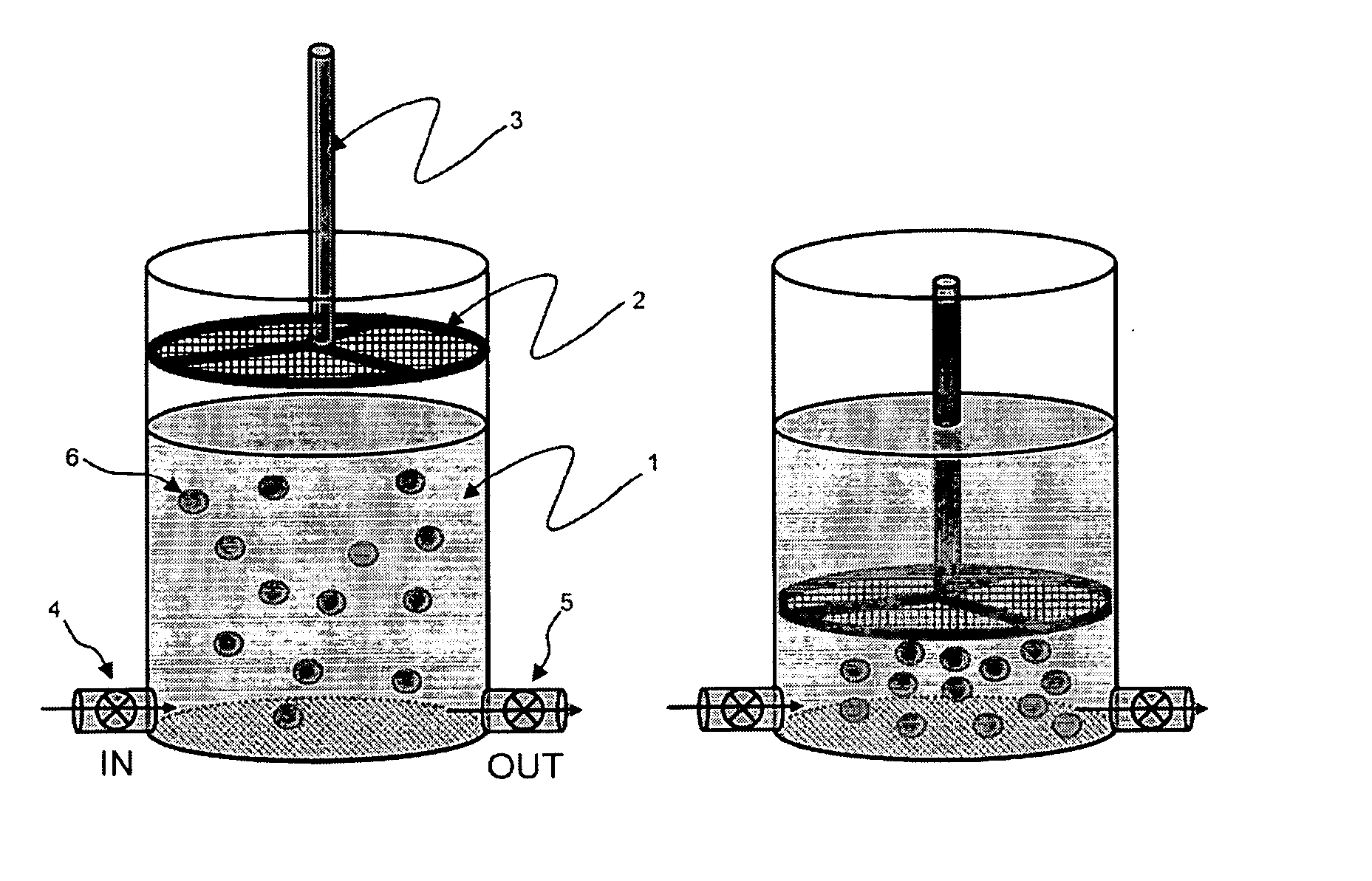
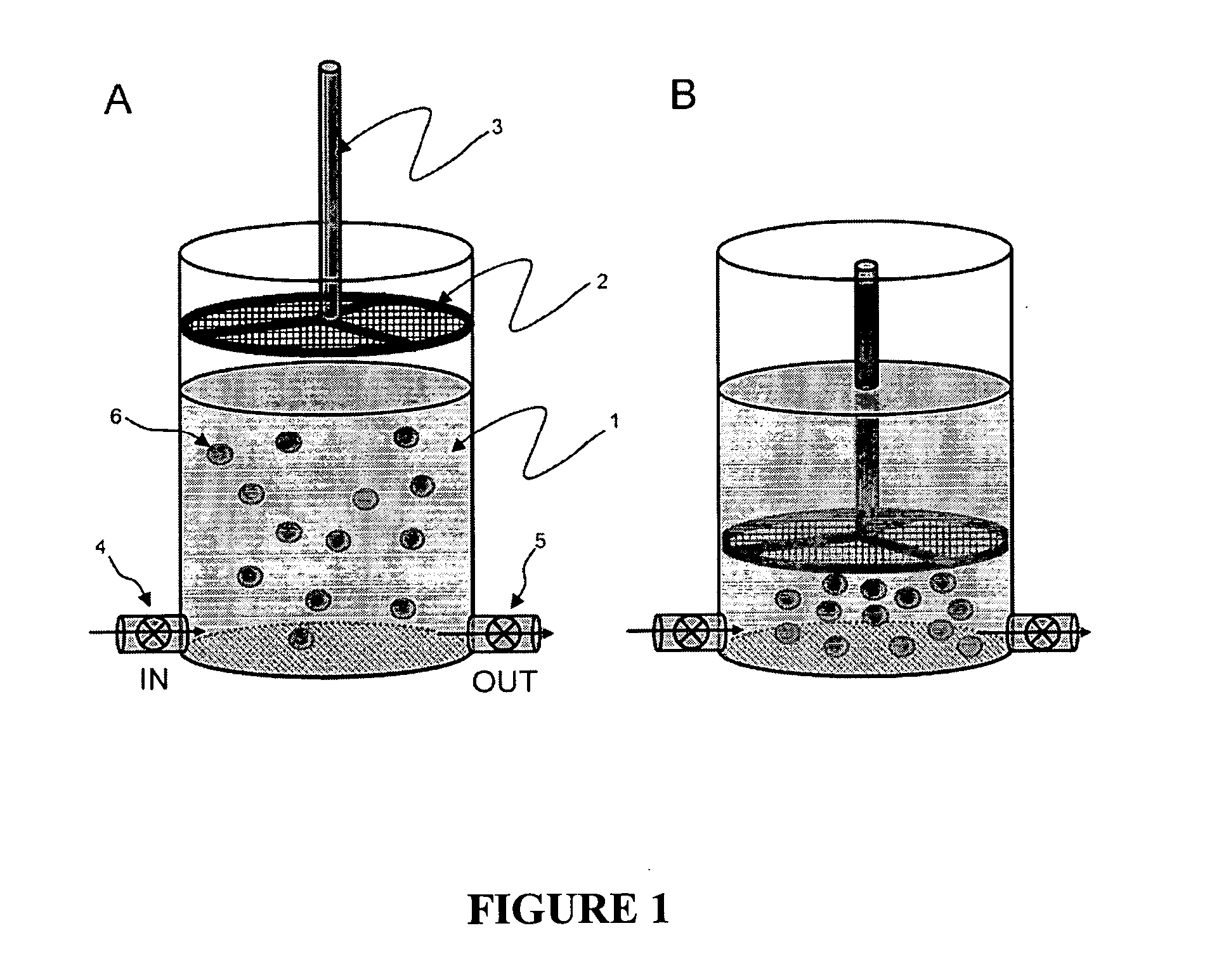
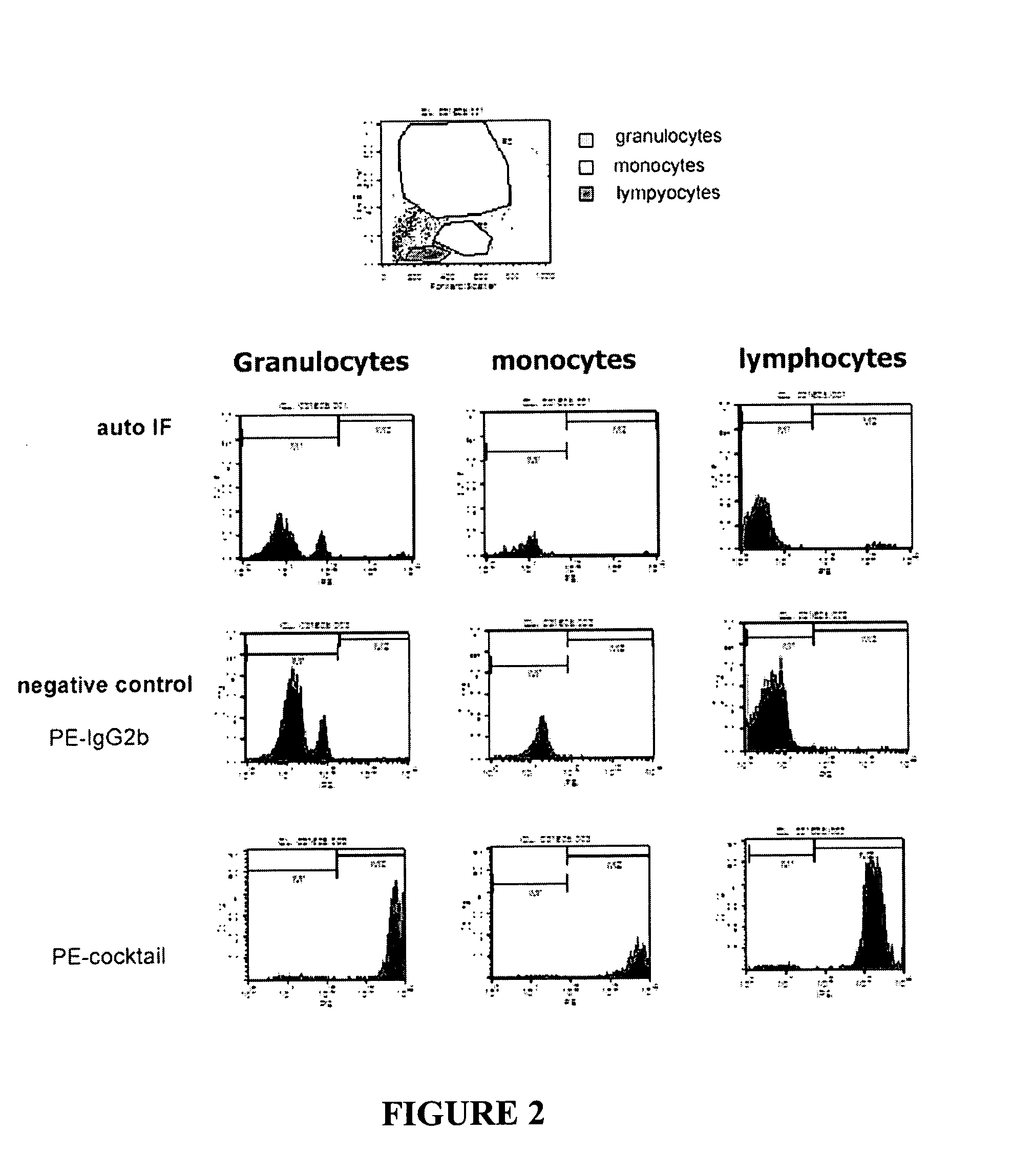
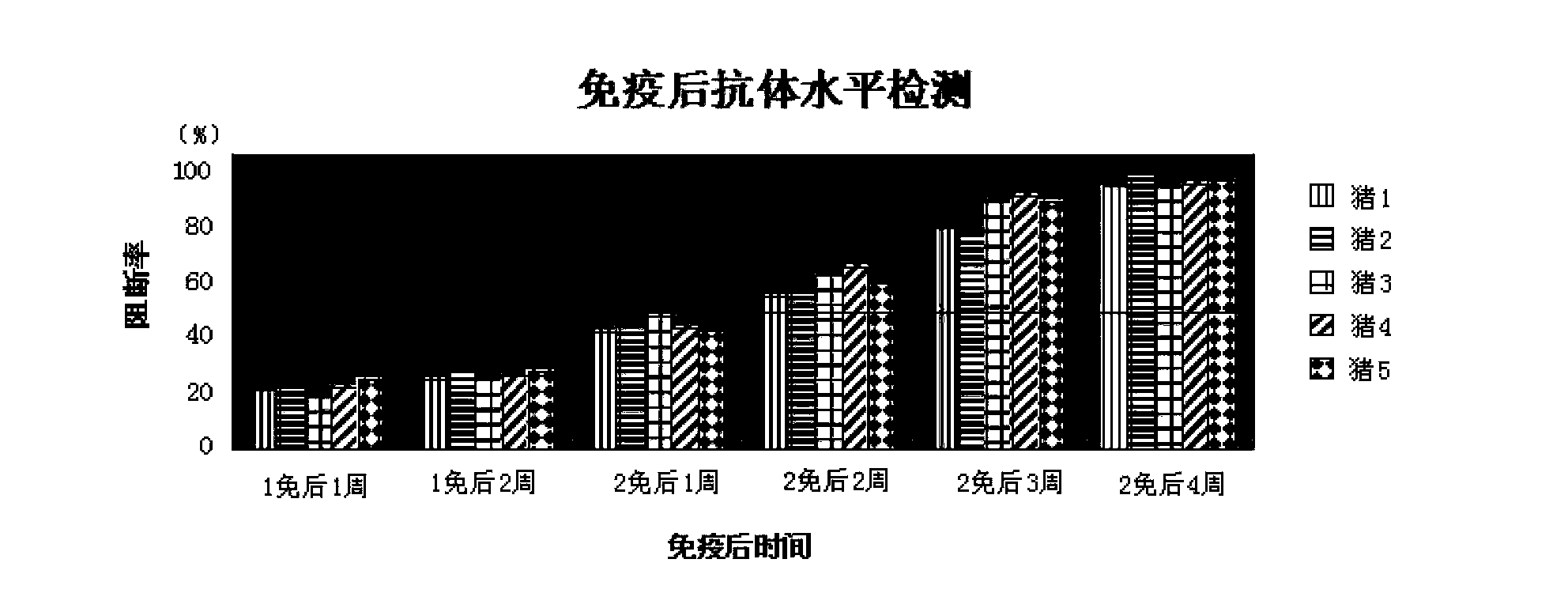
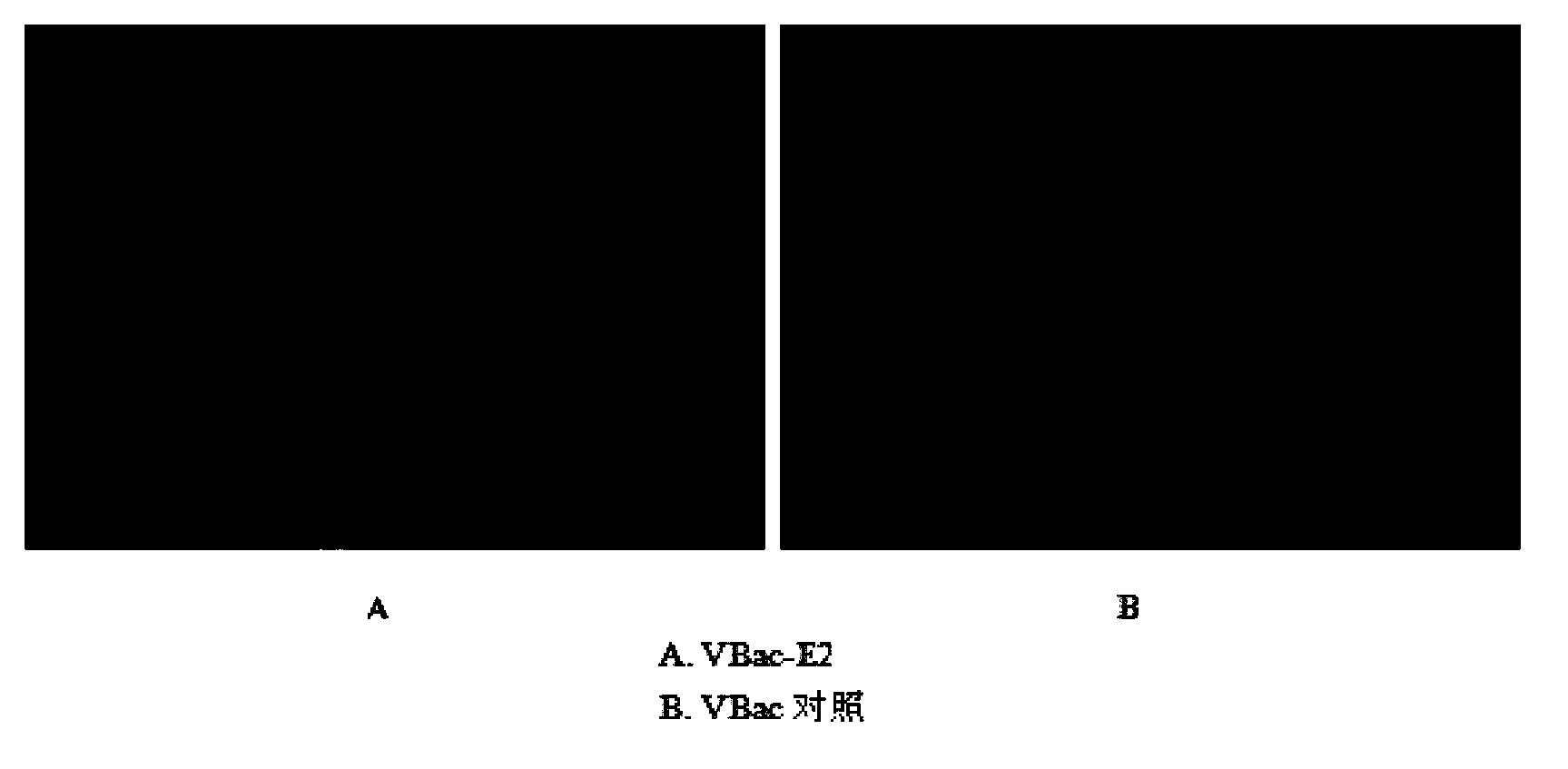
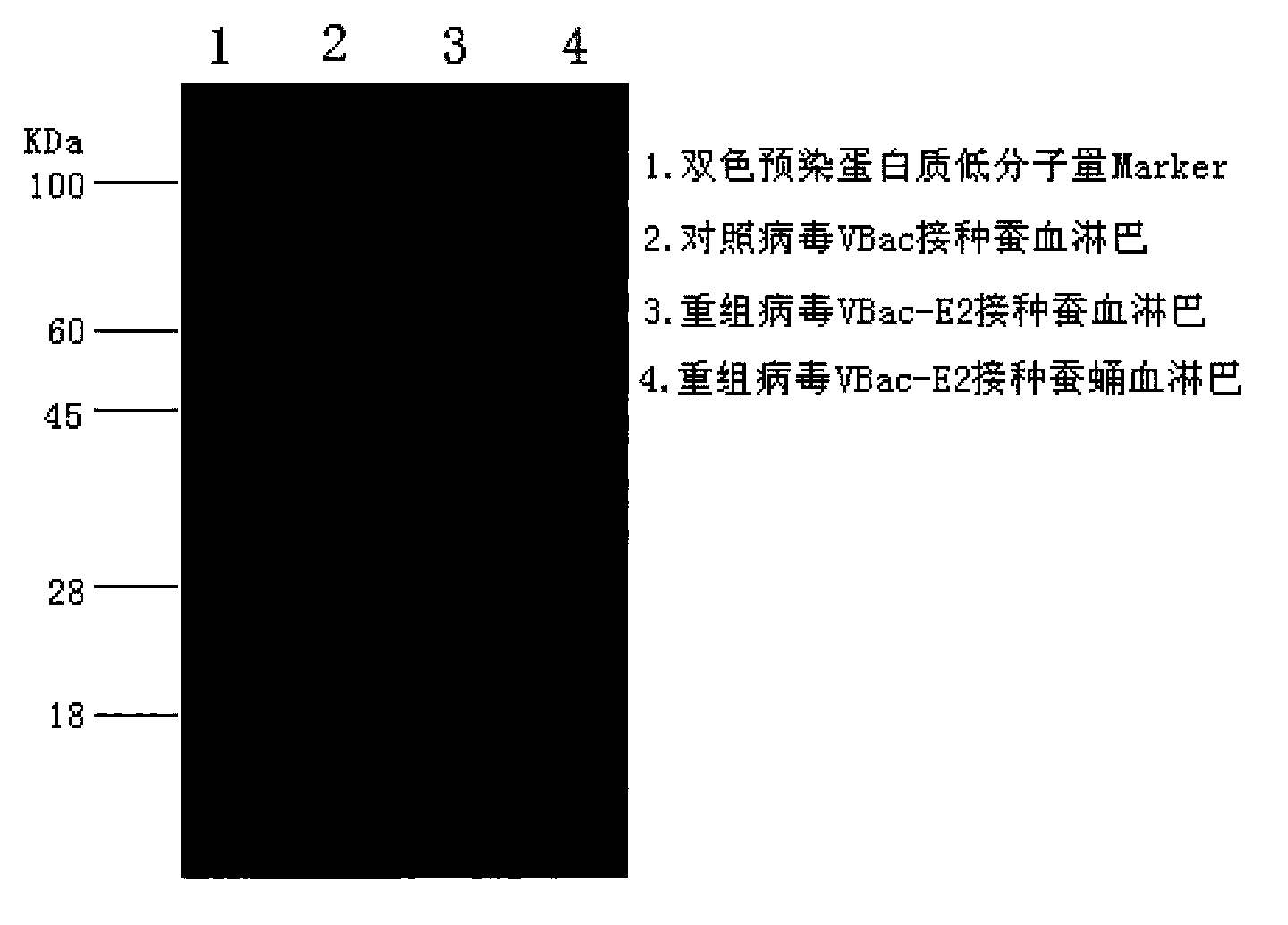
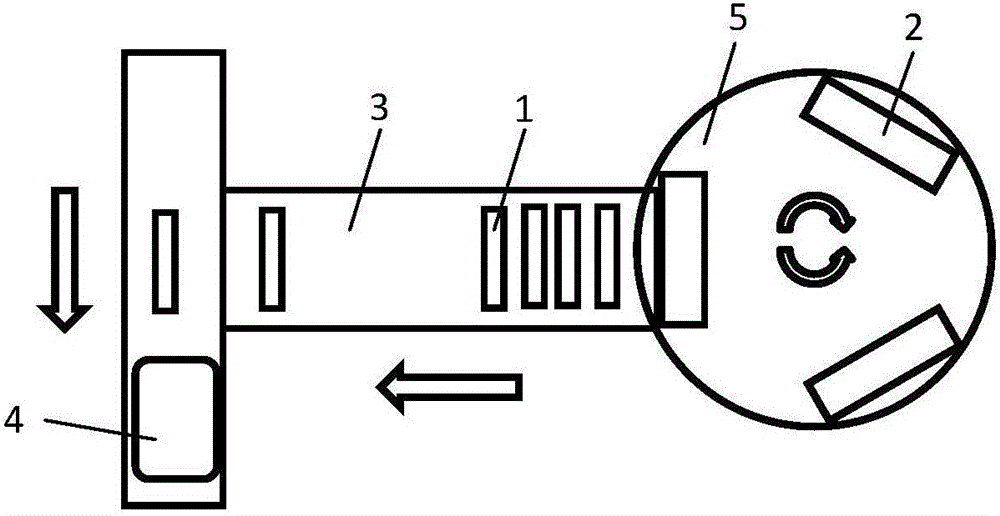
Patents
Literature
121 results about "IMMUNE FLUORESCENCE" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Methods and compositions for detecting rare cells from a biological sample
InactiveUS20080057505A1Strong specificityEasy to identifyMicrobiological testing/measurementBiomass after-treatmentHematopoietic cellWhite blood cell
The present invention provides methods and compositions for isolating and detecting rare cells from a biological sample containing other types of cells. In particular, the present invention includes a debulking step that uses a microfabricated filters for filtering fluid samples and the enriched rare cells can be used in a downstream process such as identifies, characterizes or even grown in culture or used in other ways. The invention also include a method of determining the aggressiveness of the tumor or of the number or proportion of cancer cells in the enriched sample by detecting the presence or amount of telomerase activity or telomerase nucleic acid or telomerase expression after enrichment of rare cells. This invention further provides an efficient and rapid method to specifically remove red blood cells as well as white blood cells from a biological sample containing at least one of each of red blood cells and white blood cells, resulting in the enrichment of rare target cells including circulating tumor cells (CTC), stromal cells, mesenchymal cells, endothelial cells, fetal cells, stem cells, non-hematopoietic cells etc from a blood sample. The method is based upon combination of immuno-microparticles (antibody coated microparticles) and density-based separation. The final enriched target cells can be subjected to a variety of analysis and manipulations, such as flowcytometry, PCR, immunofluorescence, immunocytochemistry, image analysis, enzymatic assays, gene expression profiling analysis, efficacy tests of therapeutics, culturing of enriched rare cells, and therapeutic use of enriched rare cells. In addition, depleted plasma protein and white blood cells can be optionally recovered, and subjected to other analysis such as inflammation studies, gene expression profiling, etc.
Owner:AVIVA BIOSCI
Integrated method for enriching and detecting rare cell in biological fluid sample
ActiveCN101587043APromote universal applicationLow costMicrobiological testing/measurementPreparing sample for investigationRed blood cellFluorescence
Owner:CYTTEL BIOSCI BEIJING
Preparation method for PCV-II Cap protein monoclonal antibody, antibody and application
InactiveCN101768218AAvoid distortionThe ability to secrete antibodies is strong and stableImmunoglobulins against virusesFluorescence/phosphorescenceBALB/cIndirect elisa
The invention discloses a preparation method for a PCV-II Cap protein monoclonal antibody, an antibody and application. The invention adopts ultracentrifuged and purified PCV-II as an immunogen to immunize a BALB / c mouse by the conventional method, takes spleen cells of the immunized BALB / c mouse to fuse with SP2 / 0 cells, obtains two strains of hybridoma cells secreting the PCV2-Cap protein monoclonal antibodies by indirect ELISA screening, respectively names the two strains of hybridoma cells as 8-60 and 10-48, identifies biological characteristics of the two strains 8-60 and 10-48, and usesthe two strains 8-60 and 10-48 as the first antibodies to establish an indirect immunofluorescence diagnostic method. The result of the indirect immunofluorescence diagnostic method is basically consistent with that of the PCR diagnostic method, and the positive and negative coincidence rates are respectively 93.75 percent and 100 percent so as to provide reference for preventing and treating theporcine circovirus disease.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY FUJIAN ACADEMY OF AGRI SCI +7
Anti-flavivirus envelope E protein monoclonal antibody and application thereof
ActiveCN101891806AGood broad-spectrum anti-flavivirus effectInfection fromFungiBacteriaCell strainProtein.monoclonal
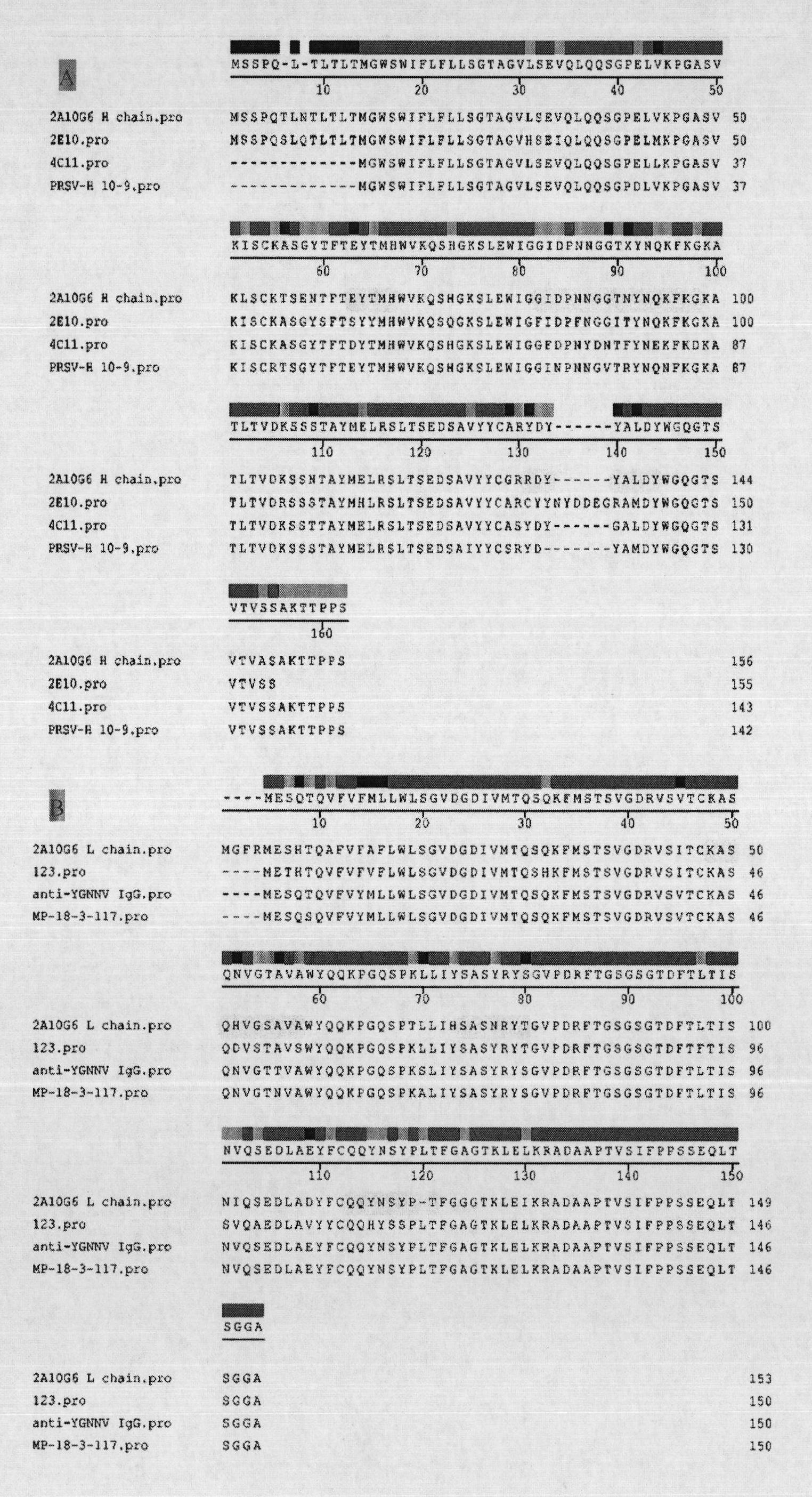
The invention discloses an anti-flavivirus envelope E protein monoclonal antibody and application thereof. The anti-flavivirus envelope E protein monoclonal antibody of the invention is secreted by a mouse source hybrid tumour cell strain D2-2A10G6 with the preservation number of CGMCC No. 3292. The anti-flavivirus monoclonal antibody of the invention can be specifically combined with the function epitope of flavivirus envelope E protein. The amino acid sequence of the functional epitope of envelope E protein is SEQ ID NO: 17. The monoclonal antibody of the invention is subject to screening by indirect immunofluorescent method, and indirect enzyme-linked immunization is used for evaluating the specificity and affinity when being combined with antigen. By adopting the monoclonal antibody or immunoconjugate of the invention, flavivirus infection cell can be blocked and suckling mouse can be protected from virus attack, thus achieving the effect of inhibiting virus infection. The monoclonal antibody or immunoconjugate of the invention also can be used for flavivirus envelope E protein detection.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Method for discriminating non-blood-borne nucleated cells enriched from human or animal biological fluid
ActiveCN103091491AHigh recovery rateAccurate identificationMicrobiological testing/measurementPreparing sample for investigationHuman bodyStaining
Owner:有限会社林平
HCMVPP65 antigenemia indirect immunofluorescence method detection reagent kit
InactiveCN101261272AImprove featuresIncreased sensitivityFluorescence/phosphorescenceBALB/cFluorescence
The invention discloses a HCMVPP65 antigenemia indirect immune fluorescence method detection kit, which uses cytomegalovirus-AD169 virus strain pp65 protein as the immunogen to immune a Balb / c mouse. The spleen cells of the immunized mouse and the myeloma cells of the mouse which belongs to the same type with the immune mouse are conventionally integrated, by indirect ELISA screening and finite dilution cloning, the hybridoma cell lines of the mouse cytomegalovirus pp65 protein cloning antibody are obtained, and the characteristics of the hybridoma cell lines are identified by ELISA, immune fluorescence experiment and other methods; two monoclonal antibodies that stably secrete the pp65 protein are established successfully and named respectively as 1A6 and 4A8. A monoclonal antibody which differs from the former report and aims at the pp65 protein of the cytomegalovirus (CMV) is prepared and a method used for preparing erythrocyte fast pyrolysis is established; compared with other detection kits which belongs to the same kind, the detection kit of the invention is faster, simpler and more convenient and has higher specificity and sensitivity.
Owner:天津市秀鹏生物技术开发有限公司
Immune fluorescent marking method for naked plant pollen tube microtubule skeleton and its use
InactiveCN101074952AOmit the closing stepStable storageFluorescence/phosphorescenceImmunofluorescent labelingIMMUNE FLUORESCENCE
An immune-fluorescence labeling method of micro-pipe frame at pollen tube on gymnosperm includes fixing pollen tube of gymnosperm on fixing liquid for 40-60min, washing pollen tube, placing pollen tube in enzyme solution for carrying out enzymolysis of 30-40 min under temperature of 20-30deg.c, washing pollen tube, setting pollen tube in Triton X-100 to penetrate there for 1-3h, washing pollen tube, adding anti-beta-tubulin single clone antibody to carry out hatching for 1-3h, washing pollen tube, adding fluorescence label to carry out shielded hatching for 1-3h for making micro-pipe frame be immune-fluorescence labeled.
Owner:INST OF BOTANY CHINESE ACAD OF SCI
Immune-fluorescence test strip component for rapidly detecting C-reactive protein quantitatively, detection card component produced by same and method for preparing same
ActiveCN102680702AHigh sensitivityImprove signal-to-noise ratioBiological testingFluorescence/phosphorescencePorphyrinIMMUNE FLUORESCENCE
The invention discloses an immune-fluorescence test strip component for rapidly detecting C-reactive protein quantitatively, a detection card component produced by the same and a method for preparing the same. The test strip component comprises a test strip and a platinum porphyrin marked specific antibody independently packed. The test strip comprises a bottom lining, a water absorption pad, coating analysis film and a sample pad. The coating analysis film is provided with a detection line and a quality control line, a specific antibody coated by the detection line is a C-reactive protein resistance monoclonal antibody, and a specific antibody coated by the quality control line is a rabbit intravenous gamma globulin (IgG) antibody; the detection card component comprises a test strip, a card box composed of a cover plate and a back plate and a latinum porphyrin marked specific antibody independently packed. The test strip component for detecting C-reactive protein in body fluid has the advantages of being simple in operation, rapid, sensitive, good in specificity and the like, and having good clinical application prospect.
Owner:GUANGZHOU HONGQI OPTICAL INSTR TECH
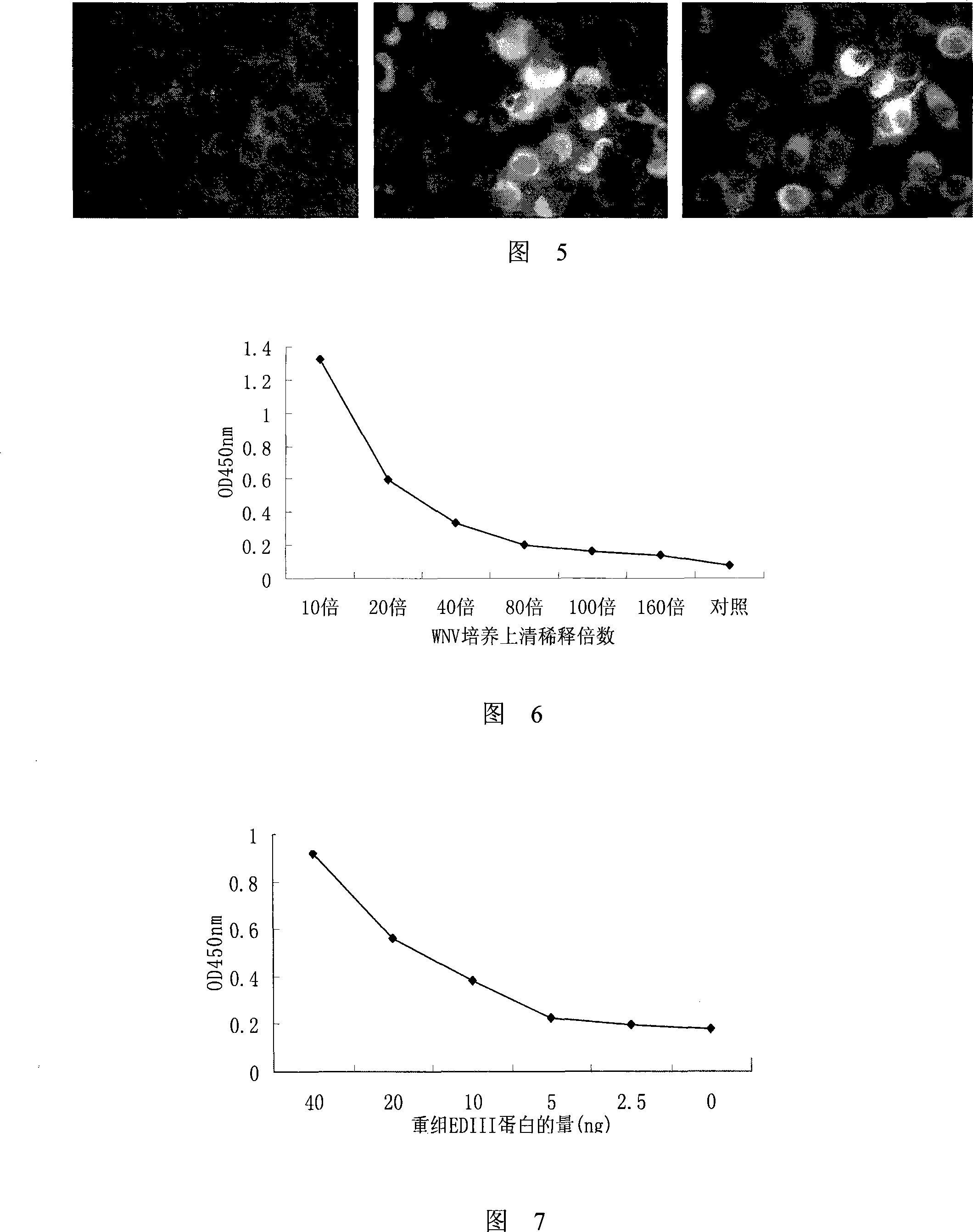
Monoclonal antibody of membrane protein E for resisting West Nile virus and application thereof
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI +1
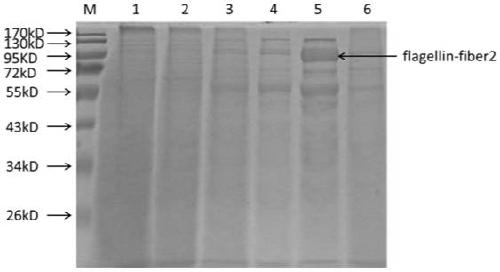
Flaggelin-fiber2 fusion protein, and preparation method and application thereof
ActiveCN109867727AEnhance immune responseStrong immune adjuvant effectAntiviralsAntibody medical ingredientsWestern blotFlagellin
The invention provides a flaggelline-fiber2 fusion protein as well as a preparation method and application thereof. The fusion protein is a fusion protein of a fowl adenovirus type 4 fiber2 protein with relatively high immunoprotection and a salmonella typhimurium flagellin. The preparation method comprises the following steps: cloning an artificially coded flaggin-fiber2 gene into a pFastBac-HA expression vector through fusion; carrying out gene transposition to form recombinant Bacmid, transfecting the recombinant Bacmid into Sf9 insect cells, expressing the fusion protein by utilizing a baculovirus system, and conducting identifying by virtue of indirect immunofluorescence IFA and Western blot. The period of obtaining the recombinant baculovirus through the system is short, and when SPFchicken are immunized by the flaggin-fibe2 fusion protein, results show that the flaggin-fibe2 fusion protein expressed by the baculovirus system has high immunoprotection capability.
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES
Near-infrared fluorescence detection kit for C-reactive protein and application of near-infrared fluorescence detection kit
InactiveCN104865390AEasy to collectSimple processing capacityBiological testingReagent stripCellulose
The invention discloses a near-infrared fluorescence detection kit for C-reactive protein and application of the near-infrared fluorescence detection kit. The near-infrared fluorescence detection kit comprises a reagent strip positioned on a back plate, wherein the reagent strip is a sample pad, a glass cellulose membrane marked with an immune fluorescent probe, a nitrocellulose membrane coated with a C-reactive protein antibody and water absorbing paper from the sampling end in sequence. The near-infrared fluorescence detection kit disclosed by the invention can quickly and accurately detect the C-reactive protein in human whole blood, serum or plasma in combination with a near-infrared fluorescence detection technology.
Owner:SHANGHAI CHEMTRON BIOTECH
Anthropogenic H5N1-resisting hemagglutinin protein broad-spectrum neutralising antibody and application thereof
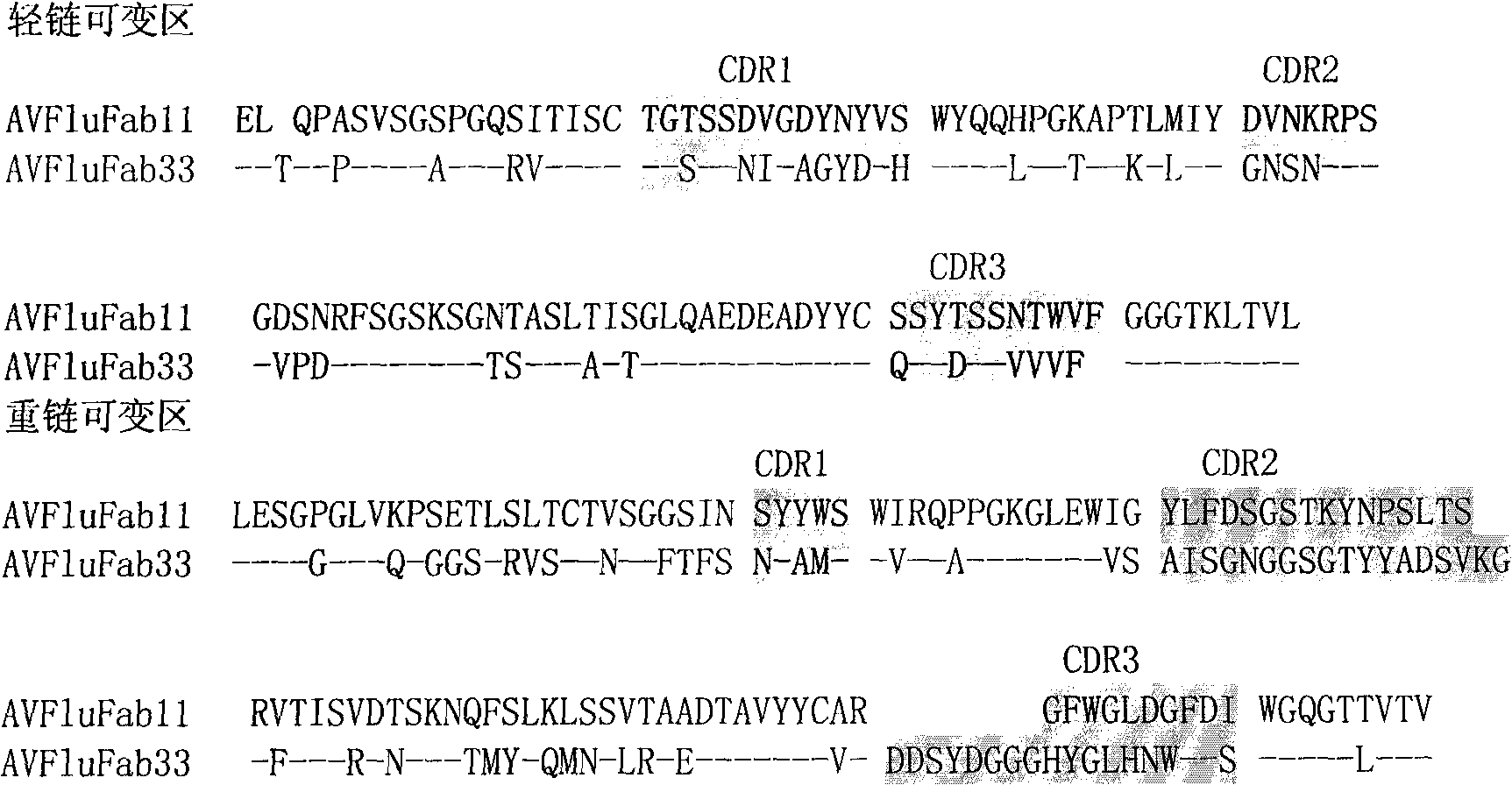
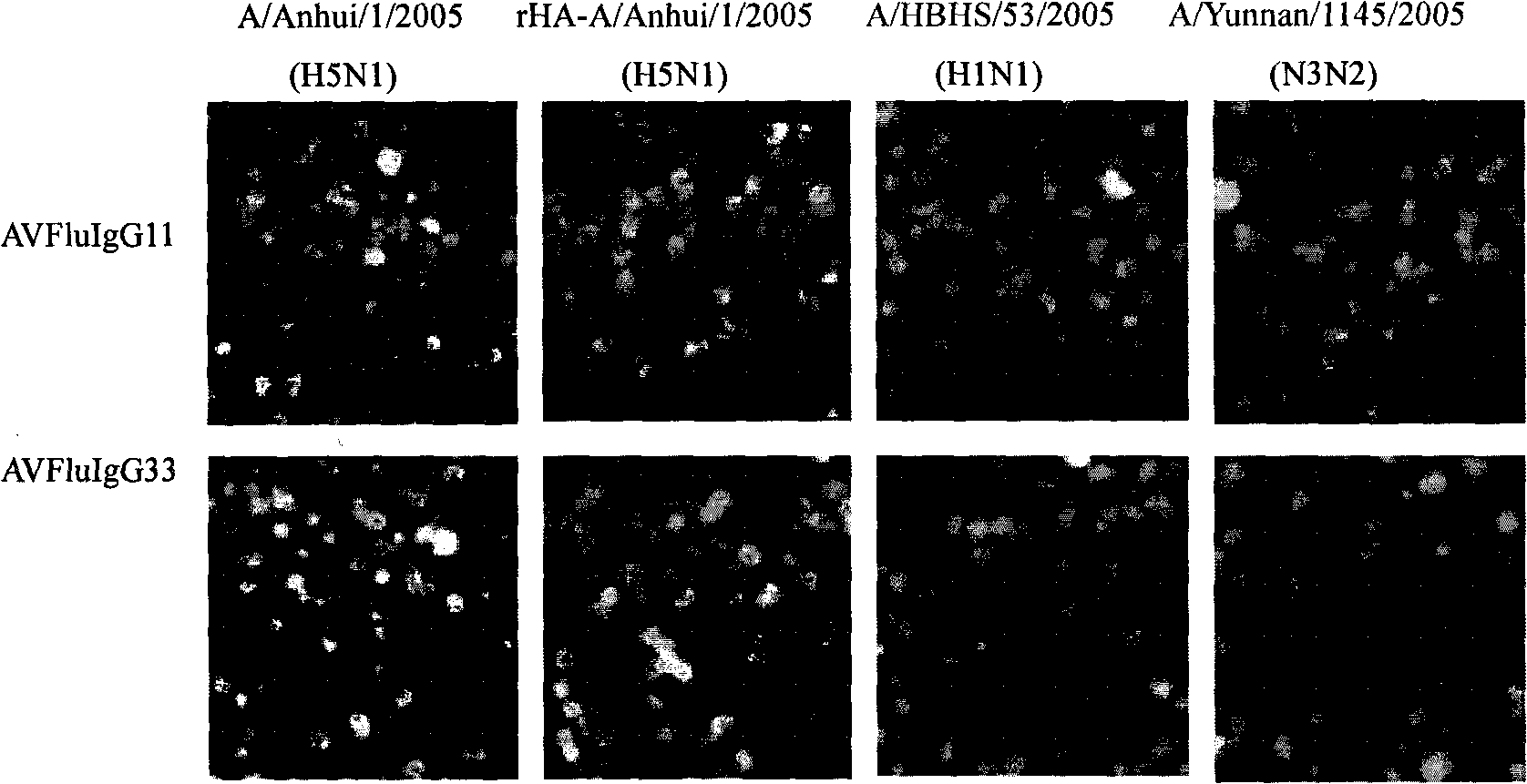
The invention provides an anthropogenic H5N1-resisting hemagglutinin protein broad-spectrum neutralising antibody. The antibody is obtained by screening through a phage display technology. The sequence of the hypervariable region (CDRs) determining the specificities of the antibody is represented by figure 1. The antibody of the invention specifically identifies H5N1 highly pathogenic avian influenza virus particle antigen, has obvious fluorescence immune reaction and enzyme-coupled immune reaction with the avian influenza virus H5N1 aiming at avian influenza virus hemagglutinin protein HA, and has the neutralising activity function of resisting the avian influenza virus H5N1. The antibody of the invention can be made into specific antibody medicines clinically used for preventing and treating human avian influenza that is caused by the H5N1 avian influenza virus, so as to clinically prevent or treat human avian influenza that is caused by the H5N1 avian influenza virus.
Owner:STATION OF VIRUS PREVENTION & CONTROL CHINA DISEASES PREVENTION & CONTROL CENT
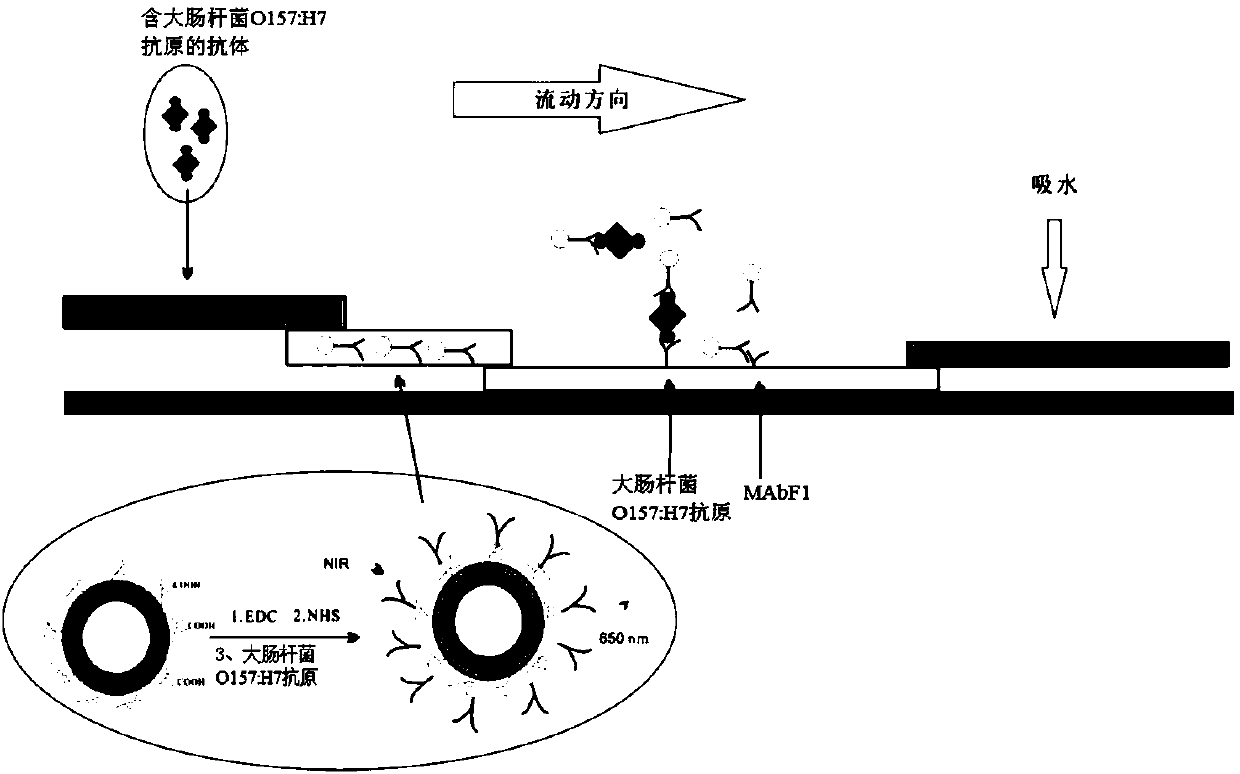
Upconversion-immunochromatography test strip used for detecting Escherichia coli O157:H7, and detection method thereof
InactiveCN107632156AReduce sensitivityWide detection rangeMaterial analysisEscherichia coliUpconversion luminescence
The invention provides an upconversion-immunochromatography test strip for detecting Escherichia coli O157:H7, and a detection method thereof. The test strip comprises a sample pad, a conjugate pad, an analysis membrane and an absorbent pad, the sample pad and the conjugate pad are glass cellulose membranes, the analysis membrane is a nitrocellulose membrane, the absorbent pad is a cellulose membrane, the analysis membrane is provided with a detection line and a quality control line, the sample pad, the conjugate pad and the absorbent pad are sequentially pasted on a PVC plate, the conjugate pad is immobilized with an upconversion luminescence nanoparticle-Escherichia coli O157:H7 monoclonal antibody IgG conjugate, the detection line on the analysis membrane is immobilized with an Escherichia coli O157:H7 antigen, and the quality control line is immobilized with MAbF1. The above upconversion nanomaterial-based immunofluorescence test strip has the advantages of low sensitivity, wide detection range, stable signal, no photobleaching, low background, sensitive signal, and accurate and reliable result.
Owner:HUNAN UNIV
Anti-human B7-H4 extracellular monoclonal antibody secreting hybridoma cell strain, anti-human B7-H4 monoclonal antibody and application thereof
ActiveCN107299085AGood passaging abilityStrong specificityBiological material analysisMicroorganism based processesAntiendomysial antibodiesWestern blot
The invention relates to the technical field of immune application, in particular to an anti-human B7-H4 extracellular monoclonal antibody secreting hybridoma cell strain, an anti-human B7-H4 monoclonal antibody and application thereof. The hybridoma cell strain comprises five hybridoma cells capable of generating high-potency monoclonal antibody and has good passaging capability. The antibody is the monoclonal antibody generated by the anti-human B7-H4 extracellular monoclonal antibody secreting hybridoma cell strain. The antibody can recognize a prokaryotic expression product, a eukaryotic expression product of human B7-H4 and expressed extracellular functional domain protein. The antibody is applied to immunological detection of the B7-H4, including detection of the B7-H4 expressed on the surfaces of the cells by a flow cytometry, detection of the B7-H4 expressed in tissues or the cells by an immunohistochemistry and a Western blot technology and detection of the soluble B7-H4 by an immunofluorescence technology, ELISA and chemiluminescence immunoassay; the antibody is also applied to antibody drug design.
Owner:GUANGDONG MEDICAL UNIV
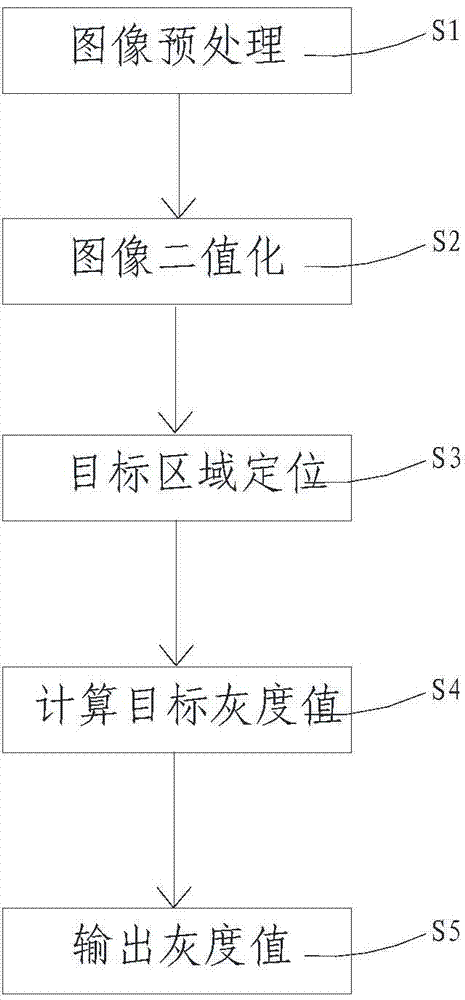
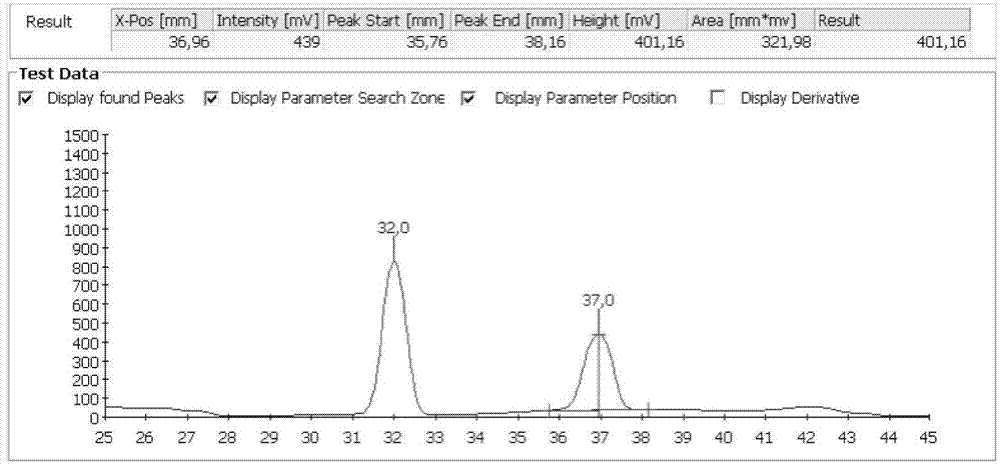
Immune complex fluorescence detection method based on image self-adaption division
InactiveCN104730050AHigh precisionGood repeatabilityFluorescence/phosphorescencePattern recognitionLuminous intensity
The invention provides an immune complex fluorescence detection method based on image self-adaption division. The immune complex fluorescence detection method comprises the following steps: step 1, pre-processing an image, namely carrying out median filtering processing on a fluorescence immune image, reducing noise point interferences and keeping the edge steep; step 2, carrying out image binaryzation, namely estimating a division threshold value according to an immune fluorescence region light intensity curve and then carrying out the binaryzation on the image; step 3, positioning a target position, namely positioning the target position based on target positioning of narrow strip characteristics; step 4, calculating a target gray value, namely after the position of the target region is obtained, calculating a gray value and a background gray value of the target region; and step 5, outputting the gray value to obtain the content of a detected object. According to the light detection method provided by the invention, the randomness and the inconsistency of shapes of an immune fluorescence composite distribution region can be eliminated and the interferences caused by background region reflection are eliminated, and the precision and the repeatability of immune complex fluorescence detection are improved; and meanwhile, the detection processing speed is also increased.
Owner:SHENZHEN KINGFOCUS BIOMDICAL ENG CO LTD
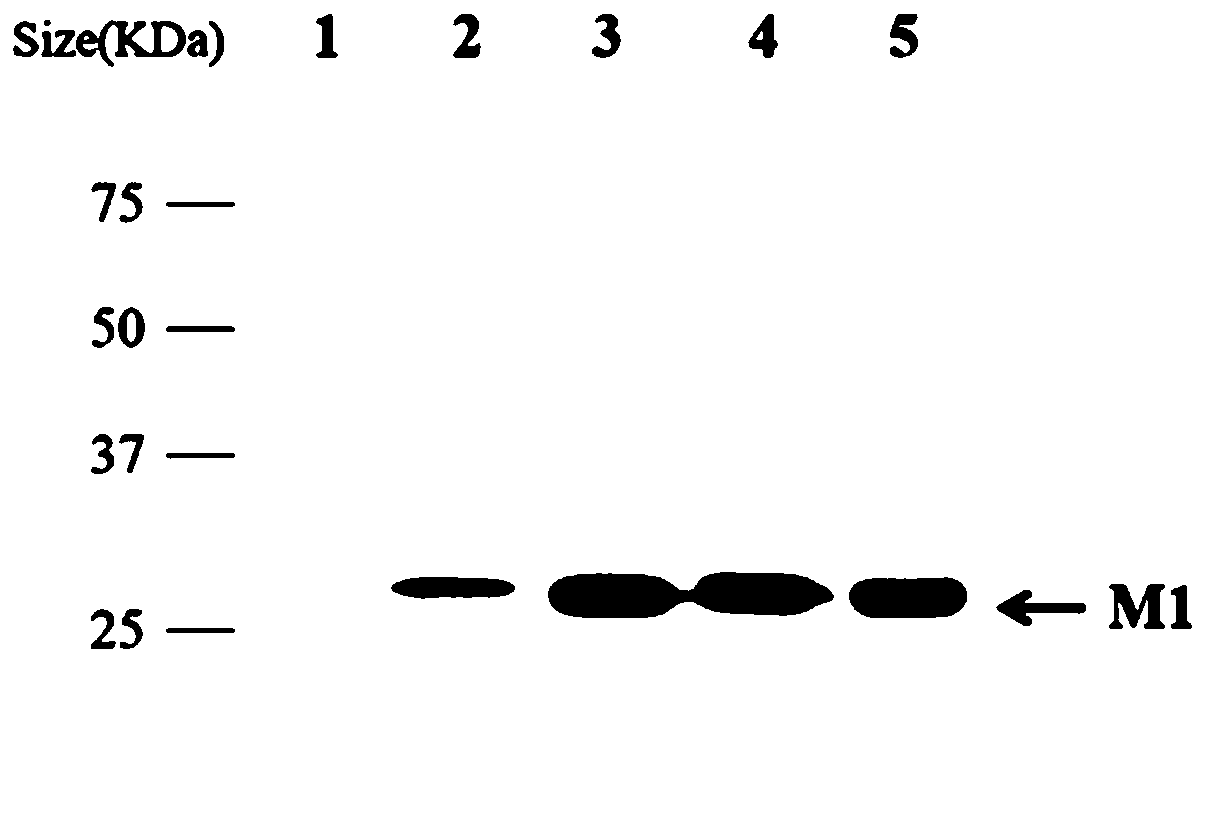
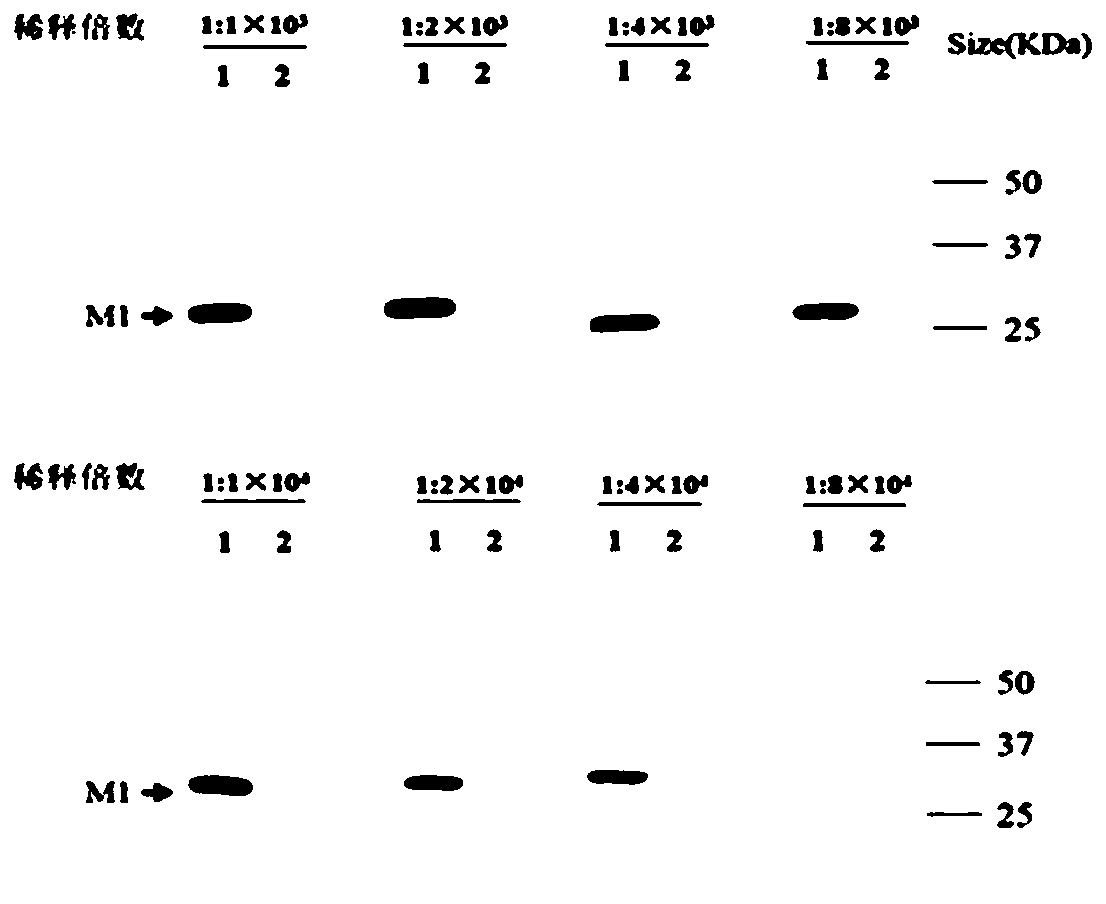
Monoclonal antibody of influenza virus matrix protein M1 and application of monoclonal antibody
ActiveCN110627901AHigh sensitivityHigh application valueBiological material analysisImmunoglobulins against virusesImmunoprecipitationIndirect immunofluorescence
The invention relates to the biology field, in particular to a monoclonal antibody of influenza virus matrix protein M1 and application of the monoclonal antibody. The monoclonal antibody of influenzavirus matrix protein comprises a heavy chain variable region and a light chain variable region, amino acid sequences of the CDR1, CDR2 and CDR3 of the heavy chain variable region are shown in SEQ IDNO.1-3 separately; and amino acid sequences of the CDR1, CDR2 and CDR3 of the light chain variable region are shown in SEQ ID NO.4-6 separately. The monoclonal antibody can specifically recognize andbind multiple subtypes of influenza virus matrix protein M1, has broad spectrum applicability among influenza virus subtypes, has higher specificity and sensitivity, can be well applied to western blotting experiments, indirect immunofluorescence experiments, immunoprecipitation technologies and the like and has higher application values.
Owner:CHINA AGRI UNIV
Monoclonal antibody with rock bream iridovirus ORF049L recombinant proteins and preparation method thereof
InactiveCN103288954AOvercoming the shortcomings of detectionStrong specificityImmunoglobulins against virusesProtein.monoclonalProkaryotic expression
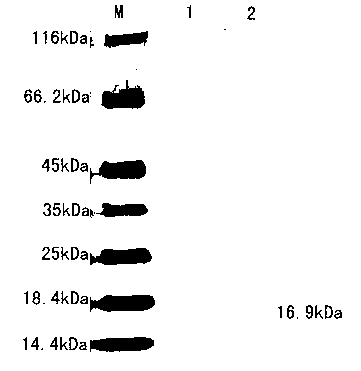
The invention discloses a monoclonal antibody with rock bream iridovirus ORF049L recombinant proteins. The monoclonal antibody is produced by secreting rock bream iridovirus ORF049L recombinant protein hybridoma of which the preservation number is CGMCC (China General Microbiological Culture Collection Center) No.7304. A preparation method of the monoclonal antibody comprises the following steps of: obtaining the rock bream iridovirus ORF049L recombinant proteins with the molecular weight of 16.9 kDa through gene cloning and prokaryotic expression; taking the obtained recombinant proteins as antigens to immune mice; performing cell fusion on mice spleens and myeloma cells, and screening and secreting the hybridoma of the rock bream iridovirus ORF049L recombinant protein monoclonal antibody by an immunoblotting method, a dot immunobinding assay and an indirect immunofluorescence; and cloning positive hybridoma by a finite dilution method.
Owner:DALIAN OCEAN UNIV +1
Polypeptide specifically binding to CD34 molecule and application thereof
ActiveCN105254717AImprove binding efficiencySmall side effectsPeptide/protein ingredientsBiological material analysisImmunofluorescenceSide effect
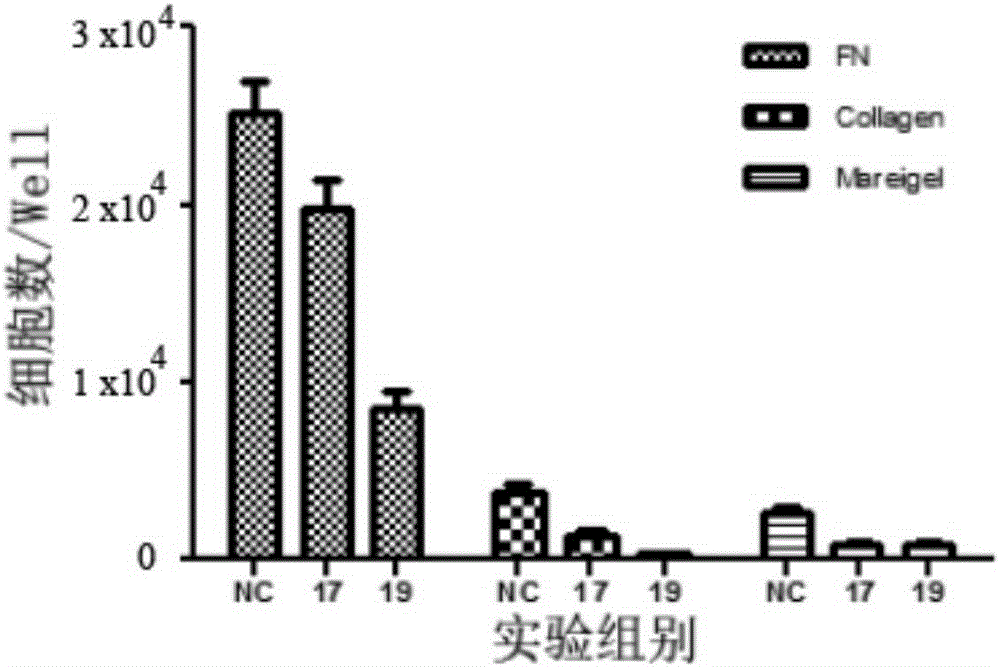
The invention provides a polypeptide specifically binding to a CD34 molecule and application thereof. The polypeptide is at least one selected from the group consisting of polypeptide No. 17 and polypeptide No. 19, wherein the amino acid sequences of the polypeptide No. 17 and the polypeptide No. 19 are respectively shown in a sequence 1 and a sequence 2 in a sequence table. The polypeptide provided by the invention can specifically bind to the CD34 molecule and can be produced by using an artificial synthesis or genetic engineering method. Compared with an antibody, the polypeptide provided by the invention has the characteristics of easiness in preparation, small molecular weight, small possibility of incurrence of immunological rejection, etc.; moreover, the polypeptide has small toxic and side effect, can bind to CD34+ cells and does not exert obvious killing and propagation inhibiting effects on target cells. The polypeptide can be used as a marker for a cell with CD34 positive expression, or as a substitute for an immunofluorescence CD34 antibody, or the like.
Owner:SUN YAT SEN UNIV +1
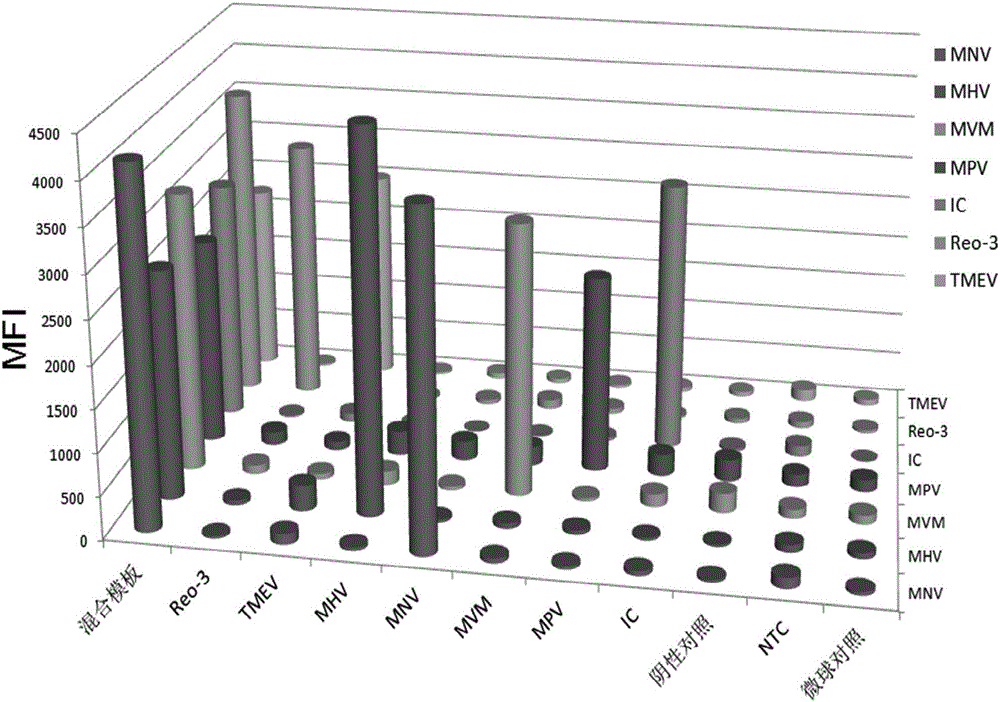
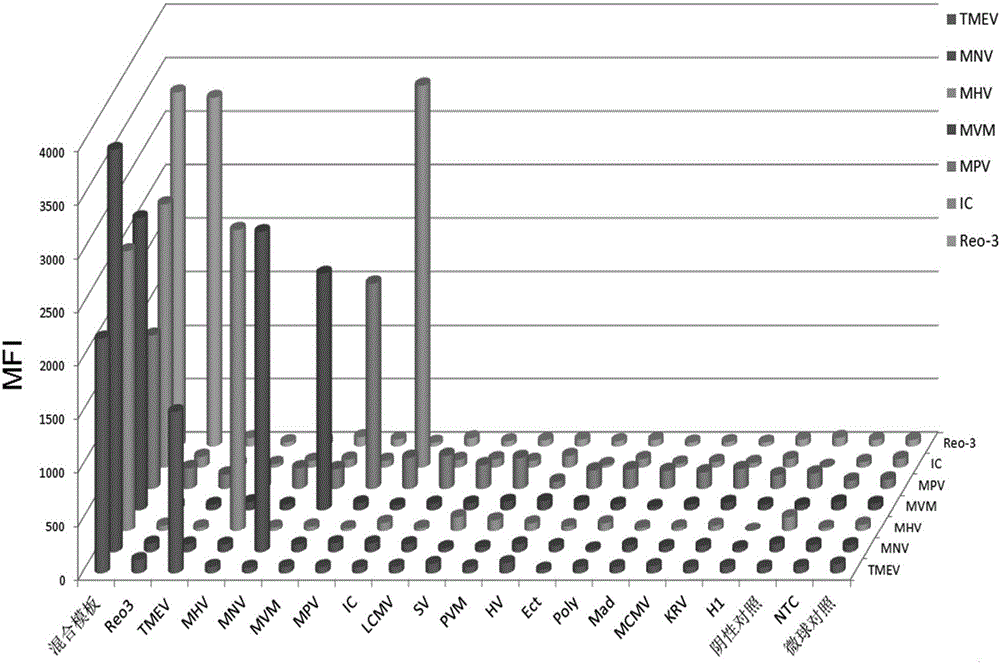
Primer group, kit and method for detecting six mouse viruses by multiple immunity fluorescence
ActiveCN106636474AHigh sensitivityStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationBiotin-streptavidin complexMicrosphere
The invention discloses a primer group, a kit and a method for detecting six mouse viruses by multiple immunity fluorescence. The primer group is designed by the aid of multiple RT-PCR (reverse transcription-polymerase chain reaction) technologies and Luminex liquid chip technologies and can simultaneously detect six high-infection viruses such as TMEV, MHV, MNV, Reo-3, MVM and MPV of mice. By the aid of the primer group, target amplification products are acquired through specific PCR, the amplification products, fluorescence encoding microspheres and streptavidin-phycoerythrin are hybridized, and different types of viruses are distinguished when a mean fluorescence intensity value is read by a Luminex detector. The method has the advantages of rapidness, high efficiency, high specificity and sensitivity, good repeatability and the like and can be applied to quality monitoring, epidemiological investigation and early warning of test mice. The primer group is good in flexibility, and varieties detecting the viruses can be increased and decreased as required on the basis.
Owner:GUANGDONG LAB ANIMALS MONITORING INST
Swine fever and porcine pseudorabies bivalent vaccine as well as preparation method and application thereof
InactiveCN103505724ASolve the problem of low early potencyImprove securityAntiviralsAntibody medical ingredientsDiseaseRabies
The invention provides a swine fever and porcine pseudorabies bivalent vaccine. The swine fever and porcine pseudorabies bivalent vaccine contains at least one swine fever virus antigen and at least one porcine pseudorabies virus antigen, wherein the two antigens coordinate well, are excellent in immune effect and can promote each other. The swine fever and porcine pseudorabies bivalent vaccine is simple in preparation method, is convenient and efficient in immunization and has the advantages that immunization cost is reduced, an immunization procedure is simplified and economy and reliability are realized compared with a vaccine which can be used for preventing and treating more than two diseases only when immunization is carried out in steps and at least two injections are taken and an immune method of the vaccine in the prior art. The immune effect of the swine fever and porcine pseudorabies bivalent vaccine is better than that of a single vaccine and better in safety and avoids adverse effects caused by multiple immunizations. Besides, the invention also provides a simple testing method for determining swine fever effect in the bivalent vaccine by adopting an indirect immunofluorescence method, so that quality of bivalent live vaccines in each batch is guaranteed, and economic benefit is obviously increased.
Owner:PU LIKE BIO ENG
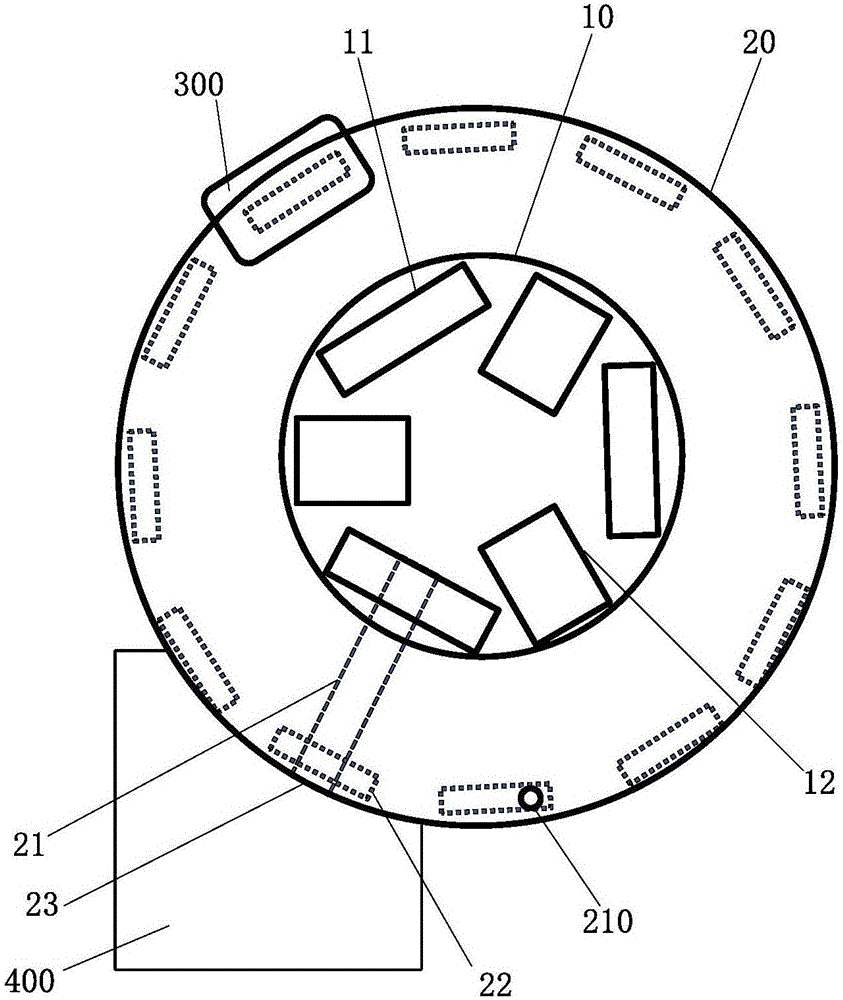
Rotary table device and full-automatic immune fluorescence analyzer with same
ActiveCN105866454AImprove work efficiencyImprove convenienceMaterial analysisPulp and paper industryIMMUNE FLUORESCENCE
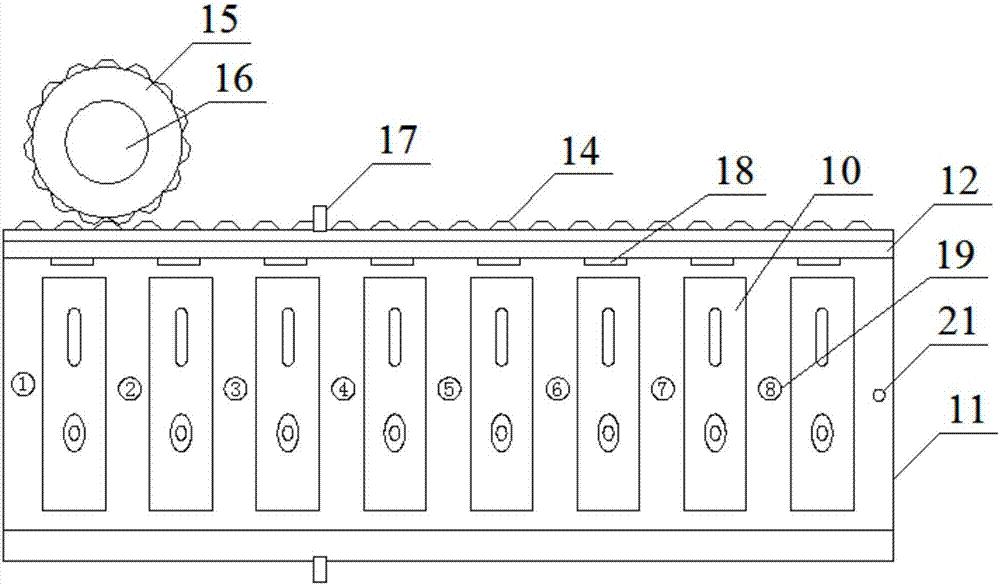
The invention discloses a rotary table device. The rotary table device comprises a test paper clip and a first rotary table of a pushing mechanism, the test paper clip feeds test paper automatically, and the pushing mechanism pushes the test paper away from the first rotary table; a second rotary table is an annular structure coaxially arranged on the outer side of the first rotary table in a sleeving mode; a guide-track groove is formed in the second rotary table in an embedded mode, and a first opening is formed in the portion, close to the outer edge and right opposite to the upper portion of the guide-track groove, of the second rotary table; one end of the guide-track groove is communicated with a test paper outlet, a second opening is formed in the other end of the guide-track groove, and an elastic piece is arranged at the end, close to the second opening, of the guide-track groove; the test paper extends out of the test paper outlet, is firstly pushed by the pushing mechanism and slides along the guide-track groove till the test paper is blocked by the elastic piece, and an area to be detected aligns to the first opening; the test paper is subjected to second pushing of the pushing mechanism, passes over the elastic piece and gets away from the second opening. According to the rotary table device and the full-automatic immune fluorescence analyzer with the same, test paper sample adding and automation of the whole detection process are achieved, a single form of queue waiting is not needed, and the work efficiency and stability are improved.
Owner:SUZHOU TOPMEDLAB MEDICAL SCI & TECH CO LTD
Monoclonal antibody of outer membrane protein of chlamydia abortus and application thereof
ActiveCN102220285AStrong specificityGood fluorescence propertiesImmunoglobulins against bacteriaTissue cultureFluorescenceIMMUNE FLUORESCENCE
The invention relates to monoclonal antibody of N-terminal protein of chlamydia abortus POMP18D as well as a preparation method and application thereof. The monoclonal antibody is secreted by a hybridoma cell strain 1F10D4 with the collection number of CGMCC No.4658. The invention also provides application of the monoclonal antibody in direct fluorescence immune or indirect fluorescence immune detection of chlamydia abortus. The monoclonal antibody of N-terminal protein of chlamydia abortus POMP18D has strong specificity and fluorescent characteristic, and is suitable for being used as a fluorescence antibody to establish a direct fluorescence detection method or indirect immune fluorescence detection method.
Owner:CHINA AGRI UNIV
Colloidal gold fluorescent quantitative analysis all-in-one machine and control method thereof
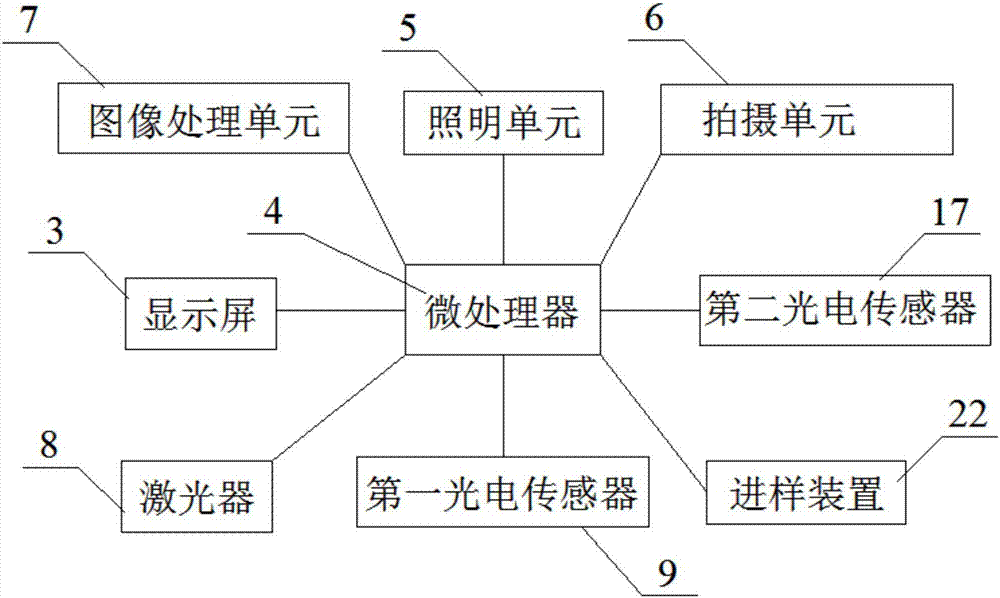
PendingCN106855515ALow costReduce experimental stepsBiological testingFluorescence/phosphorescenceFluorescenceColloidal au
The invention discloses a colloidal gold fluorescent quantitative analysis all-in-one machine and a control method thereof. According to the all-in-one machine, through the application of a microprocessor, as well as an illumination unit, a shooting unit, an image processing unit, a laser device and a first photoelectric sensor which are connected with the microprocessor, the all-in-one machine disclosed by the invention can be used as a colloidal gold card reader and also can be used as an immunity fluoroanalyzer, so that the cost is reduced. The invention also provides a control method of the all-in-one machine, and whether a test paper card is a colloidal gold test paper card or an immunity fluorescence test paper card does not need to be distinguished by an operator in the using process, so that the experimental procedures are reduced, and the experimental efficiency is improved.
Owner:WUHAN J H BIO TECH
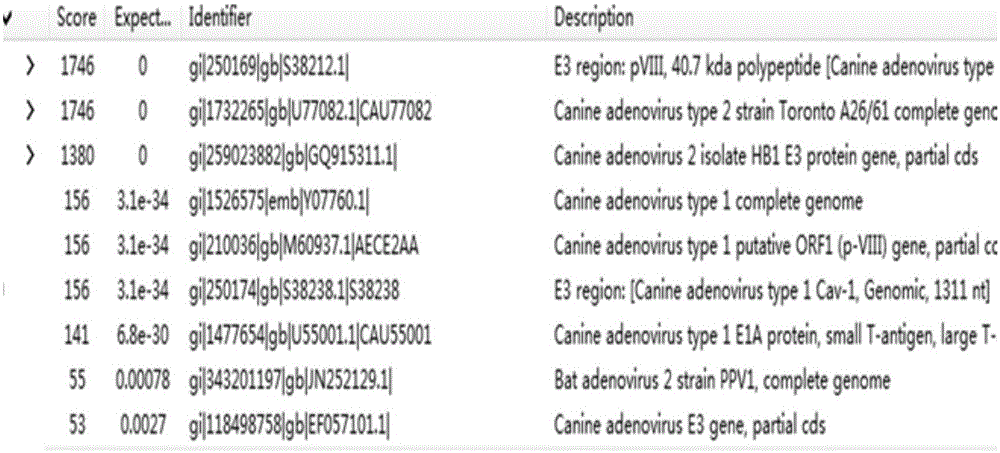
Hybridoma cell line 7D7 secreting CAV-2 monoclonal antibody, monoclonal antibody secreted by hybridoma cell line 7D7, and applications of monoclonal antibody
The present invention discloses a strain of a hybridoma cell line 7D7 secreting CAV-2 monoclonal antibody, monoclonal antibody secreted by the hybridoma cell line 7D7, and applications of the monoclonal antibody, wherein the preservation number of the hybridoma cell line 7D7 is CGMCC No.10877. According to the present invention, the indirect immunofluorescence results show that: the monoclonal antibody secreted by the hybridoma cell line 7D7 can specifically recognize CAV-2 virus and do not react with MDCK cells; the superposition experiment results of the prepared monoclonal antibody secreted by hybridoma cell line 7D7 and the monoclonal antibody secreted by 2C1 show that the two monoclonal antibodies are against different epitopes of the CAV-2 virus; the prepared monoclonal antibody can well react with CAV-2, and can be used for identification of clinical isolated strains; and the prepared 7D7 monoclonal antibody and the monoclonal antibody 2C1 are the monoclonal antibodies identifying the two different antigenic determinants of the CAV-2, such that the monoclonal antibody of the present invention can be used for preparation of the CAV-2 pathogenic derection immune colloidal gold test paper strip and establishment of the double antibody sandwich ELISA method.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Method for separating and culturing RSV (Respiratory Syncytial Virus) by using OMC (Ostiomeatal Complex)-685-1 cells
InactiveCN102559608AIncreased sensitivityHigh sensitivityViruses/bacteriophagesMaterial analysisCytopathic effectIMMUNE FLUORESCENCE
The invention discloses a method for separating and culturing RSV (Respiratory Syncytial Virus) by using OMC (Ostiomeatal Complex)-685-1 cells, which comprises the following steps of: using the OMC-685-1 cells as a respiratory syncytial virus separating tool, observing a cytopathogenic effect by using an inverted microscope and detecting the virus by using an indirect immuno fluorescence method, and specifically comprises the following steps of: culturing the OMC-685-1 cells, infecting an OMC-685-1 cell line, observing a CPE (Cytopathic Effect) by using the inverted microscope and detecting the respiratory syncytial virus by using the indirect immuno fluorescence method. With the adoption of the separating and culturing method, whether a collected specimen contains RSV or not can be directly judged. The method has the advantages of simplicity, good repeatability, high sensitivity, short time consumption and low cost.
Owner:ZUNYI MEDICAL UNIVERSITY
Construction method of recombinant baculovirus for expressing serum type 4 avian adenovirus fibroid protein F2
InactiveCN111534547AImprove expression levelOptimize purification stepsFermentationDsDNA virusesCytopathic effectShuttle vector
The invention relates to a construction method of recombinant baculovirus for expressing serum type 4 avian adenovirus fibroid protein F2. The construction method comprises the following steps: (1) constructing an FAdV4F2 gene recombinant baculovirus expression vector; (2) packaging and passing baculovirus for expressing recombinant FAdV4 protein; and (3) transfecting baculovirus genome carrying FAdV4F2 gene into Sf9 cells, culturing in an incubator at 27 DEG C for 57 days, and observing cytopathic effect; when cytopathic effect reaches 70%, collecting cell lysis buffer, centrifuging to removeprecipitate, and preserving as seed virus; passing virus, and carrying out enlarged culture as seed virus when passing to the third generation. According to the invention, a baculovirus expression system is utilized to construct a recombinant baculovirus shuttle vector containing F2 gene, the recombinant baculovirus shuttle vector is transfected into Sf9 cells to obtain recombinant baculovirus rBAF2, and expression of recombinant protein F2 is identified by virtue of indirect immunofluorescence assay (IFA) and Western blot.
Owner:YANGZHOU UNIV
Preparation and application of myoglobin monoclonal antibody
ActiveCN112521497AShort manufacturing timeHigh expressionImmunoglobulins against animals/humansFermentationEscherichia coliIMMUNE FLUORESCENCE
The invention belongs to the technical field of biology. The invention provides a recombinant protein. An amino acid sequence of the recombinant protein is formed by repeatedly connecting two dominantepitopes of myoglobin (Myo) in series, an escherichia coli preferred codon is adopted to convert the amino acid sequence of the recombinant protein into a corresponding nucleotide sequence, the nucleotide sequence is chemically synthesized, and a recombinant expression vector is constructed. Therefore, the expression quantity of the recombinant protein in escherichia coli is increased. The invention also relates to a phage library established by immunizing mice with the recombinant protein, a corresponding myoglobin single-chain antibody scfv sequence is obtained through selecting and screening, the obtained scfv sequence is constructed into a complete mouse IgG1 antibody sequence expression vector, a monoclonal antibody is expressed through transient HEK293F cells, the monoclonal antibody is purified, and an optimal monoclonal antibody pairing combination is determined through an immunofluorescence orthogonal experiment.
Owner:杭州贤至生物科技有限公司
Recombinant lactobacillus casei for expressing infectivity pancreatic necrosis virus VP2 protein and production method thereof
InactiveCN101508969AEffective stimulationImprove the level ofBacteriaMicroorganism based processesProtein targetInfectious pancreatic necrosis
The invention provides a recombinant Lactobacillus casei for expressing the infectious pancreas necrosis virus (IPNV) VP2 protein and a preparation method thereof. According to the whole gene sequence of the IPNV VP2 protein and the gene fusion characteristic of expression vector plasmid, a pair of primers are designed to perform PCR to obtain target segment of 1095bp containing the main antigen site of the IPNV VP2 protein, the IPNV VP2 protein obtained through amplification is linked to the surface expressed carrier plasmid pPG1 and enters the cell of the host strain Lactobacillus casei 393 through electrotransformation, and the Lactobacillus casei containing positive recombinant plasmid pPG1-IPNV VP2 is named pPG1-IPNV VP2 / L. casei 393. The recombinant pPG1-IPNV VP2 / L. casei 393 is expressed under the induction of lactose. In the invention, the Lactobacillus casei expression carrier system of the IPNV VP2 protein is constructed and the target protein of around 40 KDa containing the main antigen site of the IPNV VP2 protein is expressed, and according to the Western blot experiment and indirect immunofluorescence experiment, the expressed foreign protein is capable of reacting with the IPVN immune serum, which shows that the recombinant VP2 protein and the IPNV natural antigen have the same antigenicity.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Influenza B virus IgA (immunoglobulin A) antibody immunofluorescence assay test strip and preparation method, detection method and application thereof
ActiveCN108152506AQuick screeningHigh sensitivityBiological testingImmunoglobulin AImmunofluorescence
The invention provides an influenza B virus IgA (immunoglobulin A) antibody immunofluorescence assay test strip and a preparation method thereof. The influenza B virus IgA antibody immunofluorescenceassay test strip comprises a sample pad, a label pad, a nitrocellulose membrane, a water absorbing paper and a back plate, wherein the nitrocellulose membrane comprises a detection line for influenzaB virus NP (nucleoprotein) antigen polypeptide segments, and a human IgA antibody contrast line; the label pad is provided with an anti-human IgA antibody-fluorescent microsphere marker. The influenzaB virus IgA antibody immunofluorescence assay test strip has the advantages that the detection sensitivity is very high, a high-cost PCR (polymerase chain reaction) detection instrument is not needed, the detection result can be known by naked eyes, and the influenza B virus can be quickly screened. The invention also provides a detection method and application of the influenza B virus IgA antibody immunofluorescence assay test strip. The detection method has the advantages that the steps are simple, the operation is easy, the results are quick, and the detection method is suitable for beingwidely popularized.
Owner:GUANGZHOU RHFAY BIOTECH CO LTD
Preparation of canine brain natriuretic peptide (BNP) monoclonal antibody
ActiveCN112608385AImprove expression levelKeep natural activityAntibody mimetics/scaffoldsMicroorganism based processesEscherichia coliSingle-Chain Antibodies
The invention belongs to the technical field of biology. The invention relates to a polypeptide containing two dominant epitopes of canine brain natriuretic peptide (BNP). The immune effect is enhanced by coupling the polypeptide with KLH protein immune mice. The invention also provides a recombinant protein. The recombinant protein comprises two dominant epitopes of canine BNP, and in order to improve the yield of the recombinant protein in a prokaryotic expression system, escherichia coli preferred codons are adopted to convert the amino acid sequence of the recombinant protein into a corresponding nucleotide sequence, the nucleotide sequence is chemically synthesized, and a recombinant protein expression vector is constructed. The invention further relates to a phage library established by coupling polypeptide with KLH protein immune mice, a corresponding canine BNP single-chain antibody scfv sequence is obtained through panning and screening, the obtained scfv sequence is constructed into a complete mouse IgG1 antibody sequence expression vector, monoclonal antibodies are expressed through transient HEK293F cells, the monoclonal antibodies are purified, and the optimal monoclonal antibody pairing combination is determined through an immunofluorescence orthogonal experiment.
Owner:杭州贤至生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com