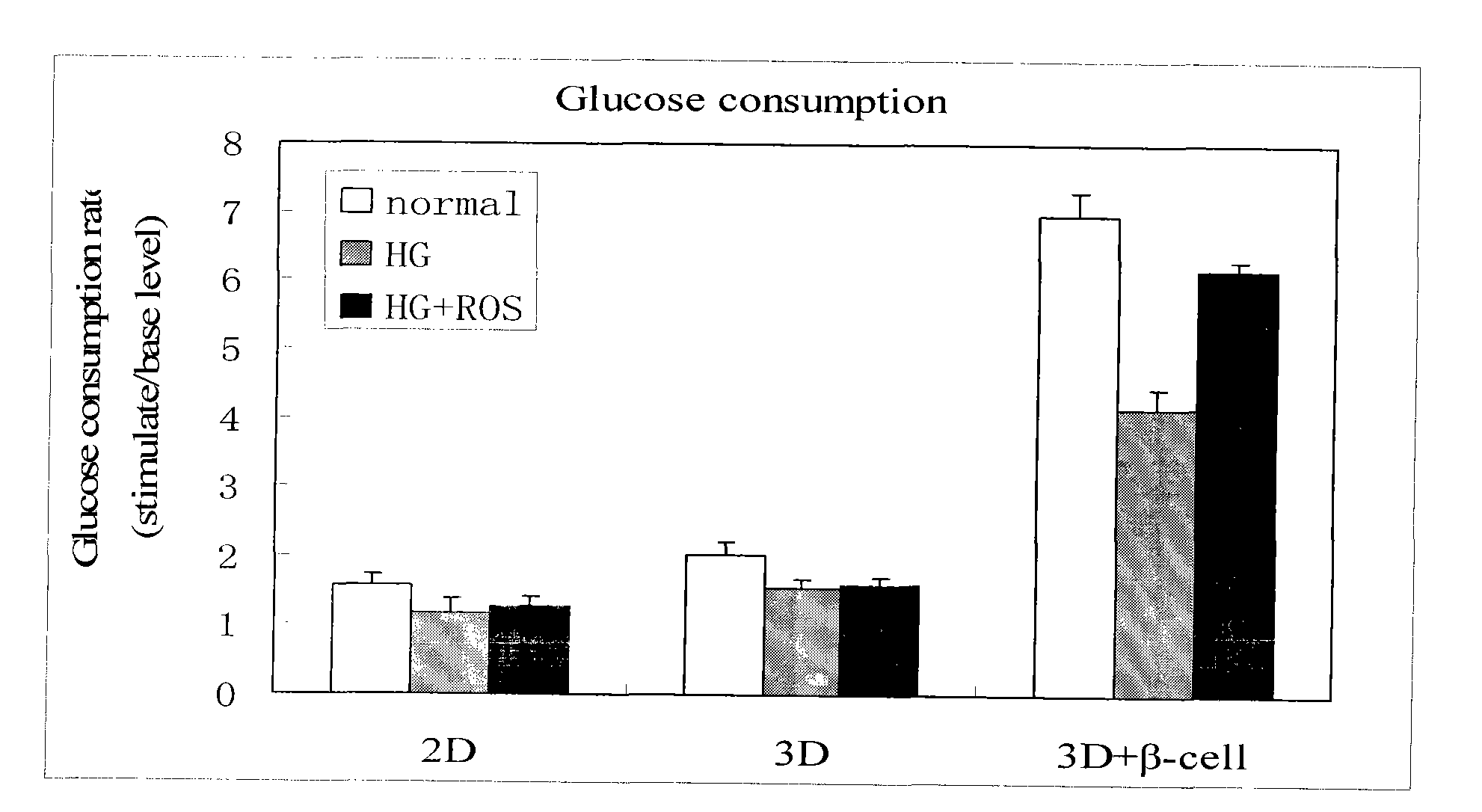
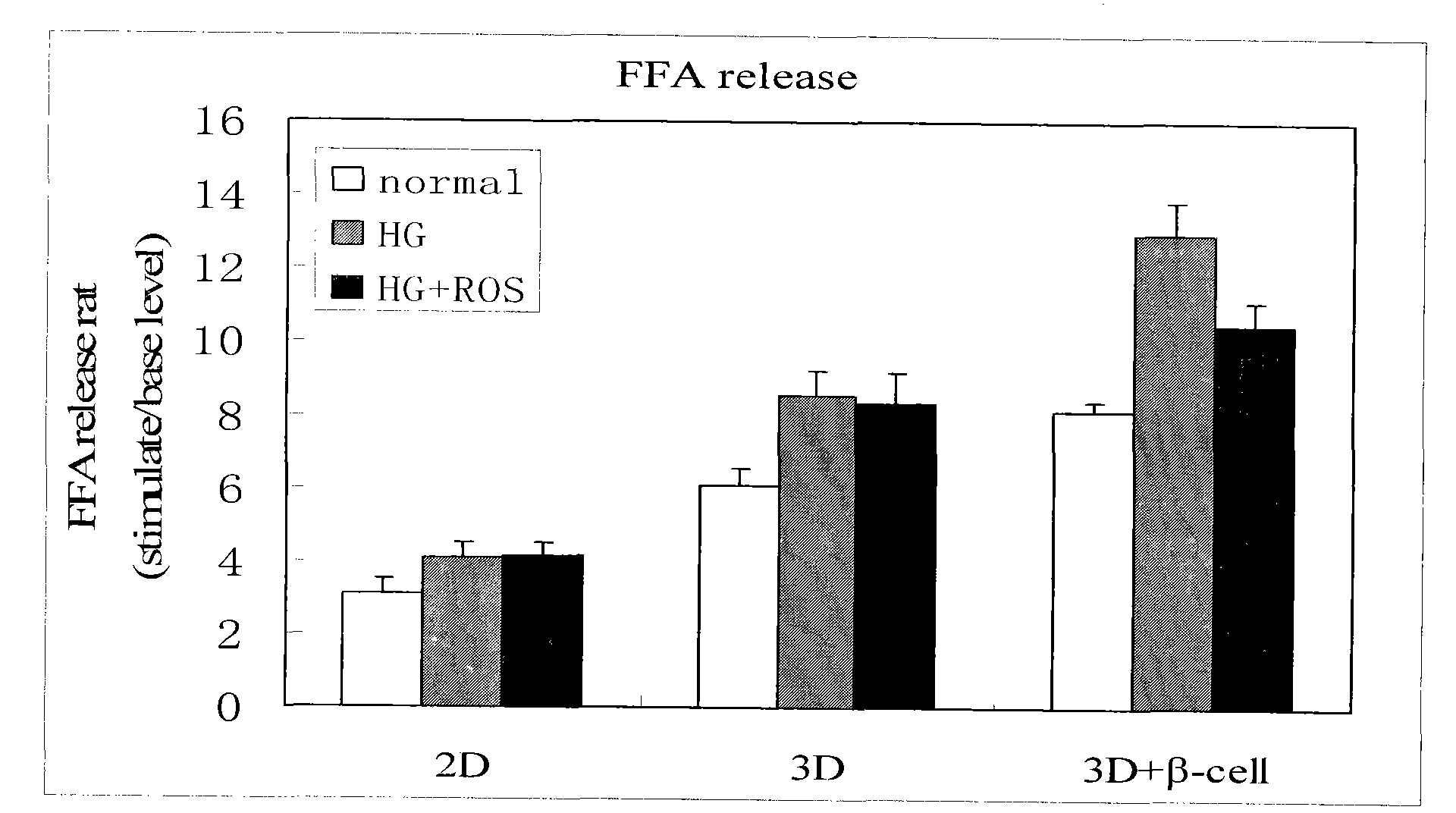
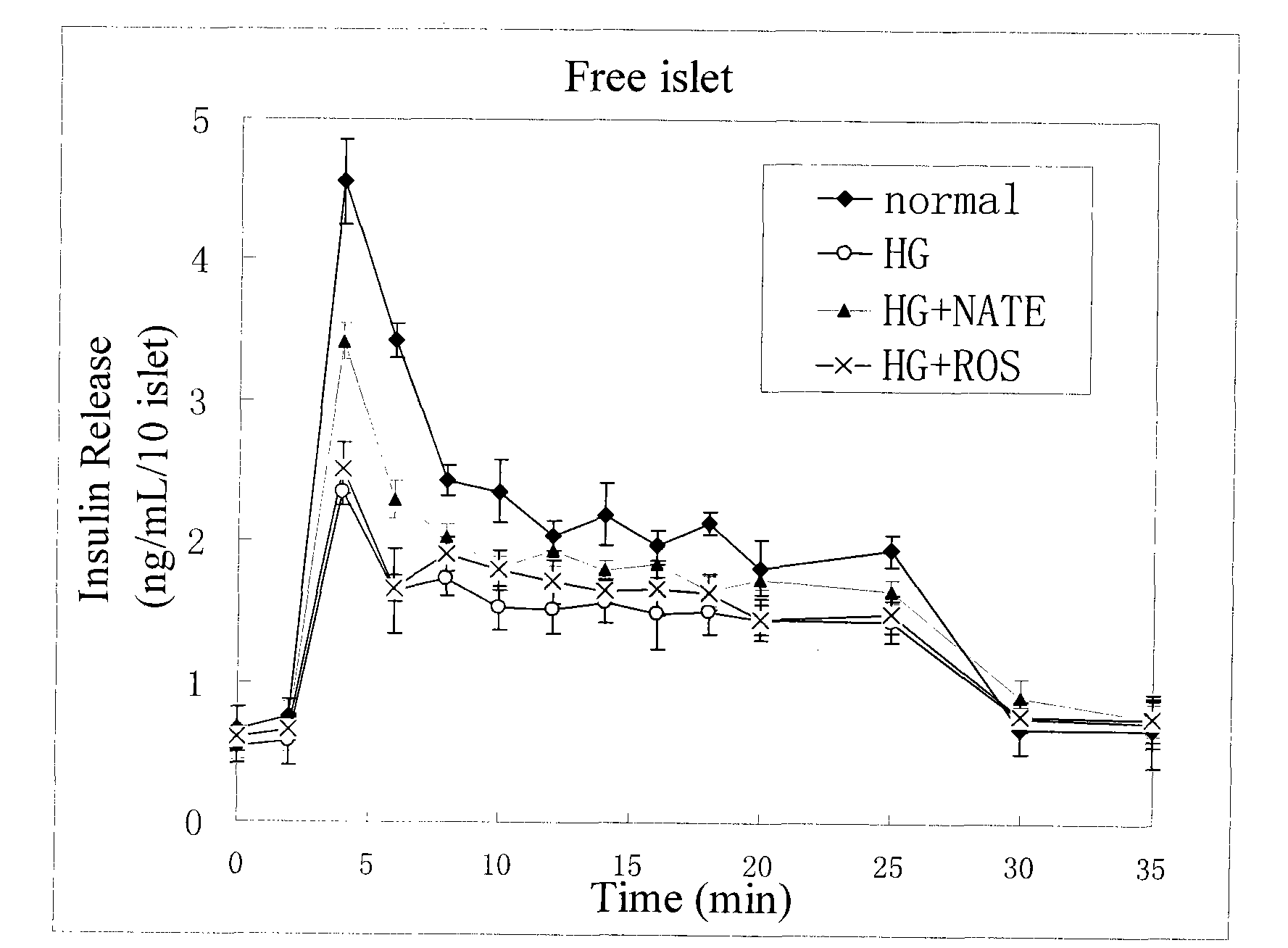
Method for high content screening of therapeutic drugs for diabetes
A screening method and technology for therapeutic drugs, applied in biochemical equipment and methods, microbiological determination/testing, etc., can solve the problems of low success rate, high-content screening of cell structure and functional integrity without major progress, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Example 1 Seed cell culture
[0074] Adipose stem cells derived from rats are selected as the seed cells forming the basis of the system. Adipose stem cells are pluripotent stem cells that can differentiate into fat cells, osteoblasts, and muscle cells.
[0075] (1) Rats (100-150 g) were anesthetized by intraperitoneal injection of pentobarbital sodium, soaked in 70% ethanol for 10 minutes, aseptically took abdominal subcutaneous fat tissue, quickly put it into a sterile beaker, and transferred it to a clean table;
[0076] (2) After the above operations are completed, remove the connective tissue and blood clots on the adipose tissue with hemostatic forceps in the ultra-clean bench, and use D-Hank’s solution ((Ca 2+ , Mg 2+ -free Hank's, pH7.4): 8.175g NaCl, 0.403g KCl, 0.114g NaCl 2 HPO4, dissolved in 950mL double-distilled water, added HEPES (4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid, 4-hydroxyethylpiperazineethanesulfonic acid) to adjust the pH value, and...
Embodiment 2
[0084] Example 2 Isolation and culture of rat islets
[0085] (1) SD rats were anesthetized by intramuscular injection of pentobarbital (30 mg / kg). Disinfection, laparotomy, pancreas and common bile duct were exposed, and a suture was threaded at the distal end of the common bile duct near the duodenum, and at the back of the common bile duct near the hilum of the liver, without ligation for now.
[0086] (2) Insert a 4.5-gauge needle (connected with a syringe equipped with pre-warmed digestion separation solution) antegrade to the far side of the middle section of the common bile duct, ligate the suture near the hepatic hilum of the common bile duct, and inject a small amount of digestion separation into the common bile duct After that, the distal end of the common bile duct was ligated with sutures, and 10-15ml of pre-warmed 5% collagenase solution was slowly retrogradely injected into the pancreatic duct to fully expand the pancreas.
[0087] (3) Quickly and completely rem...
Embodiment 3
[0090] Example 3 Multicellular system model construction process
[0091] (1) Preparation of gelatin, sodium alginate, fibrinogen and cell blend material: dissolving gelatin in PBS buffer solution to form a 20% solution, dissolving sodium alginate in PBS buffer solution to form a 5% solution, Neutralize residual acetic acid with NaOH, adjust the pH value to 7.1, and perform batch sterilization in an oven at 70°C. Dissolve fibrinogen in DMEM medium under sterile conditions to form a 5% solution. Before use, the three materials were uniformly blended at a ratio of 1:1:1. Mix the material with cultured adipose stem cells to obtain a concentration of about 1×10 7 / mL adipose stem cell-composite blend material.
[0092] (2) The assembly of the energy metabolism system consists of two steps: system basic assembly and islet assembly. Inject the adipose-derived stem cell / composite material blend into a 1mL disposable syringe, and place it in a refrigerator at 4°C for more than 30 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| porosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com