Patents
Literature
178 results about "Cytokine secretion" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cytokines are a large group of proteins, peptides or glycoproteins that are secreted by specific cells of immune system. Cytokines are a category of signaling molecules that mediate and regulate immunity, inflammation and hematopoiesis. Cytokines are produced throughout the body by cells of diverse embryological origin. Cytokine is a general name; other names are defined based on their presumed function, cell of secretion, or target of action.
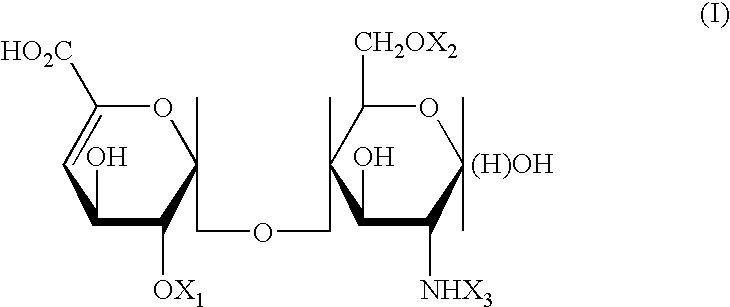
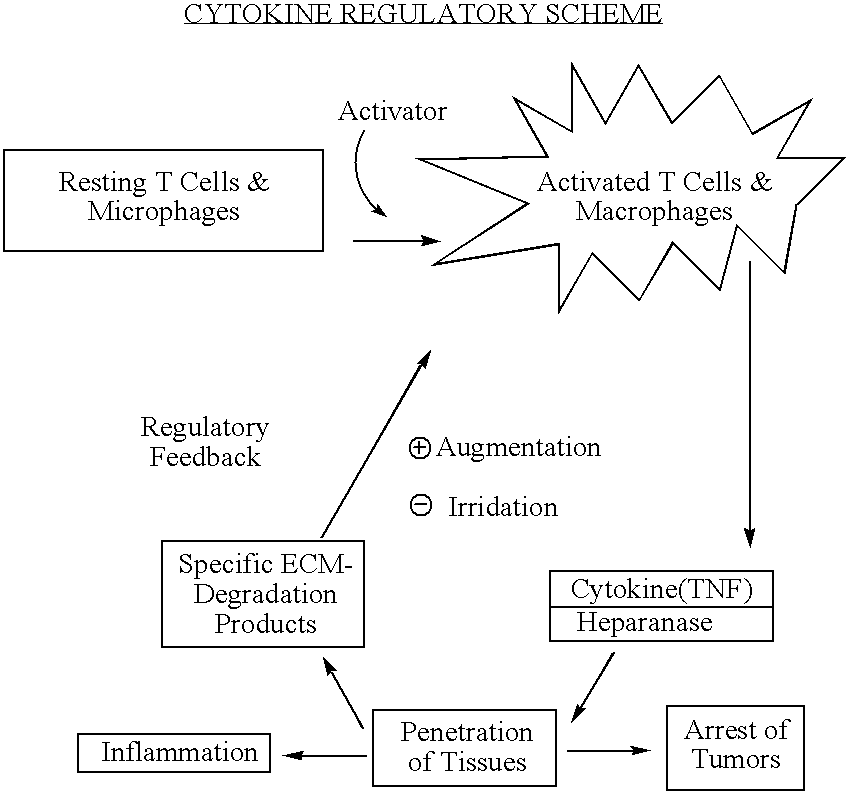
Compositions and methods for regulation of active TNF- alpha
InactiveUS6020323AReduced activityReduced availabilityEsterified saccharide compoundsBiocideSulfationMedicine
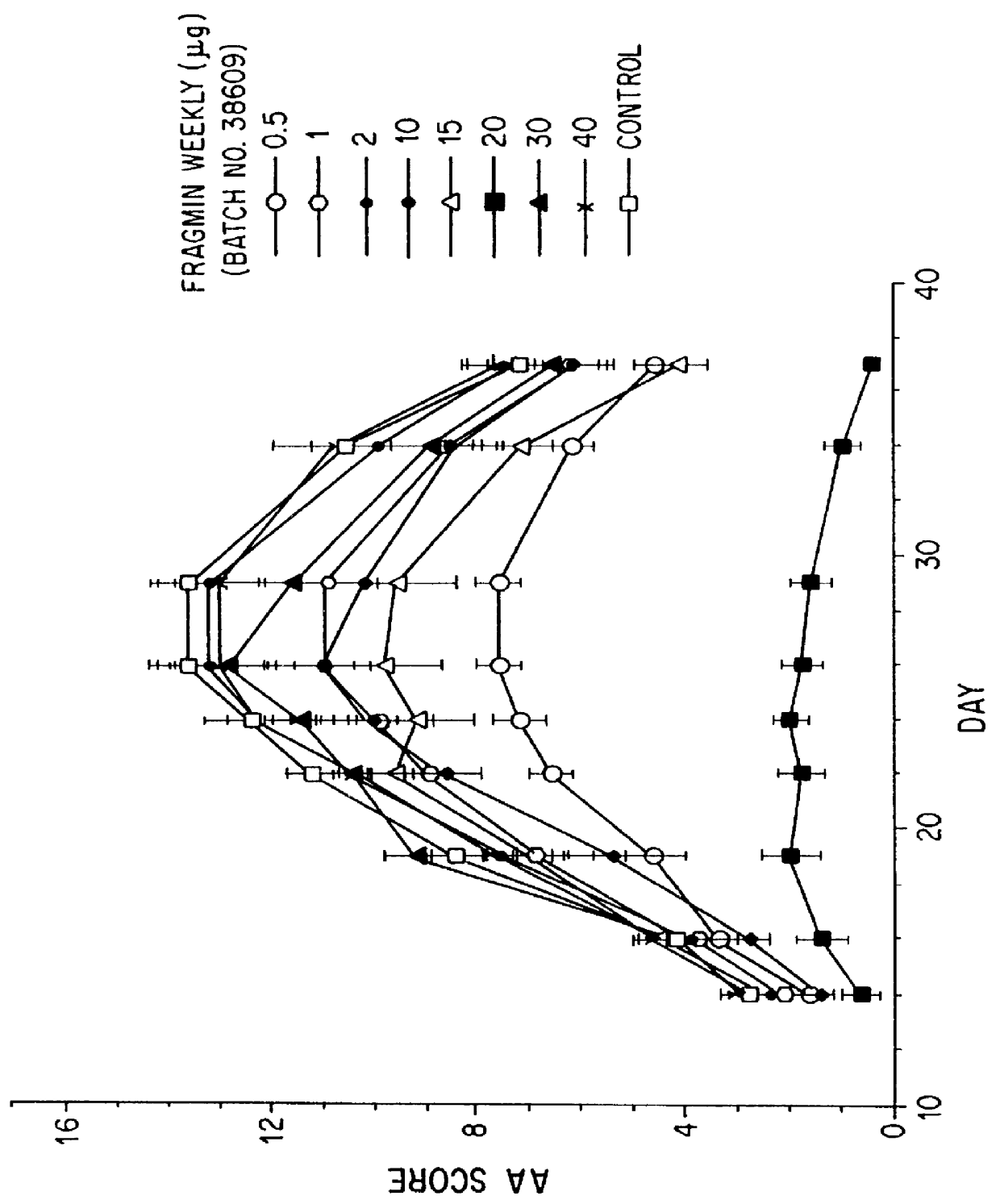
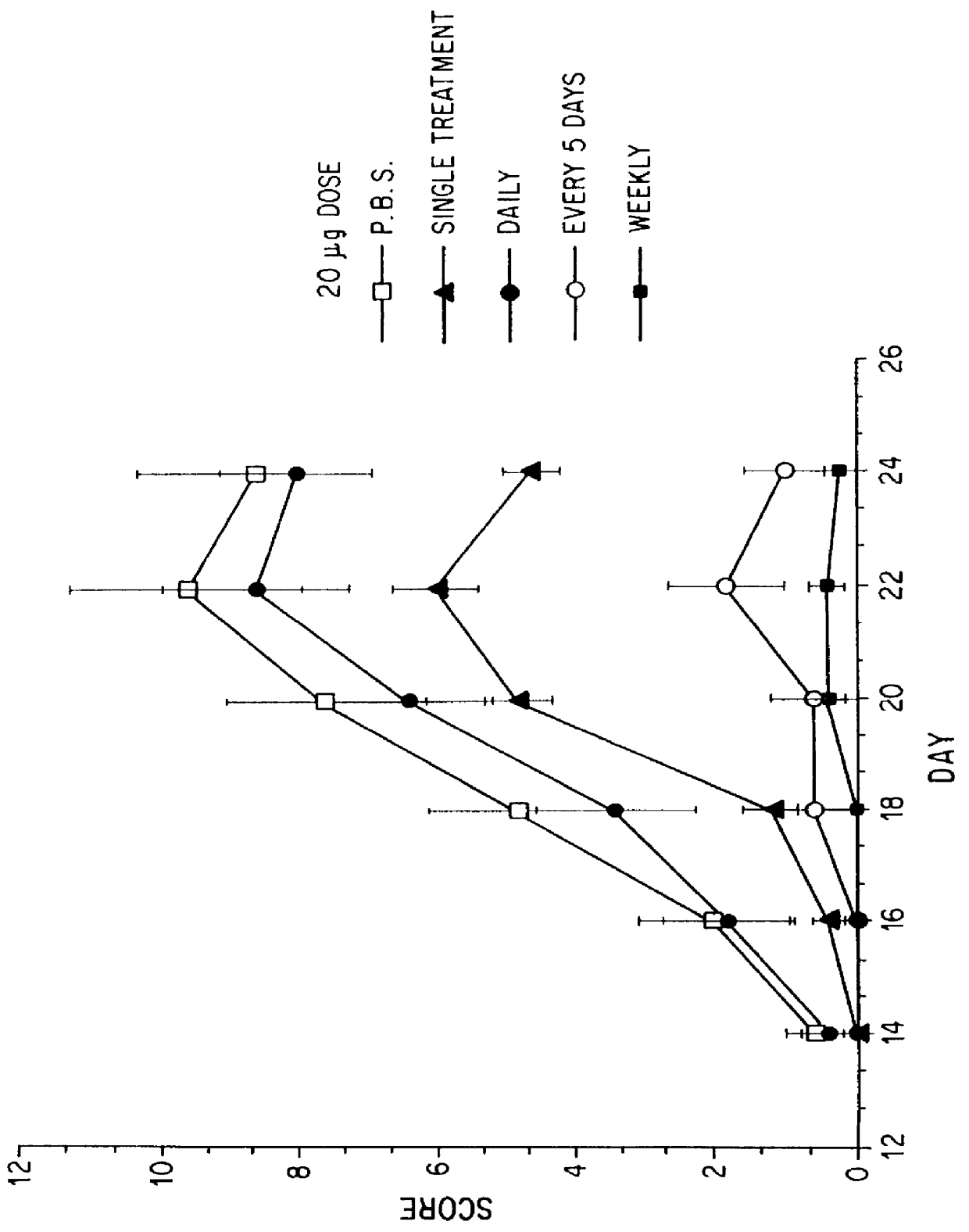
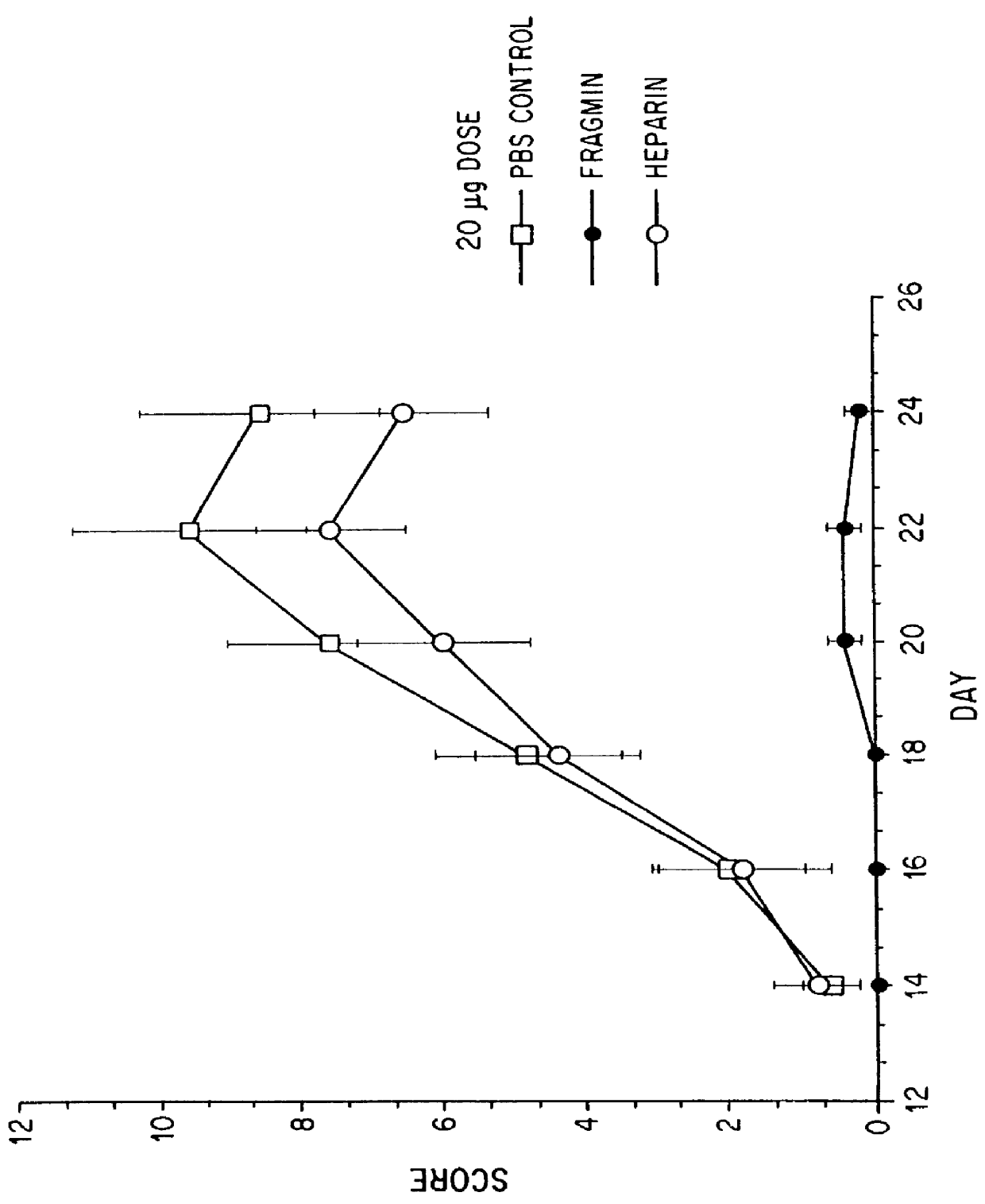
Substances comprising disaccharides and substances comprising carboxylated and / or sulfated oligosaccharides in substantially purified form, and methods of using same, are disclosed for the regulation of cytokine activity in a host. For instance, the secretion of active Tumor Necrosis Factor Alpha (TNF- alpha ) can be either inhibited or augmented selectively by administration to the host of an effective amount of a substance of the invention. Thus, the present invention also relates to pharmaceutical compositions and their use for the prevention and / or treatment of pathological processes involving the induction of active cytokine secretion, such as TNF- alpha . The invention also relates to the initiation of a desirable immune system-related response by the host to the presence of activators, including pathogens. The substances and pharmaceutical compositions of the present invention may be administered daily, at very low effective doses, typically below 0.1 mg / kg human, or at intervals of up to about 5-8 days, preferably once a week.
Owner:YEDA RES & DEV CO LTD
Methods for therapeutic administration of messenger ribonucleic acid drugs
ActiveUS20180256628A1Reducing and inhibiting accelerated blood clearanceBlood clearance is reduced and inhibitedOrganic active ingredientsNucleic acid vectorAntiendomysial antibodiesBinding site
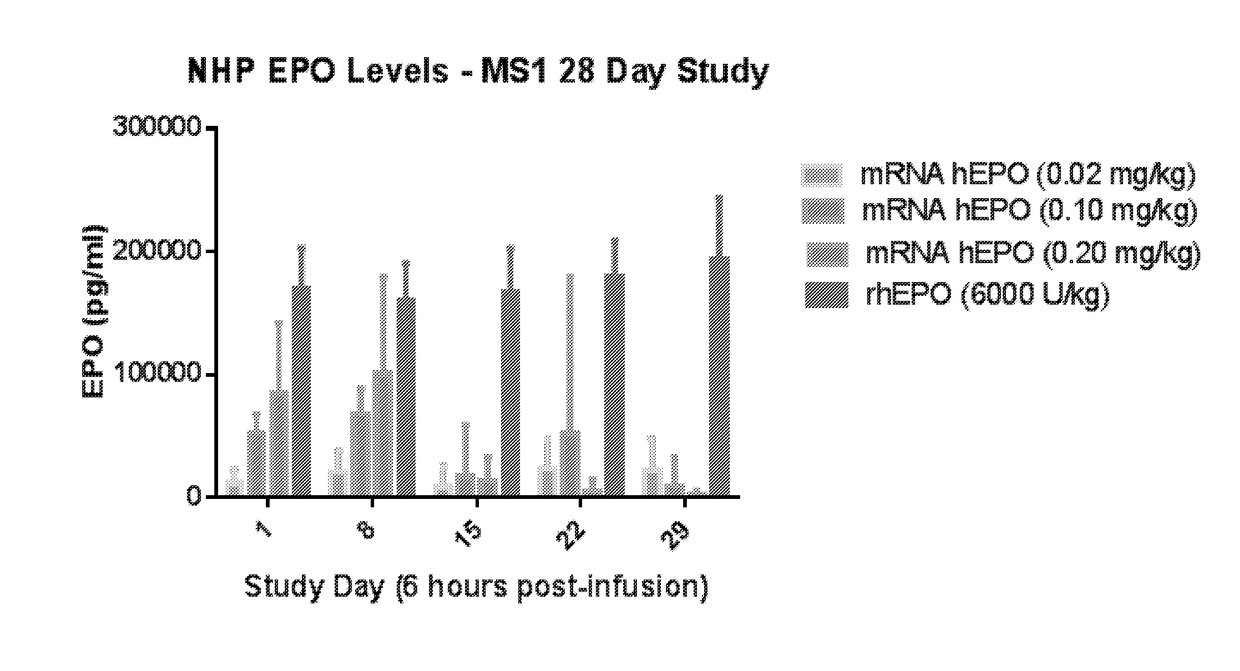
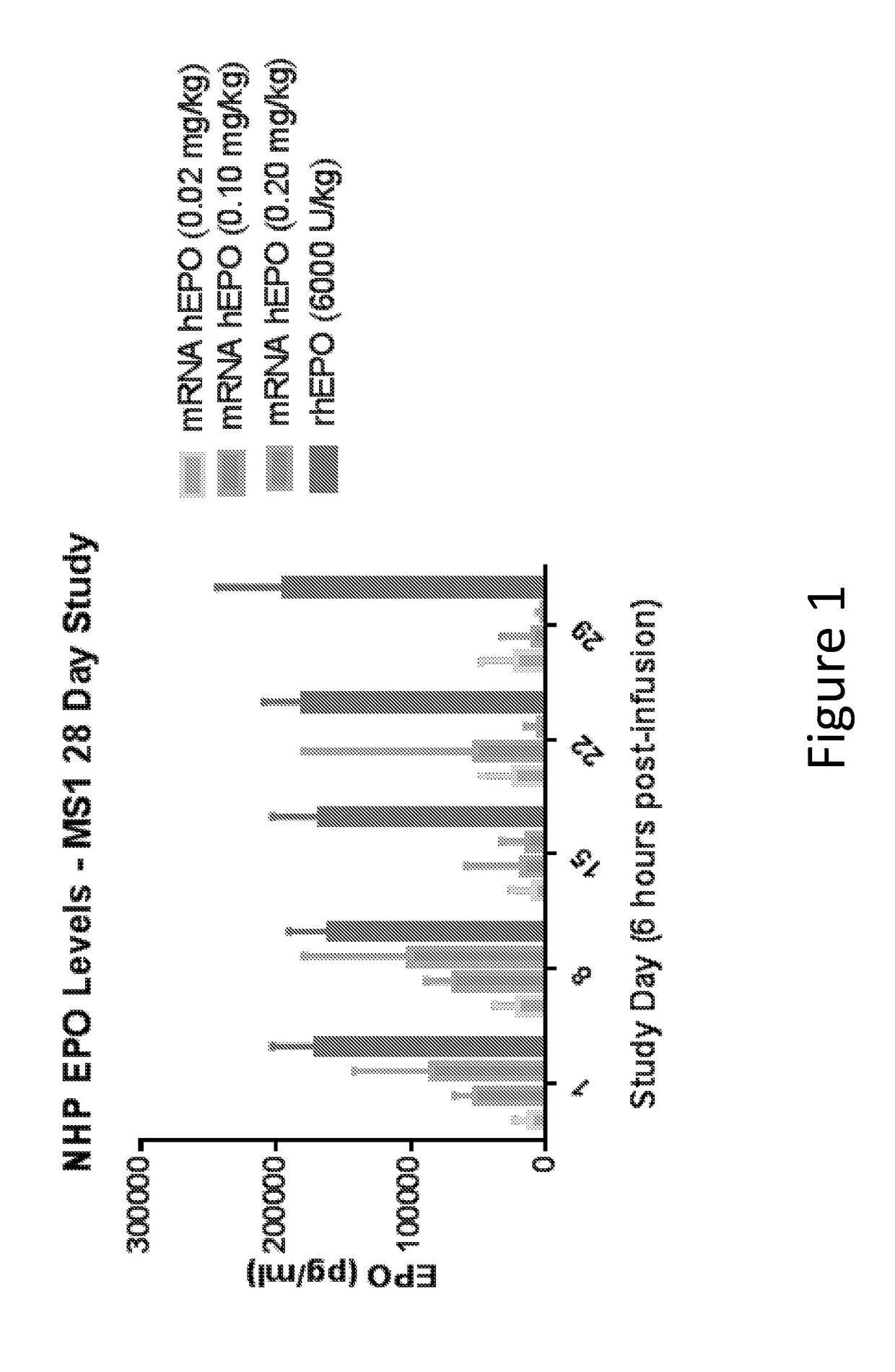
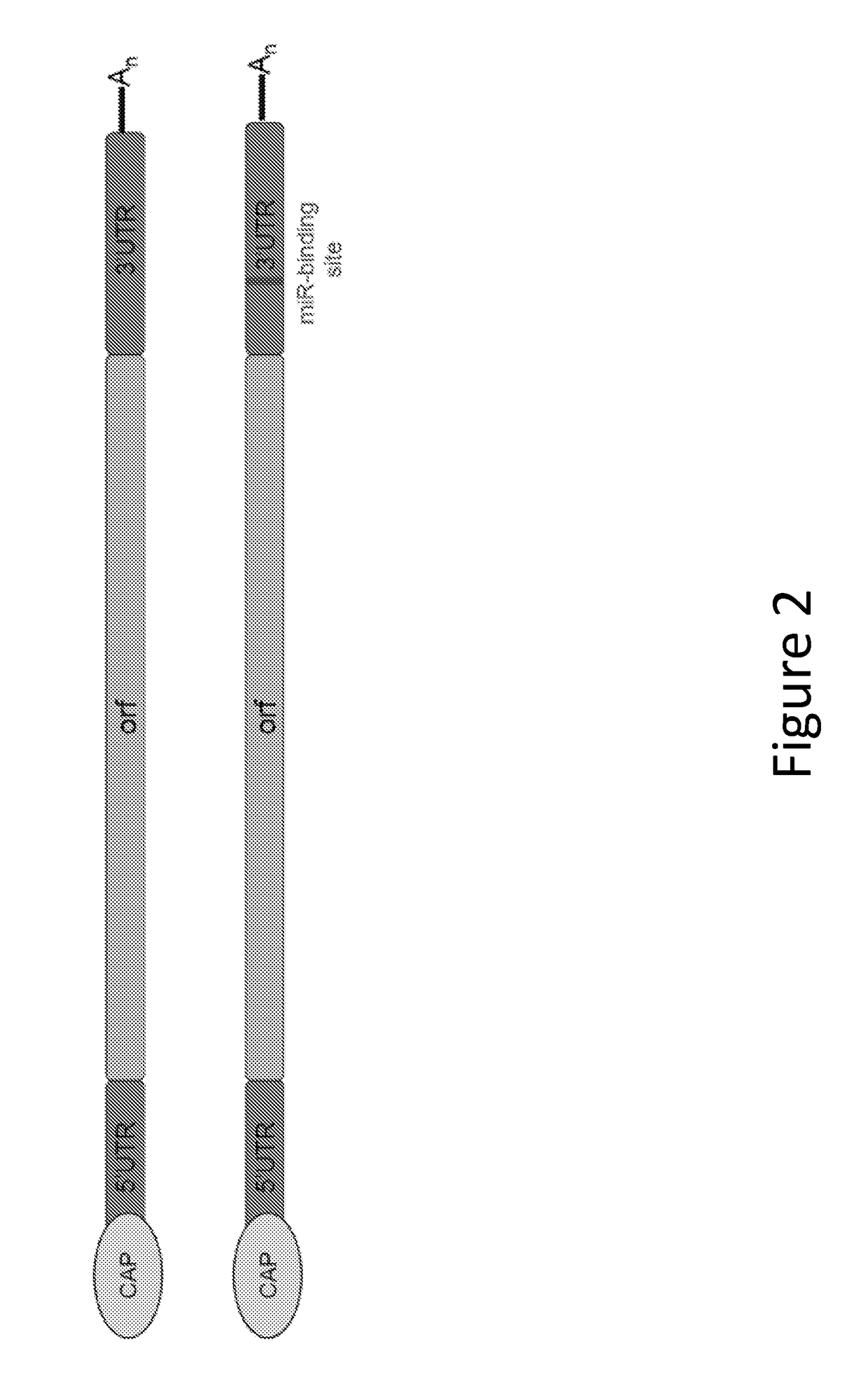
The disclosure features methods of reducing or inhibiting an anti-drug antibody response in a subject, as well as methods of reducing or inhibiting unwanted immune cell activation in a subject to be treated with a messenger RNA (mRNA), comprising administering to the subject a mRNA, e.g., a chemically modified messenger RNA (mmRNA), encoding a polypeptide of interest, wherein the mRNA comprises at least one microRNA (miR) binding site for a miR expressed in immune cells, such as miR-126 binding site and / or miR-142 binding site, such that an anti-drug antibody response to the polypeptide or interest, or unwanted immune cell activation (e.g., B cell activation, cytokine secretion), is reduced or inhibited in the subject. The disclosure further provides therapeutic treatment regimens designed to reduce or inhibit AD A or unwanted immune cell activation (e.g., B cell activation, cytokine secretion) in a subject being treated with mRNA-based therapeutics.
Owner:MODERNATX INC
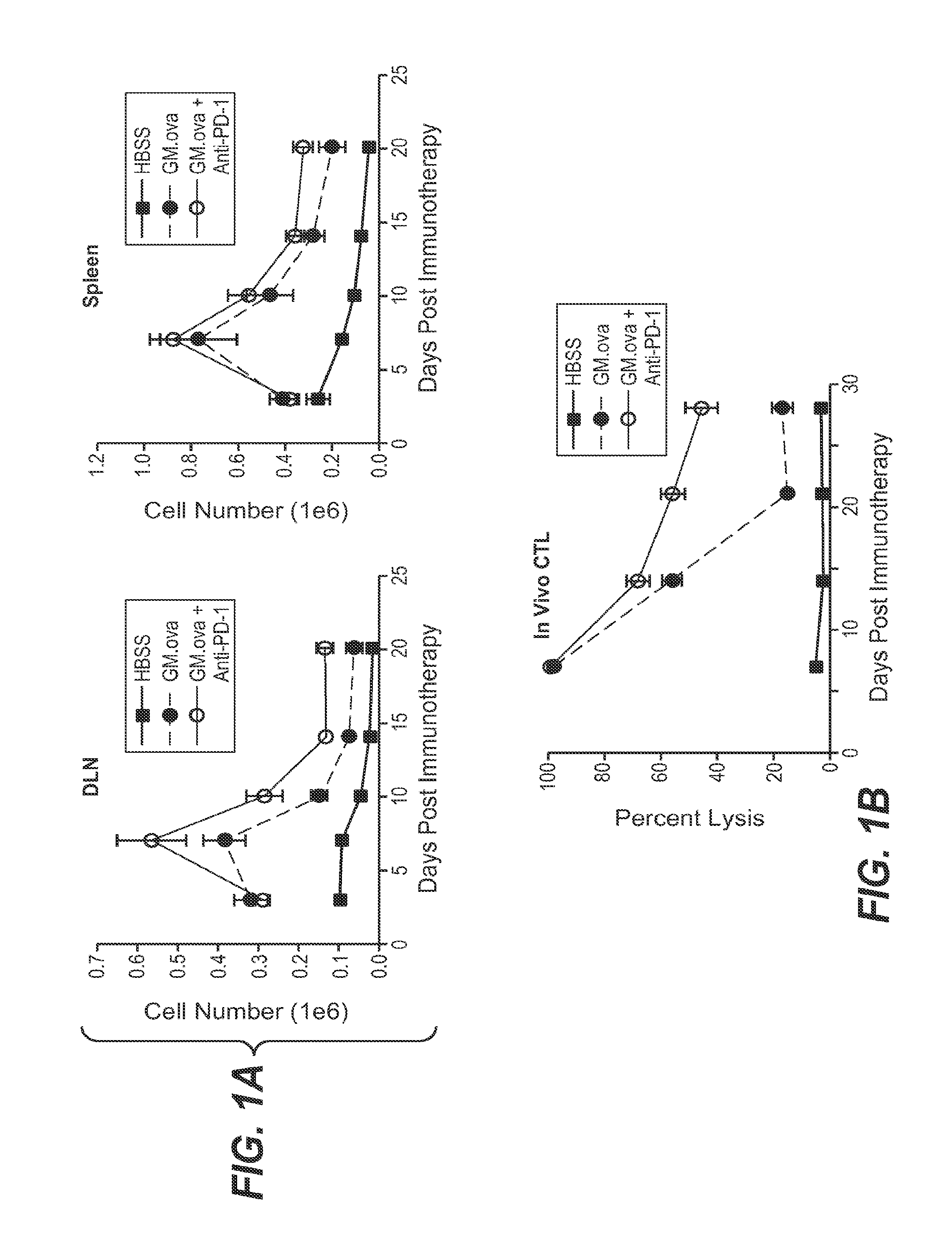
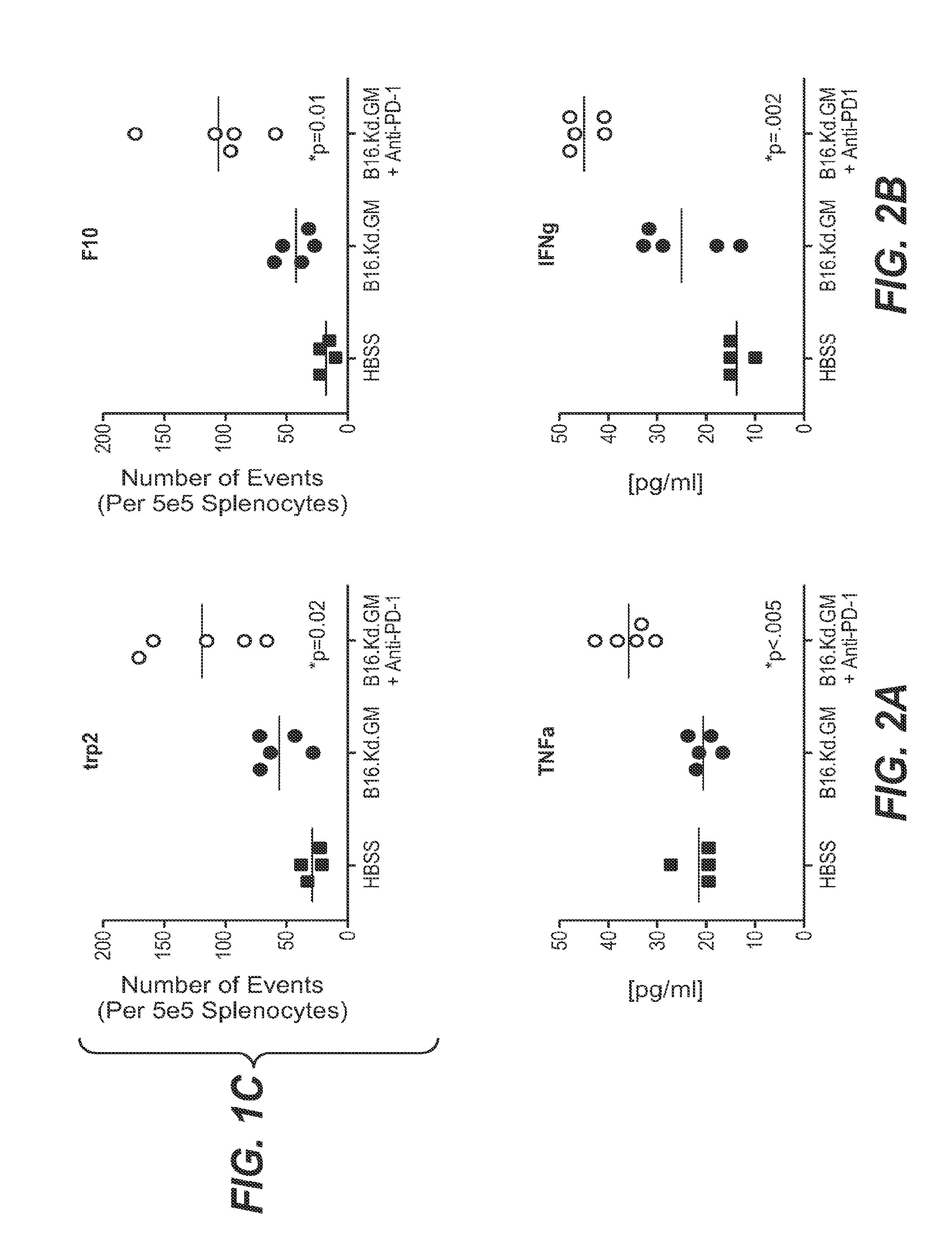
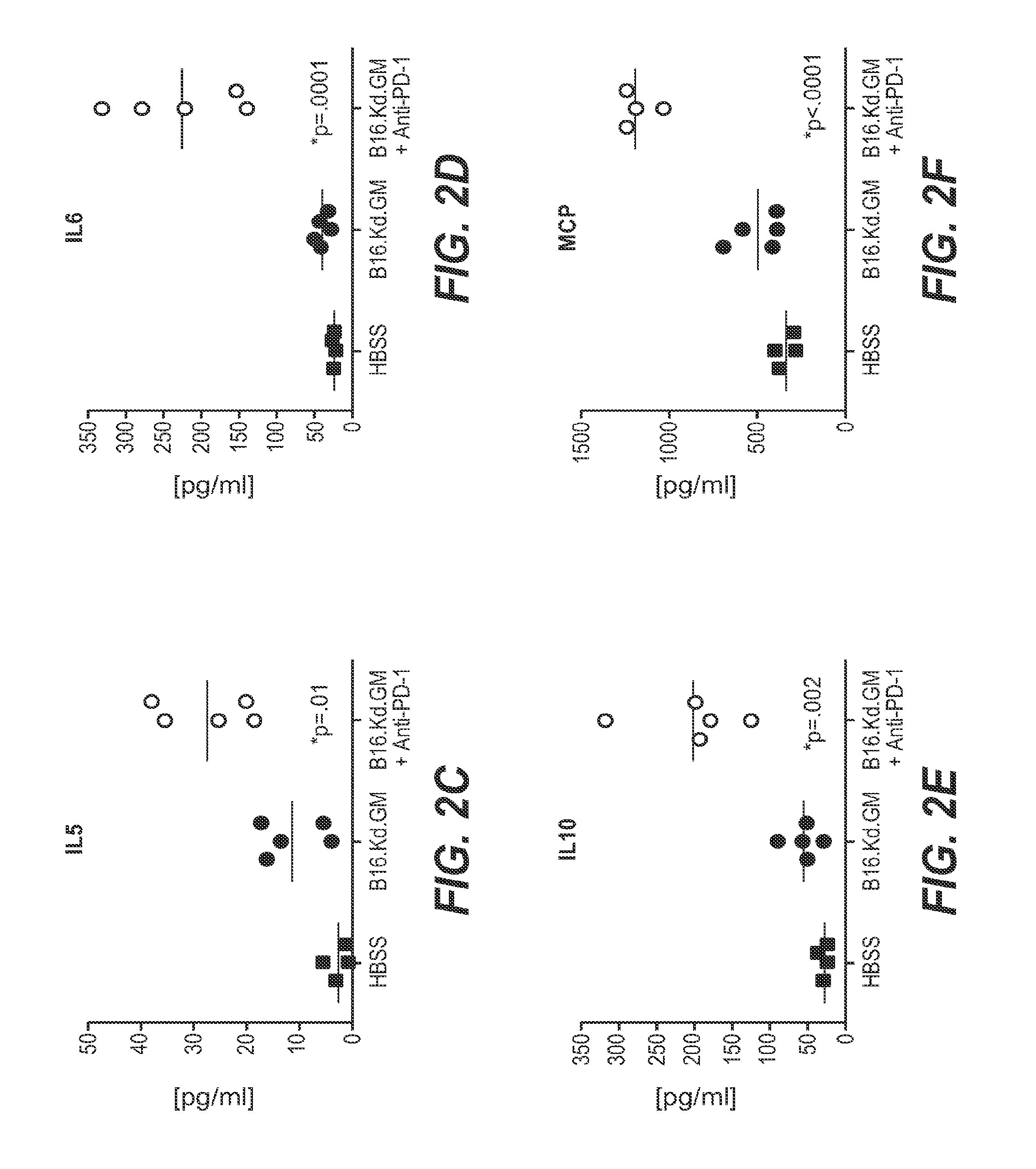
Pd-1 antibodies in combination with a cytokine-secreting cell and methods of use thereof
InactiveUS20100285013A1Good curative effectBiocidePeptide/protein ingredientsTumor responseSecreting cell
Owner:ER SQUIBB & SONS INC +1
Methods of treating spinal cord injury and minimizing scarring
ActiveUS20090136457A1Promote functional recoveryPromote healingBiocideNervous disorderProgenitorSecreting cell
The invention is directed to methods of promoting the healing of spinal cord injury. The invention is further directed to methods of minimizing the extent of scarring following spinal cord injury. Such methods utilize novel compositions, including but not limited to extraembryonic cytokine secreting cells (herein referred to as ECS cells), including, but not limited to, amnion-derived multipotent progenitor cells (herein referred to as AMP cells) and conditioned media derived therefrom (herein referred to as amnion-derived cellular cytokine solution or ACCS), each alone or in combination with each other and / or other agents.
Owner:STEMNION
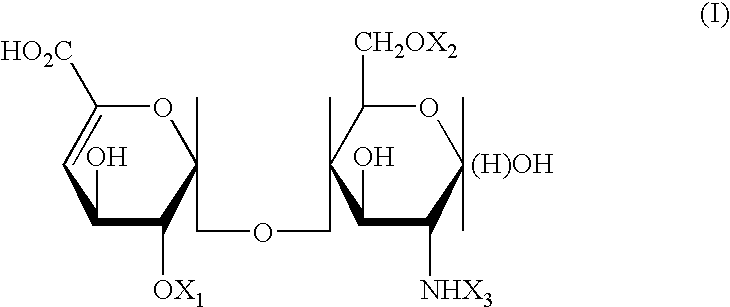
Compositions for the regulation of cytokine activity
InactiveUS20040198697A1Reduced activityReduced availabilityEsterified saccharide compoundsBiocideTumor necrosis factor alphaSecreted substance
Substances comprising carboxylated and / or sulfated oligosaccharides in substantially purified form, including compositions containing same and methods of using same, are disclosed for the regulation of cytokine activity in a host. For instance, the secretion of Tumor Necrosis Factor Alpha (TNF-alpha) can be either inhibited or augmented selectively by administration to the host of effective amounts of substances or their compositions comprising specific oligosaccharides in substantially purified form. Thus, the present invention also relates to pharmaceutical compositions and their use for the prevention and / or treatment of pathological processes involving the induction of active cytokine secretion, such as TNF-alpha. The invention also relates to the initiation of a desirable immune system-related response by the host to the presence of activators, including pathogens. The substances and pharmaceutical compositions of the present invention may be daily, at very low effective doses, typically below 0.1 mg / kg human, or at intervals of up to about 5-8 days, preferably once a week.
Owner:YEDA RES & DEV CO LTD
Methods for promoting hair growth
The invention is directed to methods for promoting hair growth. Such methods utilize novel compositions, including but not limited to extraembryonic cytokine secreting cells (herein referred to as ECS cells), including, but not limited to, amnion-derived multipotent progenitor cells (herein referred to as AMP cells), conditioned media derived therefrom (herein referred to as amnion-derived cellular cytokine solution or ACCS), cell lysates derived therefrom, and cell products derived therefrom, each alone or in combination.
Owner:STEMNION
Methods related to surgery
ActiveUS20090010899A1Promote healingImproved and accelerated healingBiocideMammal material medical ingredientsProgenitorFistula
The invention is directed to methods related to surgery, for example gastrointestinal surgery. In particular, the invention is methods of treating fistulae, promoting accelerated healing of anastomoses and preventing failure of anastomoses. Such methods utilize novel compositions, including but not limited to extraembryonic cytokine secreting cells (herein referred to as ECS cells), including, but not limited to, amnion-derived multipotent progenitor cells (herein referred to as AMP cells), conditioned media derived therefrom (herein referred to as amnion-derived cellular cytokine solution or ACCS), cell lysates derived therefrom, and cell products derived therefrom, each alone or in combination.
Owner:STEMNION
Methods of treating spinal cord injury and minimizing scarring
ActiveUS8475788B2Promote healingMinimize the extent of scarringBiocideNervous disorderProgenitorSecreting cell
Owner:STEMNION
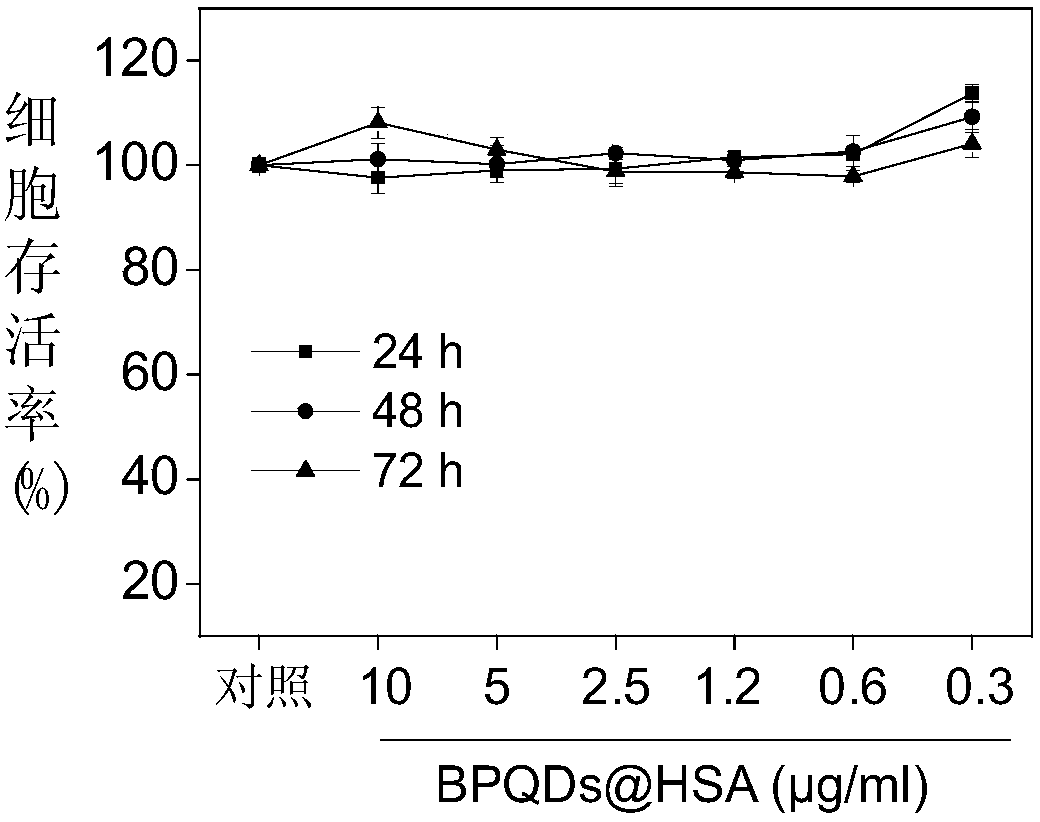
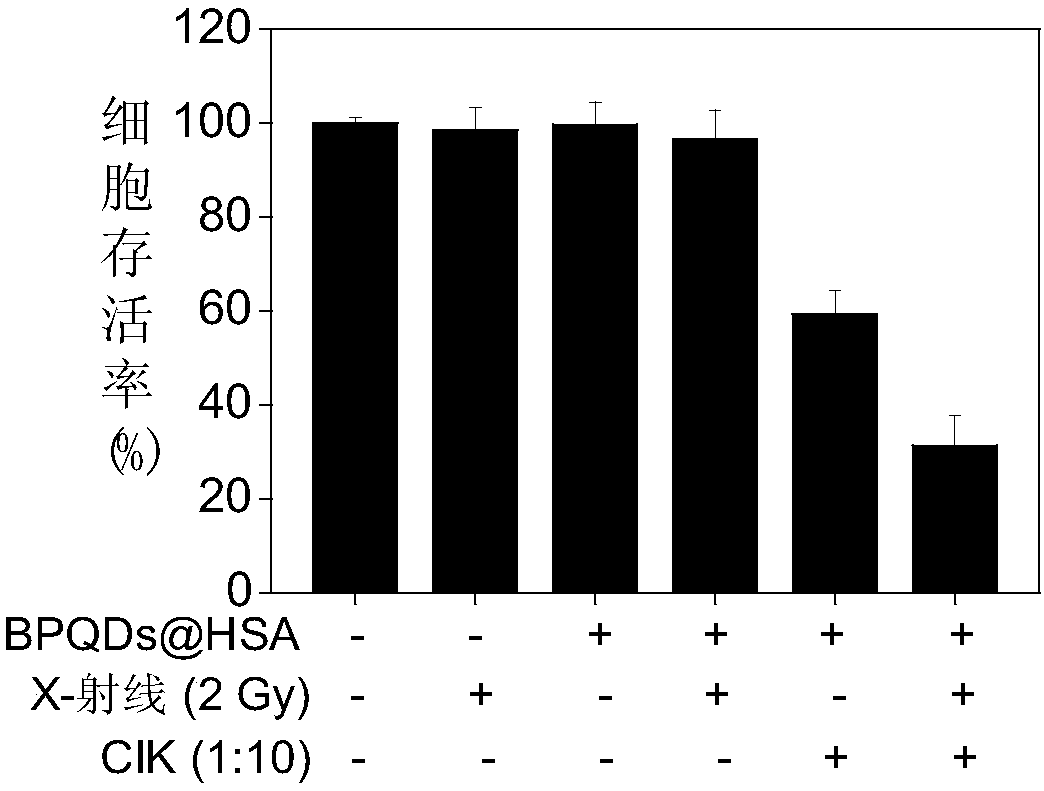
Preparation of human serum albumin modified black phosphorus quantum dots and applications of the quantum dots as a sensitizer
ActiveCN108030919AReduced bioavailabilityGood blood compatibilityInorganic phosphorous active ingredientsNanotechnologyX-rayCell therapy
Preparation of human serum albumin modified black phosphorus quantum dots and applications of the quantum dots as a sensitizer are disclosed. A preparing method of the human serum albumin modified black phosphorus quantum dots includes (1) uniformly dispersing black phosphorus quantum dots into water to obtain an aqueous solution of the black phosphorus quantum dots; and (2) adding an aqueous solution of human serum albumin into the aqueous solution of the black phosphorus quantum dots obtained in the step (1), stirring the mixture, and performing centrifugation to obtain a precipitate that isthe human serum albumin modified black phosphorus quantum dots. The obtained human serum albumin modified black phosphorus quantum dots have a CIK cell therapy sensitizing characteristic, can effectively regulate immune cell cytokine secretion of CIK immune cells, sensitize CIK cells to suppress tumor cell proliferation, have a radioactivity sensitizing characteristic, and cooperate with X rays to inhibit tumor growth. The human serum albumin modified black phosphorus quantum dots can be developed as a novel sensitizer for immunotherapy and radioactive therapy and promote development of novelclinical antitumor medicines.
Owner:JINAN UNIVERSITY
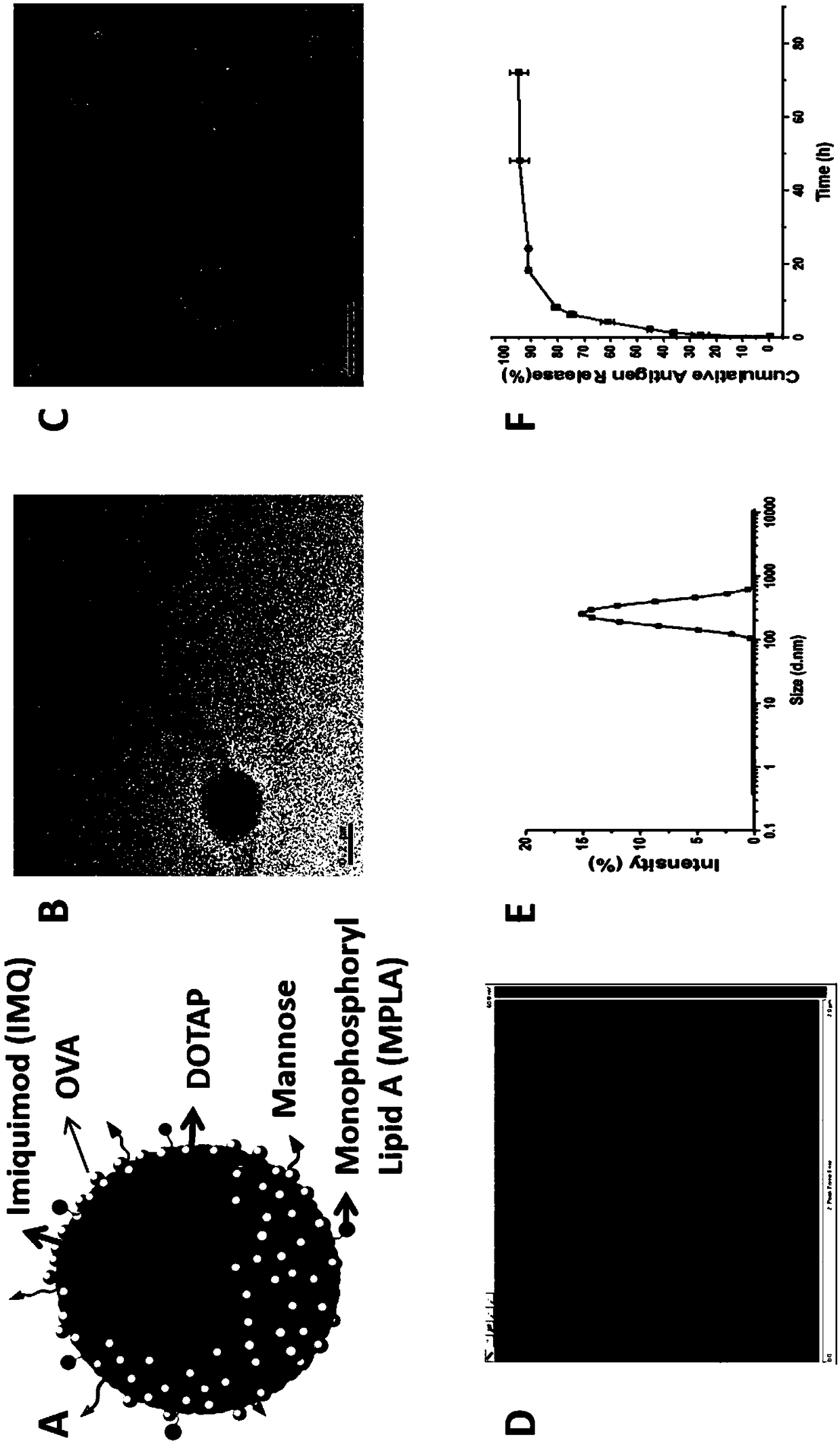
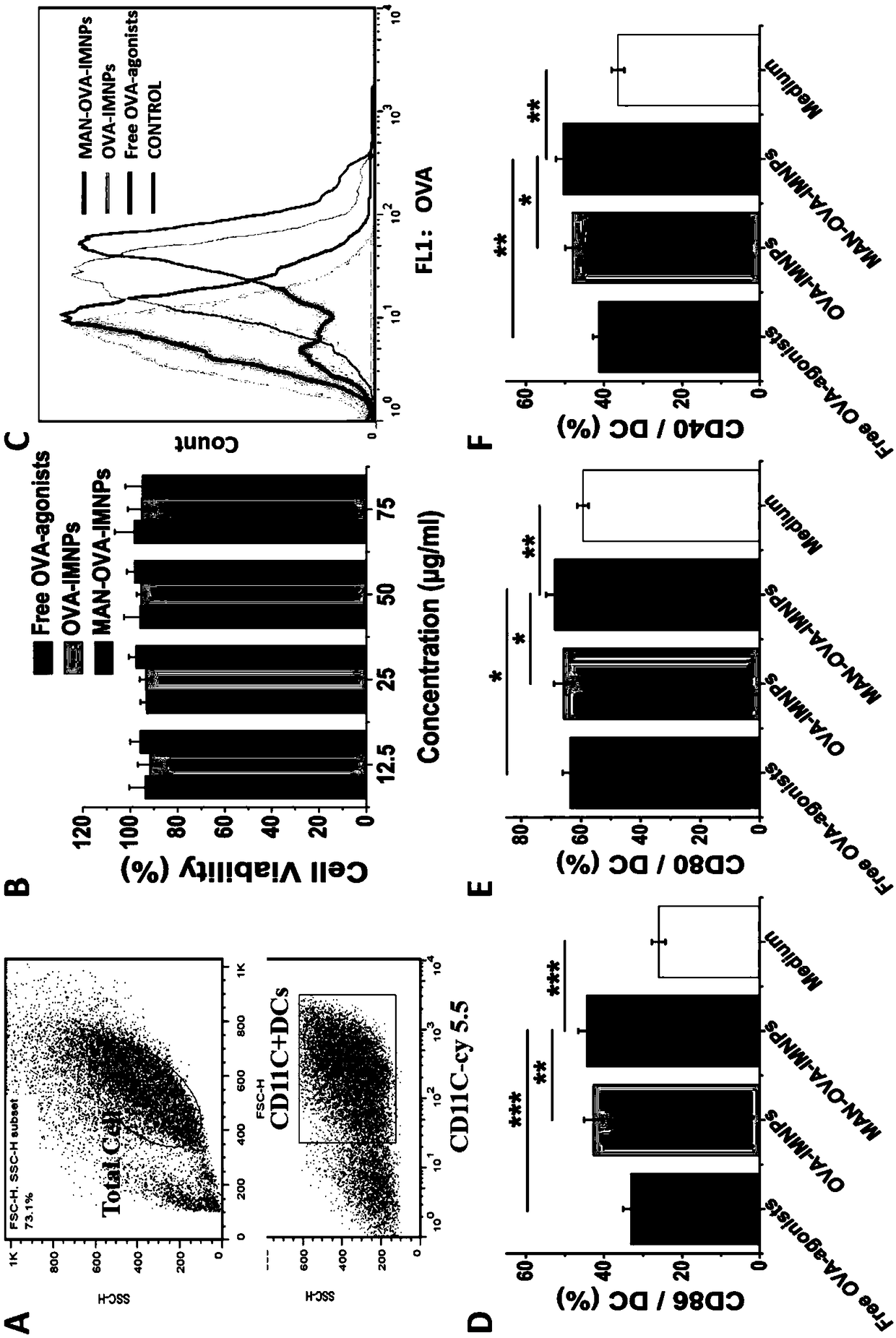
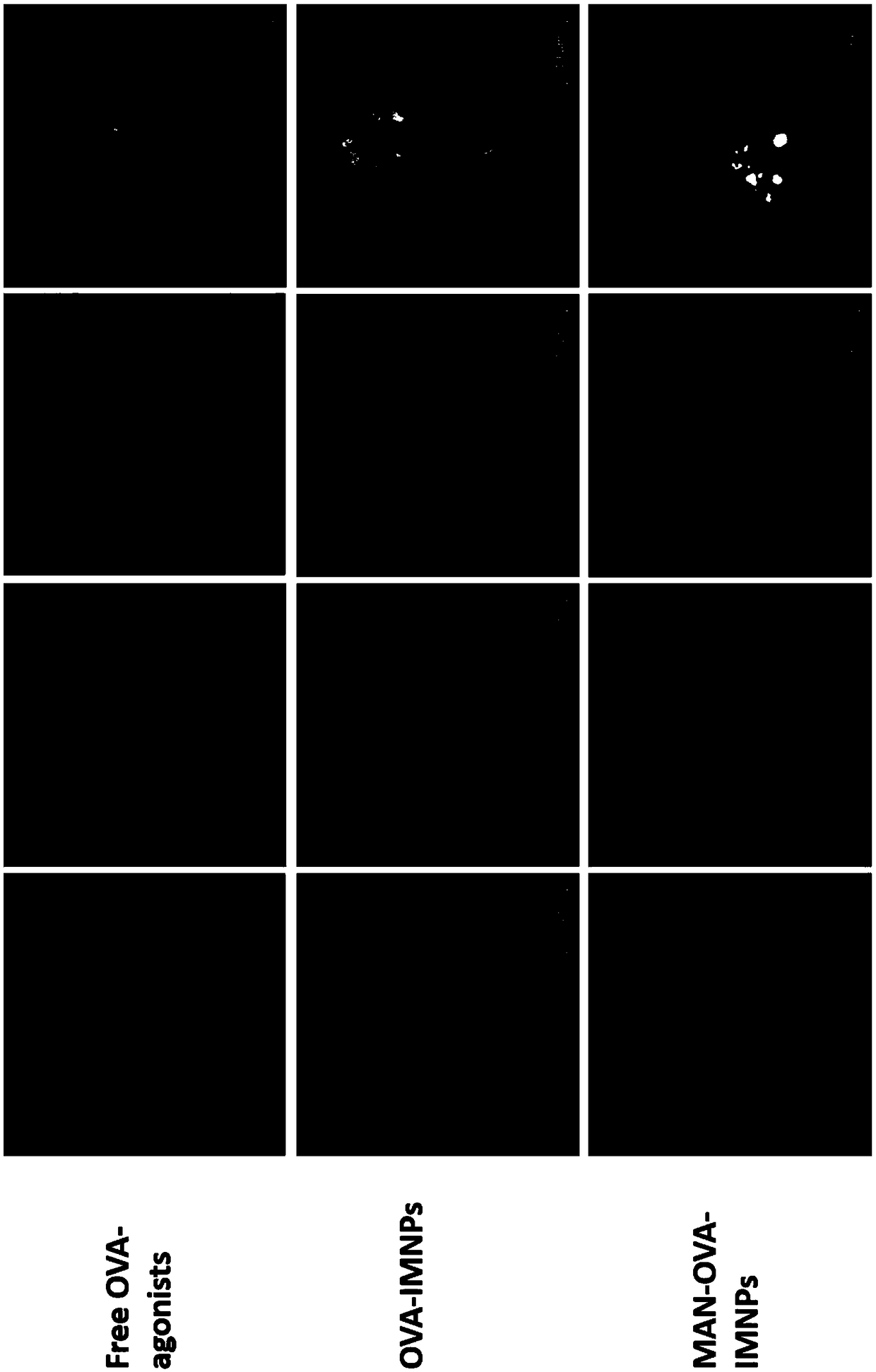
Antigen and TLR agonist targeting co-loaded cationic phospholipid-polymer hybrid nanoparticle vaccine adjuvant, and preparation method and application thereof
ActiveCN108992666AStrong activationRealize the loadCancer antigen ingredientsPharmaceutical non-active ingredientsDendritic cellPhospholipid
The invention relates to an antigen and TLR agonist targeting co-loaded cationic phospholipid-polymer hybrid nanoparticle vaccine adjuvant, and a preparation method and an application thereof. A hydrophobic inner core is encapsulated with a TLR7 agonist, a phospholipid layer is encapsulated with a TLR4 agonist, an antigen is adsorbed by cationic phospholipids in the phospholipid layer, and ligation of a mannose ligand makes the vaccine adjuvant like a specific targeting antigen to have the dendritic cell presenting ability, so the DC cell intake and the maturation promoting effect are enhanced; and the antigen is protected by hybridized nanoparticles, the antigen uptake by DC cells is improved, immune response after antigen stimulation is significantly enhanced by the TLR agonist, and thecross-presentation of the antigen is significantly improved, so vaccine adjuvant has a strong potent T cell killing effect, can induce cytokine secretion, has a long-acting memory T cell response, andhas an excellent prevention effect on tumors.
Owner:INST OF BIOMEDICAL ENG CHINESE ACAD OF MEDICAL SCI
Methods for treating pustular conditions of the skin
InactiveUS20090004161A1Promote wound healingLess scarringCosmetic preparationsOrganic active ingredientsProgenitorPustulosis
The invention is directed to methods for treating pustular conditions of the skin, for example, acne. Such methods utilize novel compositions, including but not limited to extraembryonic cytokine secreting cells (herein referred to as ECS cells), including, but not limited to, amnion-derived multipotent progenitor cells (herein referred to as AMP cells), conditioned media derived therefrom (herein referred to as amnion-derived cellular cytokine solution or ACCS), cell lysates derived therefrom, and cell products derived therefrom, each alone or in combination.
Owner:STEMNION
Methods of diagnosing and treating inflammatory bowel disease
InactiveUS20150376707A1Reduce probabilityBiocideMicrobiological testing/measurementEnteropathyCytokine secretion
The present invention also provides various methods, kits and compositions for diagnosing, prognosing, and treating various conditions including but not limited to inflammatory bowel diseases, such as ulcerative colitis and Crohn's disease. Also, the present invention provides various methods, kits and compositions for determining susceptibility to or a low probability of various conditions including but not limited to inflammatory bowel diseases, such as ulcerative colitis and Crohn's disease. These methods, kits and compositions may involve detecting risk / protective variants or haplotypes, serological markers, increased or decreased gene methylation, and increased or decreased cytokine secretion.
Owner:CEDARS SINAI MEDICAL CENT
Methods and reagents for the treatment of immunoinflammatory disorders
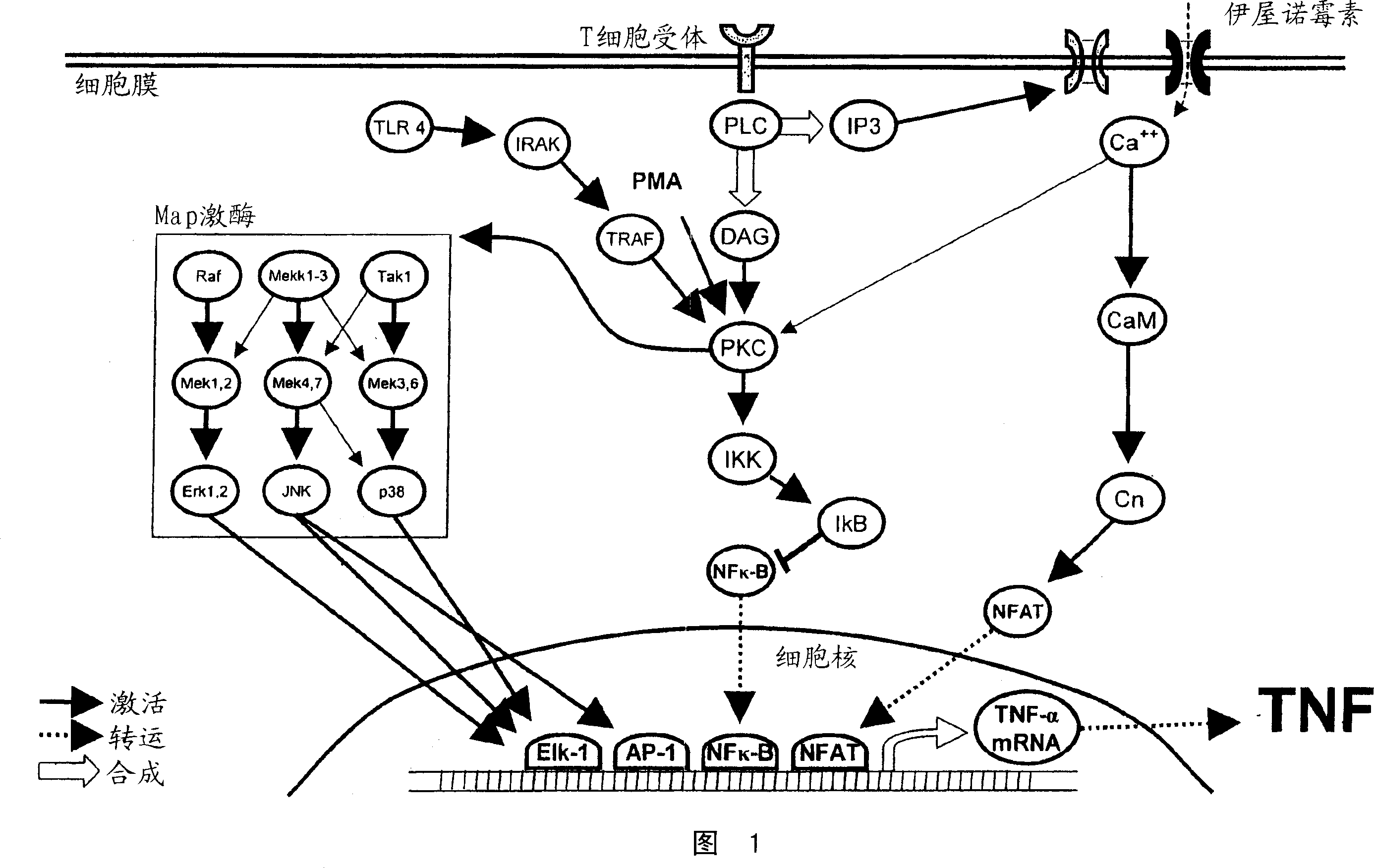
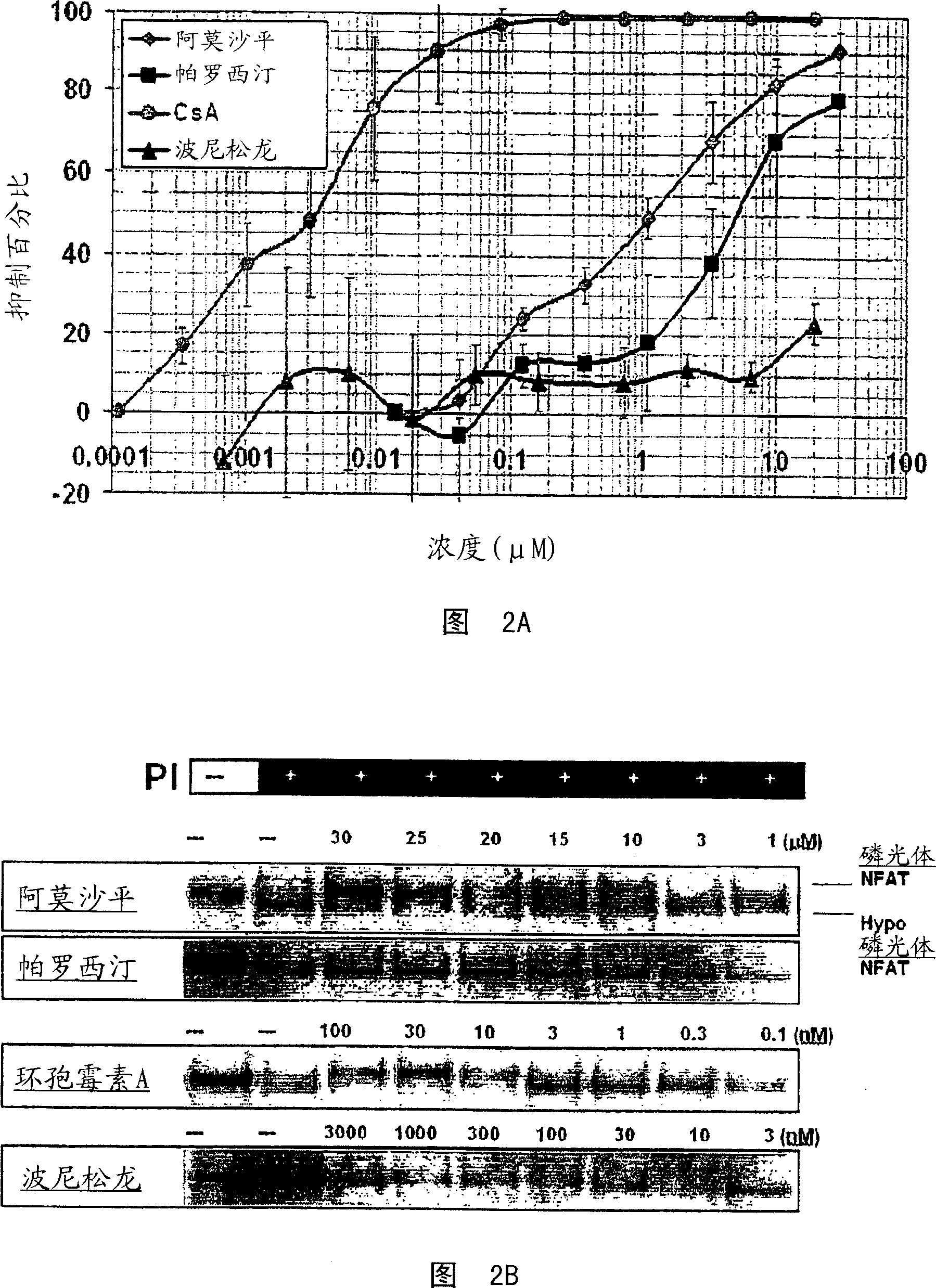
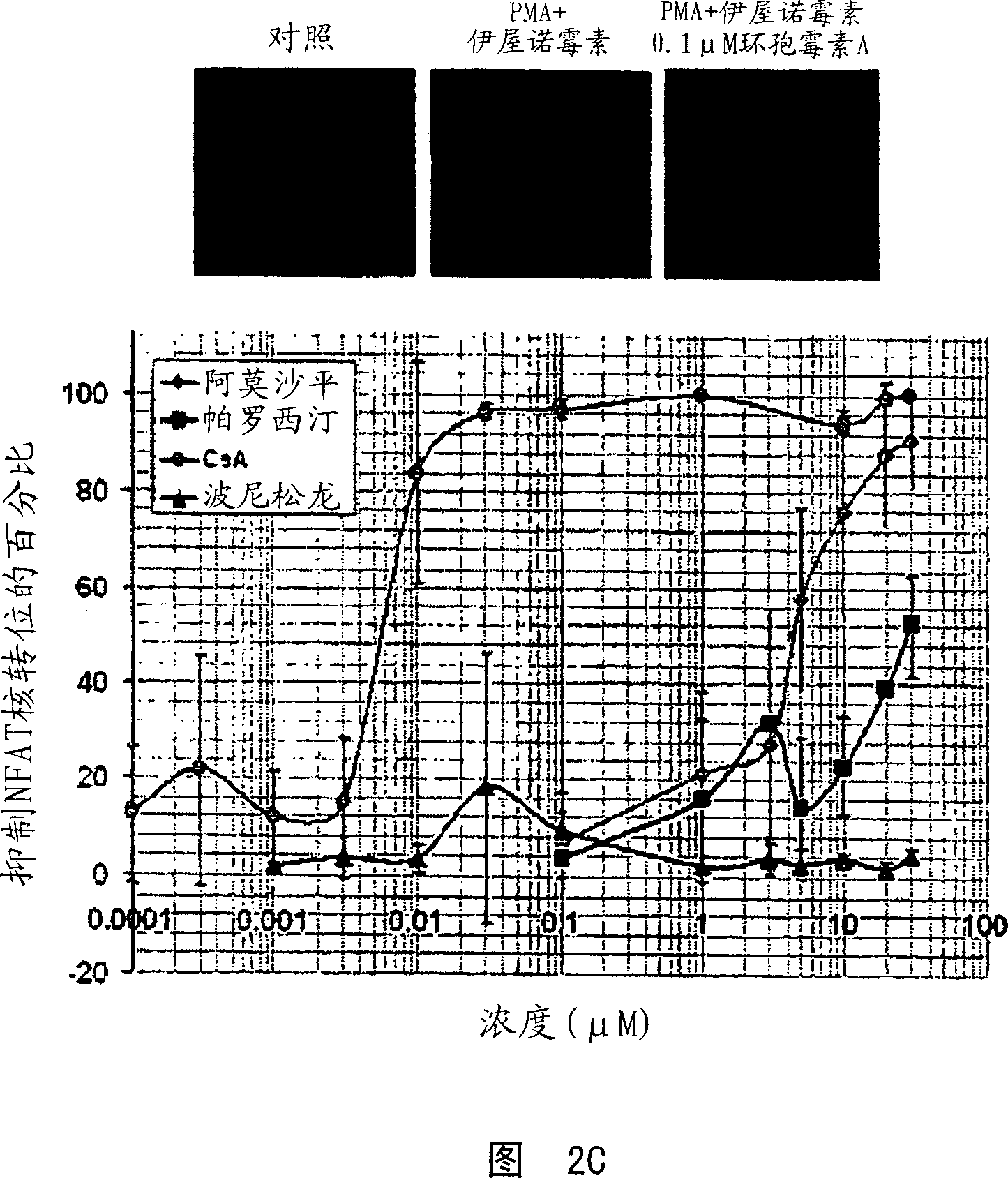
The invention involves the treatment, prevention, and reduction of immunoinflammatory disorders involving the combination of an agent that increases the signal activity of a glucocorticoid receptor (e.g., glucocorticoid receptor agonist) and an agent that modulates the signaling activity of one or more signaling pathways selected from the NF-kappaB pathway, NFAT pathway, AP-1 pathway, and Elk-1 pathway such that proinflammatory cytokine secretion or production, or any other inflammatory response, is reduced. Further, screening methods are provided for identifying candidate compounds and strategies useful for treating, preventing, or reducing such conditions.
Owner:COMBINATORX
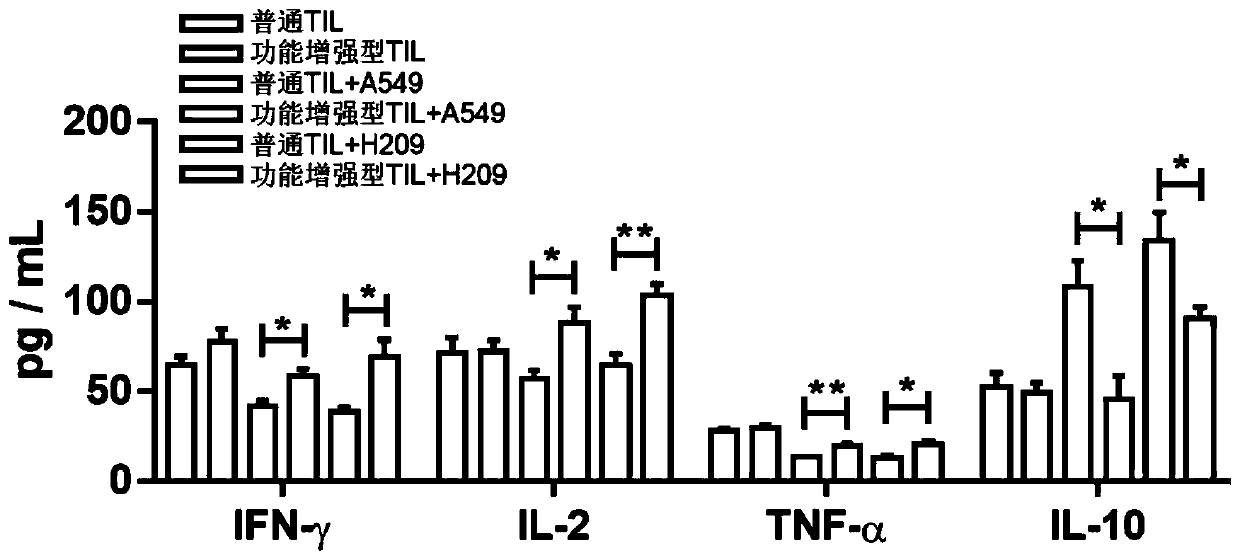
Culture method for functionally enhanced TILs
PendingCN110938594AHigh activityPromote secretionBlood/immune system cellsCell culture active agentsLymphocyte cultureTumor cells
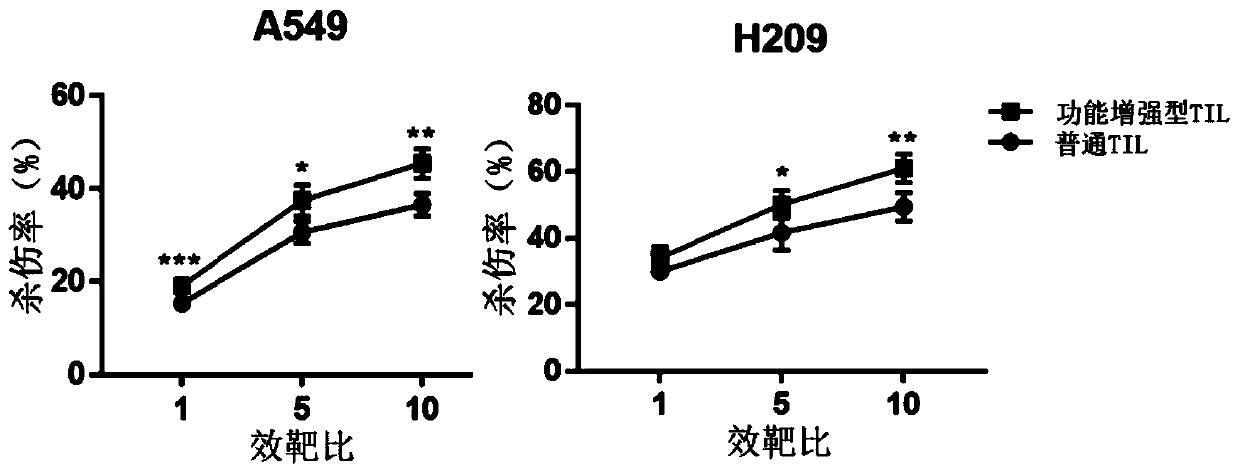
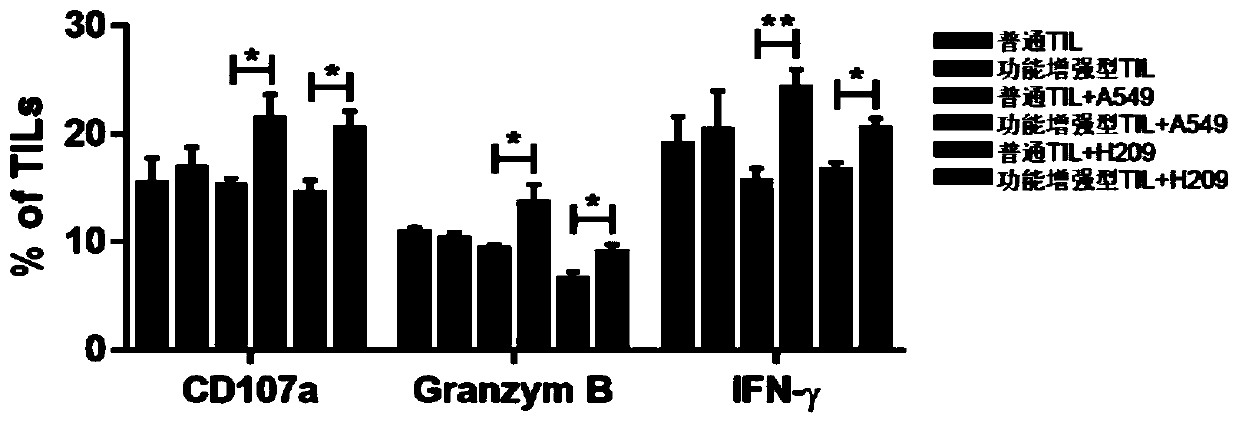
The invention discloses a culture method for functionally enhanced tumor infiltrating lymphocytes (TILs). The method includes the following steps: separating lymphocytes from tumor tissue, adding a start culture medium, performing inoculation in a 12-well culture plate (4 ml / well), performing start lymphocyte culture for 10 days or 14 days to obtain start TILs, and performing cryopreservation on the obtained start TILs for standby application; suspending the lymphocytes in a 25 cm<2> culture flask (20 ml / flask) by using an induction culture medium, placing the culture flask in an incubator having 5% CO2 at 37 DEG C, and performing TIL induction culture for 1 day; performing half quantity change by using an expansion culture medium, and performing expansion flask culture and expansion bag culture for 13 days or 14 days; on the 14th or 15th day of culture, collecting TILs, performing washing by using normal saline, and resuspending the TILs by using a function enhancement culture medium,and performing incubation for 30 min; and collecting TILs, and performing functional test to obtain the functionally enhanced TILs. The culture method described in the invention can obtain the functionally enhanced TILs with stronger tumor cell killing activity and higher-level anti-tumor cytokine secretion ability.
Owner:SUN YAT SEN UNIV CANCER CENT
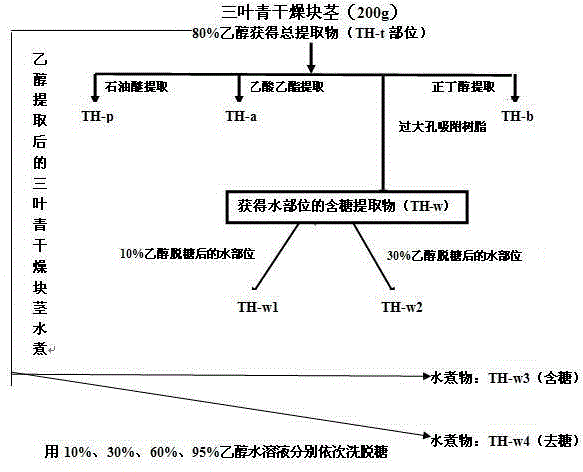
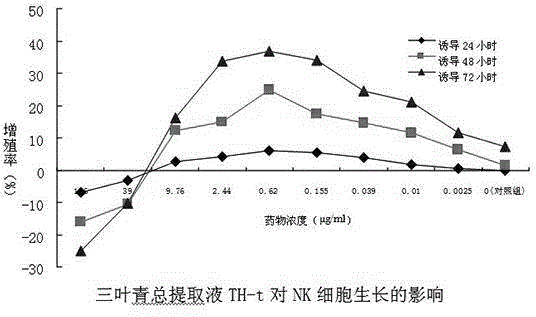
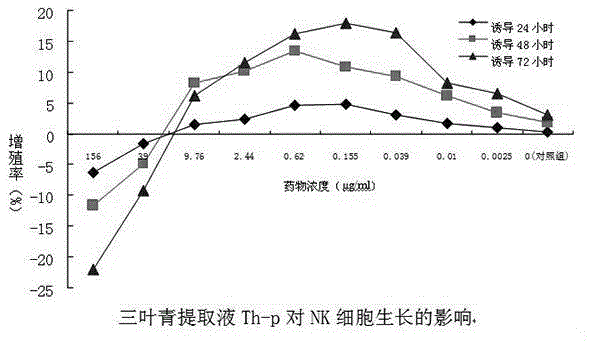
NK cell in-vitro induced amplification method
InactiveCN104651310AOptimizing the best protocol for inducing NK cellsHigh purityBlood/immune system cellsHigh cellSecreted substance
The invention provides an NK cell in-vitro induced amplification method which comprises the following step: adding a radix tetrastigme extract into an NK cell culture solution, wherein the concentration of the radix tetrastigme extract is 0.00625-9.76 mu g / ml, and the radix tetrastigme extract is radix tetrastigme total extract Th-t, boiled desugarized part Th-w3, petroleum ether part Th-p or water part desugarized part Th-w2. According to the method, abundant NK cells with higher application safety, higher purity and higher killing activity can be obtained under in-vitro culture conditions, so that the cells achieve the quality indexes for safe and effective clinical application. The method is simple to operate, low in cost and suitable for large-scale application. The NK cells obtained by the method have obviously higher cell number, purity, killing activity and cell factor secretion level. When the method is used for culture, the cell number is enhanced by thousands of times.
Owner:浙江卫未生物医药科技有限公司
Methods for treating inhalation injury
InactiveUS20120207717A1Promote healingEasy to useBiocidePeptide/protein ingredientsProgenitorInhalation
The invention is directed to methods for treating inhalation injuries. Such methods utilize novel cell compositions such extraembryonic cytokine secreting (ECS) cells and Amnion-derived Multipotent Progenitor (AMP) cells and novel cellular factor-containing solution compositions such as extraembryonic cell-derived cellular cytokine solution, Amnion-derived Cellular Cytokine Solution (ACCS), and physiologic cytokine solution (PCS) compositions. The compositions may be used alone or in combination with each other and / or other agents.
Owner:STEMNION
Methods for tailoring the immune response to an antigen or immunogen
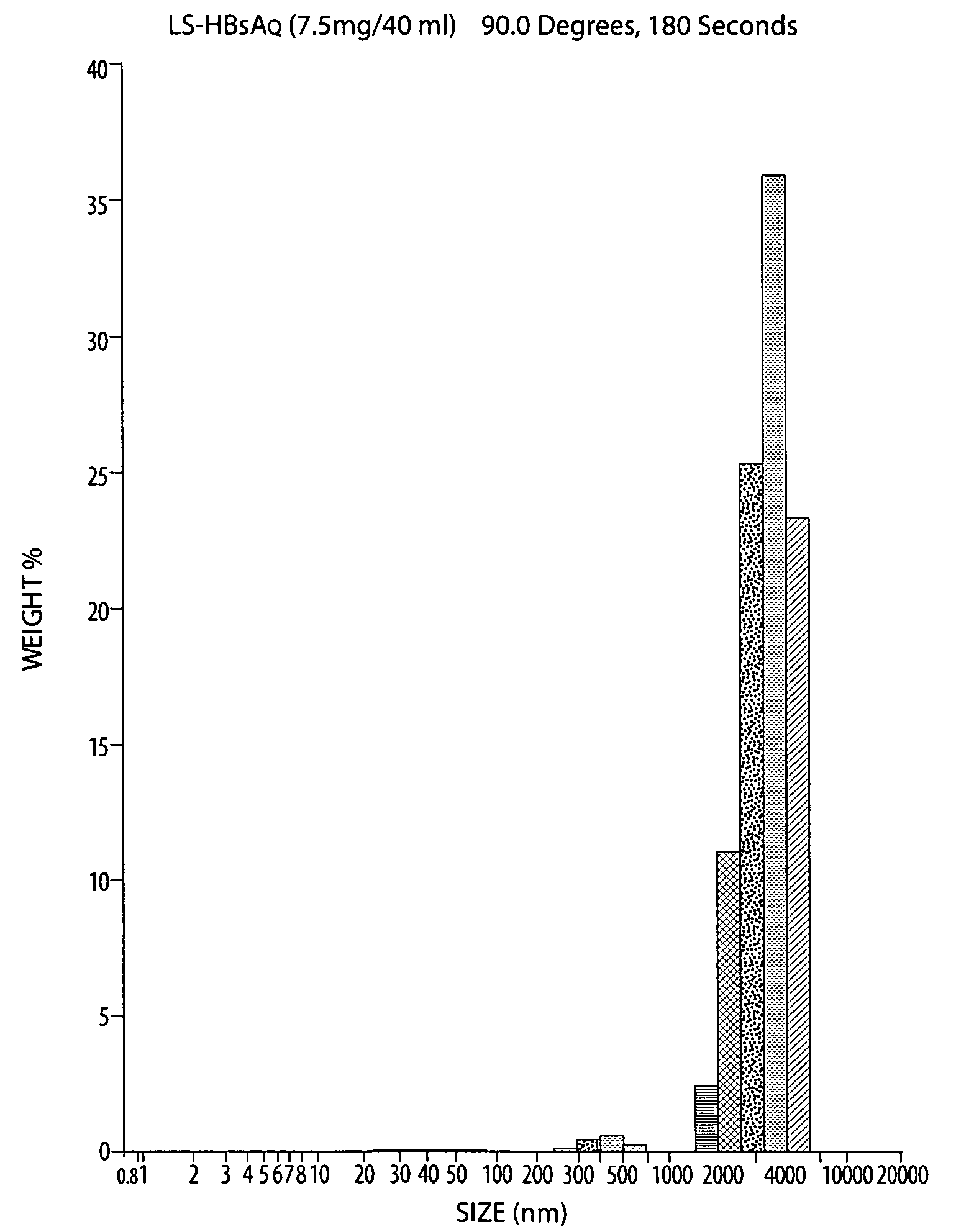
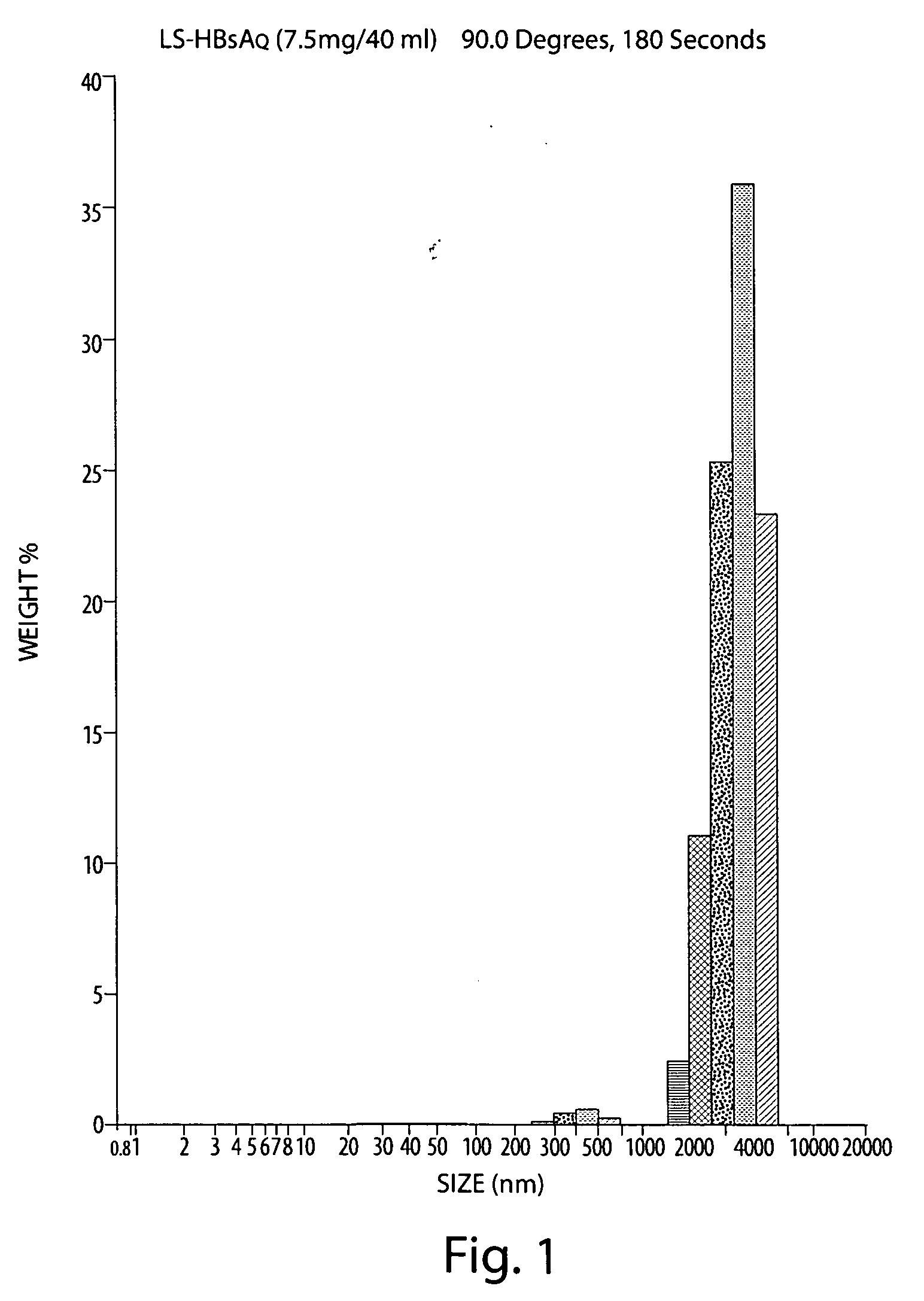
The invention relates to methods and reagents for immunizing animals to elicit specific cellular and humoral immune-responses against specific antigens, such as viral antigens, including HBsAg antigen. The invention provides methods of using specifically prepared immunogen in fresh or lyophilized liposome, proper routes of administration of the immunogen, proper doses of the immunogen, and specific combinations of heterologous immunization including DNA priming in one administration route followed by liposome-mediated protein antigen boost in a different route to tailor the immune responses in respects of enhancing cell mediated immune response, cytokine secretion, humoral immune response, immune protection and selective skewing of T helper responses to be Th1, Th2, or a mixed or balanced Th response.
Owner:MUCOSAL VACCINE TECH LLC
Natural killer cell lines and methods of use
InactiveUS20140099714A1Peptide/protein ingredientsGenetically modified cellsCancer cellWhite blood cell
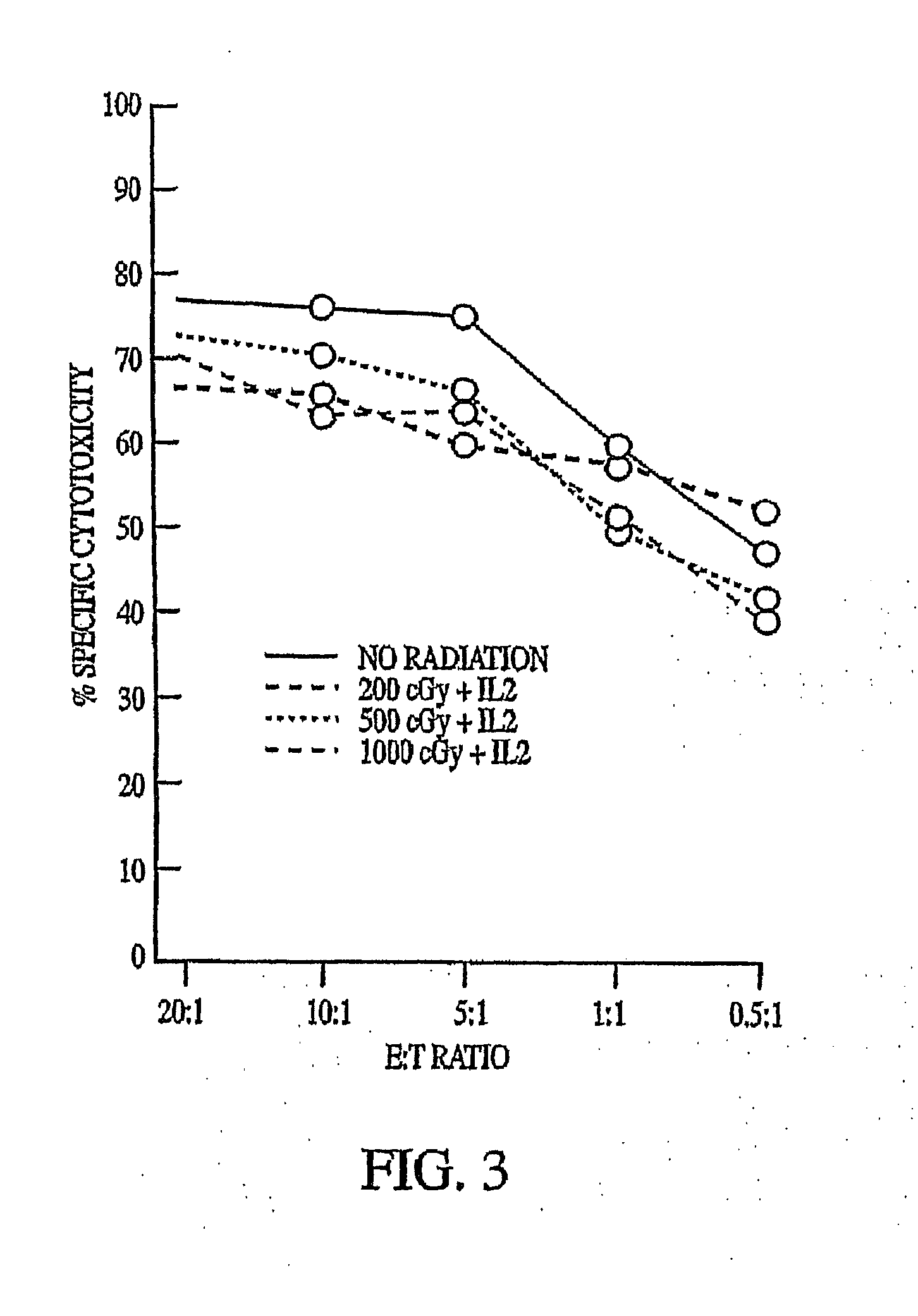
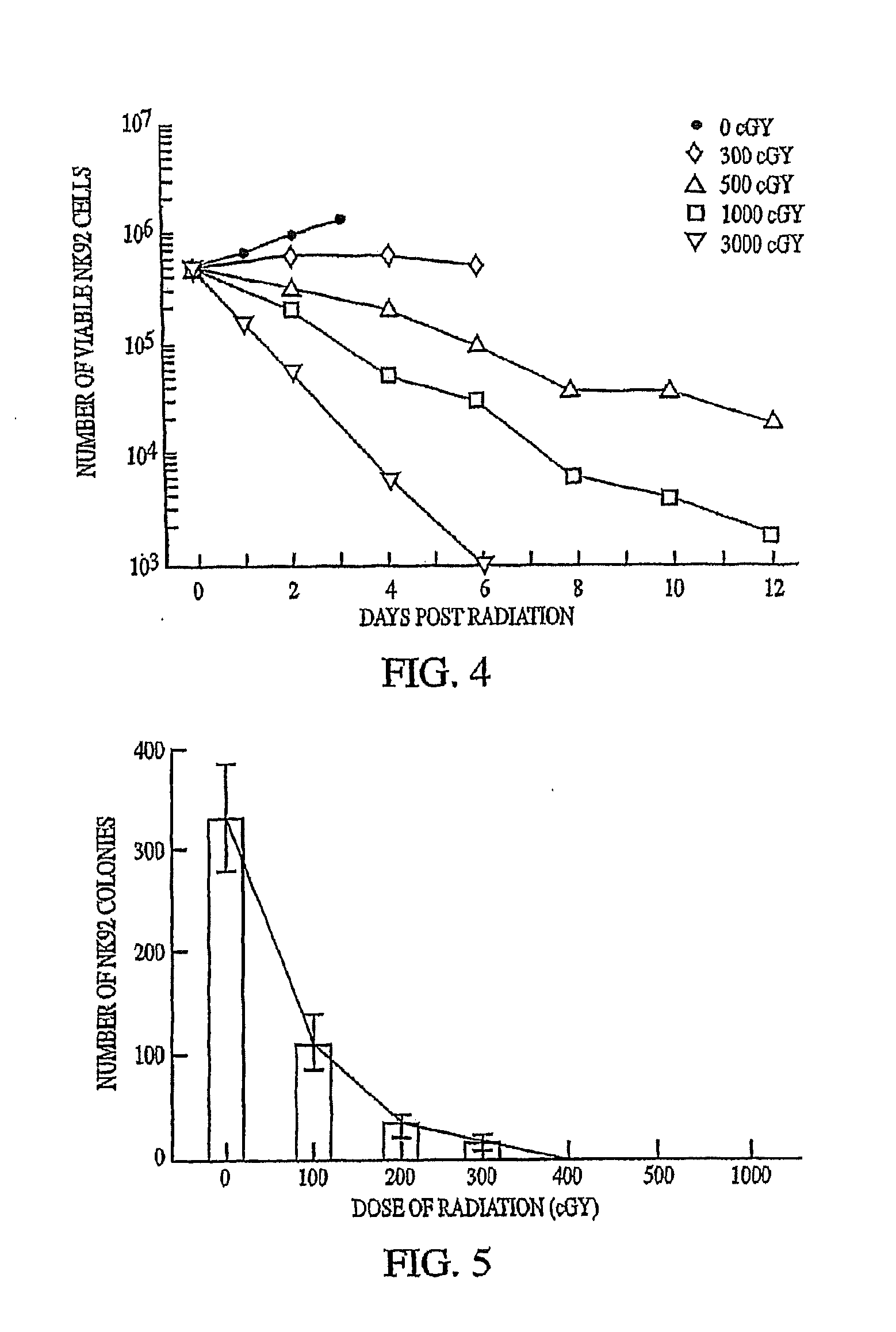
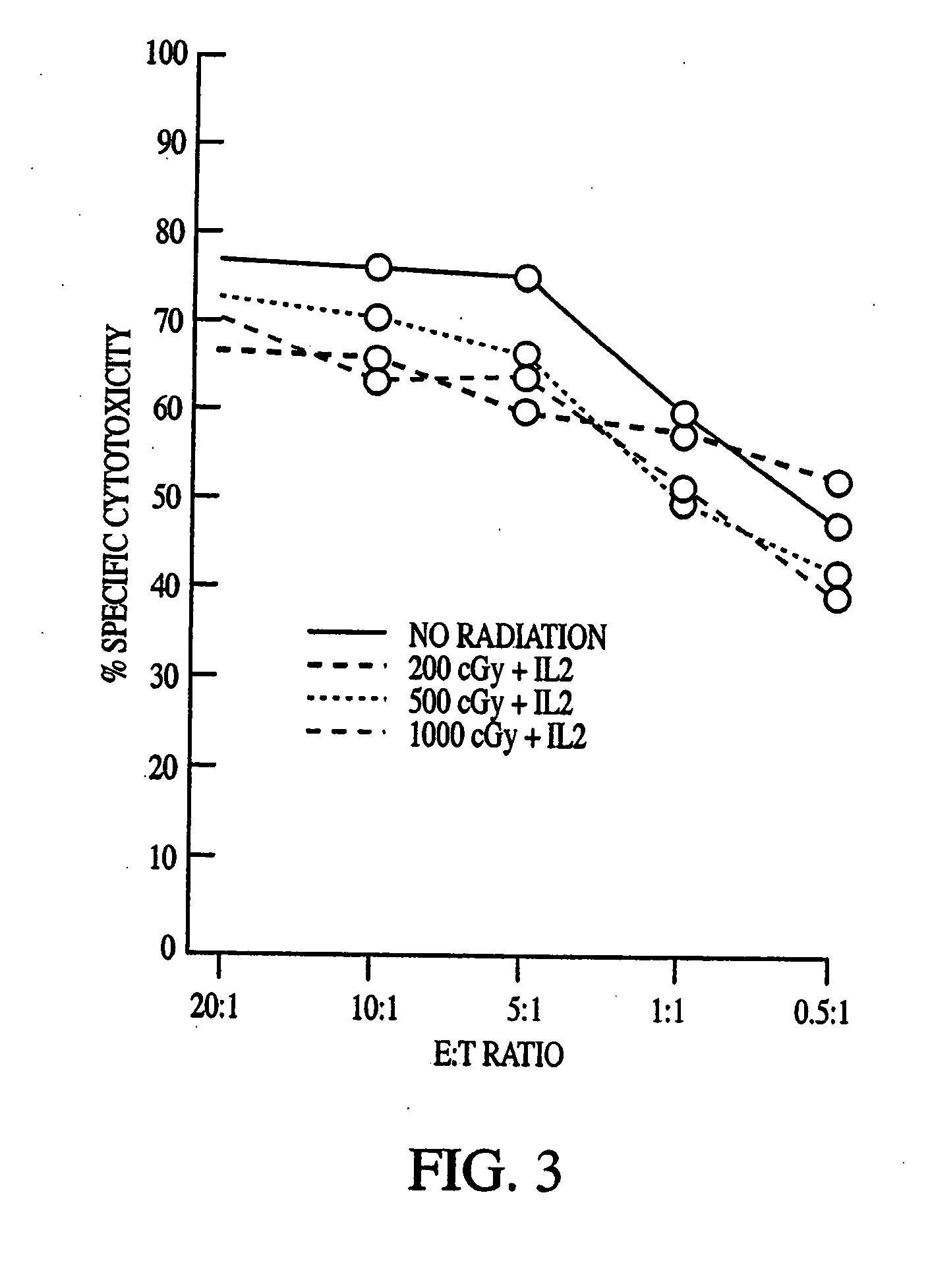
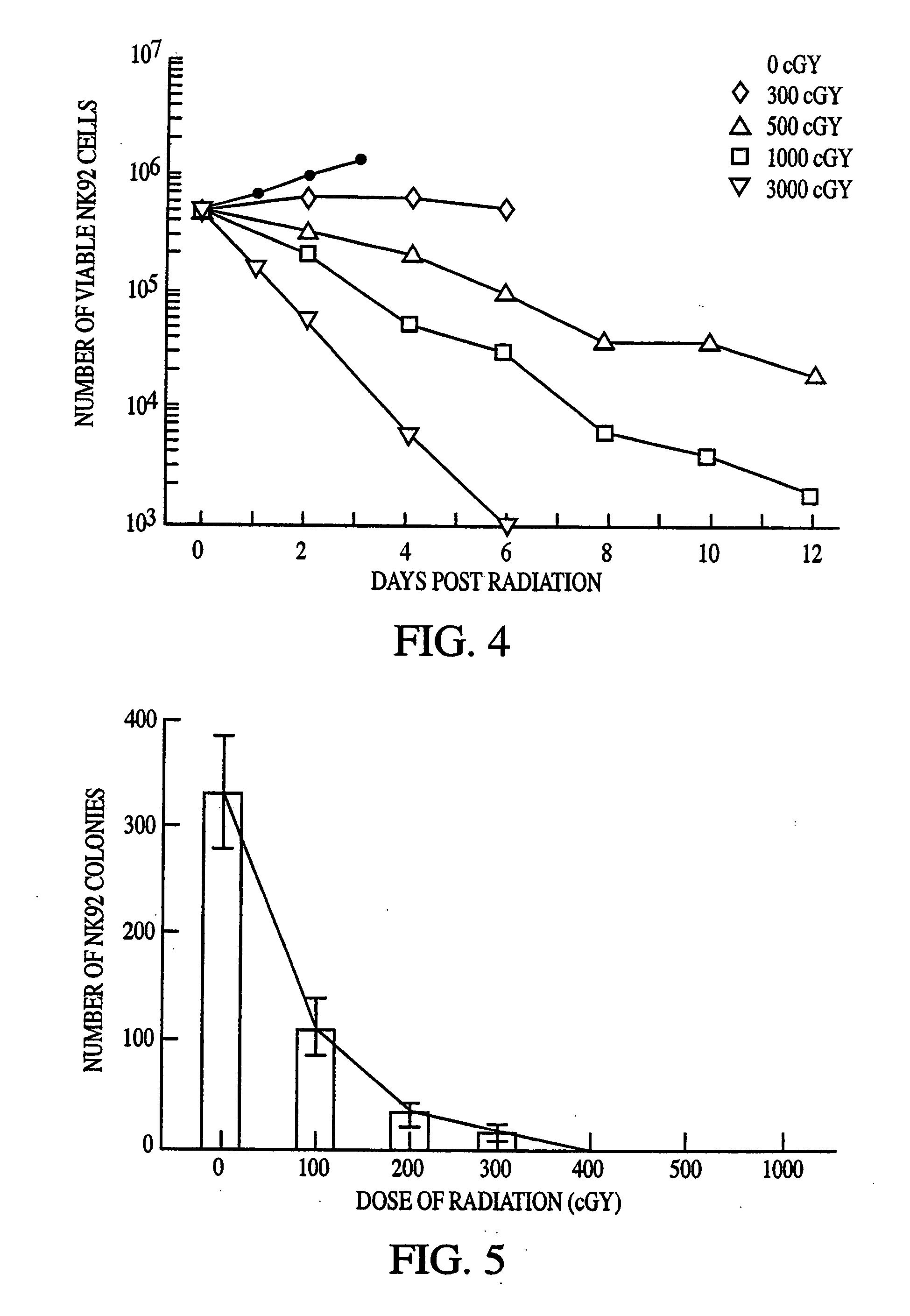
This invention relates to a natural killer cell line termed NK-92. The invention provides a vector for transfecting a mammalian cell which includes a nucleic acid sequence encoding a cytokine that promotes the growth of NK-92. Additionally, the invention provides an NK-92 cell, or an NK-92 cell modified by transfection with a vector conferring advantageous properties, which is unable to proliferate and which preserves effective cytotoxic activity. The invention further provides a modified NK-92 cell that is transfected with a vector encoding a cytokine that promotes the growth of NK-92 cells. The cell secretes the cytokine upon being cultured under conditions that promote cytokine secretion, and furthermore secretes the cytokine in vivo upon being introduced into a mammal. In a significant embodiment, the cytokine is interleukin 2. The present invention also provides methods of purging cancer cells from a biological sample, of treating a cancer ex vivo in a mammal, and of treating a cancer in vivo in a mammal employing a natural killer cell, such as NK-92 itself, an NK-92 cell which is unable to proliferate and which preserves effective cytotoxic activity, or natural killer cells transfected with a vector encoding a cytokine.
Owner:CONKWEST
Apc activators in combination with a cytokine-secreting cell and methods of use thereof
InactiveUS20090130054A1Good curative effectBiocidePeptide/protein ingredientsSecreting cellTumor cells
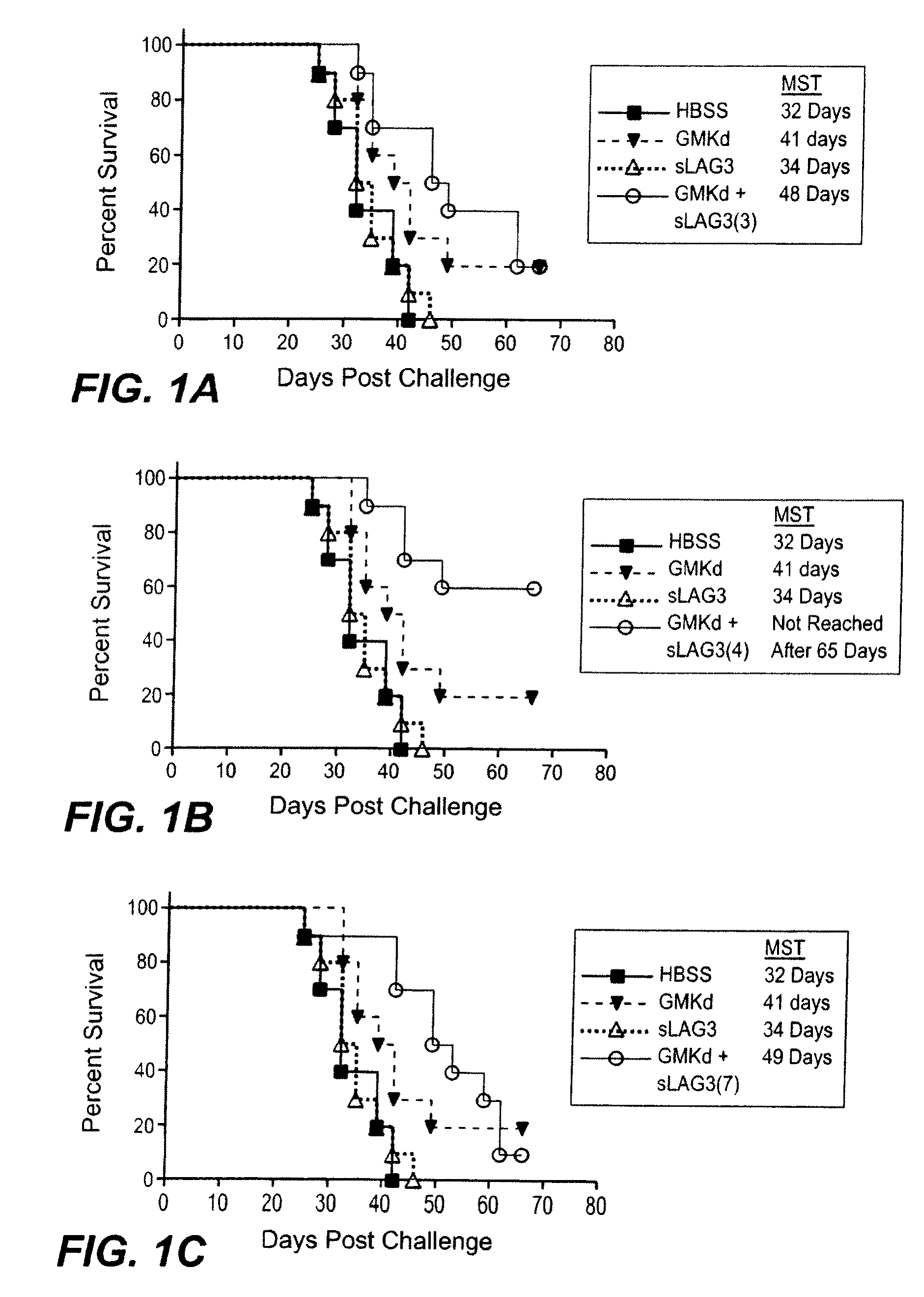
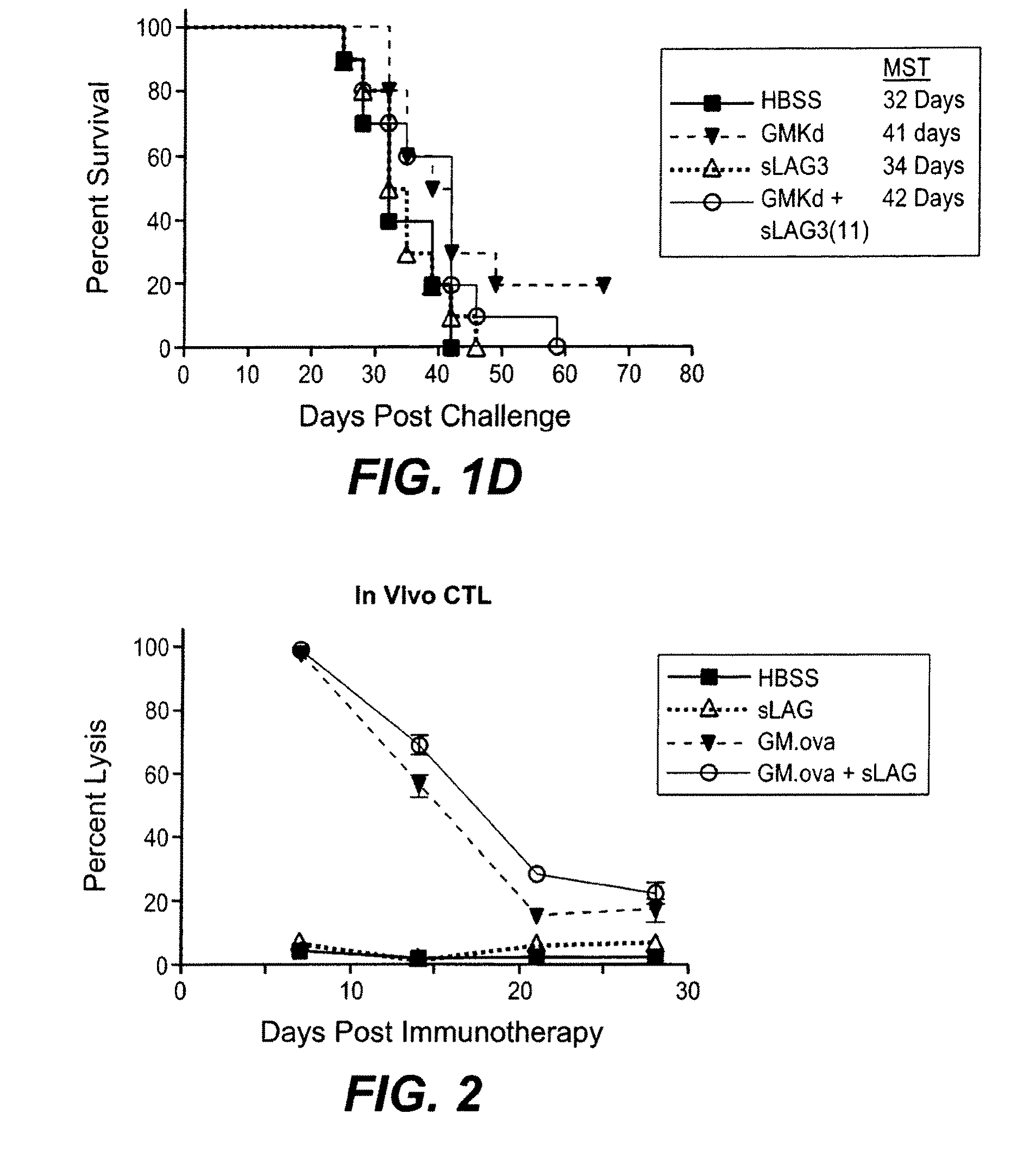
The present invention relates to a method of enhancing anti-tumor protection in a mammal. More particularly, the invention is concerned with combinations comprising antigen presenting cell (APC) activators and a cytokine-secreting cell and methods of administering the combination for enhanced immune response to tumor cells in a patient with a cancer.
Owner:IMMUTEP
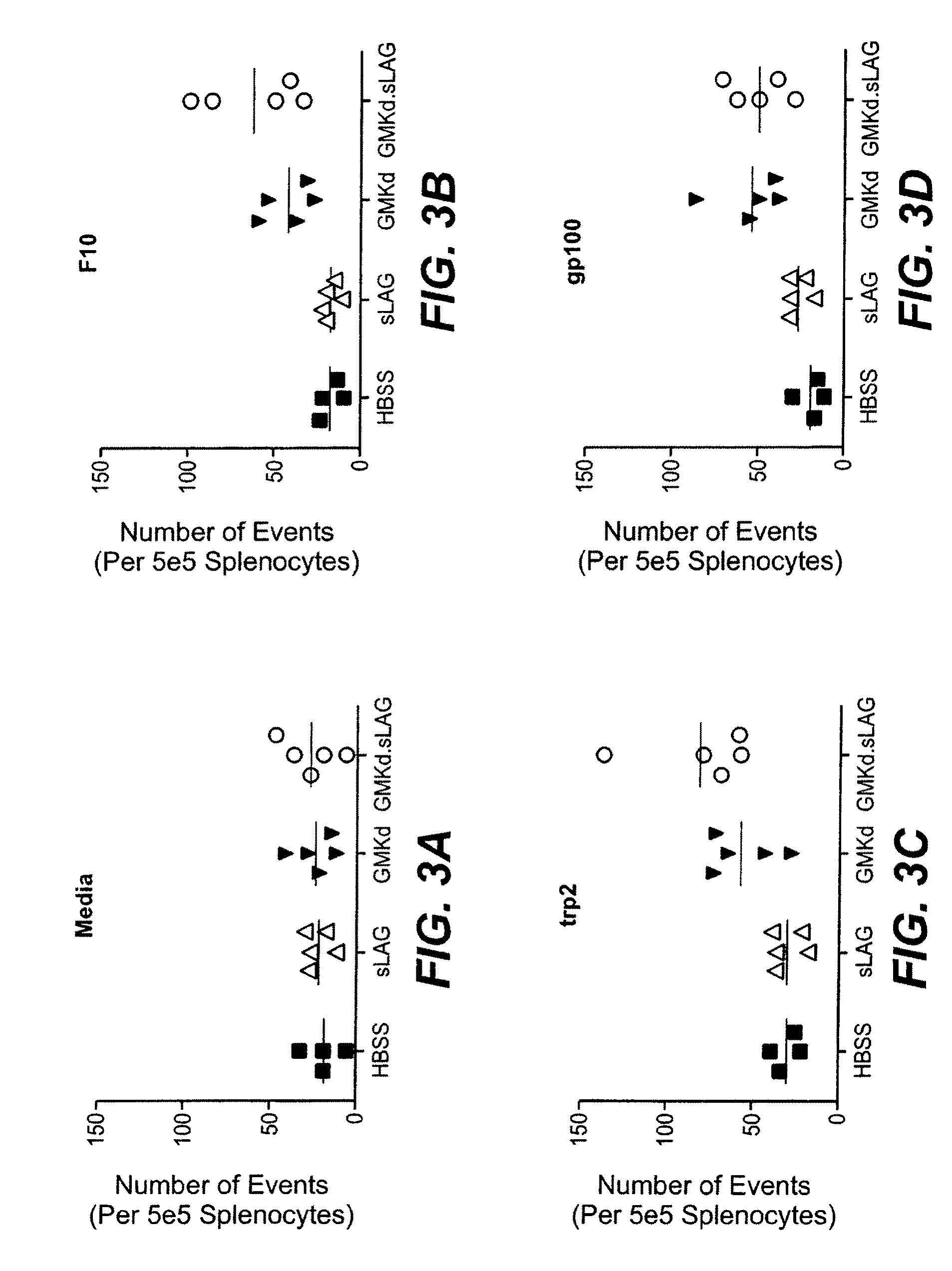
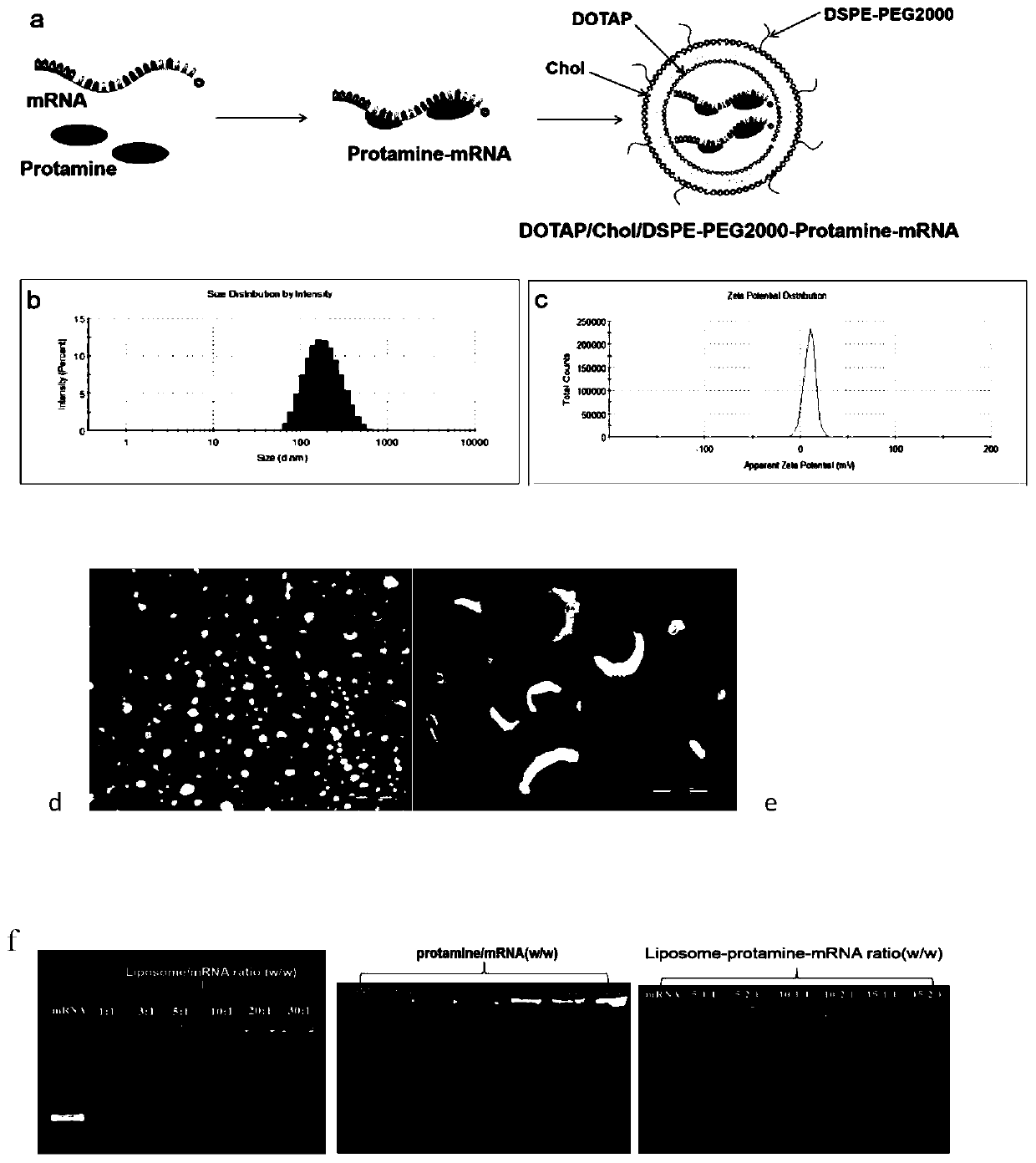
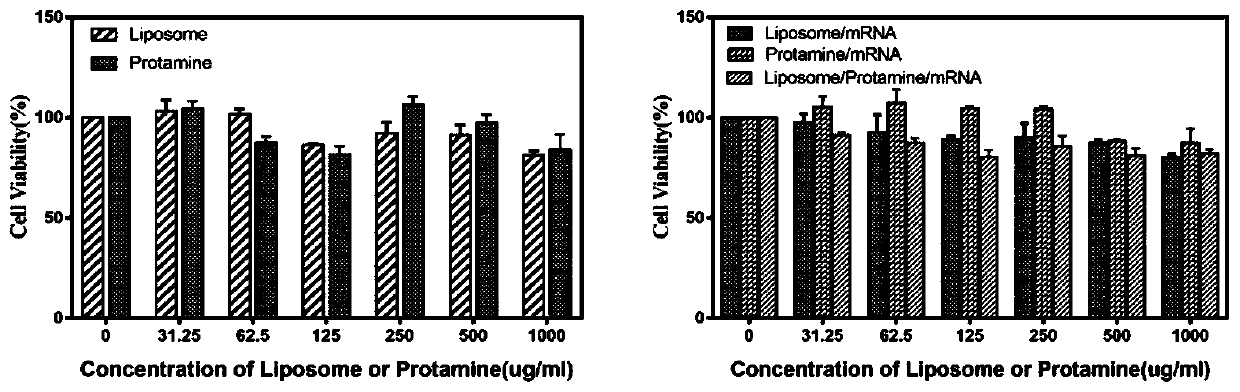
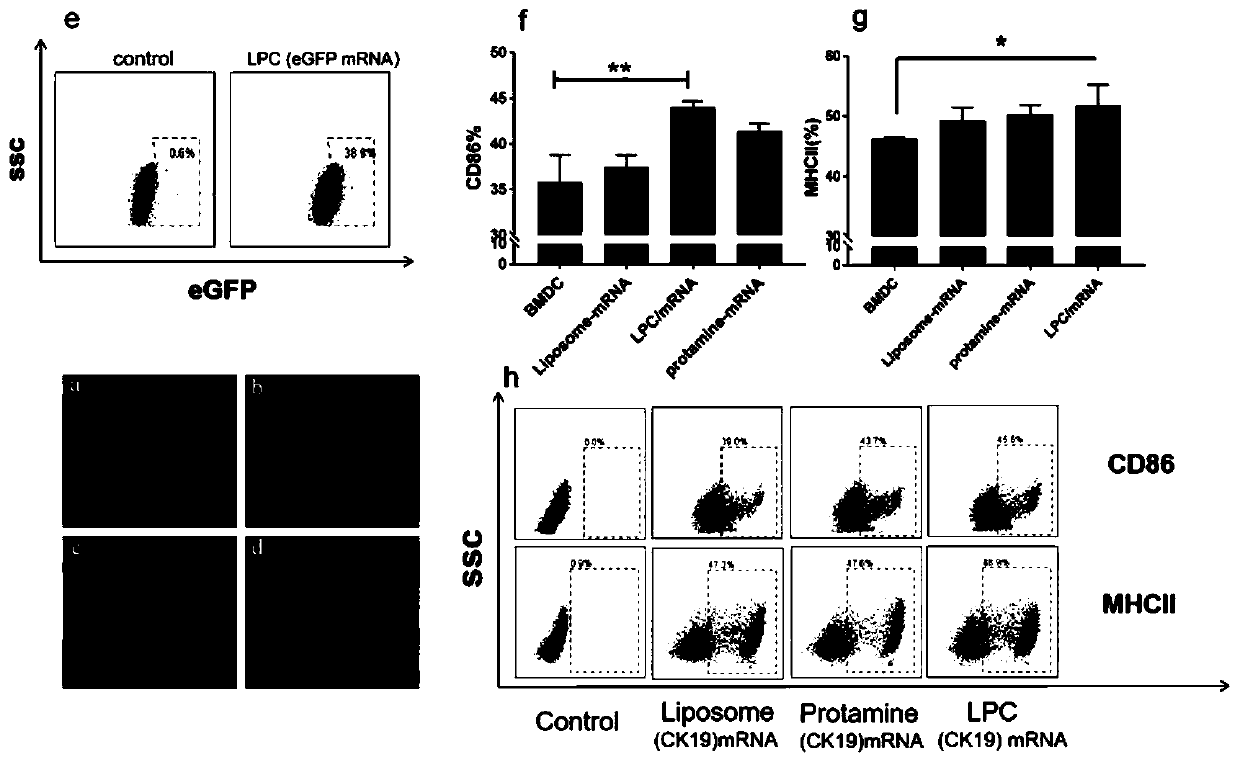
Cationic liposome-protamine-mRNA tumor vaccine and preparation method and application method thereof
PendingCN110882383ASynthesis fastRapid purificationSkin cancer vaccineCancer antigen ingredientsAdjuvantTumor therapy
The invention relates to a cationic liposome-protamine-mRNA tumor vaccine and a preparation method and an application method thereof. According to the tumor vaccine, a cationic liposome-protamine compound is used as a delivery carrier and adjuvant of mRNA, wherein the mass ratio of the cationic liposome to protamine to mRNA is 5:1: 1 to 15: 2:1, and the optimal mass ratio is preferably 10:1:1; theparticle size of the constructed cationic liposome-protamine-mRNA tumor vaccine is 50 to 800nm; the Zeta potential is 10 to 60mv; and the encapsulation efficiency is 70% to 95%. The cationic liposome-protamine-mRNA tumor vaccine prepared by the invention can effectively promote antigen uptake of APCs, induce DC stimulation and maturation, promotes secretion of cytokines and cause anti-tumor immune response. The tumor vaccine is used for immunotherapy through intramuscular and subcutaneous injection or nasal mucosa administration, wherein nasal mucosa administration can show a more excellent tumor treatment effect. The tumor vaccine has a wide application prospect in the aspect of tumor treatment.
Owner:NINGXIA MEDICAL UNIV
Method for screening molecules that restore NOD1 activity in cells containing an NOD2 mutation that reduces or eliminates NOD1 activity
InactiveUS20060251659A1Effective stimulationInduce cytokine responseMicrobiological testing/measurementGenetic material ingredientsGramCytokine secretion
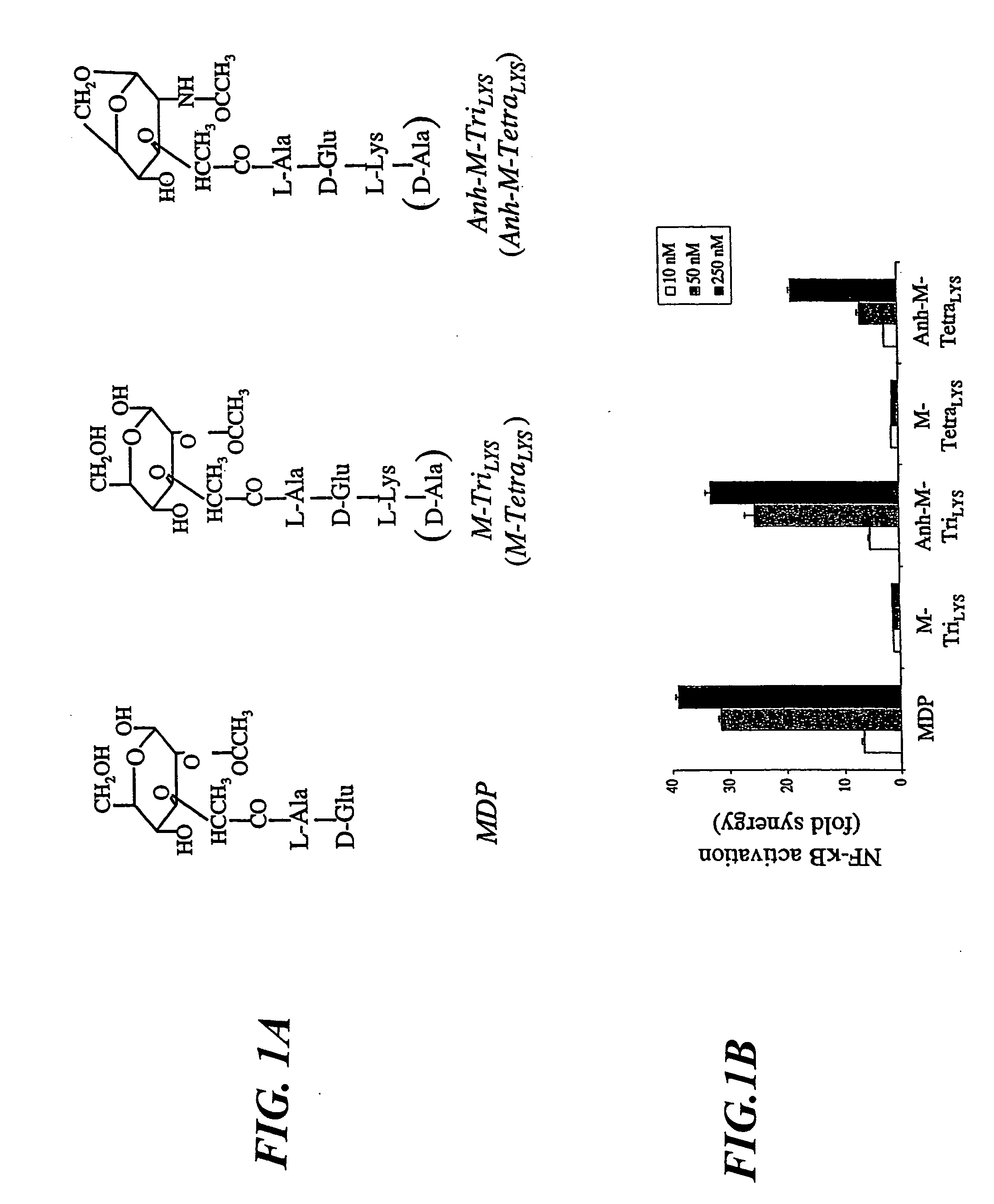
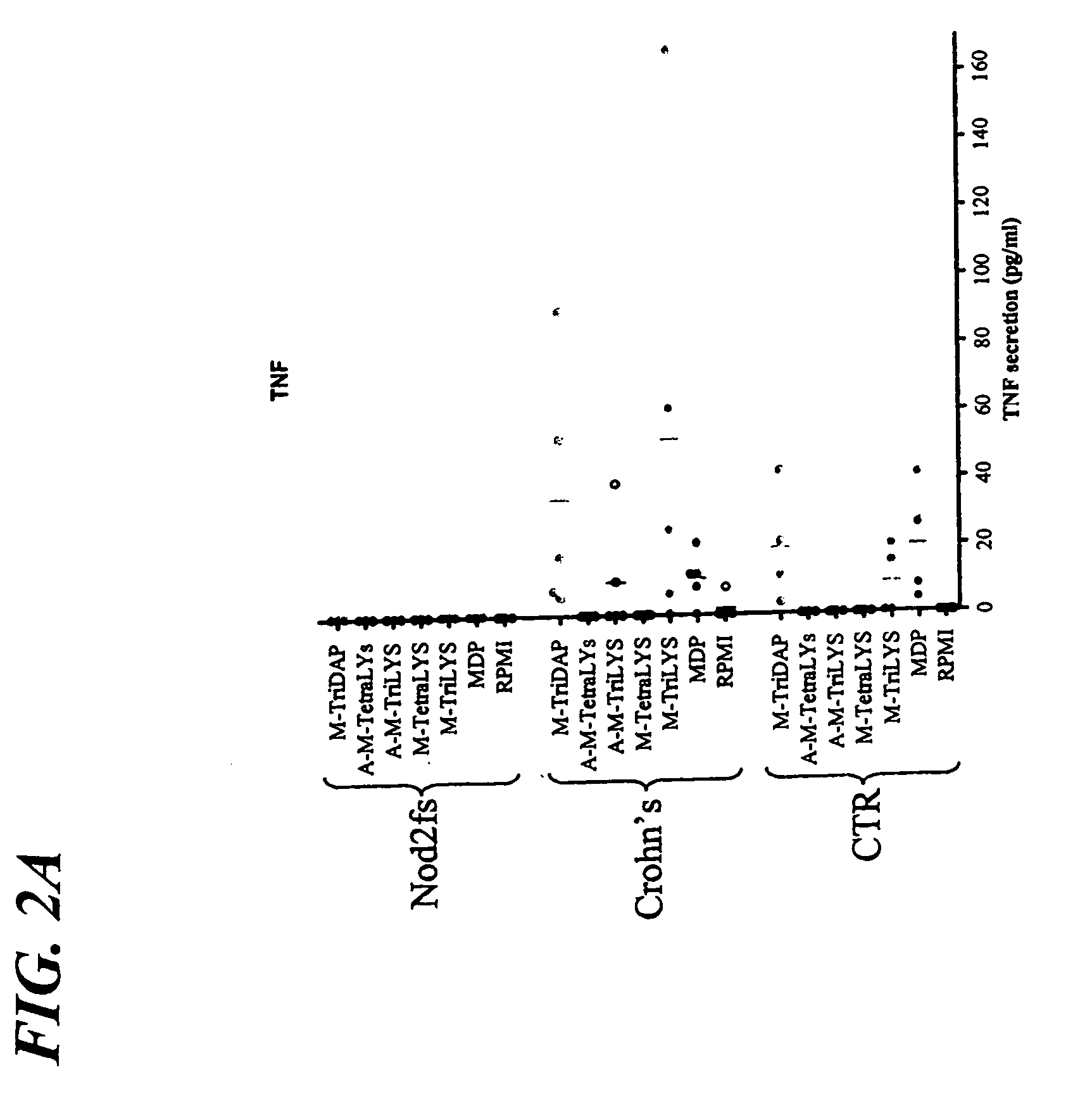
A method for identifying a molecule that restores Nod1 activity in cells which contain a Nod2 mutation that reduces or eliminates Nod1 activity. Nod2 / CARD15 is the first characterized susceptibility gene in Crohn's disease. The Nod2 1007fs (Nod2fs) frameshift mutation is the most prevalent in Crohn's disease patients. Muramyl dipeptide (MDP) from bacterial peptidoglycan is the minimal motif detected by Nod2 but not by Nod2fs. The inventors investigated the response of human peripheral blood mononuclear cells (PBMCs) from Crohn's disease patients not only to MDP, but also to several other muramyl peptides. Unexpectedly, it was observed that patients homozygous for the Nod2fs mutation were totally unresponsive to MurNAc-L-Ala-D-Glu-mesoDAP (M-TriDAP), the specific agonist of Nod1. Accordingly, Gram-negative bacterial peptidoglycan, which can be detected by both Nod1 and Nod2, was unable to stimulate cytokine secretion from Nod2fs PBMCs. While M-TriDAP acts in synergy with both LTA and LPS to induce cytokine secretion from PBMCs of healthy donors, this phenomenon is attenutated in cells from Nod2fs patients.
Owner:GIRARDIN STEPHEN +1
Methods of treating tumors using natural killer cell lines
InactiveUS20060110360A1Diminish cytotoxicity of cellPromote cell growthBiocideGenetically modified cellsAbnormal tissue growthCancer cell
This invention relates to a natural killer cell line termed NK-92. The invention provides a vector for transfecting a mammalian cell which includes a nucleic acid sequence encoding a cytokine that promotes the growth of NK-92. Additionally, the invention provides an NK-92 cell, or an NK-92 cell modified by transfection with a vector conferring advantageous properties, which is unable to proliferate and which preserves effective cytotoxic activity. The invention further provides a modified NK-92 cell that is transfected with a vector encoding a cytokine that promotes the growth of NK-92 cells. The cell secretes the cytokine upon being cultured under conditions that promote cytokine secretion, and furthermore secretes the cytokine in vivo upon being introduced into a mammal. In a significant embodiment, the cytokine is interleukin 2. The present invention also provides methods of purging cancer cells from a biological sample, of treating a cancer ex vivo in a mammal, and of treating a cancer in vivo in a mammal employing a natural killer cell, such as NK-92 itself, an NK-92 cell which is unable to proliferate and which preserves effective cytotoxic activity, or natural killer cells transfected with a vector encoding a cytokine.
Owner:CONKWEST
Preparation and application of morinda root water extract, oligosaccharides and polysaccharides
ActiveCN108752497APromote proliferationPromote secretionSugar derivativesDigestive systemLiver and kidneyOrganism
The invention relates to a morinda root water extract, coarse polysaccharides and oligosaccharide and polysaccharide components and preparation methods and application thereof. The morinda root waterextract and the coarse polysaccharides are extracted from morinda roots by a mode of water extraction, then the coarse polysaccharides are separated to obtain the oligosaccharide and polysaccharide components in the coarse polysaccharides, and properties are measured. Experiments show that both the morinda root water extract and the coarse polysaccharides provided by the invention can promote theproliferation of spleen cells and the secretion of cell factors in mice, promote the proliferation of human liver cells, reduce the damage of toxic agents on cells, inhibit the expression of hepatitisB surface antigens and core antigens, inhibit the proliferation of liver cancer cells as well as inhibit the damage of ConA to the livers and kidneys of the mice, have obviously better biological activity than that of the morinda root oligosaccharides, polysaccharides or morinda root polysaccharide components prepared by other methods, and have the prospect of being developed into immunomodulators, anti-liver injury and anti-tumor drugs or health-care food. Preparation steps are simple, and preparation processes have less pollution to the environment and are suitable for industrial production.
Owner:SHUGUANG HOSPITAL AFFILIATED WITH SHANGHAI UNIV OF T C M +2
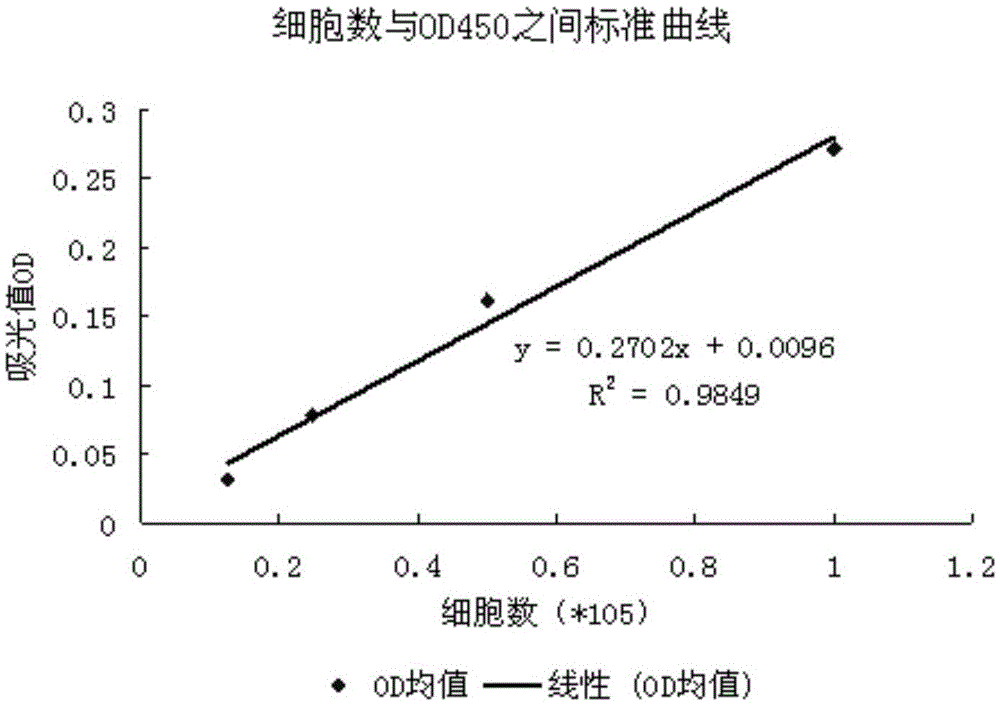
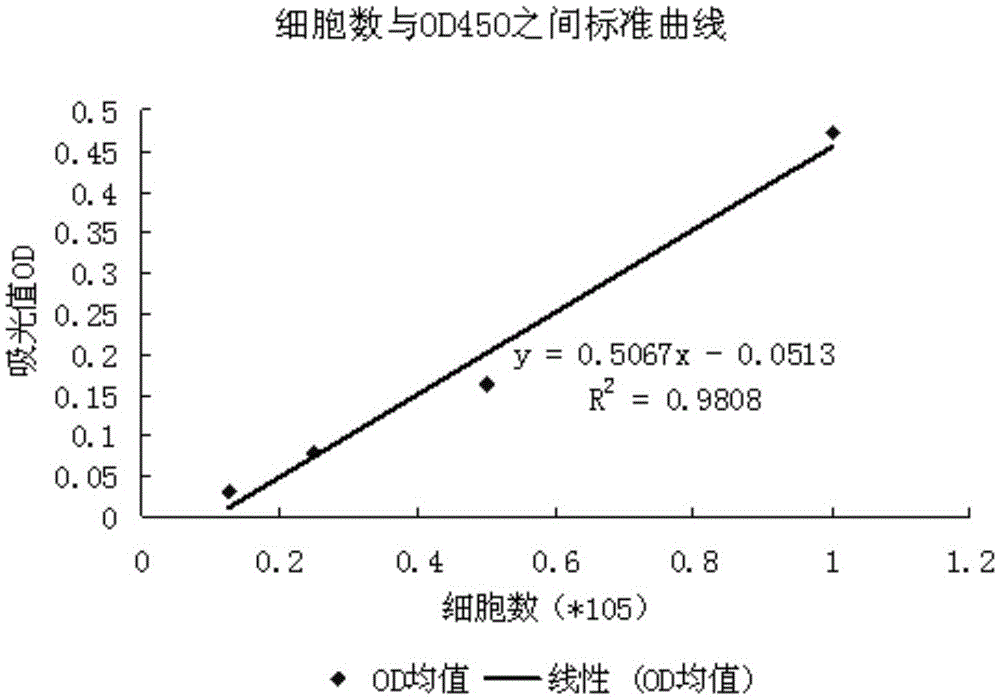
Quantitative method for detecting angiogenesis promotion ability of MSC
InactiveCN105463058AMicrobiological testing/measurementMaterial analysisAngiogenesis growth factorMesenchymal stem cell
The invention discloses a quantitative method for detecting angiogenesis promotion ability of mesenchymal stem cells (MSC). The quantitative method is characterized by comprising the steps that a co-culture system is established, wherein MSC promote proliferation of endothelial cells by means of cytokine secretion, the proliferation number of the endothelial cells is detected, the ability of the MSC in promoting proliferation of the endothelial cells is reflected by the proliferation number of the endothelial cells, and the angiogenesis promotion ability of the MSC is quantified and evaluated. The quantitative method is suitable for detecting the angiogenesis promotion ability of MSC samples with different sources.
Owner:BEIJING HEALTH & BIOTECH (H&B) CO LTD
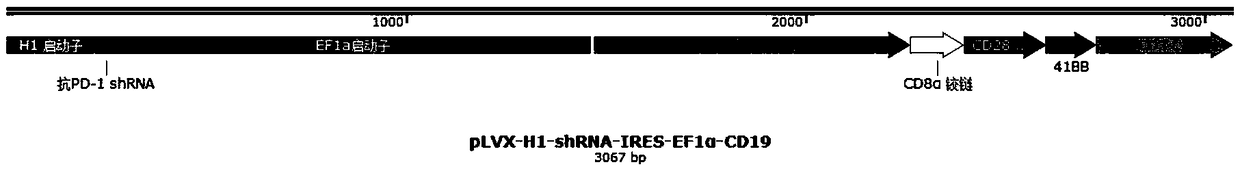
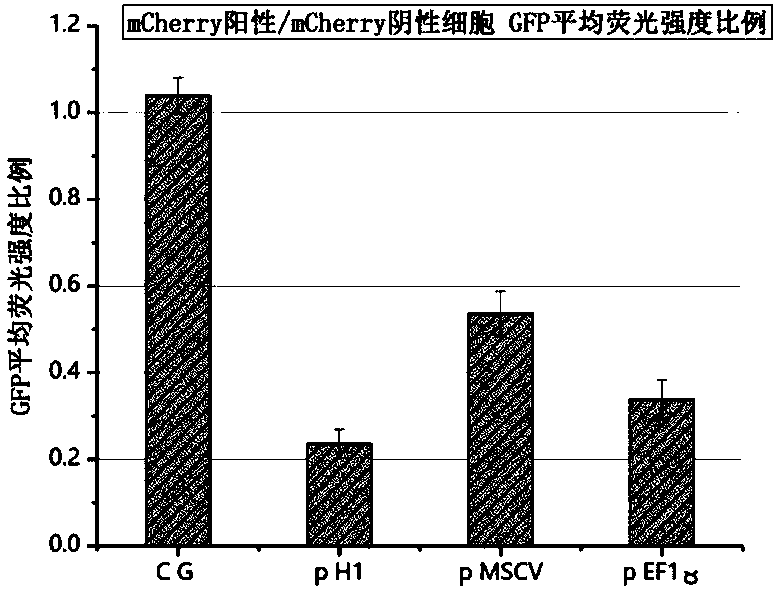
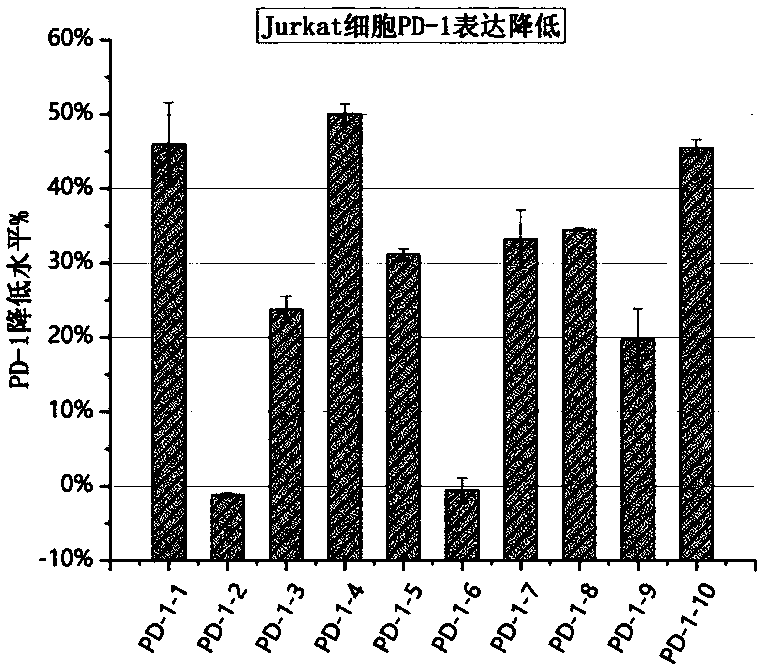
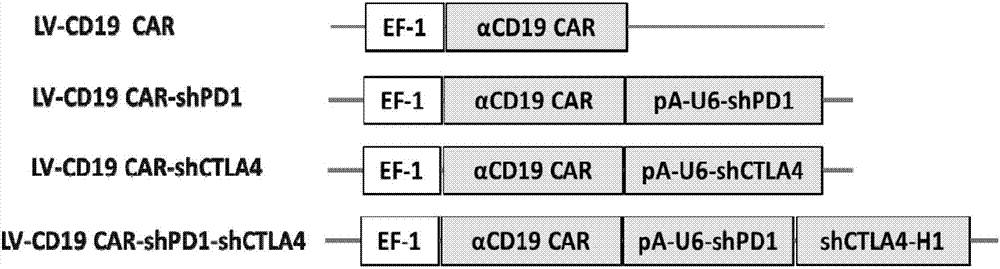
Plasmid structure for expressing CD19CAR and knocking down T cell surface PD-1 expression simultaneously and construction method thereof
InactiveCN108424927ALow efficiencyEnhanced killing effect in vitroVector-based foreign material introductionHinge regionWilms' tumor
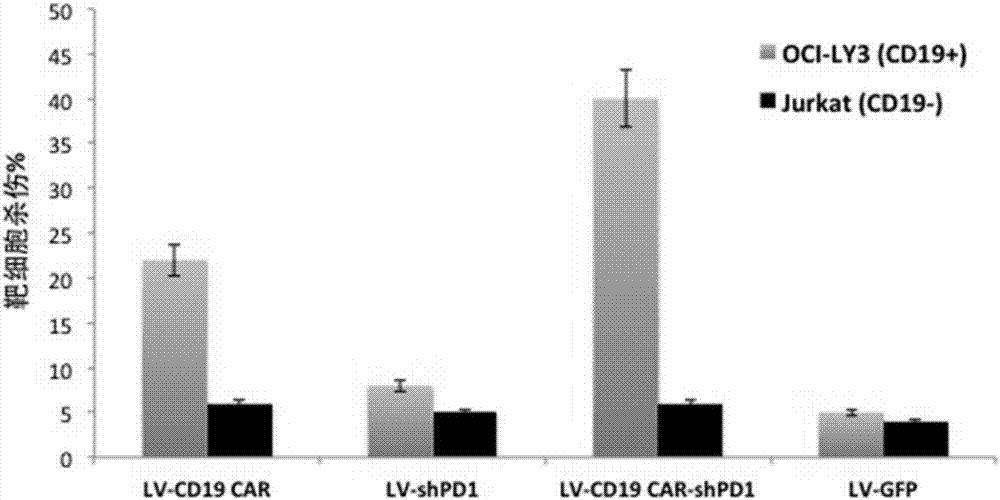
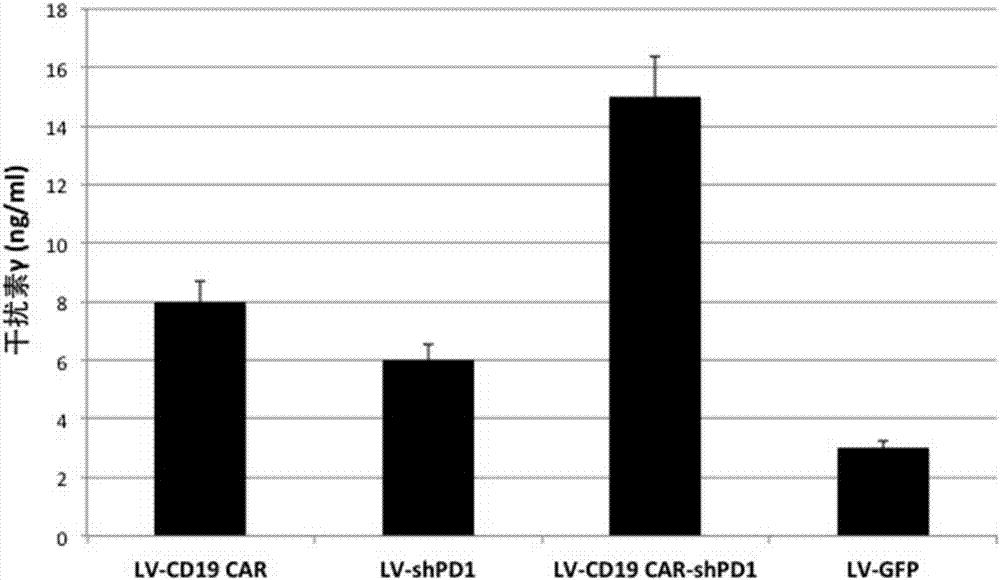
The invention relates to a plasmid structure for expressing CD19CAR and knocking down T cell surface PD-1 expression simultaneously. The plasmid structure comprises an H1 promoter, an anti-PD-1shRNA sequence started by the H1 promoter, an EF1alpha promoter and a three-generation CD19CAR sequence started by the EF1alpha promoter that are connected in series in order. The three-generation CD19CAR sequence consists of a CD19 single chain variable region, a human CD8alpha hinge region, a human CD28 transmembrane region and an intracellular region, a human 41BB intracellular region and a human CD3zeta intracellular region that are connected in series. The plasmid structure provided by the invention is transduced into T cells by a lentivirus, and the CART cell production process is significantlysimplified than using CRISPR / Cas9 for knockout of PD-1. Moreover, the CART cells show enhanced tumor cytotoxicity killing ability and increased persistent cell repair and cytokine secretion ability after antigenic stimulation in vitro.
Owner:WUHAN BIO RAID BIOTECH CO LTD
Cytokine controlling device, treating device, and treating method
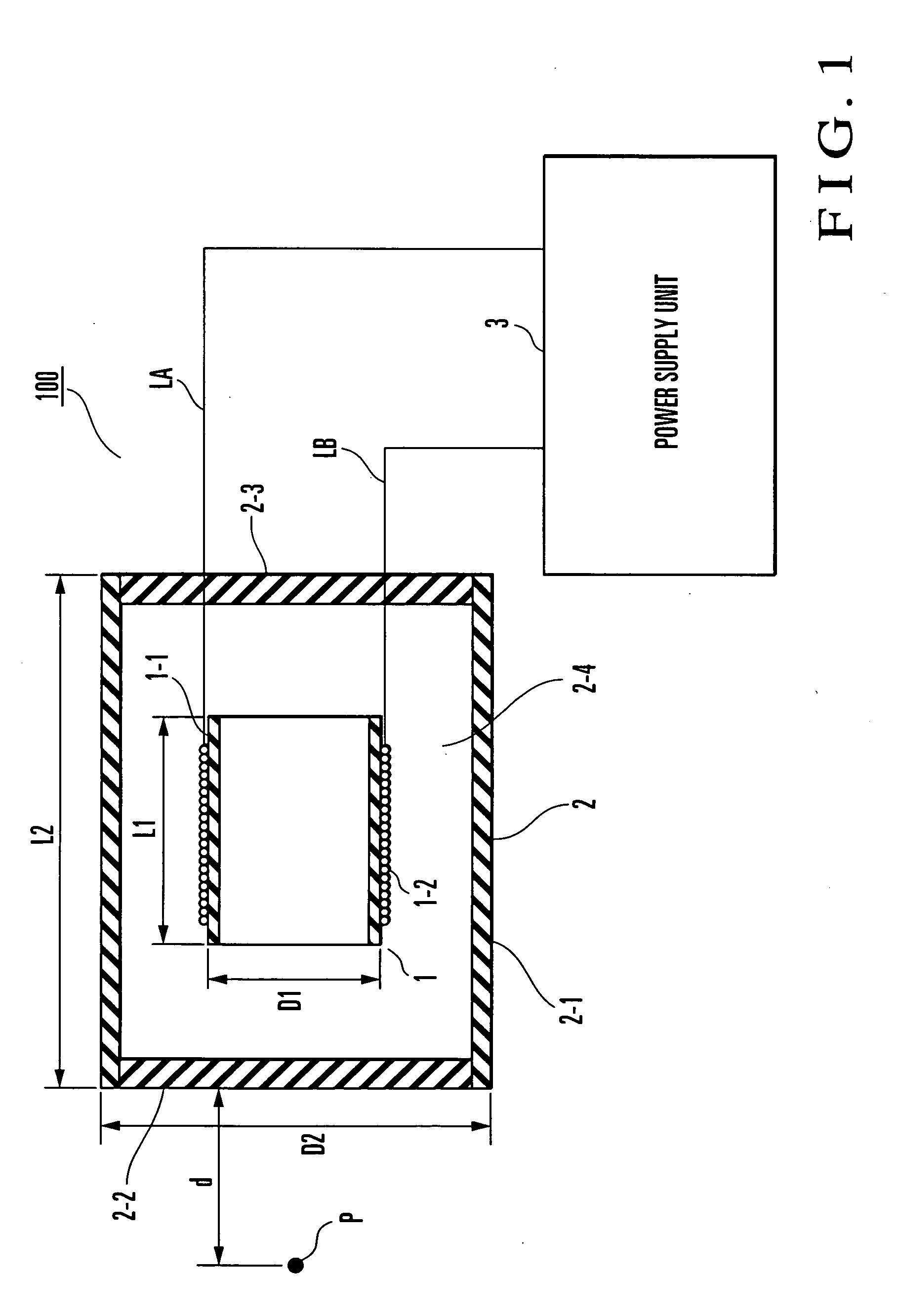
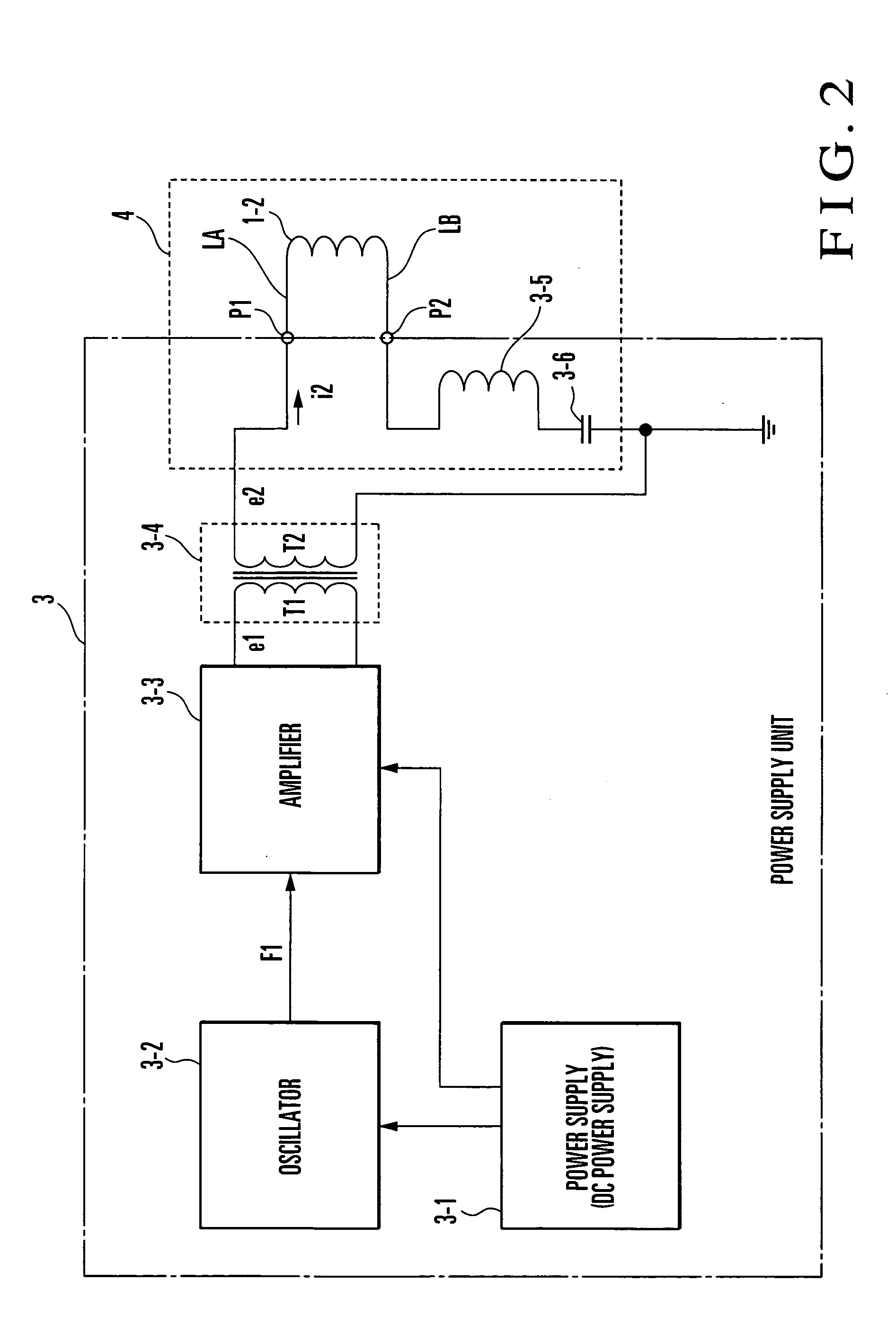
InactiveUS20050010163A1Avoid side effectsEffect phytotoxicityElectrotherapyMagnetotherapy using coils/electromagnetsBobbinResonance
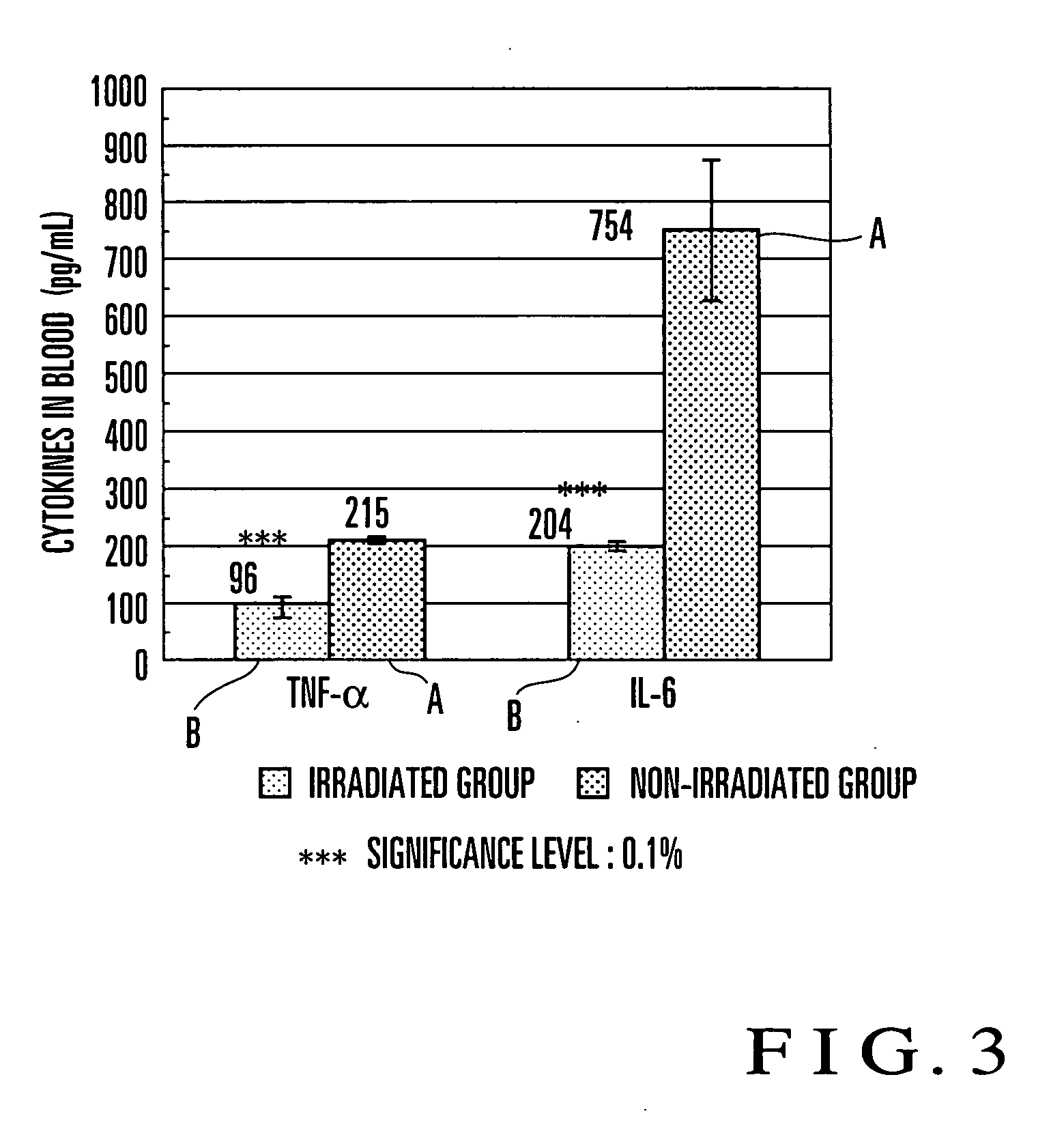
An electromagnetic wave emitting unit (1) formed by winding an emission coil (1-2) around a coil bobbin (1-1) is provided in a case (2). A current (for example, resonance frequency of 60 kHz, resonance current of 0.95 mA) is supplied from a power supply unit (3) to the emission coil (1-2) via a resonance circuit (4). With this operation, weak electromagnetic waves are emitted from the emission coil (1-2) to form an electromagnetic field (for example, magnetic flux density≈6.7 nT, electric field strength≈0.42 V / m) suitable for treating at a P-point. When an affected part is set at the P-point and in the vicinity thereof, a cytokine secretion is controlled. It is confirmed that inflammatory cytokines in an animal subjected to inflammation, for example, are significantly controlled. Efficacy is recognized in a range of 20 to 180 kHz, and especially a preferable result is obtained in the vicinity of 60 kHZ.
Owner:NIPPON SIGMAX
Humanized monoclonal antibody against hIL-33 and application of monoclonal antibody
ActiveCN111378037AAffinity ensuresLow heterogeneityNervous disorderAntipyreticAntiendomysial antibodiesDrug biological activity
In order to provide a antibody against hIL-33 with clinical application prospect, the invention provides a high-affinity mouse anti-human IL-33 antibody by adopting a panning technology of immunizingmouse B cells, based on the action mechanism of IL-33 in inflammation and tumors in the prior art, the function of IL-33 in regulating cytokine secretion, and cytokines involved in inflammation and tumors. After recombinant expression of mouse-derived antibodies, preliminary screening of biological activity, humanization transformation, affinity maturation and biological activity rescreening, a humanized monoclonal antibody against hIL-33 is obtained, and the monoclonal antibody has the characteristics of high stability, high affinity, good biological activity, broad clinical application prospects, etc.
Owner:NANJING NOVOACINE BIO-TECH CO LTD
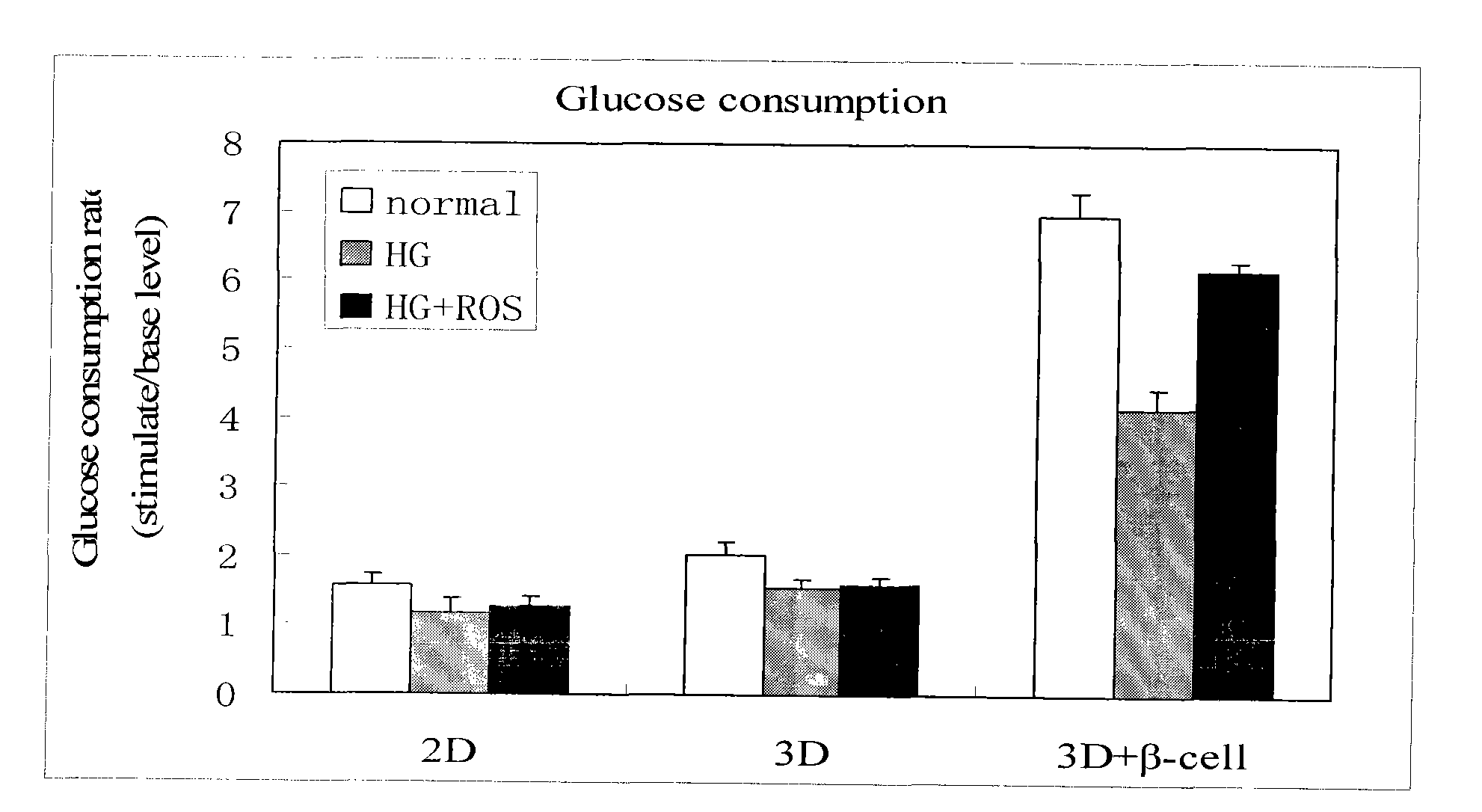
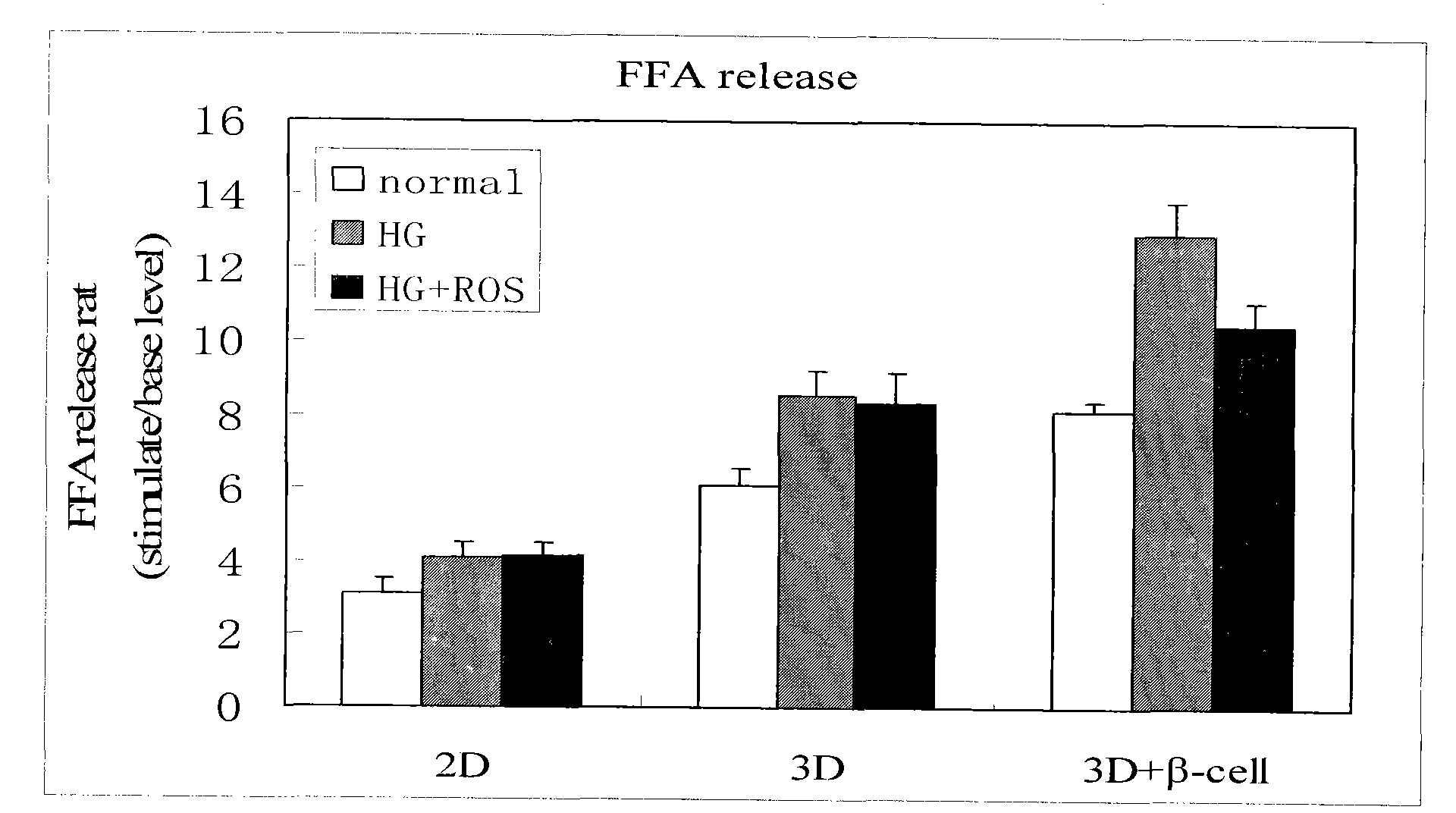
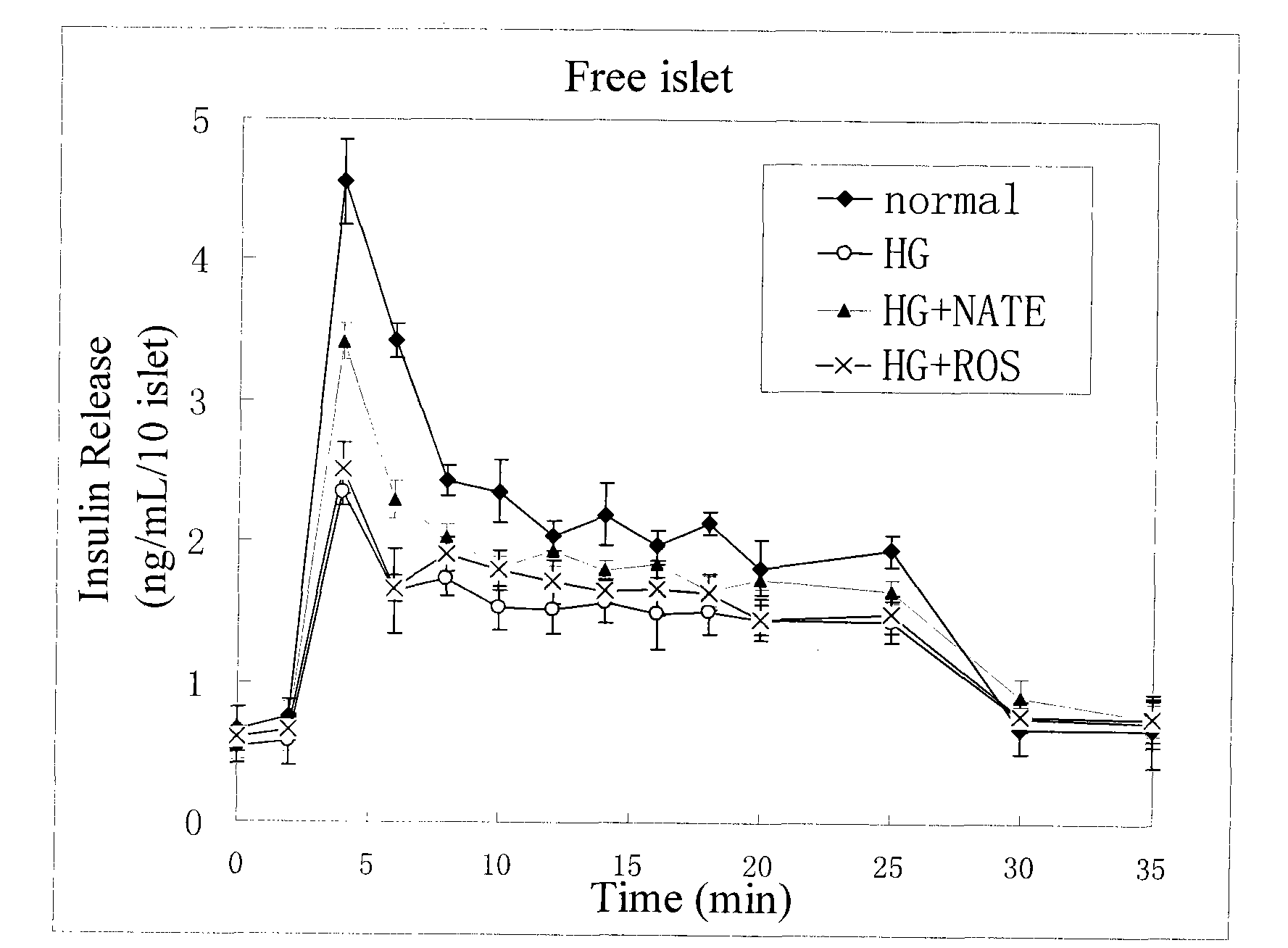
Method for high content screening of therapeutic drugs for diabetes
ActiveCN101775431AStable and reliable biological cross-linking technologyControl curingMicrobiological testing/measurementPorosityHigh concentration
The invention relates to a diabetes pathological model based on cell assembly technology and application thereof in high content drug screening. Adipose-derived stem cells and simulated extracellular matrix material are assembled to form a cell-containing three-dimensional structure with certain porosity; the inducing differentiation condition is controlled to enable the adipose-derived stem cells on the surface of the inner passage of the three-dimensional structure to differentiate into endothelial cells and to enable the adipose-derived stem cells inside the matrix material to differentiate into fat cells; and pancreas islet separated out from the body of an animal is directly assembled in the gaps of the three-dimensional structure. The system model is excited for a long time by high-concentration dextrose, fatty acid insulin, TNF or cytokine secretion of fat cells and the like so as to induce the system model to produce pathology and symptom of diabetes. The drug to be tested is put into a culture system, and by detecting the level of the relevant markers of the model under normal condition and pathological condition, the pharmacological activity of the drug to be tested can be analyzed and thereby high content screening can be carried out to the drug.
Owner:HANGZHOU DIANZI UNIV
Therapeutic composition for treating B cell leukemia and B cell lymphoma
ActiveCN107034193AImprove physical functionInhibitory responseOrganic active ingredientsViral/bacteriophage medical ingredientsLymphocyteChimeric antigen receptor
The invention provides a transgenic lymphocyte, a construct and a therapeutic composition for treating cancers. A cell surface immune checkpoint of the transgenic lymphocyte provided by the invention is silenced and is applied to expression of a chimeric antigen receptor. The transgenic lymphocyte provided by the invention is high in cytokine production and strong in targeted killing effect on CD19+ tumor cells; and especially, a directional killing effect on the CD19+ tumor cells of B cell leukemia and B cell lymphoma is obviously improved.
Owner:BEIJING MARINO BIOTECH PTY LTD
Spleen mesenchymal stem cell with immune adjustment function, preparation method of cell and application of cell
PendingCN106282108AImprove purityLess hematopoietic pollutionCell dissociation methodsSkeletal/connective tissue cellsHigh cellHematopoietic cell
The invention discloses a spleen mesenchymal stem cell with an immune adjustment function, a preparation method of the cell and an application of the cell. By the aid of the strategy of removing hematopoietic cell-digestion matrix tissues to obtain Sp-MSCs-passage purification, MSCs are cultured in an isolated manner from the spleen, and a large number of Sp-MSCs with high cell purify are obtained by isolation. Further, the Sp-MSCs have high immune adjustment function, heterogenetic antigen and homologous antigen activated T lymphocyte proliferation and activation can be inhibited, and inflammatory cytokine secretion of T lymphocyte is inhibited.
Owner:CHINESE PEOPLES LIBERATION ARMY AIR FORCE GENERAL HOSPITAL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com