Patents
Literature
35 results about "Indirect Immunofluorescence Assays" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Indirect immunofluorescence assay: A laboratory test used to detect antibodies in serum or other body fluid. The specific antibodies are labeled with a compound that makes them glow an apple-green color when observed microscopically under ultraviolet light.
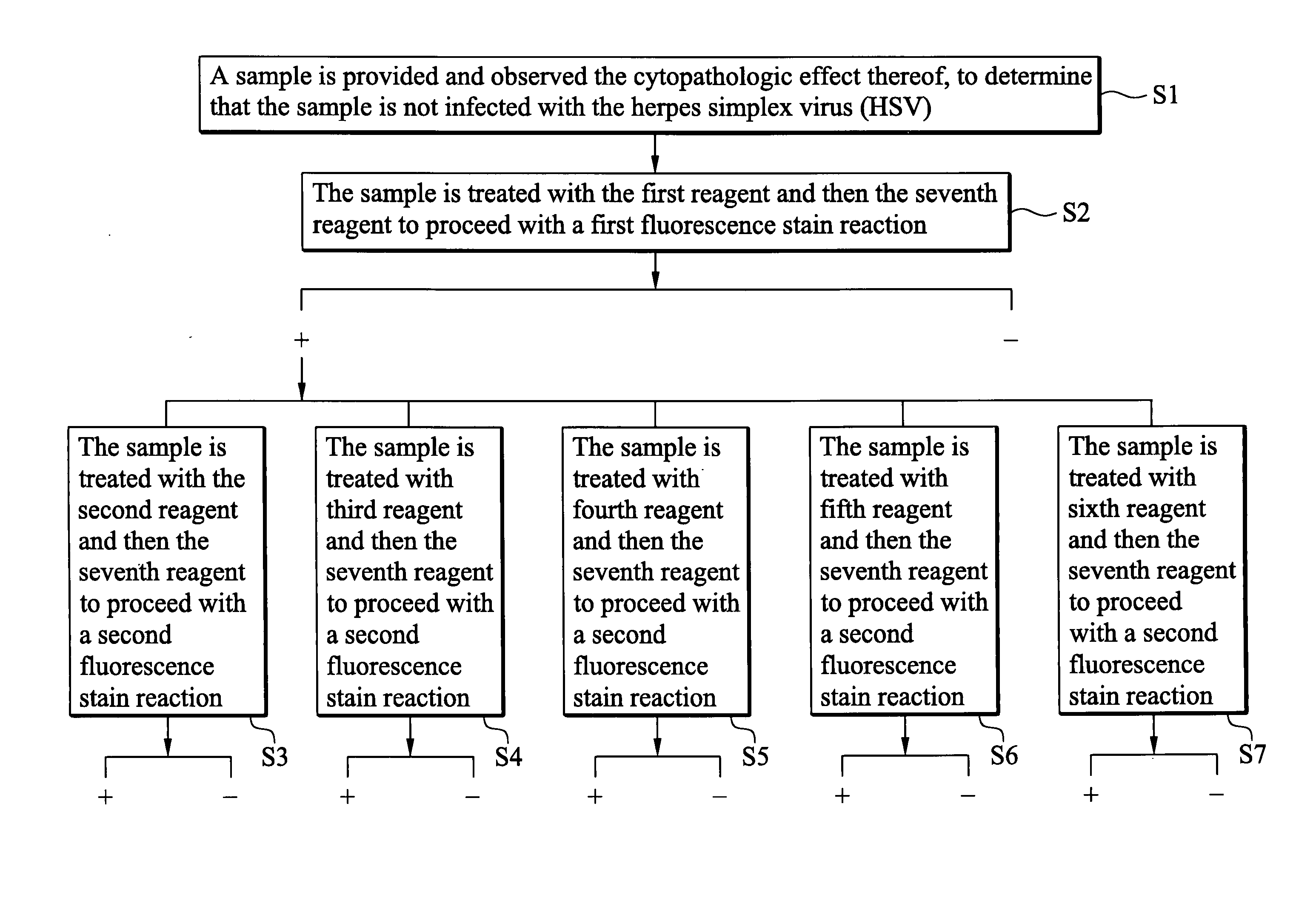
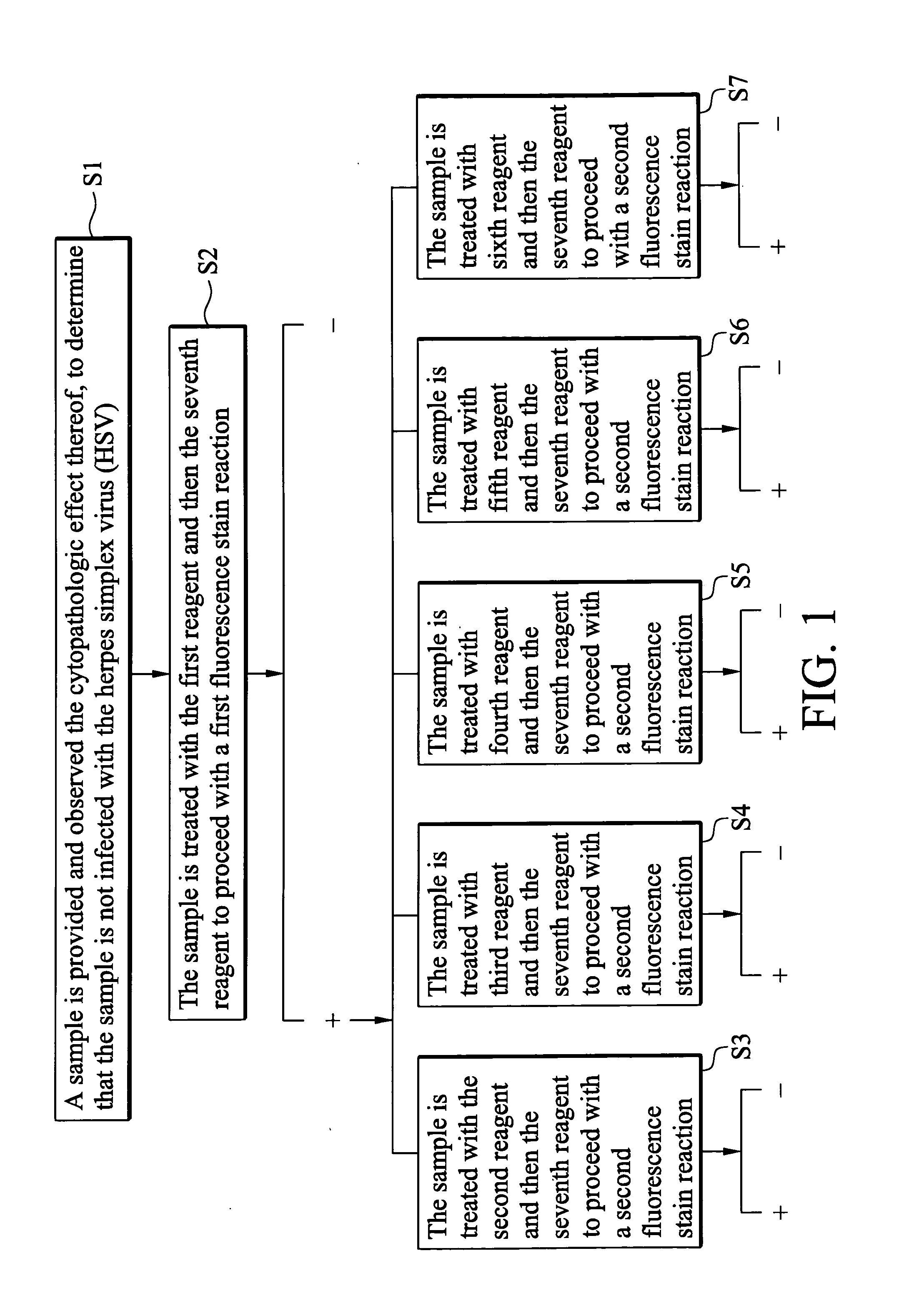
Indirect immunofluorescence assay typing kit for coxsackievirus A group and method for typing coxsackievirus A group
InactiveUS20090317796A1Microbiological testing/measurementBiological material analysisImmunofluorometric AssaysTyping
An indirect immunofluorescence assay typing kit for coxsackievirus, comprising: a first reagent comprising a mixture of an anti-coxsackievirus A2 polyclonal antibody, an anti-coxsackievirus A4 polyclonal antibody, an anti-coxsackievirus A5 polyclonal antibody, an anti-coxsackievirus A6 polyclonal antibody, and an anti-coxsackievirus A10 polyclonal antibody; a second reagent comprising the anti-coxsackievirus A2 polyclonal antibody; a third reagent comprising the anti-coxsackievirus A4 polyclonal antibody; a fourth reagent comprising the anti-coxsackievirus A5 polyclonal antibody; a fifth reagent comprising the anti-coxsackievirus A6 polyclonal antibody; a sixth reagent comprising the anti-coxsackievirus A10 polyclonal antibody; and a seventh reagent comprising a secondary antibody labeled with a fluorescence compound, wherein the secondary antibody is used for detecting the antibody anti-coxsackieviruses A2, A4, A5, A6 and A10 polyclonal antibodies and a titer of the anti-coxsackieviruses A2, A4, A5, A6 or A10 polyclonal antibody is about 1:5000-151:70000.
Owner:CENTS FOR DISEASE CONTROL DEPT OF HEALTH
Antiviral serotype shared monoclonal antibody of foot-and-mouth disease and distinguished epitope thereof
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
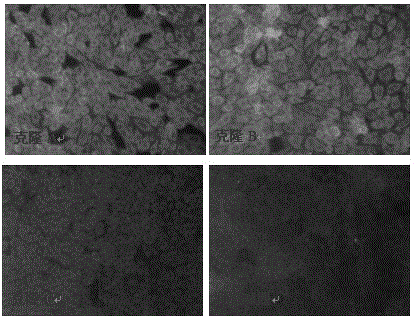
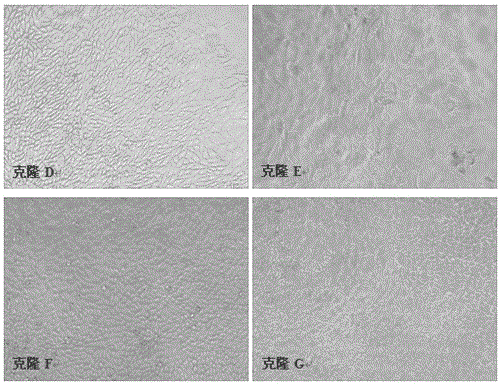
Method for performing screening in virus-sensitive cell cloning by applying indirect immunofluorescence assay technology
ActiveCN102994445AShort detection timeEasy to operateVertebrate cellsArtificial cell constructsVirulent characteristicsFluorescence
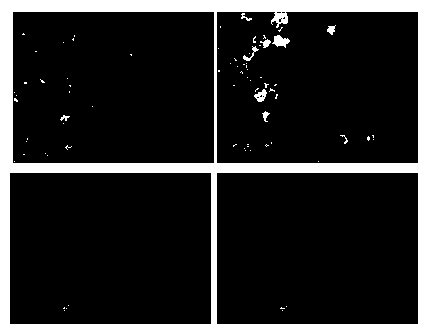
The invention relates to the field of biotechnology, and particularly relates to a method for performing screening in virus-sensitive cell clonal strains by applying an indirect immunofluorescence assay technology. The method comprises the following steps of: identifying the purity of a cell line; obtaining monoclonal cell strains in the cell line; and identifying the sensitivity of the monoclonal cell strains to virus. The method disclosed by the invention has the beneficial effects of being short in detection time, capable of identifying the sensitivity of single-cell cloning to virus only by 3 days, relative simple to operate, easy, capable of being used for screening lots of non-cytopathic virus-sensitive cell strains, stable in result, strong in repeatability, and consistent with the effect of a virulence determination method.
Owner:山东滨州沃华生物工程有限公司
Monoclonal antibody BTV16-3G10 resistant to bluetongue virus serum 16 type VP2 protein, B-cell epitope polypeptide identified by monoclonal antibody BTV16-3G10, and applications of monoclonal antibody BTV16-3G10
The invention discloses monoclonal antibody BTV16-3G10 resistant to bluetongue virus serum 16 type VP2 protein, B-cell epitope polypeptide identified by the monoclonal antibody BTV16-3G10, and applications of the monoclonal antibody BTV16-3G10, and belongs to the field of bluetongue prevention. The invention also relates to a hybridoma cell strain which is capable of secreting the monoclonal antibody BTV16-3G10, and monoclonal antibody secreted by the hybridoma cell strain. It is shown by indirect immunofluorescence assay that the specific reaction is observed between the monoclonal antibody and BTV16, and no cross reaction is observed between the monoclonal antibody and other serum types of bluetongue virus, Ibaraki virus or Chuzan disease virus. Further, BTV16-VP2 protein B-cell epitope polypeptide which can be identified by the monoclonal antibody is determined via cutting expression antigen short peptide and peptide scanning technology. The monoclonal antibody, and the BTV16-VP2 protein B-cell epitope polypeptide identified by the monoclonal antibody can be used for preparation of BTV16 specific differential diagnosis reagents, and foundation is provided for development of BTV epitope labeled vaccines.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Recombinant expression protein of 1301 ORF136 genes of herpesvirus cyprinid type 3, antibody and application of antibody
InactiveCN107056898AHigh purityMaintain biological activitySerum immunoglobulinsVirus peptidesNucleotideWestern blot
The invention discloses a recombinant expression protein of 1301 ORF136 genes of herpesvirus cyprinid type 3, a polyclonal antibody and an application of the polyclonal antibody. A full-length amino acid sequence of the recombinant expression protein of 1301 ORF136 genes of the herpesvirus cyprinid type 3 is as shown in SEQ ID NO:2, and a full-length nucleotide sequence encoding the protein is as shown in SEQ ID NO:1. A pET32a-ORF136 recombinant prokaryotic expression vector is constructed by selecting one part of gene sequence of ORF136; the recombinant expression protein is obtained through IPTG-induced expression; the obtained polyclonal antibody is subjected to western blot analysis by adopting a purified CyHV-3 virus and a KS cell infected by the CyHV-3; and the detection application of the antibody is further verified by adopting an indirect immunofluorescence assay. An important material is provided for construction of an ORF136 protein function research and CyHV-3 serological diagnosis method through preparation of the polyclonal antibody of the recombinant expression protein of the ORF136 genes.
Owner:PEARL RIVER FISHERY RES INST CHINESE ACAD OF FISHERY SCI
Preparation method of PCV2 (Porcine Circovirus2)-D
The invention discloses a preparation method of PCV2 (Porcine Circovirus2)-D. The microorganism preservation number of PCV2 (Porcine Circovirus2) is CGMCC (China General Microbiological Culture Collection Center) No.7245. According to the preparation method, the preference of genetic codon of the PCV2 is modified, and the preferred codon is modified into non-preference codon, so that the expressions of the components of the virus are reduced, further, the copy rate of the virus in host cells is lowered, the virus is weakened, and the PCV2-D is obtained by genetic modification. Furthermore, the proliferation speed of the PCV2-D is compared with that of wild type strains at a cellular level. The detection of PCR (Polymerase Chain Reaction) and IFA (Indirect Immunofluorescence Assay) prove that the PCV2-D can be infected and copied in cells, and has certain infection.
Owner:SHANGHAI ACAD OF AGRI SCI
Monoclonal antibody BTV16-2B4 resistant to bluetongue virus serum 16 type VP2 protein, B-cell epitope identified by monoclonal antibody BTV16-2B4, and applications of monoclonal antibody BTV16-2B4
InactiveCN103740649AVirus peptidesImmunoglobulins against virusesIbaraki virusMonoclonal antibody 14G2A
The invention discloses monoclonal antibody BTV16-2B4 resistant to bluetongue virus serum 16 type VP2 protein, B-cell epitope identified by the monoclonal antibody BTV16-2B4, and applications of the monoclonal antibody BTV16-2B4, and belongs to the field of bluetongue prevention. The invention also relates to a hybridoma cell strain which is capable of secreting the monoclonal antibody BTV16-2B4, and monoclonal antibody secreted by the hybridoma cell strain. It is shown by indirect immunofluorescence assay that the specific reaction is observed between the monoclonal antibody and BTV16, and no cross reaction is observed between the monoclonal antibody and other serum types of bluetongue virus, Ibaraki virus or Chuzan disease virus. Further, BTV16-VP2 protein B-cell epitope polypeptide which can be identified by the monoclonal antibody is determined via cutting expression antigen short peptide and peptide scanning technology. The monoclonal antibody, and the BTV16-VP2 protein B-cell epitope polypeptide identified by the monoclonal antibody can be used for preparation of BTV16 specific differential diagnosis reagents, and foundation is provided for development of BTV epitope labeled vaccines.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
A kind of porcine circovirus type Ⅱ nucleic acid vaccine and its preparation method
InactiveCN102274523AViral antigen ingredientsGenetic material ingredientsRestriction enzyme digestionFluorescence
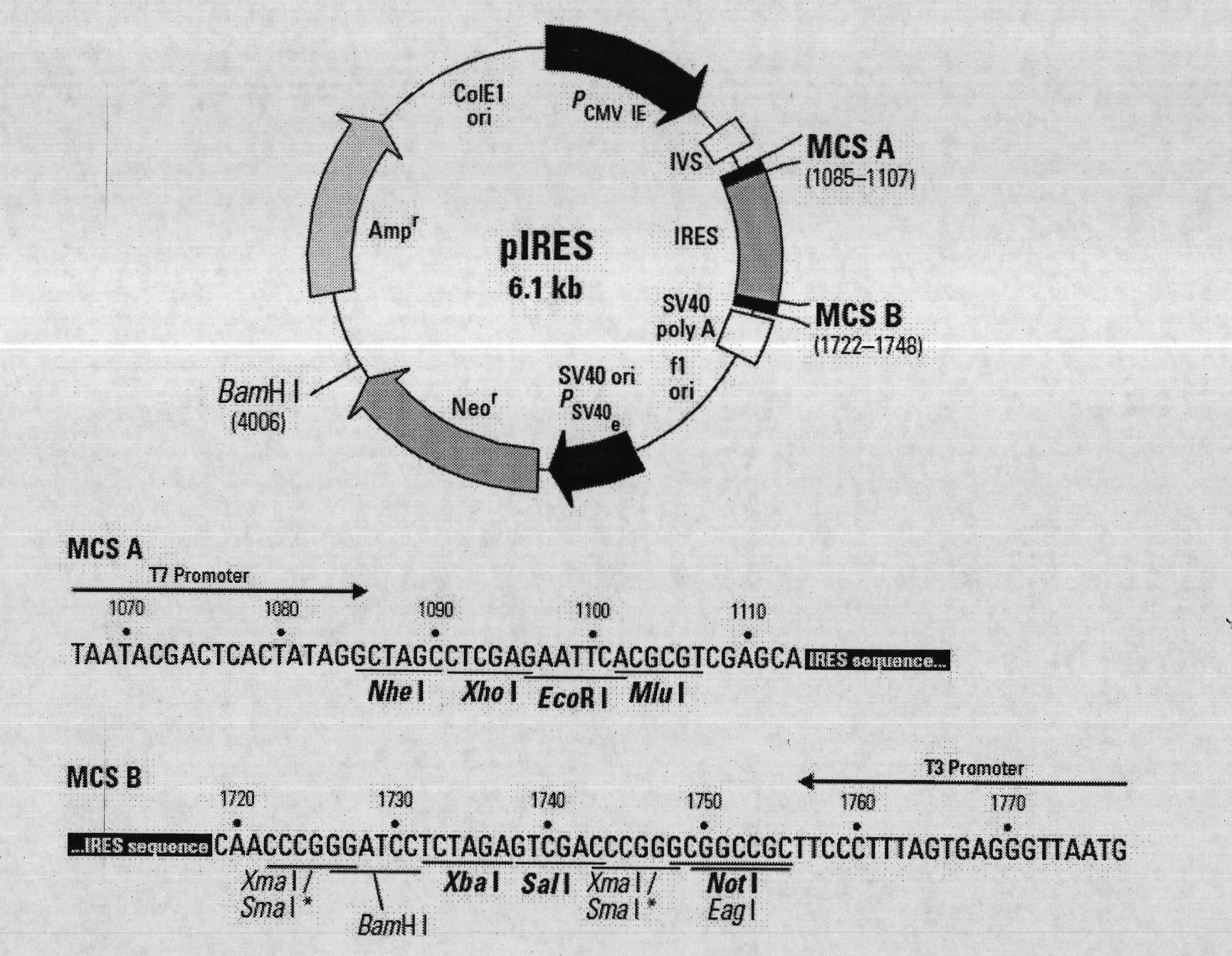
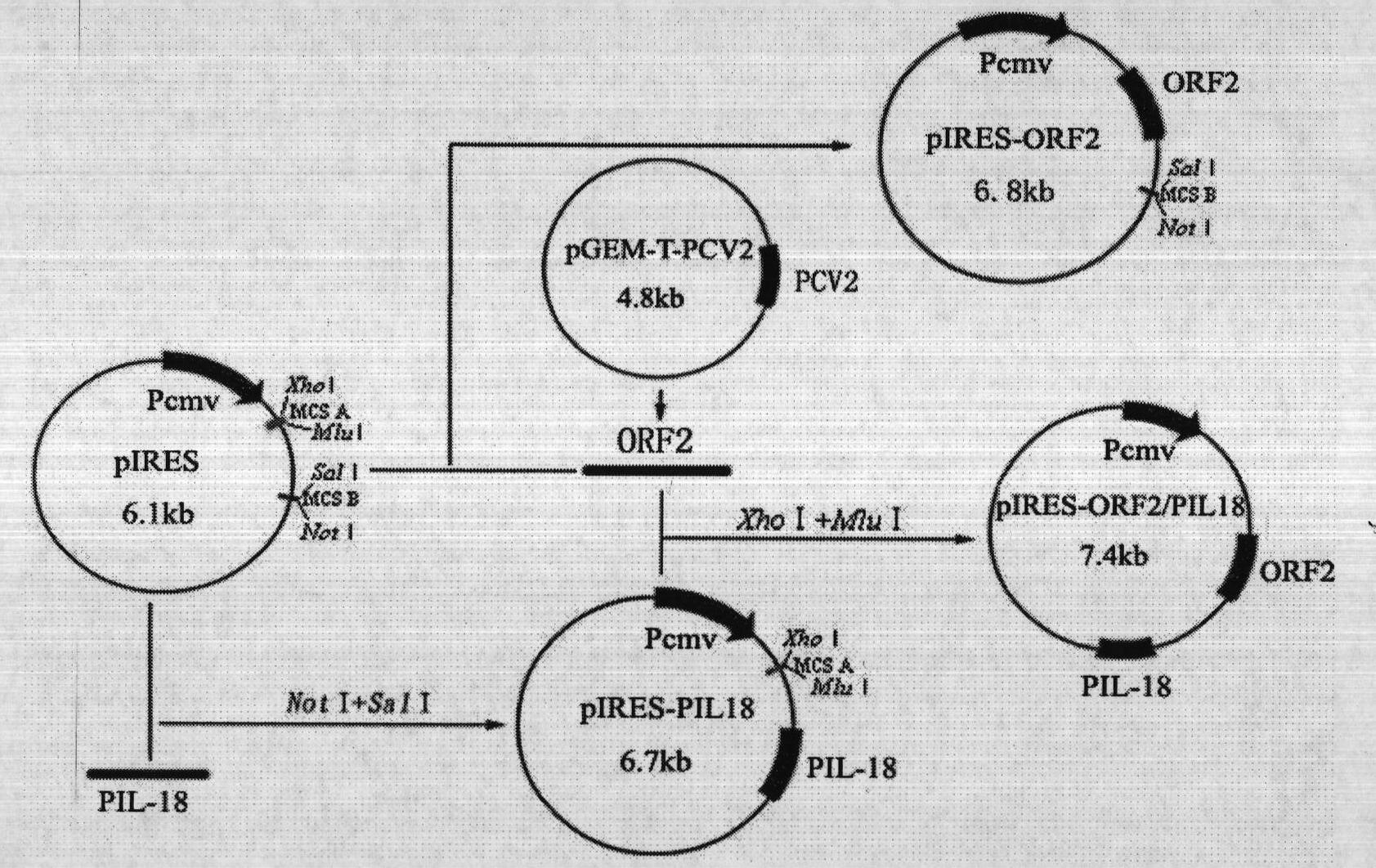
The technical scheme of the present invention is: a kind of porcine circovirus type II nucleic acid vaccine, respectively inserting porcine circovirus type II LY strain ORF2 and porcine IL-18 gene into two multiple cloning sites of the eukaryotic expression vector pIRES, The nucleic acid vaccine co-expresses porcine circovirus type II LY strain Cap and porcine IL-18 protein. The present invention is identified by PCR, double enzyme digestion and sequencing, which proves that the co-expression plasmid pIRES-ORF2 / PIL18 has been successfully constructed. The successfully constructed recombinant plasmid pIRES-ORF2 / PIL18 was transfected into porcine kidney PK-15 cells by liposome method, and the expression of the target protein was detected by RT-PCR and indirect immunofluorescence. The transcription of the two target genes was detected by RT-PCR; the specific bright green fluorescence of ORF2 protein was observed in the cytoplasm and nucleus by indirect immunofluorescence test, which proved that the protein was expressed.
Owner:HENAN AGRICULTURAL UNIVERSITY
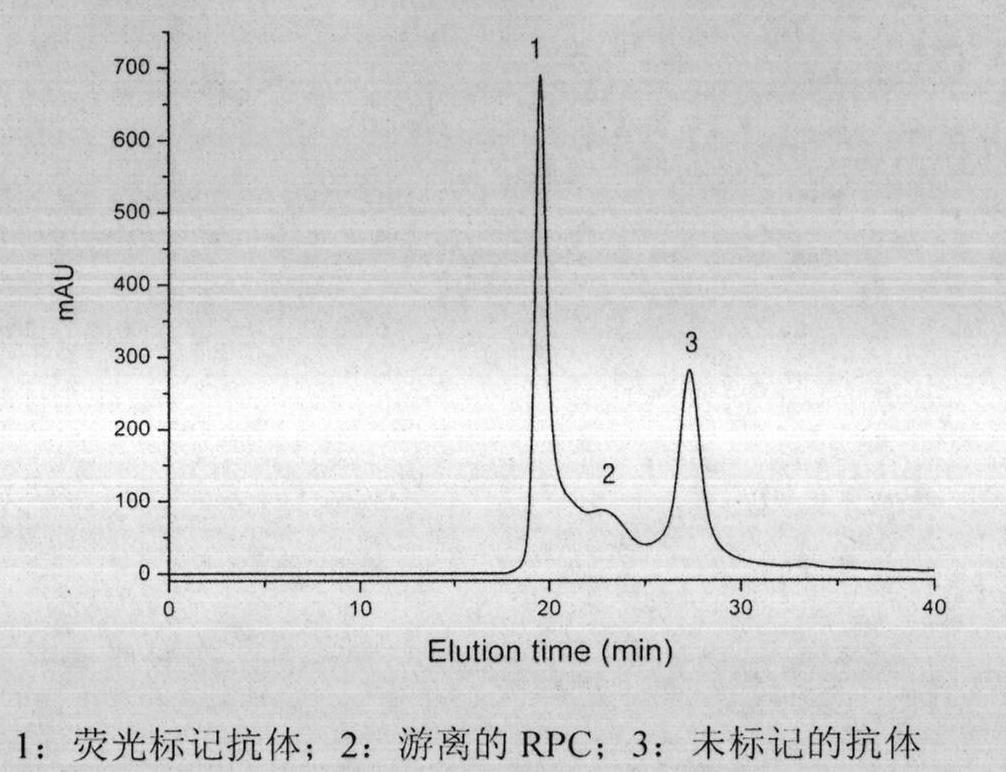
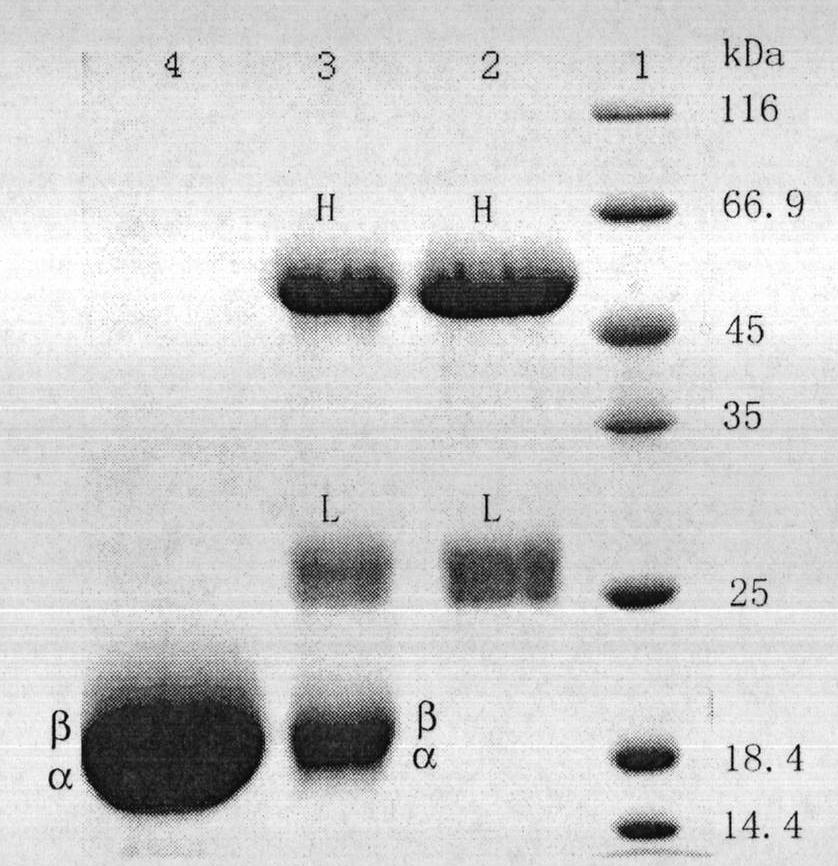
Preparation method of R-phycocyanin (RPC)-marked fluorescent anti-antibody
The invention relates to a preparation method of an R-phycocyanin (RPC)-marked fluorescent anti-antibody. A preparation process comprises the following steps of: preparing the RPC by an anion exchange chromatographic method; deriving the RPC and the anti-antibody with difunctional chemical cross-linking agents, namely, N-succinimidyl-3-(2-pyridyldithiol)propionate (SPDP) in molar ratios of 20-30:1 and 50-100:1; crosslinking a derivative with a liquid phase in an appropriate molar ratio; and performing high-pressure liquid phase chromatography purification so as to prepare the RPC-marked fluorescent anti-antibody. The RPC-marked fluorescent anti-antibody prepared by the method has high crosslinking efficiency, purity reaching electrophoretic purity, bright red fluorescence and stable fluorescent anti-antibody activity and can be taken as the general fluorescent probe for performing indirect immunofluorescence assay on zoonosis.
Owner:QILU UNIV OF TECH
Construction method and application of DNA (Deoxyribonucleic Acid) vaccine for avian leukosis virus subgroup J
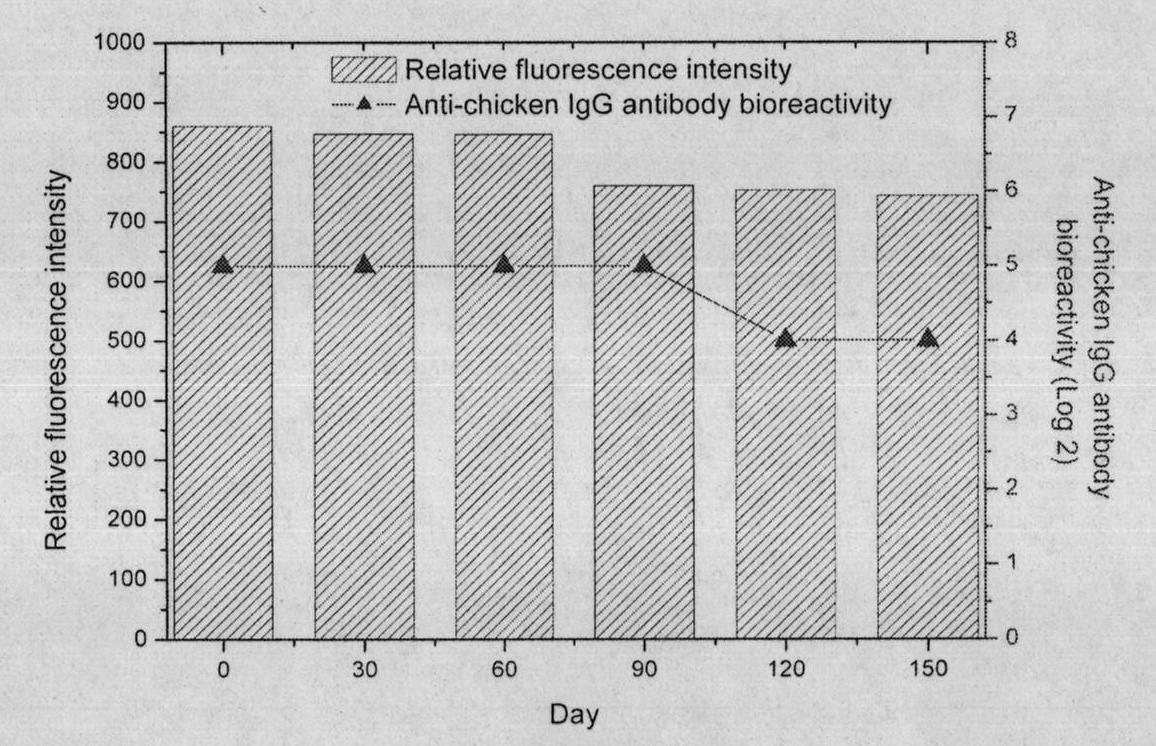
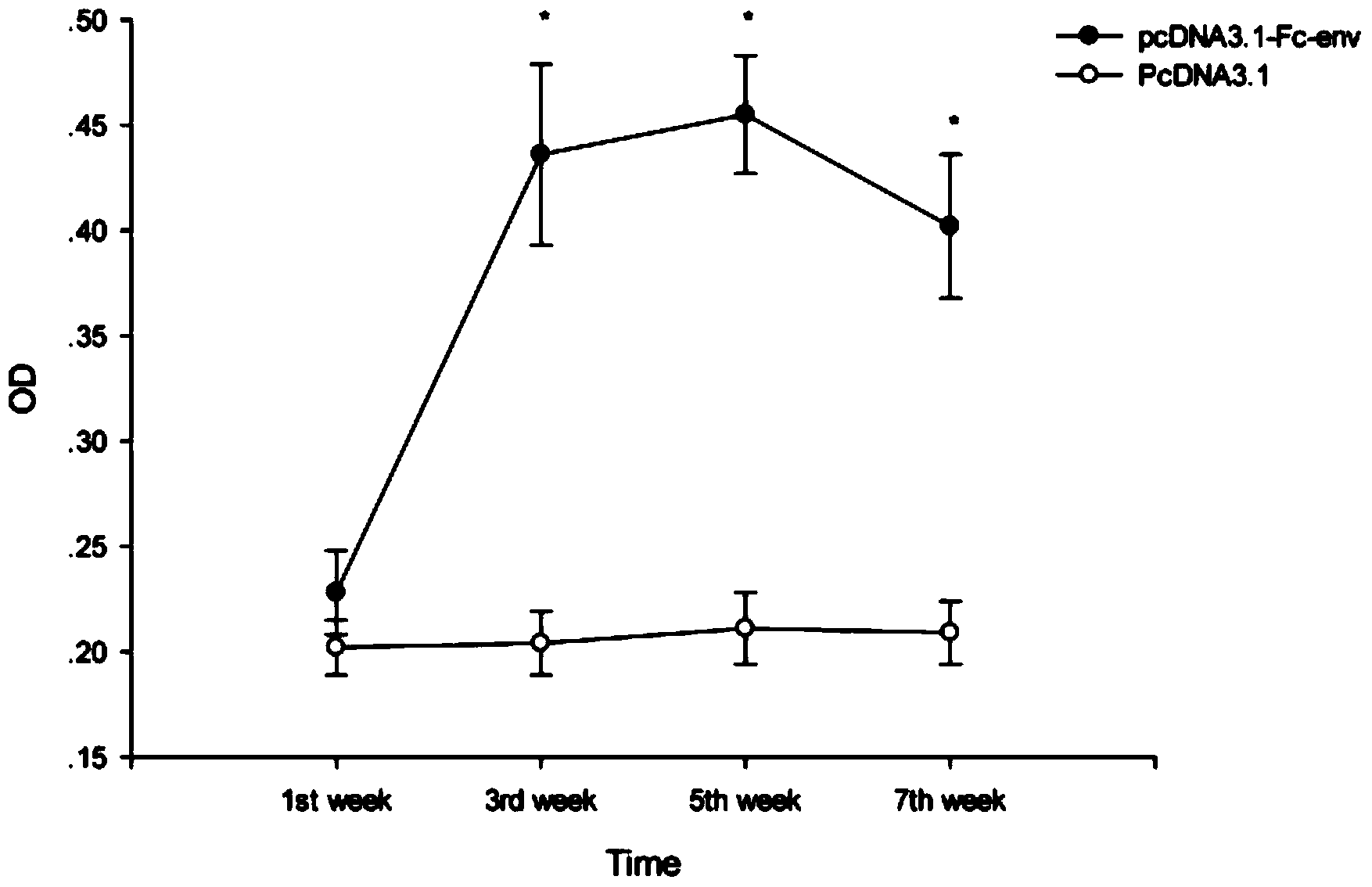
The invention relates to a construction method and application of a DNA (Deoxyribonucleic Acid) vaccine for avian leukosis virus subgroup J. A recombinant eukaryotic expression plasmid pcDNA3.1-Fc-env of an Fc fragment gene for expressing chicken immunoglobulin G and an envelope protein (env protein) gene of the avian leukosis virus subgroup J is constructed; transient transfection and indirect immunofluorescence assay prove that the pcDNA3.1-Fc-env can be accurately expressed in a 293T cell; a great number of plasmids are extracted, purified and quantified to 1mg / ml, then, the recombinant plasmids are used for immunizing mice, each mouse is immunized three times, 100mu g of recombinant plasmids are used in each immunization, and one immunization is carried out every two weeks; the level of an ALV-J env protein-specific antibody in serum is detected to show that the pcDNA3.1-Fc-env has the effect of preventing the avian leukosis virus subgroup J.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
Construction method of recombinant baculovirus for expressing serum type 4 avian adenovirus fibroid protein F2
InactiveCN111534547AImprove expression levelOptimize purification stepsFermentationDsDNA virusesCytopathic effectShuttle vector
The invention relates to a construction method of recombinant baculovirus for expressing serum type 4 avian adenovirus fibroid protein F2. The construction method comprises the following steps: (1) constructing an FAdV4F2 gene recombinant baculovirus expression vector; (2) packaging and passing baculovirus for expressing recombinant FAdV4 protein; and (3) transfecting baculovirus genome carrying FAdV4F2 gene into Sf9 cells, culturing in an incubator at 27 DEG C for 57 days, and observing cytopathic effect; when cytopathic effect reaches 70%, collecting cell lysis buffer, centrifuging to removeprecipitate, and preserving as seed virus; passing virus, and carrying out enlarged culture as seed virus when passing to the third generation. According to the invention, a baculovirus expression system is utilized to construct a recombinant baculovirus shuttle vector containing F2 gene, the recombinant baculovirus shuttle vector is transfected into Sf9 cells to obtain recombinant baculovirus rBAF2, and expression of recombinant protein F2 is identified by virtue of indirect immunofluorescence assay (IFA) and Western blot.
Owner:YANGZHOU UNIV
Application of porcine circovirus type 2 double-copy infectious clone to indirect ELISA detection method
InactiveCN106701793AImprove connection efficiencyImprove efficiencyMicrobiological testing/measurementFermentationCircovirusGene engineering
The invention relates to a porcine circovirus type 2 (PCV2) double-copy infectious clone virus rescue method and application thereof to an indirect ELISA detection method. PCV2 genome DNA is obtained on the basis of clinical separation by adopting a proper gene engineering technology method, specific primers are designed, full-length genes are obtained through polymerase chain reaction (PCR), two copied PCV2 full-length genes are in end-to-end connection to be connected on a pEGFP-N1 vector in series to form PCV2 double-copy whole-gene recombinant plasmid, ST cells are transfected, blind passage is conducted continuously for 60 generations, the infectivity on the ST cells and the virus reproduction stability are authenticated by virtue of a PCR detection method and an indirect immunofluorescence assay (IFA) technology, and foundation is laid for further development of the pathogenesis of a circovirus and research of a diagnosis technology.
Owner:广东永顺生物制药股份有限公司
Monoclonal antibody for identifying PCV2 virus-like particles and application thereof in qualitative and quantitative detection of PCV2 virus-like particles
ActiveCN109536456AAccurate measurementSpecific determinationImmunoglobulins against virusesTissue cultureFluorescenceVirus-like particle
The invention discloses a monoclonal antibody for identifying PCV2 virus-like particles and an application thereof in qualitative and quantitative detection of PCV2 virus-like particles, wherein the monoclonal antibody capable of specifically identifying porcine circovirus type 2 virus-like particles is secreted by a hybridoma cell line with the preservation number of CGMCC NO.15793. The specificity of the obtained monoclonal antibody 3H9 is identified by indirect immunofluorescence assay, and the result indicates that the monoclonal antibody can specifically identified the PCV2 virus-like particles and can react with the PCV2 VLPs, but not with an ELISA plate coated with a linear cap protein. Therefore, a simple method for simultaneous qualitative and quantitative analysis of the PCV2 VLPs is established, and the problems of expensive instruments for detecting a structure of PCV2 VLP particles, difficult operation and inability of quantitative methods to distinguish non-specific proteins from broken subunits are solved.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI +1
Canine primary bronchial epithelial cell and application thereof in preparation of immortalized cells
ActiveCN104531607AHigh purityOvercome the disadvantage of very limited proliferative abilityArtificial cell constructsVertebrate cellsTelomeraseBeagle
The invention provides a canine primary bronchial epithelial cell and an application thereof in preparation of immortalized cells, and belongs to the technical field of biology. The canine primary bronchial epithelial cell is prepared by taking 45-day-old clean male beagles; separating canine tracheal tissues, cutting into pieces, adding collagenase and trypsin to digest, so as to obtain intact bronchiole tissues; cultivating the obtained cells, and further carrying out indirect immunofluorescence assay to detect keratin 18 expression, wherein an experiment proves that the canine primary bronchial epithelial cell is successfully obtained. A eukaryotic expression vector pEGFP-hTERT containing telomerase genes (hTERT) is built; after the canine primary bronchial epithelial cell is transfected, the hTERT gene is highly expressed; the bronchial epithelial cell can be stably subcultured for at least 20 generations. According to the canine primary bronchial epithelial cell provided by the invention, important biological materials are provided for research of infection mechanism of canine pathogens such as canine influenza viruses.
Owner:NANJING AGRICULTURAL UNIVERSITY
Anti-IBRV single-chain antibody and preparation method and application thereof
InactiveCN107312087ASmall molecular weightEasy constructionImmunoglobulins against virusesAntiviralsEscherichia coliWAS PROTEIN
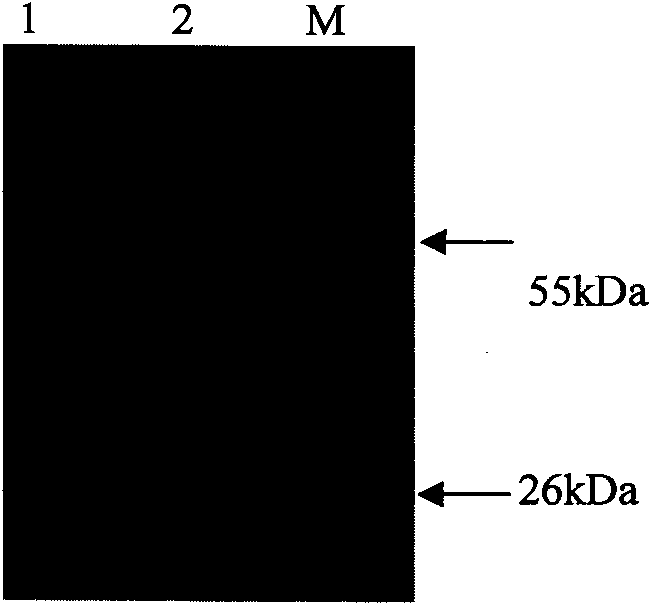
The invention provides an anti-IBRV single-chain antibody which is protein consisting of a heavy chain variable region shown as SEQ ID NO.1, a light chain variable region shown as SEQ ID NO.2 and a connecting peptide for connecting the two regions and having the characteristic of resisting an infectious bovine rhinotracheitis peplos gD protein antibody. The invention also provides a preparation method of the single-chain antibody. The single-chain antibody can be specifically combined with IBRV and can be also specifically combined with IBV gD protein in an escherichia coli expression product. An indirect immunofluorescence assay shows that the single-chain antibody can identify the IBRV infected in the bovine kidney cell MDBK, and the single-chain antibody has the good application value when applied to infectious bovine rhinotracheitis detection and development of treatment preparations.
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES
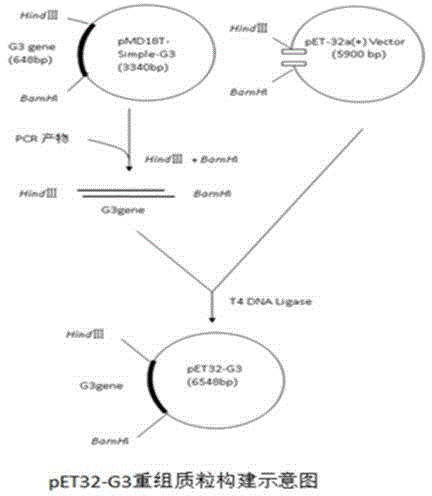
Expressed rabies virus glycoprotein optimized through gene modification and monoclonal antibody and application thereof
InactiveCN104928302AImproving immunogenicityEnsuring Response SpecificityImmunoglobulins against virusesDepsipeptidesNucleotideGene Modification
The invention discloses a modified G protein gene G3 of rabies virus. The nucleotide sequence of the G protein gene G3 is as shown by SEQ ID NO.1; the amino acid sequence of modified rabies virus G protein obtained through expression of the G protein gene G3 is as shown by SEQ ID NO.2; the rabies virus is a rabies virus HEP-Flury strain. Expression and preparation of a monoclonal antibody are performed by using the modified G protein gene G3 of the rabies virus HEP-Flury strain to construct a recombinant plasmid carrier. The monoclonal antibody can specifically recognize rabies virus glycoprotein and rabies virus, can be applied to indirect ELISA, Western-blotting tests and indirect immunofluorescence assay, can be used for detecting rabies virus and has good sensitivity and specificity.
Owner:SOUTH CHINA AGRI UNIV
Method for indirect immunofluorescence assay (IFA) detection of eperythrozoonosis by use of monoclonal antibody
InactiveCN103869071AReliable detectionQuick checkDisease diagnosisFluorescence/phosphorescenceAntigenPositive control
The invention discloses a method for indirect immunofluorescence assay (IFA) detection of eperythrozoonosis by use of a monoclonal antibody. The method includes establishment of a standard positive control and a standard negative control, antigen coating, fluorescence second antibody dilution, IFA determination and other steps. An antibody used in the method is a monoclonal antibody against porcine eperythrozoon, and compared with general antibodies used in IHA (indirect hemagglutination assay), ELISA (enzyme-linked immuno sorbent assay) and other detection methods, the antibody used in the method has the advantages of high specificity, high sensitivity, high accuracy and reliability, can fast, simply, reliably and accurately detect eperythrozoon in a sample, and can also be used for epidemiological investigation and curative effect detection of the eperythrozoonosis.
Owner:QINGDAO ZHONGREN PHARMA
Monoclonal antibody against natural cow gamma-interferon, hybridoma cell strain secreting antibody and application
ActiveCN104560885AImmunoglobulins against cytokines/lymphokines/interferonsMicroorganism based processesFluorescenceInterferon alpha
The invention discloses a monoclonal antibody against natural cow gamma-interferon, a hybridoma cell strain secreting the antibody and application. The hybridoma cell strain capable of stably secreting the monoclonal antibody against the natural cow gamma-interferon is named as 3D7 and is preserved in China General Microbiological Culture Collection Center, the strain preservation number is CGMCC No.9329, and the preservation data is June 25, 2014. According to the invention, a eukaryotic expression vector of the cow gamma-interferon is used for immunizing a mouse to screen the hybridoma cell strain capable of generating the antibody against the natural cow 1FN gamma-interferon by means of indirect immunofluorescence assay technology, the problem that the traditionally prepared IFN gamma-interferon can only react with a prokaryotic expression product and cannot react with natural IFN gamma is solved successfully, and a new technological means is provided for the detection of the natural cow gamma-interferon.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Chicken interleukin-18 monoclonal antibody as well as preparation method and application thereof
InactiveCN103589690AImmunoglobulins against cytokines/lymphokines/interferonsMicroorganism based processesMicroorganismProkaryotic expression
The invention discloses a chicken interleukin-18 monoclonal antibody as well as a preparation method and an application thereof. According to the method, recombinant protein ChIL-18 for prokaryotic expression is taken as an immunogen, and ChIL-18 protein for eukaryotic cell line expression is taken as a screening source; and after cell fusion, hybridoma cells are screened with an IFA (indirect immunofluorescence assay), and eight cell strains which secrete the chicken interleukin-18 monoclonal antibody are obtained through screening, wherein the stability of a hybridoma cell strain 4E11 is the best, the titer and the specificity of the monoclonal antibody secreted by the hybridoma cell strain 4E11 are the highest, and the microbial preservation number is CGMCC NO. 7658. The invention further provides the hybridoma cell strain 4E11 and an application of the monoclonal antibody secreted by the hybridoma cell strain 4E11 in detection or purification of the chicken interleukin-18 protein.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI +1
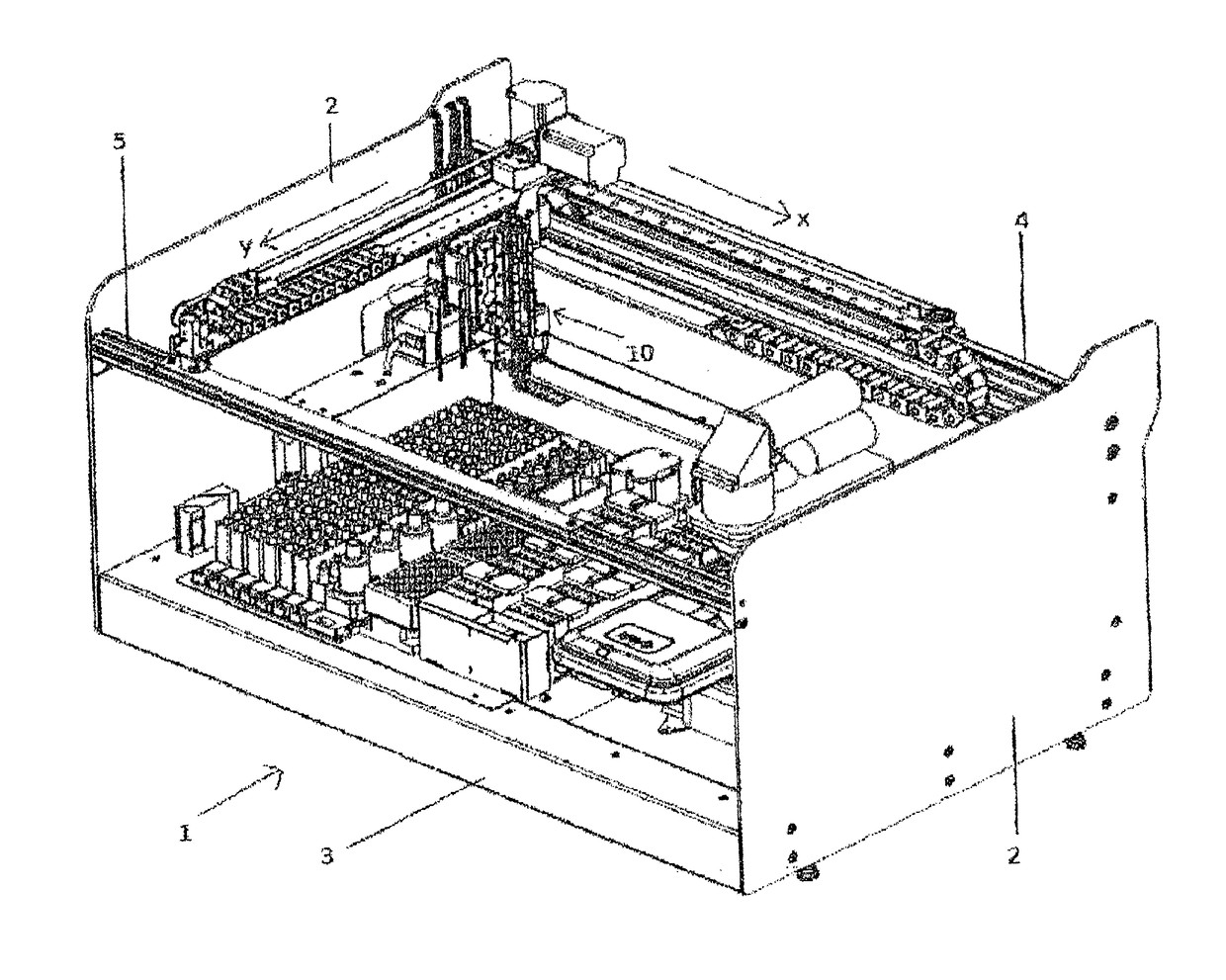
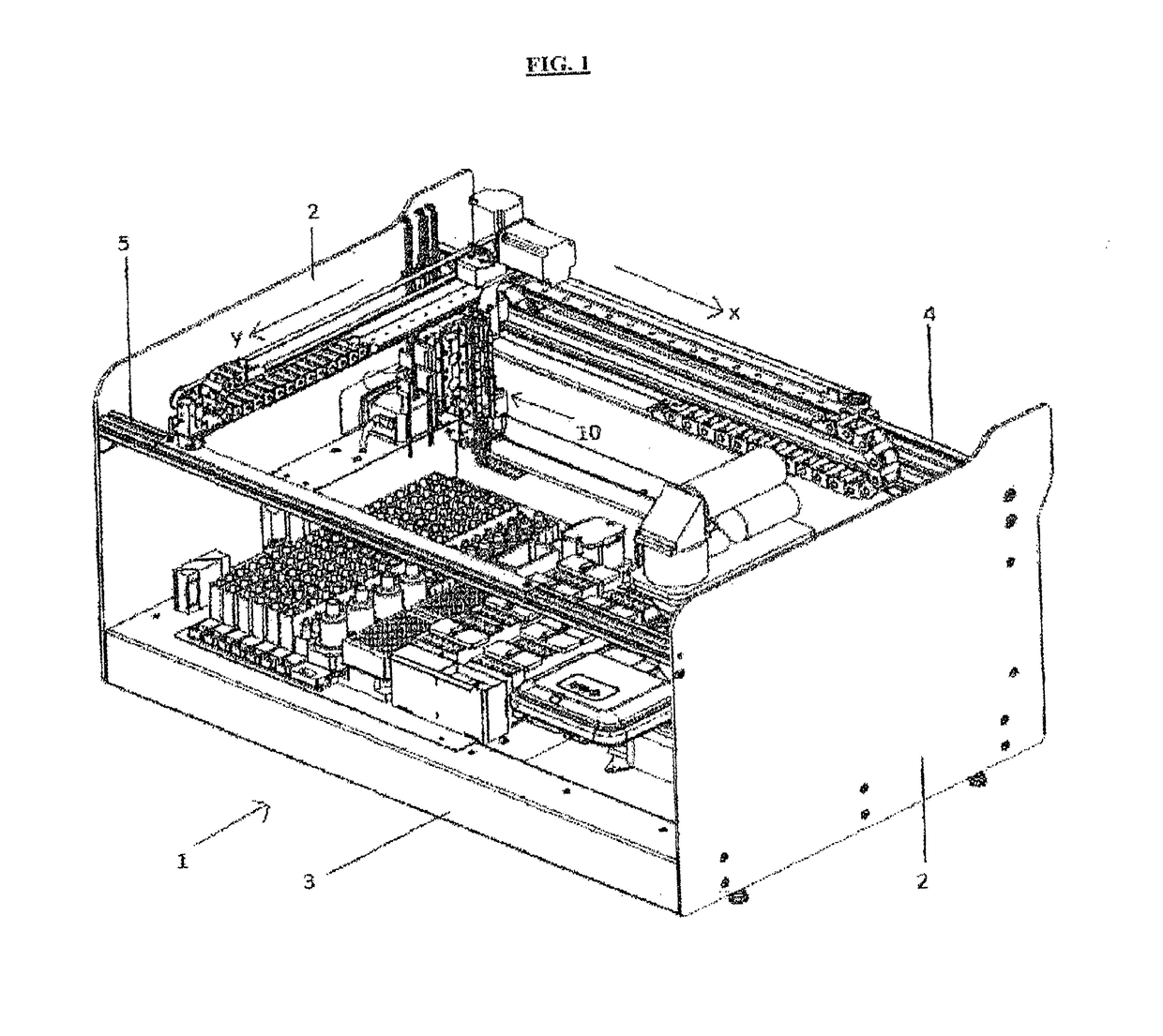
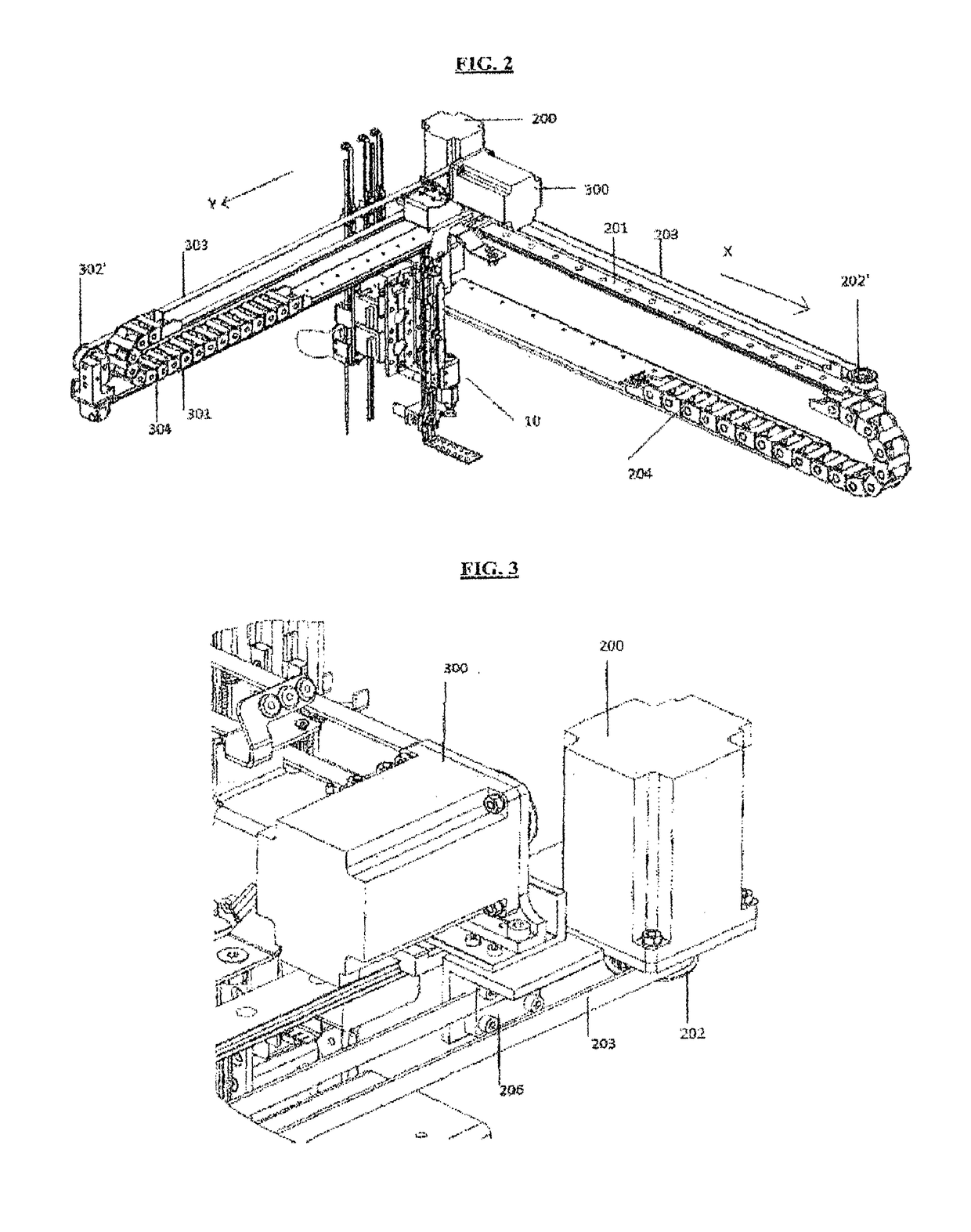
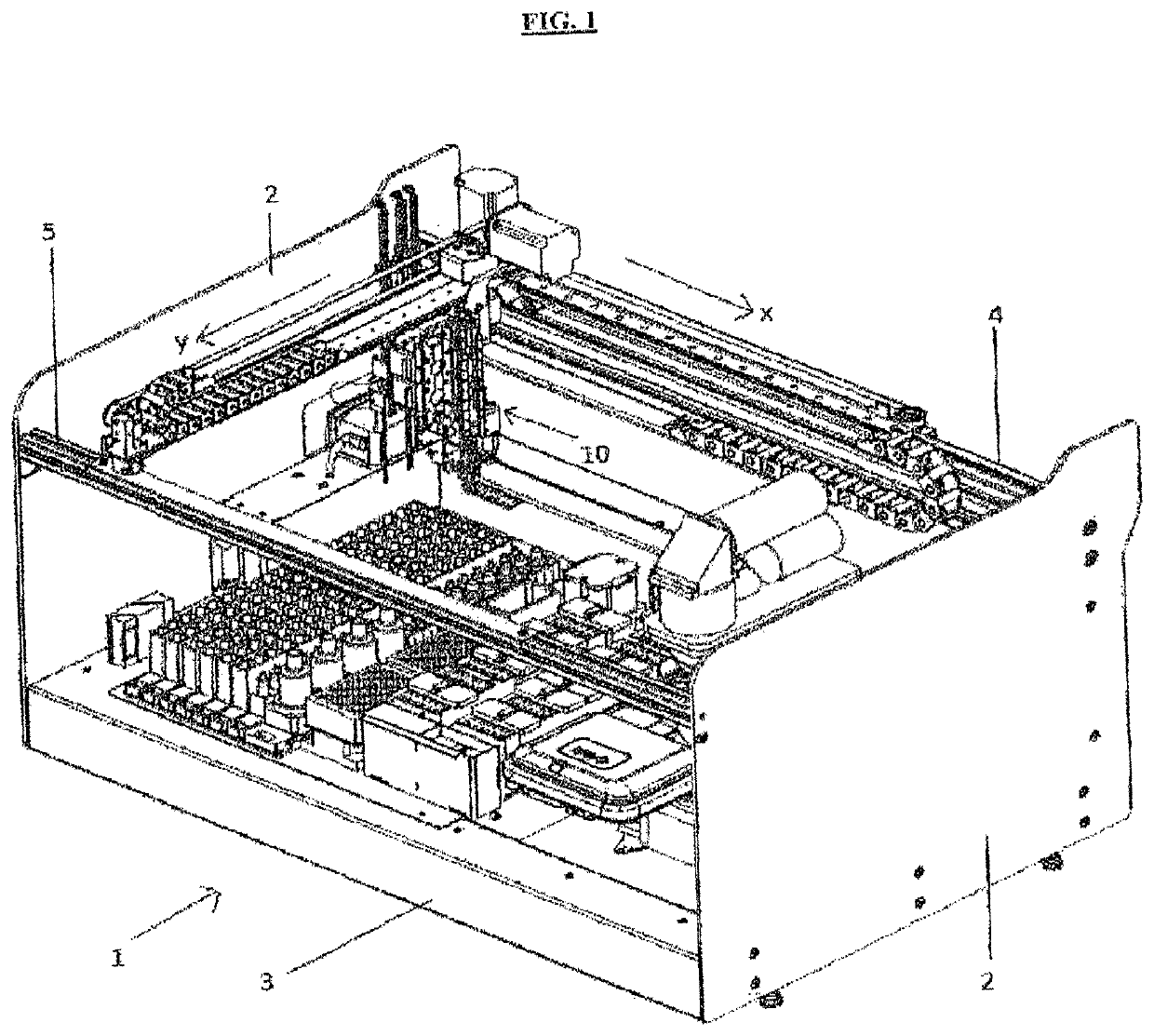
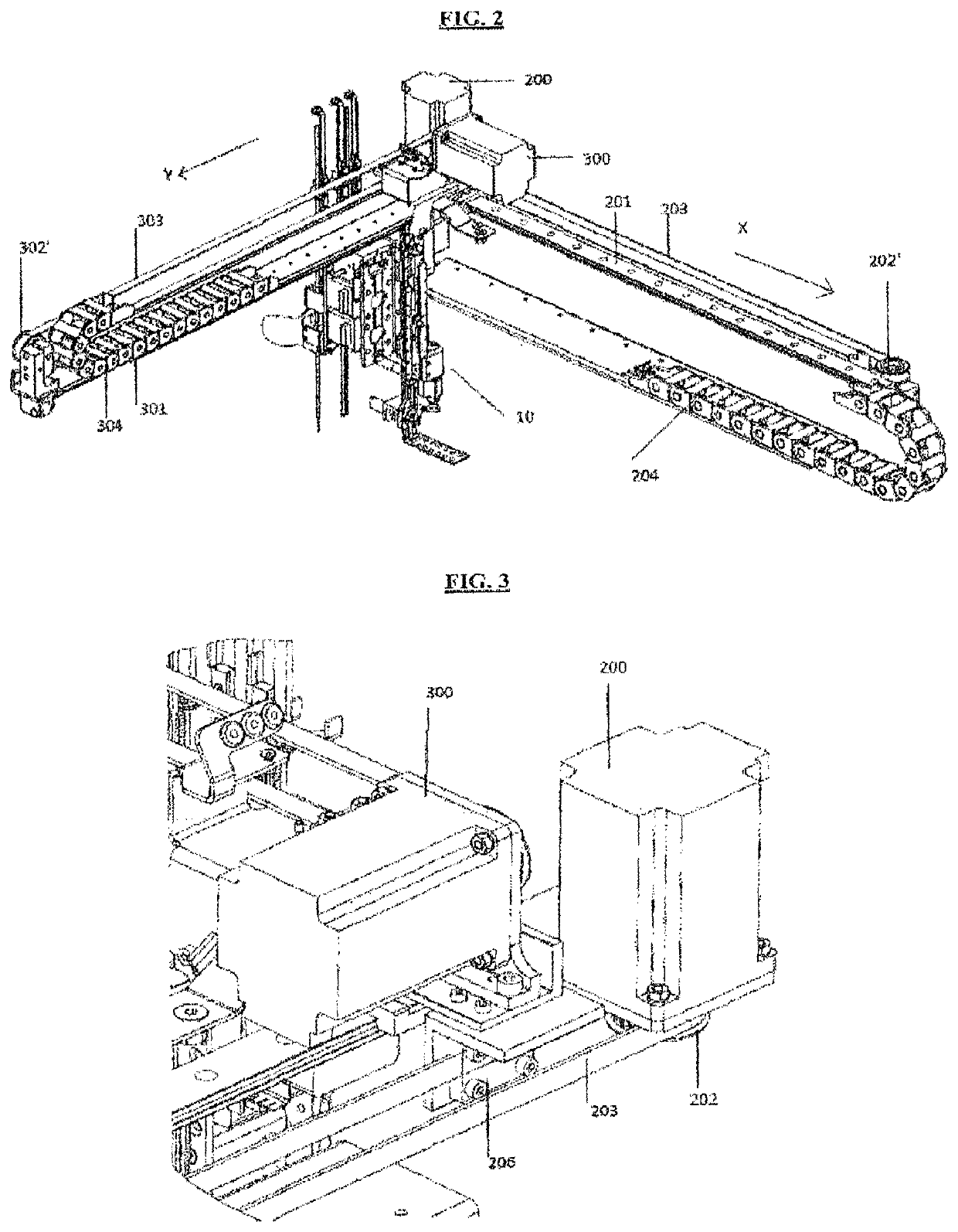
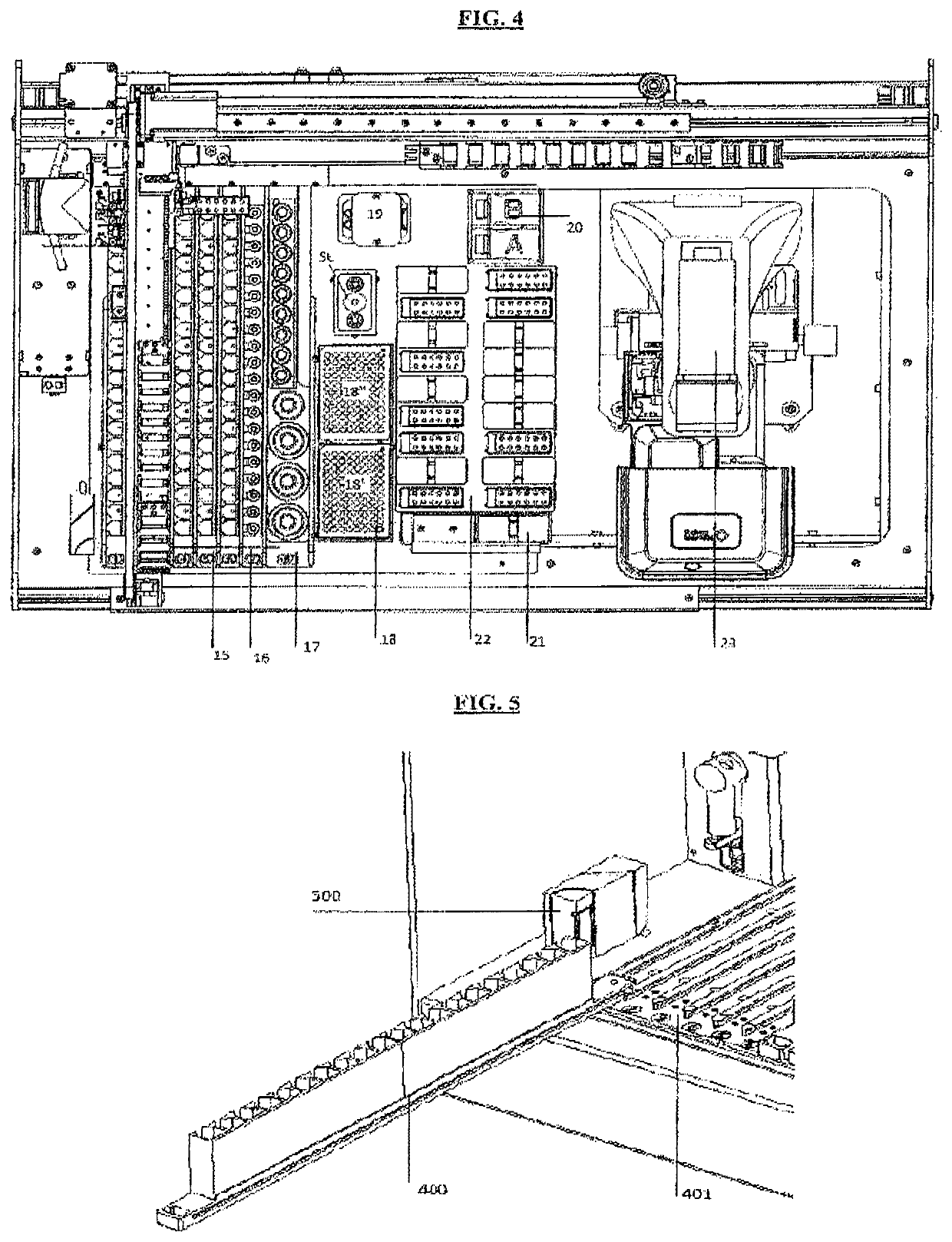
A machinery for an automated analysis of the slides in accordance with the indirect immunofluorescence assay - IFA
ActiveUS20180136096A1Quick and precise workingNo risk of decayWithdrawing sample devicesPreparing sample for investigationMicroscope slideImmunofluorescence
A machinery for preparation and analysis of one or more slides for a biological material according to an immuno-fluorescence technique includes a loading station for one or more containers of test liquids and / or samples of product; a positioning station of the slides; a containment station of one or more slide covers; an analysis station having a microscope that acquires digital images at one or more magnifications; an assembly, mobile inside the area defined by the machinery and controlled to move through the stations to prepare one or more slides according to a predefined protocol and arrange them in a corresponding analysis station for subsequent acquisition of digital images; one or more covers for each slide arranged in the seat; a container of the covers, the assembly being controlled to remove the covers once the phase of pre-dilution of the samples has been completed and arrange them inside the container.
Owner:AMT CO LTD
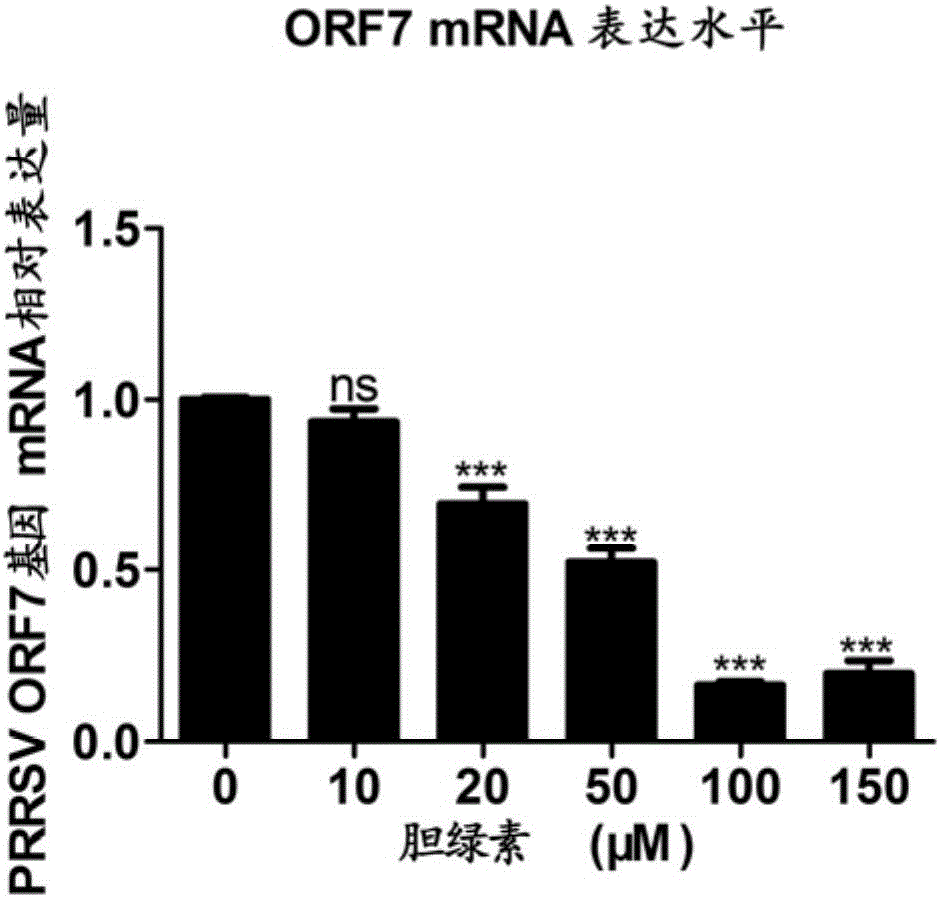
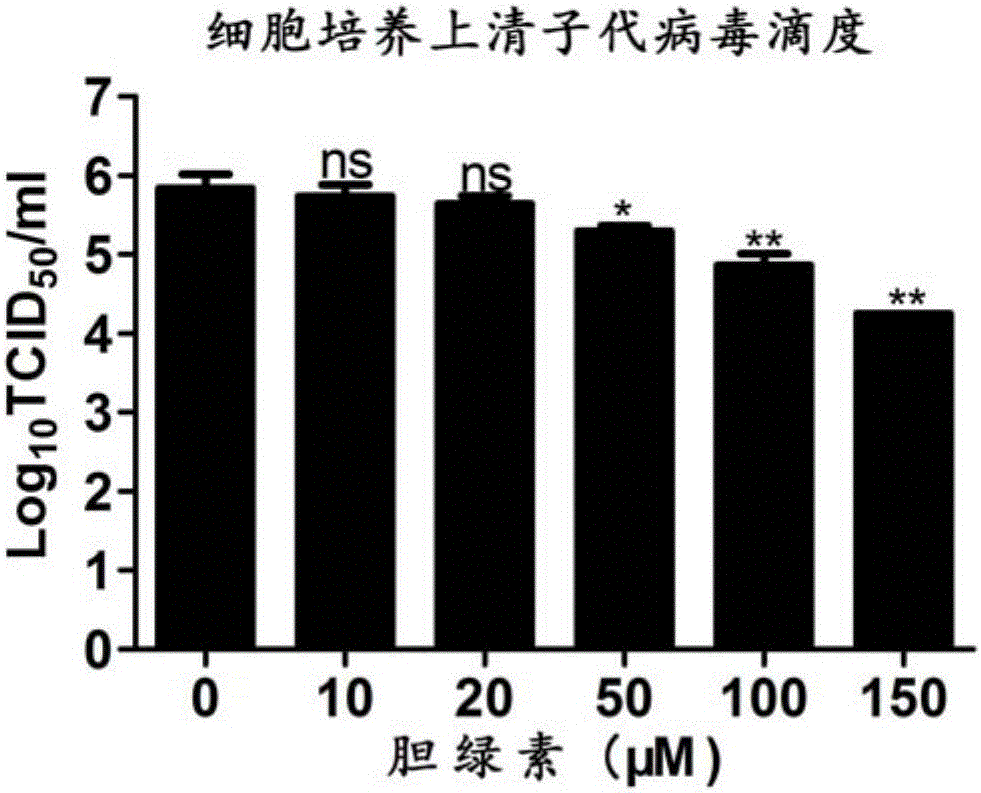
Biliverdin preparation and application thereof in prevention and treatment of porcine reproductive and respiratory syndrome, and detection method
ActiveCN106474111ASufficient raw materialsGood effectOrganic active ingredientsPowder deliveryFluorescenceWestern blot
The invention discloses a biliverdin preparation and an application thereof in prevention and treatment of PRRSV, and a detection method. An antiviral effect of exogenous biliverdin on the PRRSV is researched through qRT-PCR, western blot, cell supernatant TCID50 detection and indirect immunofluorescence assay and other methods in Marc-145 and PAM cell levels. Results show that biliverdin significantly inhibits proliferation of the PRRSV in susceptible cells and host target cells. The biliverdin preparation disclosed by the invention has good antiviral effect, and is expected to become a novel drug for prevention and control of porcine reproductive and respiratory syndrome.
Owner:NORTHWEST A & F UNIV
A method for screening virus-sensitive cell clones using indirect immunofluorescence detection technology
ActiveCN102994445BShort detection timeEasy to operateArtificial cell constructsVertebrate cellsVirulent characteristicsFluorescence
The invention relates to the field of biotechnology, and particularly relates to a method for performing screening in virus-sensitive cell clonal strains by applying an indirect immunofluorescence assay technology. The method comprises the following steps of: identifying the purity of a cell line; obtaining monoclonal cell strains in the cell line; and identifying the sensitivity of the monoclonal cell strains to virus. The method disclosed by the invention has the beneficial effects of being short in detection time, capable of identifying the sensitivity of single-cell cloning to virus only by 3 days, relative simple to operate, easy, capable of being used for screening lots of non-cytopathic virus-sensitive cell strains, stable in result, strong in repeatability, and consistent with the effect of a virulence determination method.
Owner:山东滨州沃华生物工程有限公司
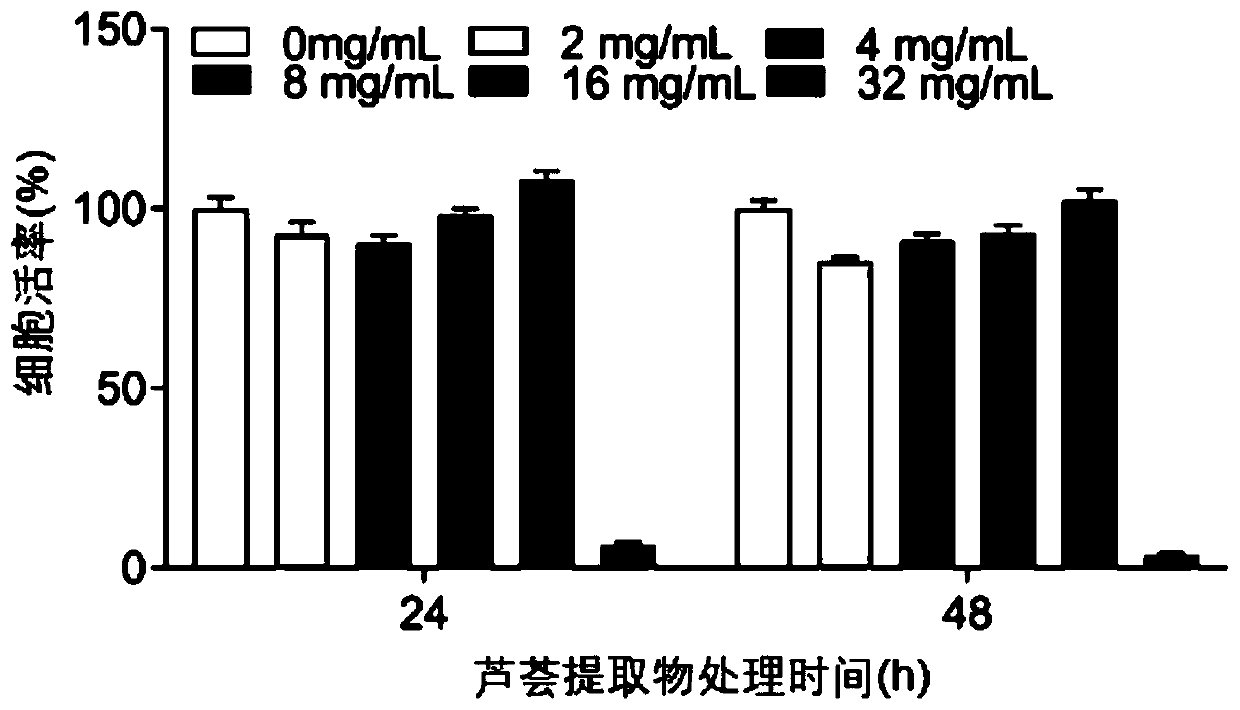
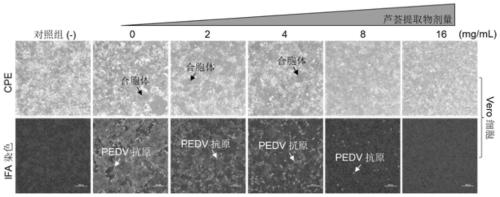
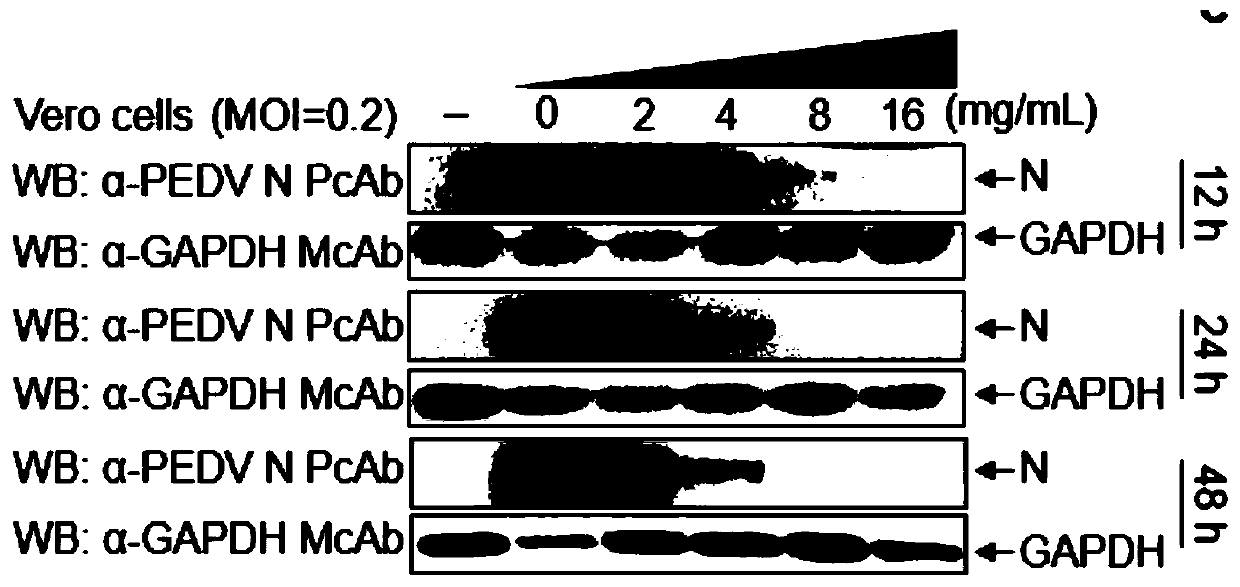
Application of aloe extract in preparation of medicine for preventing and treating porcine epidemic diarrhea
ActiveCN111110778AImprove innate immune antiviral functionPrevention and Treatment of Porcine Epidemic DiarrheaDigestive systemAntiviralsEpidemic diarrheaImmunofluorescence
The invention relates to an application of an aloe extract in preparation of a medicine for preventing and treating porcine epidemic diarrhea. An aloe extract preparation and PEDV are mixed and then inoculated into Vero and IPEC-J2 cells, and after cytopathy occurs, an indirect immunofluorescence assay (IFA), western blotting (WB) and a virus titration method are used for detection. Results show that: the aloe extract can effectively inhibit the proliferation of the PEDV in the Vero cells and the IPEC-J2 cells, has a dose-dependent effect, and can completely inhibit the proliferation of the PEDV under a high-dose condition (16mg / ml).
Owner:SUN YAT SEN UNIV
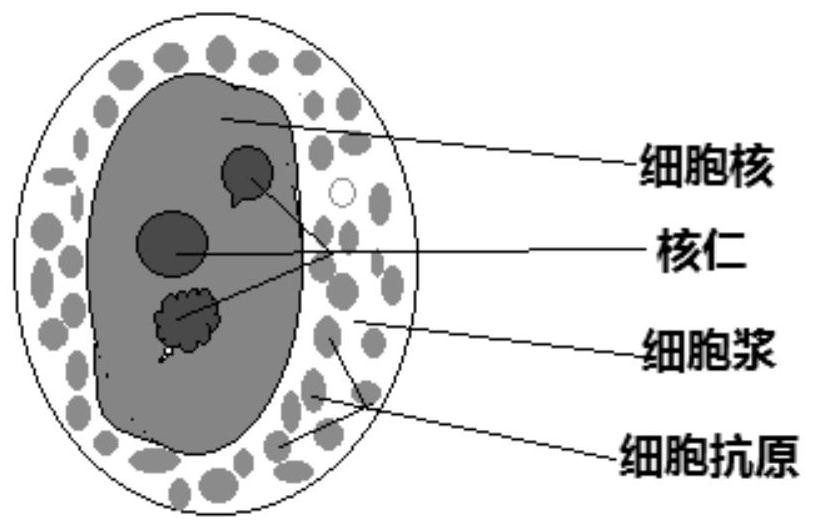
Human cell whole form immunofluorescence staining method and kit
The invention discloses a human cell holomorphic immunofluorescent staining method and a kit. Human cells are subjected to holomorphic staining by direct immunofluorescence assay or indirect immunofluorescence assay by using antibodies of cell nuclear antigens, cell nucleolus antigens and nucleoplasm antigens and antibodies of related antigens in cytomembrane and cytoplasm. The cell nucleus, particularly nucleolus capable of calibrating the nucleus can be clearly and completely displayed, and the quantity, form, size and distribution of the nucleolus are clearly visible. In addition to characteristic display of the antigens of the cells by immunofluorescent staining, the human cells can be subjected to holomorphic observation and analysis.
Owner:青岛言鼎生物医疗科技有限公司
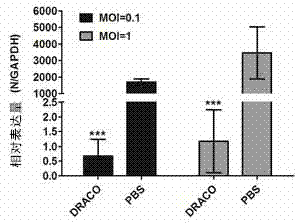
Novel pharmaceutical protein DRACO and applications thereof in prevention and treatment of porcine reproductive and respiratory syndrome
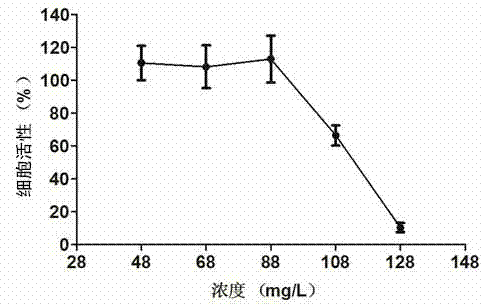
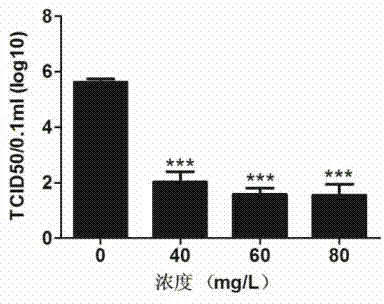
InactiveCN103571863AActiveGood antiviral effectBacteriaPeptide/protein ingredientsHighly pathogenicProkaryotic expression
The invention discloses a novel pharmaceutical protein DRACO and applications thereof in prevention and treatment of porcine reproductive and respiratory syndrome. Correlation sequences of pigs on NCBI are utilized to design the protein coding gene of DRACO which is provided with activity and capable of entering cells, then the DRACO protein is expressed and purified in a prokaryotic expression system, the antivirus activity of DRACO protein to HP-PRRSV (Highly Pathogenic Porcine Reproductive and Respiratory Syndrome Virus) is researched at the Marc-145 cellular level by qRT-PCR (Quantitative Real-Time Polymerase Chain Reaction), Western-Blot, cellular supernatant TCID50 (Tissue Culture Infectious Dose 50) detection, IFA (Indirect Immunofluorescence Assay) and other methods, and the antiviral mechanism of DRACO in two processes of absorbing viruses and entering cells are further explained. The DRACO produced by the method disclosed by the invention has good antiviral effect to HP-PRRSV, and is expected to be a novel drug for preventing and treating porcine reproductive and respiratory syndrome.
Owner:SUN YAT SEN UNIV
An indirect immunofluorescence detection kit for mink enteritis parvovirus
ActiveCN104950109BStrong specificityMicroorganism based processesImmunoglobulins against virusesImmunofluorescenceAssay
The invention discloses an indirect immunofluorescence detection kit for mink enteritis parvovirus. The kit includes the anti-mink enteritis parvovirus monoclonal antibody secreted by the hybridoma cell line with the preservation number CGMCC NO.10881. The invention also discloses an indirect immunofluorescence detection method for rapid detection of mink enteritis parvovirus established by using the monoclonal antibody. The method improves the specificity of the diagnosis of the disease and can realize the specific diagnosis of the disease. Sites of viral infection of cells are directly observed at the cellular level. The establishment of MEV indirect immunofluorescence detection method provides a new method for the diagnosis of MEV.
Owner:INST OF SPECIAL ANIMAL & PLANT SCI OF CAAS
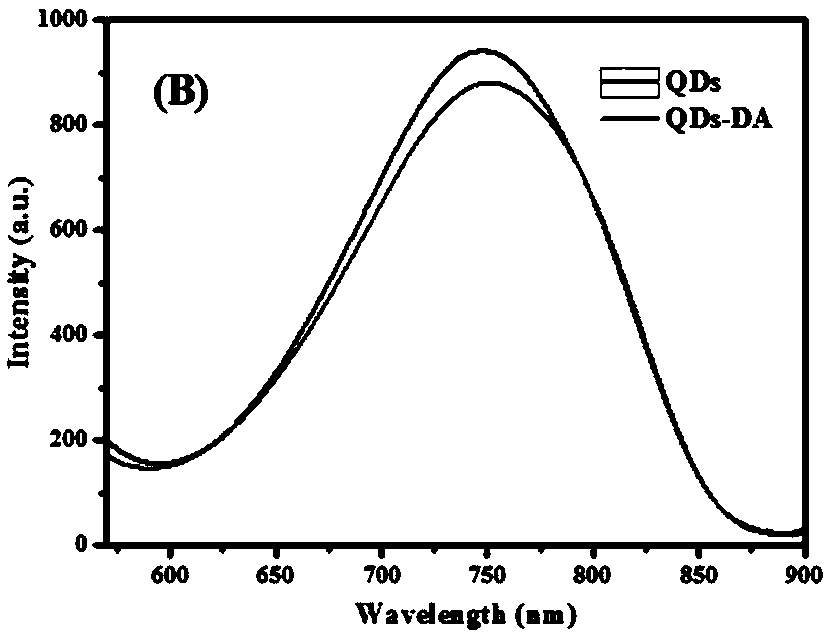
Disease marker indirect immunofluorescence assay method based on dopamine modified ternary quantum points
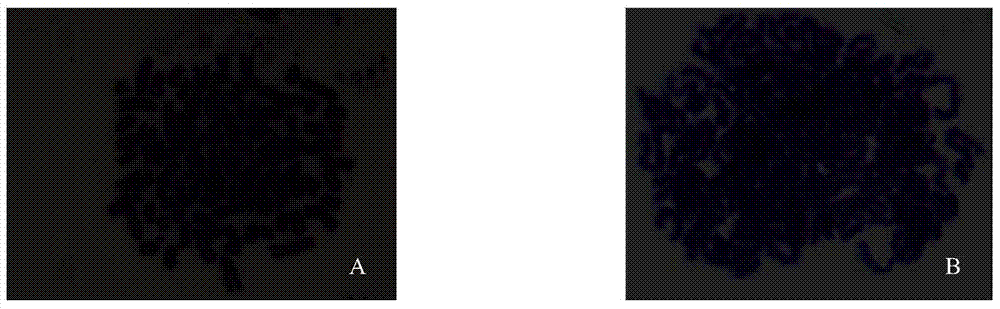
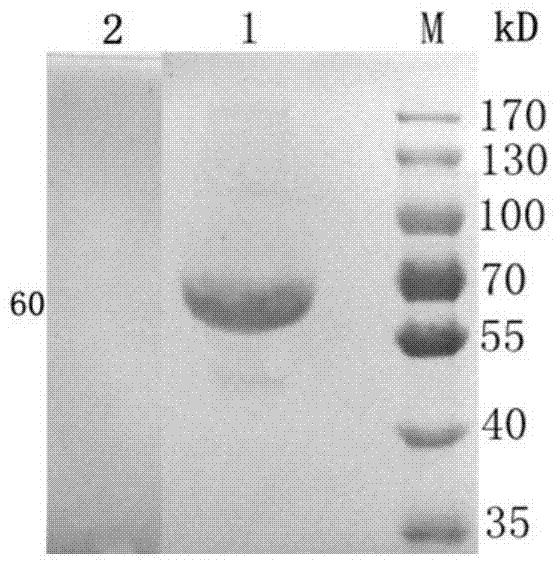
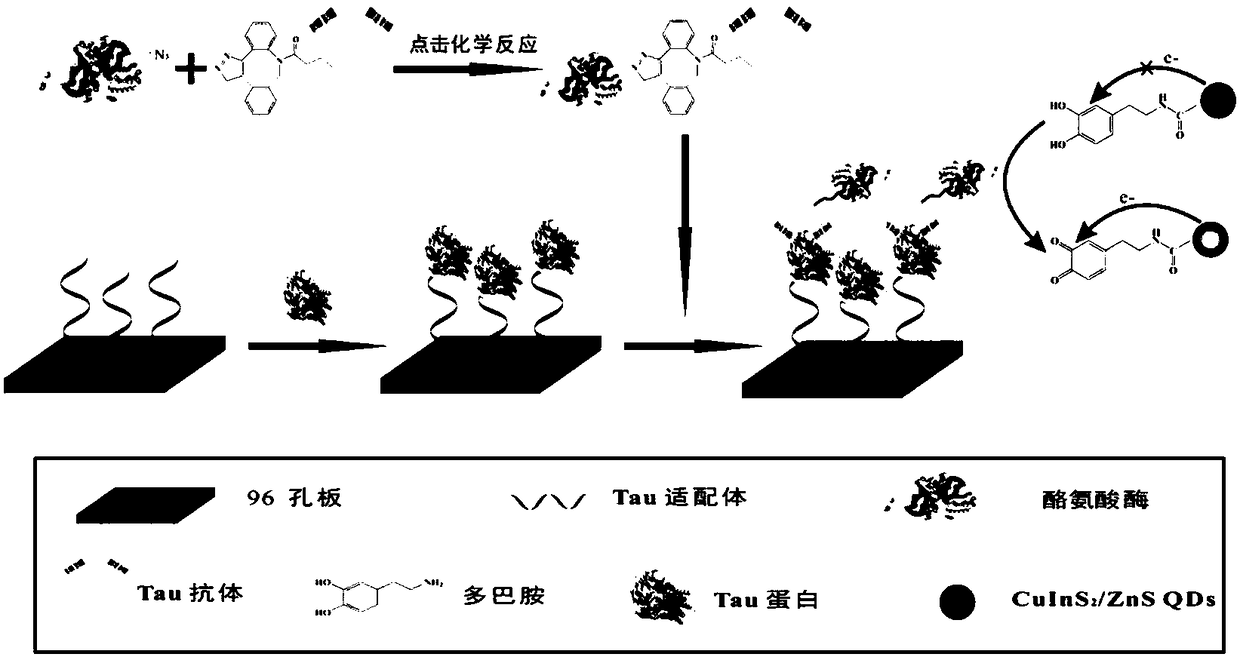
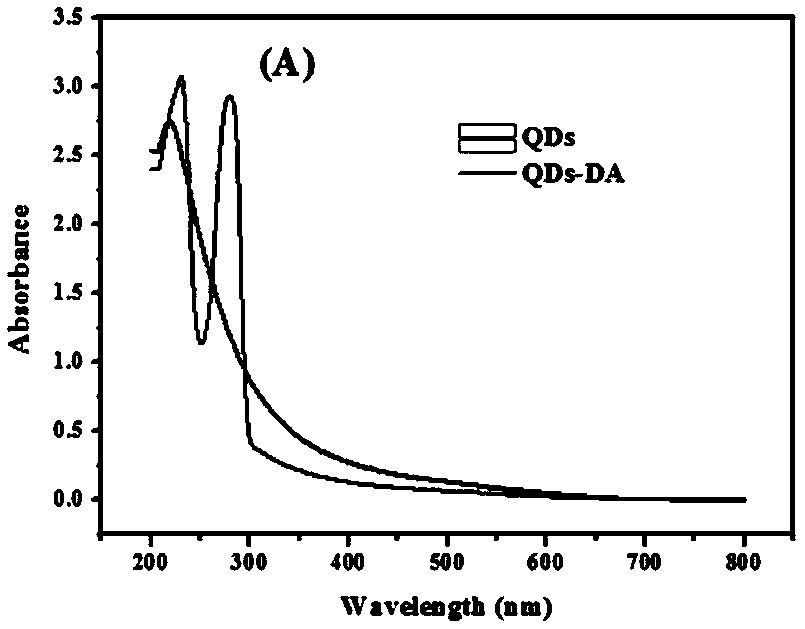
The invention discloses a disease marker indirect immunofluorescence assay method based on dopamine modified ternary quantum points. According to the method, firstly, tyrosinase (TYR) is combined withdetection antibodies of disease biomarkers through copper-free click reaction; in addition, biotin modified corresponding aptamers are combined with avidin coated 96 hole plates; the disease biomarkers and TYR-detection antibodies are sequentially added; after washing excessive TYR-detection antibodies, CuInS2 / ZnS QDs-DA is added; under the existence of the disease biomarkers, the TYR can realizethe catalytic oxidation on dopamine parts in CuInS2 / ZnS QDs-DA so that the dopamine parts can be converted into dopamine quinone to cause fluorescence quenching. Through detecting the change of fluorescence signals due to electron transferring caused by reaction of CuInS2 / ZnS QDs-DA catalytic oxidation reaction by TYR, the high-sensitivity detection on the disease biomarkers can be realized.
Owner:FUJIAN MEDICAL UNIV
Machinery for an automated analysis of the slides in accordance with the indirect immunofluorescence assay—IFA
ActiveUS10704996B2Quick and precise workingNo risk of decayWithdrawing sample devicesPreparing sample for investigationImmunofluorescenceAutoanalysis
A machinery for preparation and analysis of one or more slides for a biological material according to an immuno-fluorescence technique includes a loading station for one or more containers of test liquids and / or samples of product; a positioning station of the slides; a containment station of one or more slide covers; an analysis station having a microscope that acquires digital images at one or more magnifications; an assembly, mobile inside the area defined by the machinery and controlled to move through the stations to prepare one or more slides according to a predefined protocol and arrange them in a corresponding analysis station for subsequent acquisition of digital images; one or more covers for each slide arranged in the seat; a container of the covers, the assembly being controlled to remove the covers once the phase of pre-dilution of the samples has been completed and arrange them inside the container.
Owner:AMT CO LTD
Preparation method of PCV2 (Porcine Circovirus2)-D
The invention discloses a preparation method of PCV2 (Porcine Circovirus2)-D. The microorganism preservation number of PCV2 (Porcine Circovirus2) is CGMCC (China General Microbiological Culture Collection Center) No.7245. According to the preparation method, the preference of genetic codon of the PCV2 is modified, and the preferred codon is modified into non-preference codon, so that the expressions of the components of the virus are reduced, further, the copy rate of the virus in host cells is lowered, the virus is weakened, and the PCV2-D is obtained by genetic modification. Furthermore, the proliferation speed of the PCV2-D is compared with that of wild type strains at a cellular level. The detection of PCR (Polymerase Chain Reaction) and IFA (Indirect Immunofluorescence Assay) prove that the PCV2-D can be infected and copied in cells, and has certain infection.
Owner:SHANGHAI ACAD OF AGRI SCI
A kind of anti-ibrv single-chain antibody, its preparation method and application
InactiveCN107312087BSmall molecular weightEasy to buildImmunoglobulins against virusesAntiviralsEscherichia coliSingle-Chain Antibodies
The invention provides an anti-IBRV single-chain antibody which is protein consisting of a heavy chain variable region shown as SEQ ID NO.1, a light chain variable region shown as SEQ ID NO.2 and a connecting peptide for connecting the two regions and having the characteristic of resisting an infectious bovine rhinotracheitis peplos gD protein antibody. The invention also provides a preparation method of the single-chain antibody. The single-chain antibody can be specifically combined with IBRV and can be also specifically combined with IBV gD protein in an escherichia coli expression product. An indirect immunofluorescence assay shows that the single-chain antibody can identify the IBRV infected in the bovine kidney cell MDBK, and the single-chain antibody has the good application value when applied to infectious bovine rhinotracheitis detection and development of treatment preparations.
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com