Preparation method of coxsackievirus antigen and rapid detection kit prepared by utilizing antigen and used for detecting coxsackievirus antibody
A coxsackie virus and antigen technology, which is applied in the field of clinical medical detection to achieve the effects of strong specificity, high antigen yield and obvious effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1 Recombinant Expression, Structure Renaturation and Purification of Recombinant Coxsackievirus VP1 Protein Antigen
[0050] Coxsackievirus type A VP1 gene was synthesized with reference to the GenBank sequence GQ279371.1. The rare codons of Escherichia coli were optimized during the synthesis. The expression vector pET30a was connected with HindII / XhoI enzyme cutting sites. The total target gene was 894bp (297aa), transformed into BL21(DE3), the total expressed recombinant protein was 345aa, the molecular weight was 38.7 kDa, and the isoelectric point was 8.32. The recombinant protein is expressed in the form of inclusion body and can be purified by Ni column.
[0051] gene synthesis sequence
[0052] >CA16, VP1 optimized sequence
[0053] GCGGTGATCCTATCGCCGACATGATCGATCAGACCGTGAACAACCAGGTTAATCGCAGTCTGACCGCACTGCAGGTTCTGCCGACCGCAGCCAATACCGAAGCCAGCAGCCATCGTCTGGGTACCGGTGTGGTTCCGGCCTTACAGGCAGCCGAAACCGGCGCCAGCAGCAACGCAAGCGACAAAAACCTGATCGAGACCCGCTGTGTTCTGAATCACCATAG...
Embodiment 2
[0057] Example 2 Preparation of a Coxsackie virus antibody IgG / IgM gold-labeled rapid detection kit
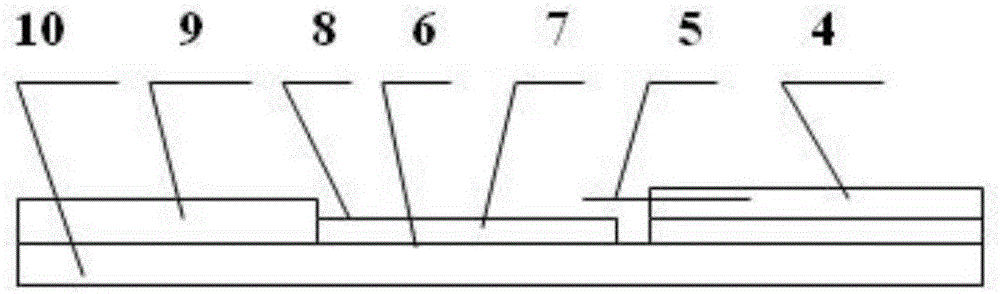
[0058] The recombinant Coxsackie virus VP1 protein obtained above was used as a detection antigen, and a detection line was coated on a nitrocellulose membrane; refer to figure 2 , prepare Coxsackie virus antibody gold label rapid detection reagent, its composition comprises: be provided with on lining board 10 and be provided with sample loading end water-absorbing layer 4, detection layer 8 and water-absorbing layer 9, be provided with detection layer and sample-loading end water-absorbing layer 4 A gold-labeled anti-Coxsackie virus antibody layer 5 is provided between them, and a detection line 7 and a quality control line 6 are coated on the detection layer 8 . Wherein, the water-absorbing layer 4 at the sample loading end and the water-absorbing layer 9 at the water-absorbing end are made of multi-layer filter paper: the detection layer 8 is a nitrocellulose membrane; th...
Embodiment 3
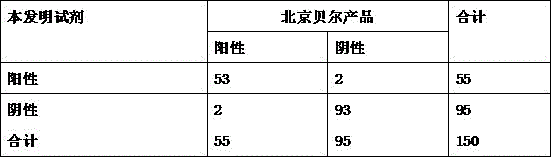
[0062] Example 3 Determination of Coxsackie virus antibody
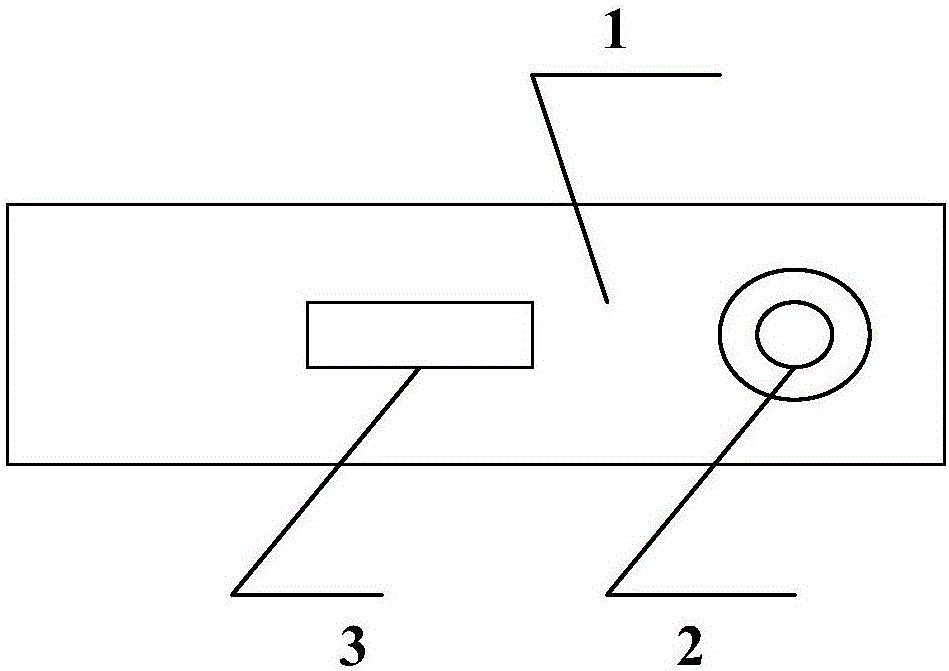
[0063] Take 10 μL of serum or plasma sample and drop it into the sample well 2 of the test plate 1, then add 100 μL of the sample diluent into the sample well 2, and observe the test result in the observation window 3, and the observation result is valid within 20 minutes. If the sample contains anti-Coxsackie virus antibody, two red lines appear in the detection line and quality control line in the observation window, and the test result is judged as positive; if the serum does not contain anti-Coxsackie virus antibody, then If a red line is seen at the quality control line of the observation window, the test result is judged as negative; if no red line is visible in the observation window, the test result is invalid.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com