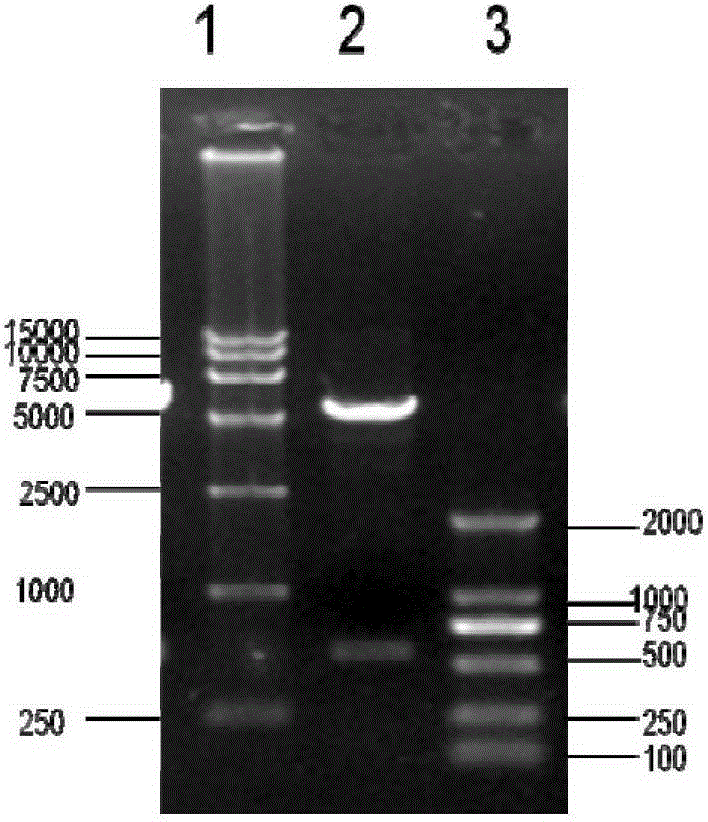
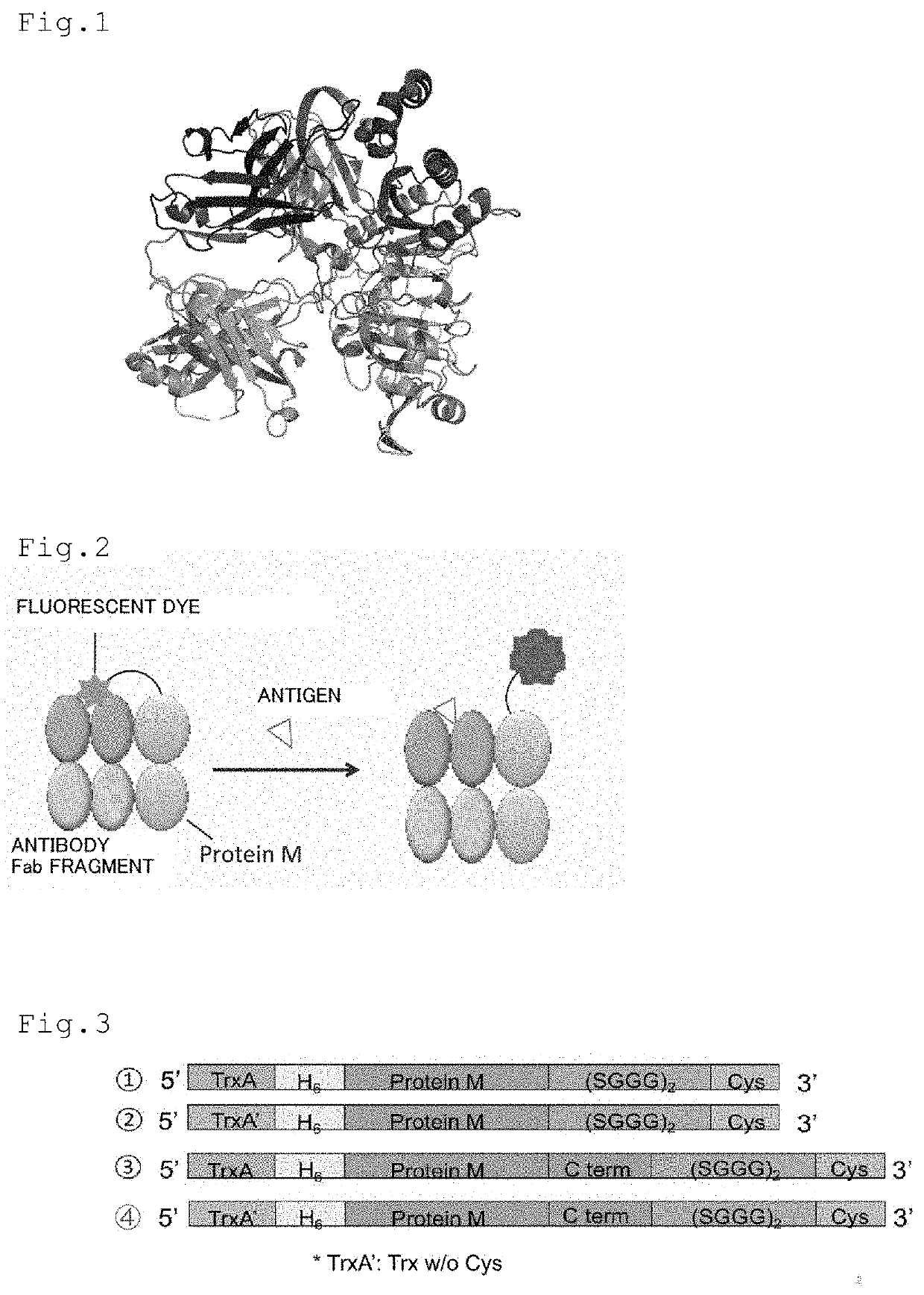
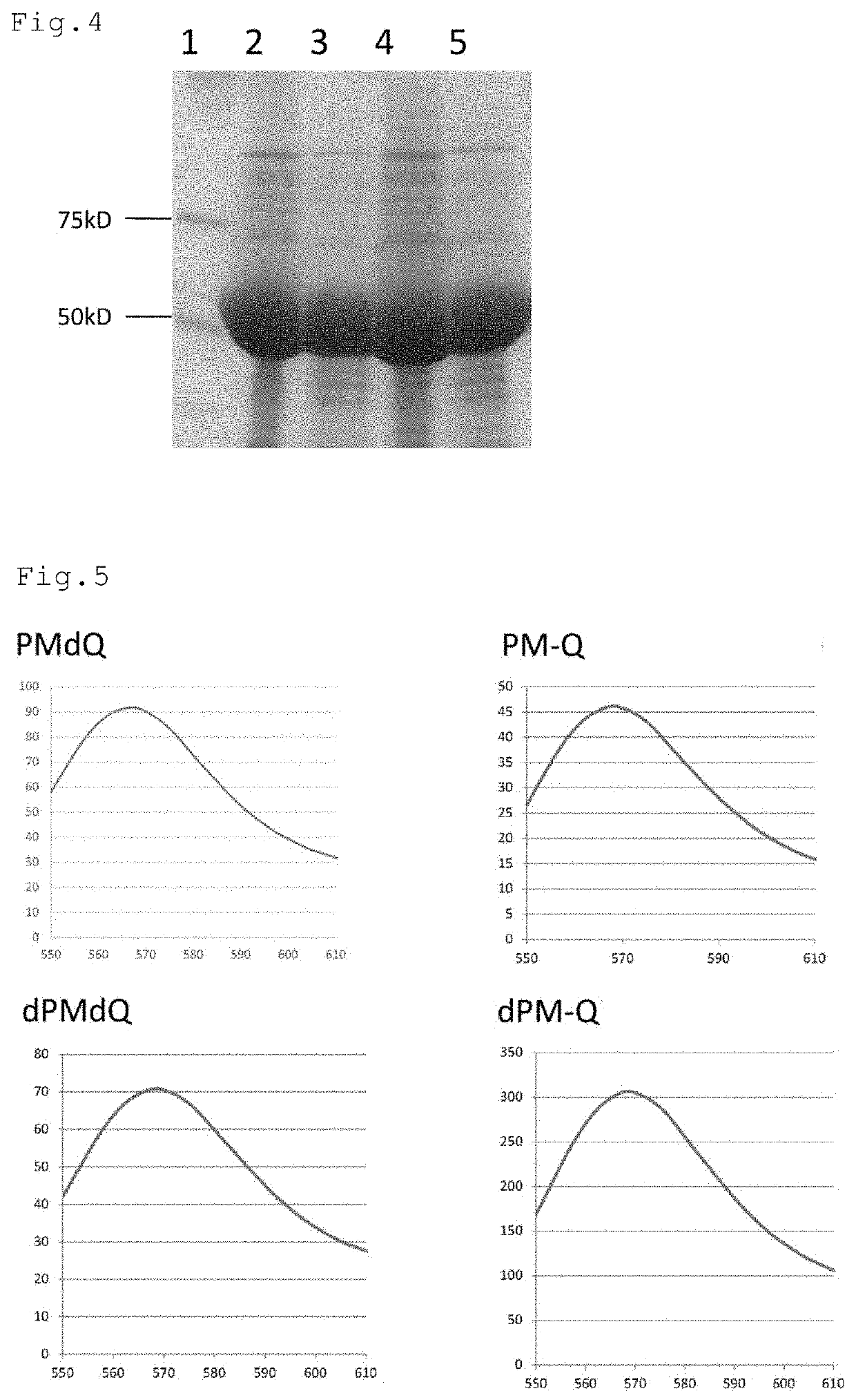
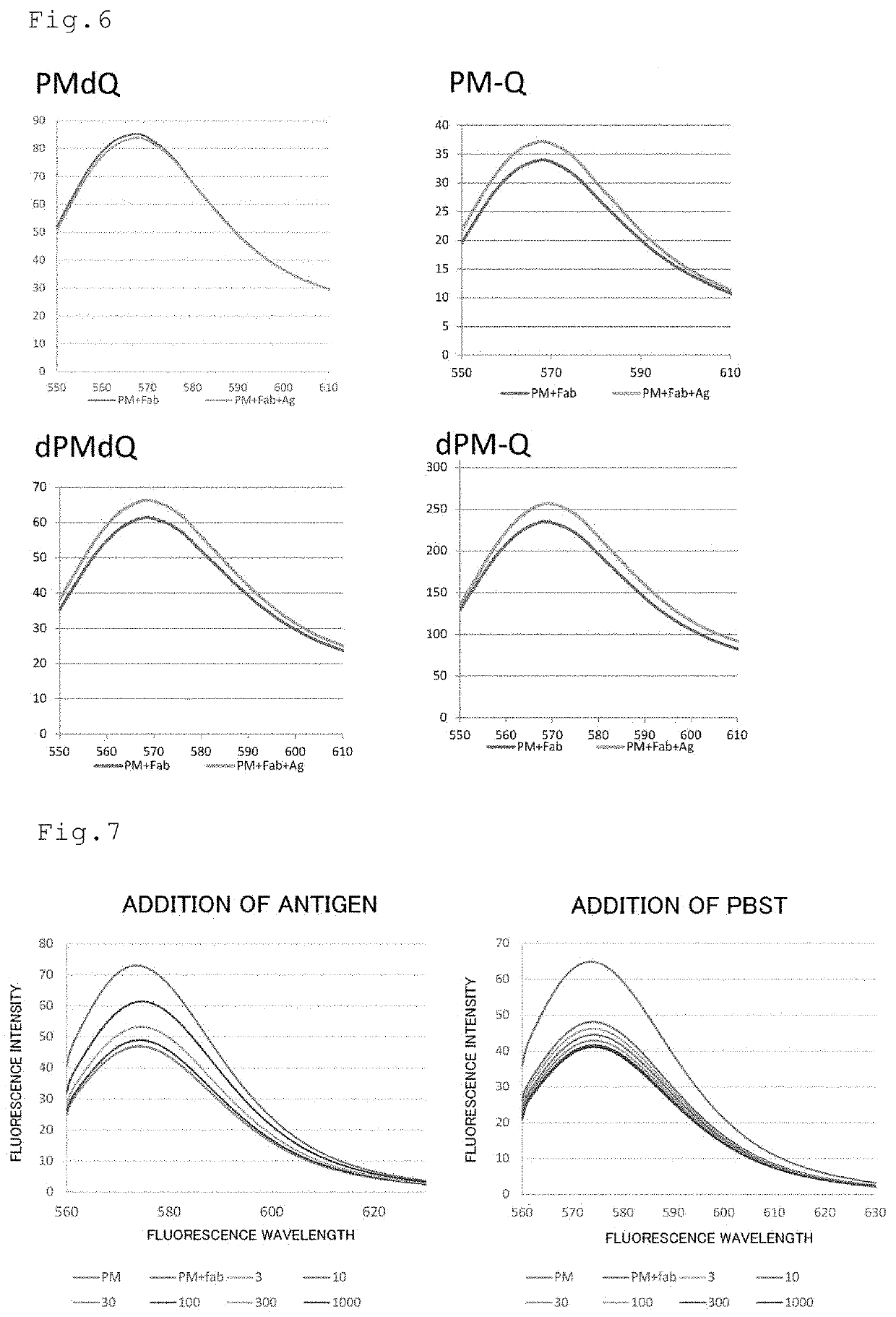
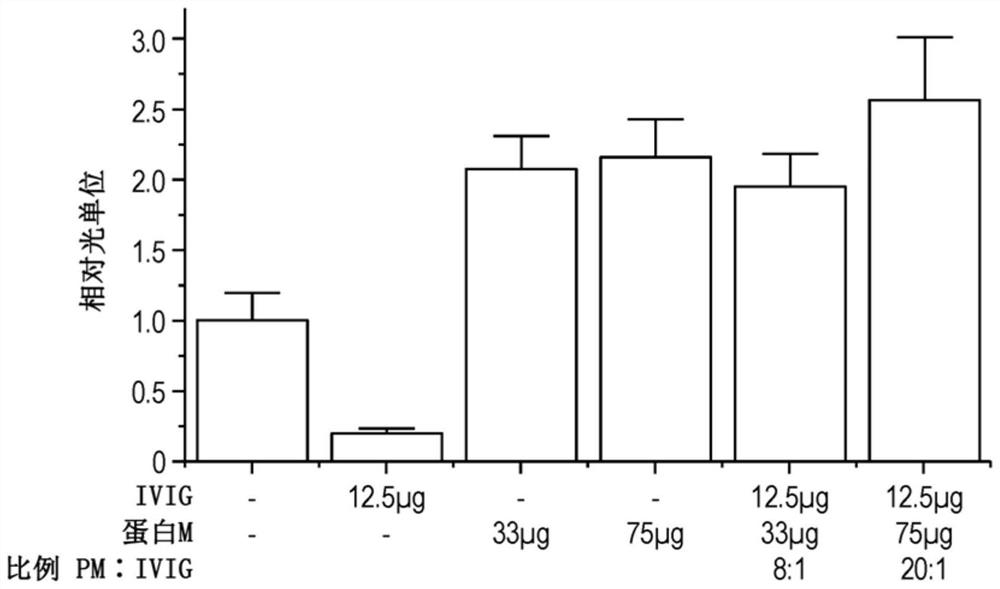
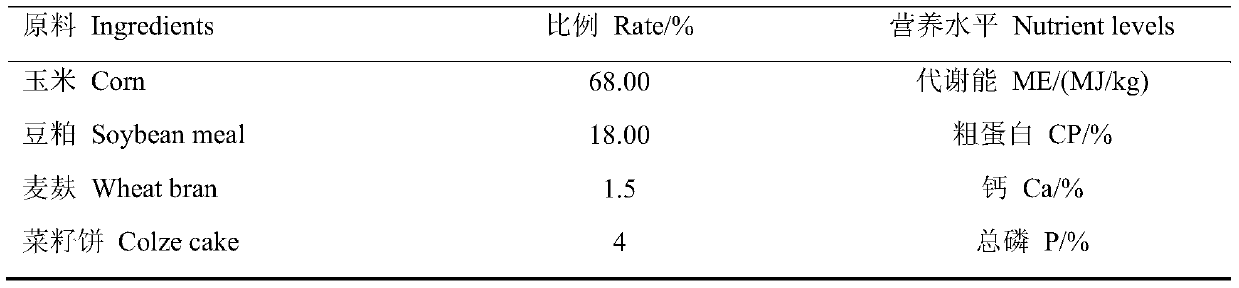
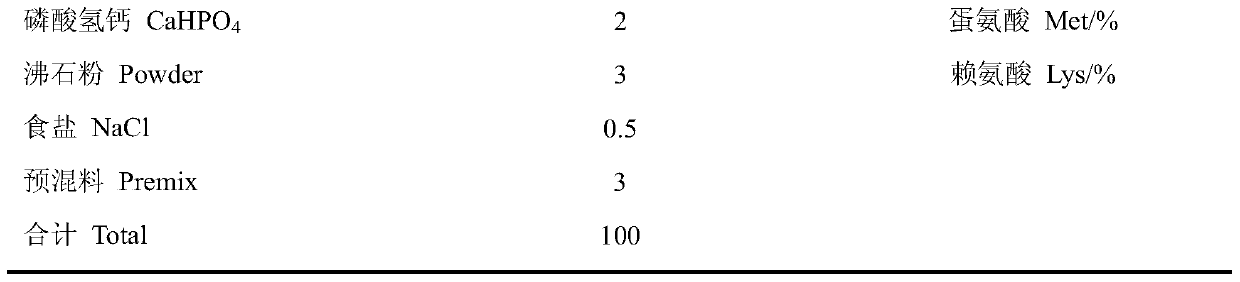
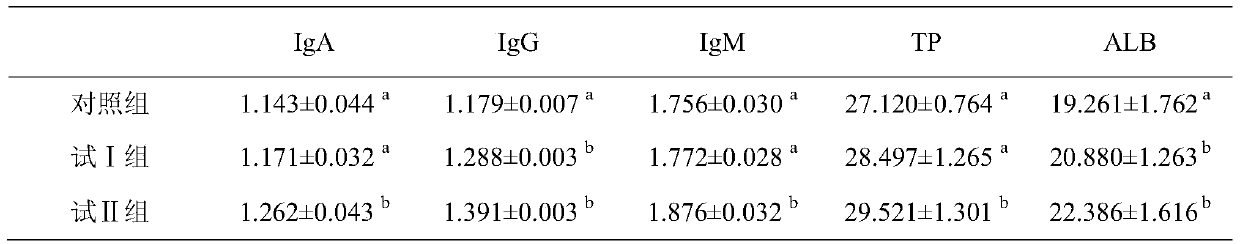
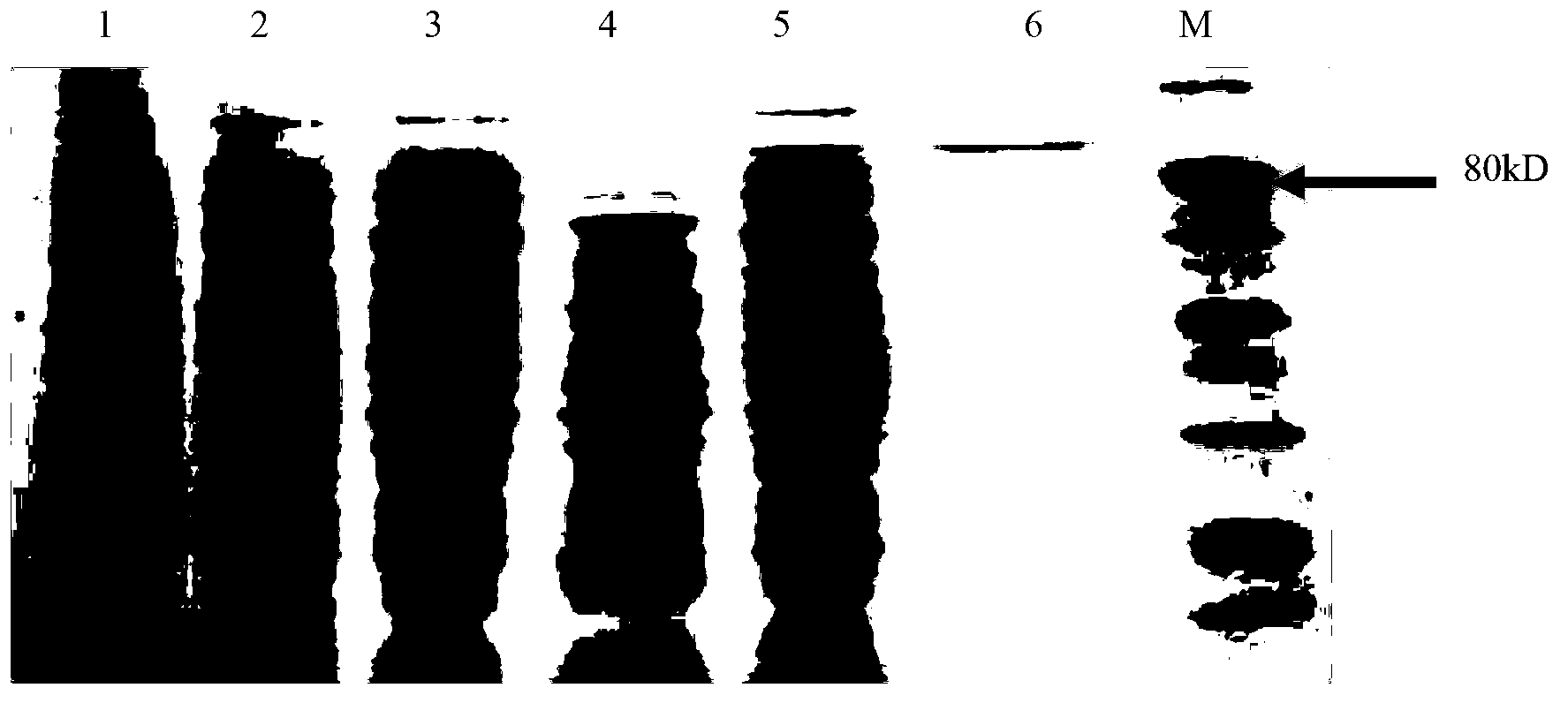Patents
Literature
34 results about "Protein M" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Protein M is an immunoglobulin-binding protein found on the cell surface of the human pathogenic bacterium Mycoplasma genitalium. It is presumably a universal antibody-binding protein, as it is known to be reactive against all antibody types tested so far. It is capable of preventing the antigen-antibody interaction due to its high binding affinity to any antibody. The Scripps Research Institute announced its discovery in 2014. It was detected from the bacterium while investigating its role in patients suffering from a cancer, multiple myeloma.
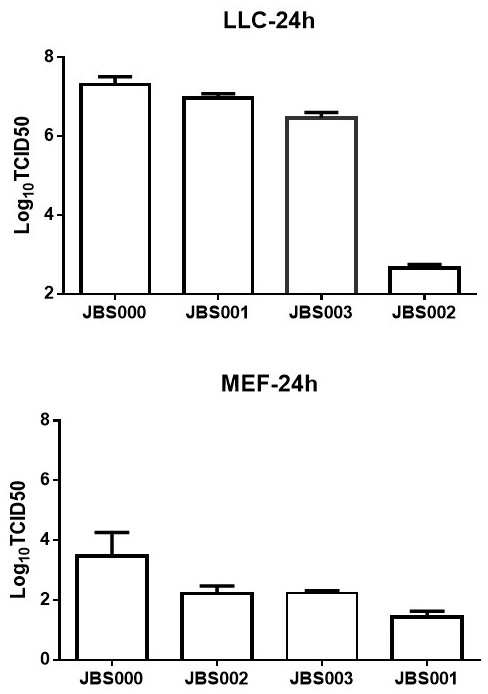
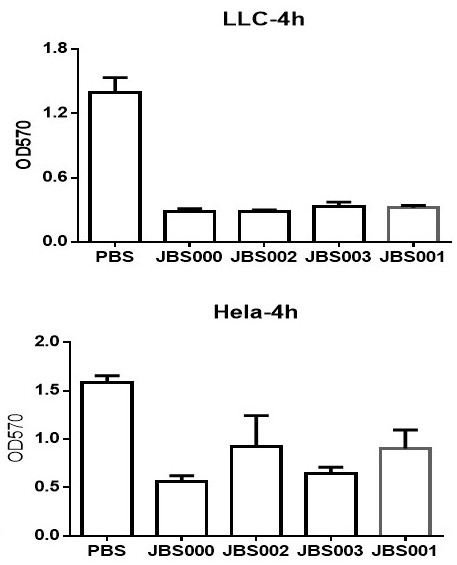
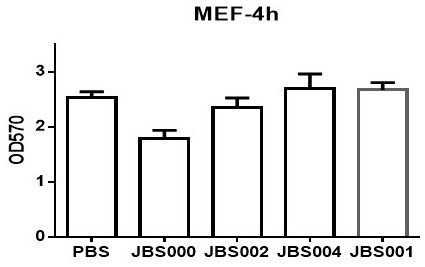
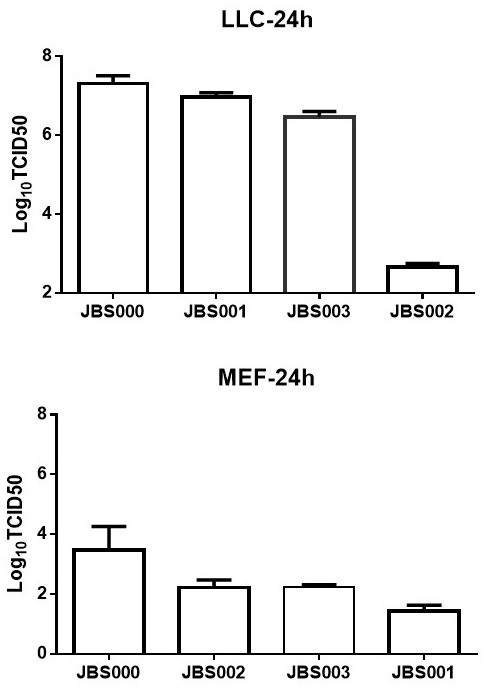
Oncolytic virus vaccine and medicine for treating tumors by combining oncolytic virus vaccine with immune cells
ActiveCN111286493AEffective treatmentHigh cure rateSsRNA viruses negative-senseMammal material medical ingredientsTumor therapyOncology
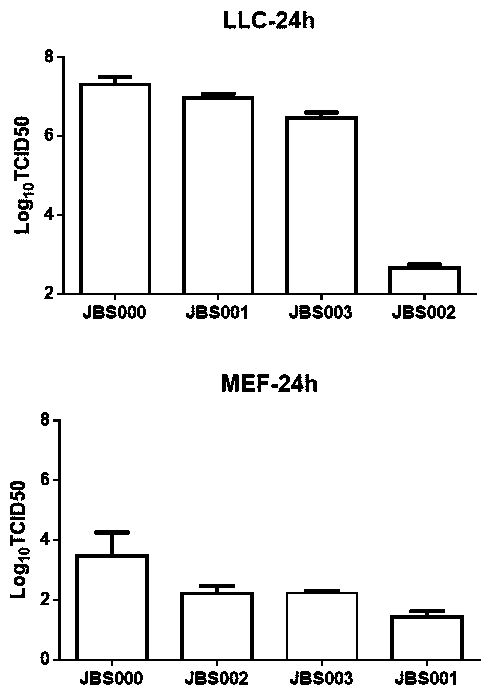
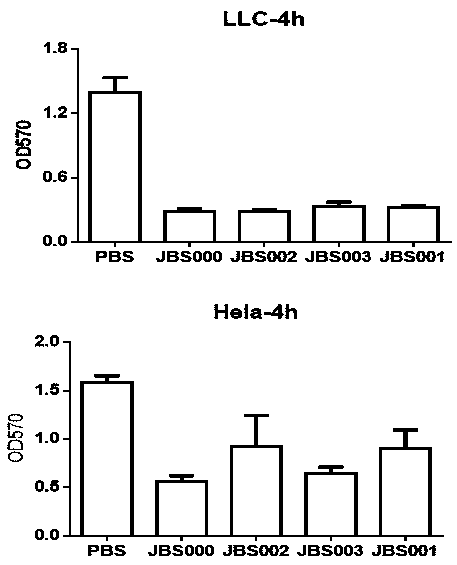
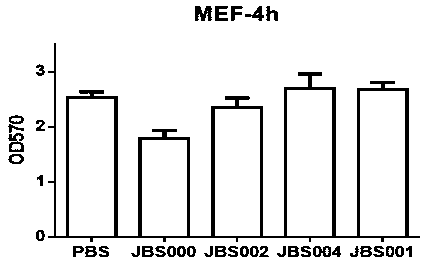
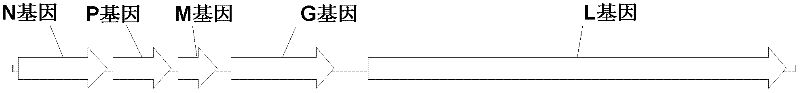
The invention belongs to the technical field of biology, and particularly relates to an oncolytic virus vaccine and a medicine for treating tumors by combining the oncolytic virus vaccine with immunecells. The invention provides a brand-new oncolytic virus attenuated strain by carrying out site-specific mutagenesis on the VSV wild type virus matrix protein M. The gene sequence of the matrix protein M is shown as SEQ ID NO 3. The attenuated strain can be independently used as a drug for treating tumors, and is superior to wild type viruses and other known attenuated strains in safety and curerate. On the basis of the oncolytic virus attenuated strain, NY-ESO-1 is inserted into the attenuated strain, and the invention further provides a vaccine capable of being applied to tumor treatment.The vaccine is high in cure rate and high in biological safety. On the basis of the vaccine, the vaccine and TCR-T cells are combined for application, and a medicine capable of efficiently treating various tumors is provided. On a mouse lung cancer model, the cure rate can reach the surprising rate of 95 percent.
Owner:JOINT BIOSCIENCES (SH) LTD
Virus-like particles for pseudorabies virus and preparation method for same
ActiveCN102533680AAvoid quality risksEasy to design and manufactureInactivation/attenuationAntiviralsRabiesGlycoprotein G
The invention provides virus-like particles for pseudorabies virus. The virus-like particles consist of pseudorabies virus glycoprotein G and pseudorabies virus matrix protein M, and have stable and homogenous spatial structures; and the outer membrane protein of the virus-like particles contains all or partial fragments of the pseudorabies virus glycoprotein G, and can induce an organism to generate protective-level cellular immunity and humoral immunity. The virus-like particles do not comprise any nucleic acid component of chromosome of the pseudorabies virus, and avoid quality risks caused by inactivator addition. The virus-like particles can be conveniently purified by the conventional purification technology, and the quality risks possibly caused by inactivator addition in the traditional inactivated vaccines are avoided on principle. Meanwhile, due to a simple and quick construction and preparation mode, novel vaccines aiming at new strains can be conveniently and quickly designed and prepared. Based on the principle, novel polyvalent vaccines and multi-vaccines are easily formed on the basis of the particles; and the particles can widely replace related critical raw materials in the conventional practical technologies such as related antigen and antibody detection, functional protein vectors and the like.
Owner:WUHAN INST OF BIOLOGICAL PROD CO LTD
Microbial analysis
ActiveUS20160237469A1Samples introduction/extractionMicrobiological testing/measurementLipid formationMicroorganism
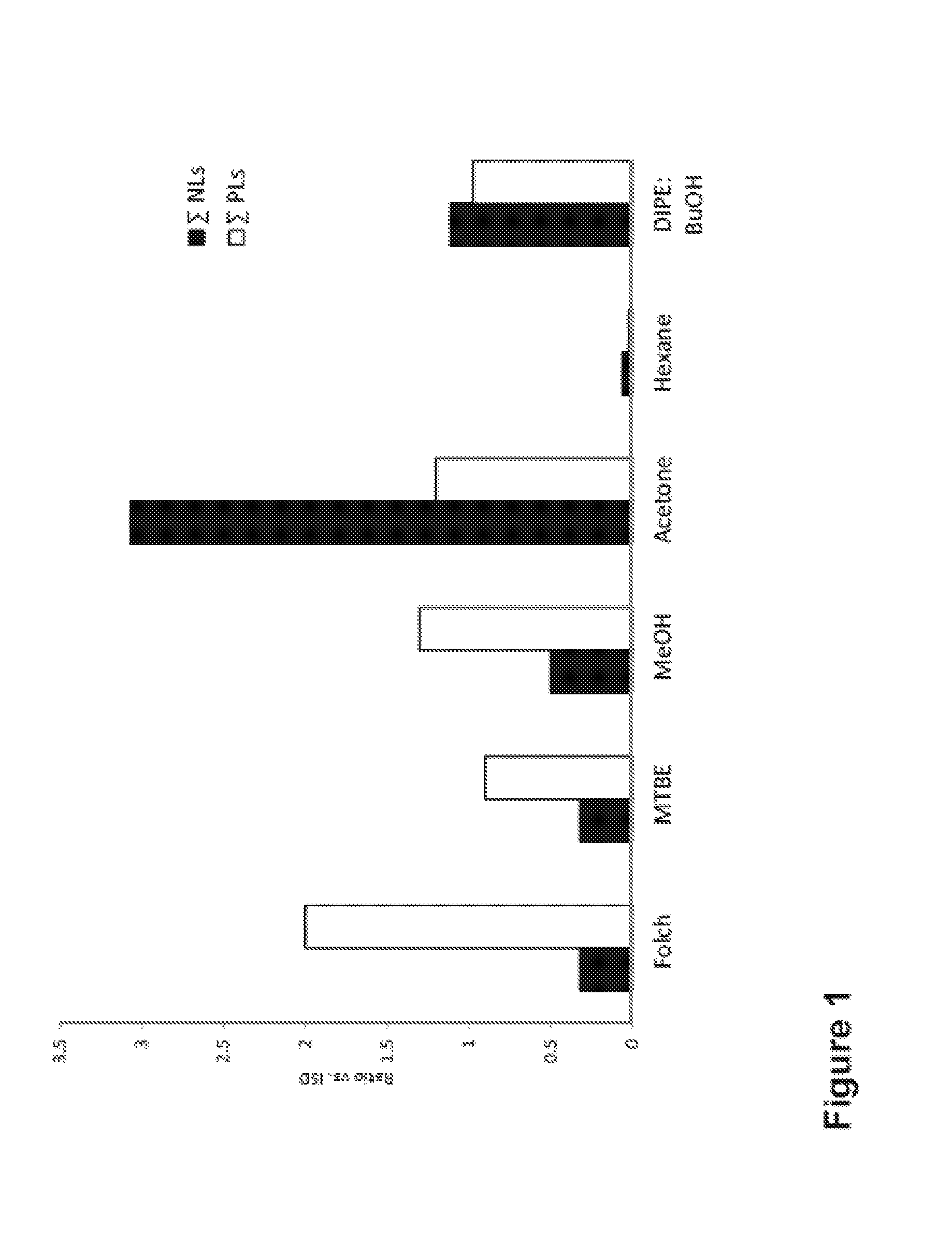
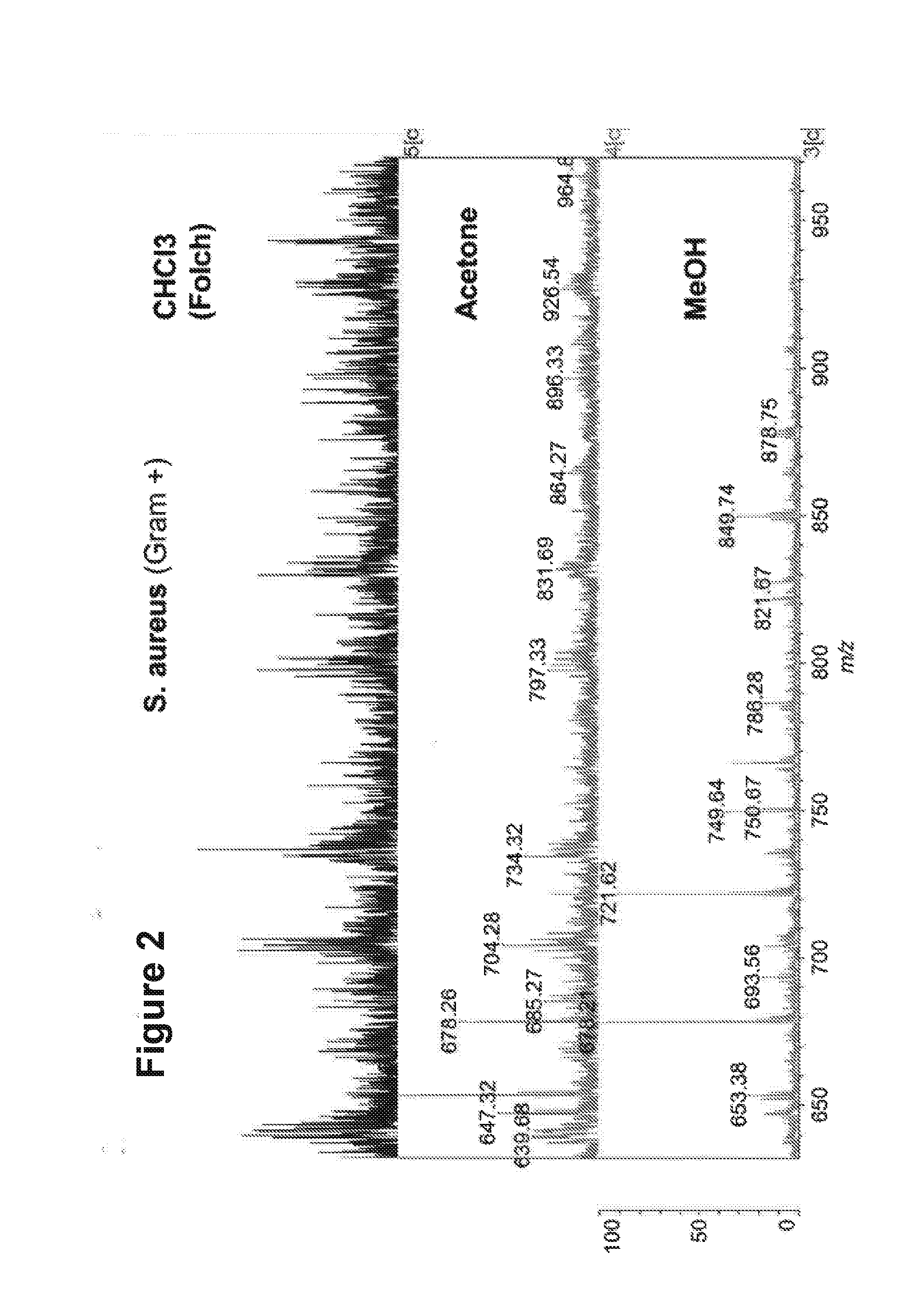
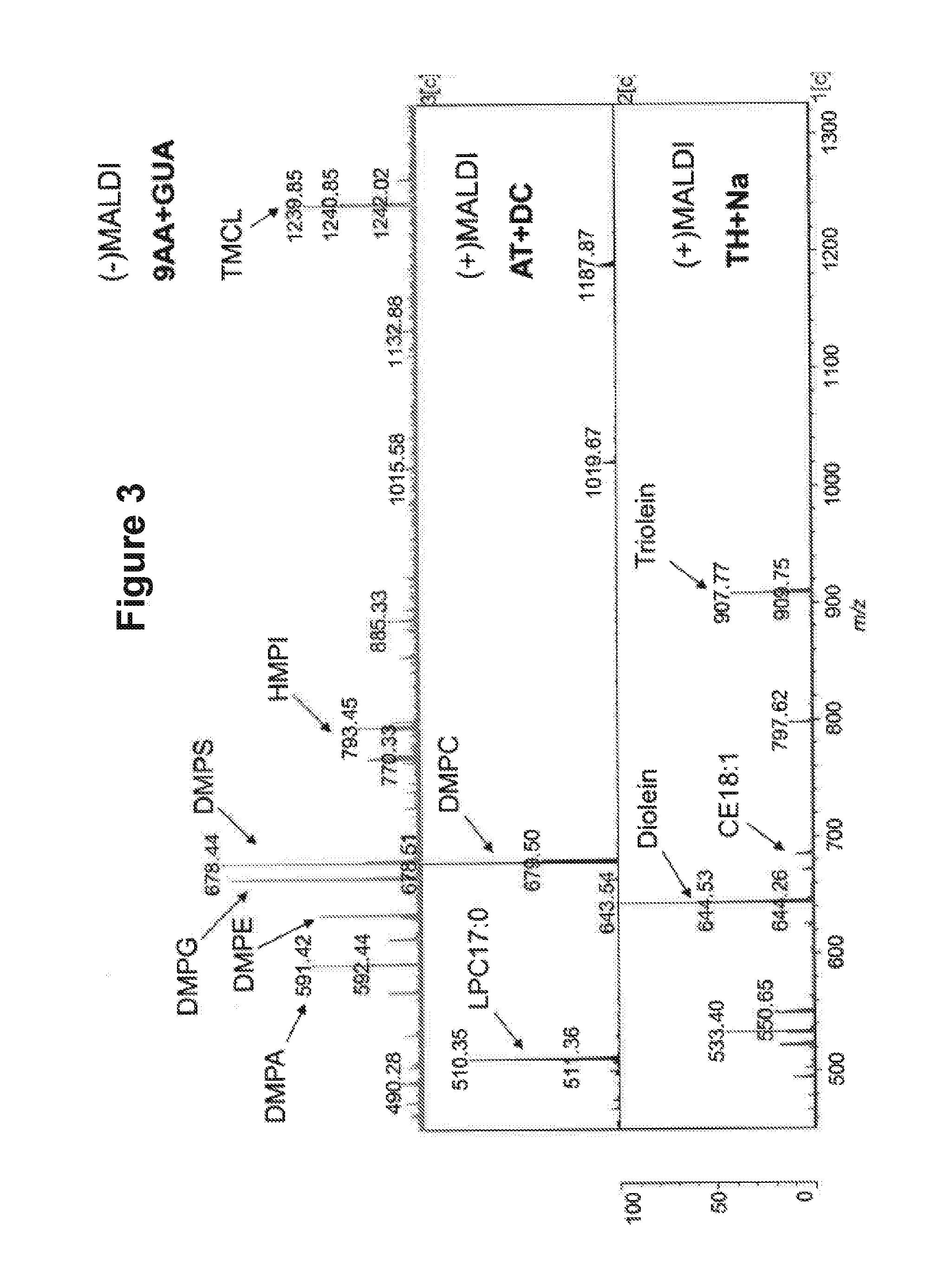
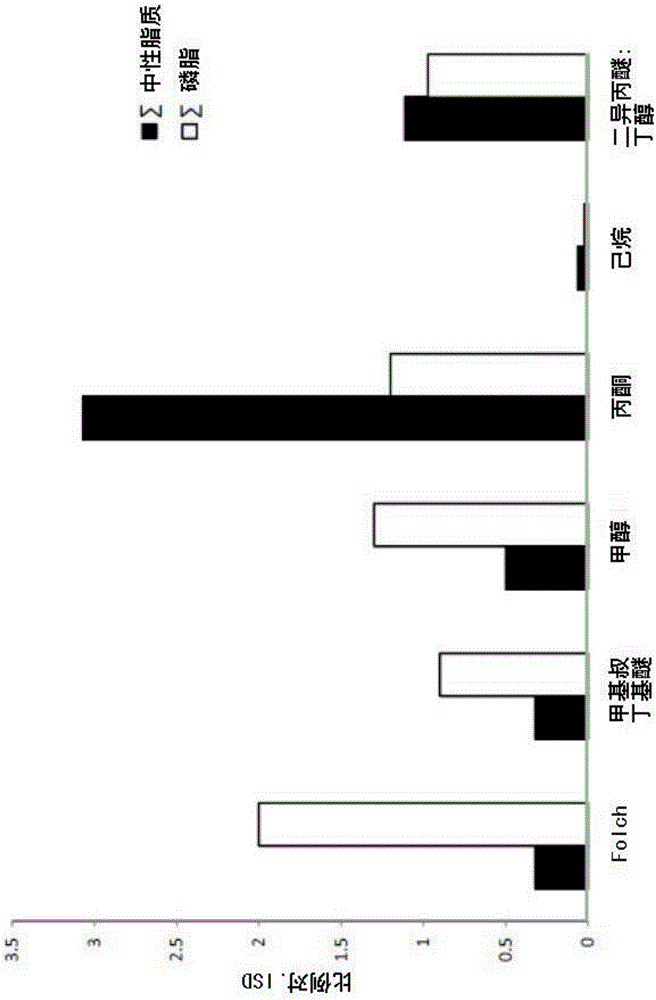
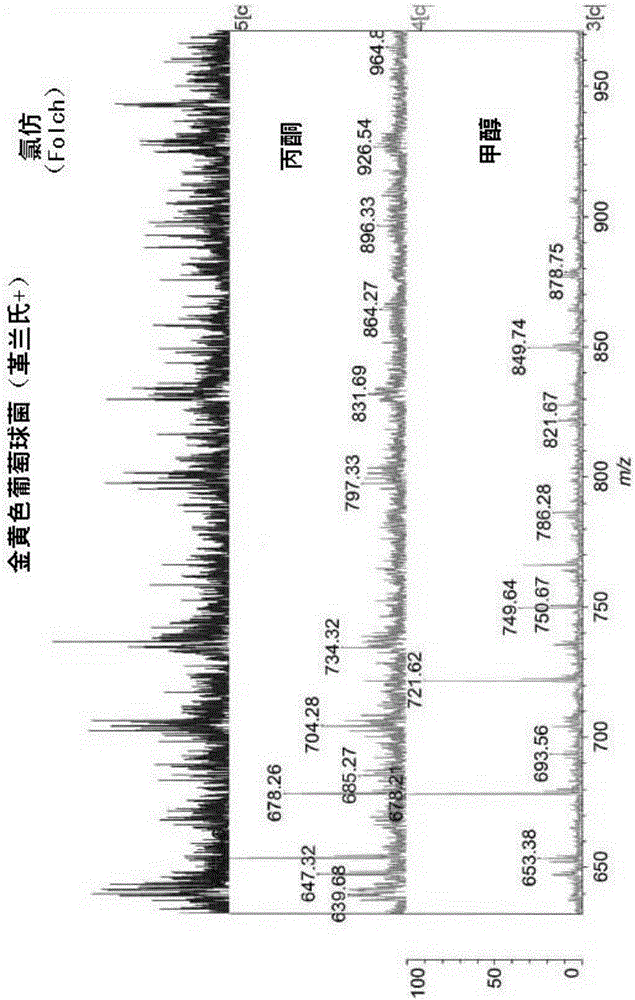
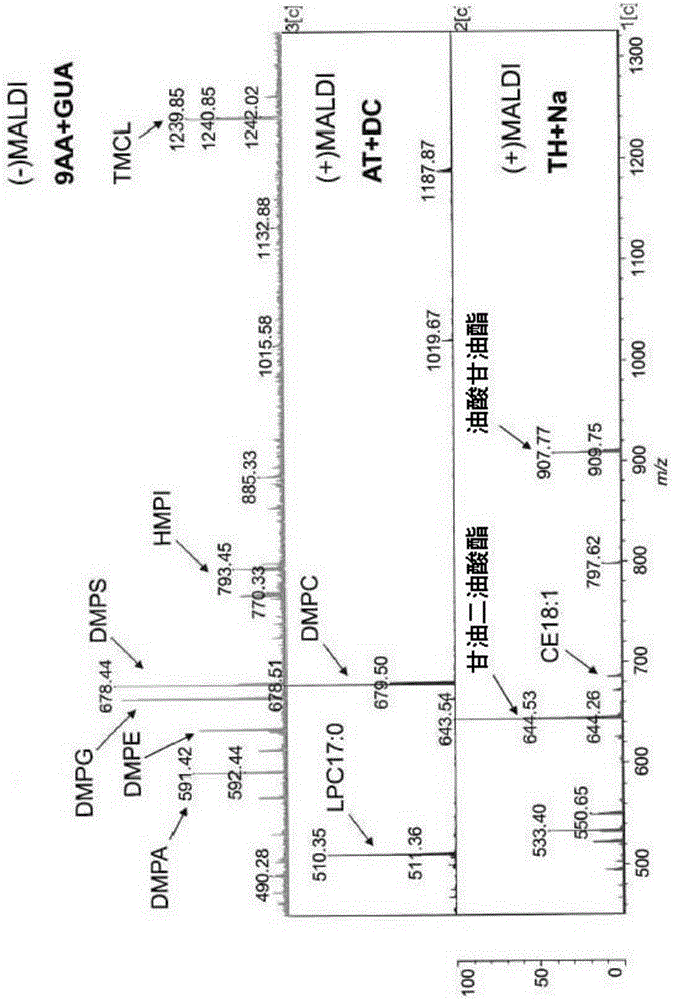
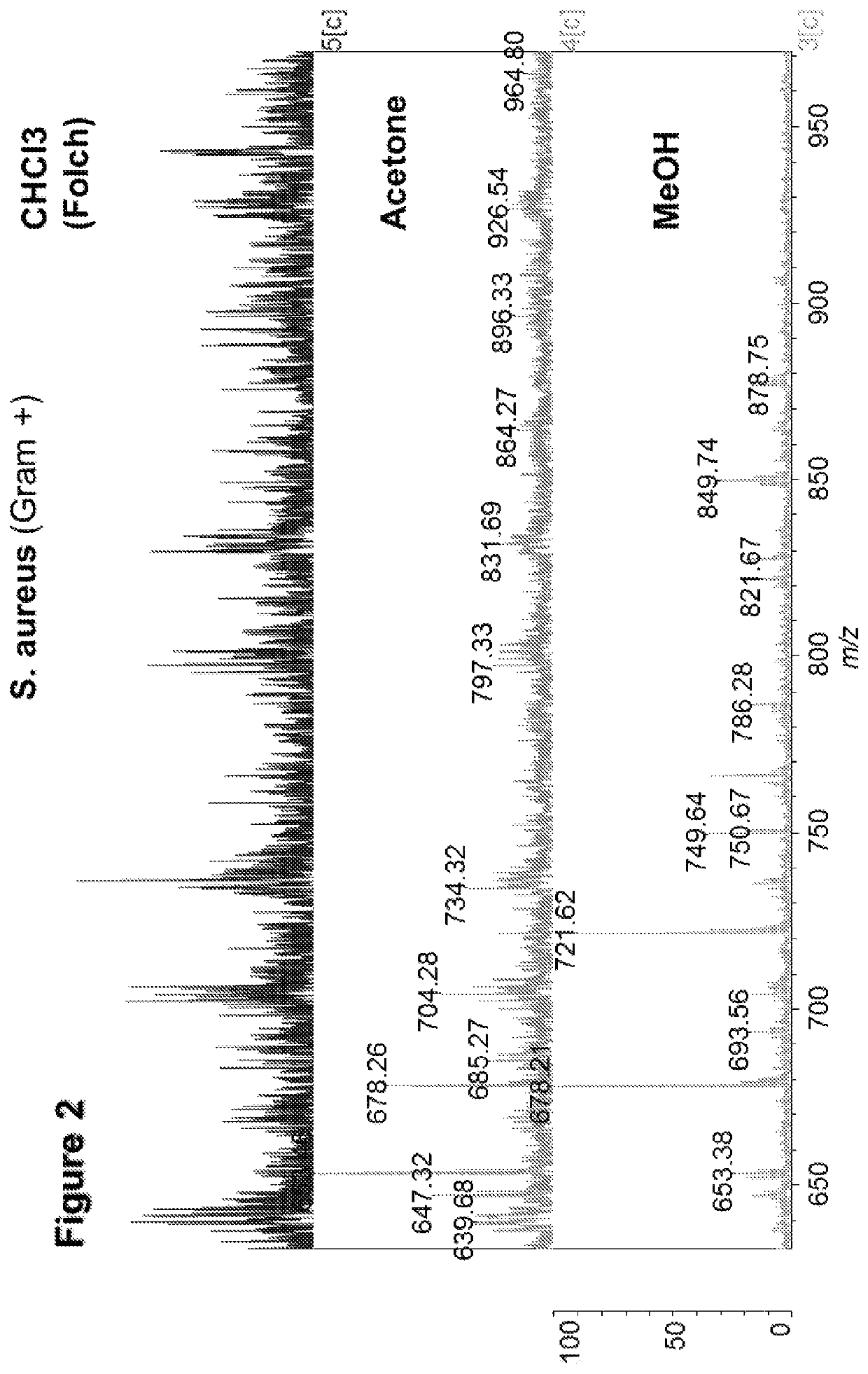
The present invention is concerned with a method of identifying microbial strains (e.g. from a cell culture), the method comprising; i) a lipid extraction step, comprising extraction of phospholipids from the microbe, suitably with an extraction composition comprising more than 50 vol % MeOH; ii) a sample preparation step, comprising preparation of a MALDI sample incorporating the extracted lipids; iii) a data gathering step, comprising performing MALDI-based mass spectrometry on the MALDI sample, and iv) a microbe identification step, comprising analysis of the mass spectrometry data to characterise or identify the microbial strain. Suitably the method also includes extracting proteins from the microbes and analysing the extracted proteins using MALDI-based mass spectrometry so as to obtain not only lipid m / z data but also protein m / z data.
Owner:KRATOS ANALYTICAL
Microbial analysis
ActiveCN106133149AParticle separator tubesMicrobiological testing/measurementLipid formationData acquisition
The invention relates to microbial analysis. The present invention is concerned with a method of identifying microbial strains (e.g. from a cell culture), the method comprising; i) a lipid extraction step, comprising extraction of phospholipids from the microbe, suitably with an extraction composition comprising more than 50vol% MeOH; ii) a sample preparation step, comprising preparation of a MALDI sample incorporating the extracted lipids; iii) a data gathering step, comprising performing MALDI-based mass spectrometry on the MALDI sample, and iv) a microbe identification step, comprising analysis of the mass spectrometry data to characterise or identify the microbial strain. Suitably the method also includes extracting proteins from the microbes and analysing the extracted proteins using MALDI-based mass spectrometry so as to obtain not only lipid m / z data but also protein m / z data.
Owner:KRATOS ANALYTICAL
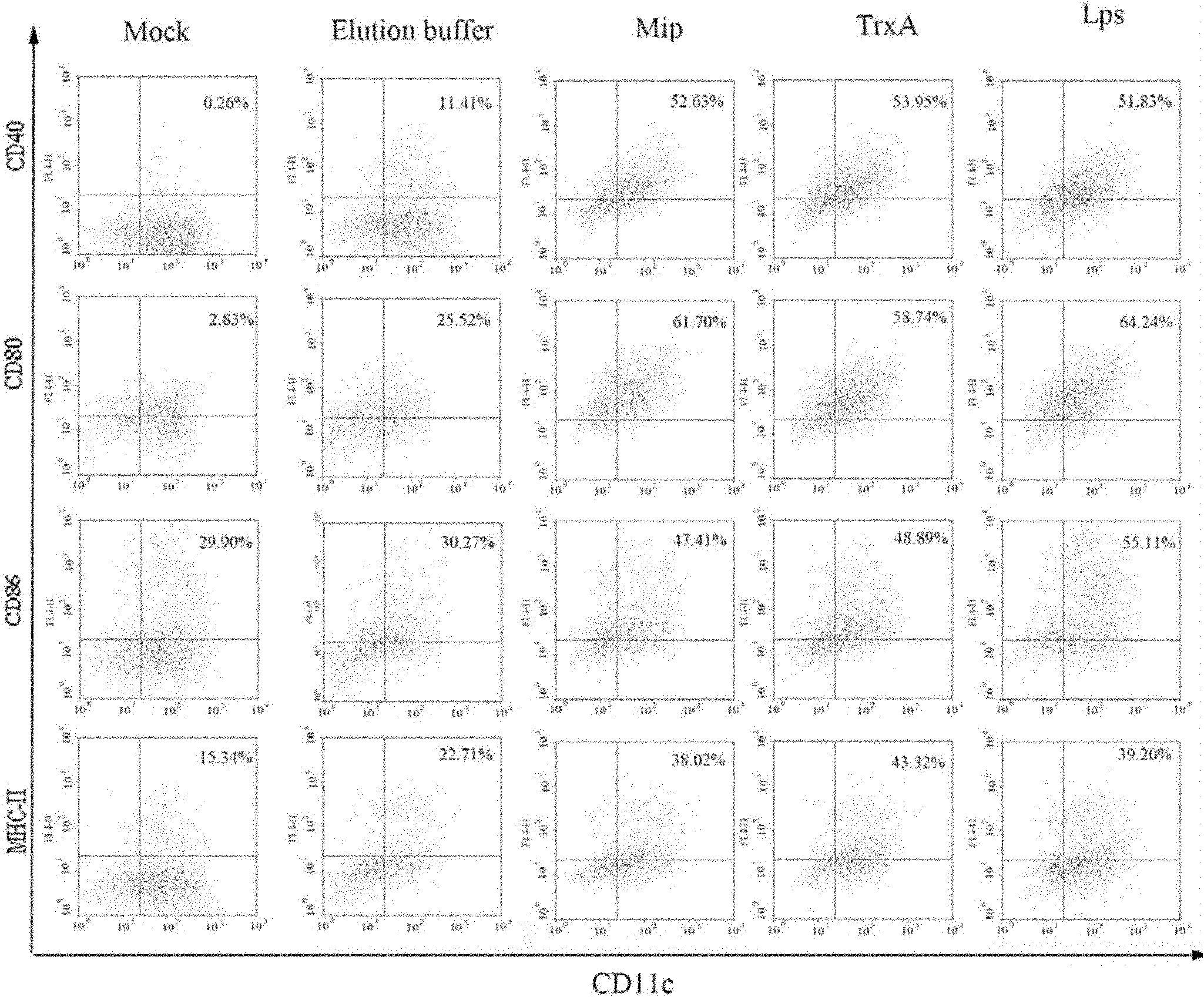
Application of protein Mip in immune protection of coxiella burnetii
InactiveCN102139100AOvercoming the defects of difficult preparationSimple and fast operationAntibacterial agentsBacterial antigen ingredientsProtective antigenCell Membrane Proteins
The invention discloses application of protein Mip in the immune protection of coxiella burnetii. The application means the application of protein shown as SEQ IDNO: 1 in the preparation of a Q fever vaccine. The application proves that coxiella burnetii recombinant membrane protein Mip can be used as antigenic protein, can stimulate bodies to produce immune response to attack anti-coxiella burnetii strains and is a protective antigen with Q fever vaccine functions. The protein Mip is prepared by a genetic engineering method, and the method is easy to operate, low in cost and high in yield. The protein Mip is used as the Q fever vaccine, so that the defect of difficulty in preparation of vaccines in the prior art is overcome.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
PRRSV-1 virus-like particles and preparation method thereof
InactiveCN109721643AEasy to operateEasy to scale up productionViral antigen ingredientsVirus peptidesCompetent cellShuttle vector
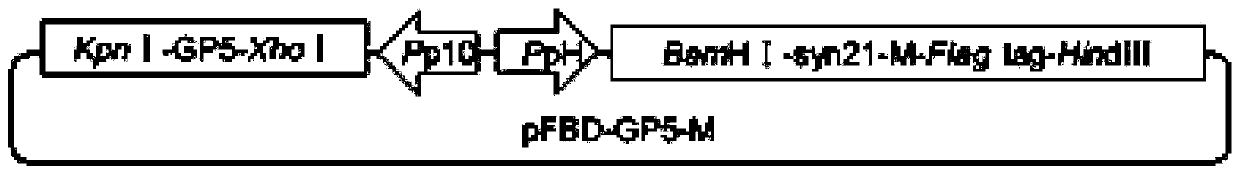
The embodiment of the invention discloses PRRSV-1 virus-like particles. The particles comprise PRRSV LV strain structural protein GP5, PRRSV LV strain matrix protein M, expressed PRRSV LV matrix protein M and co-expressed PRRSV LV structural protein GP5 of the virus-like particles. The embodiment further provides a method for preparing the PRRSV-1 virus-like particles. The method comprises the following steps: respectively cloning genes expressing GP5 protein and matrix protein M in a PRRSV LV strain; cloning the gene expressing the GP5 protein and matrix protein M to a baculovirus shuttle vector to acquire a recombinant baculovirus shuttle vector; and transforming the recombinant baculovirus shuttle vector to a DH10Bac competent cell to acquire a recombinant baculovirus genome. The methodis suitable for large-scale amplified preparation of anti-European porcine reproductive and respiratory syndrome vaccines, and has high safety and excellent development and application prospect.
Owner:ACAD OF MILITARY SCI PLA CHINA ACAD OF MILITARY MEDICAL SCI INST OF MILITARY VETERINARY MEDICINE
Lung adenocarcinoma serum marker protein
InactiveCN101923080AStable diagnostic modelGood distinctionComponent separationPreparing sample for investigationProtein insertionScreening method
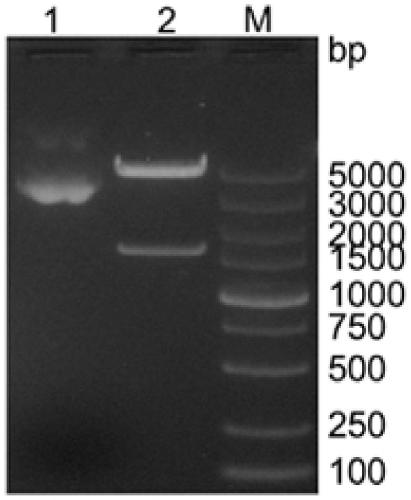
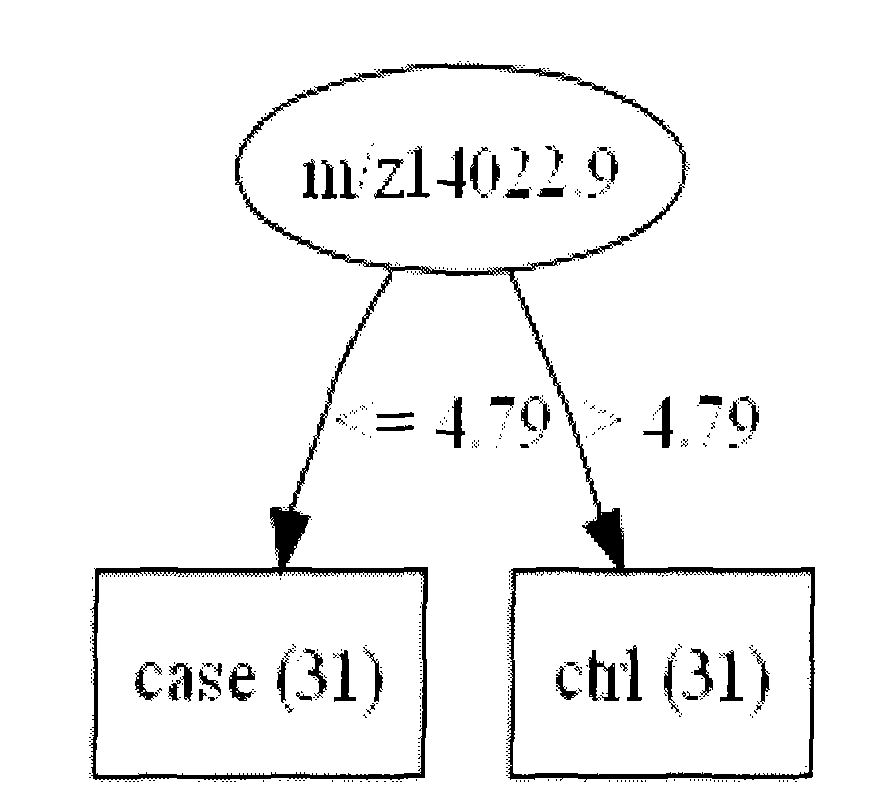
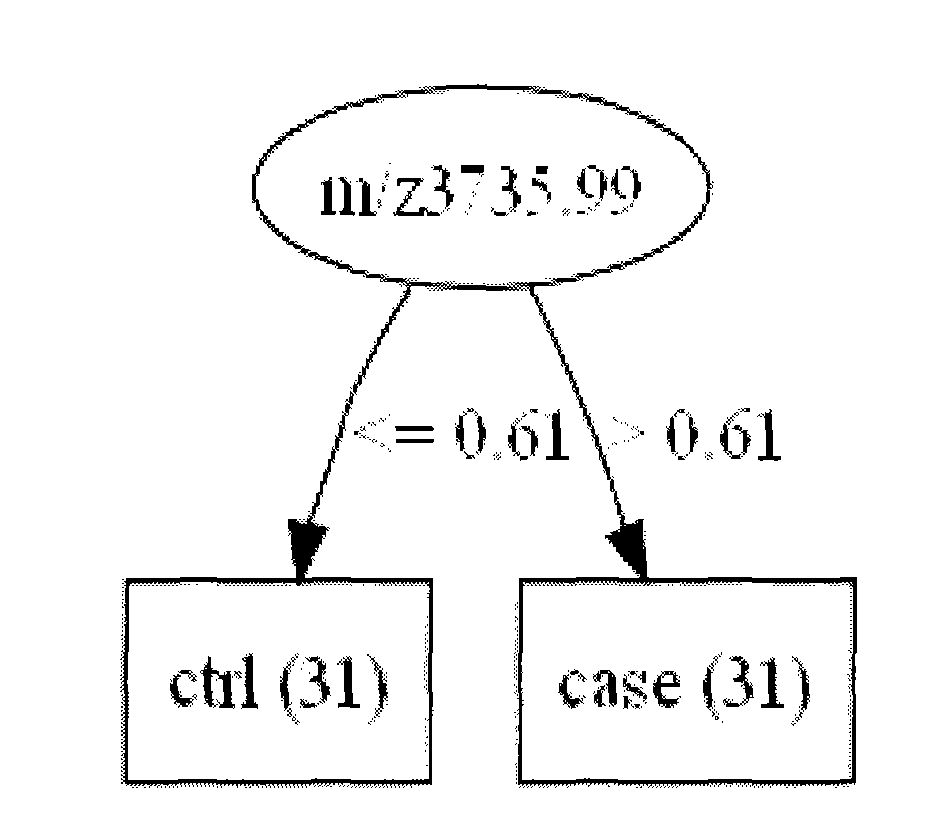
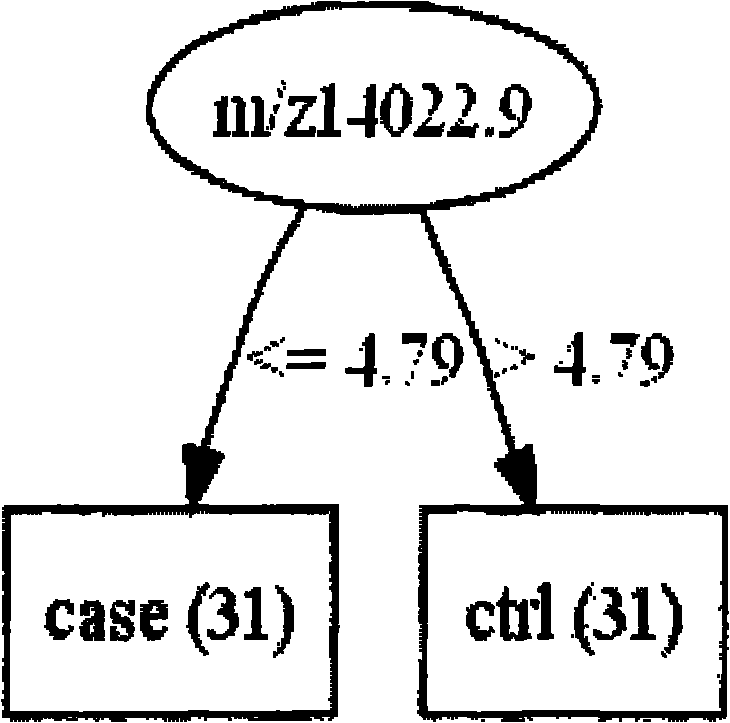
The invention relates to early diagnosis of lung adenocarcinoma, in particular to a lung adenocarcinoma serum marker protein, and meanwhile provides a screening method, a related decision tree model and a detection method thereof. The screening method comprises the following steps of: detecting serum protein differential expressions of a lung adenocarcinoma patient and a healthy control by adopting an SAX-2 protein chip, screening 102 differential mass spectrum peaks (p is less than 0.001), establishing a lung adenocarcinoma protein mass spectrogram, screening two proteins m / z14022.9 and m / z3735.99 with least p values, and establishing the decision tree model for the two proteins, wherein both the two proteins have very good distinguishing capabilities, the diagnosed lung adenocarcinoma can be obtained by the participation of the two proteins in the decision tree model, the accuracy of the two proteins is 98.387 percent, and a blind test discovers that the accuracy, sensitivity and specificity of diagnosing the lung adenocarcinoma are 100 percent.
Owner:THE FIRST AFFILIATED HOSPITAL OF SOOCHOW UNIV
Tumor treatment drug
ActiveCN111467489AAdaptableHigh cure rateSsRNA viruses negative-senseVirus peptidesTumor therapyTherapeutic effect
The invention belongs to the field of biotechnology, and particularly relates to an oncolytic virus vaccine and a tumor treatment drug combing oncolytic virus vaccine with an immune checkpoint inhibitor. A brand-new oncolytic virus attenuated strain is provided by site-directed mutagenesis of wild-type virus matrix protein M of VSV (vesicular stomatitis virus). The attenuated strain can be used asa drug alone to treat tumors, and has safety and cure rate higher than those of wild-type viruses and other known attenuated strains. On the basis of the oncolytic virus attenuated strain, a vaccinethat can be used in tumor treatment is also provided by inserting NY-ESO-1 into the attenuated strain. The vaccine has high cure rate and high biological safety. On the basis of the vaccine, the invention also combines the vaccine with the immune checkpoint inhibitor to provide the drug capable of efficiently treating various tumors. In mouse lung cancer models, the cure rate can be astonishing, up to 87.5%, and the treatment effect on large tumors is also good.
Owner:JOINT BIOSCIENCES (SH) LTD
Porcine epidemic diarrhea virus M protein affinity peptides and screening method thereof
InactiveCN104774249AInhibition of replicationCombined antiviral effect is goodPeptide preparation methodsScreening methodBacteriophage
The invention discloses porcine epidemic diarrhea virus M protein affinity peptides and a screening method thereof. The amino acid sequence of the porcine epidemic diarrhea virus M protein affinity peptides is AGWYCTEVLCVQ or AYCTRHVCYLDN. According to the invention, a short peptide capable of being specifically binding with PEDV (porcine epidemic diarrhea virus) and a recombinant protein M of PEDV can be obtained through a phage display technology; moreover, the short peptide can be synthesized artificially and then is analyzed in term of antiviral biological function. Results show that two PEDV M protein affinity peptides screened and synthesized for the first time are both capable of inhibiting the reproduction of PEDV, which provides a certain theoretical basis and test basis for micromolecular diagnosis and treatment preparations for PEDV in the further.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Method for protein affinity purification for IgY
InactiveCN104761638ASimple processImprove purification efficiencyEgg immunoglobulinsPeptide preparation methodsYolkDistilled water
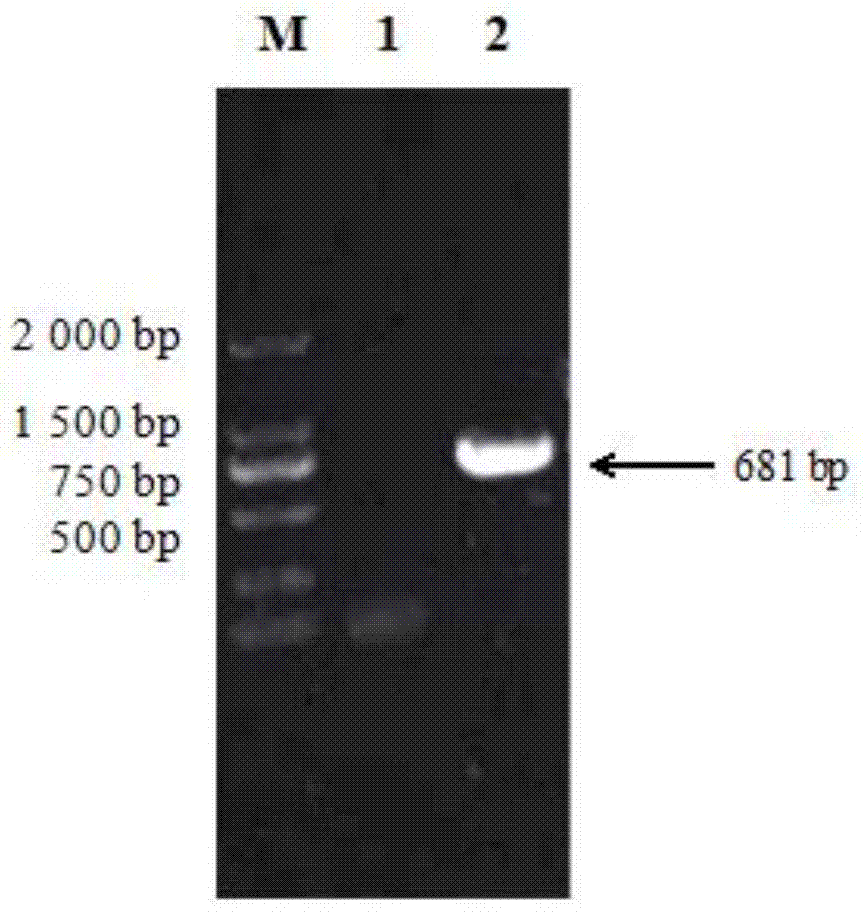
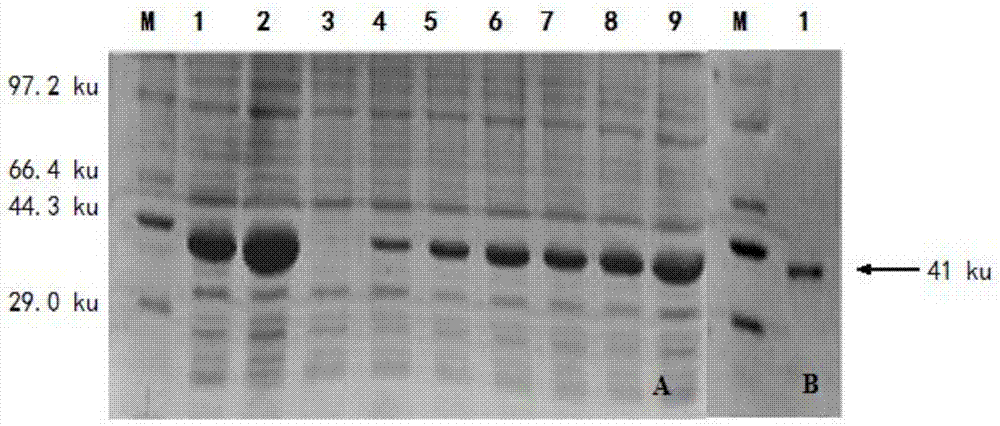
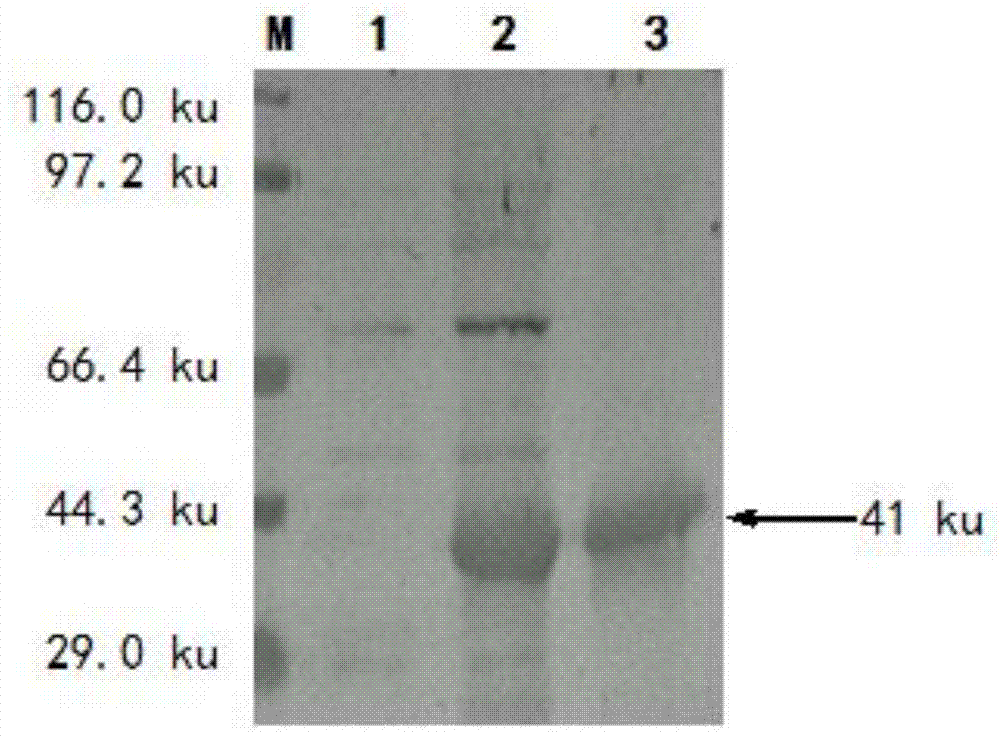
The invention specifically relates to a method for protein affinity purification for IgY. The method comprises the following steps: 1. carrying out gene cloning for protein M; 2. constructing an expression vector, carrying out soluble expression on the protein M through the expression vector, and purifying the soluble protein M; 3. coupling the purified soluble protein M with NHS-activated agarose gel 4FF to produce an affinity purification medium; 4. carrying out affinity purification on an IgY antibody primary product through a protein M agarose gel column to obtain high-purity IgY, wherein the IgY antibody primary product is the mixture of equal-mass yolk solution and distilled water; 5. dialyzing the IgY antibody solution obtained by eluting after purification in PBS solution overnight at 4 DEG C, wherein the pH value of the PBS solution is 7.0-7.4; 6. collecting a liquid, that is, a high-purity IgY antibody in a dialysis bag. The method disclosed by the invention has the following beneficial effects: 1. the working procedures are simple; 2. the IgY purification efficiency is up to 95%; 3. the method is more environment-friendly, green and healthy.
Owner:NORTHWEST A & F UNIV
Method for detecting four kinds of tumor serum proteins
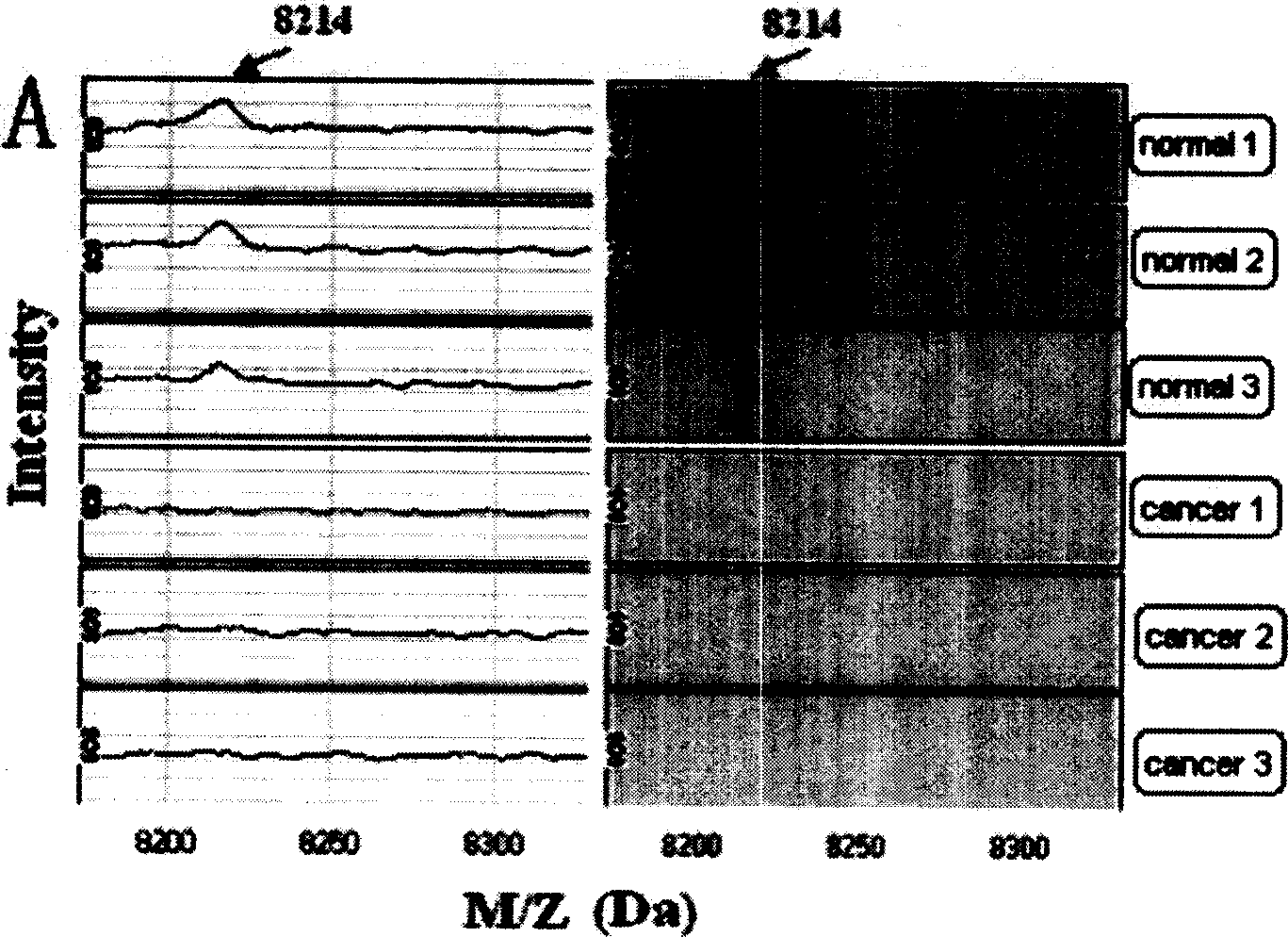
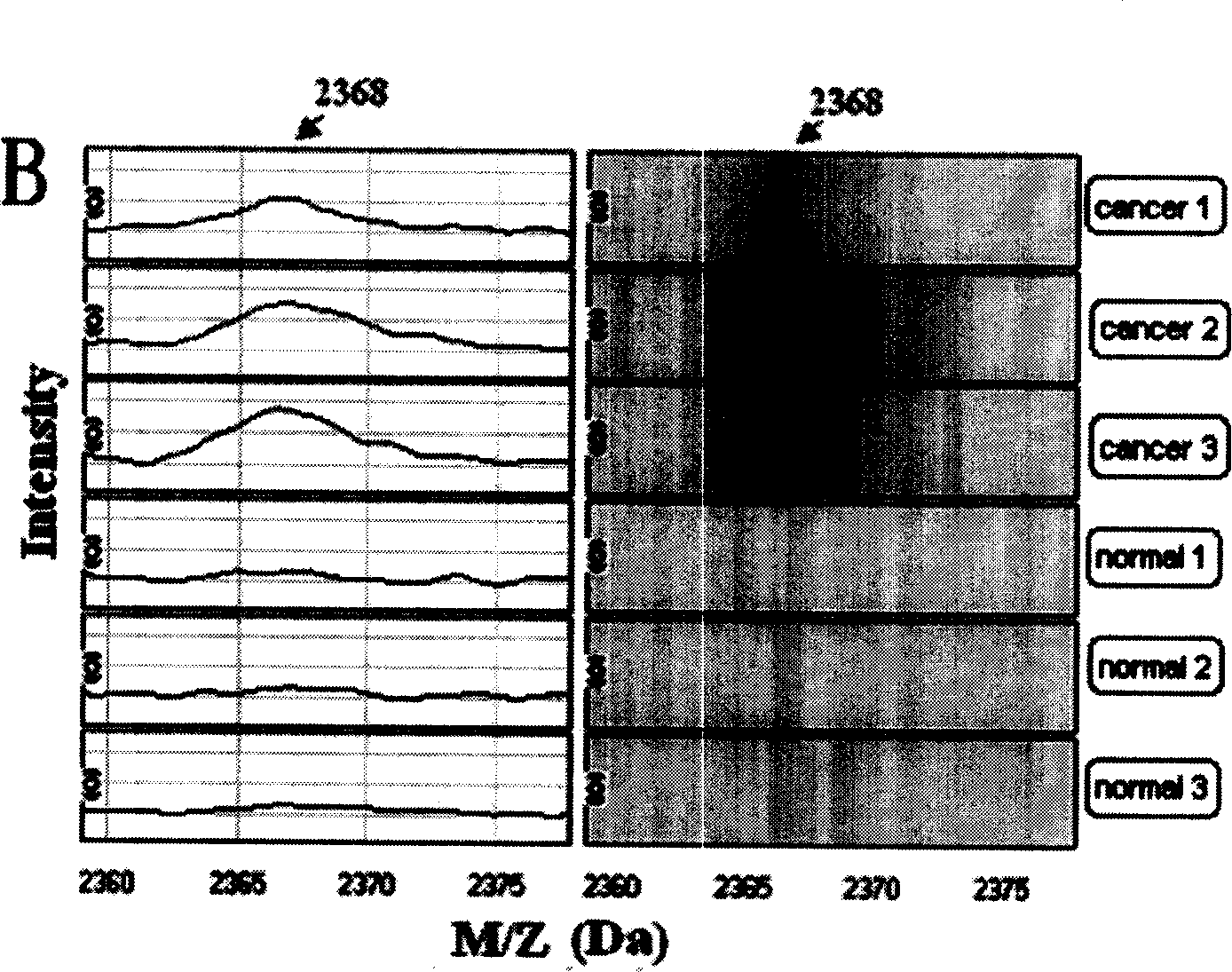
The invention provides a detecting method for the blood serum protein of four kind of familiar tumours which are, tumour, meningioma tumour, mammary tumour and hepatic tumour by the following steps: (1) using the surface concentrated laser SELDI to determine the protein group graphoes of the blood serum specimen of the tumour patient and the healthy person. (2) screening the corresponding tumour marker and establishing the detecting model for analysis integrated the biological informatics technique. The method of the invention is adopted in the process of selecting the tumour medicine and provides a new way and method for early discovery and detection of the tumour. The discovered new seriial protein m / z peak also provides a base and resource for seeking new better tumour marker, meanwhile, provides a new thought for exploring the generation and development of tumours.
Owner:ZHEJIANG UNIV
Fusion protein for novel coronavirus variant strain Delta, nasal spray vaccine as well as preparation method and application thereof
PendingCN113621076AEffectively prevent infectionImprove securitySsRNA viruses positive-senseAntibody mimetics/scaffoldsAdjuvantViral Vaccine
The invention provides a fusion protein for a novel coronavirus variant strain Delta, a nasal spray vaccine as well as a preparation method and application thereof, and belongs to the technical field of virus vaccines. The fusion protein for the novel coronavirus variant strain Delta is formed by fusing spike protein S, transmembrane protein M and nucleocapsid protein N; and coding genes of the protein S, the protein M and the protein N are sequentially shown as SEQ ID NO: 1-3. The nasal spray vaccine prepared by mixing the fusion protein and an adjuvant has good immunogenicity, safety and biological activity, and can induce an organism to generate an effective antibody, so that the growth of the novel coronavirus variant strain Delta is inhibited, and the Delta infection can be effectively prevented; by adopting the form of the nasal spray vaccine, injection is not needed, the operation is simple and convenient, and adverse reactions after use of an injection vaccine are avoided; and the preparation method of the vaccine is simple, and the vaccine is easy to purify and high in safety, and can be quickly applied to clinical tests.
Owner:NANHUA UNIV
Neutral alpha-galactosidase M-GALC, and coding gene and application thereof
ActiveCN103320414AIncrease enzyme activityFungiBacteriaAgricultural scienceAlpha-galactosidase activity
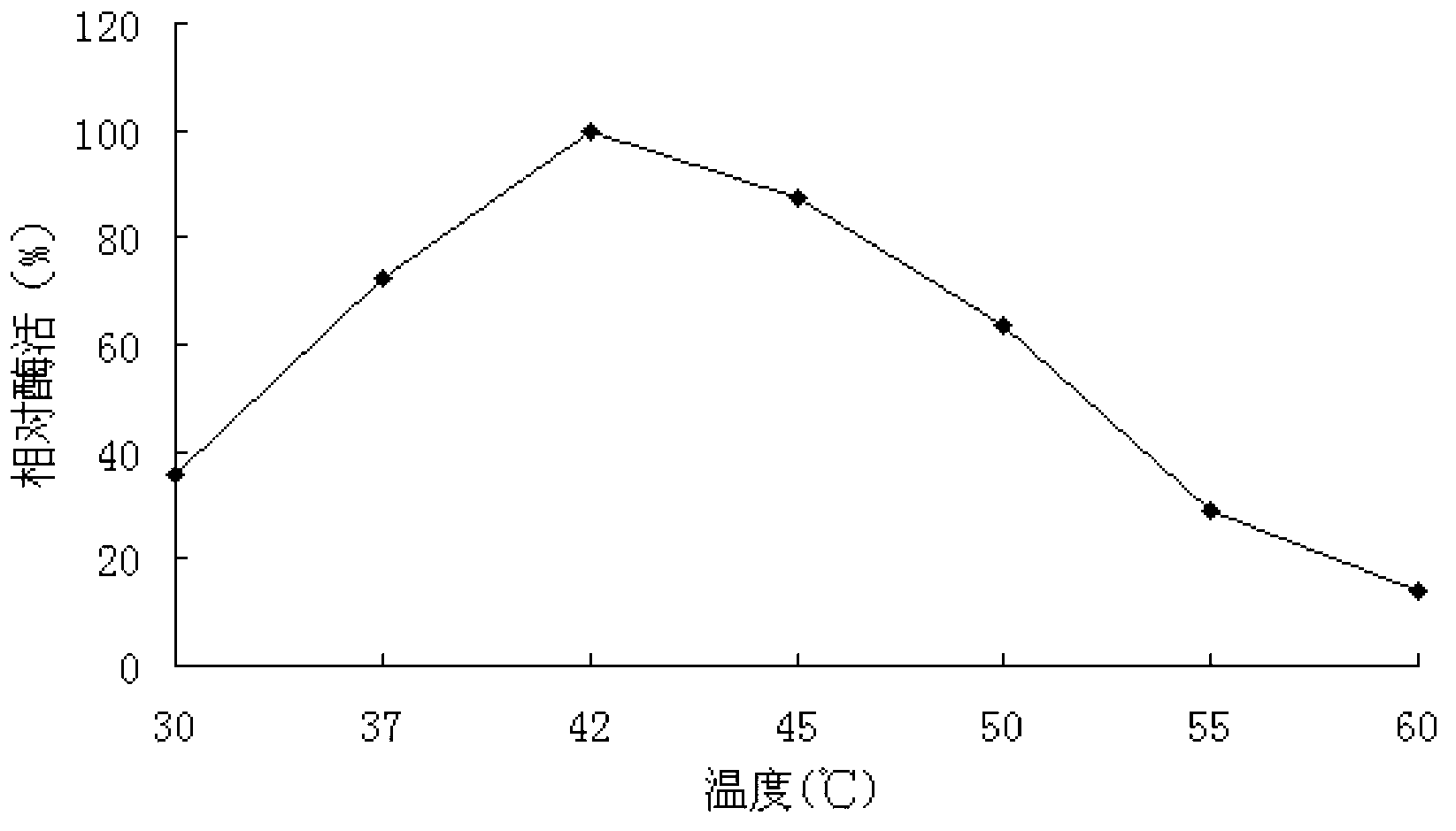
The invention discloses a neutral alpha-galactosidase M-GALC, and a coding gene and application thereof. The protein M-GALC has alpha-galactosidase activity, is derived from chaetomium globosum, and is (a) or (b) as follows: (a) protein composed of amino acid sequence disclosed as Sequence 1 in the sequence table; or (b) protein derived from Sequence 1 in the sequence table with alpha-galactosidase activity, which is subjected to substitution and / or deletion and / or addition of one or more amino acid residues of the amino acid sequence disclosed as Sequence 1 in the sequence table. The specific activity of the protein M-GALC disclosed by the invention as the alpha-galactosidase is 1.91U / mg; the optimum pH value is 7.0; the optimum temperature is 42 DEG C; and the protein Z-GALC has high enzyme activity at the temperature of 37-50 DEG C under the pH value of 6.0-9.0. The invention provides a new way for preparing alpha-galactosidase, and has important application value.
Owner:TIANJIN BIOFEED TECH CO LTD
Canine distemper virus replication defect strain and establishing method thereof
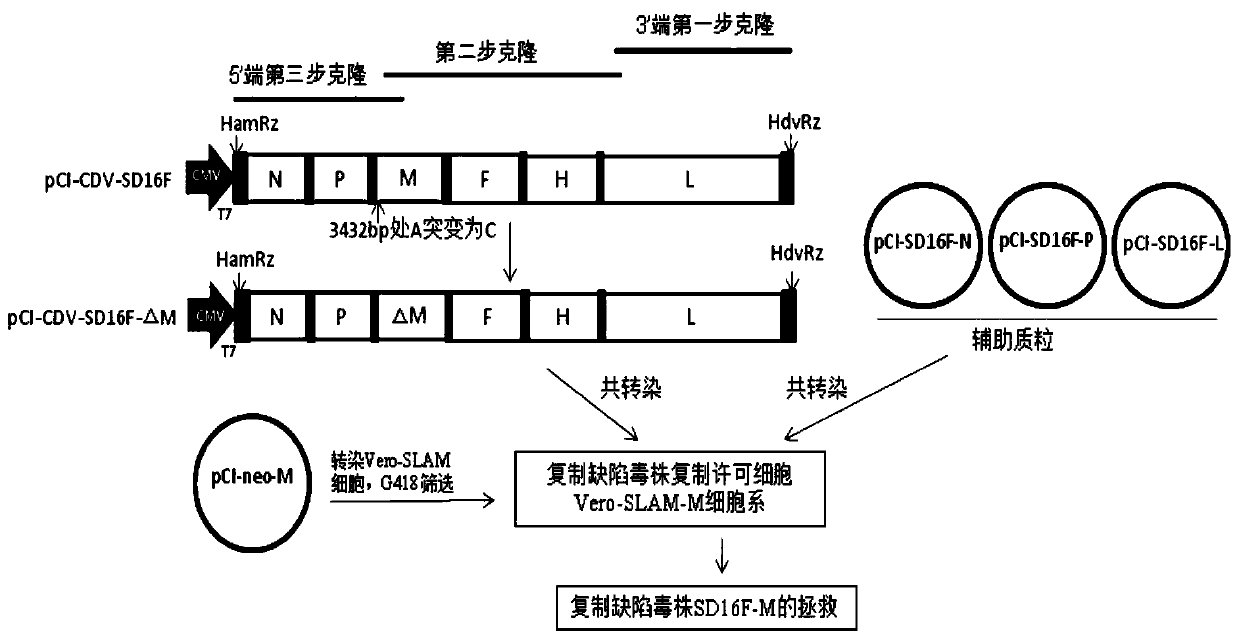
The invention relates to rescuing and verifying of a canine distemper virus replication defect strain of a canine distemper virus. A system comprises a transcription plasmid, one or more auxiliary plasmids and a Vero-SLAM-M cell line, wherein the transcription plasmid pCI-CDV-SD16F can express the genome full-length cDNA sequence of the canine distemper virus prevalent strain SD16F, the plasmid pCI-CDV-SD16F-M subjected to fixed-point mutation is a recombinant plasmid not expressing the protein M, the auxiliary plasmids can express the nucleoprotein (NP), phosphoprotein (P) and large polymerase protein (L) of the canine distemper virus prevalent strain SD16F, and the Vero-SLAM-M cell line can stably express proteins SD16FM. Through the reverse genetic operation system, the recombinant replication defect canine distemper virus is successfully rescued. Through the research, the canine distemper virus prevalent replication defect strain creates convenient conditions for a novel canine distemper virus genetic engineering biological control preparation and provides an excellent technological platform for the canine distemper virus related basic research.
Owner:QINGDAO AGRI UNIV
A method for protein affinity purification of Igy
InactiveCN104761638BSimple processImprove purification efficiencyEgg immunoglobulinsPeptide preparation methodsDistilled waterSepharose
The present invention specifically relates to a method for protein affinity purification of IgY, which comprises the following steps: 1. gene cloning of protein M; 2. constructing an expression vector, performing soluble expression of protein M through the expression vector, and purifying soluble protein M; 3. The soluble protein M was coupled with NHS-activated Sepharose 4FF to make an affinity purification medium; 4. The primary product of the IgY antibody was affinity purified through a protein M Sepharose column to obtain high-purity IgY, and the primary IgY antibody The product is a mixture of egg yolk liquid and distilled water of equal mass; 5. Dialyze the IgY antibody solution obtained after purification in PBS solution at 4°C overnight, and the pH of the PBS solution is 7.0-7.4; 6. Collect the liquid in the dialysis bag to obtain high purity IgY antibody. The invention has the following beneficial effects: 1. The process is simple; 2. The IgY purification efficiency is as high as 95%; 3. It is more environmentally friendly, green and healthy.
Owner:NORTHWEST A & F UNIV
Artificially synthesized polyepitope gene of porcine reproductive and respiratory syndrome virus and its application
The invention relates to the technical field of animal virology, epizootiology and genetic engineering, in particular to a synthesis method and application of an mMEP of a PRRSV (Porcine Reproductive and Respiratory Syndrome Virus) with high pathogenicity. The synthesis method is characterized in that the T-cell epitopes of the GP3, GP4, GP5, protein M and protein N of a PRRSVWUH3 strain are connected with the epitope of a modified GP5B cell through linkers; according to mammal codon preference, codons are modified to synthesize the mMEP artificially. The nucleotide sequence of the mMEP is shown in SEQ ID NO:1; the protein sequence of the mMEP is shown in SEQ ID NO:2. The synthetic mMEP is contained in an eukaryotic expression plasmid pcDNA3.1-mMEP. Escherichia coli DH5(Alpha) / pcDNA3.1-mMEP containing the eukaryotic expression plasmid pcDNA3.1-mMEP are preserved in China Center for Type Culture Collection (CCTCC), and the preservation number of the escherichia coli DH5(Alpha) / pcDNA3.1-mMEP is CCTCC NO: M 2012171. The invention further discloses application of the mMEP in preparing an PRRS DNA vaccine.
Owner:HUAZHONG AGRI UNIV
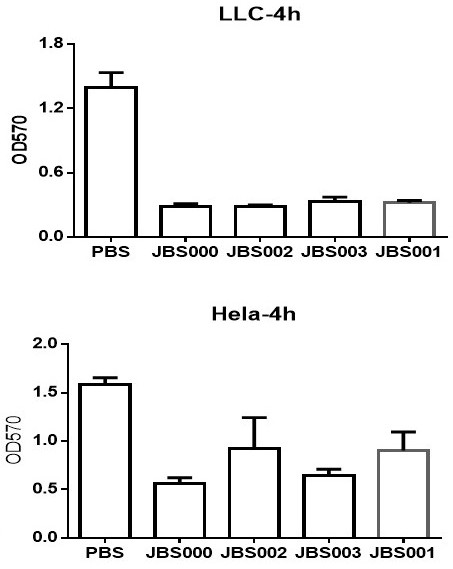
Kit for detecting antigen or measuring its amount
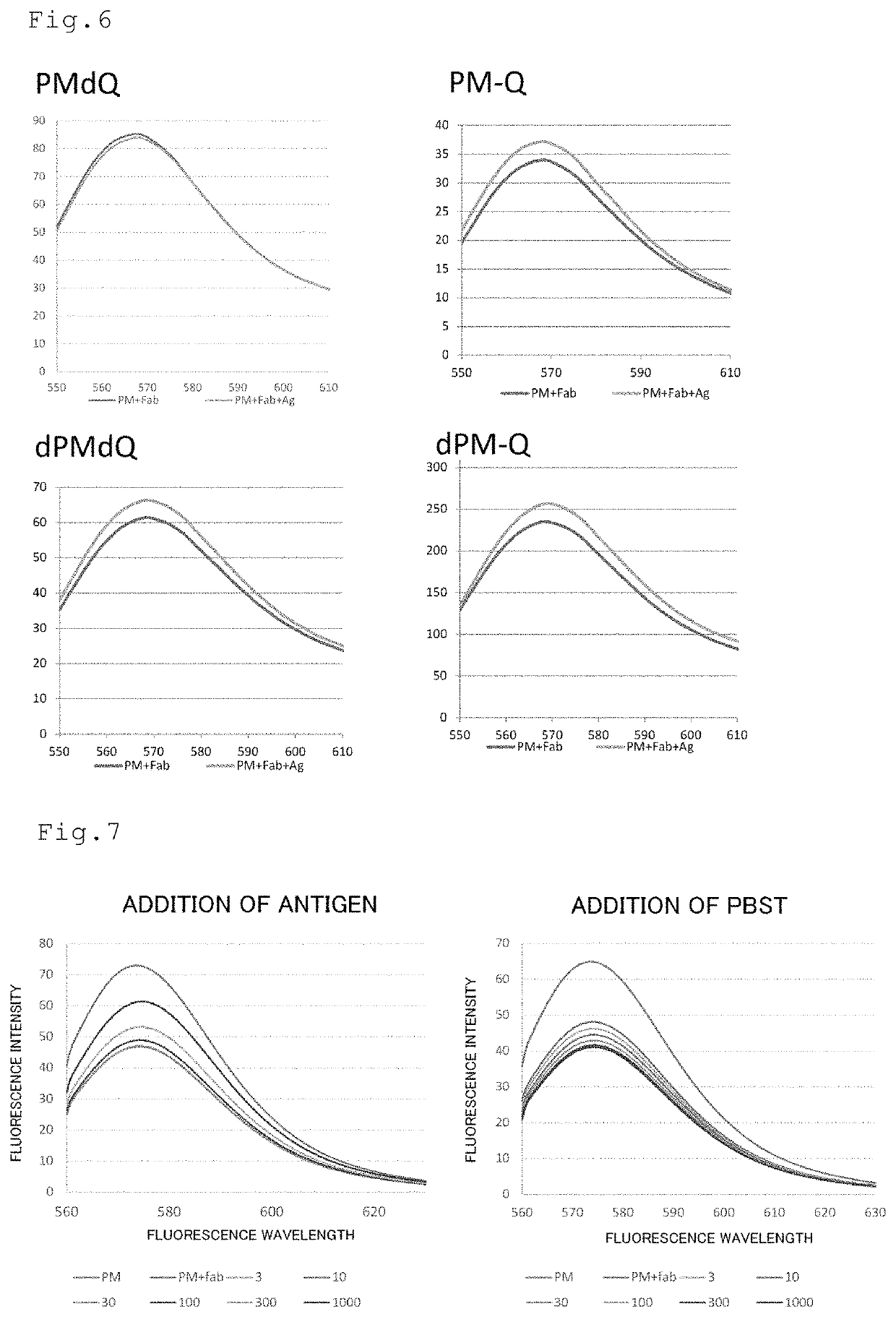
ActiveUS20190376958A1High sensitivityIncrease intensityDepsipeptidesMaterial analysisAntigenHeavy chain
The purpose of the present invention is to develop a kit capable of detecting antigen or measuring its amount with high sensitivity without requiring an immobilization step and a washing step. The present invention provides a kit for detecting antigen or measuring its amount. The kit comprises a complex of a polypeptide including an antibody light chain variable region and a polypeptide including an antibody heavy chain variable region and a protein M fragment labeled with a fluorescent dye. The protein M fragment is a fragment having a binding ability to the complex.
Owner:TOKYO INST OF TECH
An oncolytic virus vaccine and its drug combined with immune cells to treat tumors
ActiveCN111286493BEffective treatmentHigh cure rateSsRNA viruses negative-senseMammal material medical ingredientsTumor therapyT cell
The invention belongs to the field of biotechnology, and in particular relates to an oncolytic virus vaccine and a drug for treating tumors in combination with immune cells. The present invention provides a brand-new attenuated strain of oncolytic virus by performing site-directed mutation on the matrix protein M of the VSV wild-type virus, and its matrix protein M gene sequence is shown in SEQ ID NO 3. The attenuated strain can be used alone as a drug to treat tumors, and its safety and cure rate are superior to wild-type virus and other known attenuated strains. Based on the attenuated strain of oncolytic virus, the present invention also provides a vaccine applicable to tumor treatment by inserting NY-ESO-1 into the attenuated strain. The vaccine has a high cure rate and high biological safety. On the basis of the vaccine, the present invention also uses the vaccine in combination with TCR-T cells to provide a drug that can efficiently treat multiple types of tumors; on a mouse lung cancer model, the cure rate can reach 95%.
Owner:JOINT BIOSCIENCES (SH) LTD
Microbial analysis
ActiveUS10655157B2Samples introduction/extractionMicrobiological testing/measurementBiotechnologyPhospholipid
The present invention is concerned with a method of identifying microbial strains (e.g. from a cell culture), the method comprising; i) a lipid extraction step, comprising extraction of phospholipids from the microbe, suitably with an extraction composition comprising more than 50 vol % MeOH; ii) a sample preparation step, comprising preparation of a MALDI sample incorporating the extracted lipids; iii) a data gathering step, comprising performing MALDI-based mass spectrometry on the MALDI sample, and iv) a microbe identification step, comprising analysis of the mass spectrometry data to characterise or identify the microbial strain. Suitably the method also includes extracting proteins from the microbes and analysing the extracted proteins using MALDI-based mass spectrometry so as to obtain not only lipid m / z data but also protein m / z data.
Owner:KRATOS ANALYTICAL
Compositions and methods for binding antibodies and inhibiting neutralizing antibodies
PendingCN114514239ABacteriaPeptide/protein ingredientsAntiendomysial antibodiesNeutralising antibody
The present invention relates to methods and compositions for binding antibodies. The methods may be used to isolate antibodies, treat conditions associated with excess antibodies, acute block antibodies to prevent autoimmune or inflammatory responses, and inhibit neutralizing antibodies. In an embodiment, the present invention relates to a method of inhibiting a neutralizing antibody against a heterologous agent when the heterologous agent is administered to a subject, comprising administering to the subject an effective amount of Mycoplasma Protein M, or a functional fragment or derivative thereof, thereby inhibiting neutralization of the heterologous agent. The invention also relates to a modified Mycoplasma protein M or a functional fragment thereof having increased thermal stability relative to the wild-type protein M and its use in the method of the invention.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
A kind of traditional Chinese medicine composition for improving immunity and antioxidant power of laying ducks
ActiveCN104940548BImprove immunityImprove antioxidant capacityAntinoxious agentsAccessory food factorsSerum protein albuminAdditive ingredient
Owner:GUIYANG MUREN AGRI TECH DEV CO LTD
A kind of anti-avian infectious bronchitis virus peptide nucleic acid and its application
ActiveCN106434649BImprove stabilityGood membrane permeabilityOrganic active ingredientsAntiviralsBase JAntisense nucleic acid
Owner:南京美森生物科技有限公司
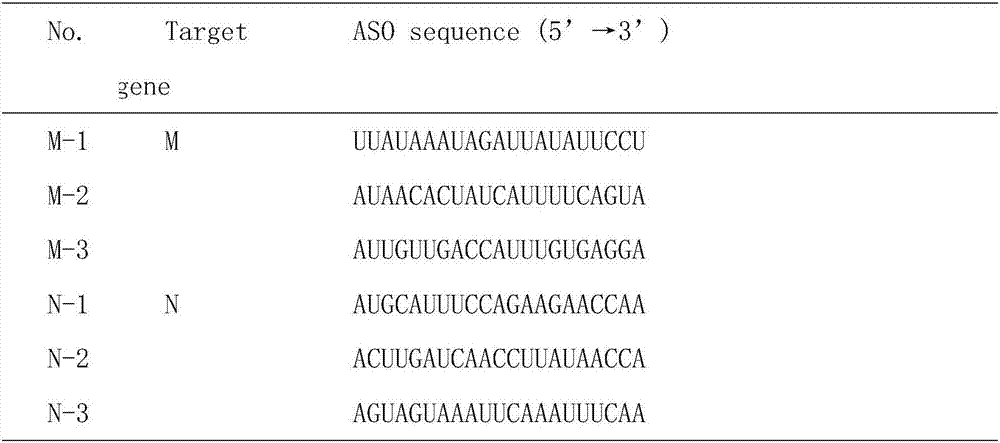
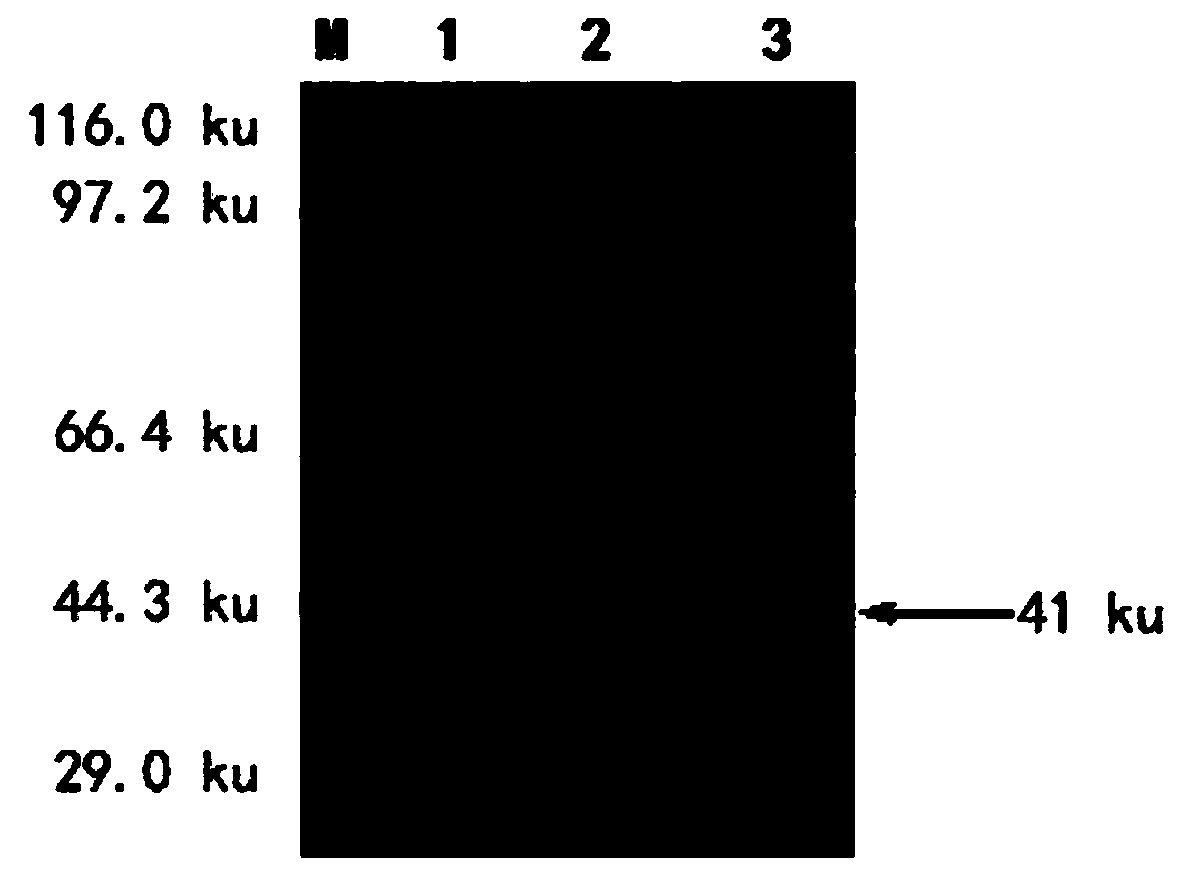
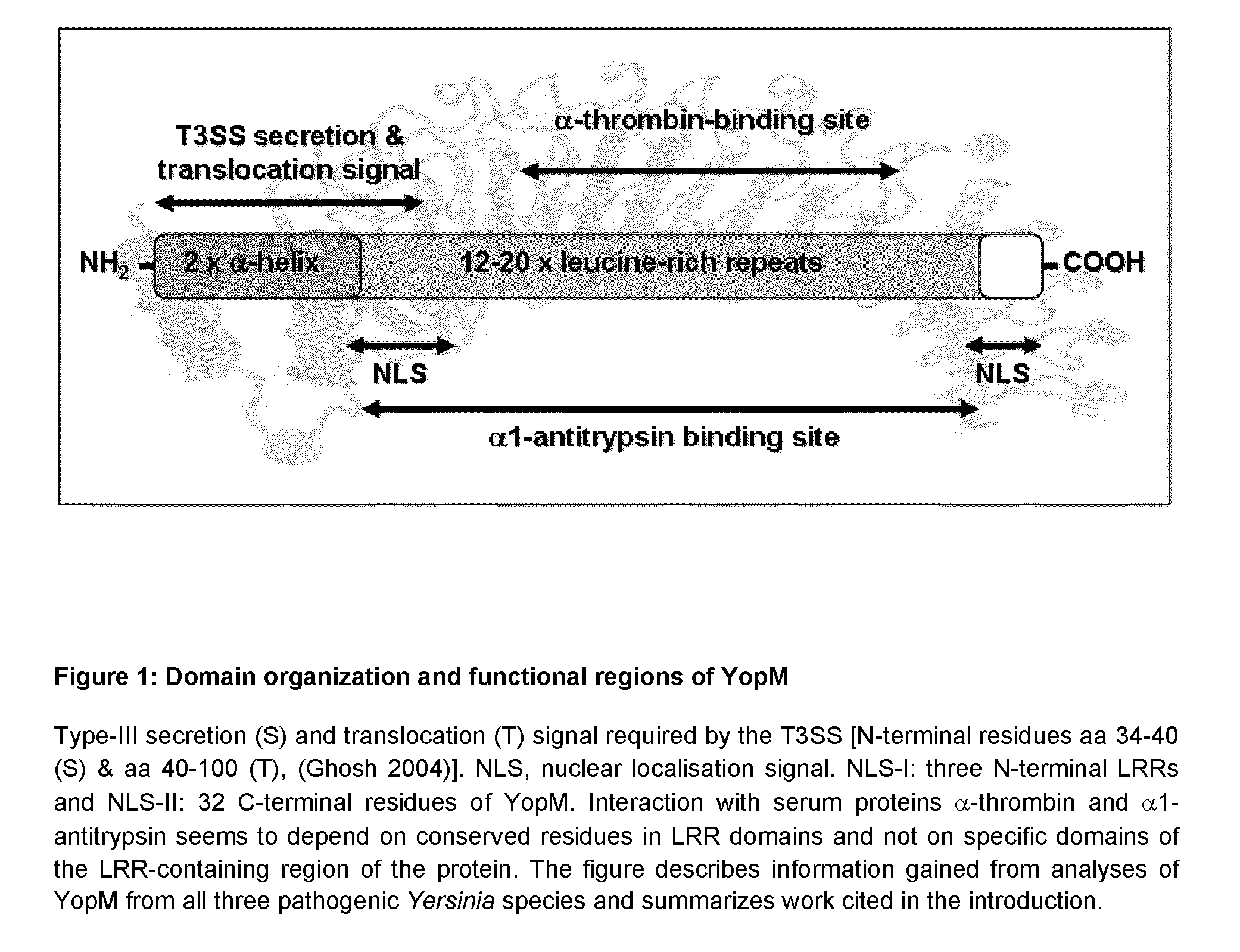
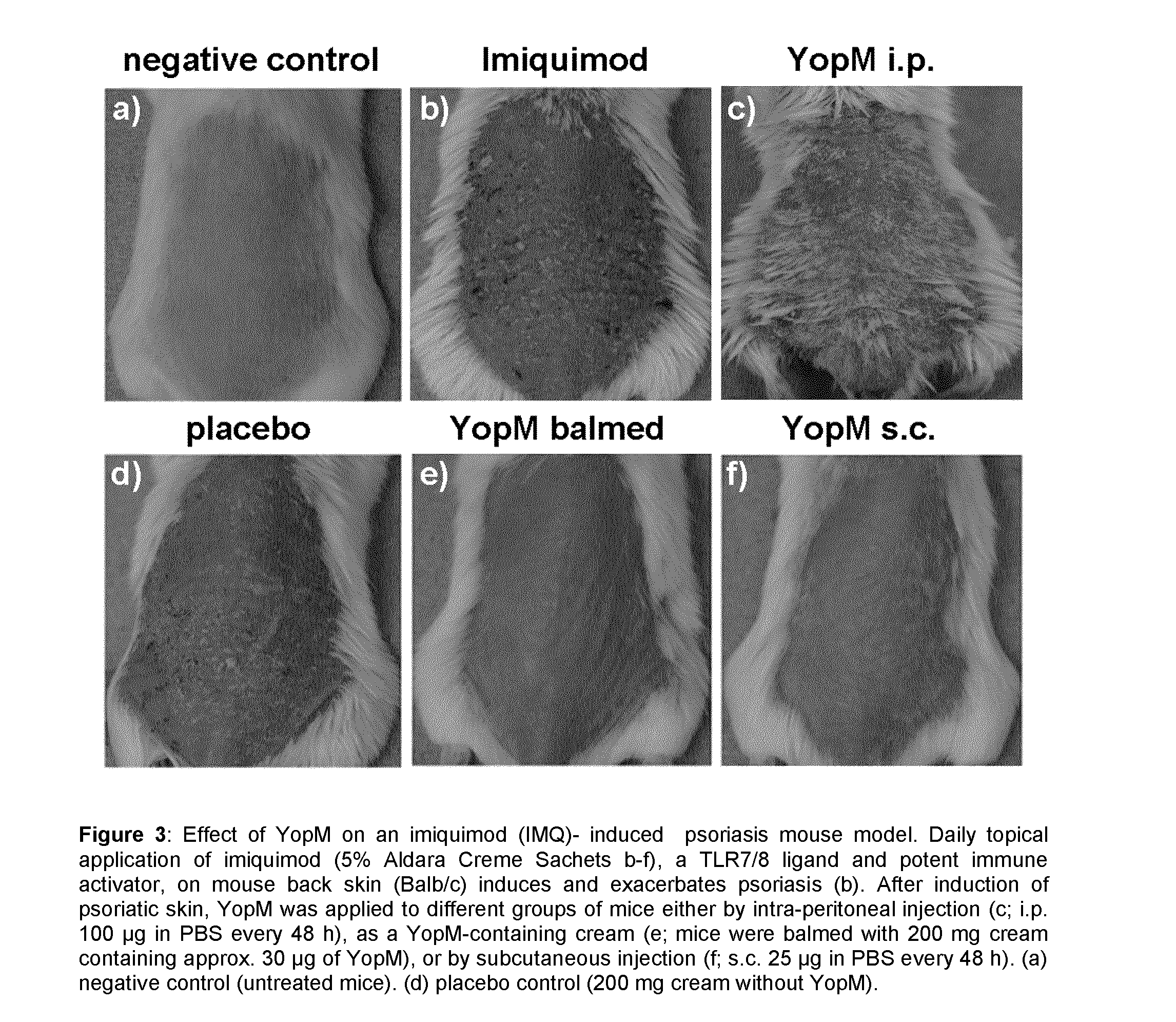
Yersinia outer protein M (YopM) in the treatment of psoriasis
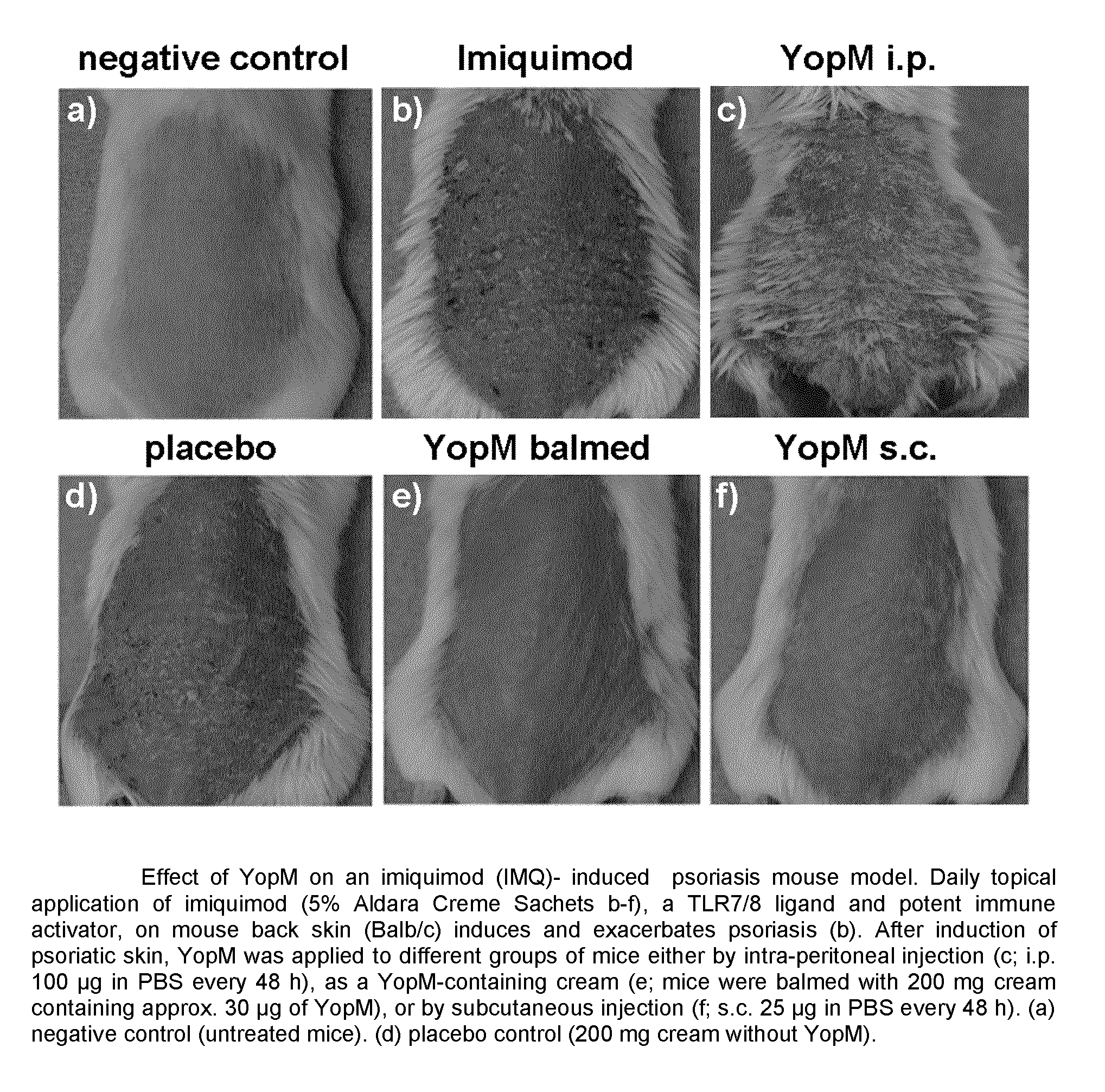
ActiveUS9155779B2Simple methodAvoid side effectsPeptide/protein ingredientsAntibody medical ingredientsArthritisPustular psoriasis
Owner:RUETER CHRISTIAN +1
Novel Ligand and Use Thereof
PendingUS20190218265A1Good alkali stabilityImprove stabilityFactor VIIAntibody mimetics/scaffoldsFc bindingMutant
The present invention is within the field of protein engineering and purification. The invention relates to a target-binding polypeptide mutant of an IgG binding polypeptide, such as Protein A, Protein G, Protein L or Protein M, comprising a metal binding motif. More closely the invention relates to an Fc binding ligand comprising an engineered protein based on the Protein A derived Z domain, to which a calcium binding EF-loop has been introduced.
Owner:CYTIVA BIOPROCESS R&D AB
Novel coronavirus virus-like particle vaccine based on vaccinia virus vector
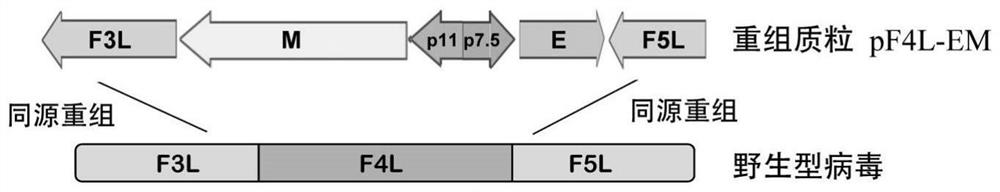
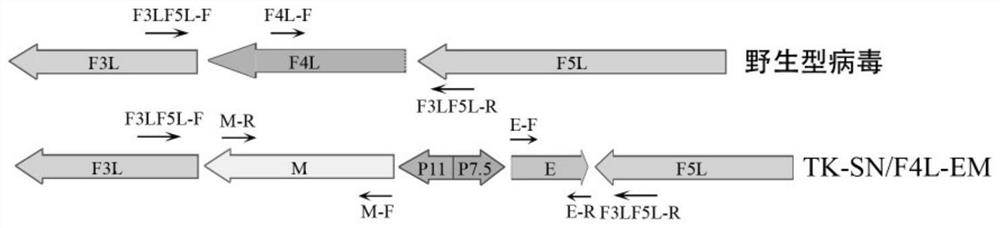
The invention provides a novel coronavirus virus-like particle vaccine based on a vaccinia virus vector. The recombinant vaccinia virus is constructed by integrating coding gene sequences of a new coronavirus envelope protein E and a membrane protein M at the position of an F4L gene in a vaccinia virus Tian Tan strain genome and integrating coding gene sequences of a new coronavirus spike protein S and a nucleocapsid protein N at the position of a TK gene; wherein the coding gene sequences of the protein E and the protein M are connected in a manner that 5'ends are opposite, and are respectively connected to the downstream of different promoters; the coding gene sequences of the proteins S and N are connected in a manner that 5'ends are opposite, and are respectively connected to the downstream of different promoters. The recombinant virus is used for immunizing mice, high S and N specific antibody titers are detected in serum of the mice, and antigen-specific T cell immune response can be induced after immunization. The novel coronavirus vaccine provided by the invention has important significance on prevention and control of novel coronavirus.
Owner:INST OF PATHOGEN BIOLOGY CHINESE ACADEMY OF MEDICAL SCI +1
Porcine reproductive and respiratory syndrome virus ELISA antibody detection kit
A porcine reproductive and respiratory syndrome virus ELISA antibody detection kit, the kit includes: a protein mixed-encapsulated enzyme label plate, a batch pretreatment device for serum samples, a sample diluent, an enzyme-labeled conjugate, a substrate color developer, and a stop solution , Porcine Reproductive and Respiratory Syndrome positive serum and Porcine reproductive and respiratory syndrome negative serum; the protein mixed-encapsulated ELISA plate is coated with antigenic proteins: envelope protein GP5, membrane matrix protein M and nucleocapsid protein N. Compared with the existing mainstream technology that coats a single antigen, the triple-coated antigen detection antibody is more effective, which can effectively reduce the problem of detection failure or missed detection. In addition, a batch pre-processing device for serum samples was invented, which can mix serum in batches, and can use a multi-channel pipette to add samples at a time, which greatly improves efficiency and reduces errors.
Owner:ZHENGZHOU ZHONGDAO BIOTECHNOLOGY CO LTD +2
Porcine epidemic diarrhea virus m protein affinity peptide and its screening method
InactiveCN104774249BInhibition of replicationCombined antiviral effect is goodPeptide preparation methodsScreening methodBacteriophage
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Yersinia outer protein m (YOPM) in the treatment of psoriasis
ActiveUS20140357842A1Simple methodAvoid side effectsPeptide/protein ingredientsPeptide preparation methodsArthritisPustular psoriasis
The present invention relates to the use of Yersinia outer protein M (YopM)in the prevention and / or treatment of psoriasis by cutaneous, intradermalor subcutaneous administration. In particular, the present invention relates to the use of YopM in the treatment of plaque psoriasis, guttate psoriasis, inverse psoriasis, pustular psoriasis, erythrodermic psoriasis, arthritis psoriasis.
Owner:RUETER CHRISTIAN +1
a drug for treating tumors
ActiveCN111467489BAdaptableHigh cure rateSsRNA viruses negative-senseVirus peptidesTumor therapyTherapeutic effect
The invention belongs to the field of biotechnology, and in particular relates to an oncolytic virus vaccine and a drug for treating tumors in combination with immune checkpoint inhibitors. The invention provides a brand-new attenuated strain of oncolytic virus by performing site-directed mutation on the matrix protein M of vesicular stomatitis virus wild type virus. The attenuated strain can be used alone as a drug to treat tumors, and its safety and cure rate are superior to wild-type virus and other known attenuated strains. Based on the attenuated strain of oncolytic virus, the present invention also provides a vaccine applicable to tumor treatment by inserting NY-ESO-1 into the attenuated strain. The vaccine has a high cure rate and high biological safety. On the basis of the vaccine, the present invention also uses the vaccine in combination with immune checkpoint inhibitors to provide a drug that can efficiently treat multiple types of tumors; on the mouse lung cancer model, the cure rate can reach an astonishing 87.5% %, and has a better therapeutic effect on large tumors.
Owner:JOINT BIOSCIENCES (SH) LTD
Kit for detecting antigen or measuring its amount
The purpose of the present invention is to develop a kit capable of detecting antigen or measuring its amount with high sensitivity without requiring an immobilization step and a washing step. The present invention provides a kit for detecting antigen or measuring its amount. The kit comprises a complex of a polypeptide including an antibody light chain variable region and a polypeptide including an antibody heavy chain variable region and a protein M fragment labeled with a fluorescent dye. The protein M fragment is a fragment having a binding ability to the complex.
Owner:TOKYO INST OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com