A kind of anti-ibrv single-chain antibody, its preparation method and application
A single-chain antibody and light chain technology, applied in the field of anti-IBRV single-chain antibody, can solve the problems of prone to false positive results, low sensitivity of detection reagents, unsuitable for clinical practice, etc., achieves good application value, small molecular weight, easy to use Effects of construction and large-scale expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
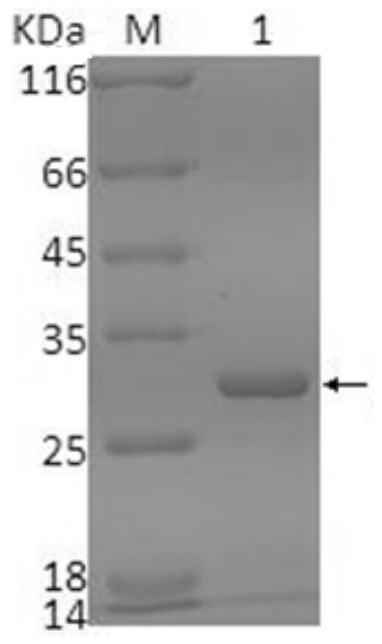
[0035] The preparation of embodiment 1 single-chain antibody VH-VL protein
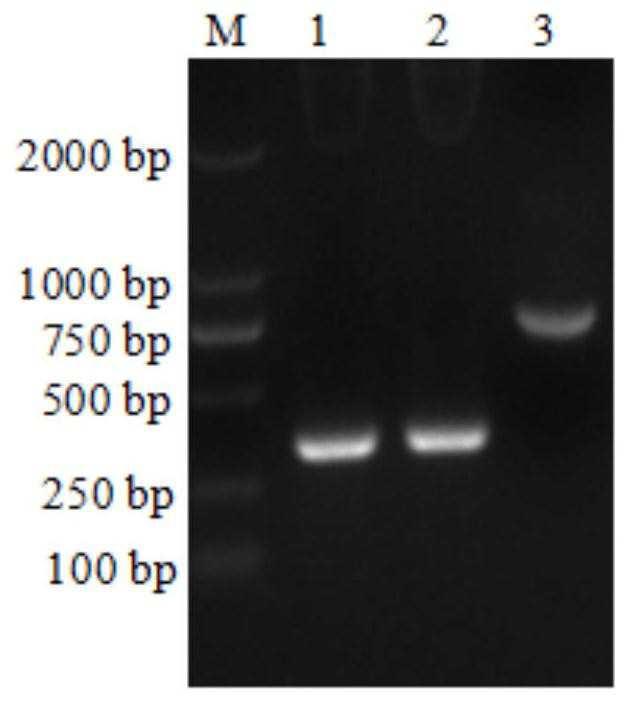
[0036]In the past, single-chain antibody genes were obtained by constructing single-chain antibody phage display libraries, which was laborious and time-consuming. In the present invention, after extracting total cellular RNA from hybridoma cells secreting monoclonal antibodies, reverse transcribe cDNA as a template, and use PCR After the primers were used to amplify the heavy chain variable region (VH) and light chain variable region (VL) of the antibody, 100 clones were selected for sequencing and comparison, and 3-4 genes with the highest repeatability were screened out , after linking VH and VL with a connecting peptide to form a complete ScFv gene, after constructing a PET28a expression vector, transforming the expression bacteria to prepare a recombinant ScFv protein.
[0037] The specific operation is as follows:
[0038] Among them, the experimental materials used in this example can be mater...
Embodiment 2
[0057] Example 2 Single-chain antibody is used for Western-blot to detect the expression of gD protein
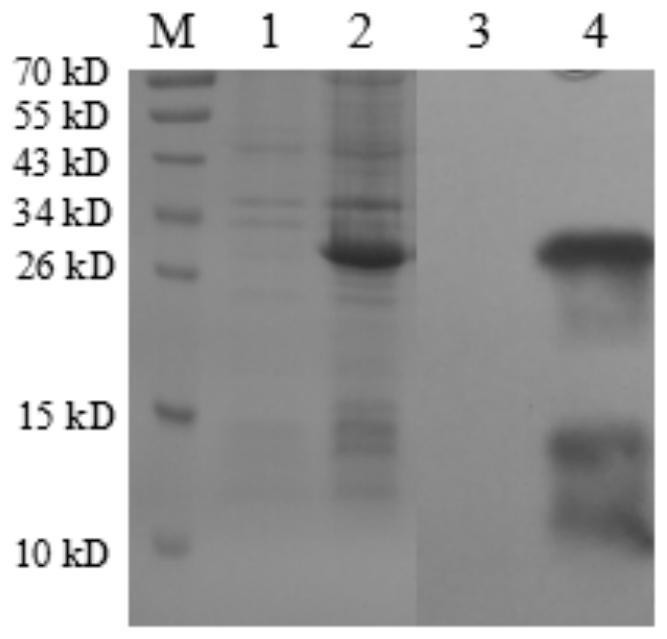
[0058] The single-chain antibody prepared in Example 1 was used for Western-blot detection of IBRV gD protein. Specifically, the lysate of E. coli cells expressing gD protein was subjected to SDS-PAGE electrophoresis to separate the protein and transferred to a PVDF membrane. After blocking with 5% skim milk, the purified HRP-labeled single-chain antibody was used for immunoblotting, and the ECL chemiluminescence kit was used for color development. And set the Escherichia coli lysate expressing pET28a empty vector as the control.
[0059] The result is as Figure 4 As shown, it shows that the single chain antibody prepared by the present invention can specifically bind to the gD protein expressed in Escherichia coli. in, Figure 4 Middle M: pre-stained protein molecular mass standard; 1: E. coli lysate expressing pET 32a empty vector; 2: E. coli cell lysate expressing ...
Embodiment 3
[0060] Example 3 Single-chain antibody is used for IFA detection of IBR virus infected in MDBK
[0061] The purified single-chain antibody prepared in Example 1 is used for indirect immunofluorescence assay (IFA) to detect IBRV in MDBK cells, specifically, MDBK cells grown on glass slides in 6-well cell plates are infected with IBRV, and when pathological changes occur After fixing the cells with 4% paraformaldehyde for 1 h, permeabilize with 0.1% Triton X-100 for 2 h, block with 0.5% BSA at 37°C for 1 h, use the recombinant single-chain antibody purified in Example 1 as the primary antibody, and use 1:100 Diluted FITC-conjugated mouse anti-His monoclonal antibody was used as the secondary antibody for immunoreaction. Observe under a fluorescent microscope, and set the gD monoclonal antibody binding to the IBRV control infected MDBK cells.
[0062] The result is as Figure 5As shown, DAPI is the blue fluorescent dye used in the figure: 4', 6-diamidino-2-phenylindole, and FIT...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com