Monoclonal antibody of influenza virus matrix protein M1 and application of monoclonal antibody
A monoclonal antibody, influenza virus technology, applied in the direction of antiviral immunoglobulin, application, antibody, etc., can solve the problems of impact and poor specificity, and achieve the effect of high application value and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1 Obtaining of Hybridoma Cell Line 2C5 and the Influenza Virus M1 Protein Monoclonal Antibody It Produces
[0049] 1. Preparation of immunogen
[0050] The immunogen used to immunize mice is the recombinant influenza virus M1 protein carrying the GST tag expressed by a prokaryotic expression system, and the specific preparation method is as follows:
[0051] (1) Construction of M1 protein prokaryotic expression vector
[0052] Using the sequence of the M1 gene of an H9 subtype influenza virus as a template, use the following primers to PCR amplify the M1 protein coding gene and connect it to the pGEX-1ZT vector.
[0053] Upstream primer: 5'-CTGGTTCCGCGTGGATCCGGATCCATGAGTCTTTCTAACCGAGGT-3' (contains BamHI restriction site)
[0054] Downstream primer: 5'-GGCCGCGATATCAAGCTTAAGCTTTCACTTGAACCGCTGCAGTT-3' (contains HindIII restriction site)
[0055] (2) Prokaryotic expression of M1 protein
[0056] The prokaryotic expression vector of the M1 protein constructed ab...
Embodiment 2
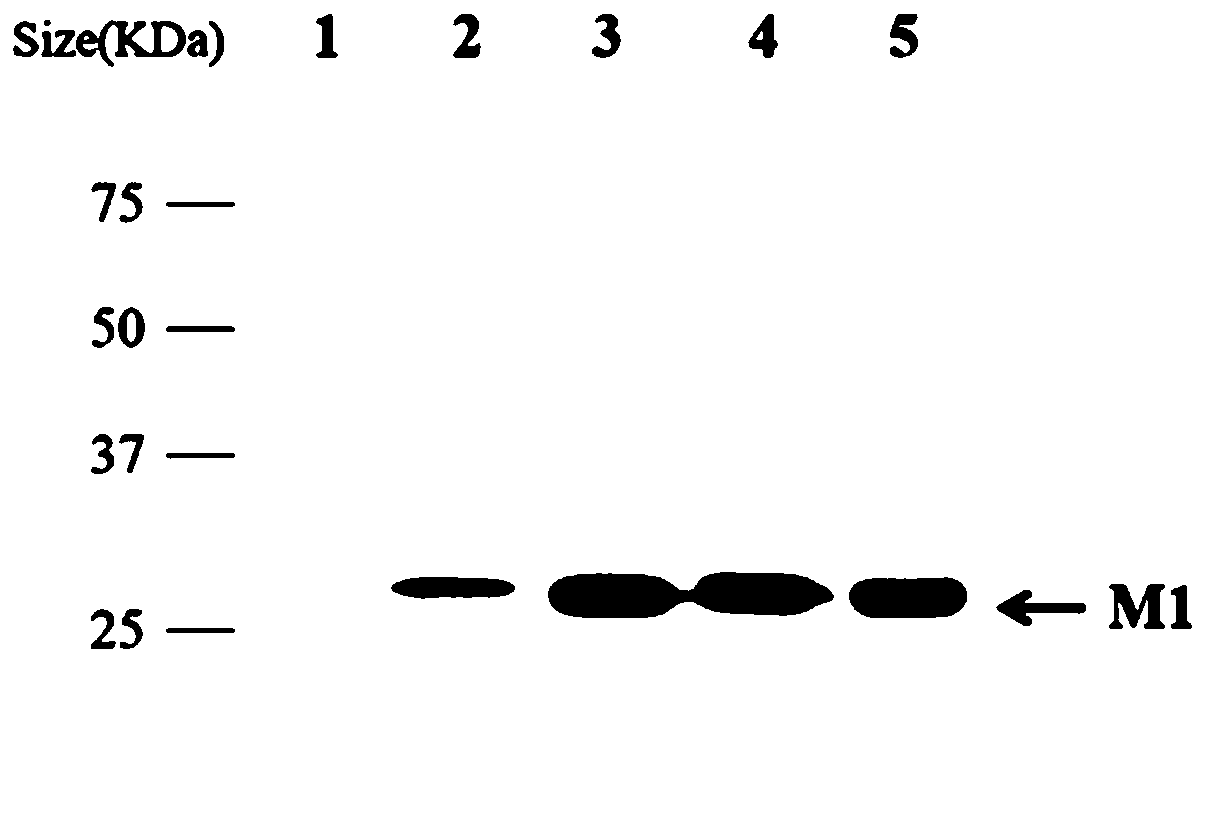
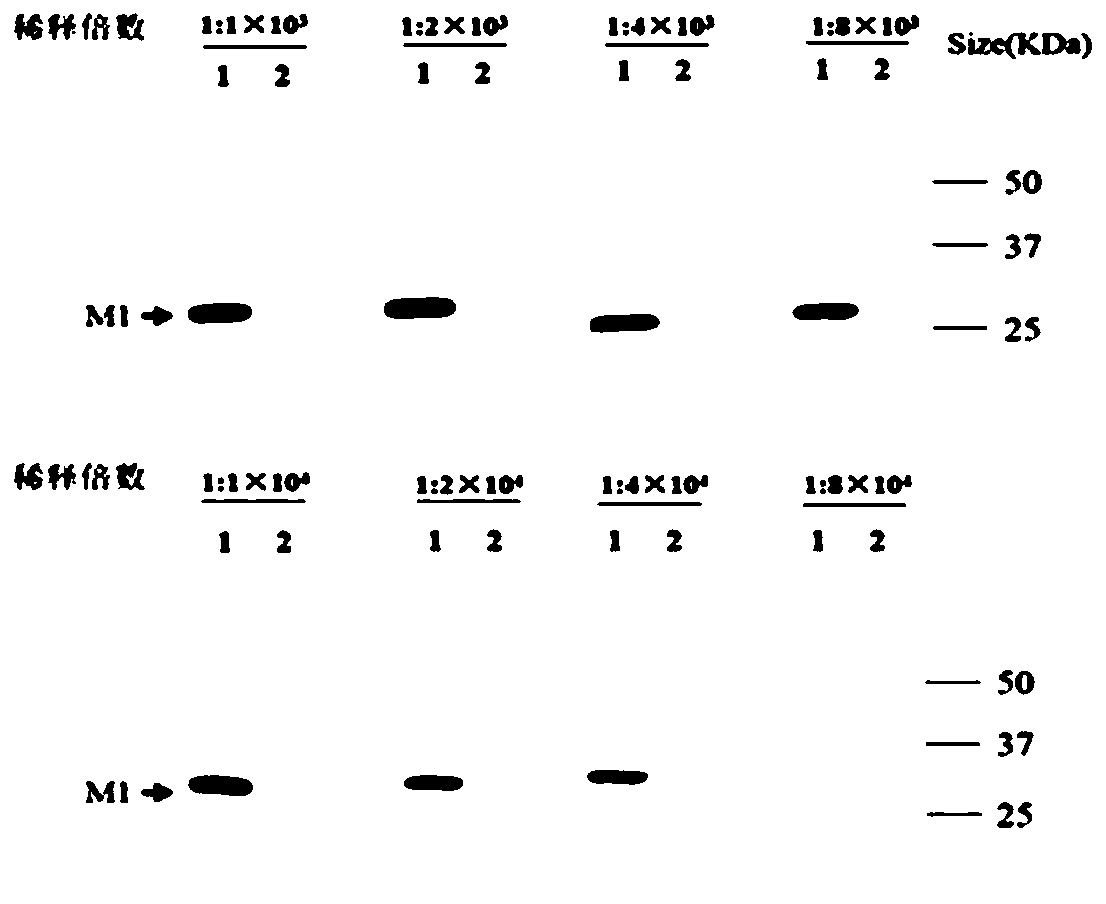
[0071] Application of Example 2 Monoclonal Antibody 2C5 in Western Blot (Western blot)
[0072] 1. Specific identification of monoclonal antibody 2C5 for Western blot detection
[0073] (1) Cell plate for A549 cells: transfer A549 cells from the large cell flask to a 12-well plate about 24 hours before the Western blot experiment, calculate the number of cell wells required for the experiment, and spread cells in one more hole for counting , Pay attention to observation. When the cells grow to 80%-90% of the cell well, gently remove the culture medium in the cell well, gently wash the cells once with PBS, and discard the washing solution;
[0074] (2) Virus dilution: take the allantoic fluid of H9N2, H7N9, H5N1, and H1N1 subtype viruses out of the -80°C refrigerator and melt at 4°C, and use a cell counter to perform cell counting according to the conventional cell counting method. Calculate the infection amount of the required virus according to MOI=0.2, and dilute the virus...
Embodiment 3
[0093] Example 3 Application of Monoclonal Antibody 2C5 in Indirect Immunofluorescence (IFA)
[0094] 1. Specific identification of monoclonal antibody 2C5 for IFA detection
[0095] (1) Infect MDCK cells with influenza viruses of four subtypes, H9N2, H7N9, H5N1, and H1N1, according to the method for infecting viruses in Example 2;
[0096] (2) Washing: Gently shake off the culture medium in the well, wash it once with PBS, and do it gently to avoid the cells falling off, and discard the PBS in the well;
[0097] (3) Fixation: add an appropriate amount of fixative solution prepared according to the ratio of absolute ethanol: acetone = 3:2 to each well, and fix at room temperature for 20 minutes;
[0098] (4) Washing: Dry the fixative in the wells, wash with PBS for 3 times, and place it on a shaker for 3-5 minutes after each wash;
[0099] (5) Sealing: add an appropriate amount of blocking solution made of 5% skim milk to each well, place it in a 37°C constant temperature in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com