the substrate used for the implantable device, in the raw condition, following a cleaning regime and physiochemical pretreatments, is coated using a biomimetic process in a supersaturated calcium phosphate solution (SCPS) to obtain the desired coating coverage and morphology maintaining a ratio of calcium to phosphorus pH, as well as solution temperature plays a major role in yielding precipitation of the proper phase of CaP so that composition, morphologies, crystal structures, and solubility characteristics are optimal for the deposition process. The biomimetic coating adds the attribute of osteoconductivity to the implant device. To maximize bone growth, the implant must also induce bone growth, or possess the attribute of osteoinductivity. This attribute is acquired by the use of therapeutic agents, i.e. bone morphogenic proteins (BMP), growth factors, stem cells, etc. The preparation of the SCPS solution is slightly altered so that during the immersion of the implant in the SCPS, the therapeutic agents are co-precipitated and bonded with the CaP directly on the underlying surface of the implant device. A final dipping process into a BMP solution provides an initial burst of cellular activity. For delivering stem and / or progenitor cell, after drying the dipped solution of BMP, the cells are cultured on the surface of the implant.
Owner:HERKOWITZ HARRY N
Method of quantifying hydrocarbon formation and retention in a mother rock
InactiveUS20080059140A1Analogue computers for chemical processesSeismologyHydrocotyle bowlesioidesSedimentary basin
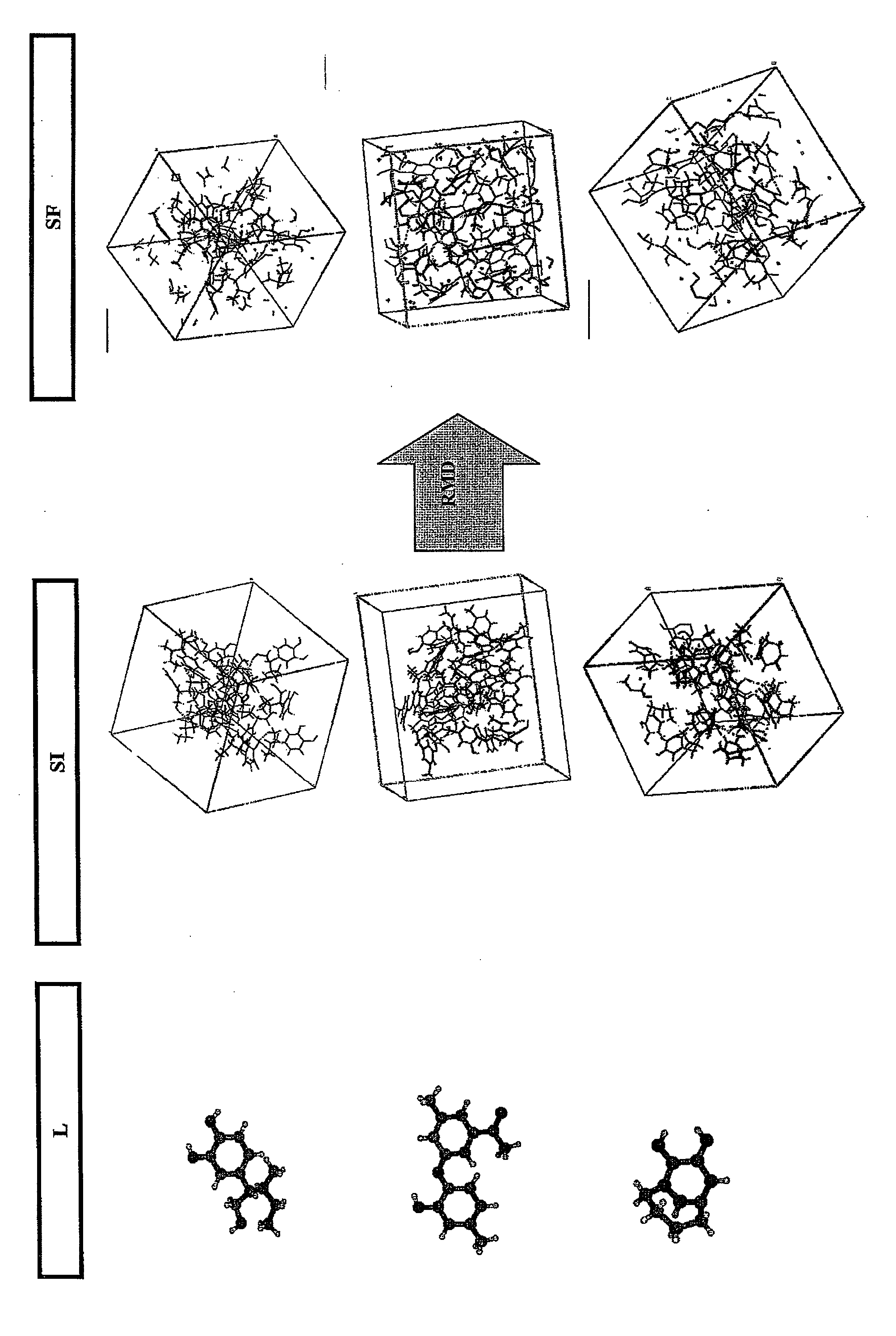
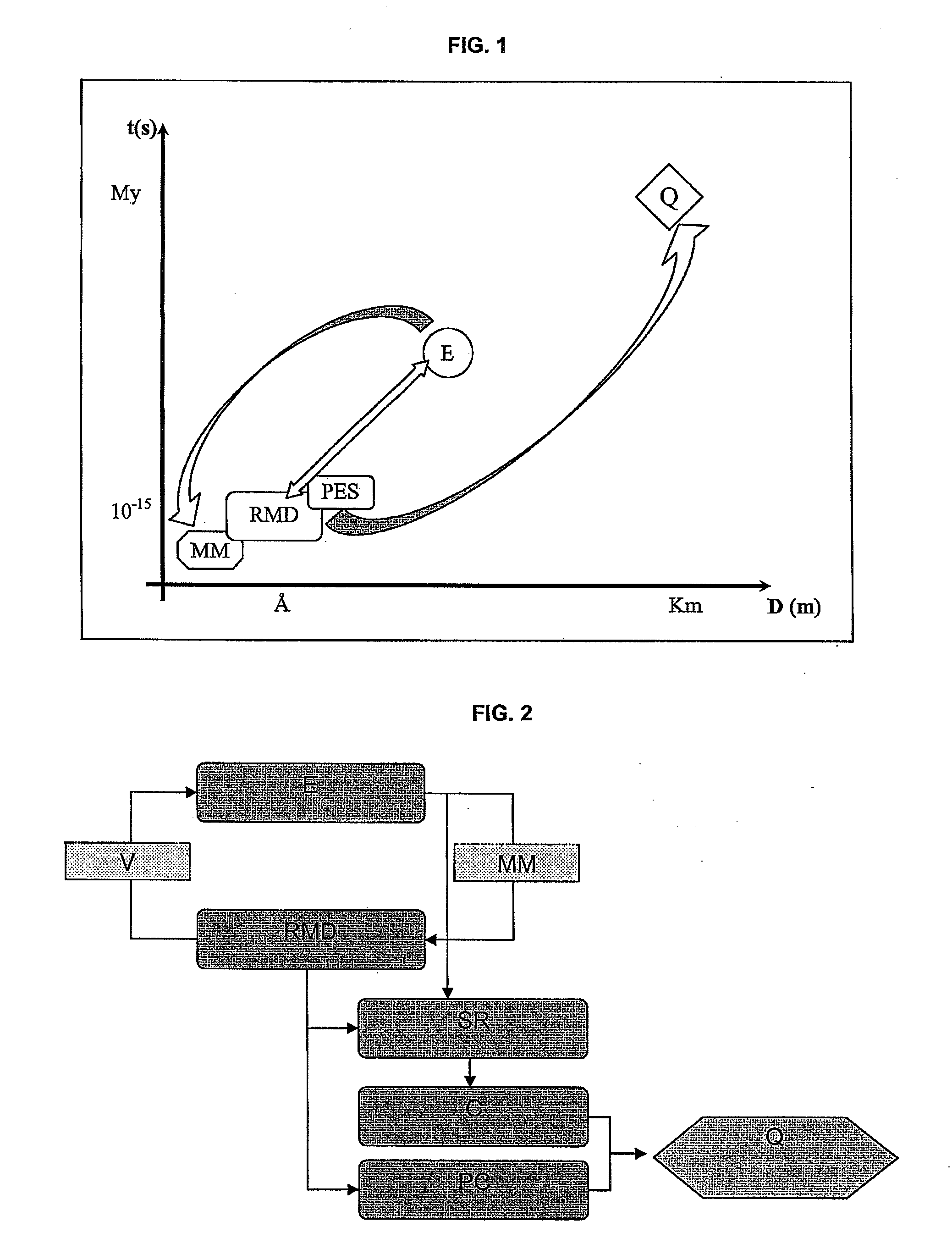
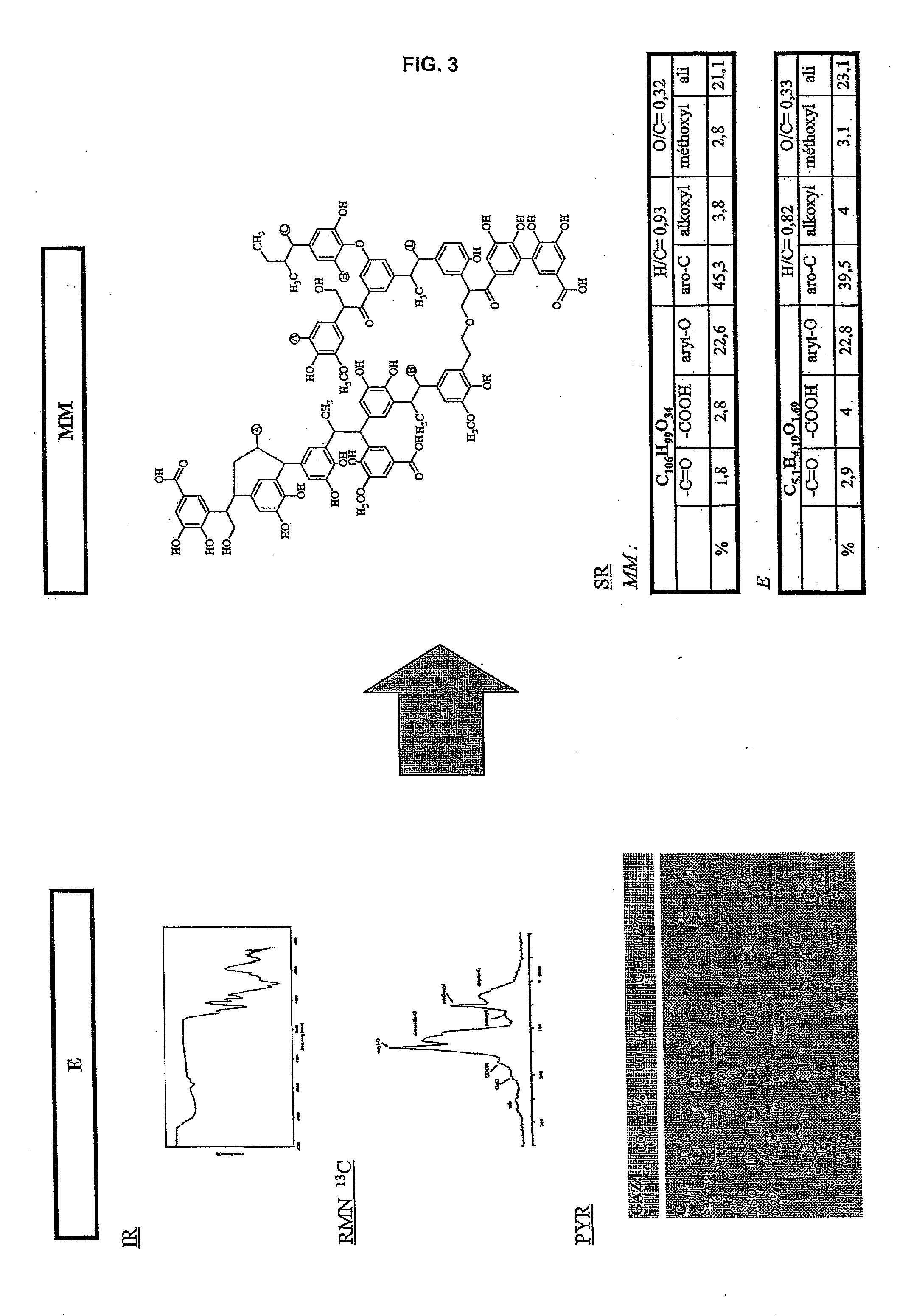
The method according to the invention allows the formation of oil and the retention phenomenon in the mother rock to be modelled. Organic matter characterization experiments are used to establish the molecular model (MM) of the initial sample (E). The thermal cracking reaction of this molecular model is reproduced by dynamic molecular simulation computations with a reactive force field (RMD) and validated by comparison with experimental data. The reaction mechanism obtained (SR) allows to carry out a kinetic study (C) by variation of the temperature parameter. The phase equilibria (PES) of the reaction medium are determined at any time from dynamic simulation. The successive phase equilibrium assessments at various progress stages of the cracking reaction allow following the physicochemical evolution (PC) of the thermal maturation of the organic sample studied. The free hydrocarbons (liquid and gaseous) that are not retained in the solid residue can be quantified throughout numerical modelling of the sample maturation; representing, in the sedimentary basins, the hydrocarbons that are not retained in the organic matrix of the mother rock (Q). This quantity can be used as an indicator or an input value for the retention threshold in basin models.
Owner:INST FR DU PETROLE
Boron-containing small molecules
This invention relates to compounds useful for treating fungal infections, more specifically topical treatment of onychomycosis and / or cutaneous fungal infections. This invention is directed to compounds that are active against fungi and have properties that allow the compound, when placed in contact with a patient, to reach the particular part of the skin, nail, hair, claw or hoof infected by the fungus. In particular the present compounds have physiochemical properties that facilitate penetration of the nail plate.
Owner:ANACOR PHARMACEUTICALS LLC
Method and apparatus for opto-thermo-mechanical treatment of biological tissue
InactiveUS20050119643A1Increase and decrease abilityEfficiency of influenceElectrotherapyControlling energy of instrumentOptical radiationControl signal
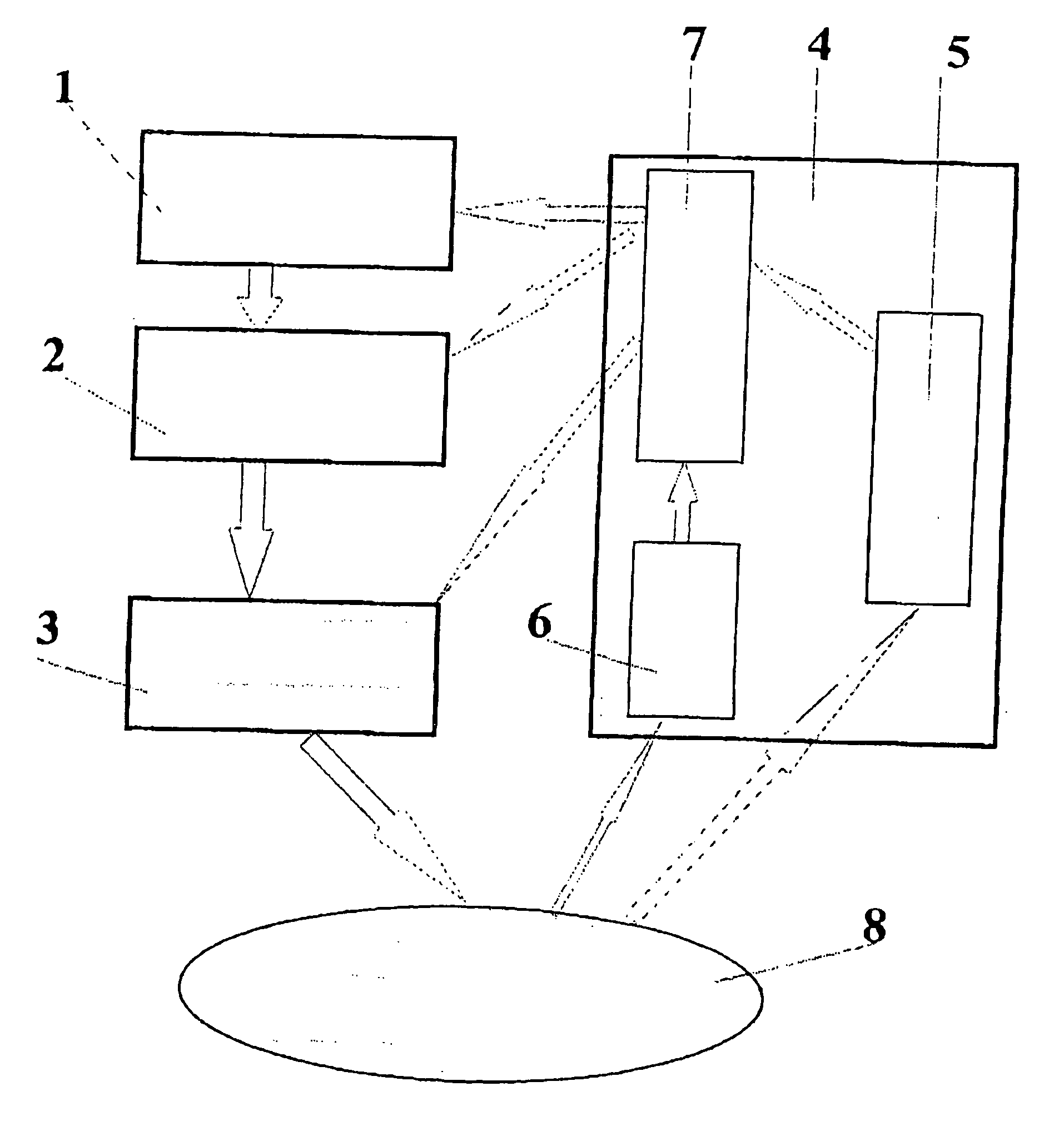
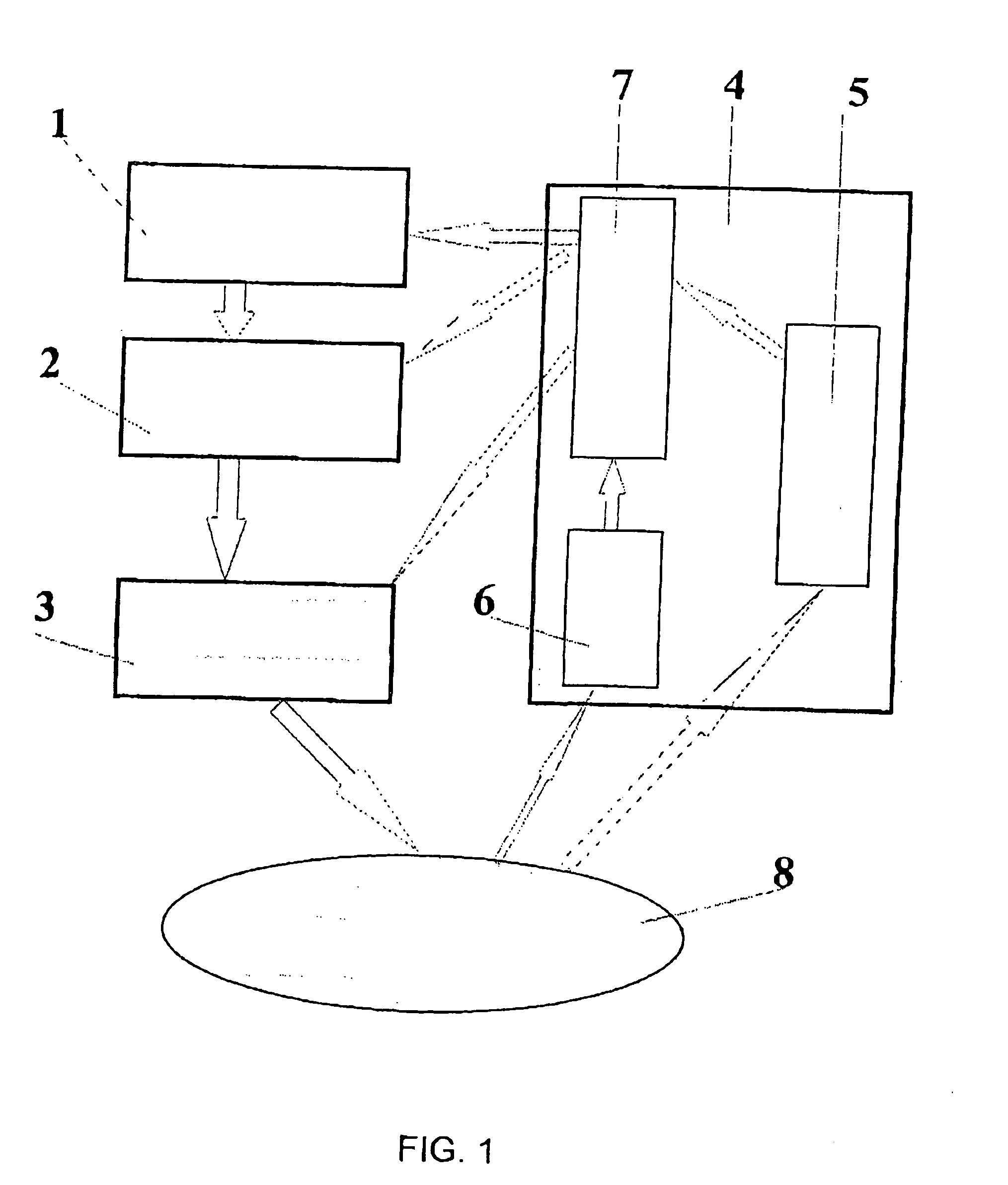
The invention relates to a method and apparatus for opto-thermo-mechanical treatment of biological tissue. A biological tissue area 8 is irradiated with a radiation in the optical wavelength range with predetermined parameters, the radiation being modulated and spatially formed under a predetermined law; the irradiation is accompanied by simultaneous thermal and mechanical treatment of the area 8; concurrently with the irradiation of the biological tissue area, spatial distribution of physico-chemical and geometrical characteristics is measured both in the zone of direct optical treatment and in close vicinity, using a control diagnostic system 4; a data processing unit 7 coordinates parameters of optical radiation spatial formation and modulation with each other and with the biological tissue characteristics and provides a control signal to an optical radiation power and time modulation control unit 2 and a device 3 for delivering optical radiation and forming spatial distribution of optical radiation power on the surface and in the bulk of the biological tissue 8. Optical radiation parameters are adjusted responsive to control signals of the control-diagnostic system 4 during irradiation as a function of continuously changing characteristics of spatial distribution of physico-chemical and geometrical characteristics both in and beyond the directly treated biological tissue area.
Owner:ARCUO MEDICAL
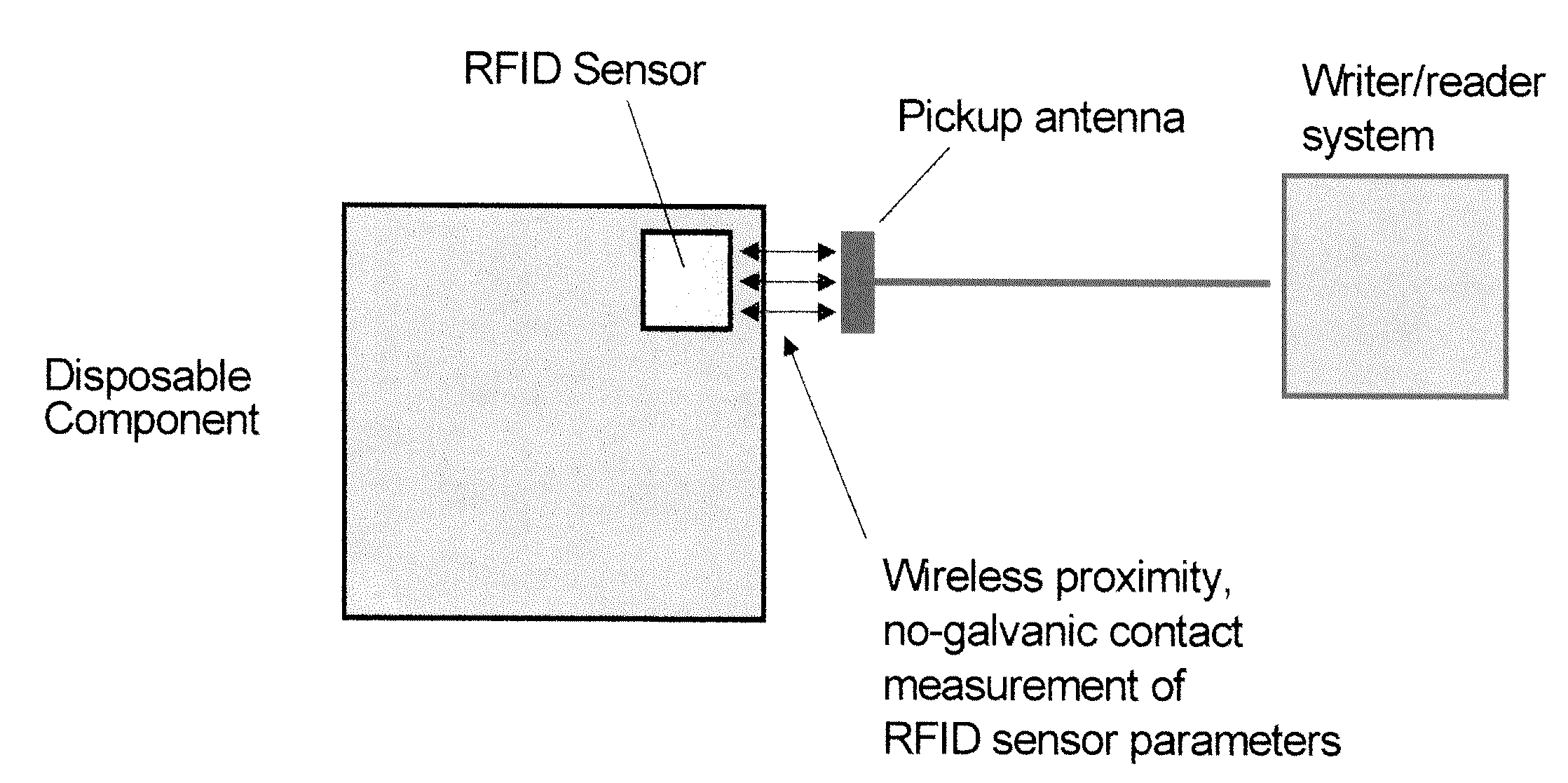
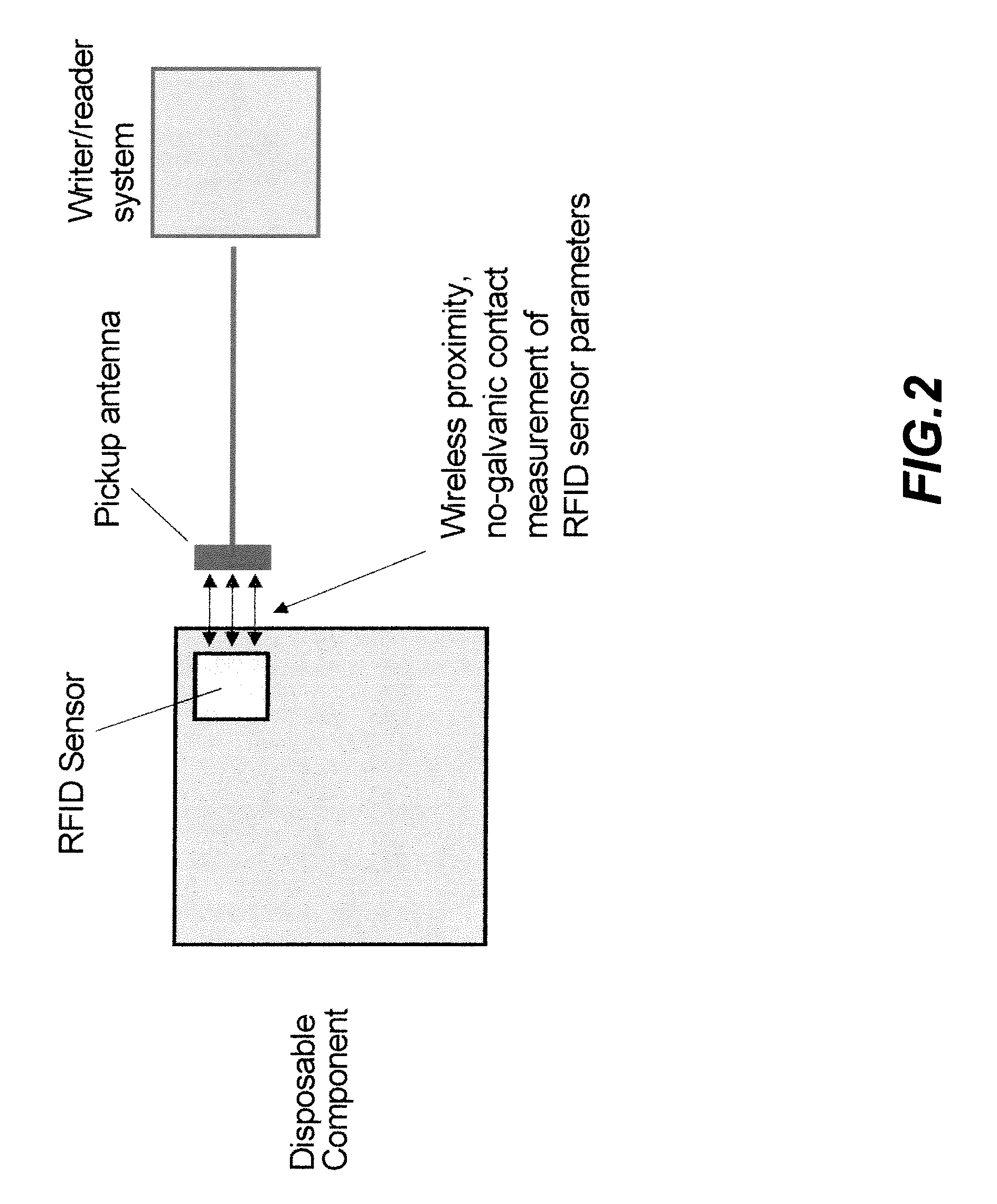
System and method for integrating RFID sensors in manufacturing system comprising single use components
InactiveUS20090204250A1Biochemistry apparatusRecording measured valuesBiological propertyEngineering
The present invention provides a system and method for measuring physical, chemical and biological properties of a manufacturing system comprising embedding a plurality of RFID sensors in a plurality of corresponding single use components wherein each of the plurality of RFID sensors is configured to provide multi-parameter measurements for at least one single use component from the plurality of single use components, and each of the plurality of RFID sensors is further configured to provide simultaneous digital identification for the single use component and for its respective RFID sensor and further comprises reading the multi-parameter measurements and the digital identification for the plurality of single use components using at least one RFID writer / reader, processing the measurements using a processor, and controlling subsequent process steps by comparing the measurements of at least one parameter to a predetermined value.
Owner:GENERAL ELECTRIC CO
Zinc salt compositions for the prevention of dermal and mucosal irritation
InactiveUS20050281762A1Minimize and prevent irritationReduce transmissionBiocideCosmetic preparationsMedicineIrritation
The present invention relates to methods and compositions which employ low concentrations of combinations of zinc salts to prevent the irritation of skin or mucous membranes that may be caused by therapeutic agents, by personal hygiene products, or by various physical, chemical, mechanical, or biological irritants, including infectious agents.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
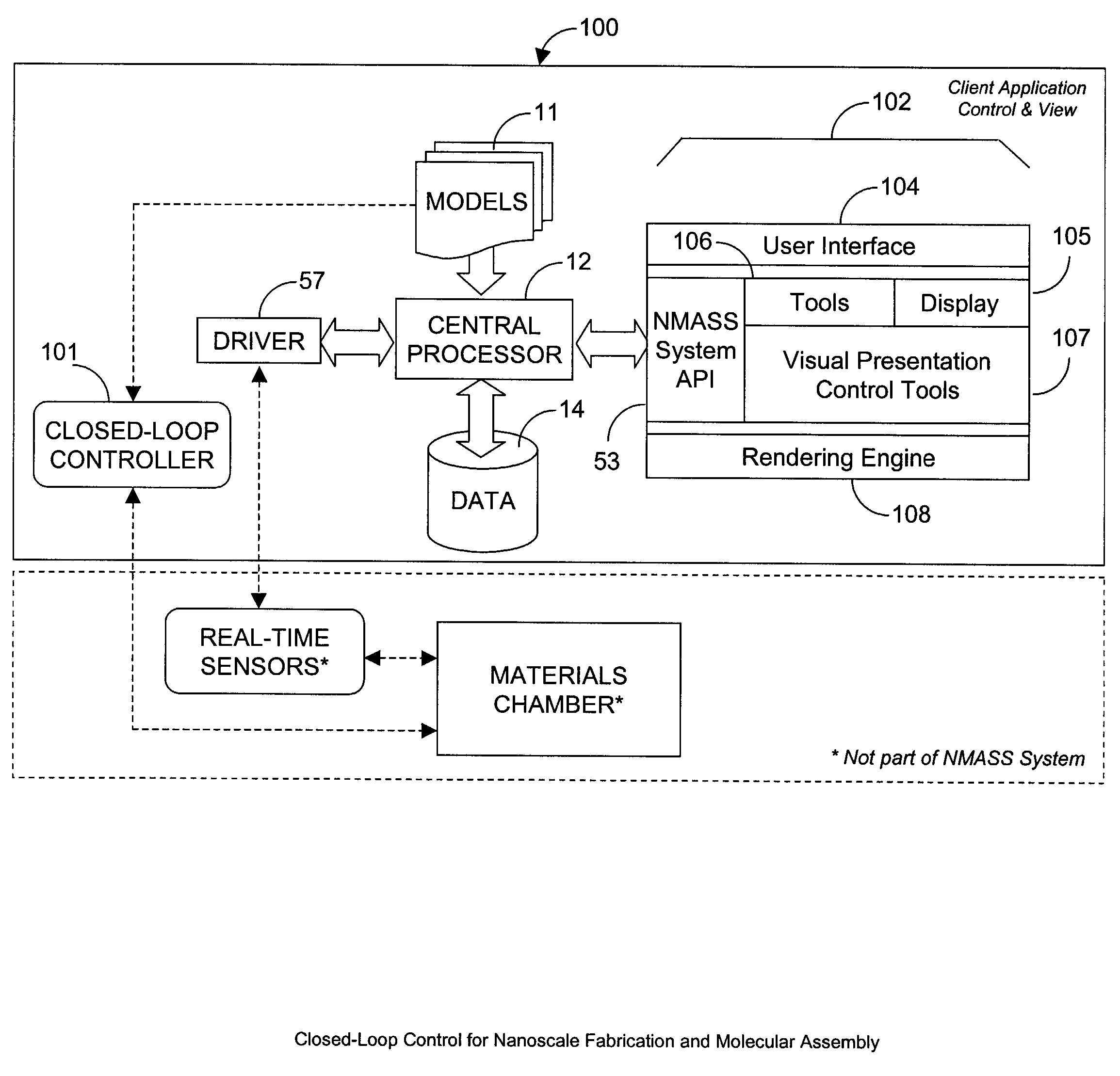
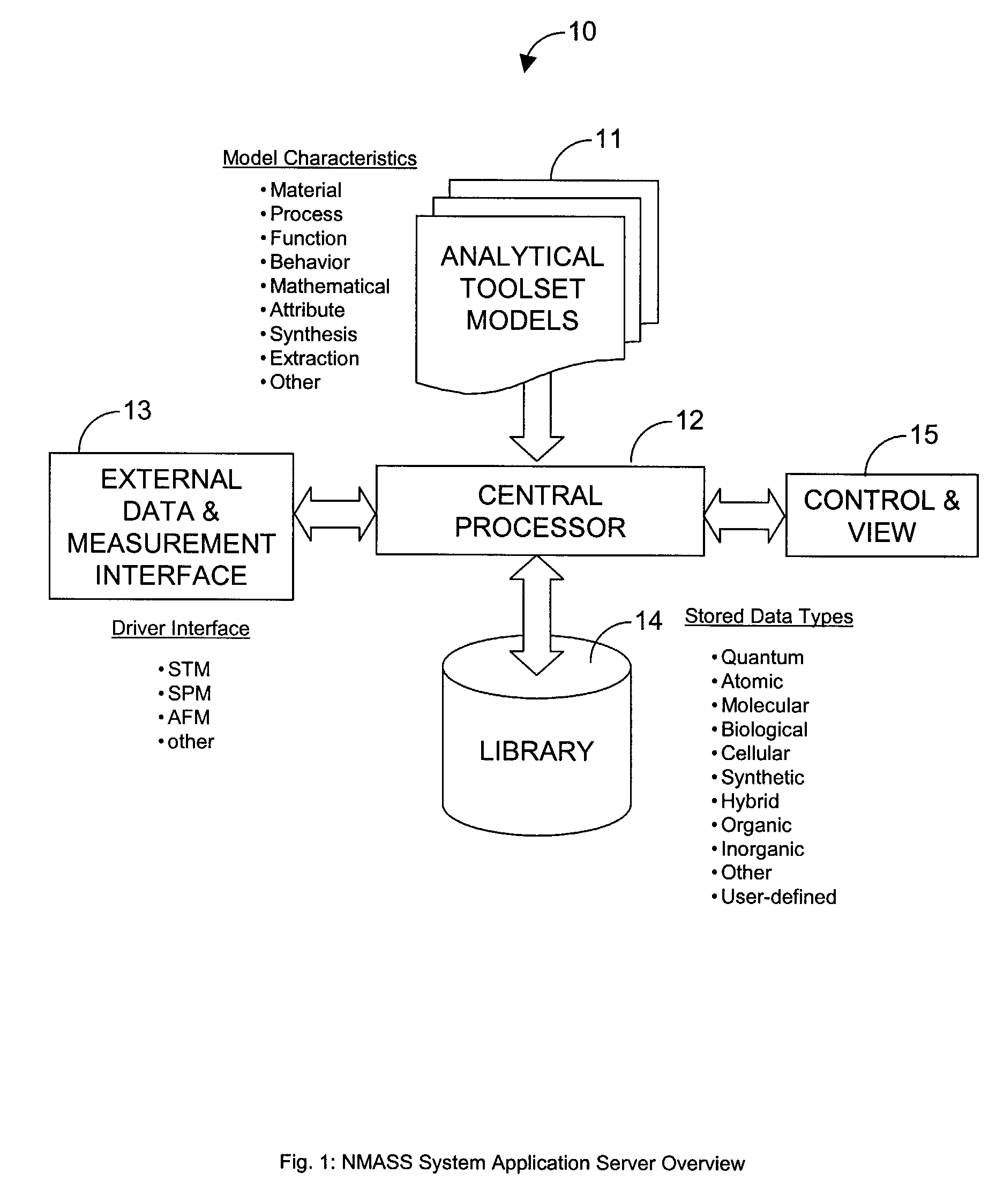
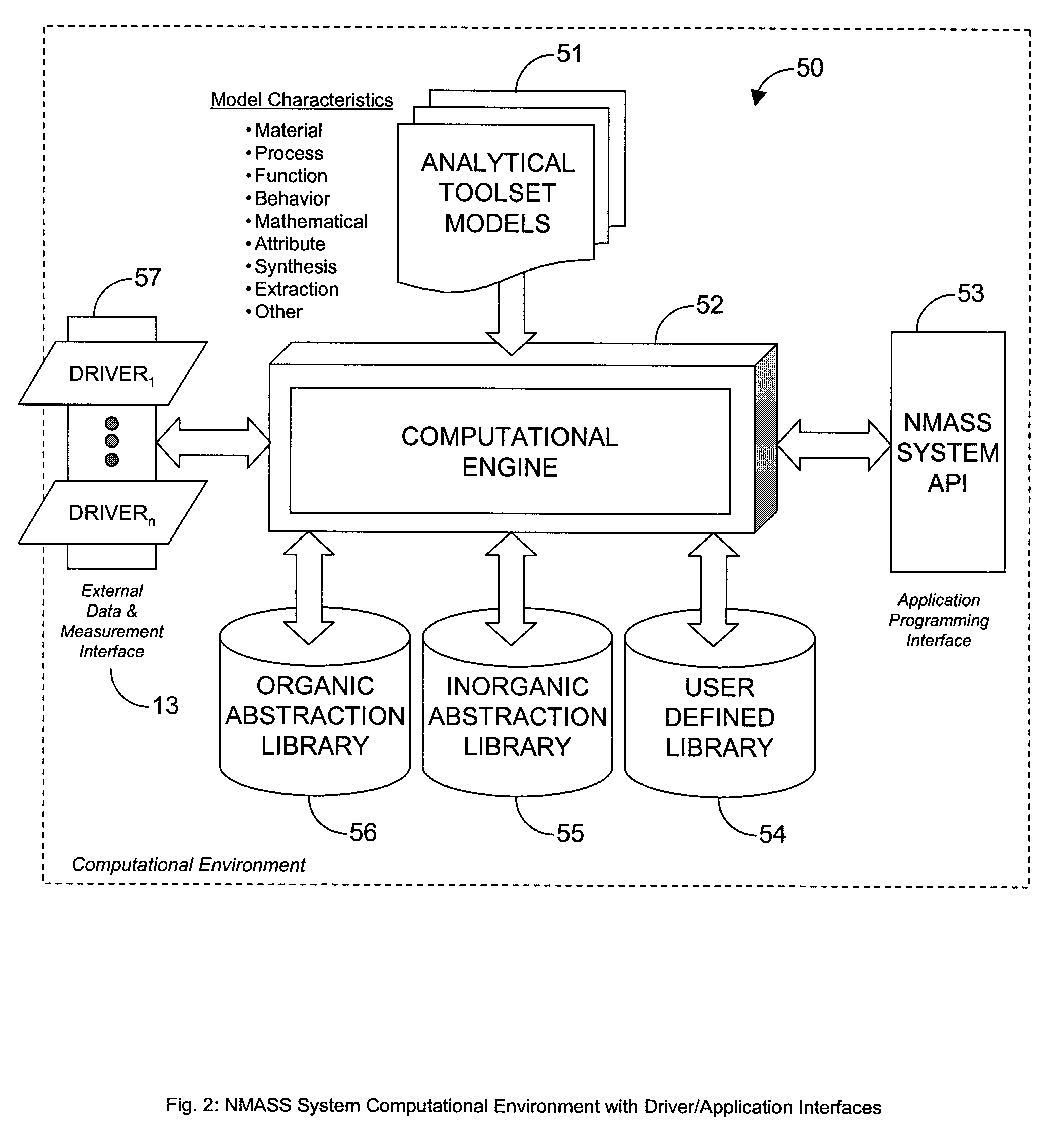
System, Method, and Product for Nanoscale Modeling, Analysis, Simulation, and Synthesis (NMASS)
InactiveUS20030139907A1Chemical property predictionSimulator controlPerformance functionLinear control
Abstract of Disclosure A computer-based system is described that provides users with the ability to develop high-fidelity digital quantitative representations of physical and chemical phenomena, and to employ an optimization-based approach to control associated physiochemical processes. The system includes a computational environment, intuitive user interface(s), integrated software libraries, analytical tools, and visualization / rendering engine that together provide an integrated framework for nanoscale modeling, analysis, simulation, and synthesis. Additionally, the system includes an optimal linear control synthesis methodology that incorporates a first order dynamic mathematical representation (of the conceptual molecular system) suitable for applying various pragmatic control system techniques including optimization of structured singular values, linear quadratic performance functions, Lyapunov criteria, or similar, for the purposes of nanoscale fabrication and molecular assembly.
Owner:MCCARTHY ROBERT J
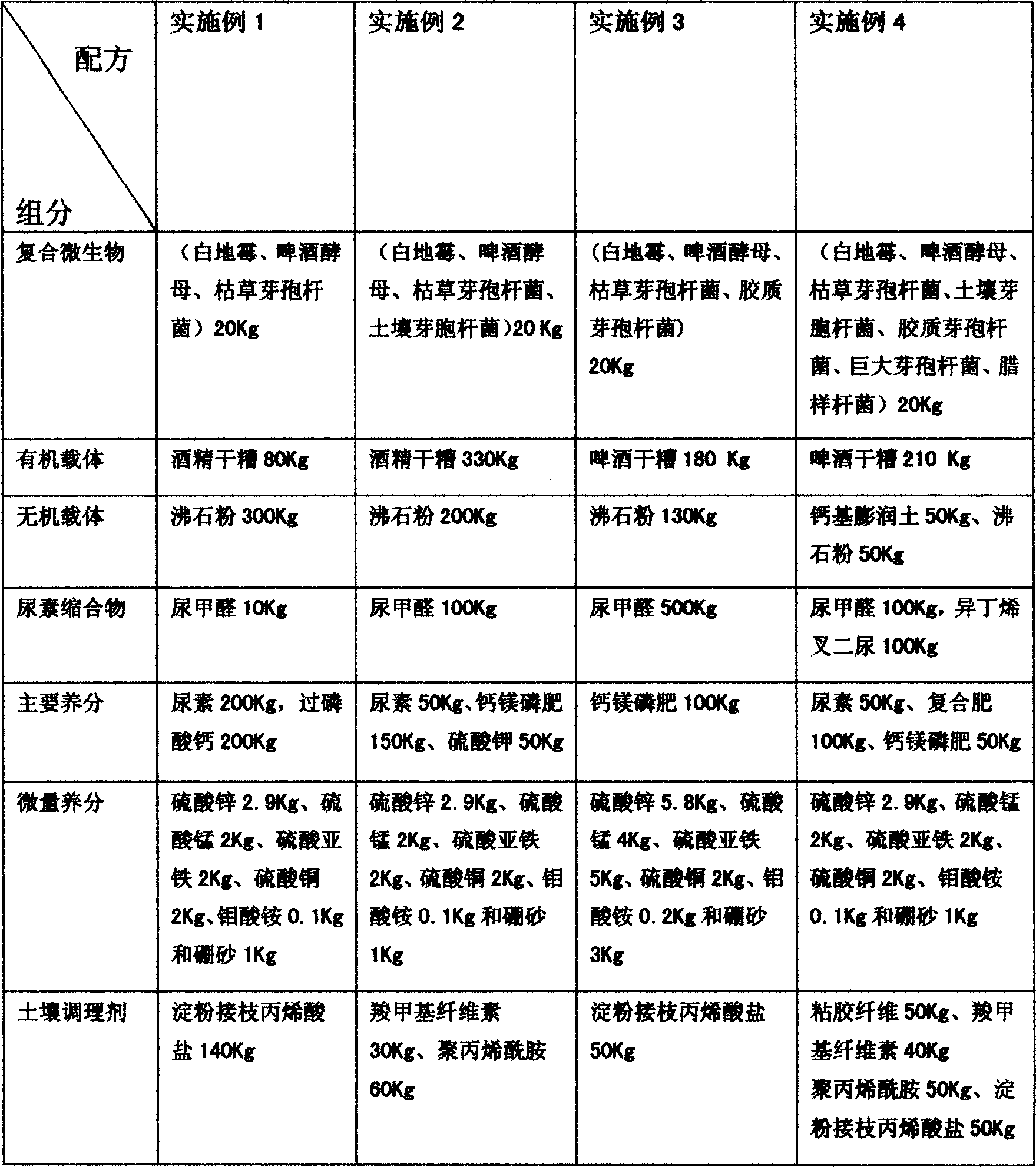
Organic slow-release fertilizer additive
InactiveCN1569758AAdapt to needsImprove microbial activityOrganic fertilisersFertilizer mixturesMicroorganismTrace element
The invention relates to a fertilizer and soil conditioner, wherein the compound fertilizer contains a slow-release fertilizer additive adjustable based on soil and plant requirement, which comprises compound microorganism - organic carrier 2-50%, inorganic carrier 1-30%, ureacondensate 1-50%, primary nutrient 1-60%, trace element 1-10%, and inorganic soil conditioner 1-50%.
Owner:朱绍林
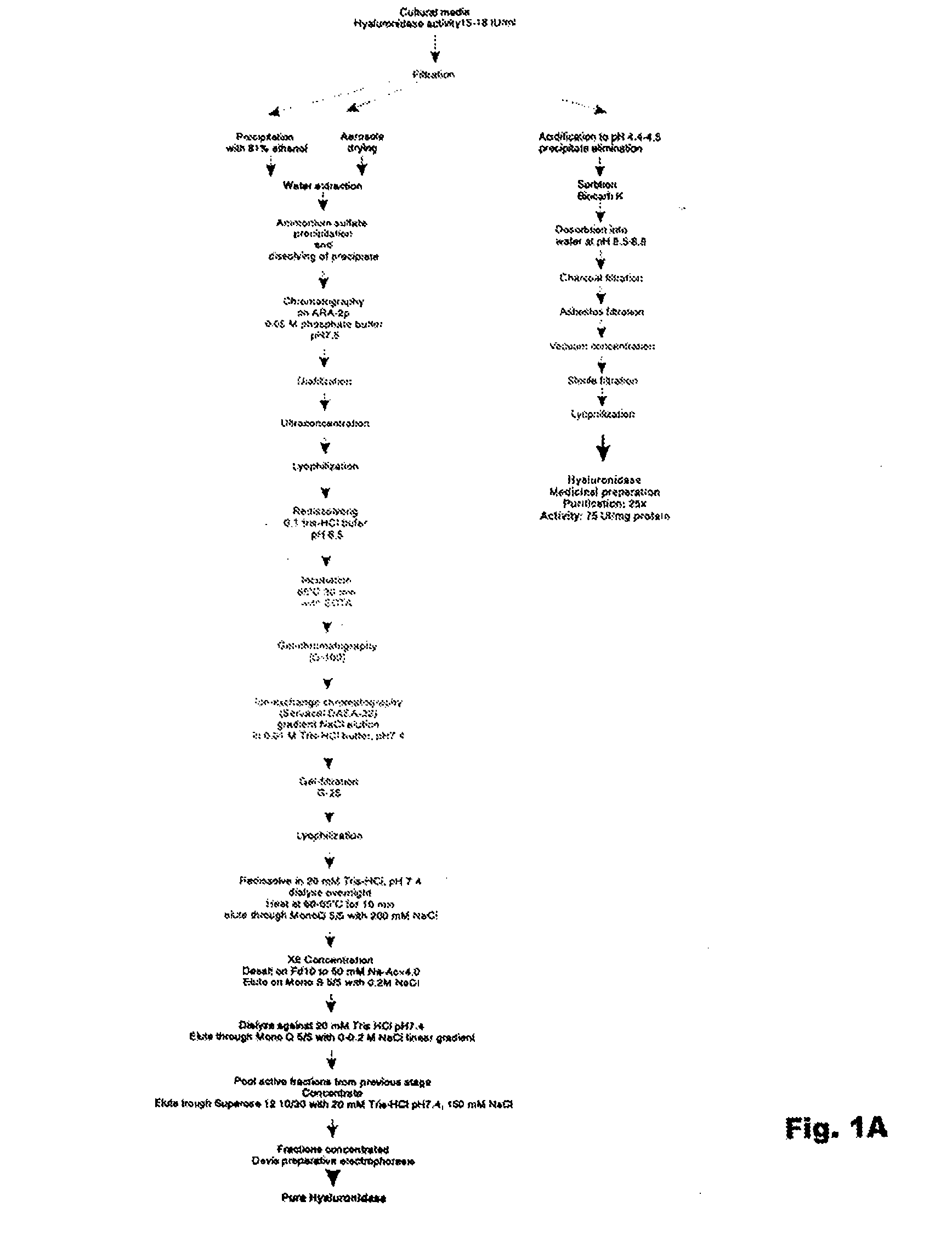
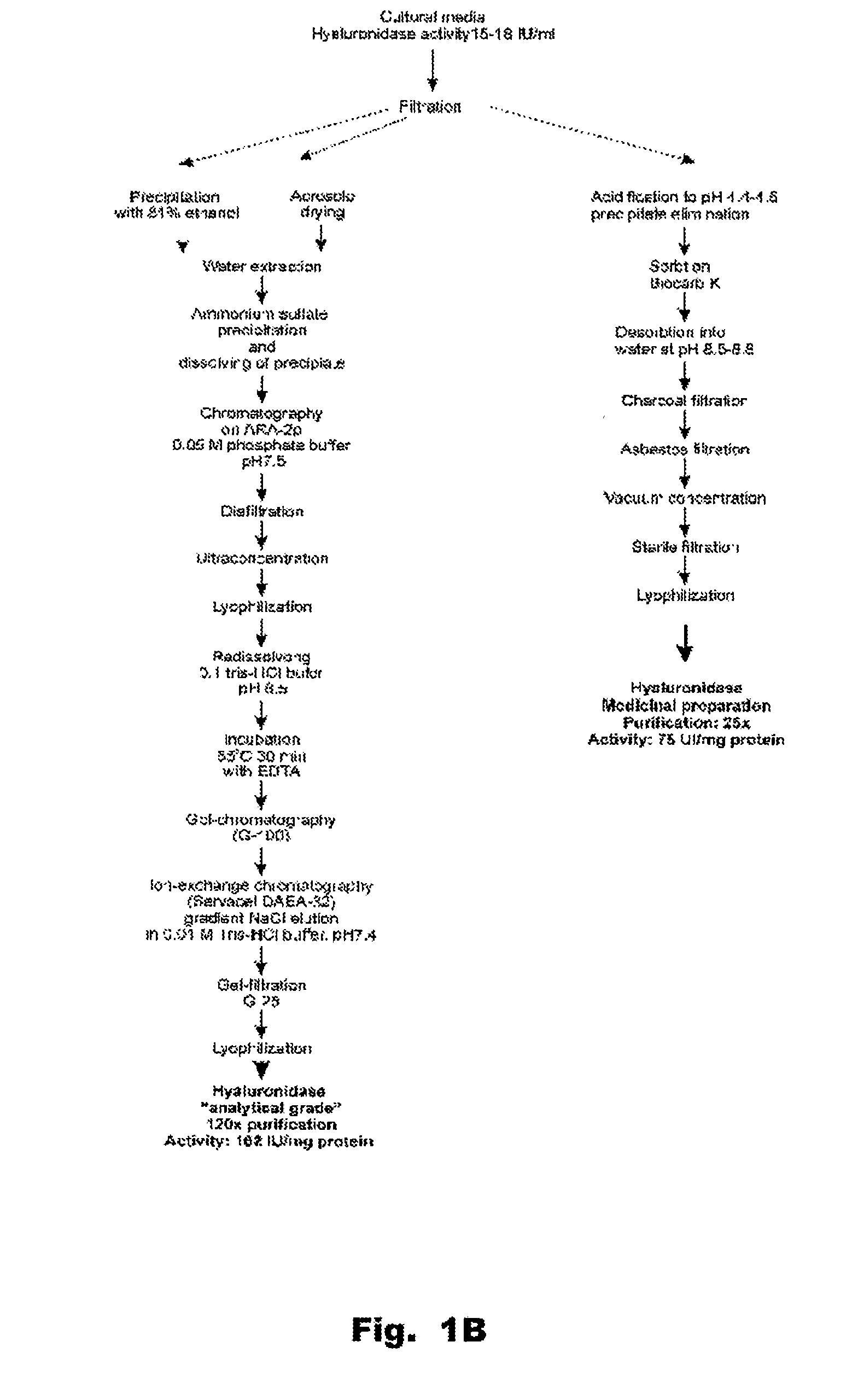
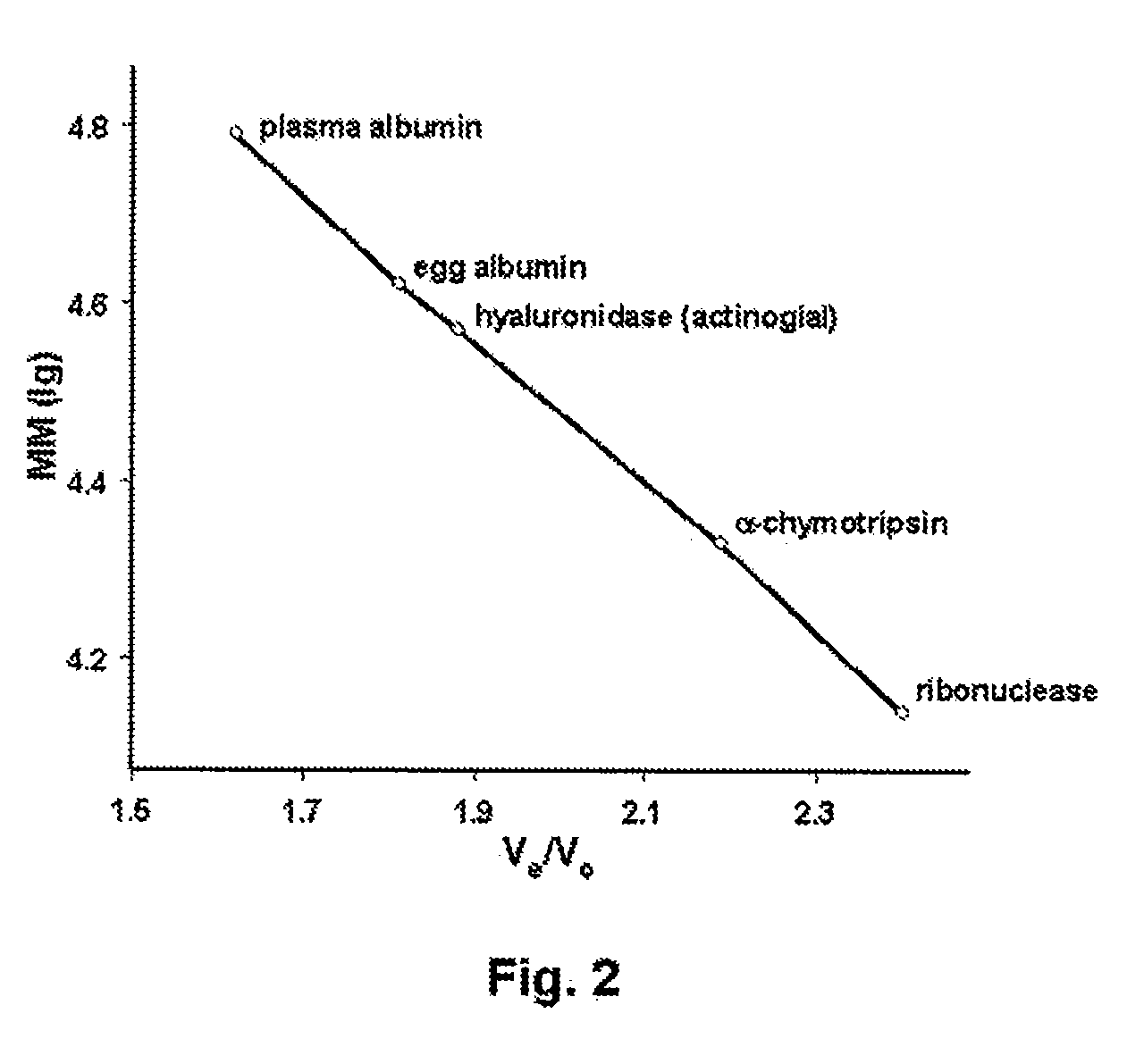
Hyaluronidase and method of use thereof
The present invention provides a tnaluronidase. The hyaluronidase can be produced by the strain Streptomyces aitinocidm 77, Exemplary characteristics of the hyaluronidase include specific C-terminal or other amino acid sequences, including full-length sequences, and improved physico-chemical and actix itj properties as compared to known h> alυronidase preparatkiiis. Described are also various uses of the hyaiurυnidasc, including topical administration of the h> aiuronidasc to improve skin penetration of a co-administered active substance.
Owner:UVARKINA TAMARA P +1
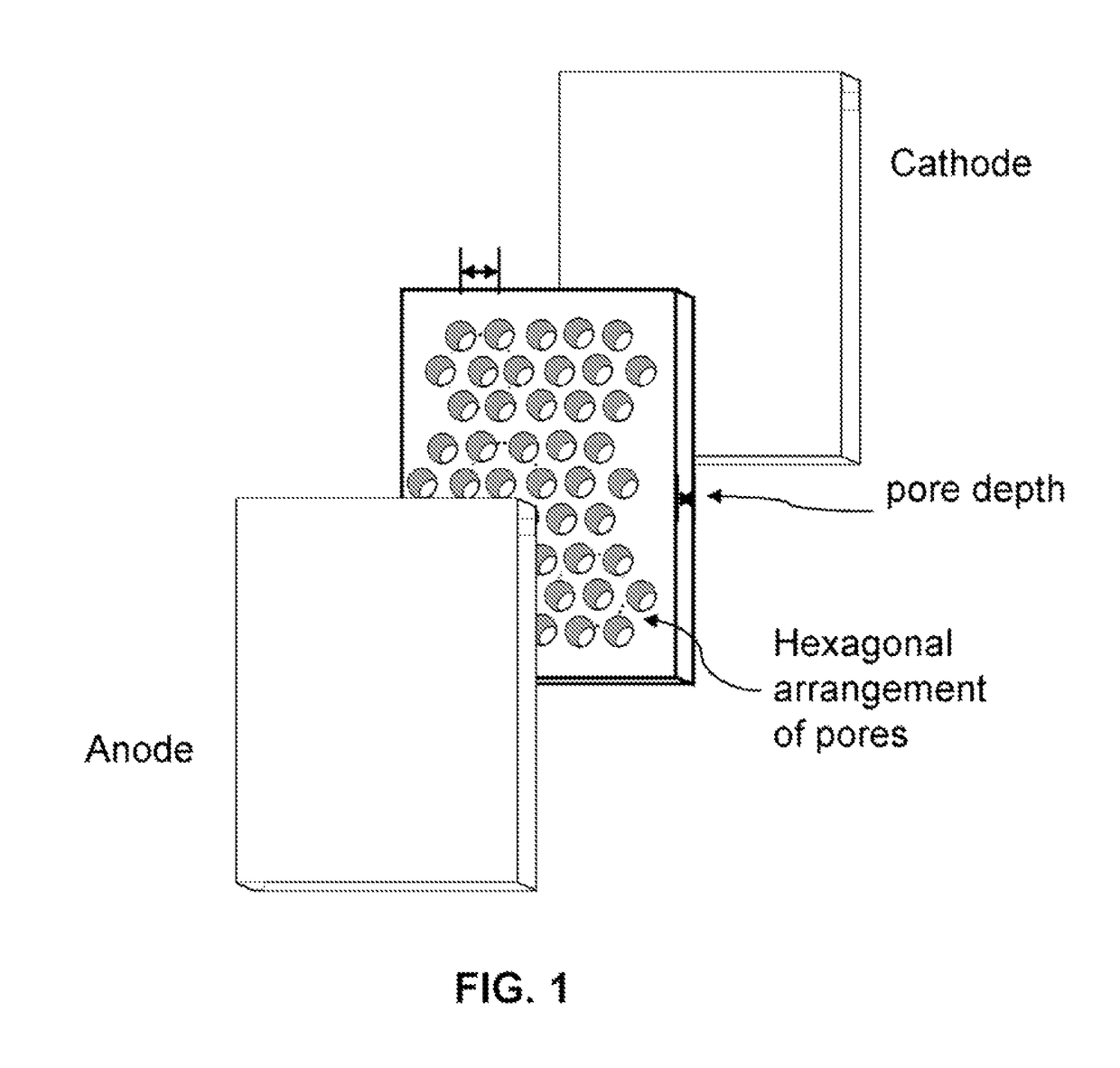
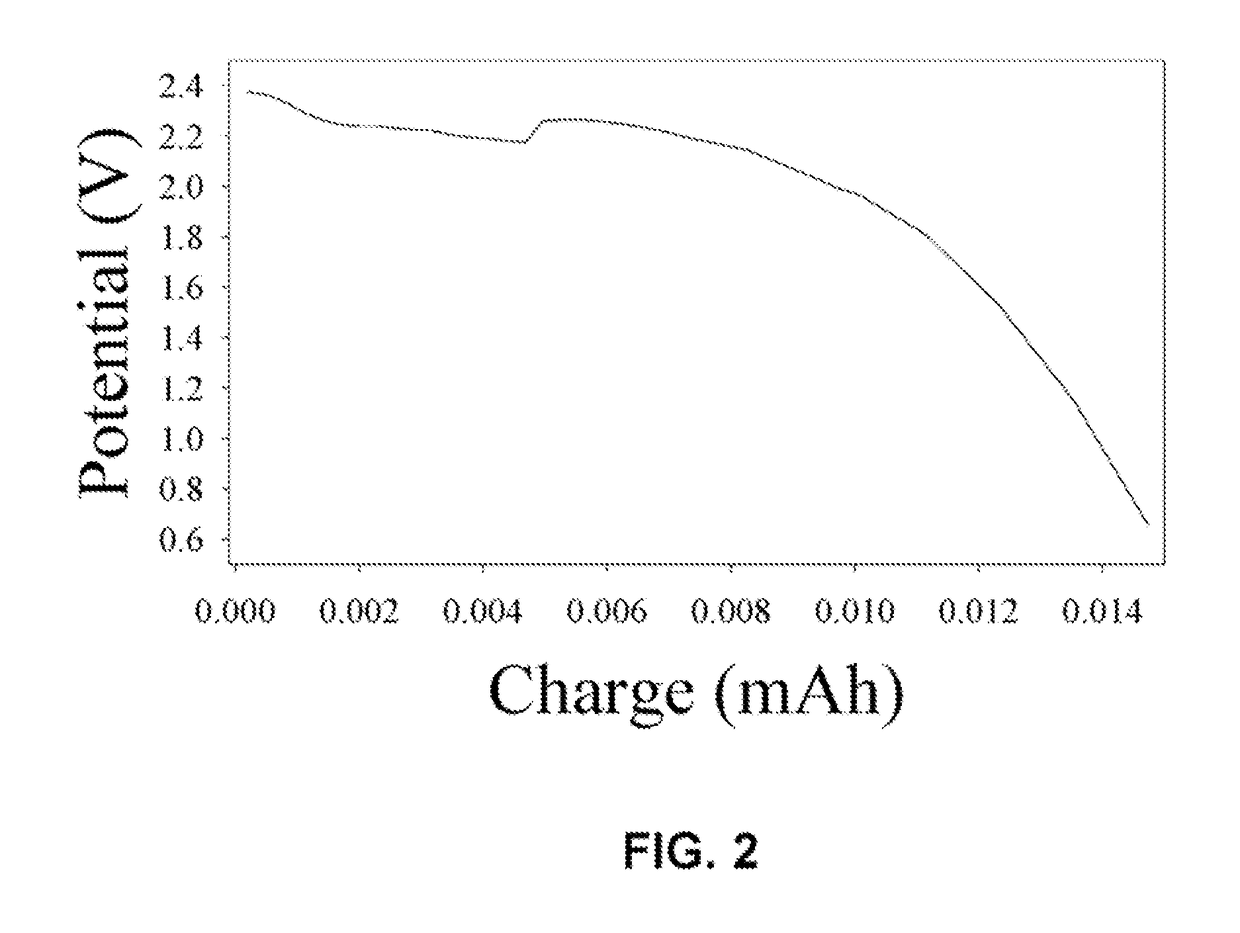
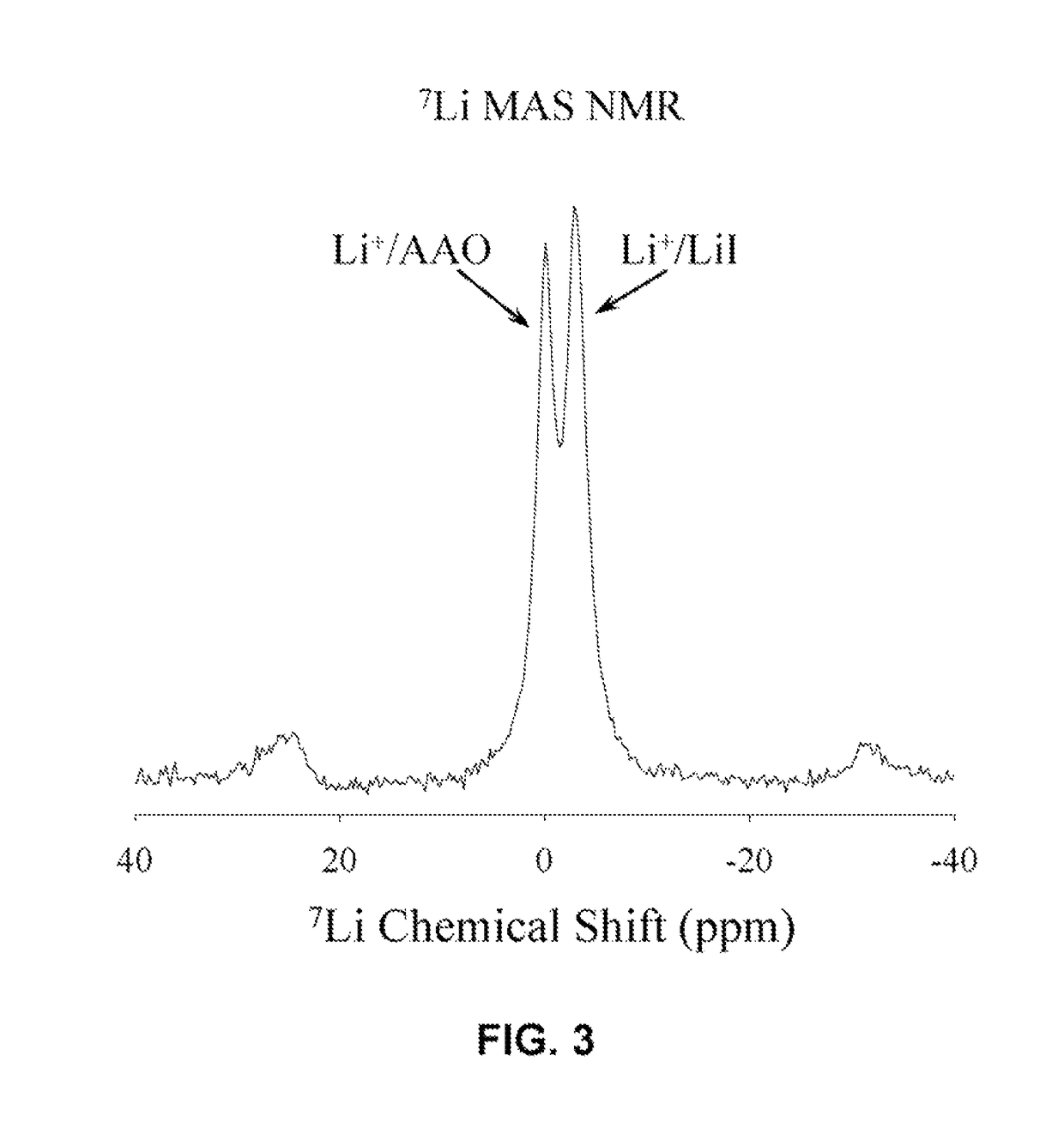
Unique battery with an active membrane separator having uniform physico-chemically functionalized ion channels and a method making the same
InactiveUS8119273B1Decrease in ion conductivityIon-conductivity can be maximizedCell seperators/membranes/diaphragms/spacersElectrode carriers/collectorsCation PumpIon transporter
The invention relates to a unique battery having an active, porous membrane and method of making the same. More specifically the invention relates to a sealed battery system having a porous, metal oxide membrane with uniform, physicochemically functionalized ion channels capable of adjustable ionic interaction. The physicochemically-active porous membrane purports dual functions: an electronic insulator (separator) and a unidirectional ion-transporter (electrolyte). The electrochemical cell membrane is activated for the transport of ions by contiguous ion coordination sites on the interior two-dimensional surfaces of the trans-membrane unidirectional pores. The membrane material is designed to have physicochemical interaction with ions. Control of the extent of the interactions between the ions and the interior pore walls of the membrane and other materials, chemicals, or structures contained within the pores provides adjustability of the ionic conductivity of the membrane.
Owner:ENERGY UNITED STATES OF AMERICA
Pesticide auxiliary agent
ActiveCN1701663AWith characteristicsHave made significant progressBiocideAnimal repellantsActive agentSolvent
Disclosed is a pesticide auxiliary agent whose raw material includes (by mass ratio) plant oil 100, auxiliary solvent 0-50, surface active agent 5-55, the auxiliary agent can be made into the dosage forms of oil miscible concentrate, oil solution, hot mist, aerosol, low-volume spraying agent, pesticide, germicidal agent, sterilizing agent, hygienic pesticide and plant growth agent.
Owner:FUJIAN NUODE BIOTECH
Sulfoalkyl Ether Cyclodextrin Compositions and Methods of Preparation Thereof
A particulate SAE-CD composition is provided. The SAE-CD composition has an advantageous combination of physical properties not found in known solid forms of SAECD. In particular, the SAE-CD composition possesses an advantageous physicochemical and morphological property profile such that it can be tailored to particular uses. The SAE-CD composition of the invention has improved flow and dissolution performance as compared to known compositions of SAE-CD.
Owner:CYDEX PHARMACEUTICALS INC
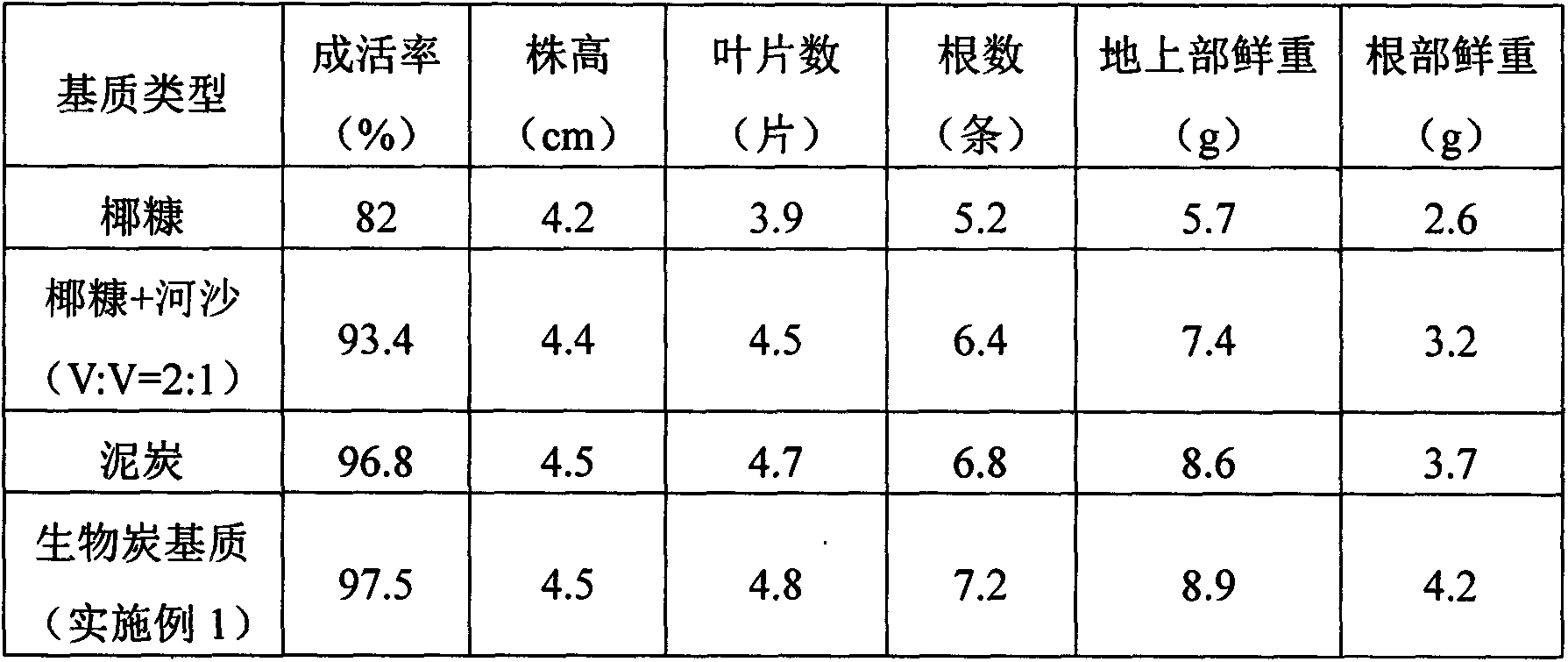
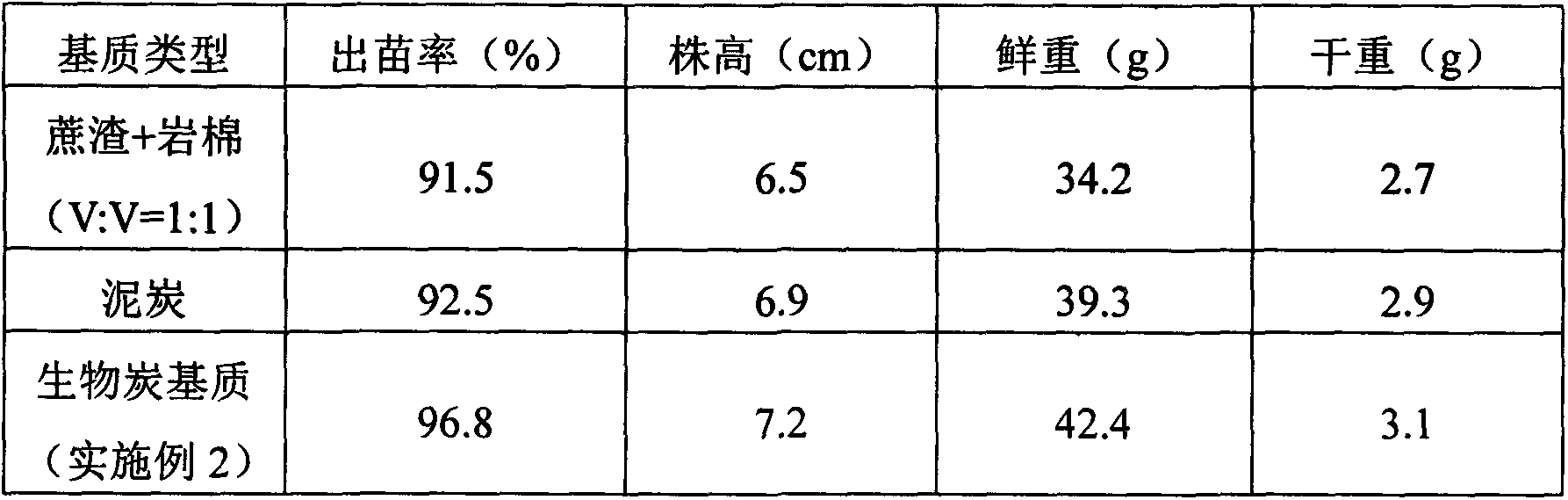
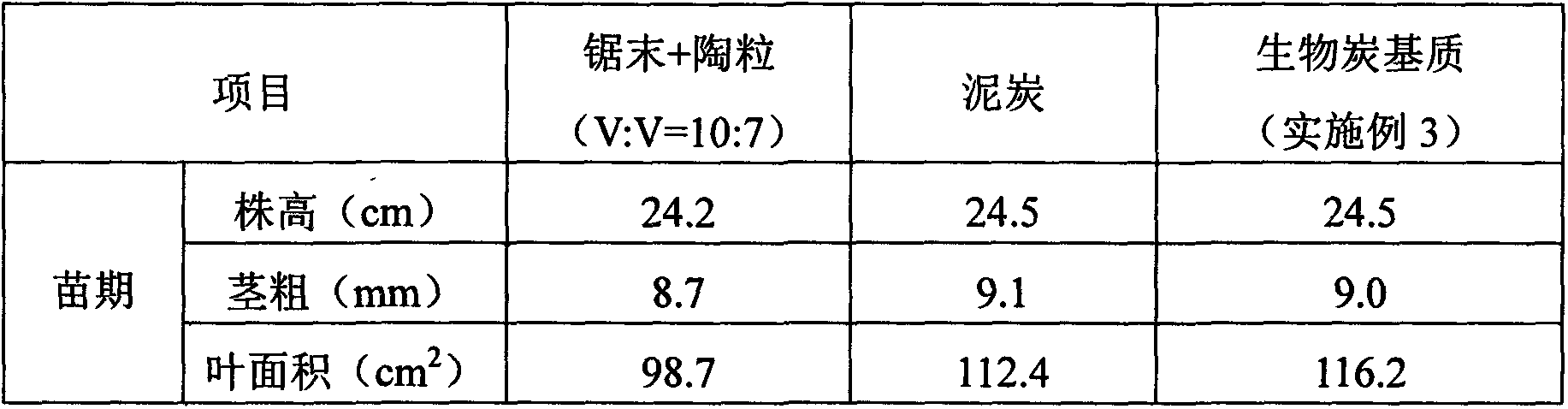
Charcoal based plant cultivation substrate and preparation method thereof
InactiveCN103435411AEnhanced ability to provide phosphorus and potassium fertilizersLight textureFertilizer mixturesPhosphatePotassium
The invention relates to a charcoal based plant cultivation substrate and a preparation method thereof, and belongs to the technical field of soilless culture. The invention adopts the specific scheme that: after being dried, biomass material is pyrolyzed to form charcoal particles under an anoxic and heating condition, potassium dihydrogen phosphate is used for neutralizing the alkalinity of the charcoal particles and filtering and drying are performed, and after a certain amount of inorganic fertilizer is added, the charcoal particles are fully mixed with inorganic filling material, organic filling material and organic fertilizer, namely the charcoal based plant cultivation substrate is obtained. The invention has the advantages that the physical chemical property is excellent, the nutrients are complete, the fertilizer efficiency is lasting, the moisture adjusting capability is strong, the cost is low, the ecological benefits are high, the suitability is strong, the application range is wide, and the charcoal based plant cultivation substrate is suitable for cultivation of various plants.
Owner:QIONGZHOU UNIVERSITY
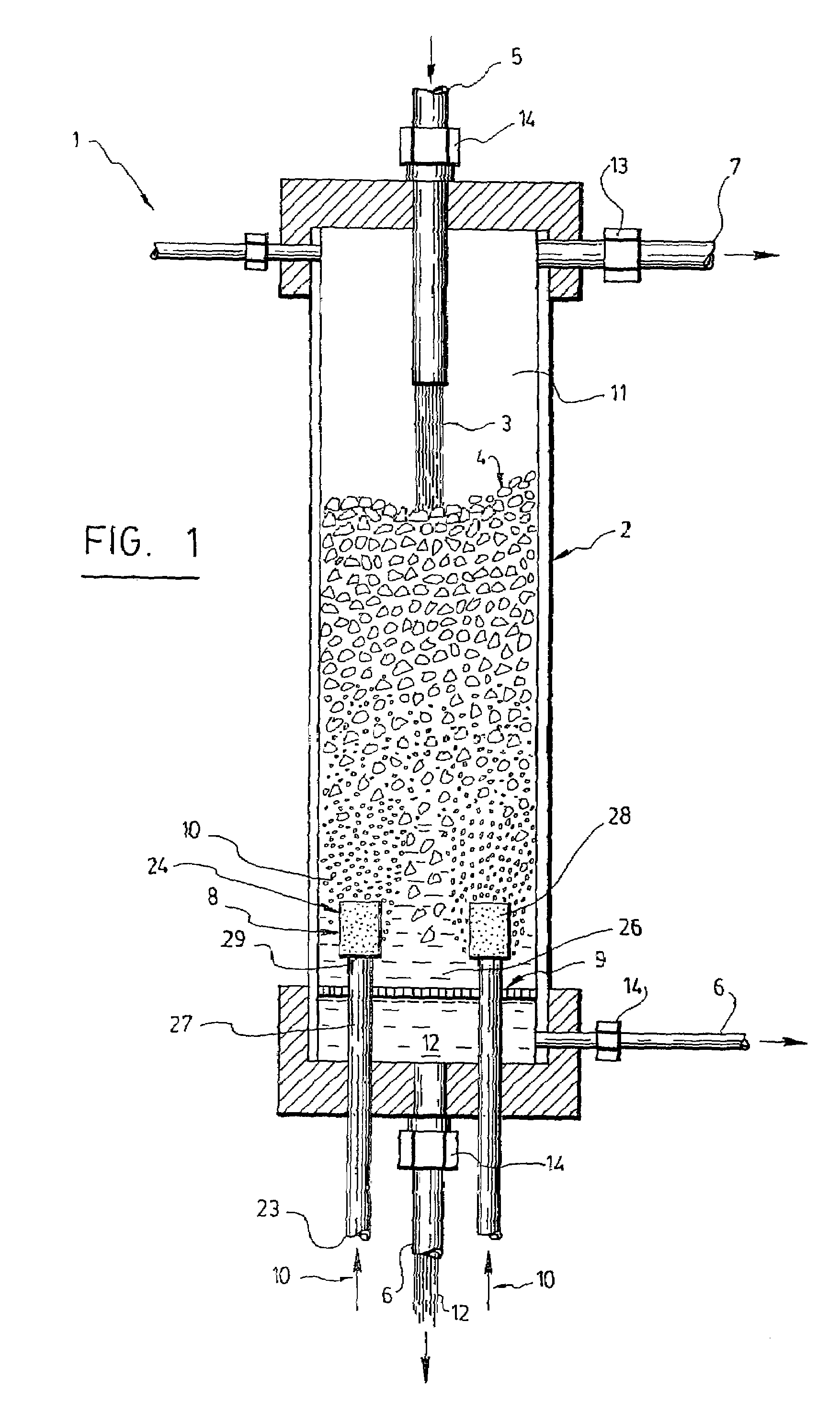
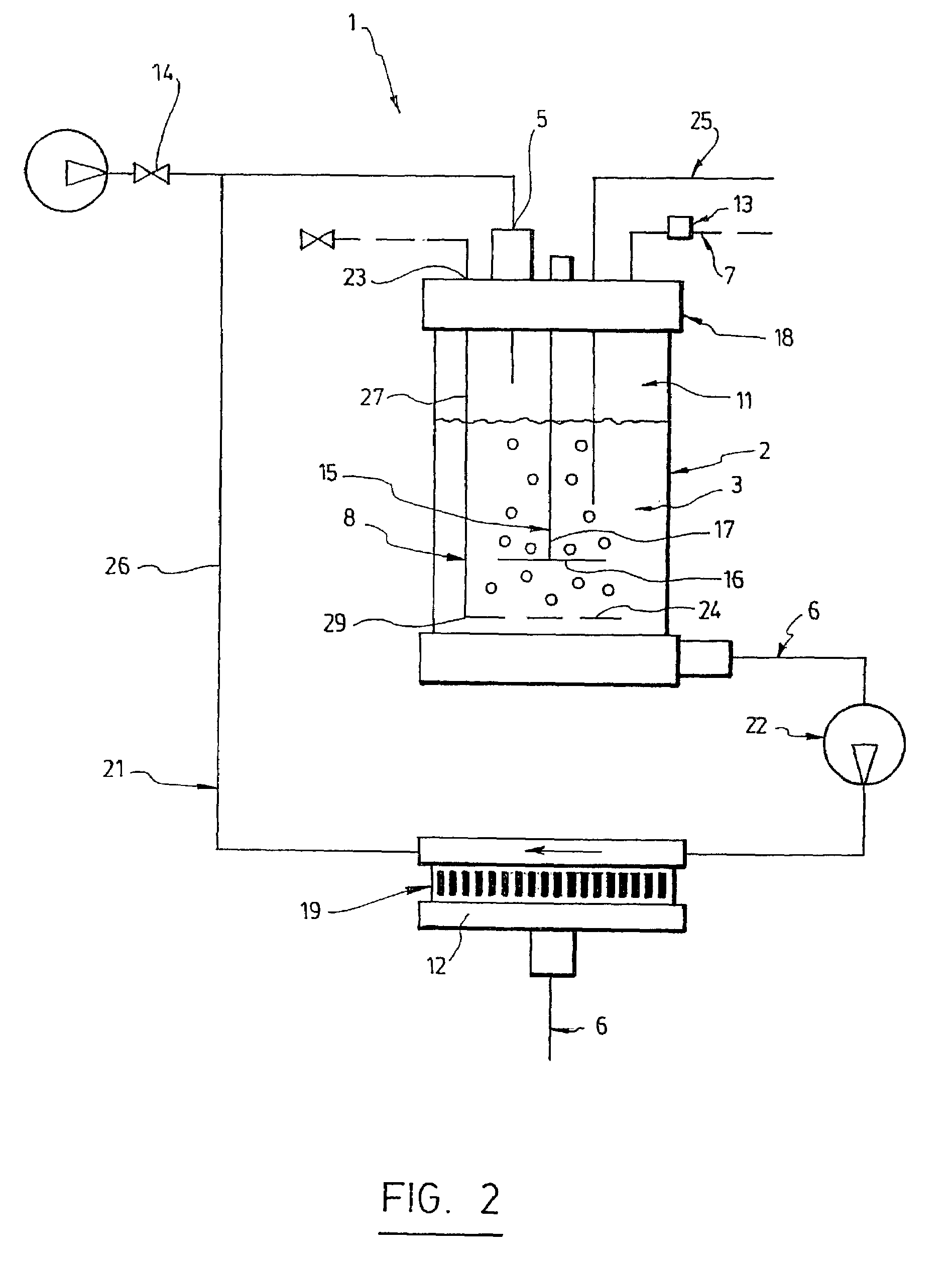
Triphasic bioreactor and process for gas effluent treatment
InactiveUS7176017B2Bioreactor/fermenter combinationsBiological substance pretreatmentsBioreactorReaction chamber
A triphasic bioreactor for physico-chemically treating a gas is disclosed. The triphasic bioreactor comprises a reaction chamber with a liquid and biocatalysts in suspension in the liquid, for catalyzing a reaction between the gas and the liquid to obtain a treated gas and a solution containing a reaction product. A gas bubbling means is provided in the reaction chamber for bubbling the gas to be treated into the liquid thereby dissolving the gas into the liquid and increasing a pressure inside the reaction chamber. The bioreactor further comprises a liquid inlet in fluid communication with the reaction chamber for receiving the liquid and filling the reaction chamber, a liquid outlet in fluid communication with the reaction chamber for releasing the solution and a gas outlet in fluid communication with the reaction chamber to release the treated gas. The bioreactor further comprises a retention device to retain the biocatalysts in the reaction chamber. The invention also concerns a process using the triphasic bioreactor. The triphasic bioreactor may advantageously be used for removing carbonic dioxide from a CO2-containing gas
Owner:CO2 SOLUTION
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com