Cationic liposome-protamine-mRNA tumor vaccine and preparation method and application method thereof
A cationic liposome and protamine technology, applied in the field of nanomaterials and nucleic acid vaccines, can solve the problems of weak immunogenicity, poor patient compliance, single vaccination route, etc., and achieve high safety, simple cost, synthesis and purification fast effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
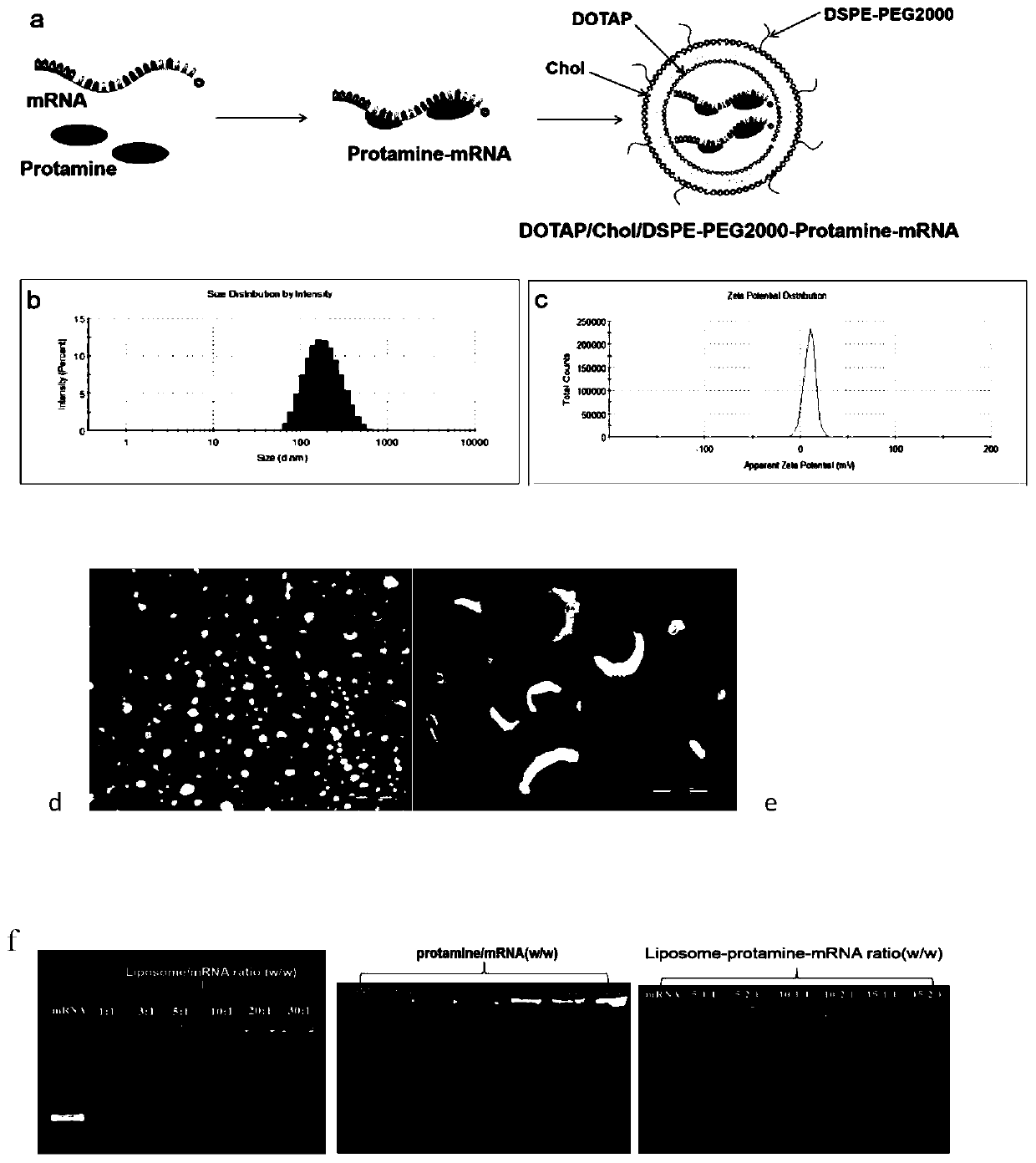
[0036] Example 1: Preparation of DDA / TDB-protamine-(CK19) mRNA vaccine by film dispersion method
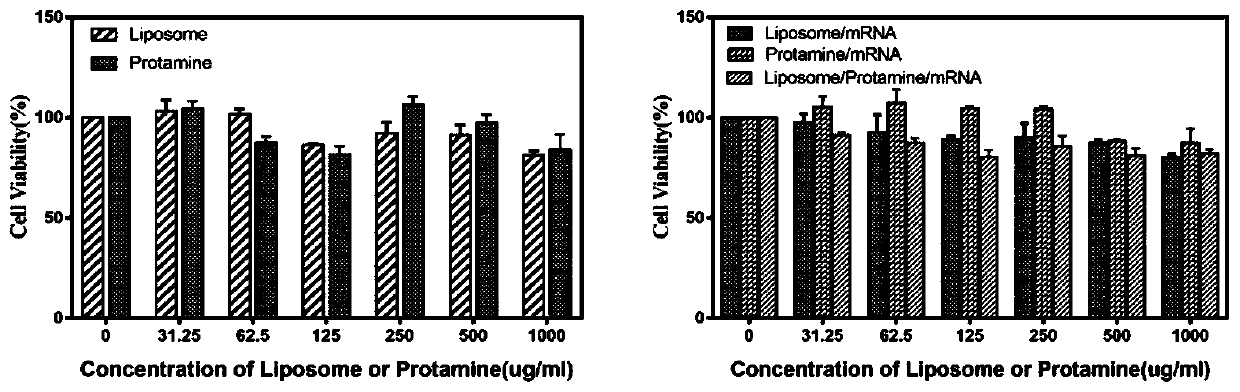
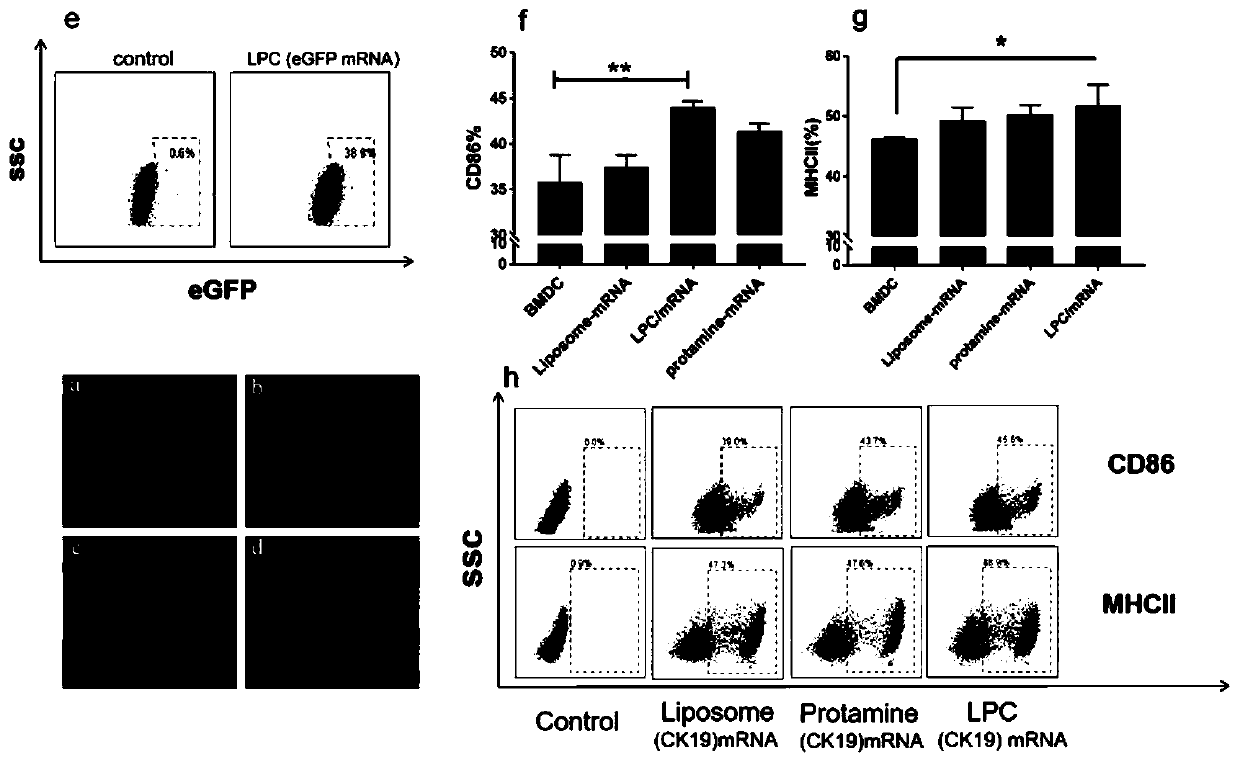
[0037] Put DDA and TDB in a 50 ml round bottom flask with a molar ratio of 6-10:1, add 1-2 ml of chloroform and methanol to dissolve (chloroform:methanol 9:1), and rotate under reduced pressure in a constant temperature water bath at 37°C Remove the organic solvent by evaporation to form a uniform lipid film, and then pass through 2 minN 2 , remove the residual solvent. Then add 5ml of Tris-HCl buffer (pH 6.8-9) with a concentration of 10 mM, and hydrate at 60°C for 30 minutes to obtain blank liposomes with a particle size of about 50-800 nm. Then according to liposome: protamine: mRNA mass ratio of 5:1:1 ~ 15:2:1, the protamine condensed mRNA (protamine is added to mRNA to condense and stand still). Add it into the above-mentioned blank liposome, make it fully fused, and let it rest for 20-40 minutes to obtain a cationic liposome-protamine-mRNA vaccine, which is ready for use....
Embodiment 2
[0038] Example 2: Preparation of DOTAP / Chol / DSPE-PEG200-protamine-(CK19) mRNA vaccine by film dispersion method
[0039] Put DOTAP, Chol and DSPE-PEG2000 in a molar ratio of 1:1:0.1 to 1:1:1 in a 50 ml round bottom flask, add 1 to 2 ml of chloroform and methanol to dissolve (chloroform:methanol 9:1), Remove the organic solvent by rotary evaporation under reduced pressure under heating in a water bath to form a uniform lipid film, and then pass it through for 2minN 2 , remove the residual solvent. Then add 5 ml of Tris-HCl buffer solution (pH 5-9) with a concentration of 10 mM, and hydrate at 60°C for 30 min to obtain blank liposomes with a particle size of about 50-800 nm. Then according to liposome:protamine:mRNA mass ratio of 5:1:1~15:2:1, protamine condensed mRNA (add protamine to mRNA to condense, stand still) add to In the above-mentioned blank liposome, make it fully fused, and stand still for 20 to 40 minutes to obtain a cationic liposome-protamine-mRNA vaccine, which...
Embodiment 3
[0041] Example 3: Preparation of DOTAP / Chol / DSPE-PEG200-protamine-(CK19) mRNA vaccine by freeze-drying method
[0042] Put DOTAP, Chol and DSPE-PEG2000 in a molar ratio of 1:1:0.1 to 1:1:1 in a 50 ml round bottom flask, add 1 to 3 ml of chloroform and methanol to dissolve (chloroform:methanol 9:1), Remove the organic solvent by rotary evaporation under reduced pressure under heating in a water bath to form a uniform lipid film, and then pass it through for 2 to 4 minutes under N 2 Remove residual solvent. Then add 2 ml of Tris-HCl buffer (pH 5-9) with a concentration of 10 mM, and hydrate at 50-70°C for 30 min-1 h to obtain blank liposomes with a particle size of about 50-800 nm. Then press liposome:protamine:mRNA mass ratio is 5:1:1~15:2:1, and add concentration and be 2% mannitol, sucrose, lactose, or trehalose as freeze-drying protection agent, make it Fully fused, then put it into the refrigerator to pre-freeze for 4, 8, and 12 hours, take it out, and freeze-dry it in a ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Electric potential | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com