Tumor treatment drug
An anti-tumor drug and drug technology, applied in anti-tumor drugs, drug combinations, microorganisms, etc., can solve the problems of low cure rate, inability to successfully package, and inability to prepare recombinant viruses, and achieve the effect of strong adaptability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
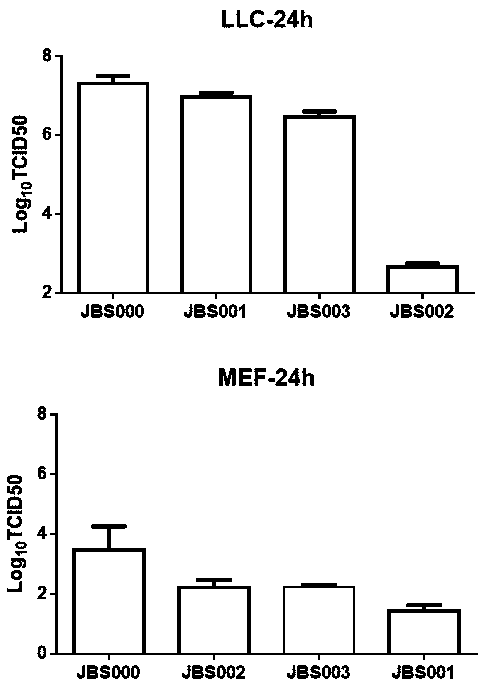
[0078] Example 1: Construction and effect display of attenuated strains with site-directed mutation
[0079] 1. According to the manner in Table 1, the M matrix protein of the Indiana strain of vesicular stomatitis virus was subjected to site-directed mutation to obtain three groups of mutated attenuated strains. The group number without gene mutation is: JBS000, as a control.
[0080] Table 1 The mutation status of each group
[0081]
[0082] The specific construction method of the attenuated strain is a conventional technique in the art, which is briefly described as follows:
[0083] (1) Construct the plasmid. Using the pVSV-XN2 plasmid as a template, the PCR method was used to introduce different mutation sites as described in Table 1. PCR was performed on the plasmid and primers for each mutation site, and then the PCR product was subjected to 1% agarose gel electrophoresis, and then the gel recovery kit was used for gel recovery to obtain plasmids with different m...
Embodiment 2
[0104] Example 2: Construction and effect display of oncolytic virus vaccine
[0105] 1. On the basis of each attenuated strain and wild-type virus prepared in Example 1, insert the NY-ESO-1 gene to construct an oncolytic virus vaccine. The construction schematic diagram is as follows Figure 5 shown. The insert fragments of each group are shown in Table 2.
[0106] Table 2 Display table of insert fragments in each group
[0107]
[0108] The specific preparation methods of JBS004-JBS007 are conventional techniques in the field, and are briefly described as follows. It should be noted that the following description does not limit JBS004 to JBS007 to be carried out according to the following method, but gives an example.
[0109] (1) Construction of attenuated strain plasmid. Artificially synthesized linking sequences with restriction enzyme sites Xho I and Mlu I, using biological technology and gene recombination technology, insert it into the non-coding between the G p...
Embodiment 3
[0126] Example 3: The pharmacokinetics and acute toxicity test results of JBS004
[0127] 1. Pharmacokinetic experiment. Select C57BL / 6 mice and subcutaneously inoculate 2×10 5 LLC cells, about 9 days after inoculation, the transplanted tumor grows to 100mm 3 Left and right, the LLC xenograft tumor model was established. Single intratumoral injection of 10 8 pfu / only JBS004, tumor tissue samples were taken at 0min (+15min), 6h, 12h, 48h, 96h, 120h and 14 days (repeat 3 times), the tissue was crushed with an automatic grinder, and the tumor tissue was extracted by Trizol The total RNA was finally analyzed by quantitative PCR (fluorescent probe method) for viral nucleic acid copy number. The result is as Figure 19 , 20 shown.
[0128] The results showed that the amount of virus in the tumor reached its peak at 6 hours after infection, which was about 500 times more than the initial dose; 48 hours after infection, the amount of virus began to be lower than the initial in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com