Herpes virus glycoprotein gE recombinant protein, vaccine, preparation method and application
A herpes virus vaccine and protein technology, applied in the biological field, can solve the problems of unfavorable large-scale production, cumbersome cultivation process, weakened immune response, etc., and achieve the effect of quality control cost advantage, wide application prospect, and improved protein expression level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0059] Preferably, the preparation method of the recombinant gE protein provided by the present invention uses the polynucleotide sequence shown in SEQ ID NO. Acidic recombinant engineering bacteria, culture, collect the bacteria, break the bacteria to obtain a lysate, and separate and purify the lysate to obtain gE protein. Specifically, the method includes the following steps:
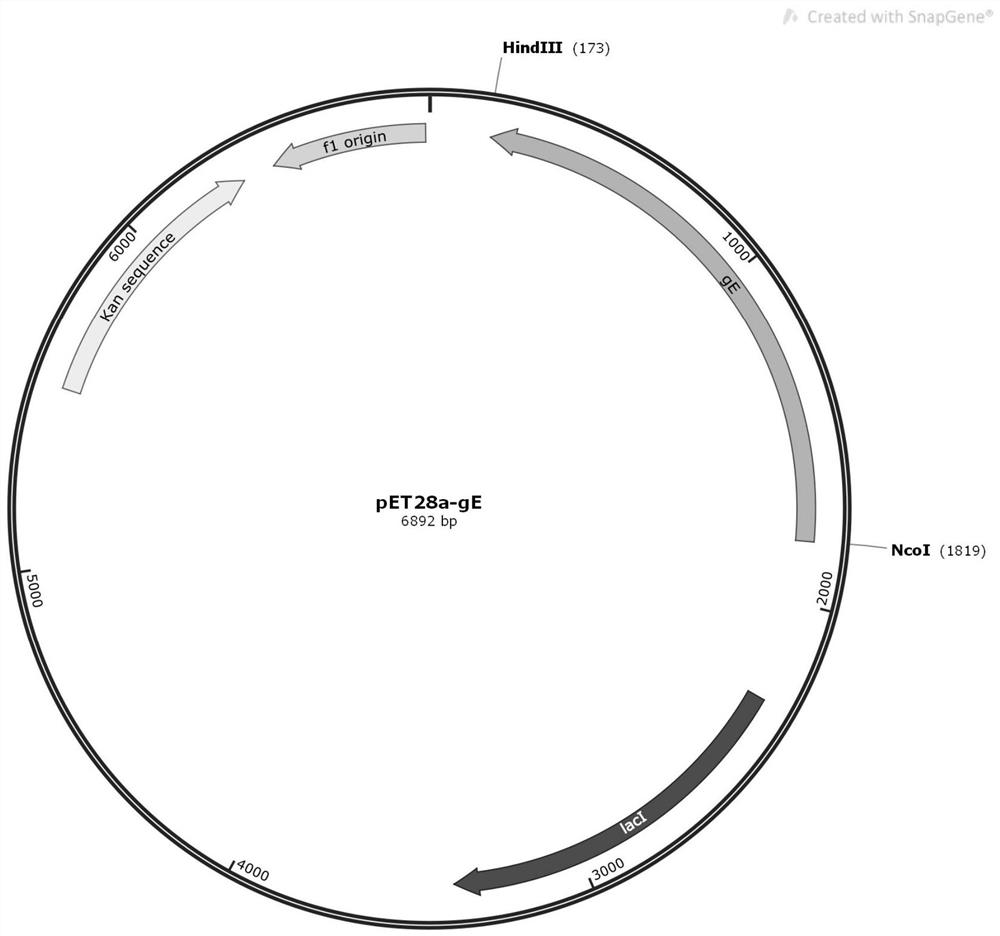
[0060] 1) construct the above-mentioned gE protein recombinant expression vector;
[0061] 2) the above-mentioned recombinant expression vector is transformed into bacteria to construct recombinant engineering bacteria;
[0062] 3) Verify the recombinant engineered bacteria and obtain the correct constructed target recombinant engineered bacteria;
[0063] 4) Cultivate the target recombinant engineered bacteria under specific conditions, collect the cultured bacteria and purify the recombinant gE protein.
[0064] In step 1), primers are designed to amplify the coding sequence of the target gE pro...
Embodiment 1
[0116] Example 1 Construction of recombinant gE protein engineering strain
[0117] 1. Selection of gE protein-coding genes and optimal design of codons
[0118] The amino acid sequence encoding the gE protein refers to the gE protein sequence of the Japanese vaccine strain Oka, as shown in SEQ ID NO.2.
[0119] The corresponding nucleotide coding sequence of the amino acid sequence shown in SEQ ID NO.2 is modified, and the codons used in the E. coli expression system are used as frequently as possible, and at the same time, the transcription factor binding region and repetition that may affect the expression are avoided. Sequence and RNA higher order structure. The gE protein coding gene sequence obtained after codon optimization is shown in SEQ ID NO.1.
[0120] 2. Construction of recombinant expression vector of gE protein
[0121] The gE protein coding gene sequence was designed and the whole gene was synthesized to obtain the nucleotide sequence shown in SEQ ID NO.1. ...
Embodiment 2
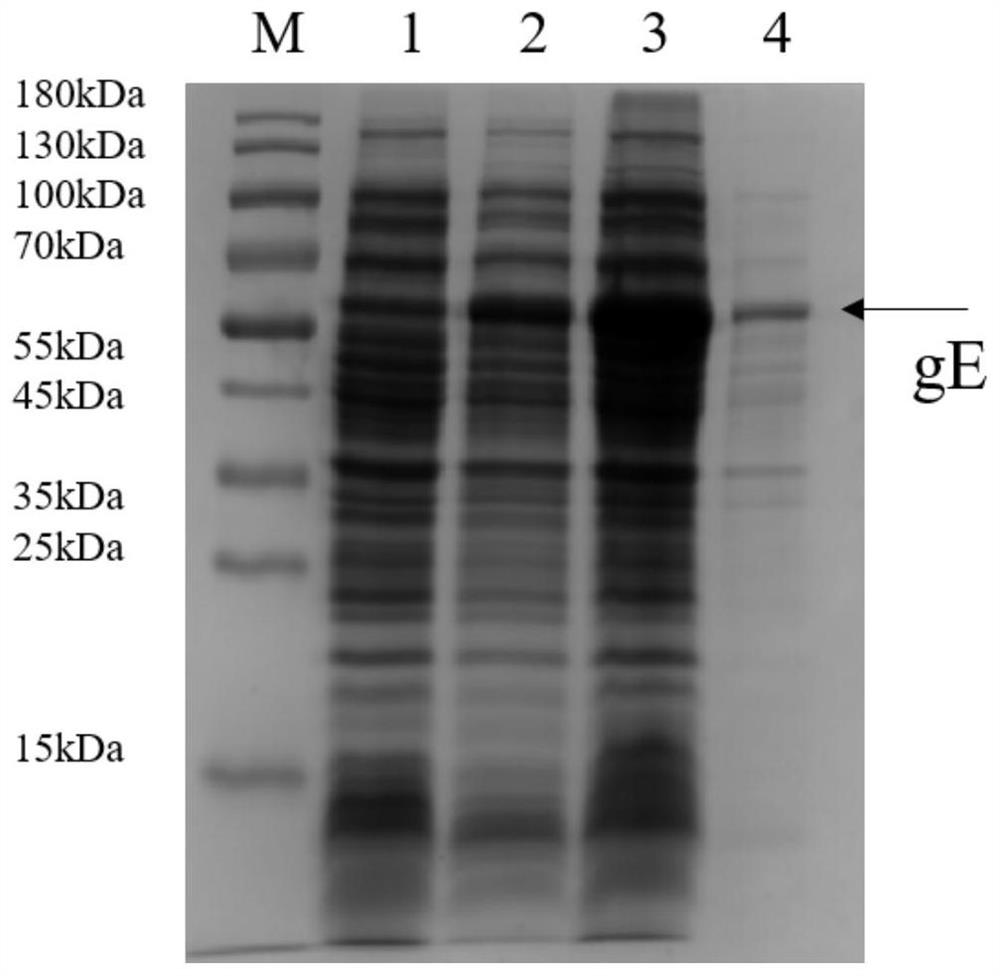
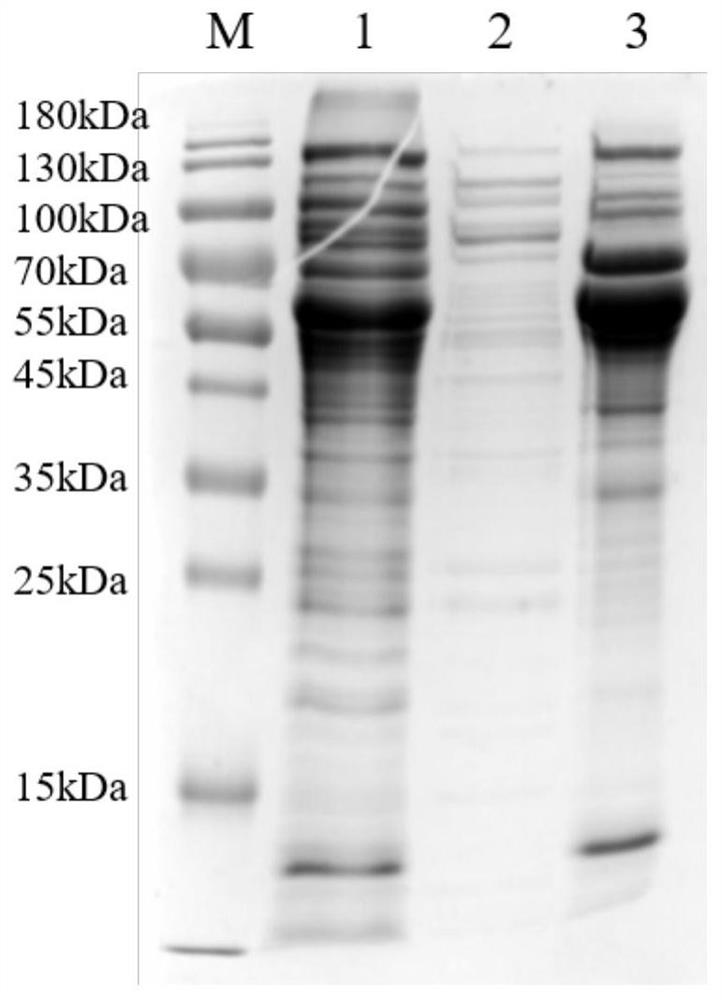
[0127] The preparation technology of embodiment 2 recombinant gE protein
[0128] 1. Fermentor culture of pET28a-gE-BL21(DE3) engineering strain
[0129] The pET28a-gE-BL21(DE3) expression strain stored in glycerol tube was inoculated into LB medium at a ratio of 1:1000 (v / v), and cultured in shake flasks for 16-24 hours to obtain seed liquid. The seed liquid was inoculated into the fermentation medium at a ratio of 1:20 (v / v) to make E. coli cells grow, and the fermentation was terminated after 4 hours of induction. The fermented cells were collected after centrifugation for subsequent purification.
[0130] The composition of the fermentation medium is: disodium hydrogen phosphate dodecahydrate 1.7%, potassium dihydrogen phosphate 0.3%, magnesium sulfate 0.0225%, anhydrous calcium chloride 0.00113%, glucose 0.5%, ammonium sulfate 0.2%, yeast powder 1%, tryptone 1%, sodium chloride 0.5%. Feed (mass ratio) during fermentation and culture: 20% yeast powder.
[0131] The fer...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Theoretical molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com