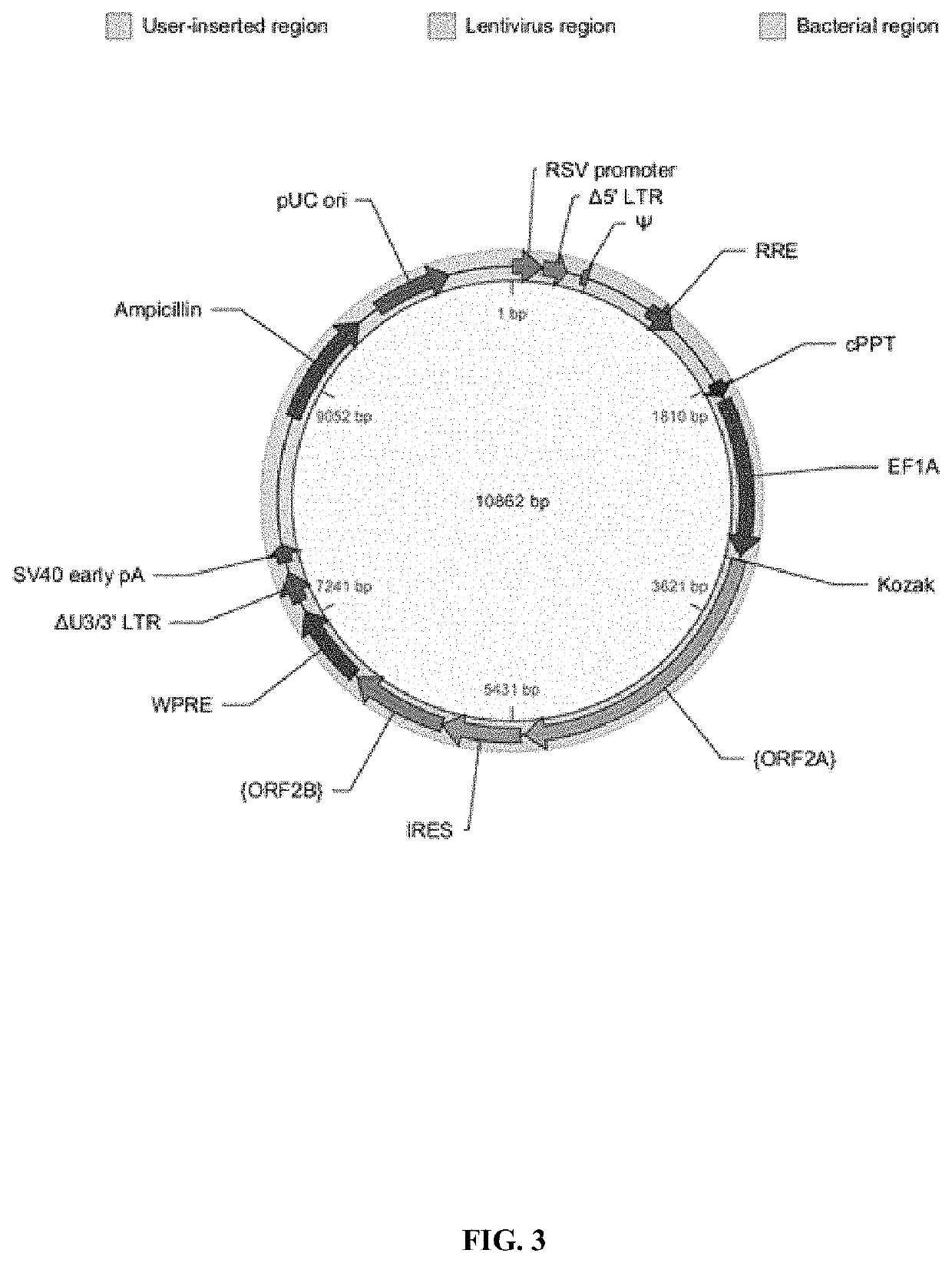
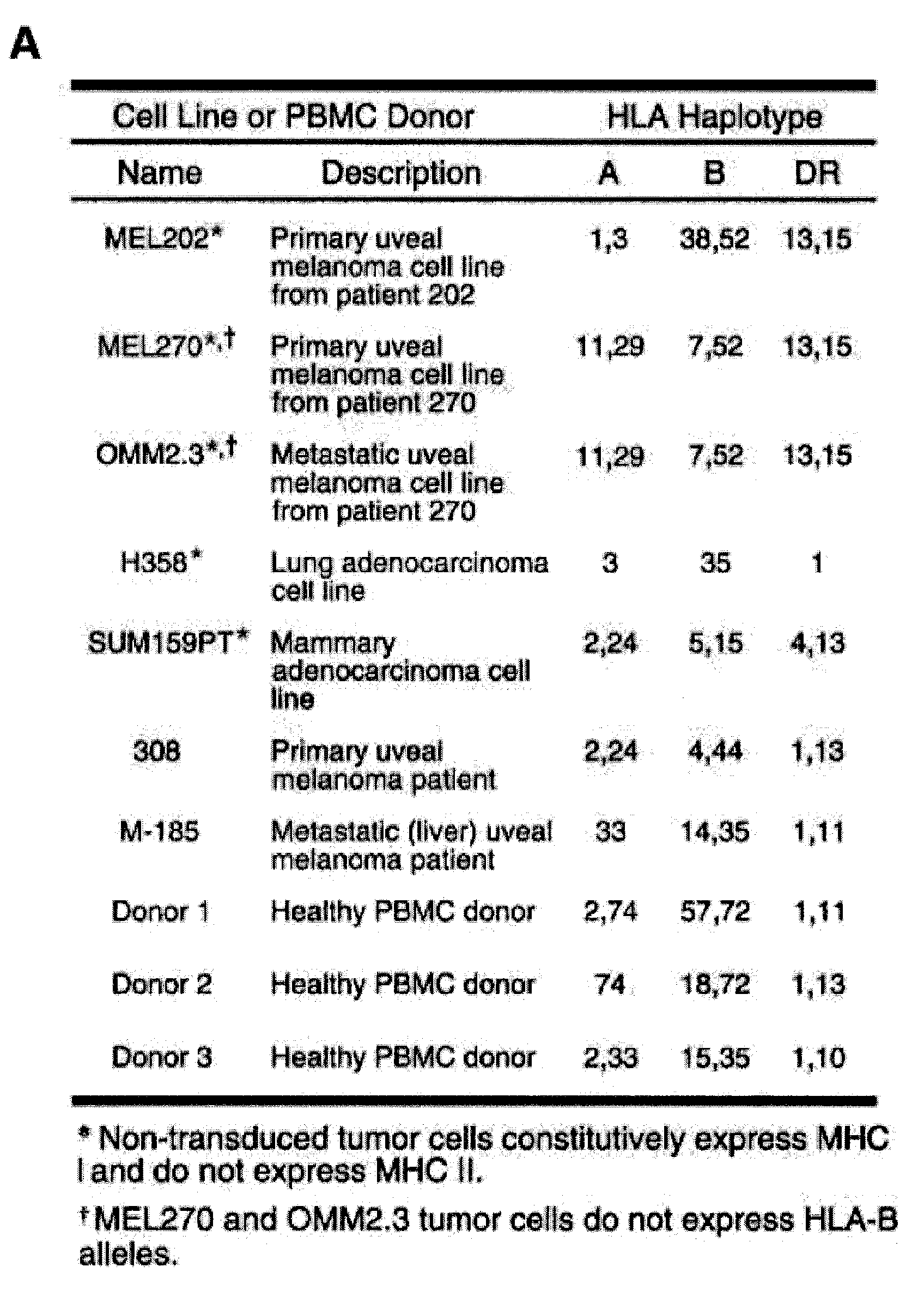
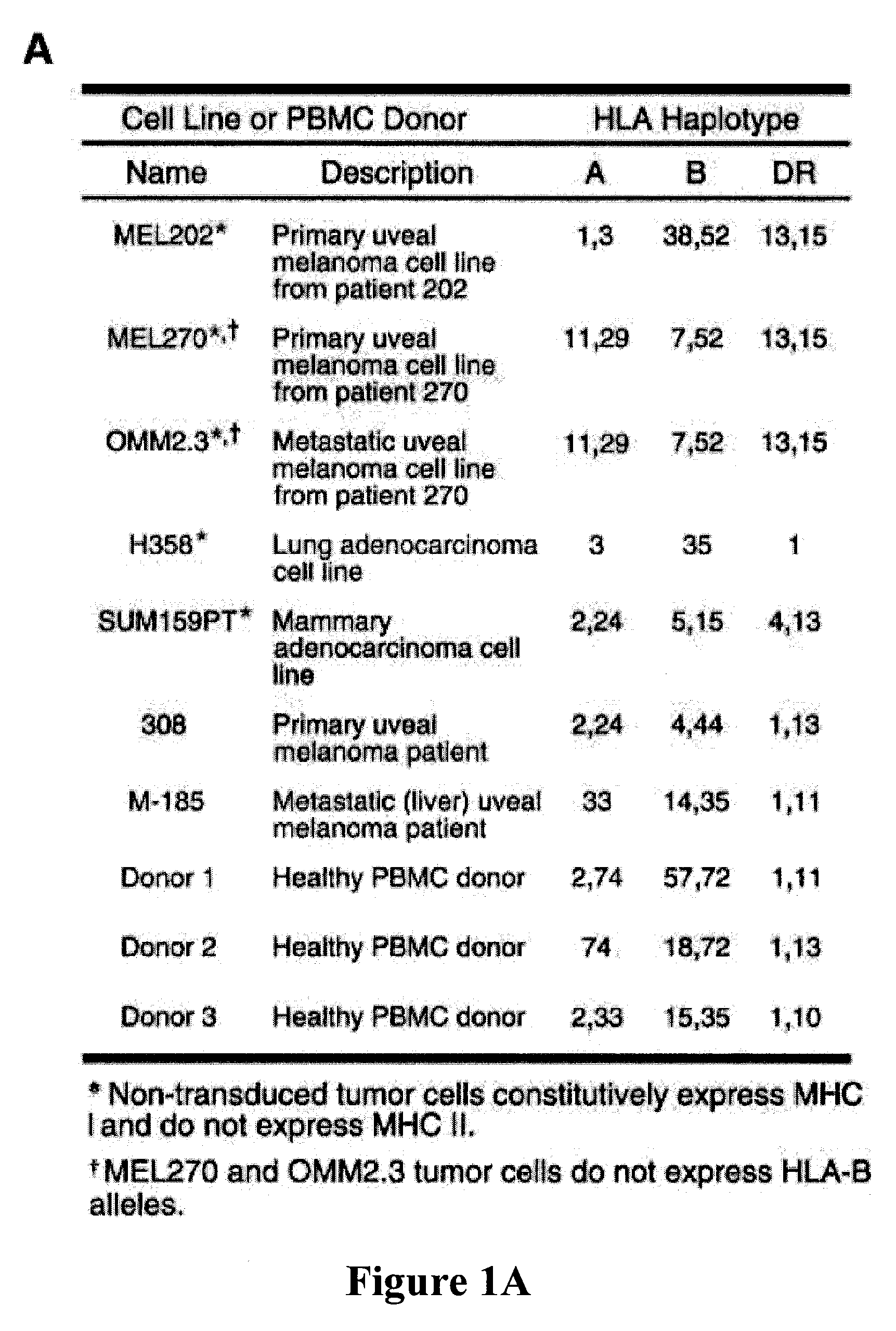
Patents
Literature
168results about "Skin cancer vaccine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
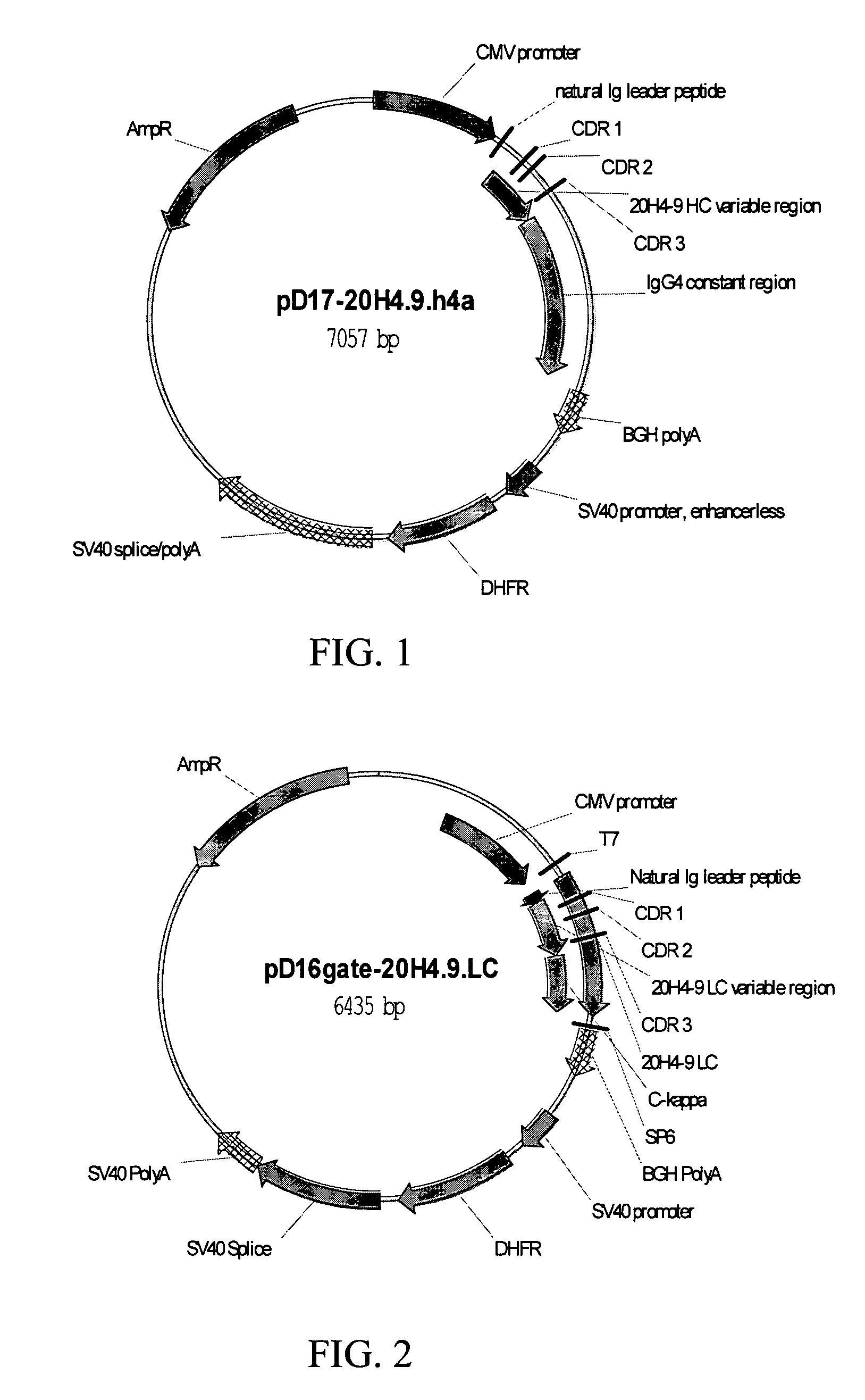
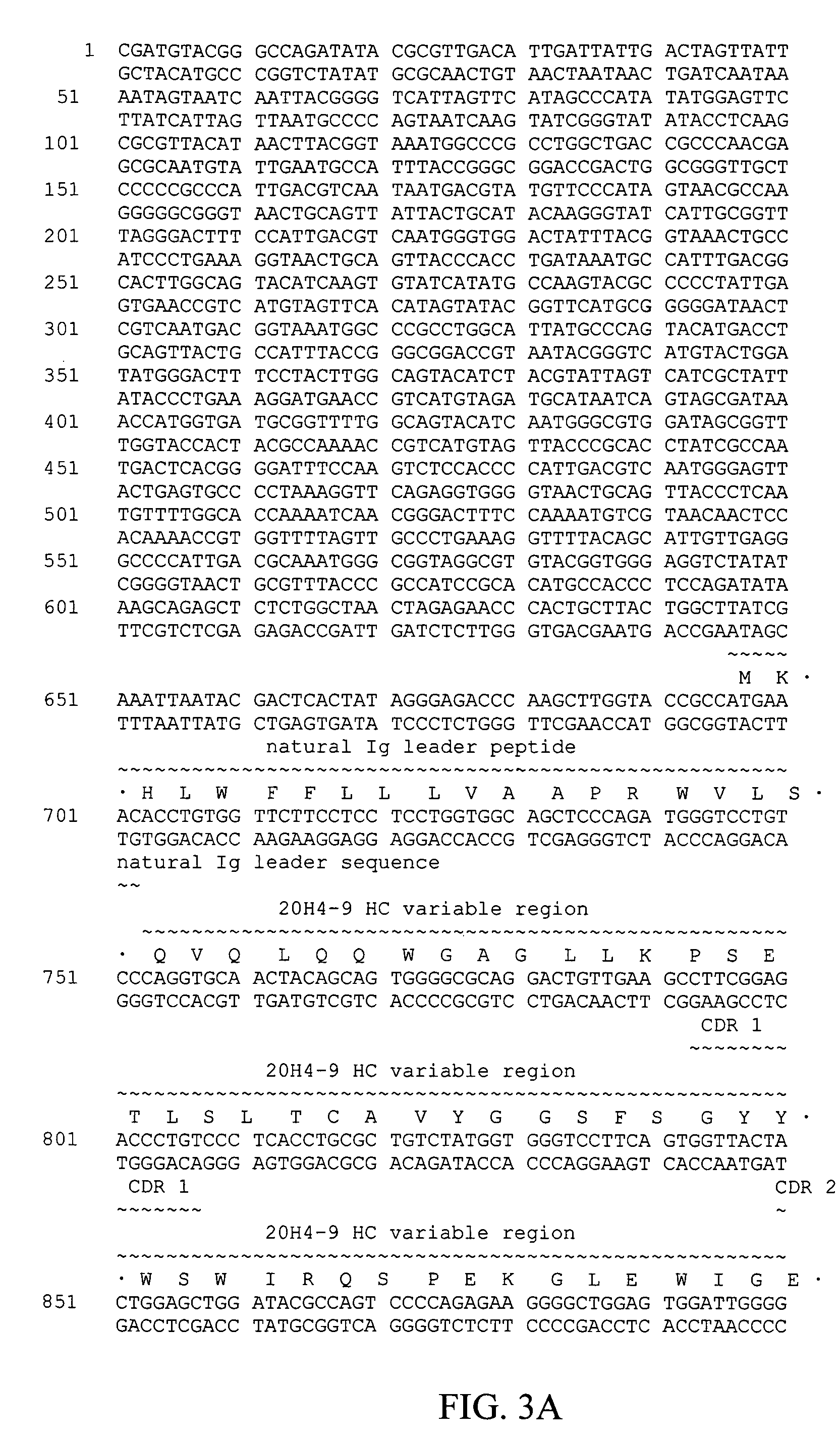
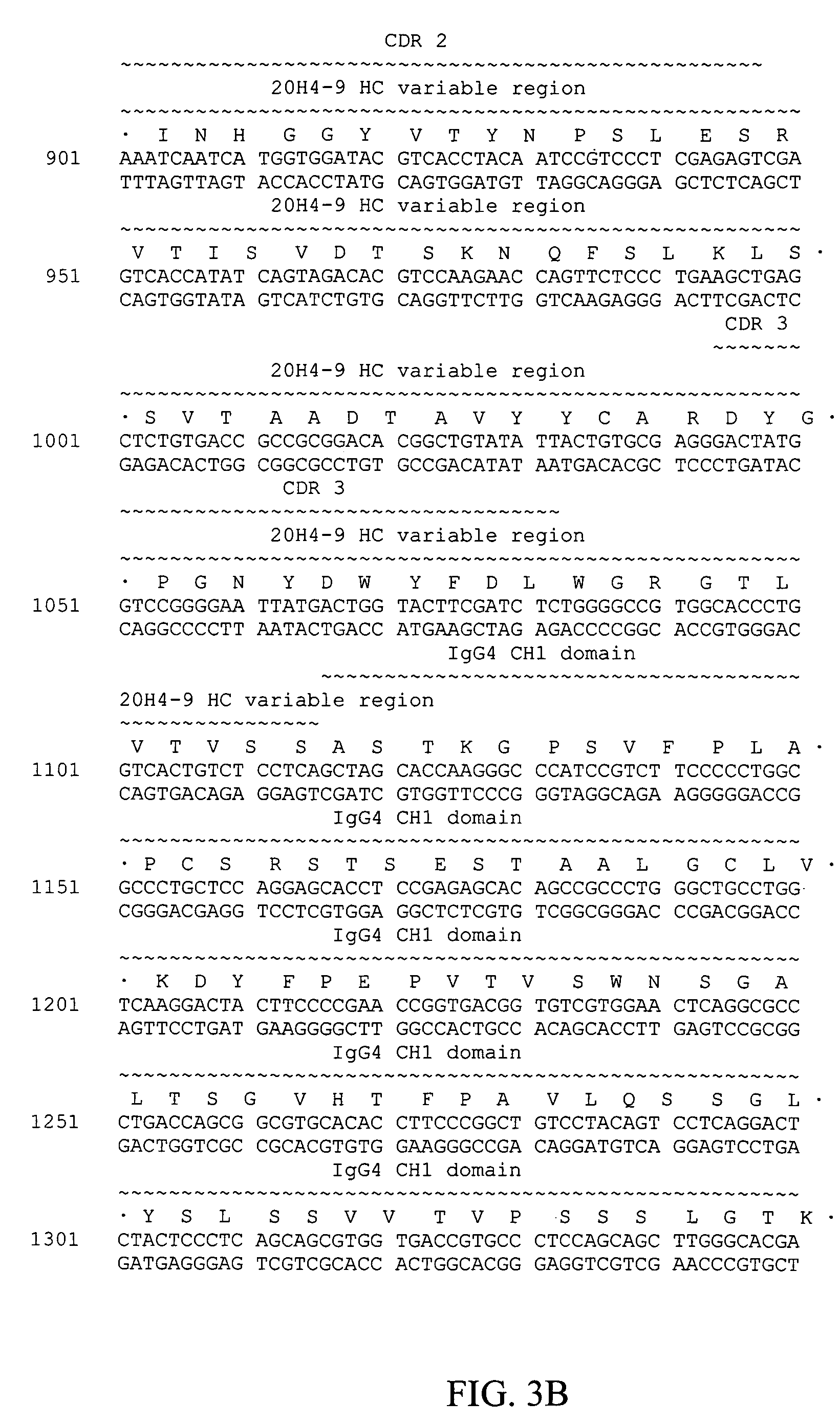
Fully human antibodies against human 4-1BB
Fully human antibodies and antigen-binding portions thereof that bind to human 4-1BB and that allow binding of human 4-1BB to a human 4-1BB ligand. In one aspect, the antibody is an IgG4 antibody. Also provided is a method for treating a disease in a subject comprising administering a therapeutically effective amount of the antibody to said subject.
Owner:BRISTOL MYERS SQUIBB CO
Reducing the Likelihood of Skin Cancer in an Individual Human Being
ActiveUS20200179460A1Encourage jammingGood for healthOrganic active ingredientsBacterial antigen ingredientsBiotechnologyMicroorganism
Compositions, systems and methods of improving the health of the microbiome of an individual's skin relate to the provision of skin contacting formulations containing beneficial bacteria and other microbe components to foster the growth and maintenance of a healthy skin microbiome.
Owner:SEED HEALTH INC
Cancer immunotherapy
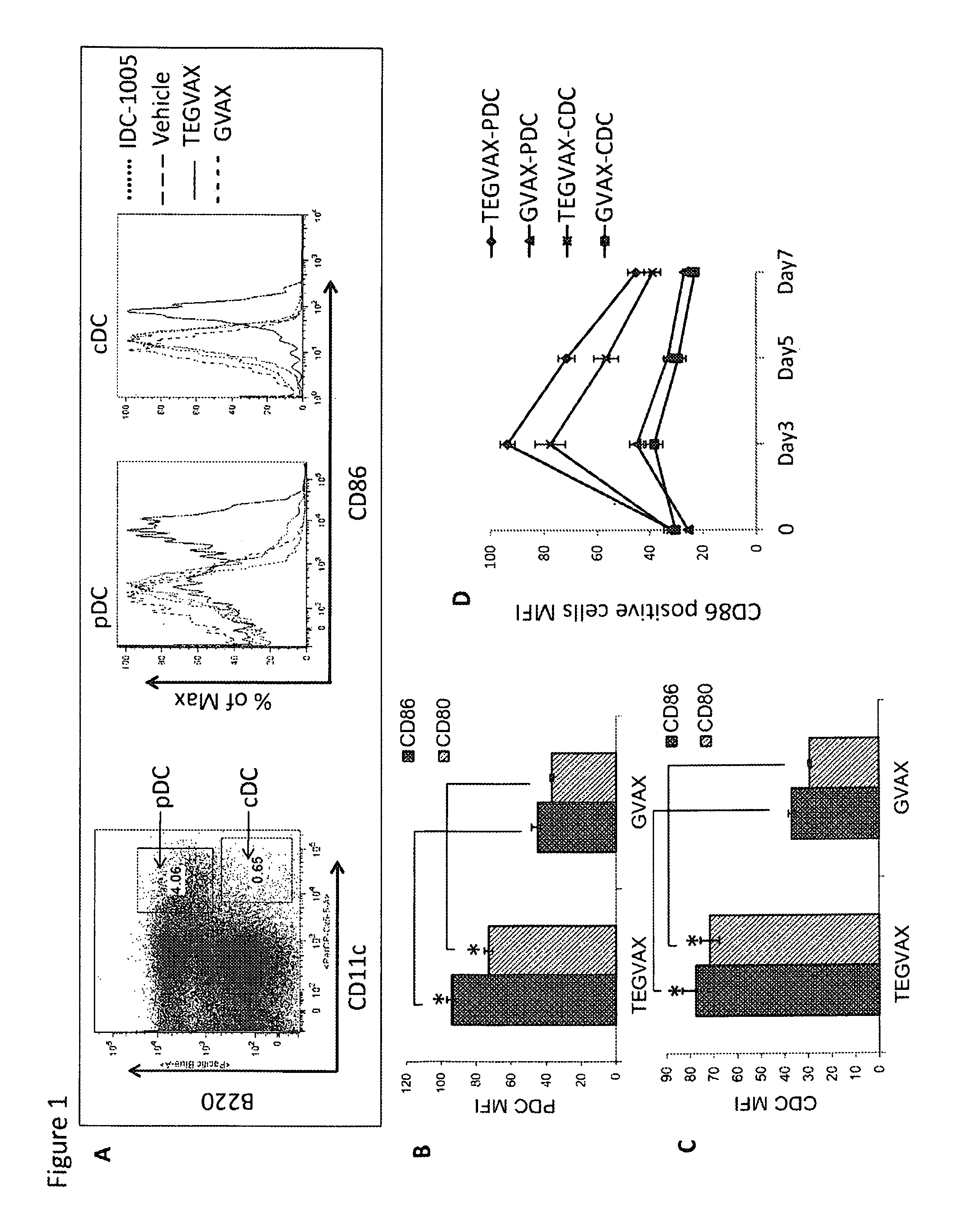
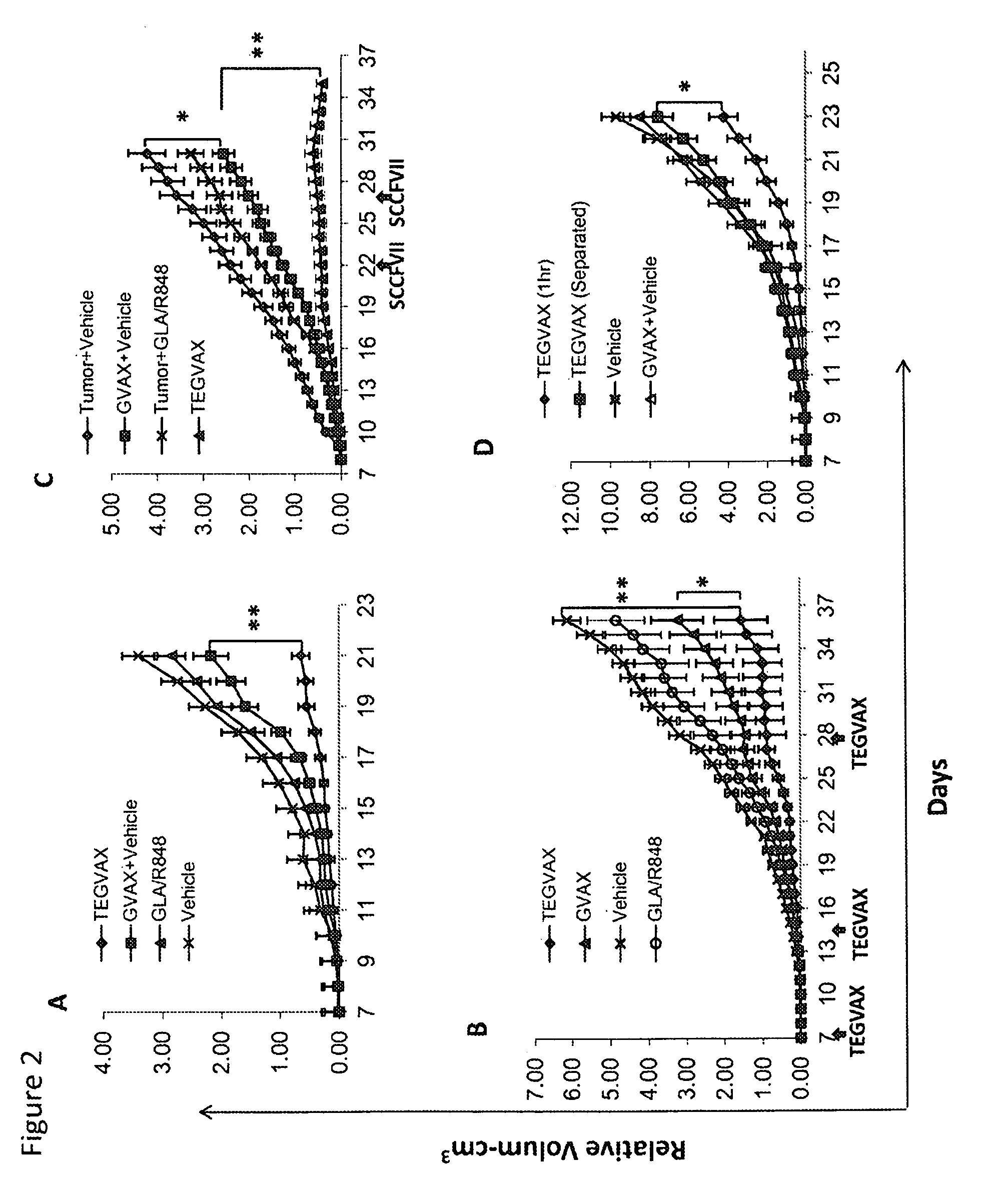
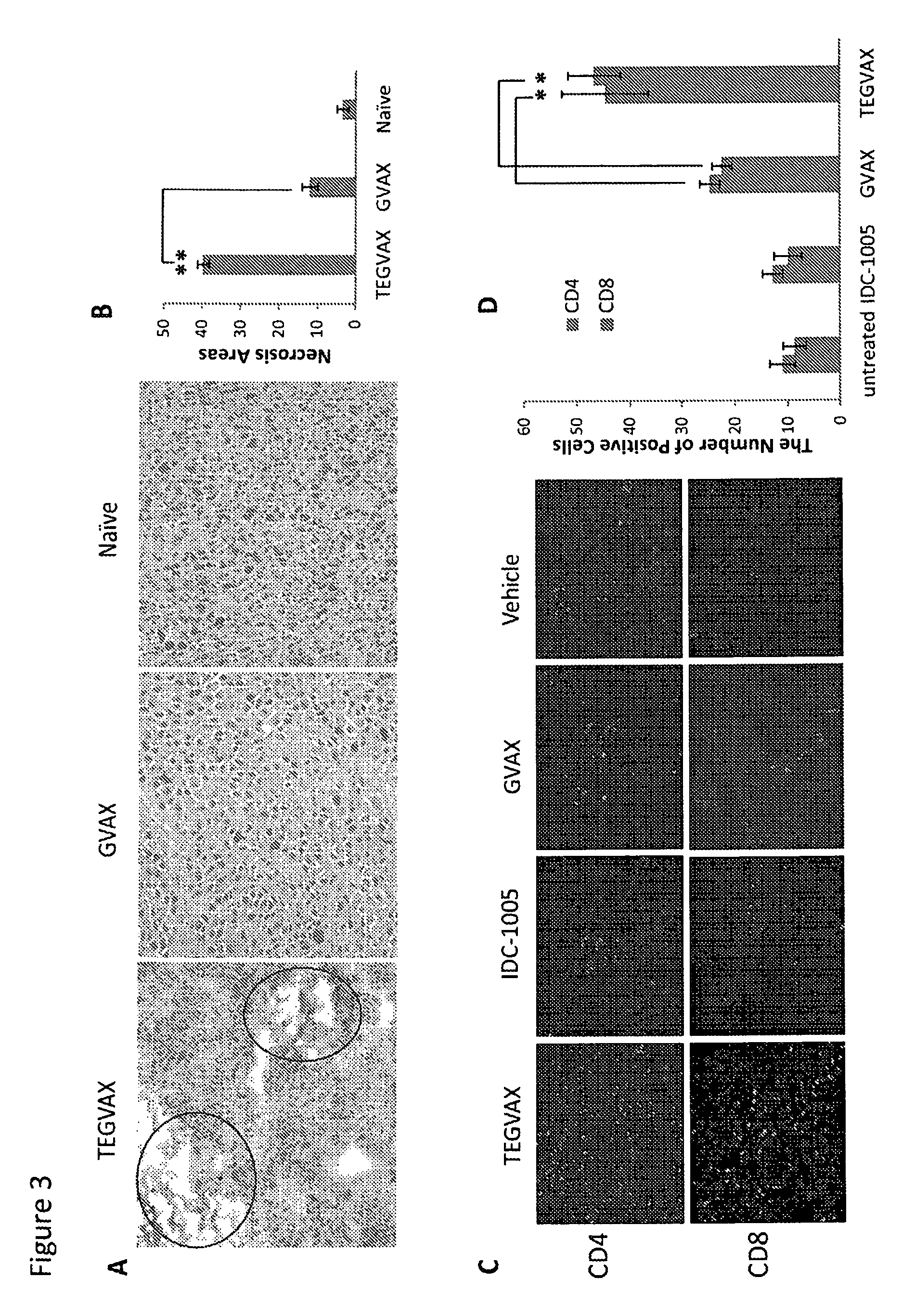
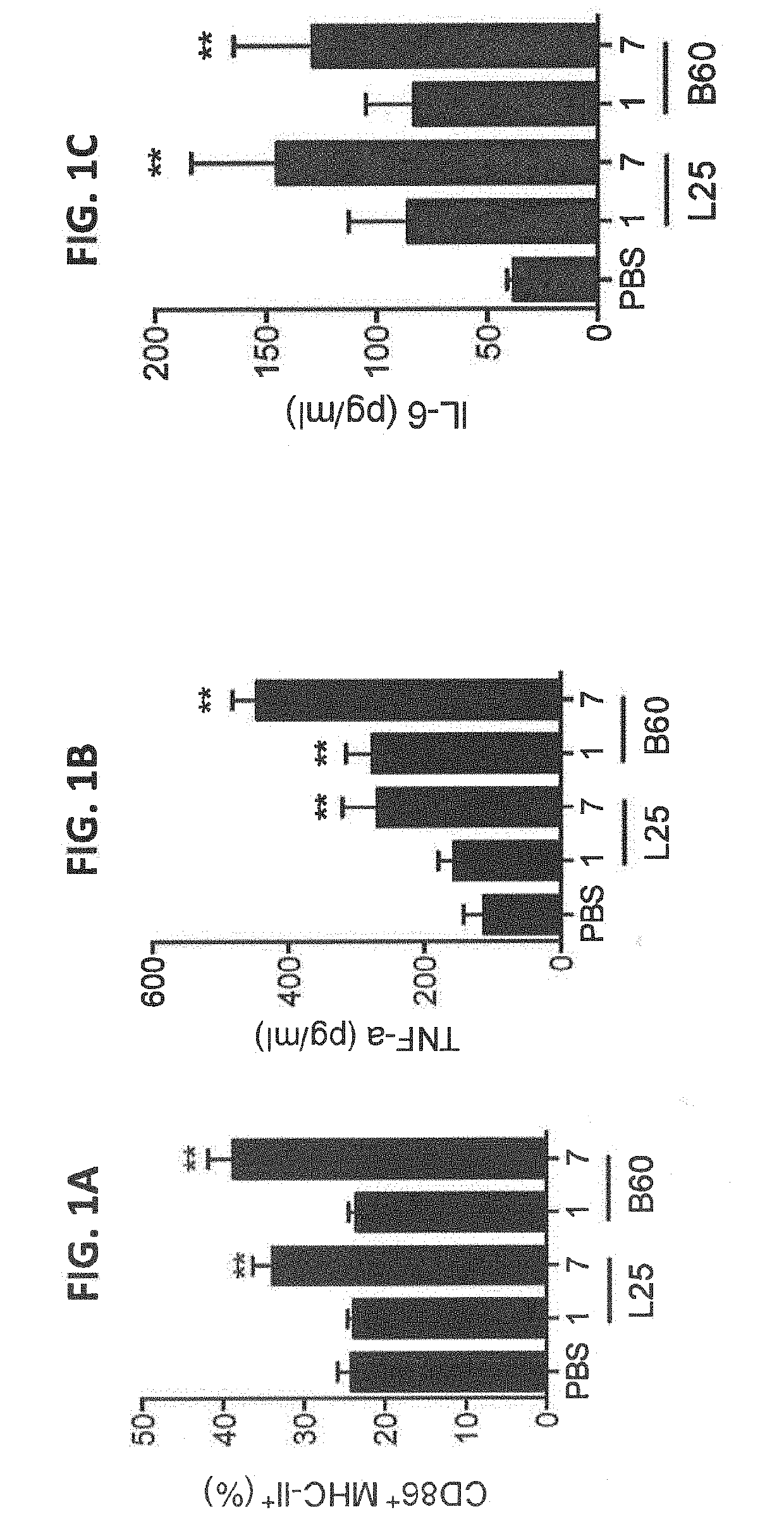
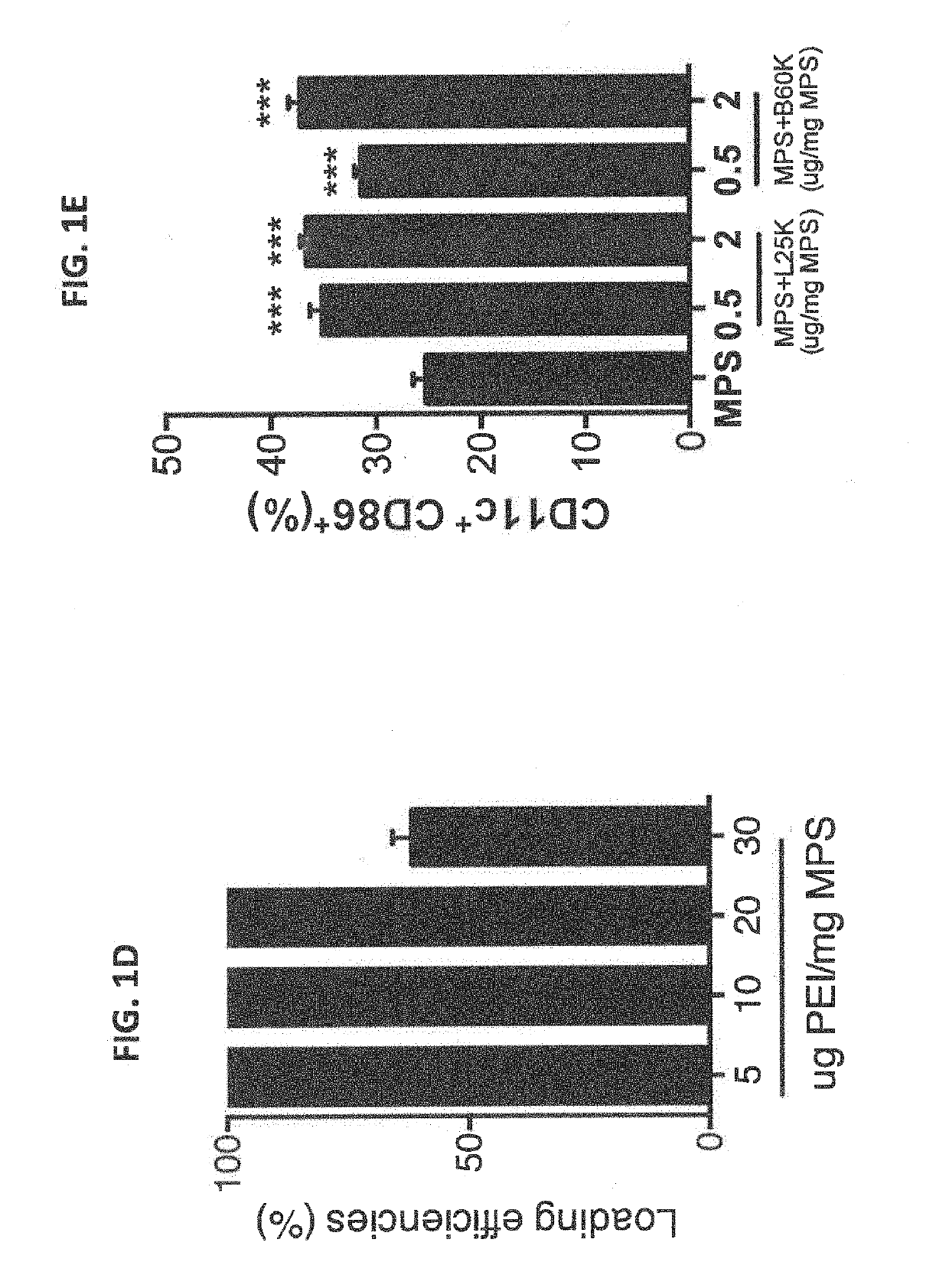
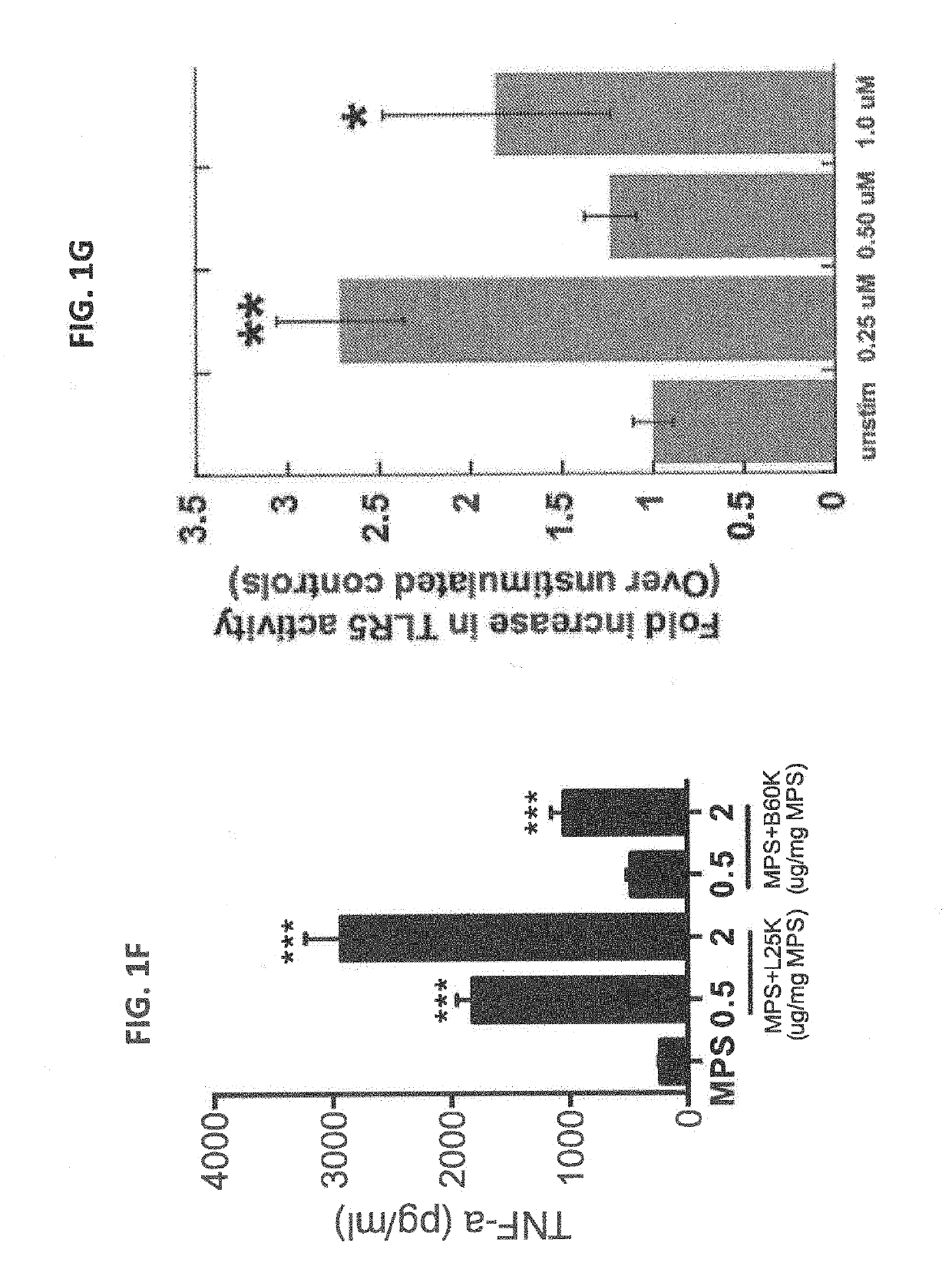
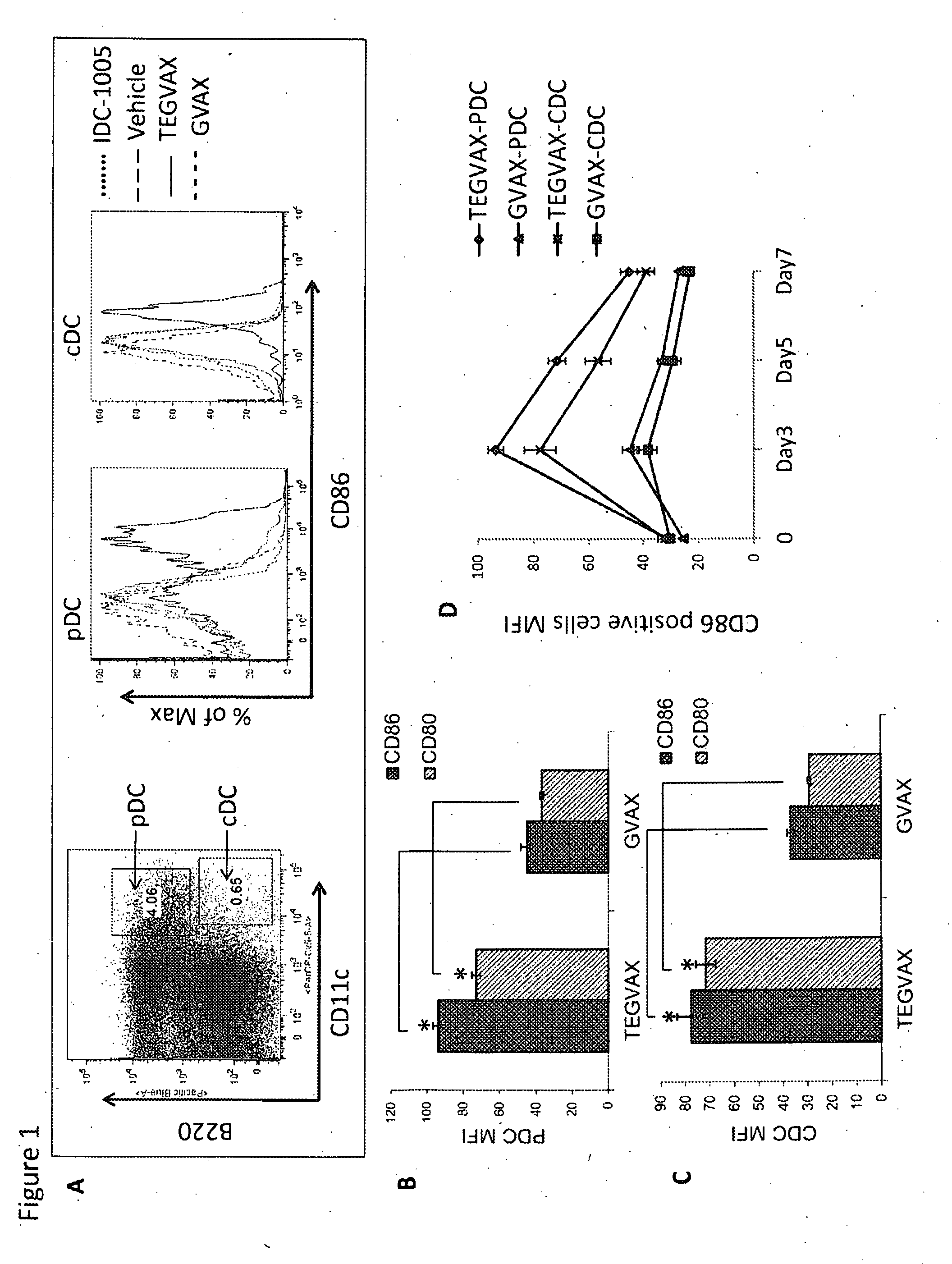
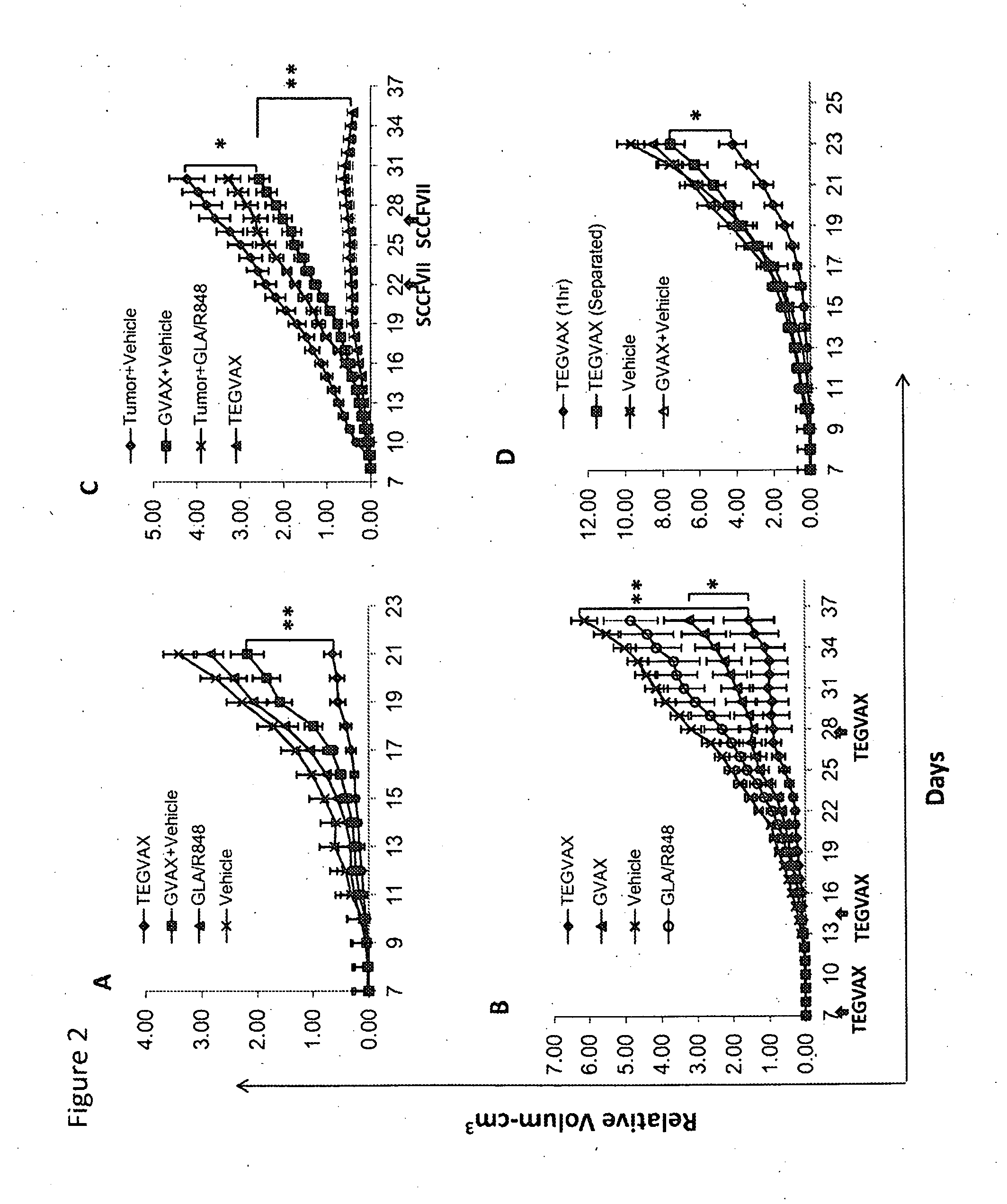
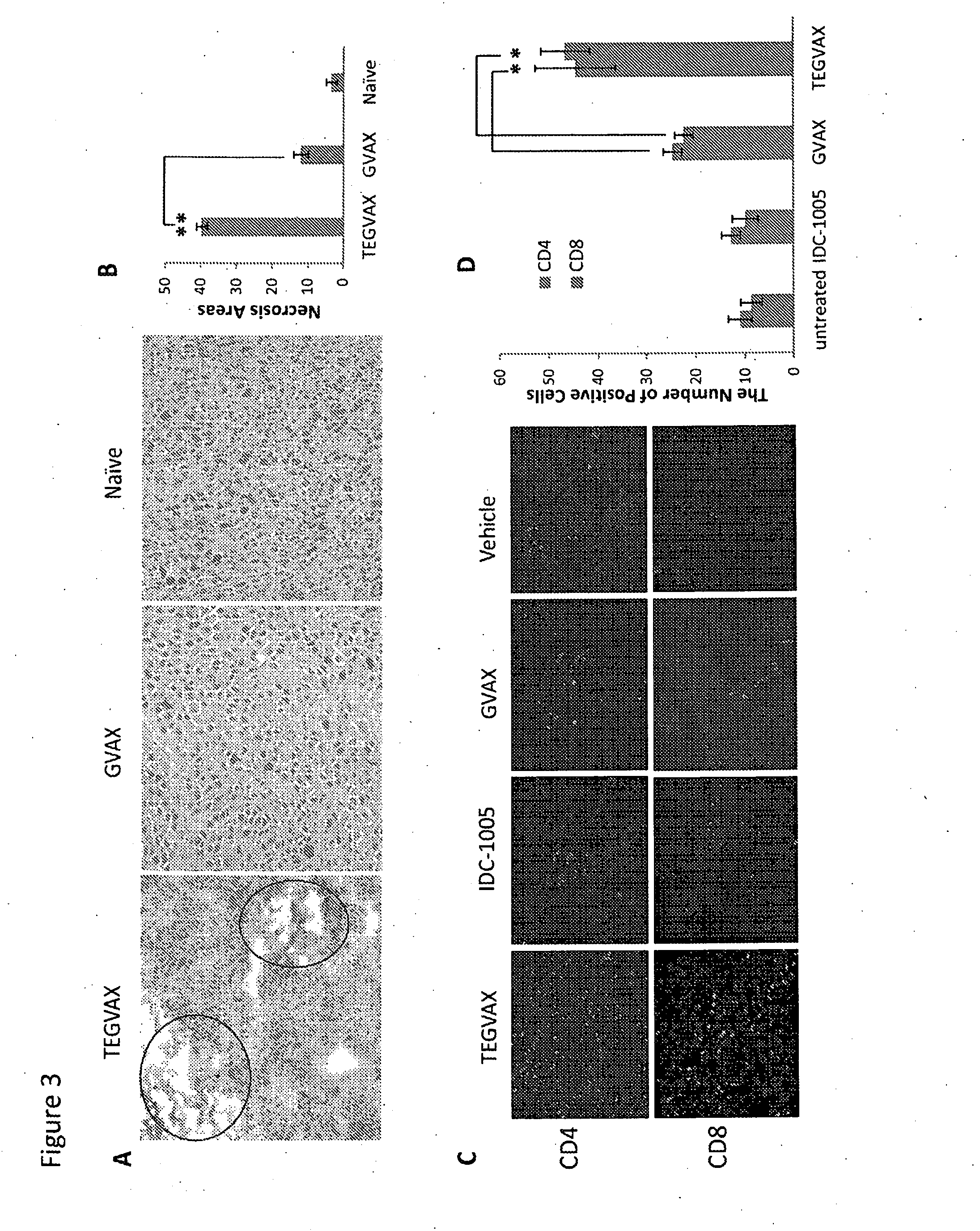
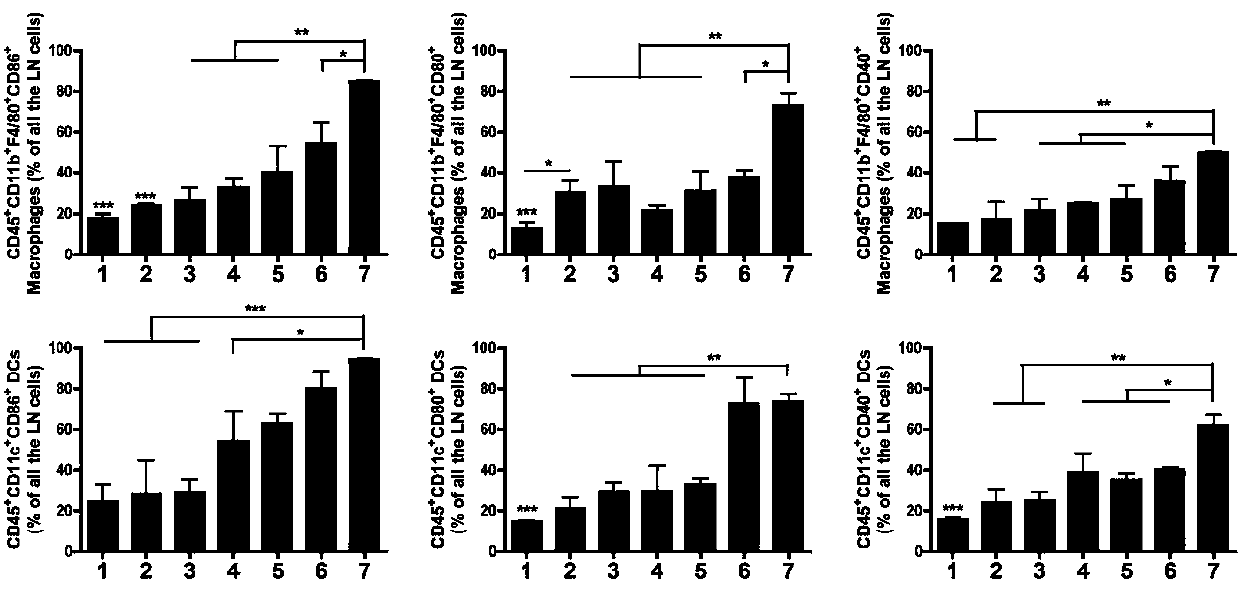
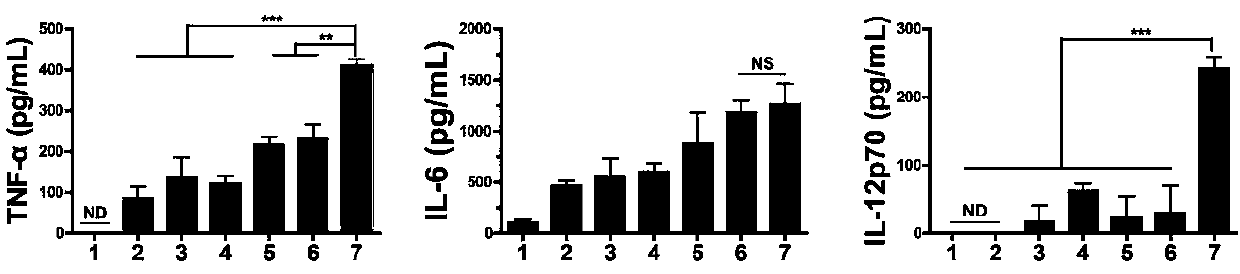
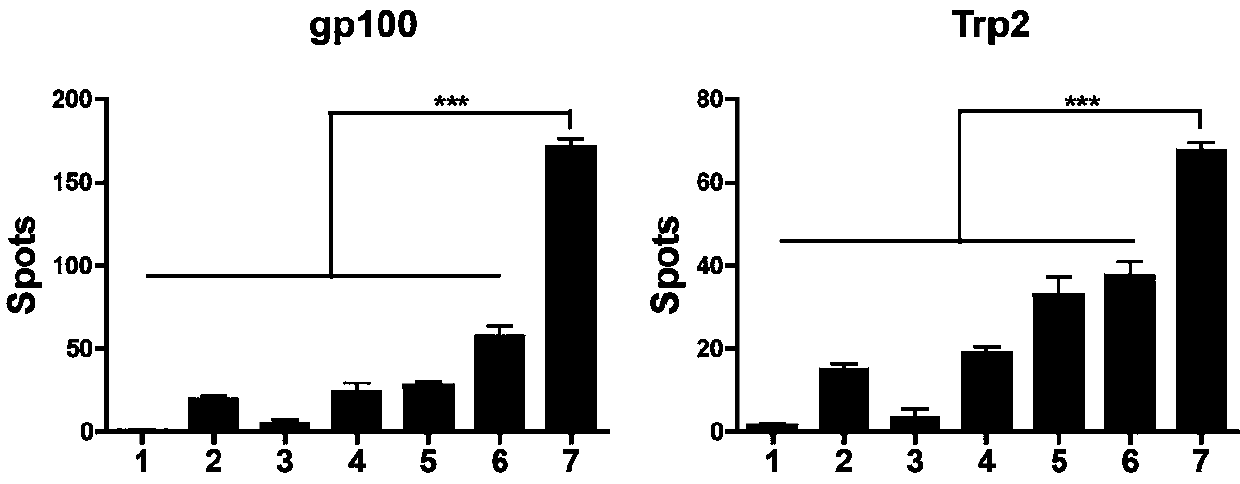
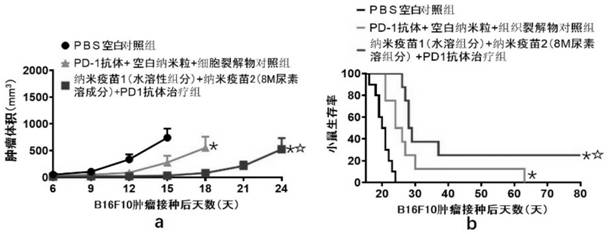
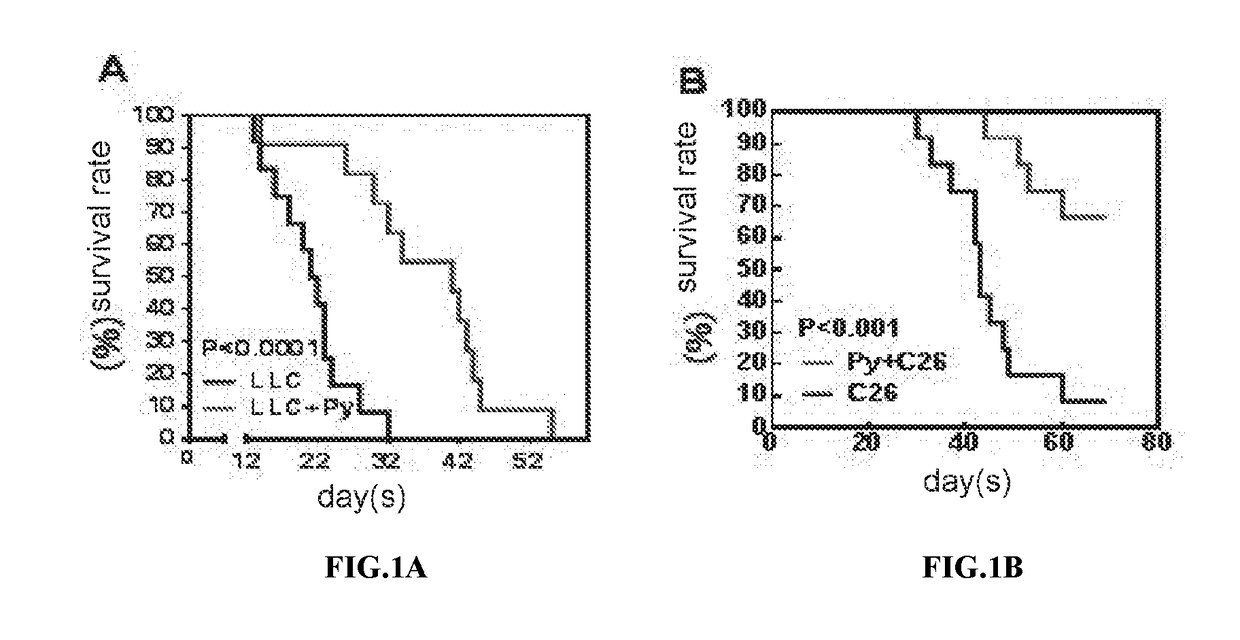
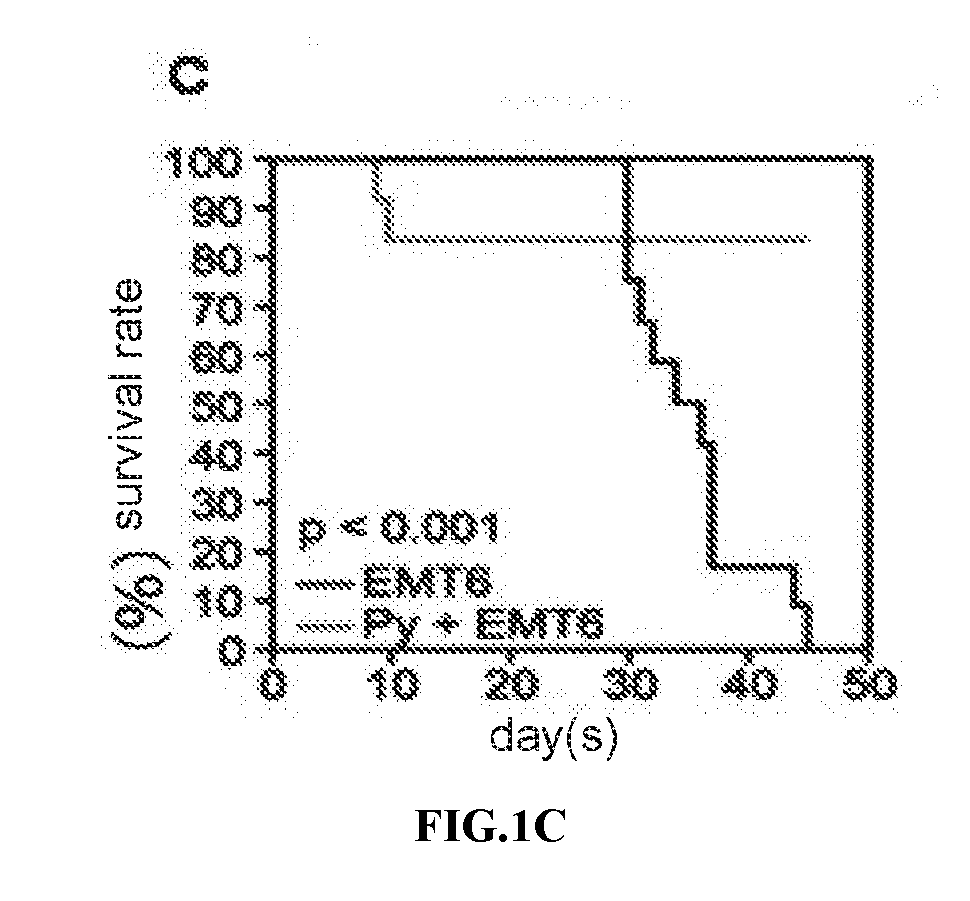
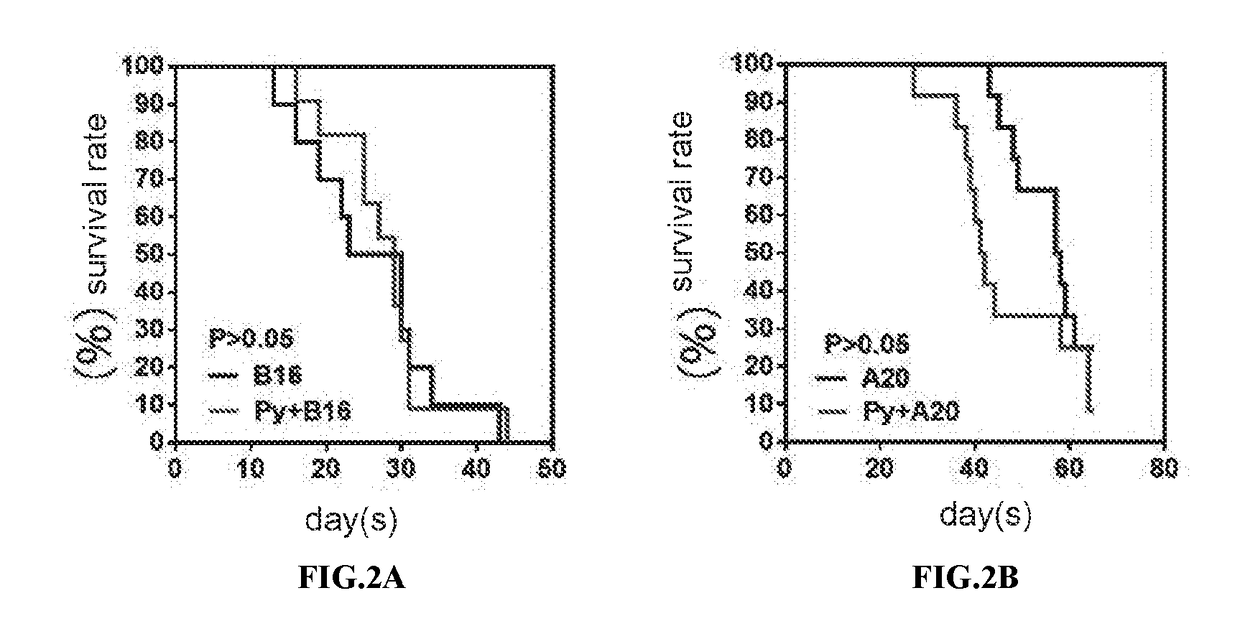
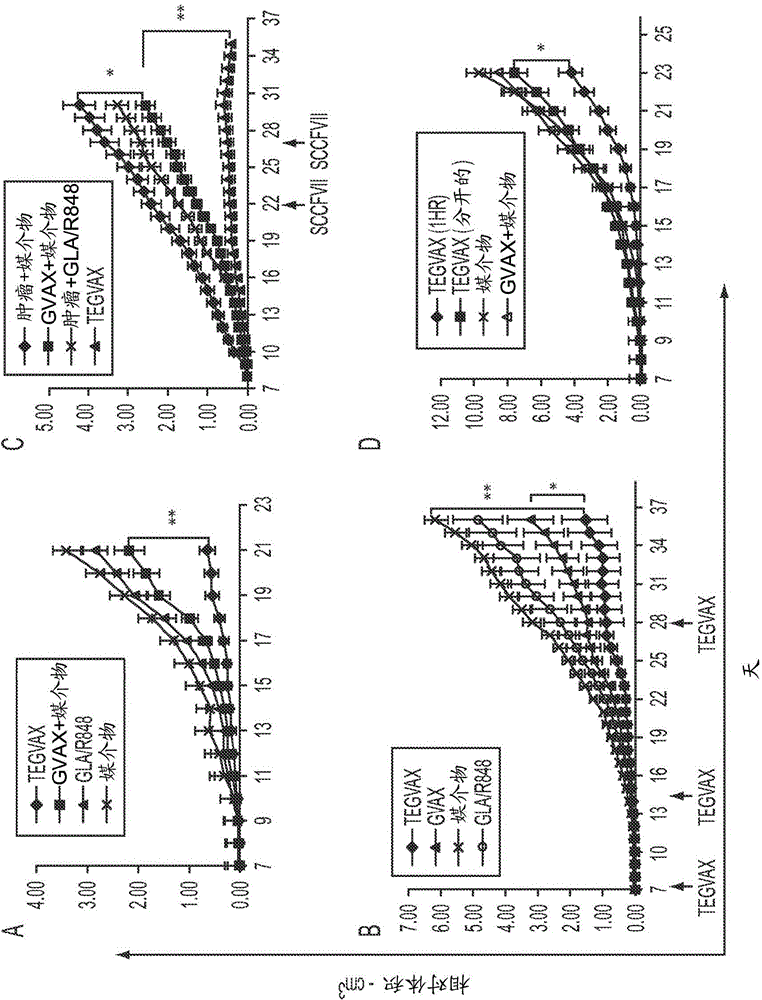
We formulated multiple TLR agonists into GVAX (lethally irradiated tumor cell vaccines engineered to secrete GM-CSF). Specifically, GLA and R848, TLR4 and TLR7 / 8 agonists found to be safe in patients, were formulated with GVAX (TEGVAX—for TLR agonists enhanced GVAX), and this formulation was effective in producing anti-tumor responses in 3 different preclinical models, including palpable B16. These anti-tumor responses were correlated with increased CD4 and CD8 T-cells that can secrete IFNγ circulating in the tumor microenvironment as well as significantly higher level of p15E specific CTL mediated cell killing in mice treated with TEGVAX in comparison to controls. When combined with anti-PD-1 antibody, TEGVAX was able to induce regression of established B16 tumors.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Biomaterials for modulating immune responses
PendingUS20190216910A1Improving immunogenicityReduce the burden onInorganic non-active ingredientsSkin cancer vaccineMicrobiologyBiological organism
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Cancer immunotherapy
InactiveUS20140341978A1Skin cancer vaccineMammal material medical ingredientsAbnormal tissue growthTumor response
We formulated multiple TLR agonists into GVAX (lethally irradiated tumor cell vaccines engineered to secrete GM-CSF). Specifically, GLA and R848, TLR4 and TLR7 / 8 agonists found to be safe in patients, were formulated with GVAX (TEGVAX—for TLR agonists enhanced GVAX), and this formulation was effective in producing anti-tumor responses in 3 different preclinical models, including palpable B16. These anti-tumor responses were correlated with increased CD4 and CD8 T-cells that can secrete IFNγ circulating in the tumor microenvironment as well as significantly higher level of p15E specific CTL mediated cell killing in mice treated with TEGVAX in comparison to controls. When combined with anti-PD-1 antibody, TEGVAX was able to induce regression of established B16 tumors.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Preparation method and application of anti-tumor vaccine based on cell microvesicles
InactiveCN110898215ASufficient quantityImprove bioavailabilitySkin cancer vaccineCancer antigen ingredientsAdjuvantCells/microL
The invention relates to a preparation method and application of an anti-tumor vaccine based on cell microvesicles and aims to effectively solve problems in preparation of anti-tumor vaccines high inyield, good in universality, strong in killing effect on tumor cells and capable of controlling tumors by improving tumor microenvironments, repairing an immune system and enhancing anti-tumor immuneresponse of organisms. The preparation method comprises the following steps: preparing tumor cell microvesicles loaded with immunomodulators and connecting the surfaces of the microvesicles with adjuvant-loaded liposome to stably form the anti-tumor vaccine. The prepared anti-tumor vaccine is high in yield, good in universality, strong in killing effect on tumor cells and capable of improving tumor microenvironments, repairing an immune system and enhancing anti-tumor immune response of organisms; the method is simple in process and high in efficiency; and the prepared microvesicles are derived from cells, are sufficient in quantity and wide in source, are easy for large-scale production, improve the bioavailability of adjuvants, are effectively applicable to preparation of anti-tumor vaccines based on the cell microvesicles, and can be applied to prevention and treatment of different types of tumors.
Owner:ZHENGZHOU UNIV
Use of anti-PD-1 antibody in treatment of tumors
ActiveCN110882385AMicrobiological testing/measurementSkin cancer vaccineAntiendomysial antibodiesAntigen Binding Fragment
The present invention relates to the use of an anti-PD-1 antibody in the treatment of tumors. The invention also relates to use of a reagent for detecting the state of CD8 + T cells and / or NK cells inperipheral blood or a reagent for detecting EMSY genes in a detection kit for predicting the treatment effect of a tumor patient on the anti-PD-1 antibody and / or an antigen binding fragment thereof.
Owner:SHANGHAI JUNSHI BIOSCI
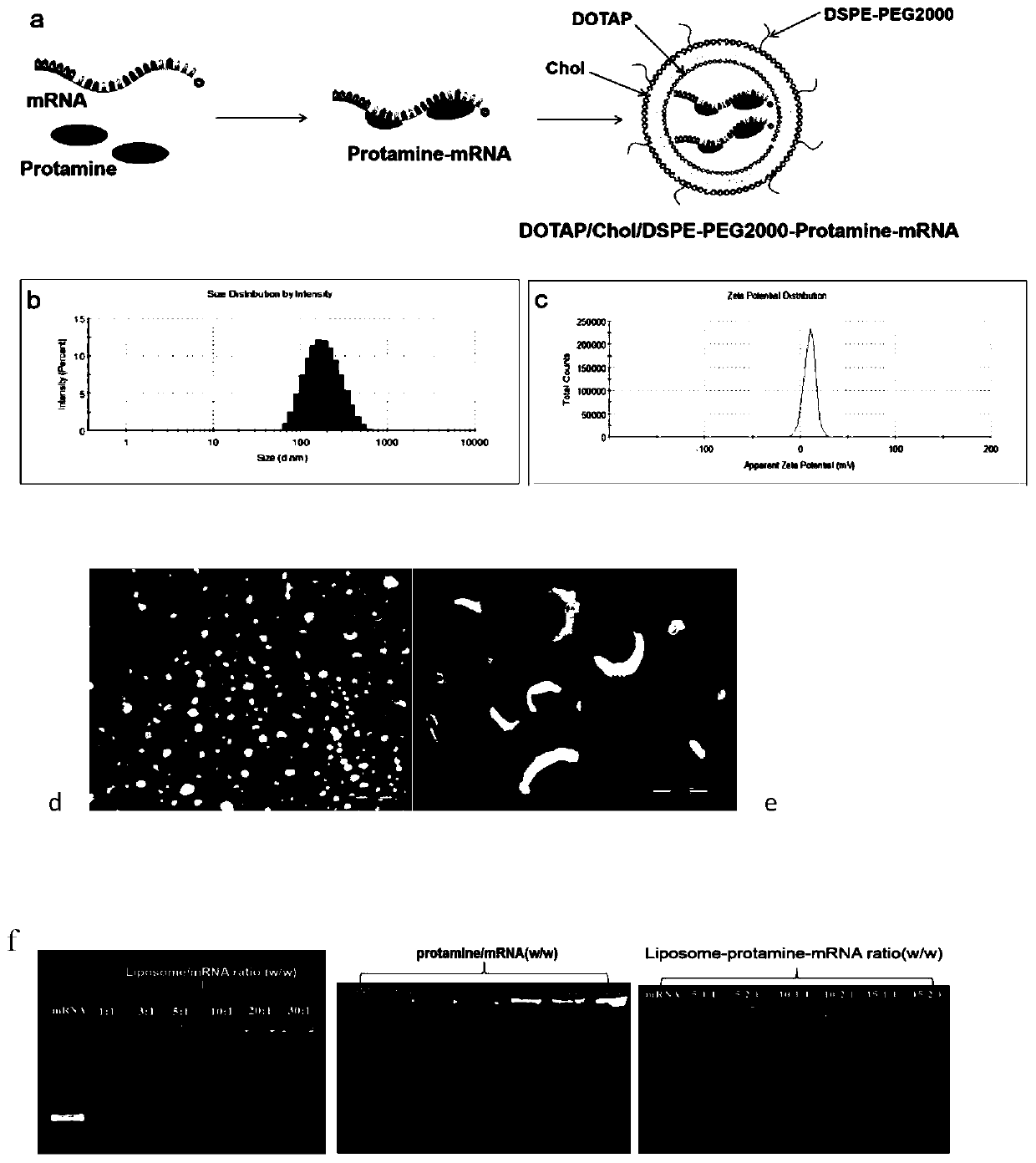
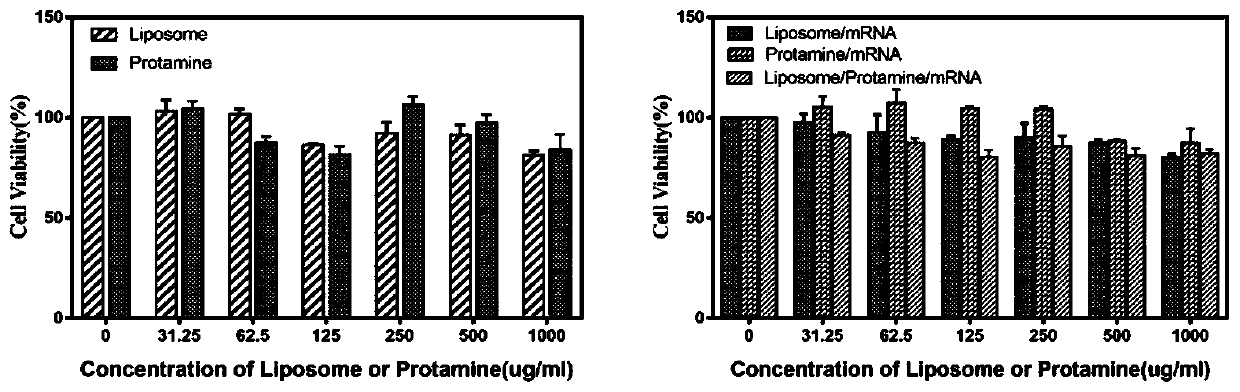
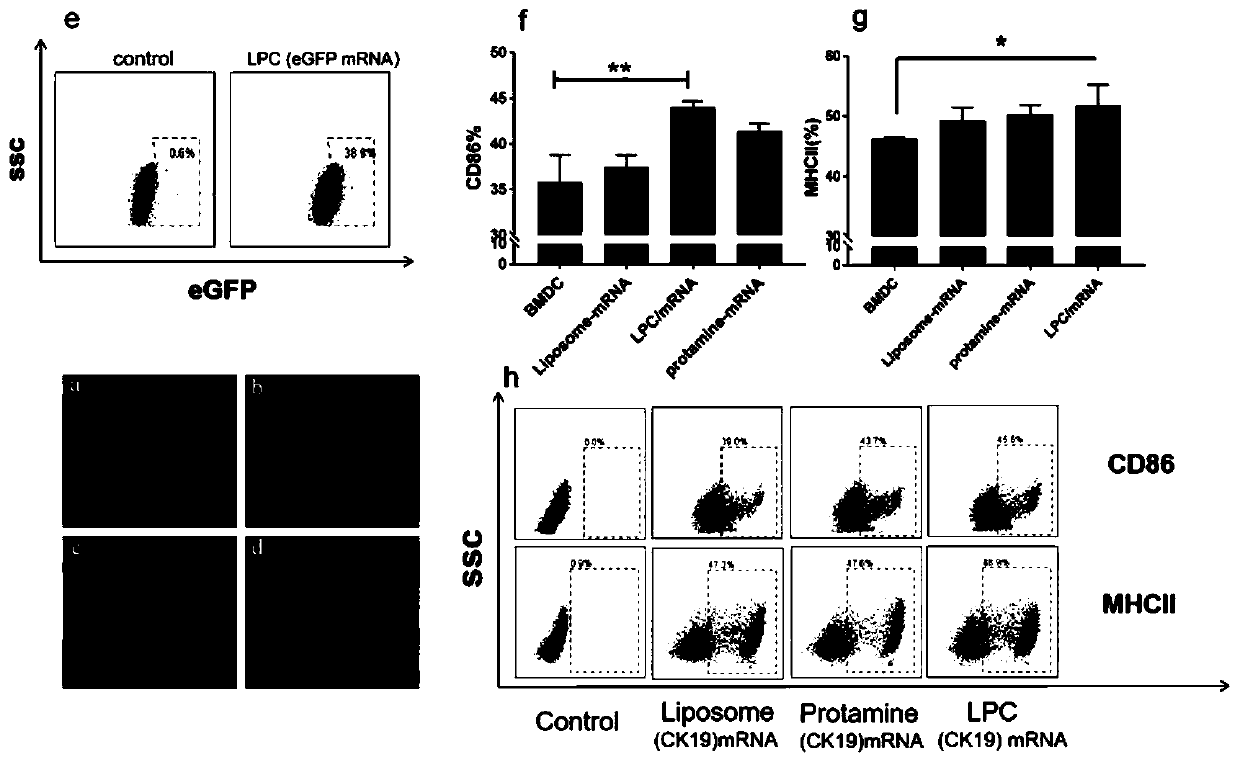
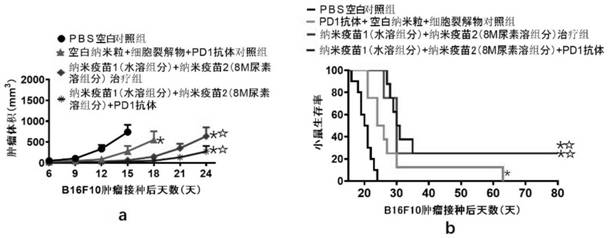
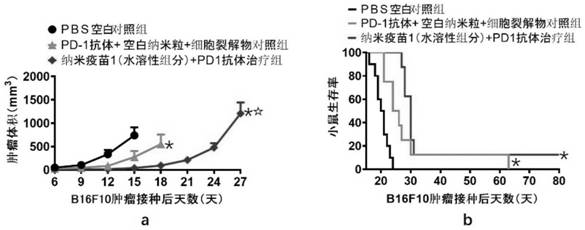
Cationic liposome-protamine-mRNA tumor vaccine and preparation method and application method thereof
PendingCN110882383ASynthesis fastRapid purificationSkin cancer vaccineCancer antigen ingredientsAdjuvantTumor therapy
The invention relates to a cationic liposome-protamine-mRNA tumor vaccine and a preparation method and an application method thereof. According to the tumor vaccine, a cationic liposome-protamine compound is used as a delivery carrier and adjuvant of mRNA, wherein the mass ratio of the cationic liposome to protamine to mRNA is 5:1: 1 to 15: 2:1, and the optimal mass ratio is preferably 10:1:1; theparticle size of the constructed cationic liposome-protamine-mRNA tumor vaccine is 50 to 800nm; the Zeta potential is 10 to 60mv; and the encapsulation efficiency is 70% to 95%. The cationic liposome-protamine-mRNA tumor vaccine prepared by the invention can effectively promote antigen uptake of APCs, induce DC stimulation and maturation, promotes secretion of cytokines and cause anti-tumor immune response. The tumor vaccine is used for immunotherapy through intramuscular and subcutaneous injection or nasal mucosa administration, wherein nasal mucosa administration can show a more excellent tumor treatment effect. The tumor vaccine has a wide application prospect in the aspect of tumor treatment.
Owner:NINGXIA MEDICAL UNIV
Whole-cell component conveying system and application thereof
The application belongs to the field of immunotherapy and discloses a conveying system for conveying water-soluble components and non-water-soluble components of whole-cell components by using nano-scale or micron-scale particles and application of the conveying system in preparation of vaccines for preventing and treating cancers. The whole-cell component conveying system is composed of the nano-scale or micron-scale particles and the whole-cell components loaded by the particles, wherein the whole-cell components are water-soluble components and non-water-soluble components of whole cells in cells or tissues. The water-soluble components and the non-water-soluble components are loaded in the nano particles or the micron particles, so that variant proteins or polypeptides generated by the cancers in the cell components are loaded in the nano particles or the micron particles. Substances with immunogenicity, which are generated due to disease mutation, in the whole-cell components can be used for preventing and treating the cancers. The whole-cell component conveying system can be used for preparing the vaccines for preventing and / or treating the cancers.
Owner:SUZHOU ERSHENG BIOPHARMACEUTICAL CO LTD
Compositions and methods for selected tumour treatment
InactiveUS20050063995A1Reduce decreaseShorten the progressEnergy modified materialsColon cancer vaccineAbnormal tissue growthMelanoma
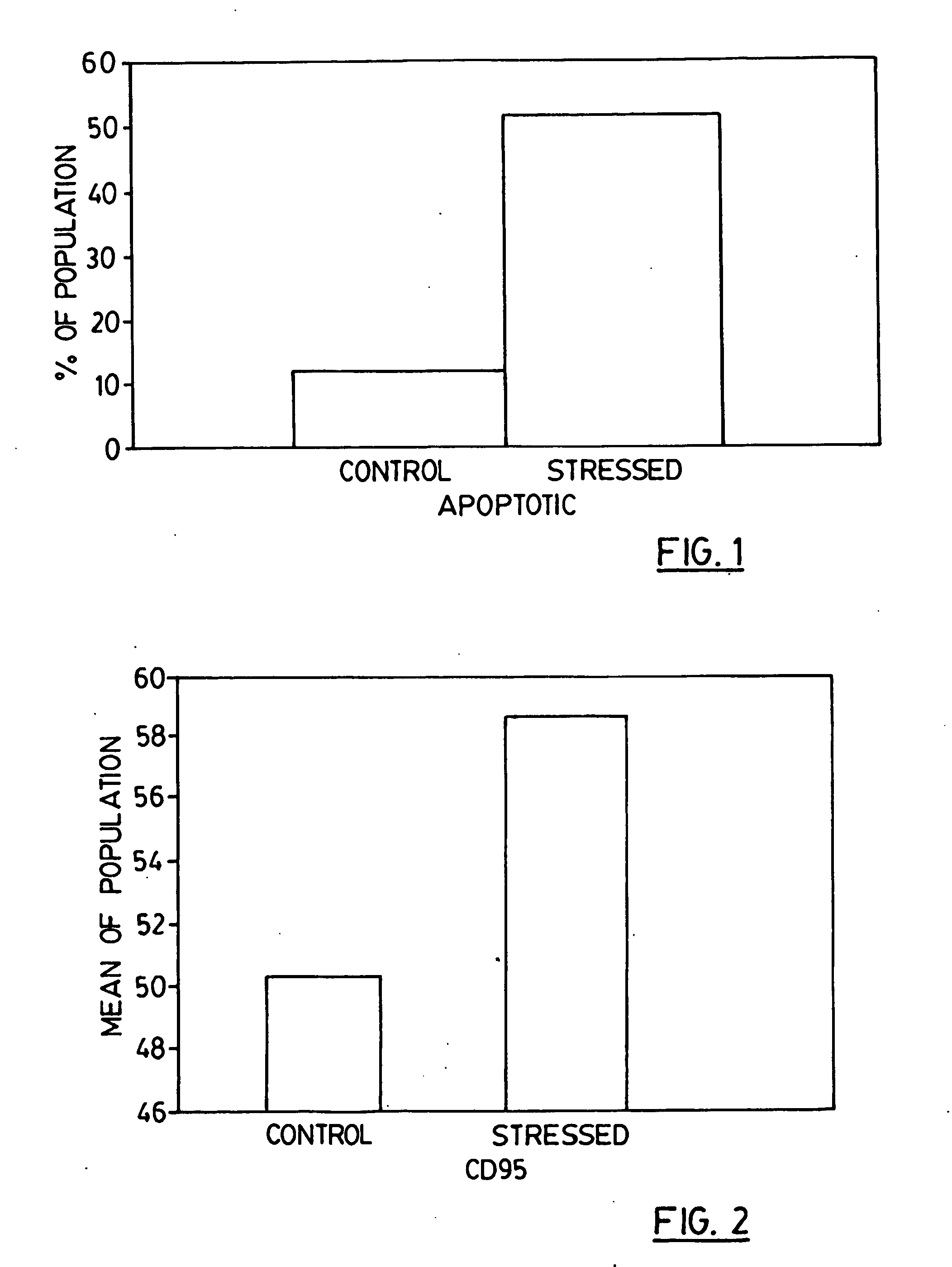
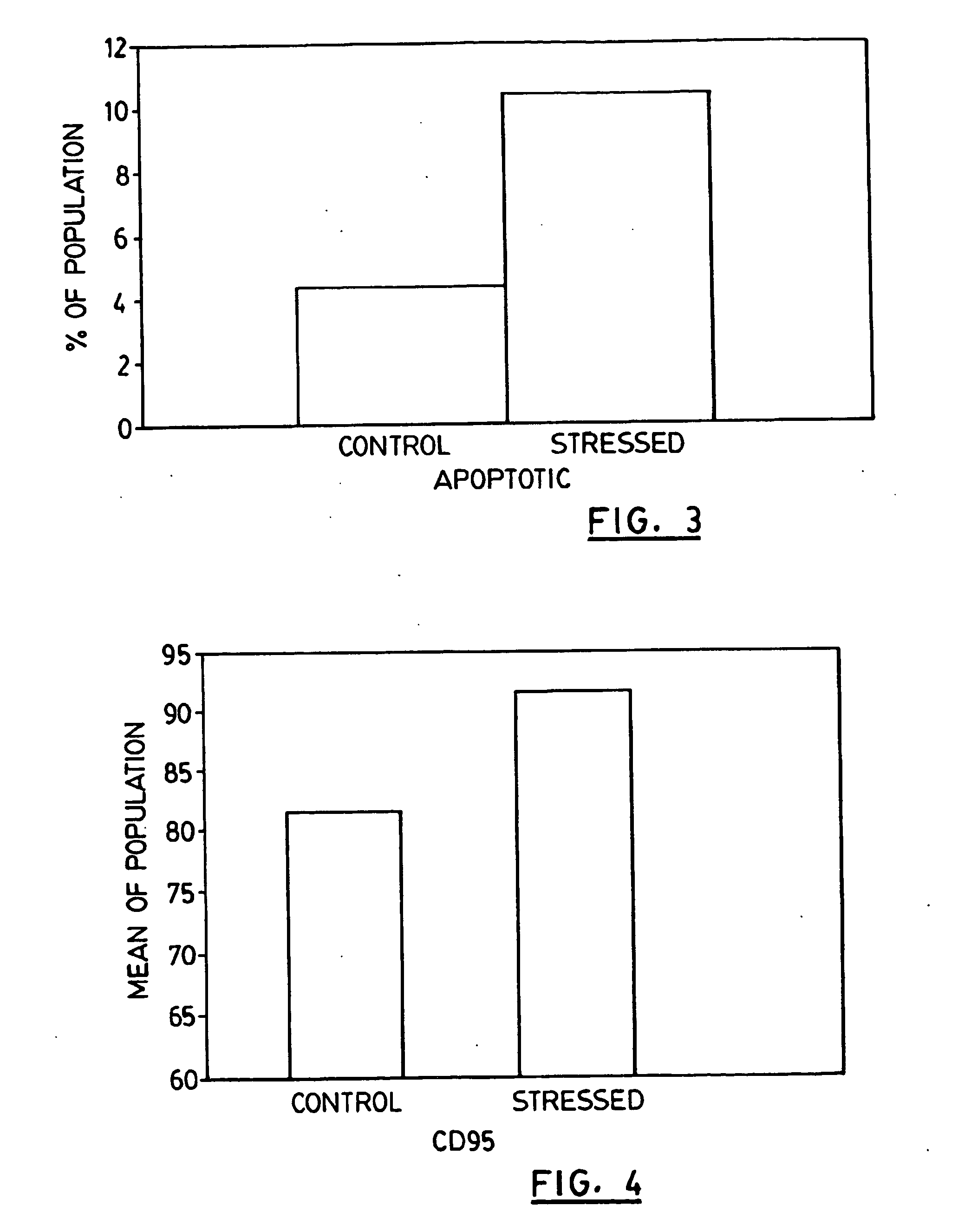
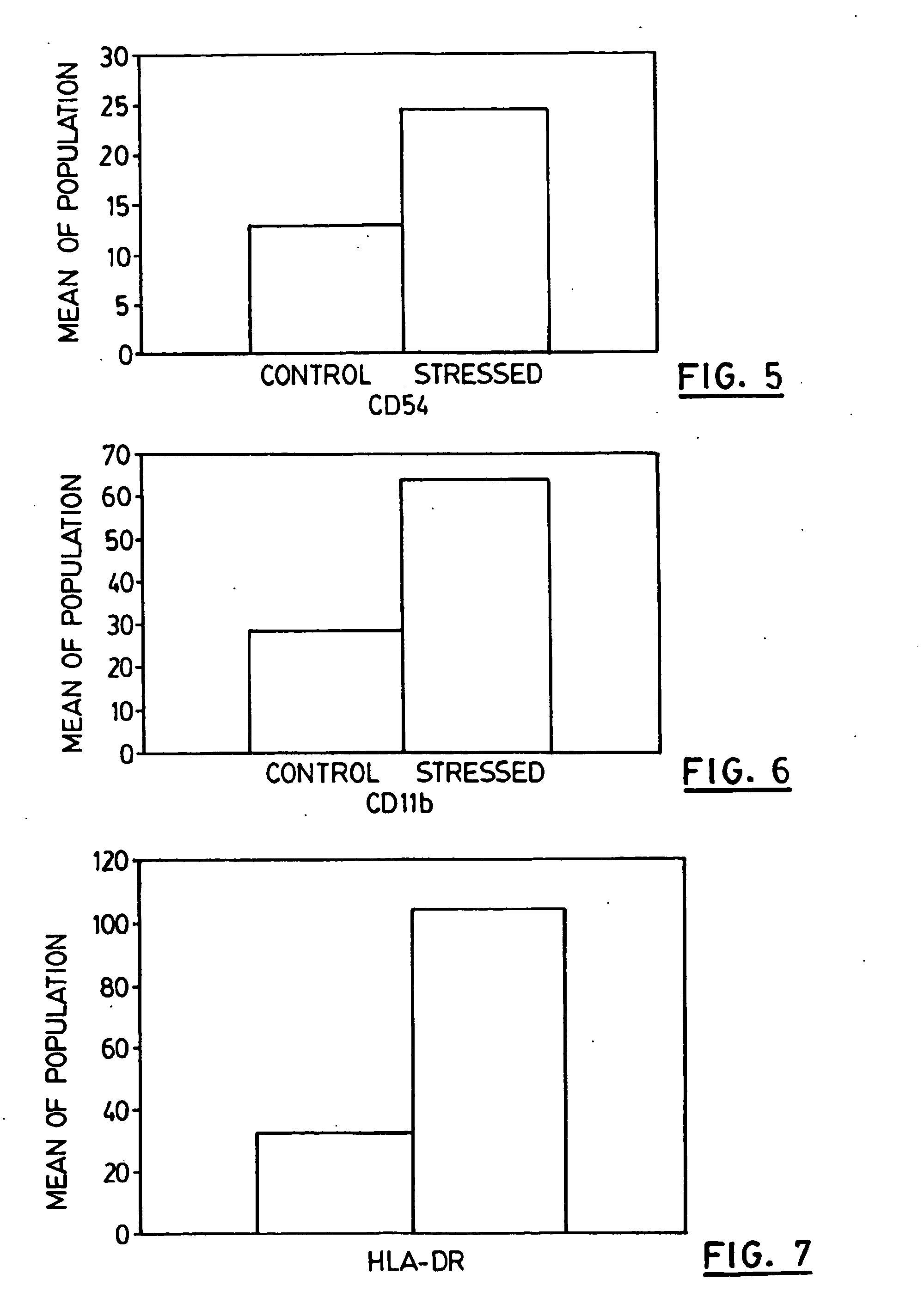
Disclosed are novel compositions, methods and vaccines, which upon administration to a patient suffering from a melanoma, colon carcinoma tumor or breast cancer, postpone and / or reduce the need for chemotherapy treatment, slow the progression of or eliminate the tumor and / or alleviate the symptoms of the tumor. The compositions comprise stressed colon carcinoma, melanoma or breast cancer cells, preferably autologous such cells.
Owner:VASOGEN IRELAND LTD
A Composition, A Treatment Method and An Application Thereof
InactiveUS20190015458A1Good effectImprove efficiencyColon cancer vaccineProtozoa material medical ingredientsMelanomaTreatment field
The present invention relates to the field of treatment of tumor, and especially to a composition comprising a plasmodium, a treatment method and an application thereof. The composition of the present invention has therapeutic effects on colorectal carcinoma, lung carcinoma, breast carcinoma, gastric carcinoma and hepatic carcinoma etc., can inhibit the growth of tumor and prolong the life of the tumor patients, whereas has no therapeutic effect on melanoma and lymphoma; meanwhile, the present invention describes that the long-term plasmodium infection has better therapeutic effect on tumors, and the plasmodium immunotherapy of the present invention does not take the fever time as a course standard when treating tumors, but should be used to extend the duration of plasmodium infection as much as possible until the progression of tumors can be controlled under the premise of protecting the organ functions and life safety of the patients.
Owner:BLUE ELEGANT BIOTECH CO LTD
Tumor cells from immune privileged sites as base cells for cell-based cancer vaccines
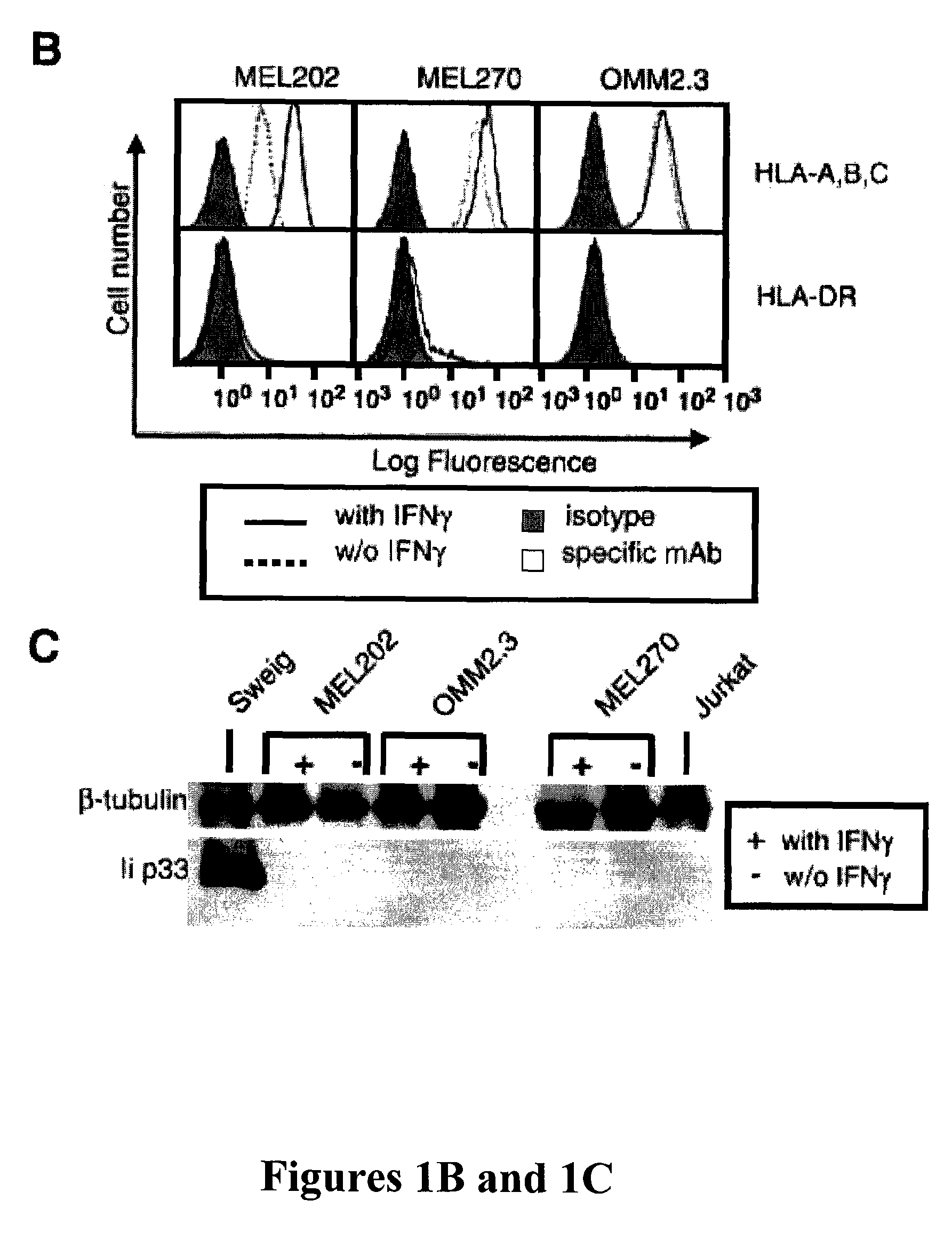
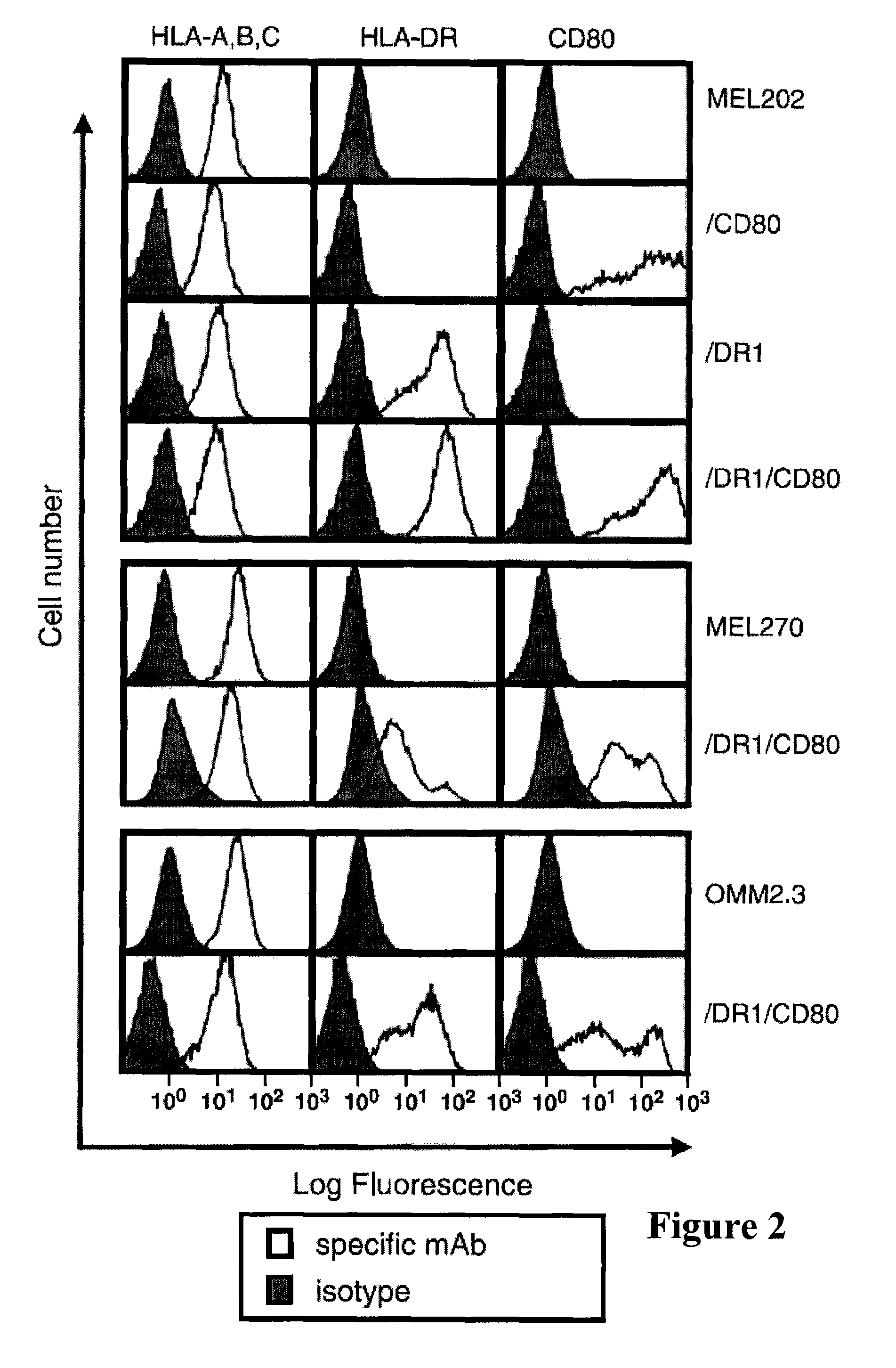
The present invention relates to tumor cell-based vaccines and methods of using same, wherein the vaccines are based on naturally immune privileged tumor cells that have been genetically modified to express MHC-II restricted peptides derived from endogenously encoded tumor antigens, activate CD4+ T-lymphocytes, provide an array of antigens to which the host is not tolerized and / or induce immunity against the originating tumor cells as well as against metastatic tumor cells.
Owner:UNIV OF MARYLAND BALTIMORE COUNTY
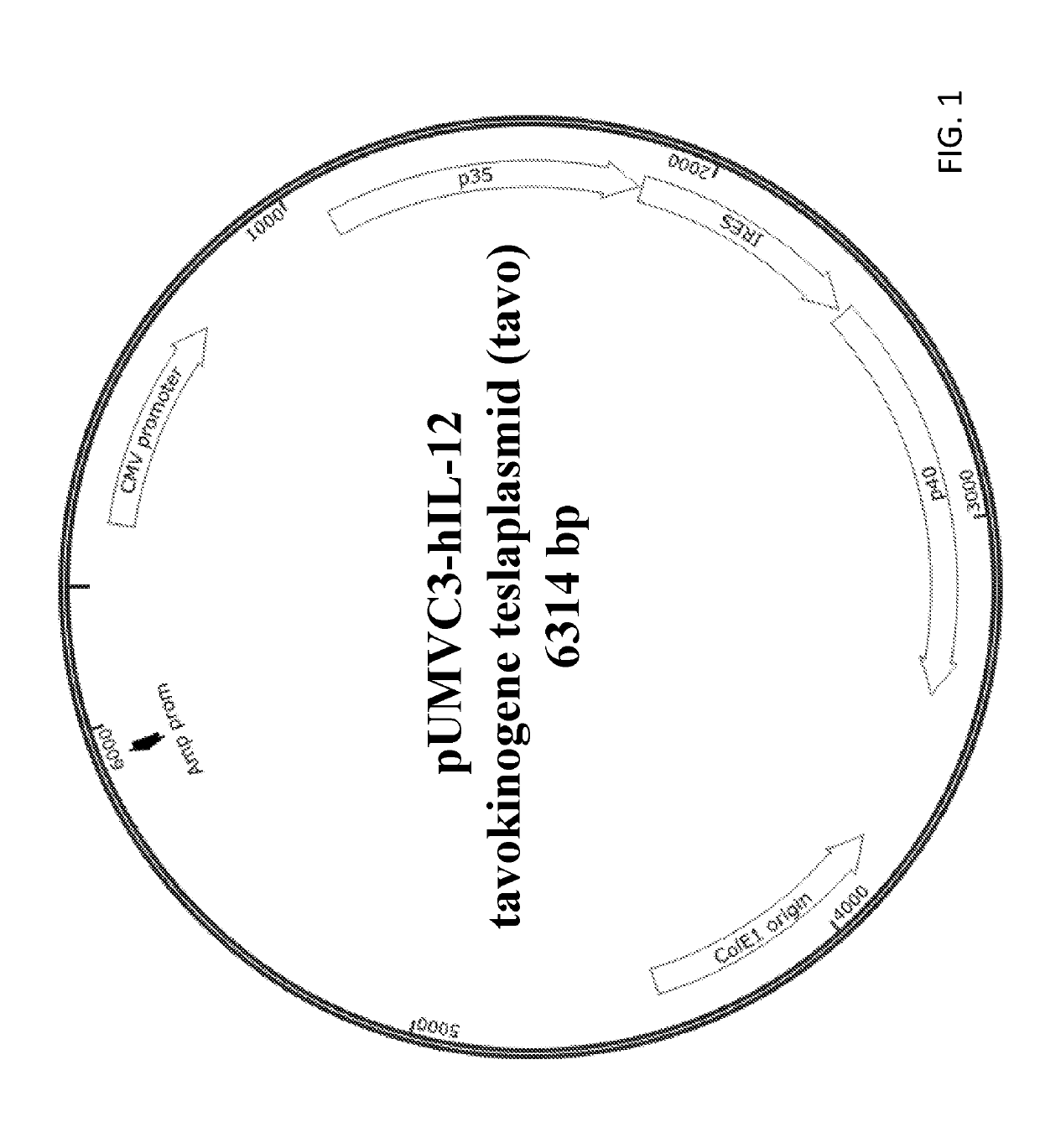
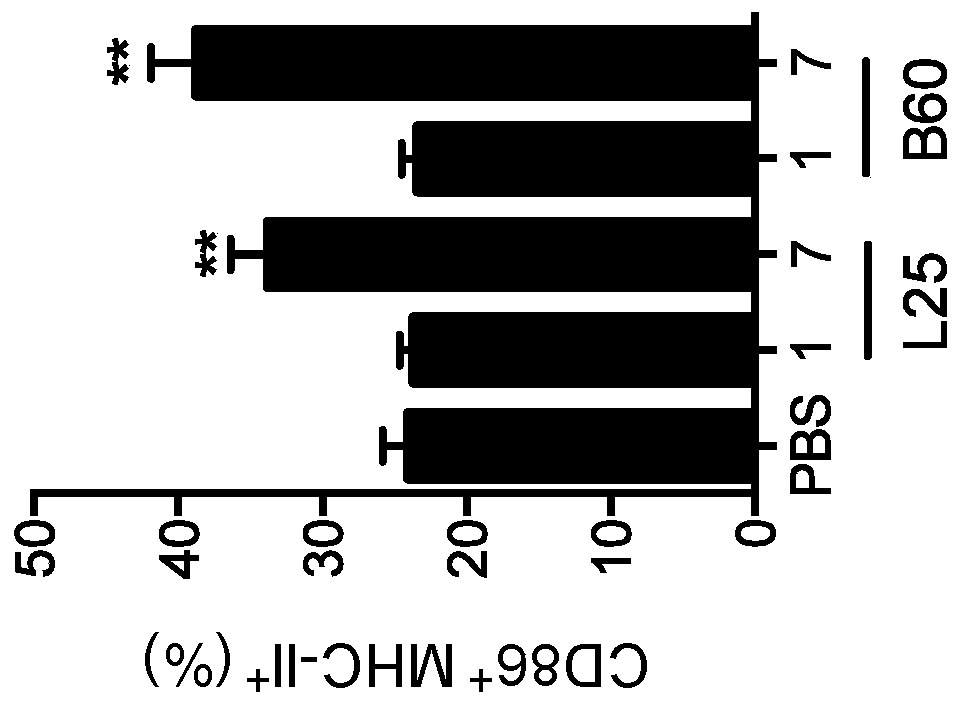
Modulating responses to checkpoint inhibitor therapy
The present invention provides for a dosing schedule for the intratumoral delivery of an immunostimulatory cytokine in combination with systemic delivery of a checkpoint inhibitor. In particular, it provides delivery of a plasmid encoding the immunostimulatory cytokine, e.g., IL-12, using intratumoral electroporation, and the systemic delivery of a PD-1 antagonist.
Owner:RGT UNIV OF CALIFORNIA +1
Biomaterials for modulating immune responses
PendingCN110418651AInorganic non-active ingredientsSkin cancer vaccineMicrobiologyBiological organism
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Improved cell compositions and methods for cancer therapy
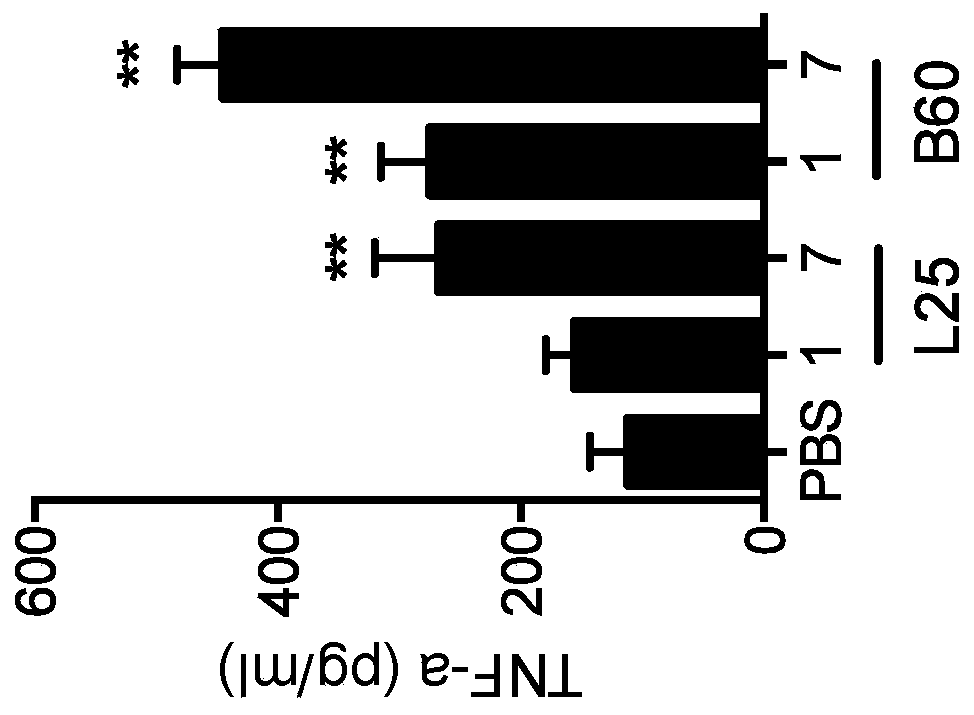
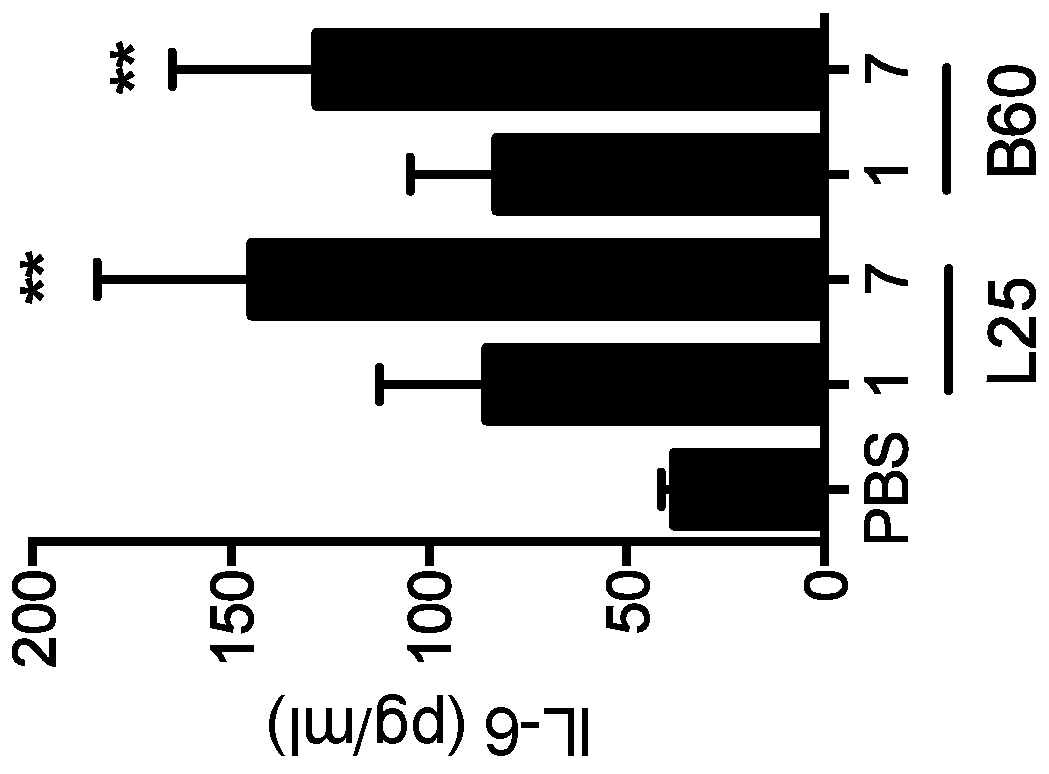
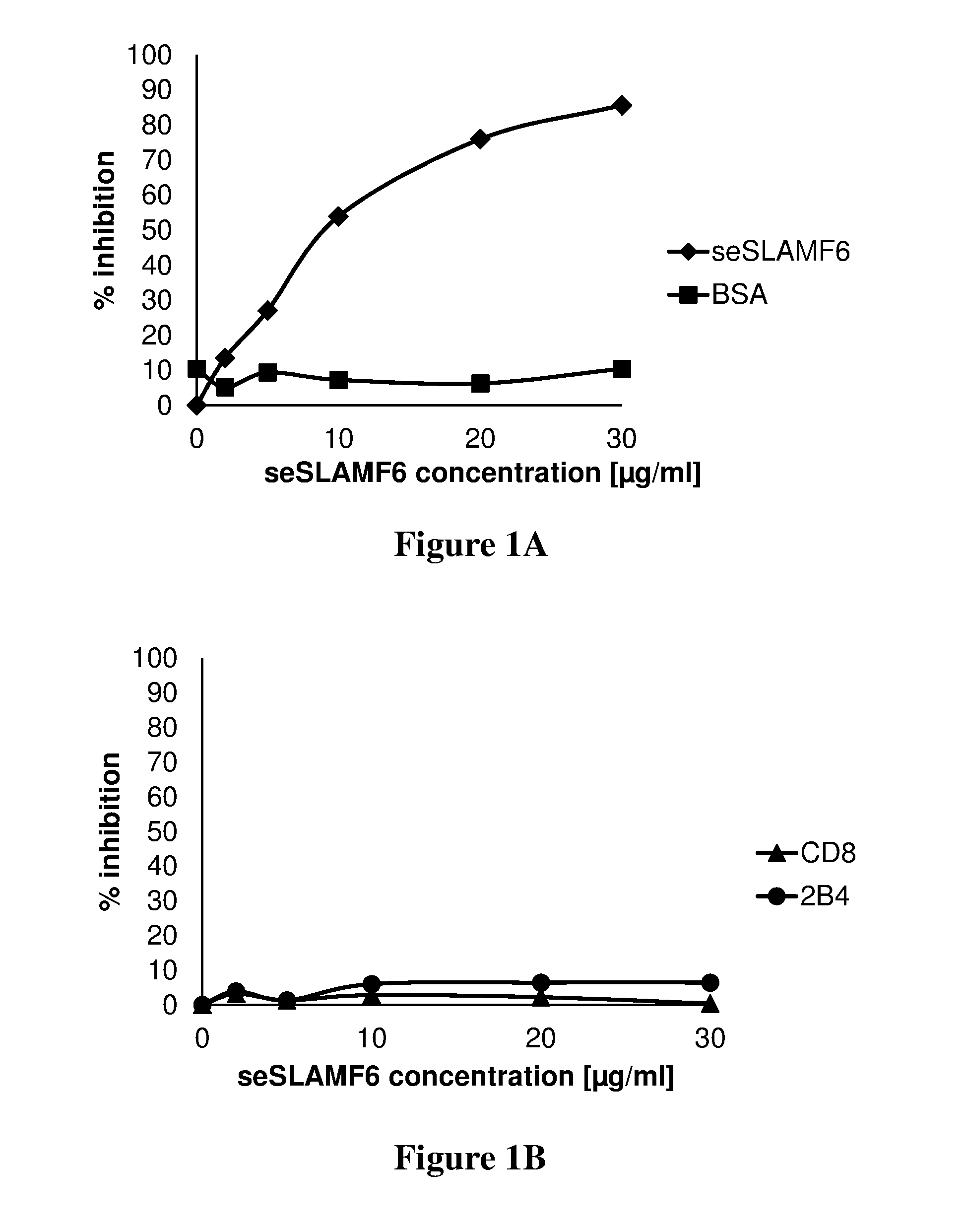
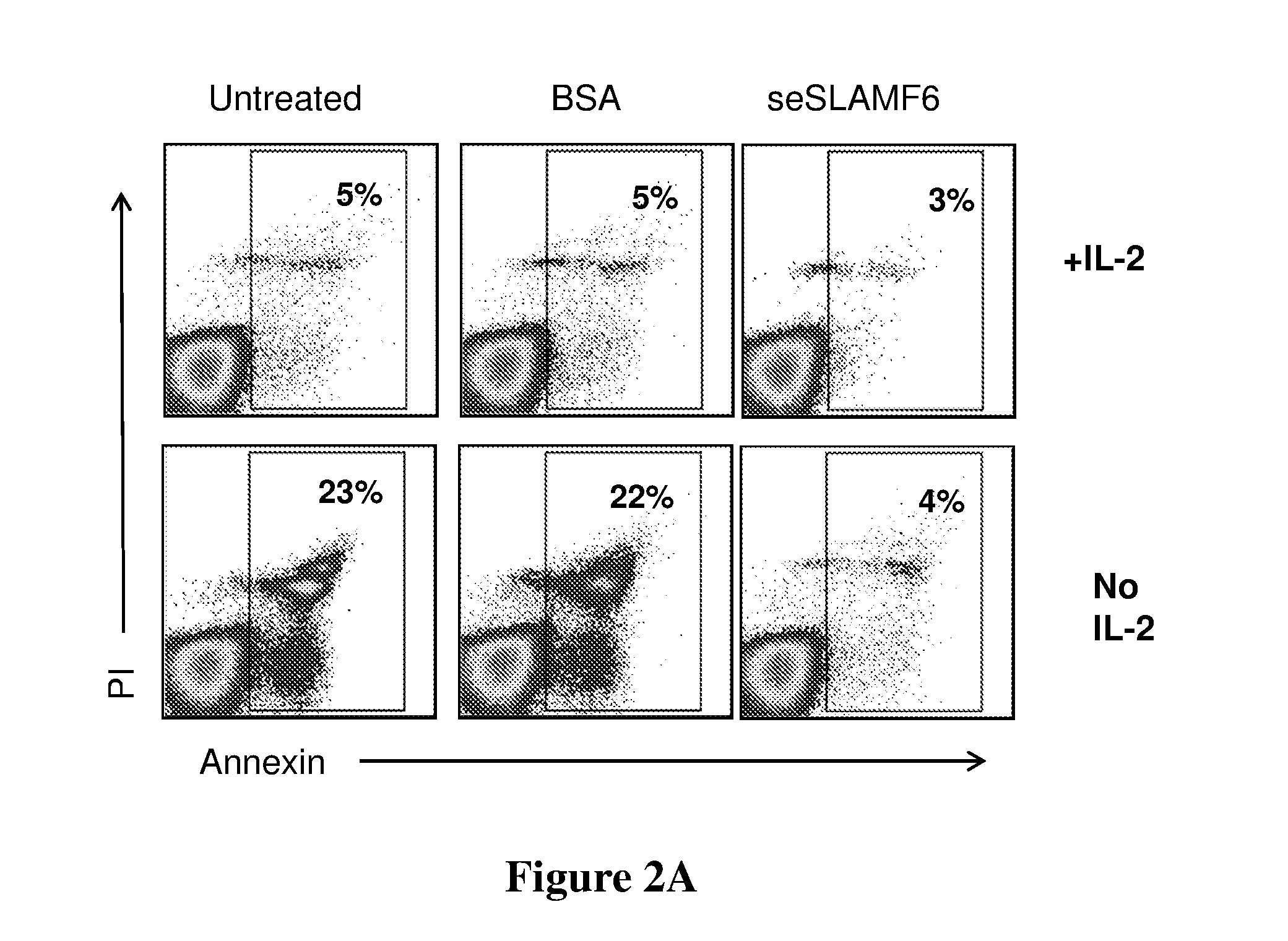
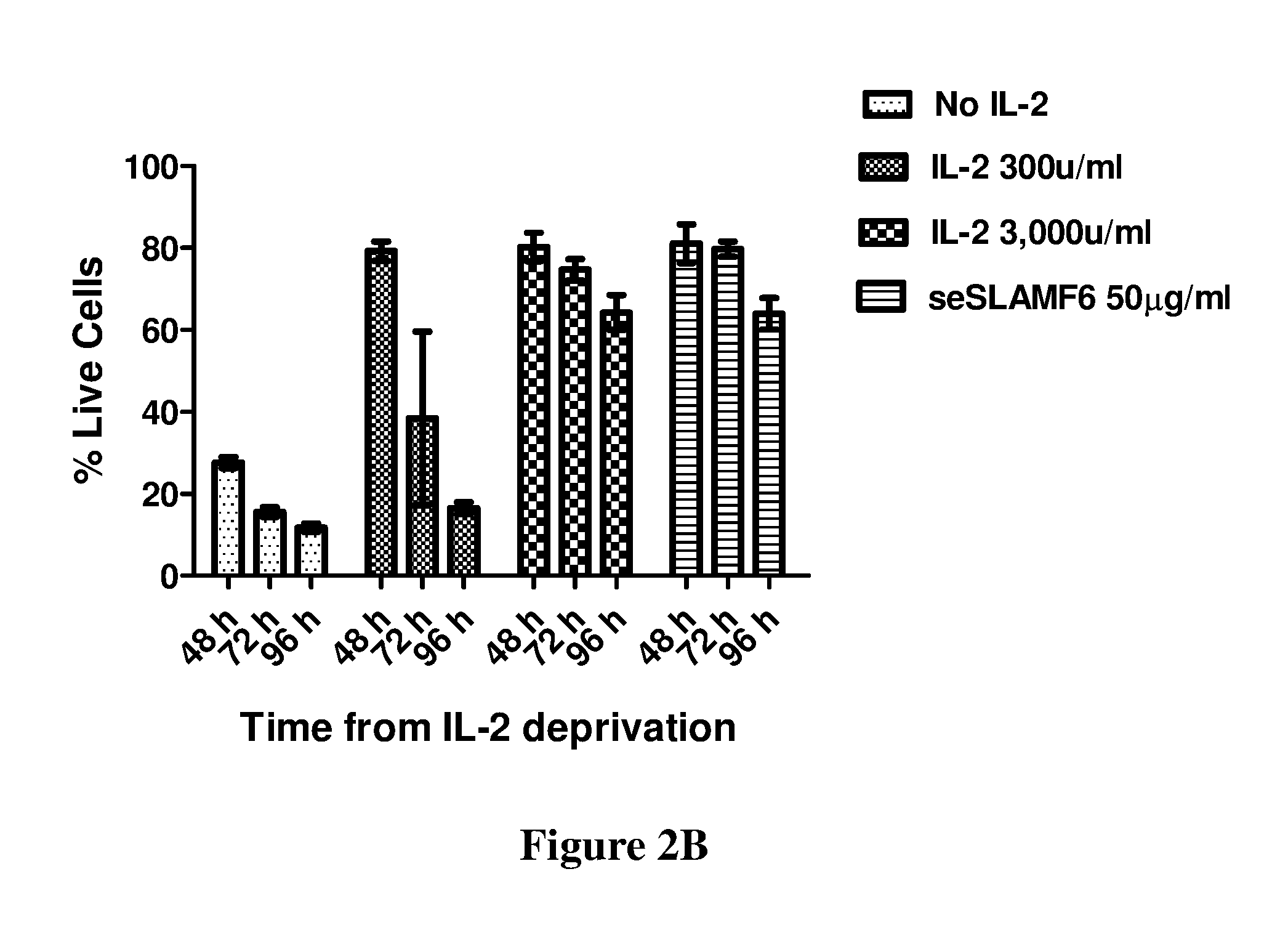
ActiveUS20160333072A1Improved T cell modulationPreventing and treating cytopeniaImmunoglobulin superfamilyPeptide/protein ingredientsTreatment fieldSubject matter
Provided is directed to the field of immunotherapy. Specifically, provided are compositions and methods for improved T cell modulation ex vivo and in vivo and for the treatment of cancer and other pathologies. More specifically, embodiments of the subject matter are directed to the use of soluble NTB-A polypeptides or agonists thereof for the treatment of cancer patients, for preventing and treating cytopenia in susceptible patients, and for the ex vivo preparation of improved cell compositions.
Owner:HADASIT MEDICAL RES SERVICES & DEVMENT
Tumor treatment drug
ActiveCN111467489AAdaptableHigh cure rateSsRNA viruses negative-senseVirus peptidesTumor therapyTherapeutic effect
The invention belongs to the field of biotechnology, and particularly relates to an oncolytic virus vaccine and a tumor treatment drug combing oncolytic virus vaccine with an immune checkpoint inhibitor. A brand-new oncolytic virus attenuated strain is provided by site-directed mutagenesis of wild-type virus matrix protein M of VSV (vesicular stomatitis virus). The attenuated strain can be used asa drug alone to treat tumors, and has safety and cure rate higher than those of wild-type viruses and other known attenuated strains. On the basis of the oncolytic virus attenuated strain, a vaccinethat can be used in tumor treatment is also provided by inserting NY-ESO-1 into the attenuated strain. The vaccine has high cure rate and high biological safety. On the basis of the vaccine, the invention also combines the vaccine with the immune checkpoint inhibitor to provide the drug capable of efficiently treating various tumors. In mouse lung cancer models, the cure rate can be astonishing, up to 87.5%, and the treatment effect on large tumors is also good.
Owner:JOINT BIOSCIENCES (SH) LTD
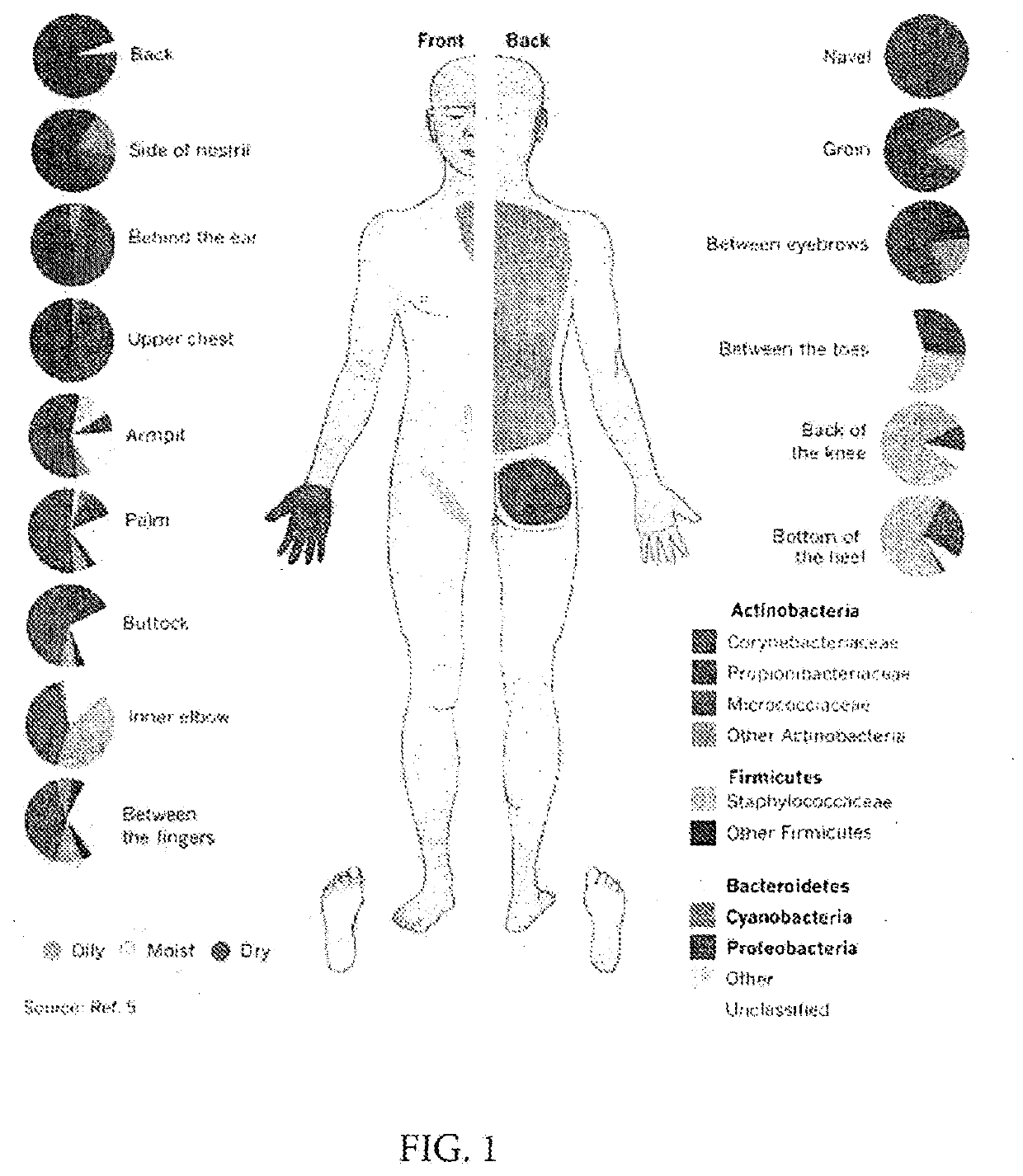
Reducing the likelihood of skin cancer in an individual human being
ActiveUS10835560B2Encourage jammingGood for healthOrganic active ingredientsBacterial antigen ingredientsMicroorganismSkin contact
Compositions, systems and methods of improving the health of the microbiome of an individual's skin relate to the provision of skin contacting formulations containing beneficial bacteria and other microbe components to foster the growth and maintenance of a healthy skin microbiome.
Owner:SEED HEALTH INC
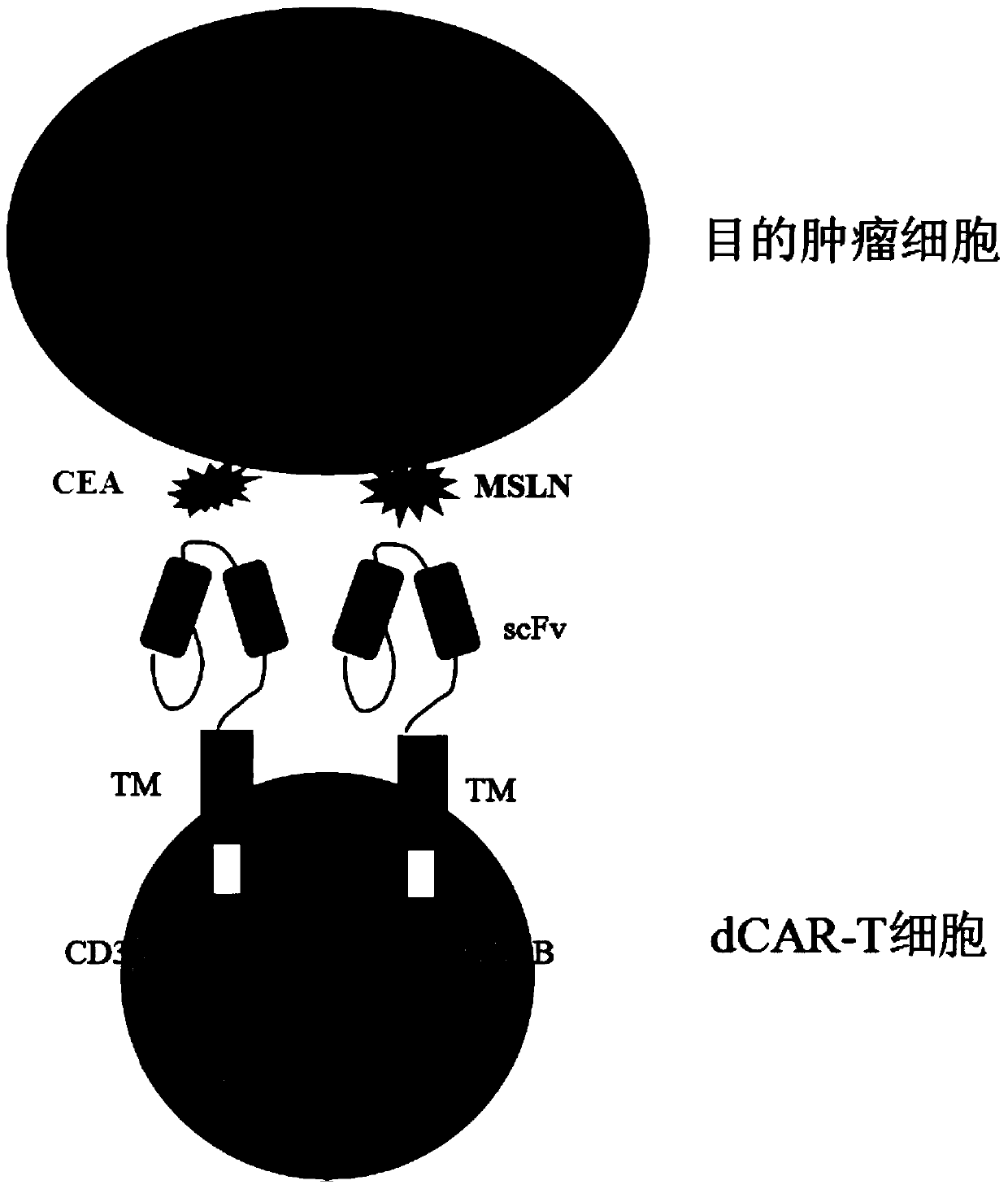
Double-chimeric antigen receptor, T cell and construction method and application thereof
PendingCN110669138AToxicityImprove securityImmunoglobulins against cell receptors/antigens/surface-determinantsBlood/immune system cellsTumor antigenSialyl tn
The invention discloses a double-chimeric antigen receptor, a T cell and a construction method and an application thereof, which belong to the field of cellular immunotherapy of tumors. The inventionspecifically relates to a specific structure and a construction method of the double chimeric antigen receptor T cell (dCAR-T cell), and preliminarily discusses the in-vivo and in-vitro activity of the dCAR-T cell. The selected tumor-associated antigens are mesothelin and carcino-embryonic antigens, and researches show that the two tumor antigens can be simultaneously expressed on the surface of asolid tumor, such as pancreatic cancer. The invention discloses an antigen receptor. The in-vitro and in-vivo tests prove that the constructed dCAR-T cell can be permanently and effectively activatedonly under the condition that two antigens are simultaneously recognized, and has efficient anti-tumor activity, so that a specific tumor killing function can be exerted, and the application of CAR-Tcell immunotherapy is improved.
Owner:CHINA PHARM UNIV
Slc45a2 peptides for immunotherapy
InactiveUS20190169262A1Effectively kill melanoma cellLow toxicityPeptide/protein ingredientsSkin cancer vaccineAntigenMetastatic melanoma
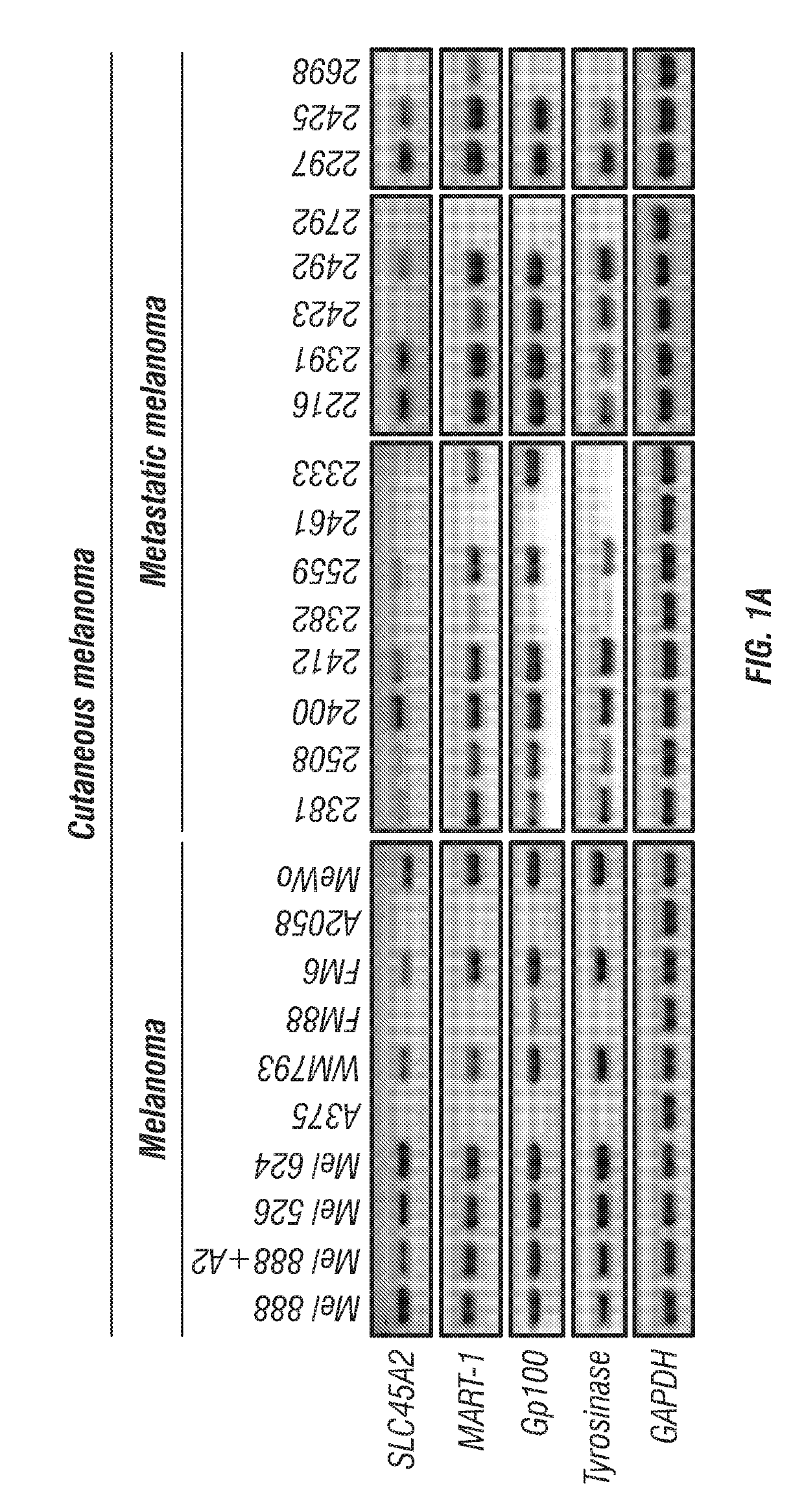
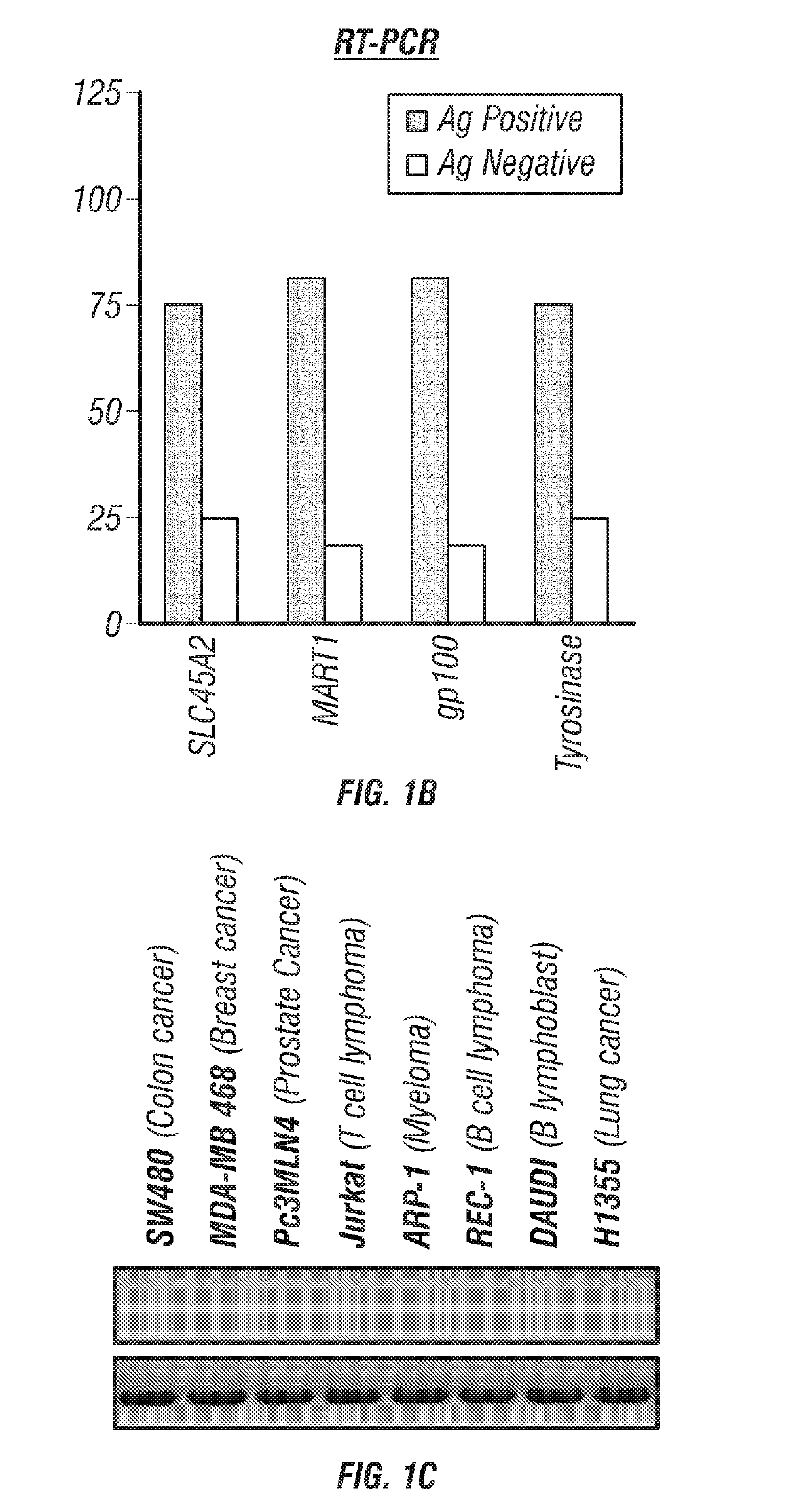
Provided are SLC45A2 peptides that bind to MHC I (HLA-A2) on melanoma cells or other antigen-presenting cells and are recognized by T-cell receptors on T cells. The SLC45 A2 peptides may be therapeutically used to treat a cancer, such as a cutaneous melanoma, uveal melanoma, a mucosal melanoma, or a metastatic melanoma. Methods for expanding a population of T cells that target SLC45A2 are also provided.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
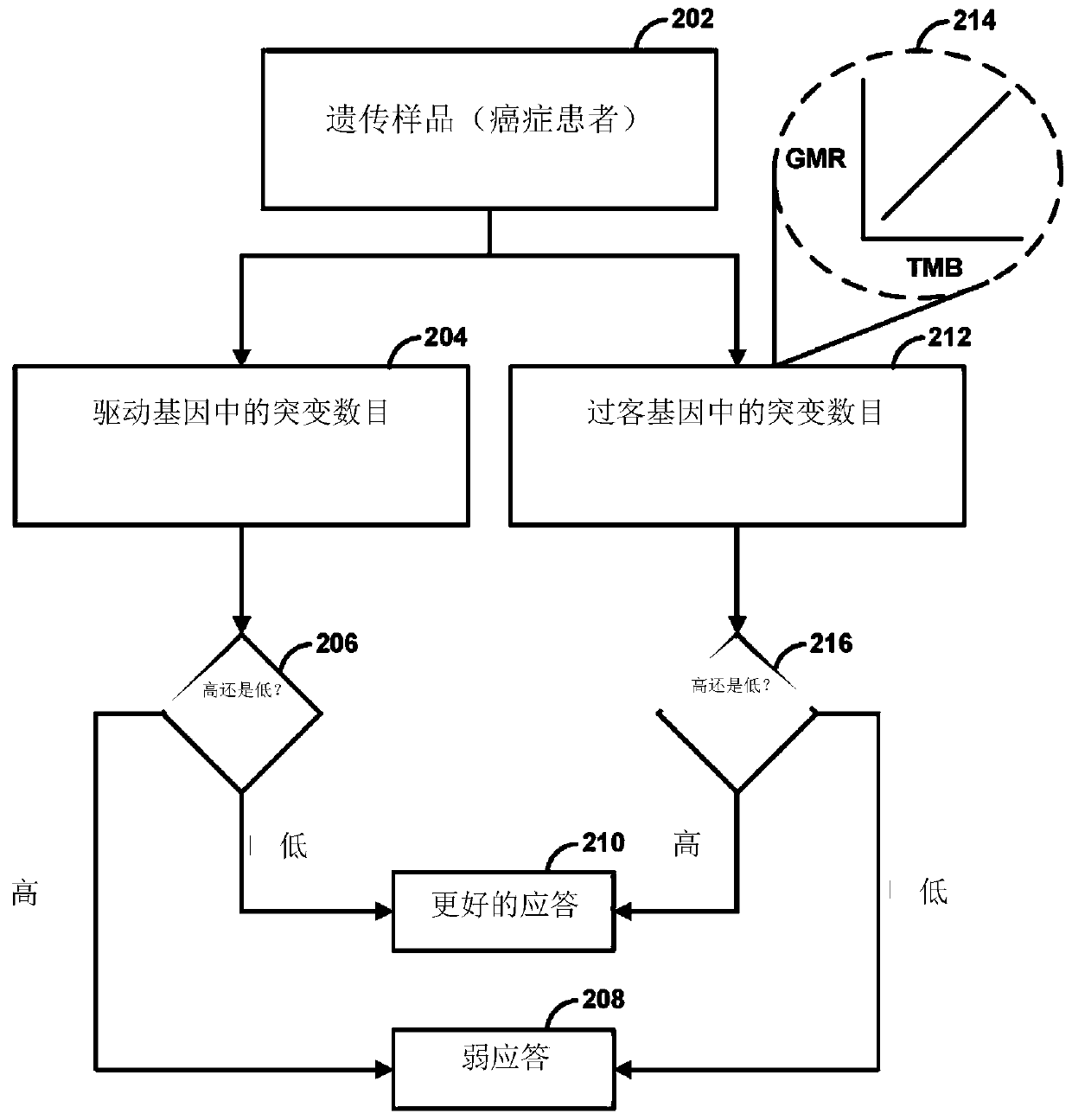
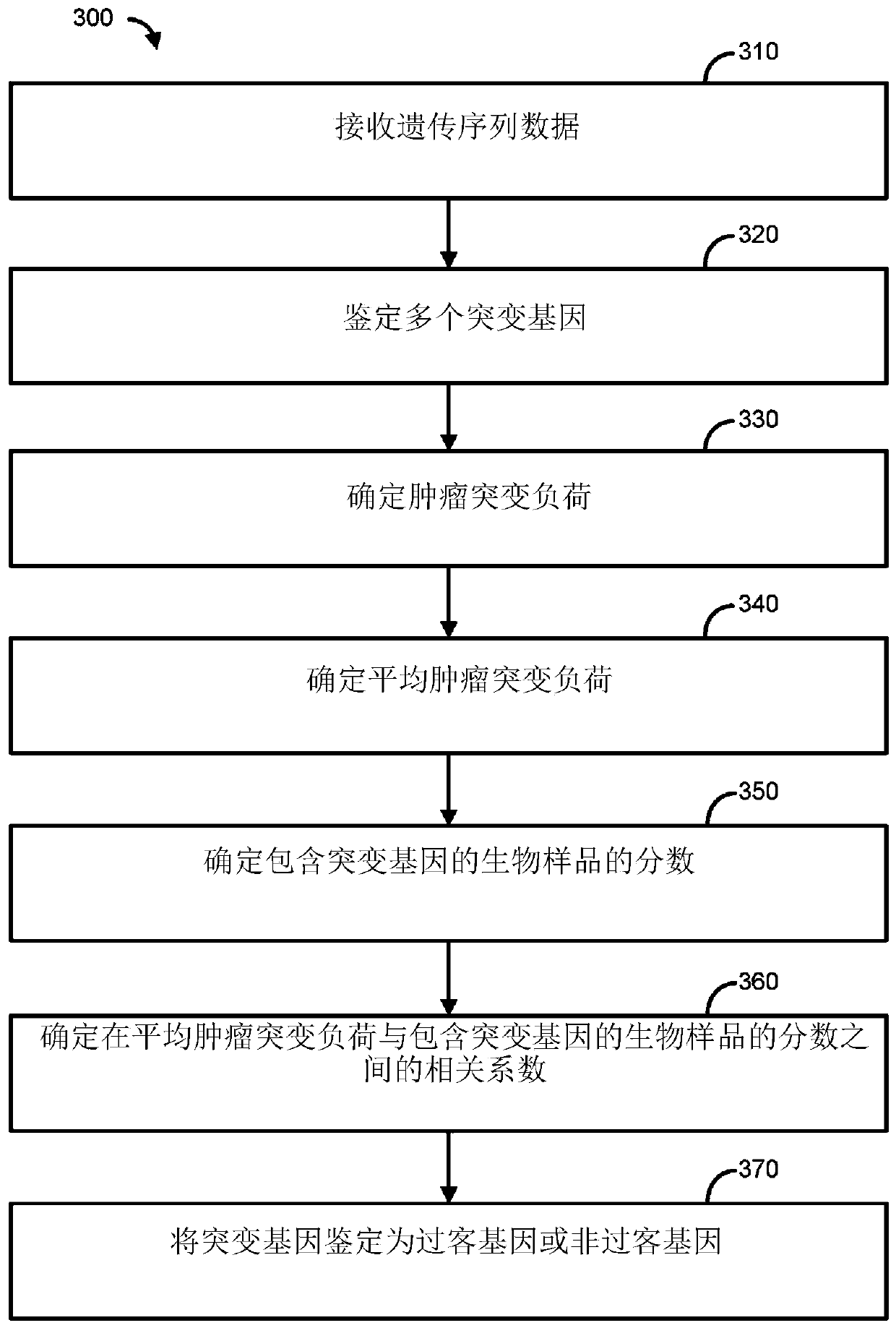
Immunotherapy methods for patients whose tumors carry a high passenger gene mutation burden
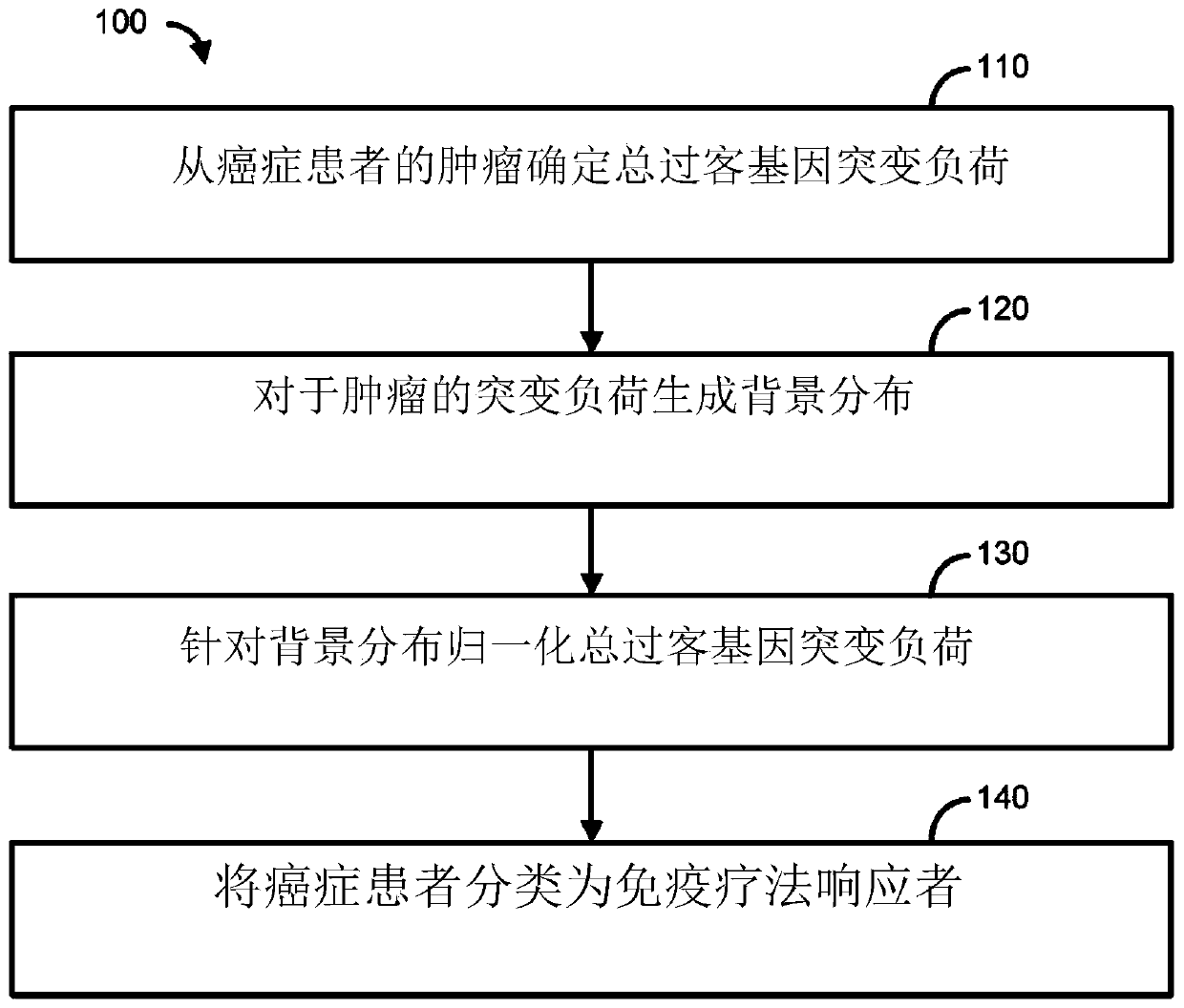
Methods for selecting a cancer patient for immunotherapy comprise establishing a total passenger gene mutation burden from a tumor of a cancer patient, generating a background distribution for the mutational burden of the tumor, normalizing the total passenger gene mutation burden against the background distribution, and categorizing the cancer patient as an immunotherapy responder when the totalpassenger gene mutation burden is greater than the mean of the background distribution. When the cancer patient is an immunotherapy responder, the patient may be administered an immunotherapy regimenthat comprises activation / inhibition of T cell receptors that promote T cell activation and / or prolong immune cytolytic activities.
Owner:REGENERON PHARM INC
Allogenic tumor cell vaccine
ActiveUS20200330596A1Reduce tumor burdenImprove clinical outcomesPolypeptide with localisation/targeting motifOrganic active ingredientsVaccinationAutologous tumor cell
The described invention provides allogeneic tumor cell vaccines comprising tumor cell lines or tumor cell line variants that are genetically engineered to express a core group of three immunomodulatory molecules, and optionally additional R immunomodulatory polypeptides for induction of one or more subpopulations of PBMCs to proliferate in response to the expressed immunomodulatory molecules and to then enter an effector phase for killing of tumor cells. According to some embodiments, the tumor cell vaccine candidate can induce an immune response in the recipient cancer patient that cross reacts with the patient's own (autologous) tumor cells, the effects of which are sufficient to result in enhanced anti-tumor immunity contributing to the increased survival of a vaccinated patient cohort compared to a matched unvaccinated patient cohort.
Owner:ALLOPLEX BIOTHERAPEUTICS INC
Antimicrobial extracts of boswellia and thyme
ActiveUS20190298787A1High antibacterial activitySynergistic and additive antibacterial activityAntibacterial agentsSkin cancer vaccineBoswelliaPolymicrobial Infections
Owner:IMAM ABDULRAHRNAN BIN FAISAL UNIVERSITY
Tumor cells from immune privileged sites as base cells for cell-based cancer vaccines
The present invention relates to tumor cell-based vaccines and methods of using same, wherein the vaccines are based on naturally immune privileged tumor cells that have been genetically modified to express MHC-II restricted peptides derived from endogenously encoded tumor antigens, activate CD4+ T-lymphocytes, provide an array of antigens to which the host is not tolerized and / or induce immunity against the originating tumor cells as well as against metastatic tumor cells.
Owner:UNIV OF MARYLAND BALTIMORE COUNTY
Cancer immunotherapy
We formulated multiple TLR agonists into GVAX (lethally irradiated tumor cell vaccines engineered to secrete GM-CSF). Specifically, GLA and R848, TLR4 and TLR7 / 8 agonists found to be safe in patients, were formulated with GVAX (TEGVAX - for TLR agonists enhanced GVAX), and this formulation was effective in producing anti-tumor responses in 3 different preclinical models, including palpable B16. These anti-tumor responses were correlated with increased CD4 and CD8 T-cells that can secrete IFN circulating in the tumor microenvironment as well as significantly higher level of p15E specific CTL mediated cell killing in mice treated with TEGVAX in comparison to controls. When combined with anti-PD-1 antibody, TEGVAX was able to induce regression of established B16 tumors.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Vaccine compositions
The invention relates to tumour therapy. In particular, the present invention relates to vaccine compositions comprising allogenic cells modified with hypercytokines for the treatment of cancer in general and for the treatment of melanoma in particular.
Owner:AGIRX
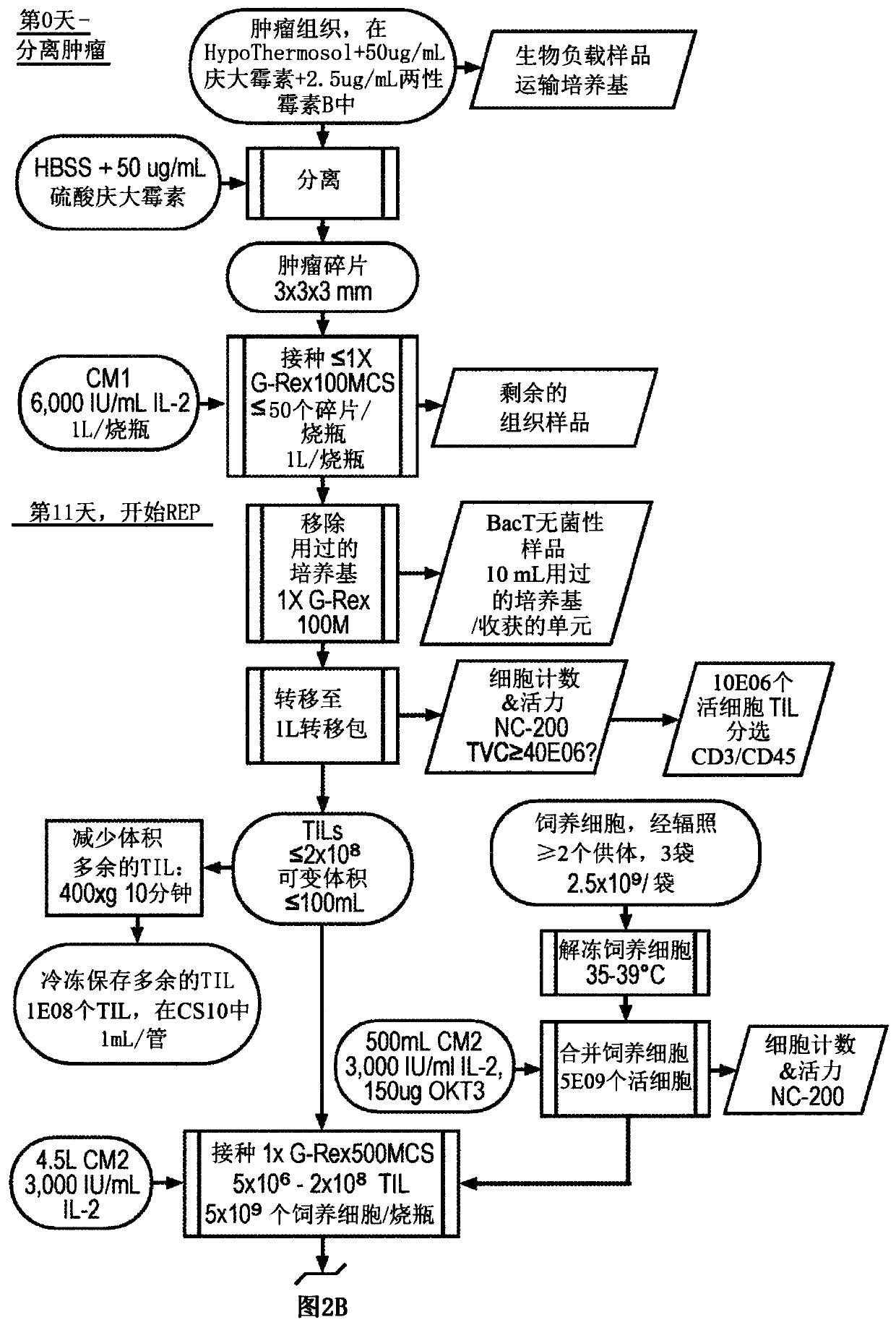
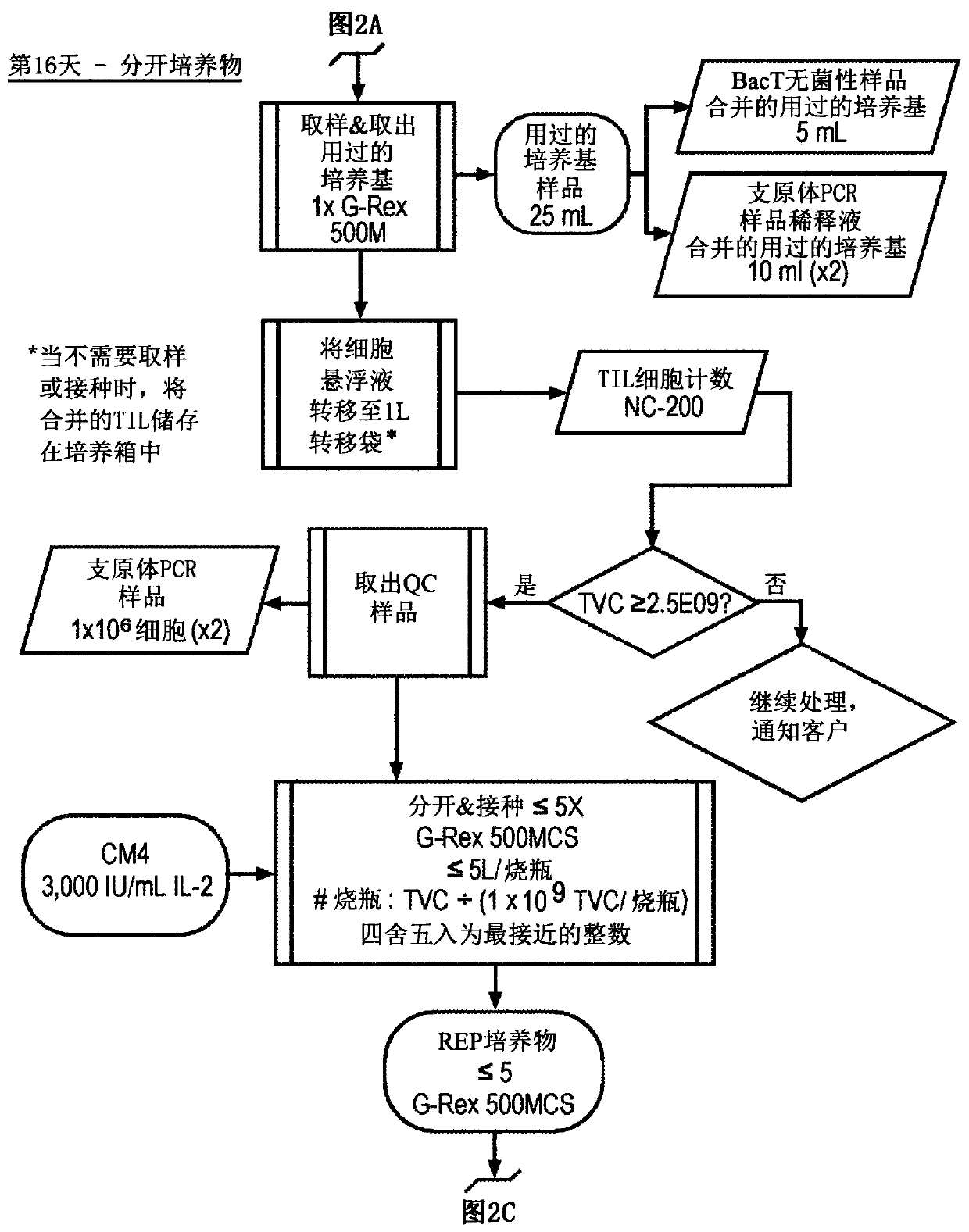
Til expansion from fine needle aspirates and small biopsies
The present disclosure provides methods for expanding TIL populations from fine needle aspirates (FN As) or small biopsies which contain low numbers of TILs, using the methods disclosed herein including in a closed system that leads to improved phenotype and increased metabolic health of the TILs in a shorter time period.
Owner:IOVANCE BIOTHERAPEUTICS INC
Immune adjuvant for cancer
ActiveUS20190160168A1Good effectImprove response rateAntibacterial agentsBacterial antigen ingredientsSingle strandPD-L1
The present invention relates to a medicament for treating cancer or infectious disease, the medicament comprising an immune checkpoint blockade and an adjuvant composition. More specifically, the present invention relates to a medicament for cancer or infectious diseases, wherein an anti-PD-1 antibody or an anti-PD-L1 antibody is used in combination with a nucleic acid adjuvant composed of a double-stranded RNA and a single-stranded ODN.
Owner:INST OF ADVANCED IMMUNOTHERAPY
Immunotherapeutic constructs and methods of their use
ActiveUS20210008198A1Reducing and even and developmentReducing and even spreadPowder deliveryOrganic active ingredientsAntigen releaseAdjuvant
Disclosed herein are immunotherapeutic constructs comprising a delivery particle, at least one adjuvant, and one or more therapeutic agents / compounds that cause antigen release and / or modulate immunosuppressive tumor microenvironment. These immunotherapeutic constructs create adaptive immunity or anti-cancer immune response(s) that can be used, for instance, to prevent and treat broad types of cancer. Further disclosed are uses of the immunotherapeutic constructs, including to prevent and treat cancer in humans and animals.
Owner:PDX PHARM INC +1
Nanometer assembly based on immune checkpoint inhibitor and preparation method and application of nanometer assembly
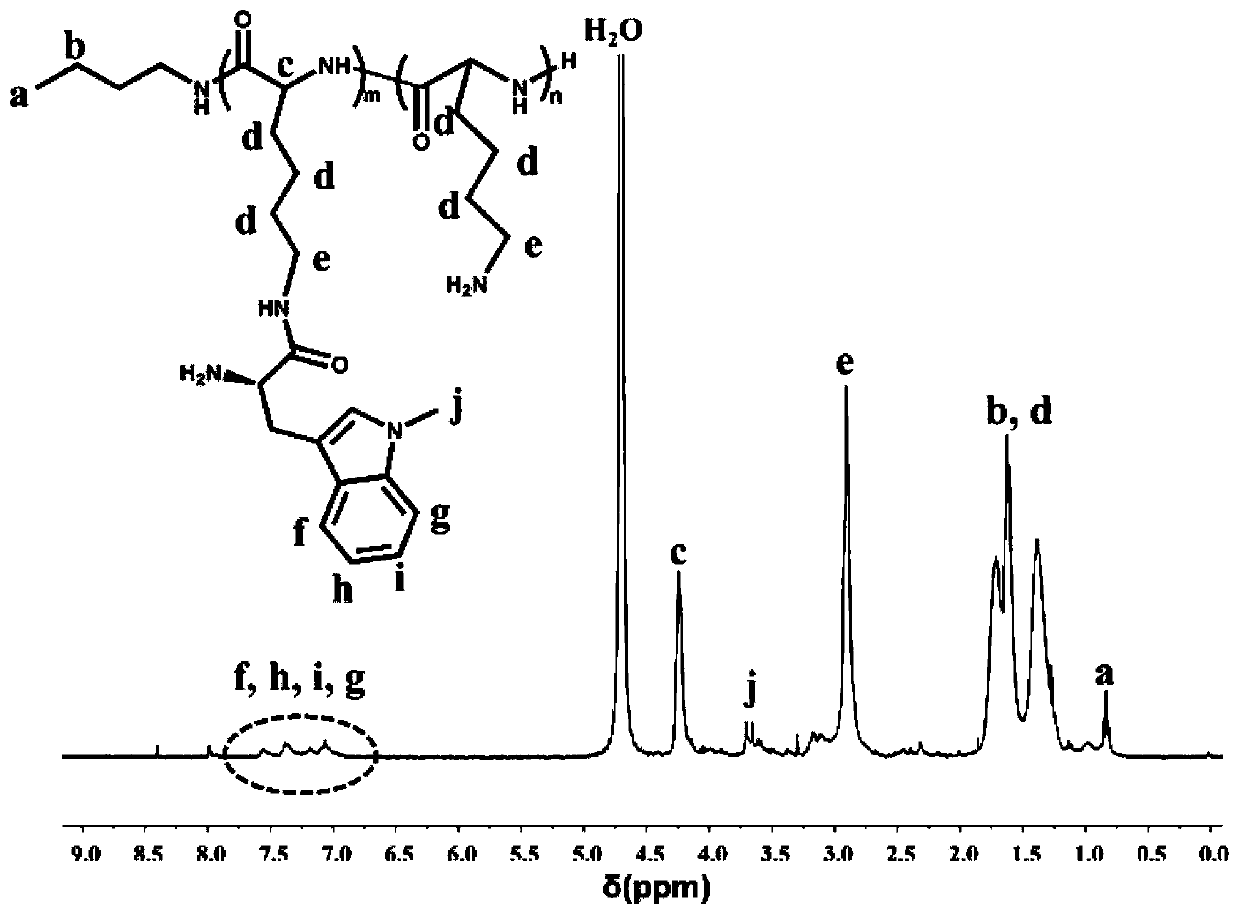
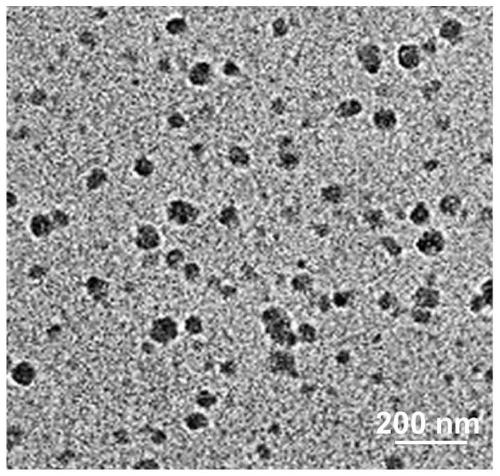
ActiveCN110066395AHigh drug loadingStrong cytotoxicityEnergy modified materialsSkin cancer vaccineAntiendomysial antibodiesTumor Purging
The invention discloses nanometer assembly based on an immune checkpoint inhibitor and a preparation method and application of the nanometer assembly. The nanometer assembly comprises a polymer, hyaluronic acid grafted with chlorin and a PD-L1 monoclonal antibody, wherein the structural formula of the polymer is shown in the description, the number-average molar mass of the polymer is 2000-6000, and the ratio of m to n is 1:(8-15). The nanometer assembly can achieve integrated combination treatment of enhancing the enhance antigen presentation stage and the lymphocyte activation, proliferationand differentiation stage and the tumor removal stage.
Owner:SHANDONG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com