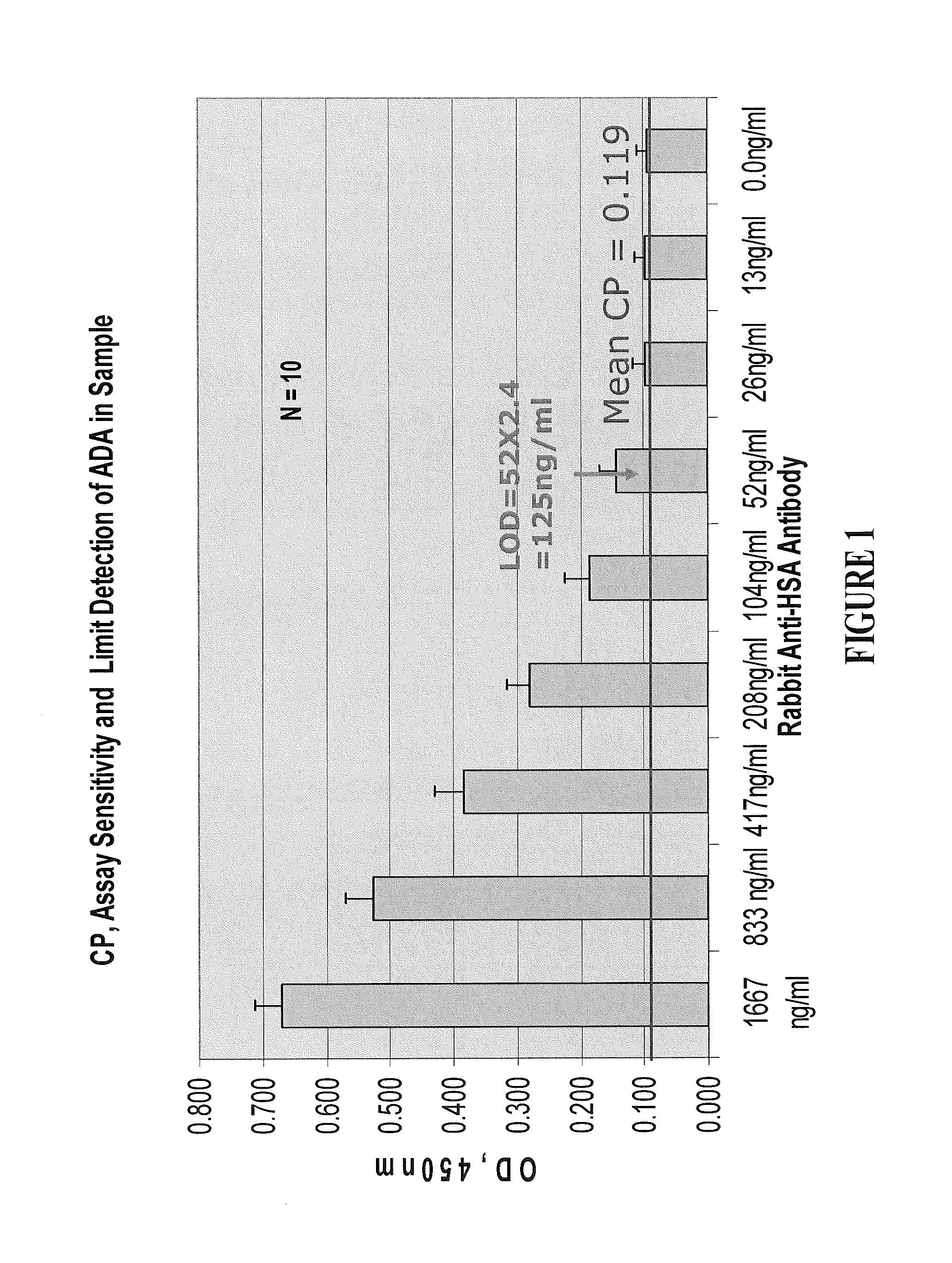
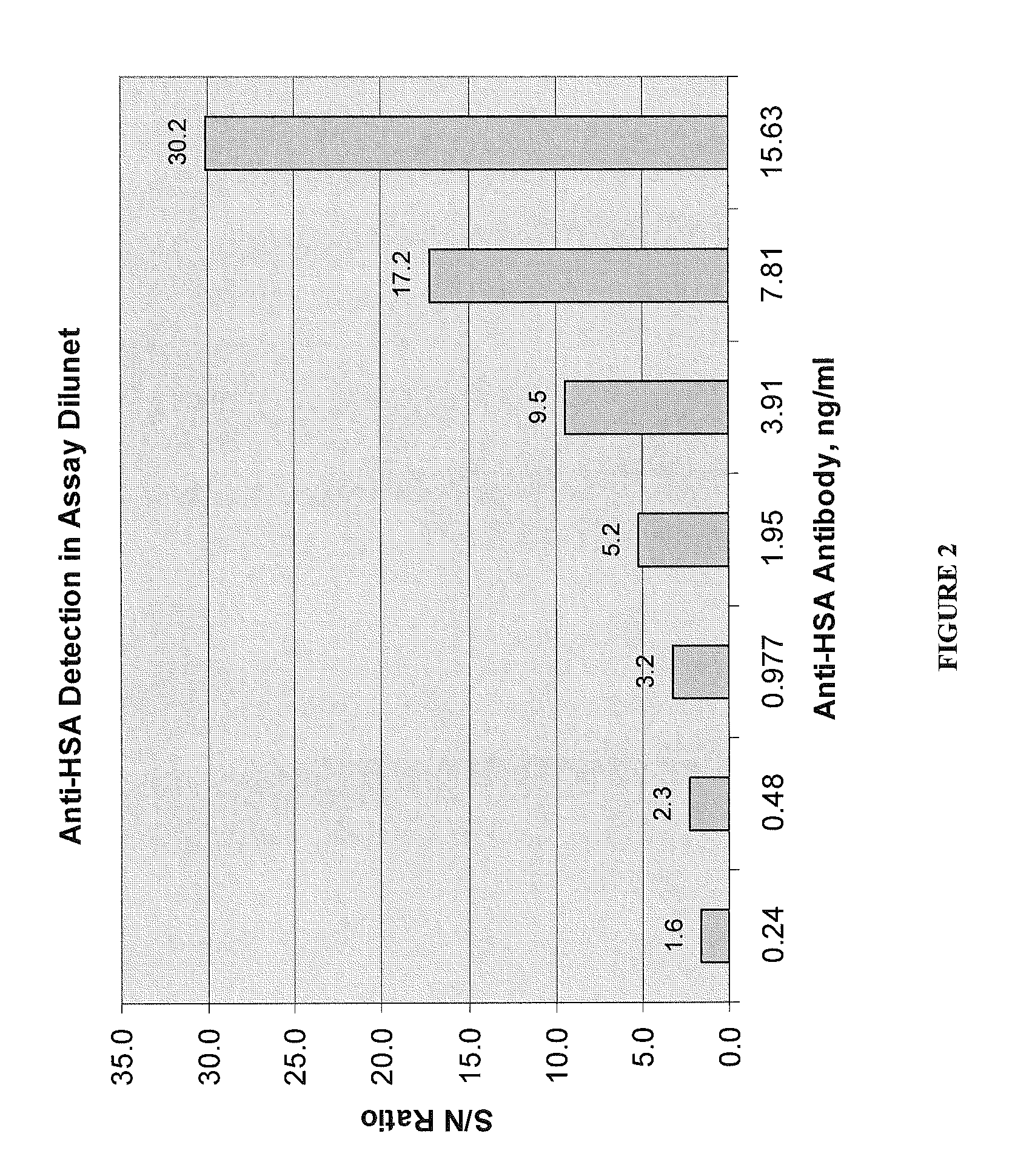
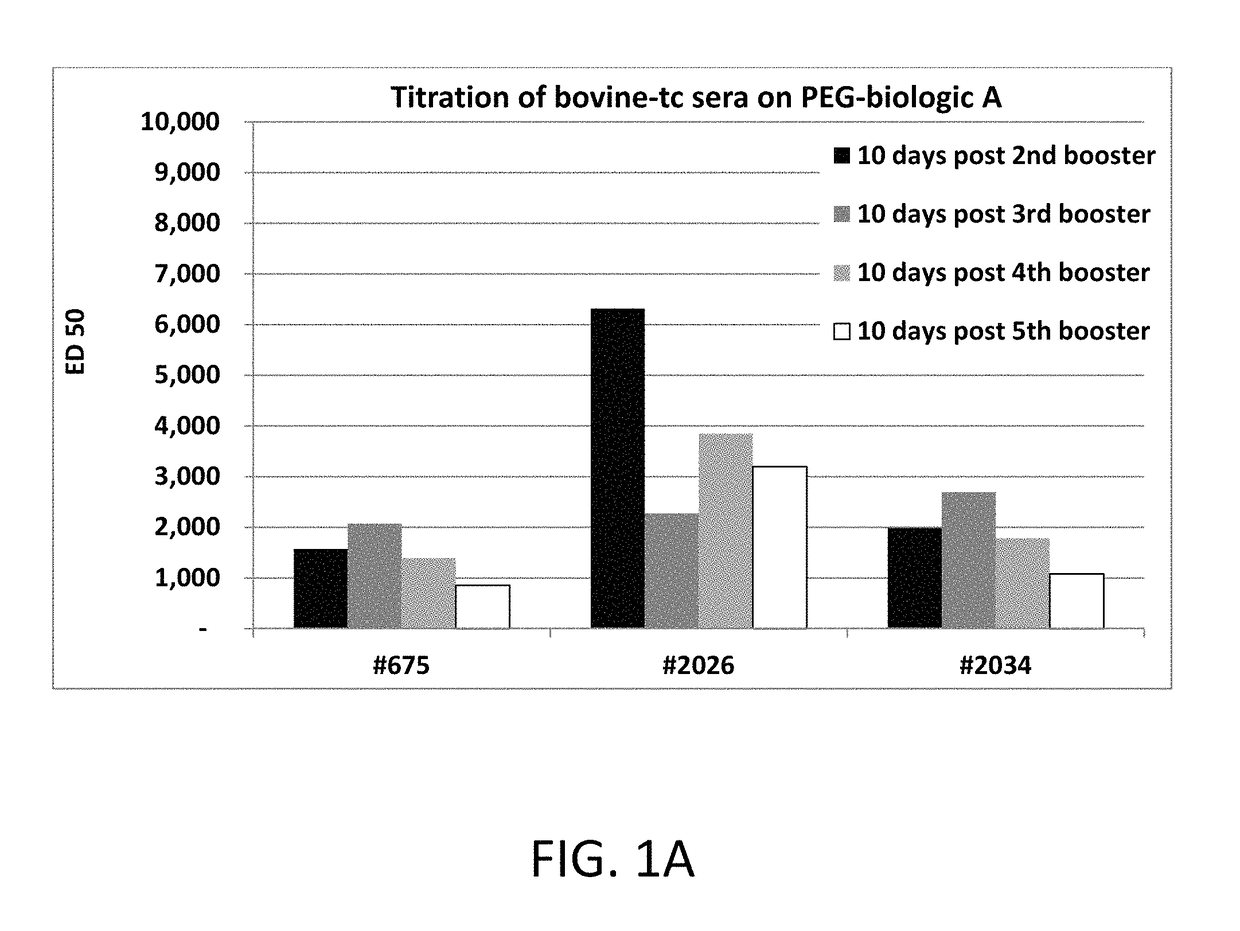
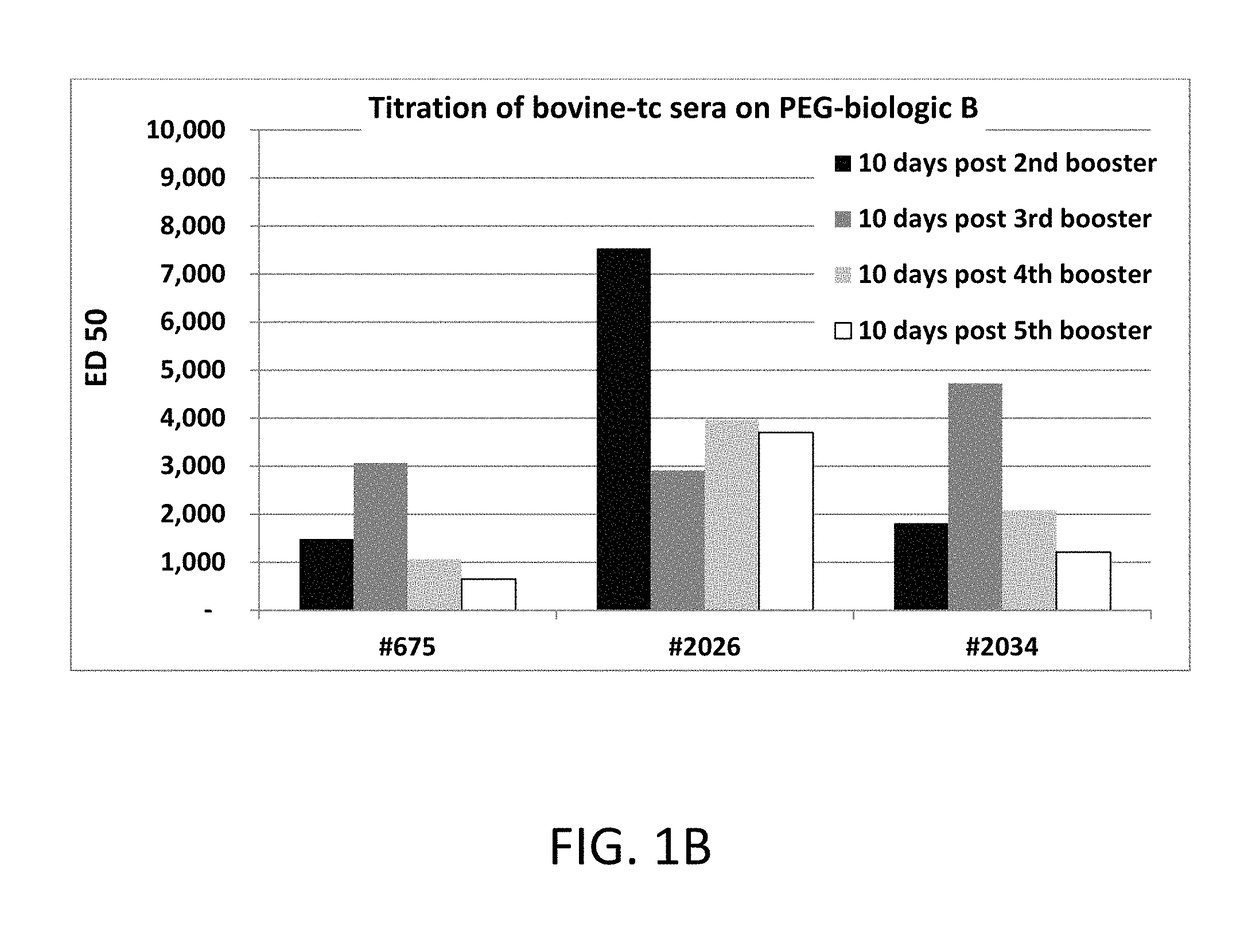
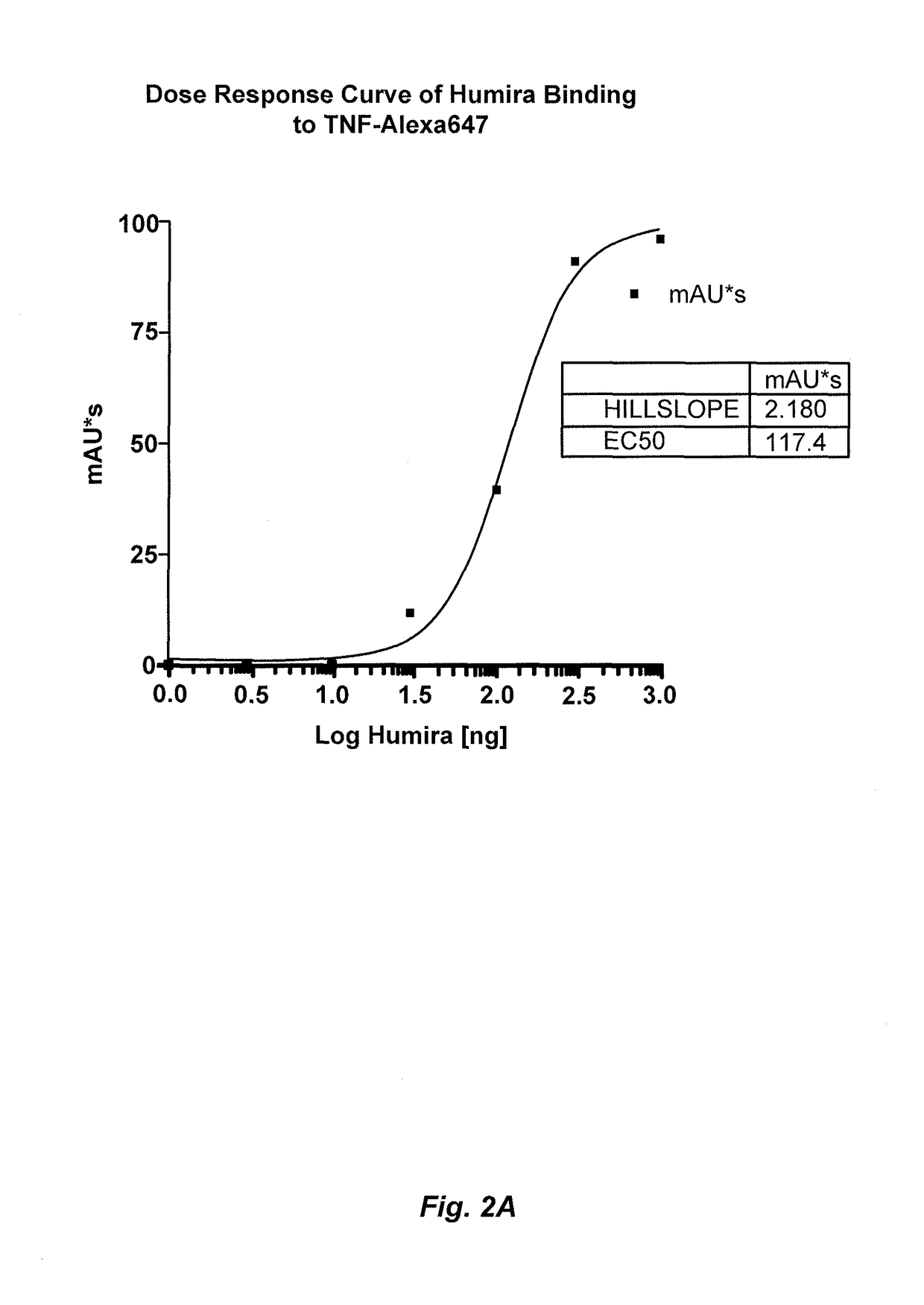
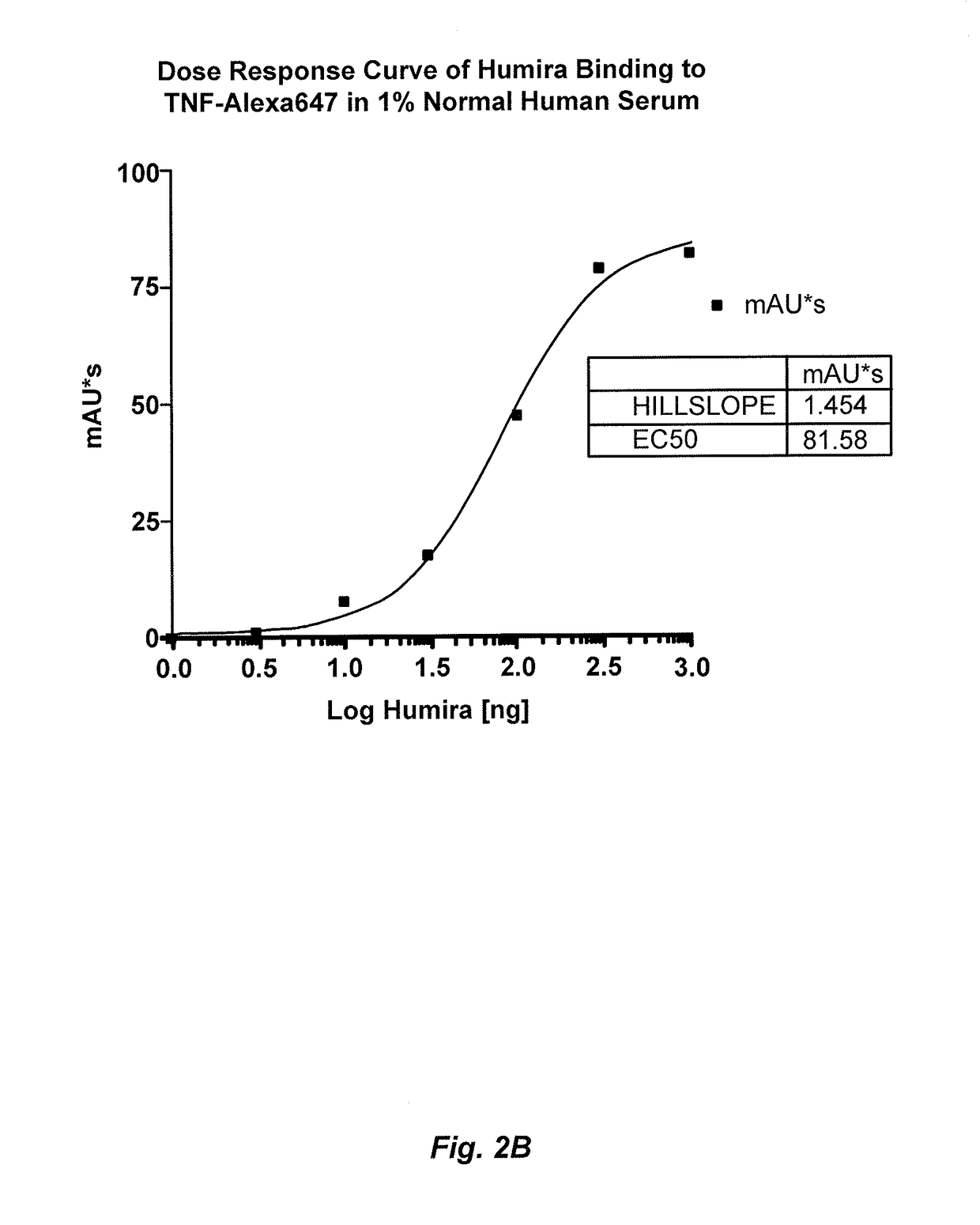
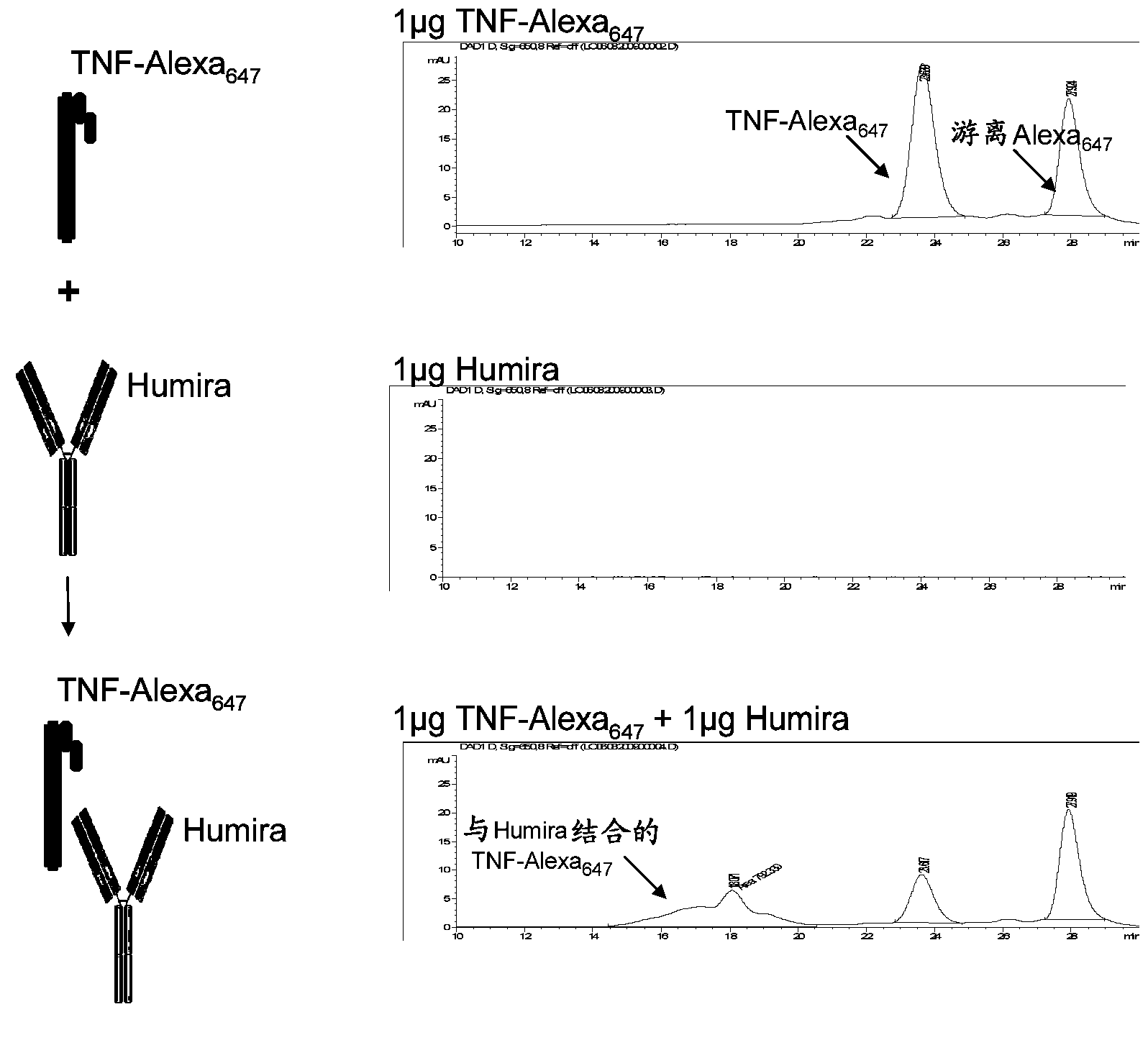
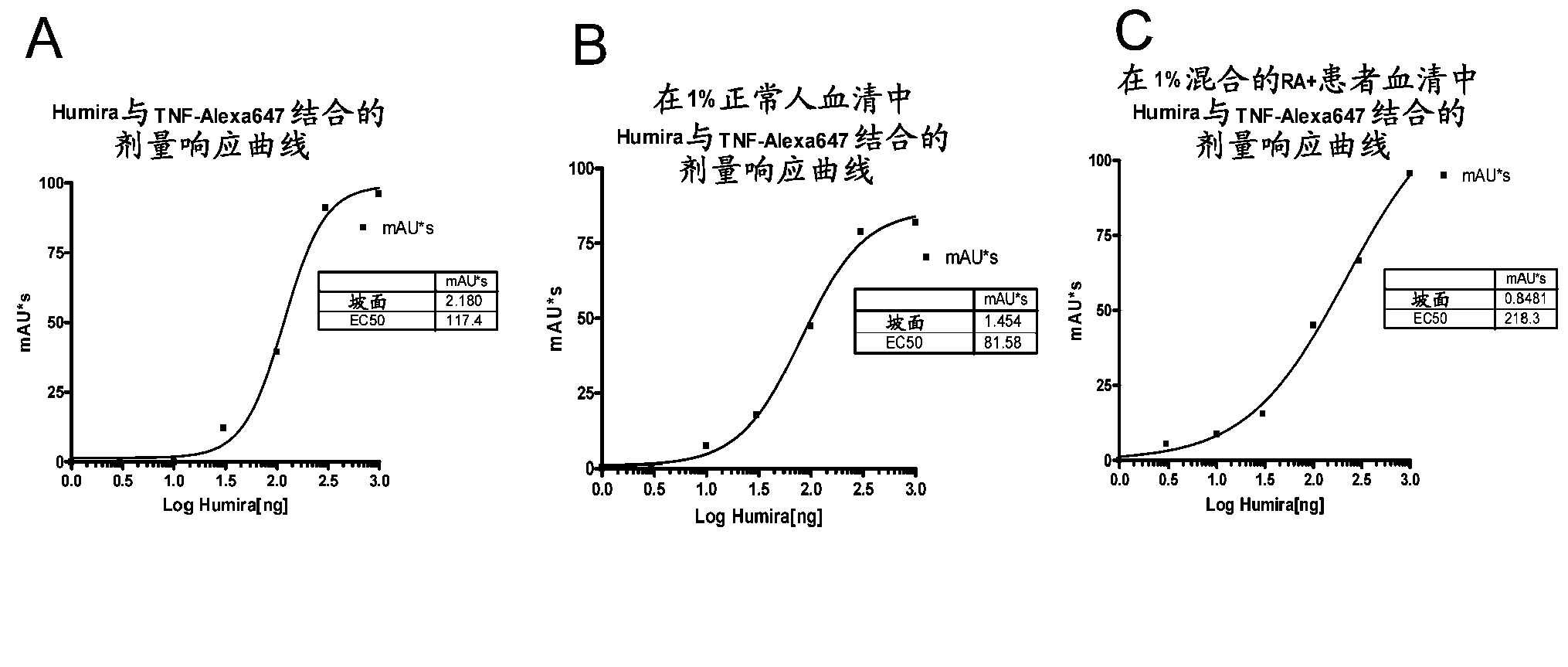
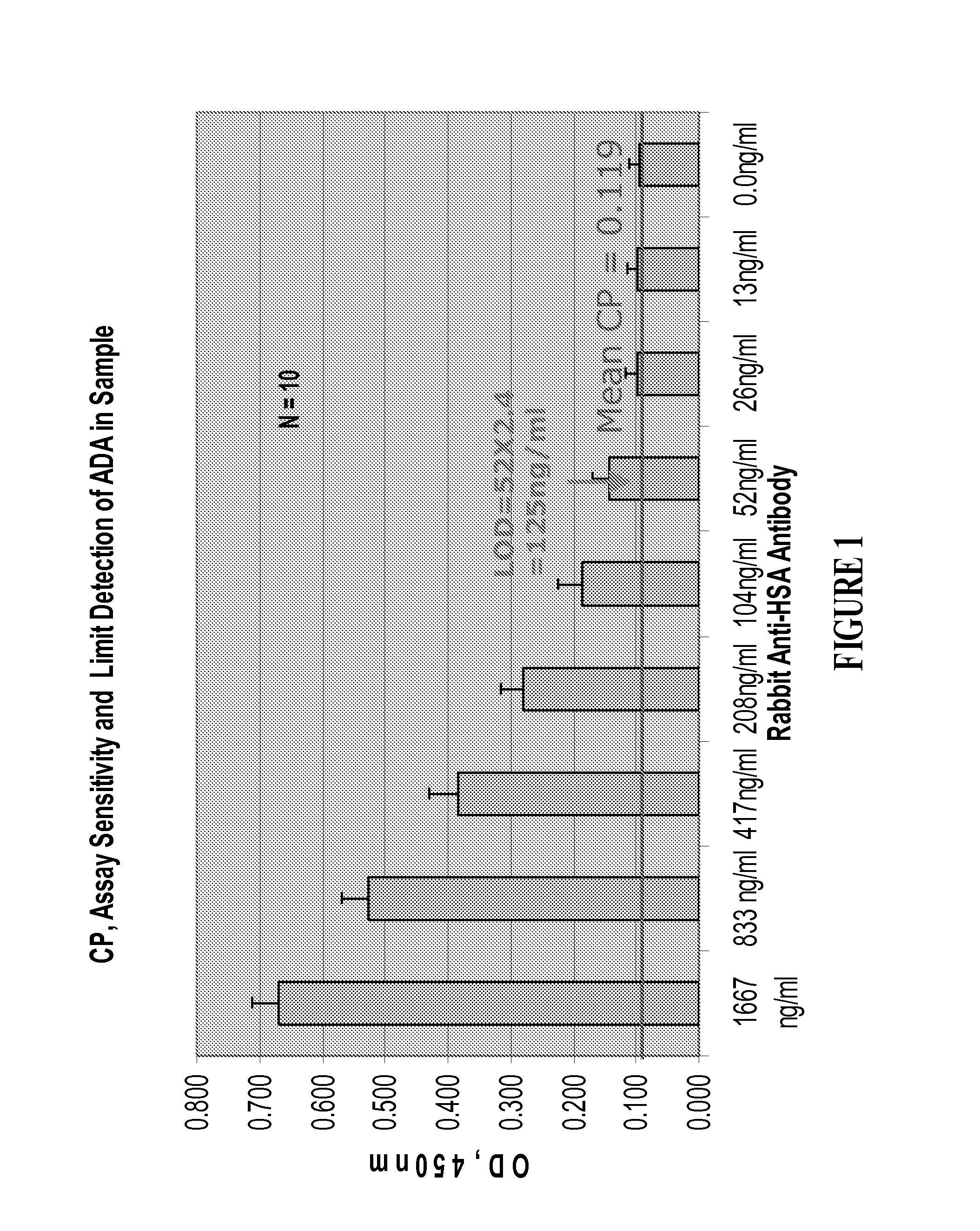
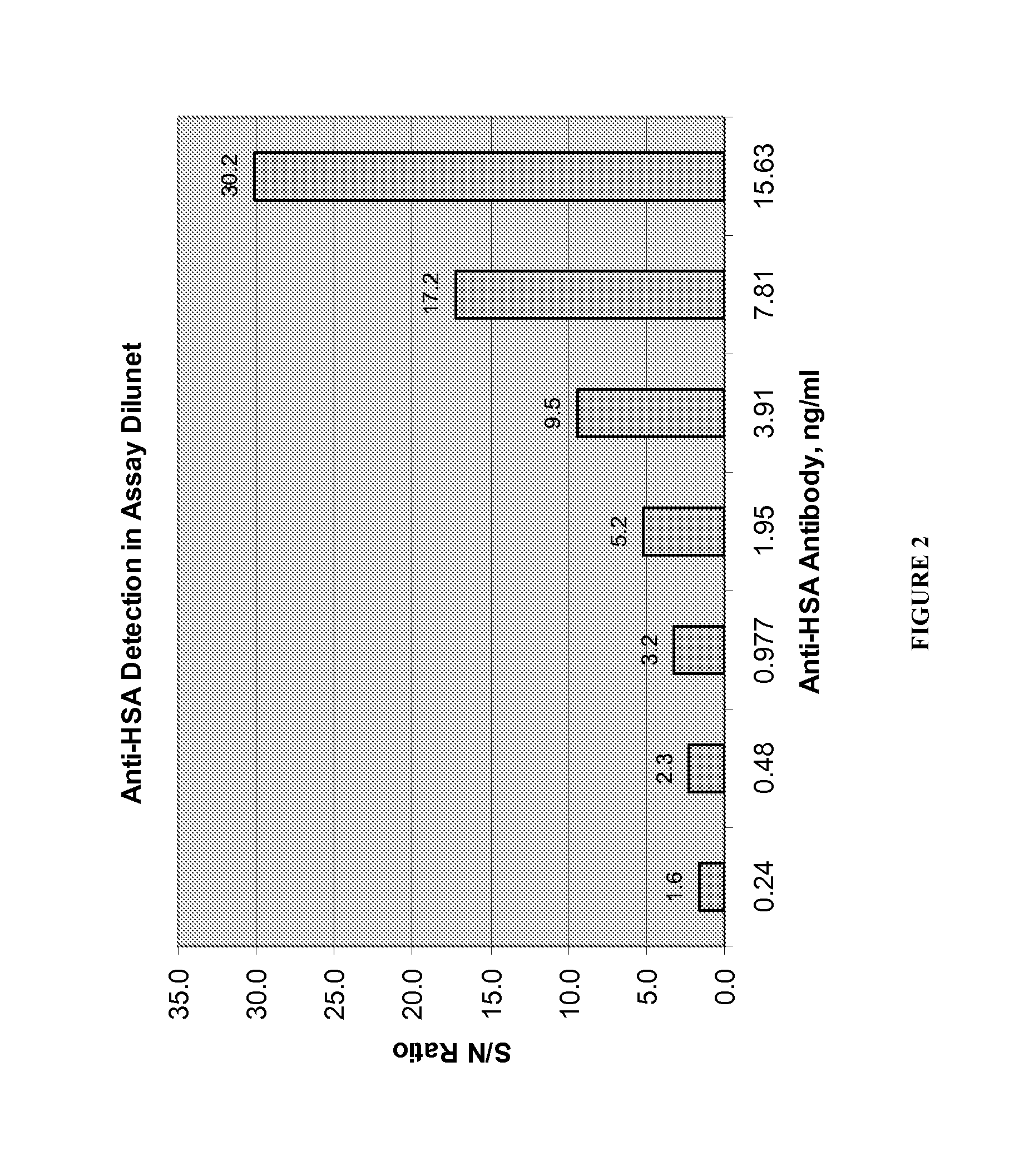
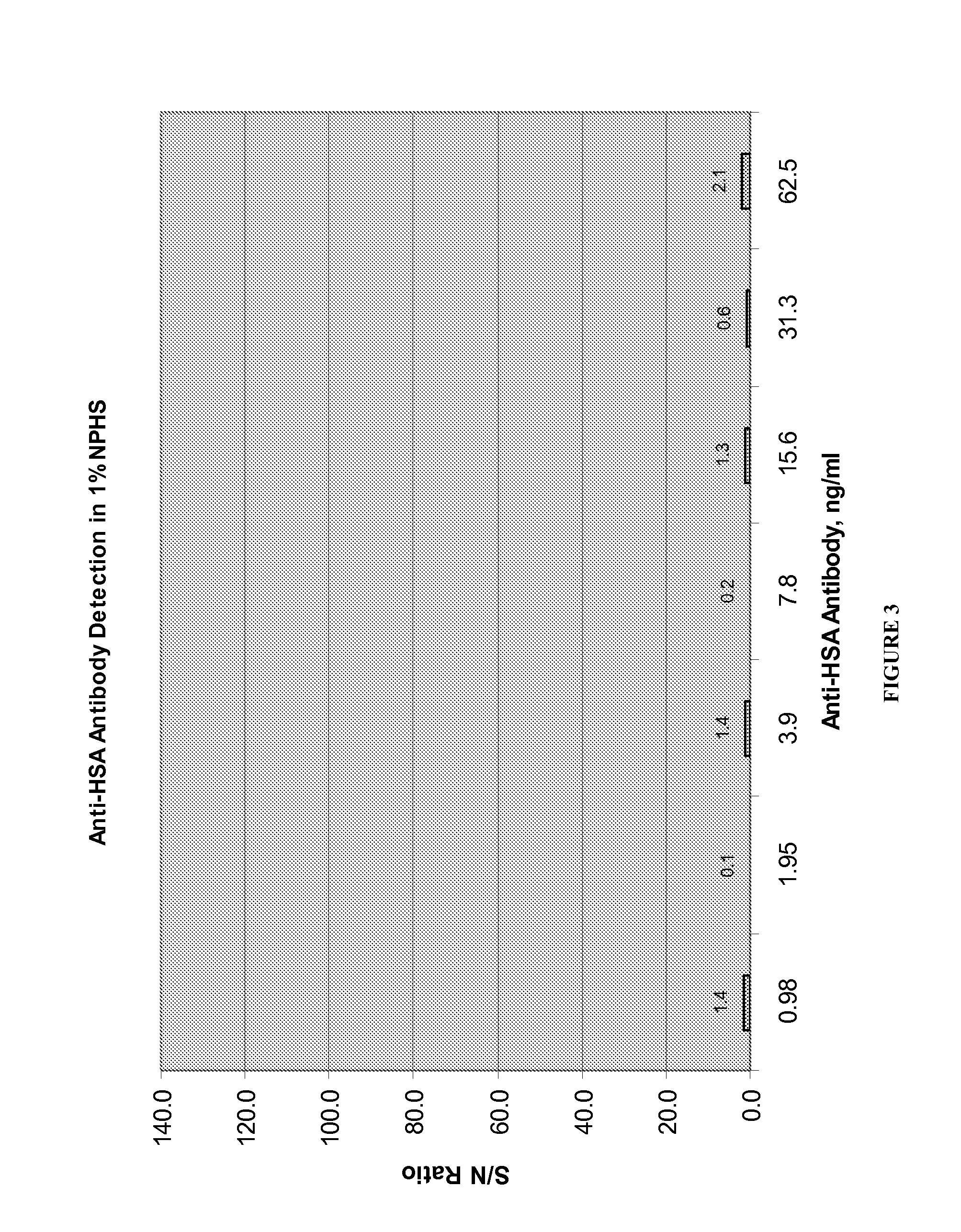
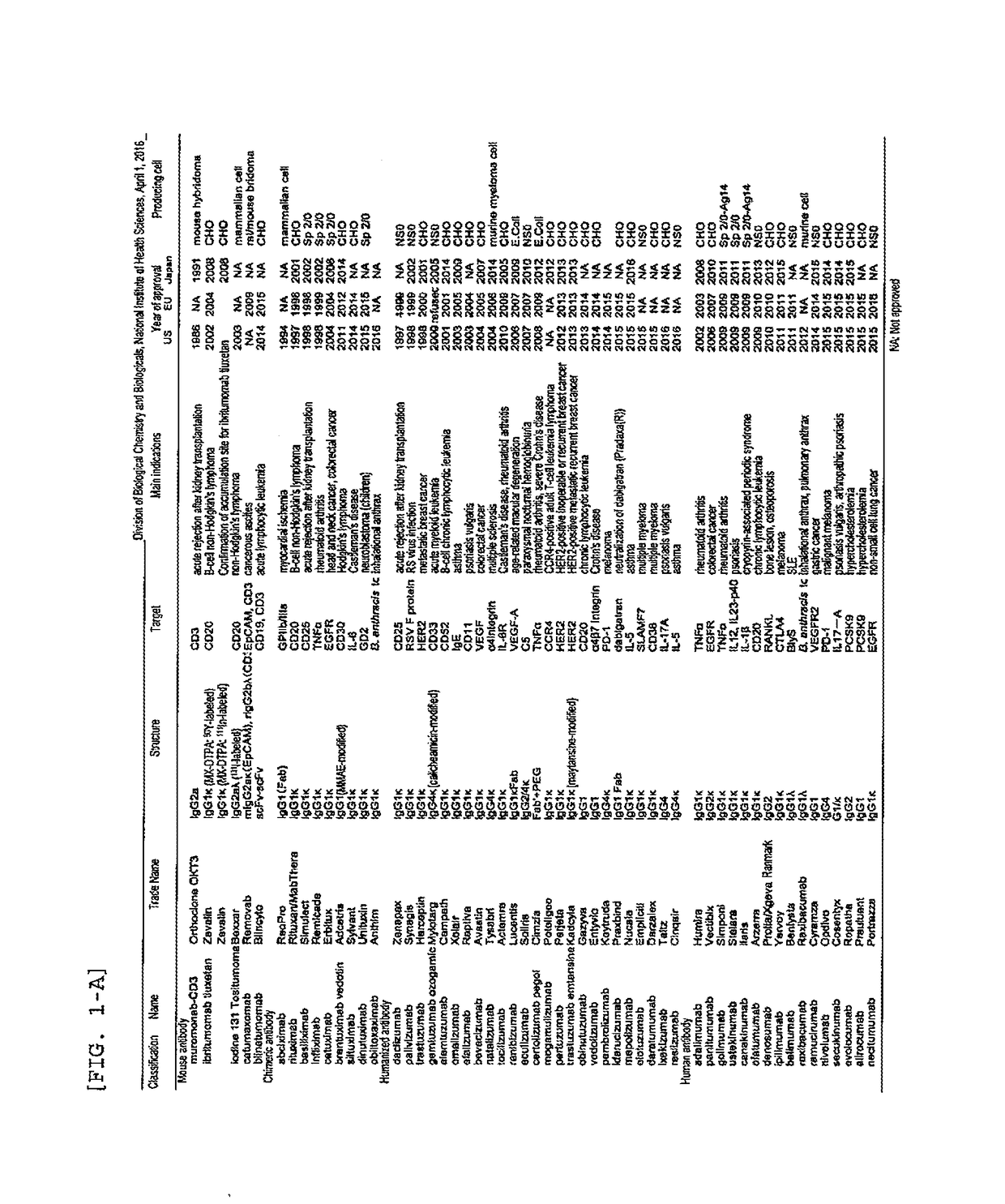
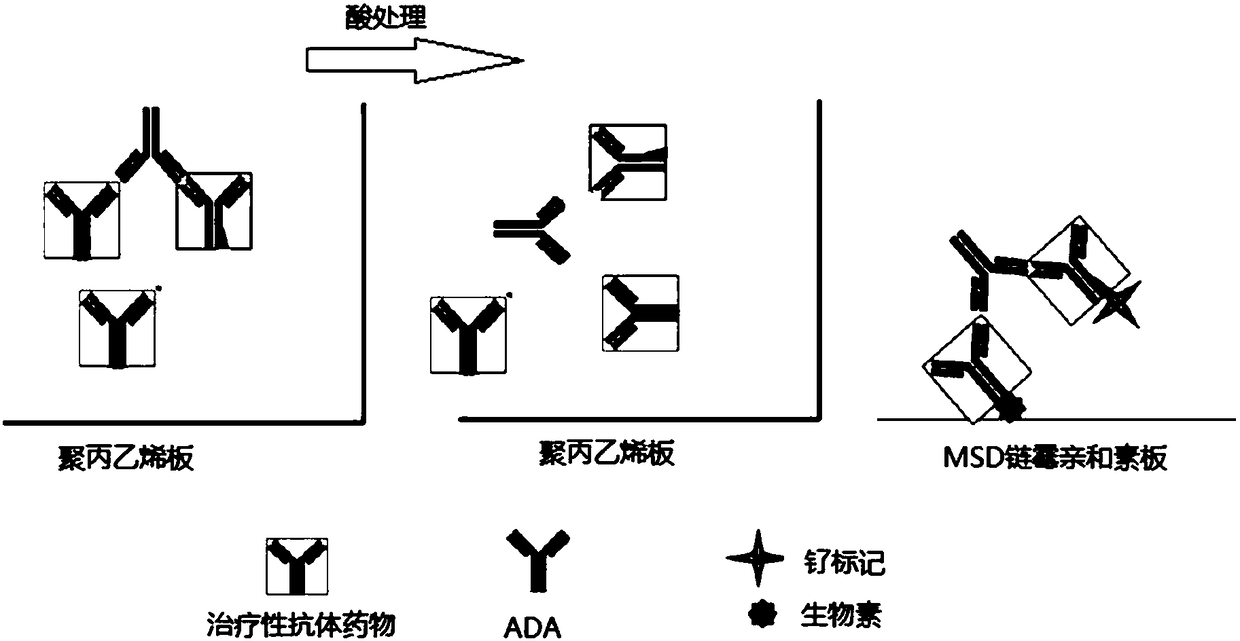
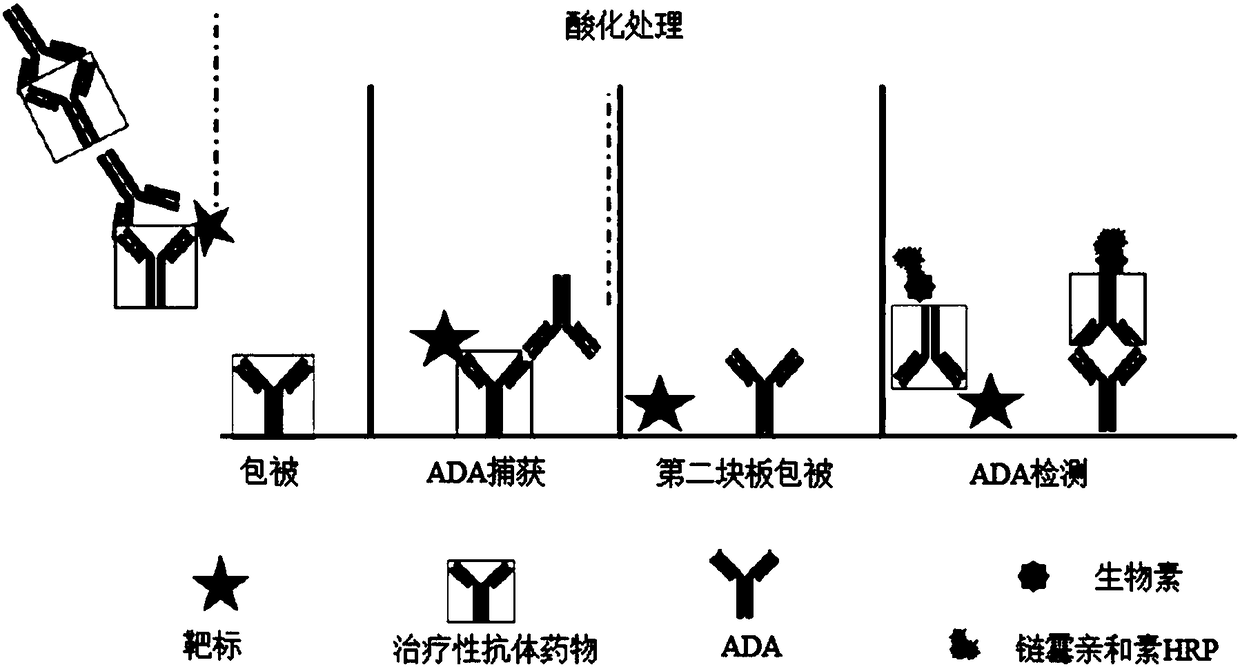
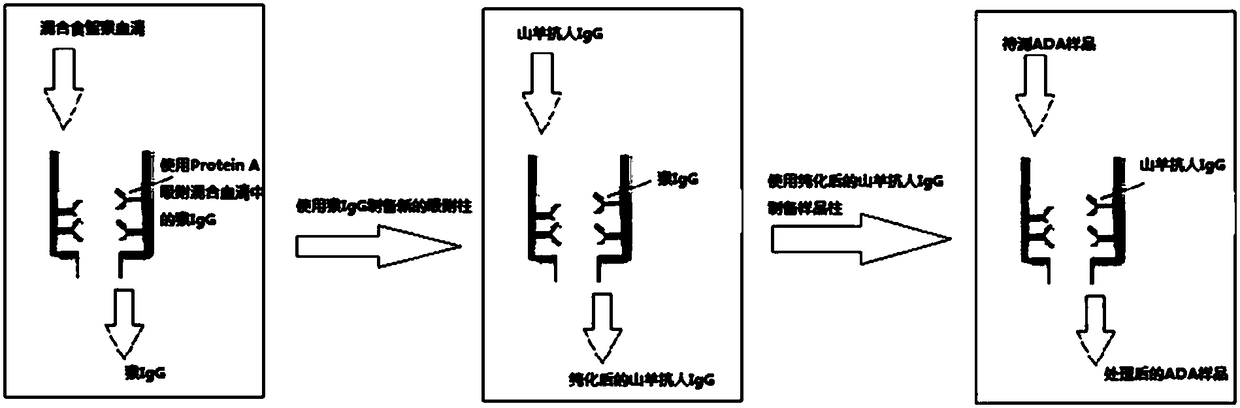
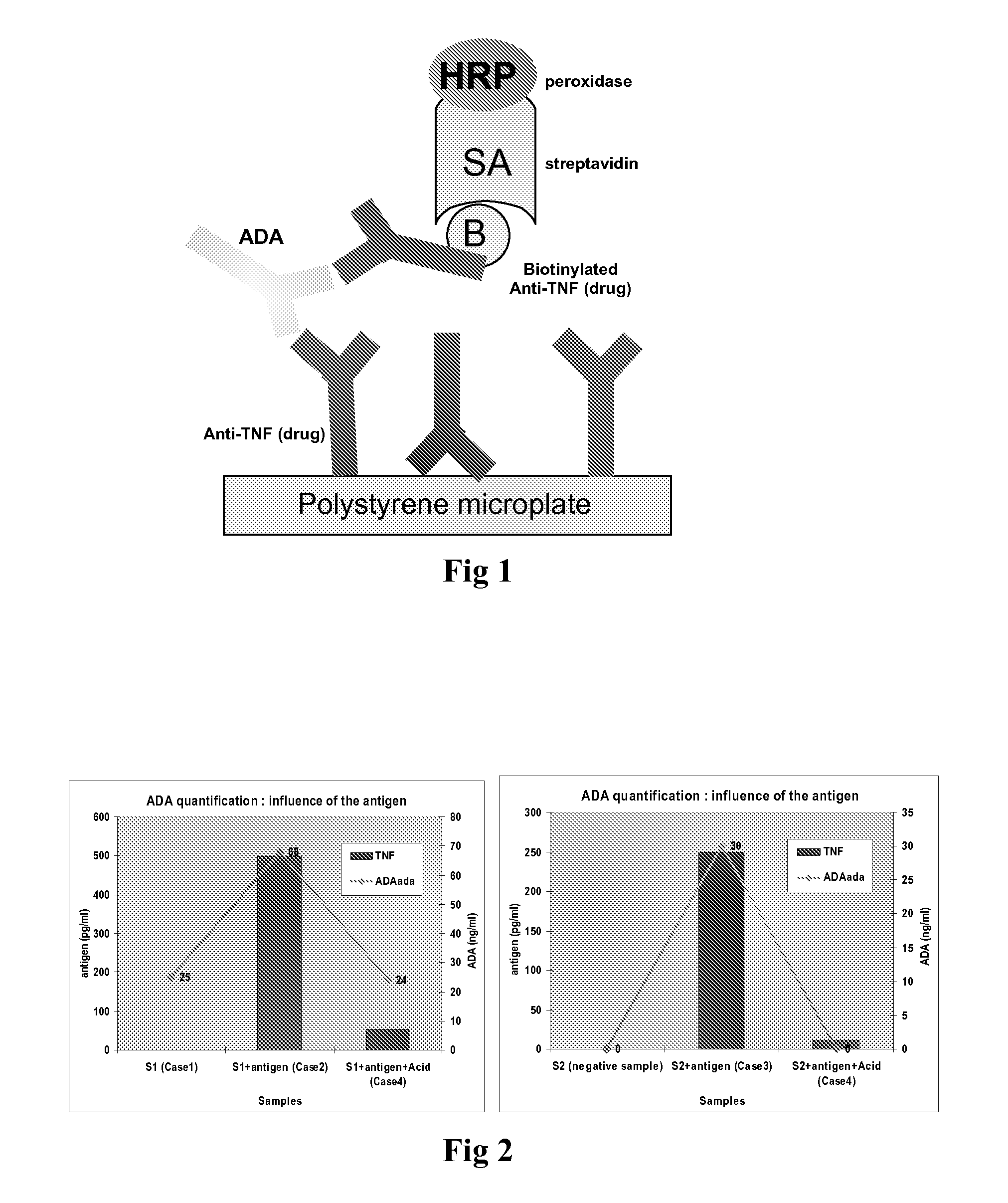
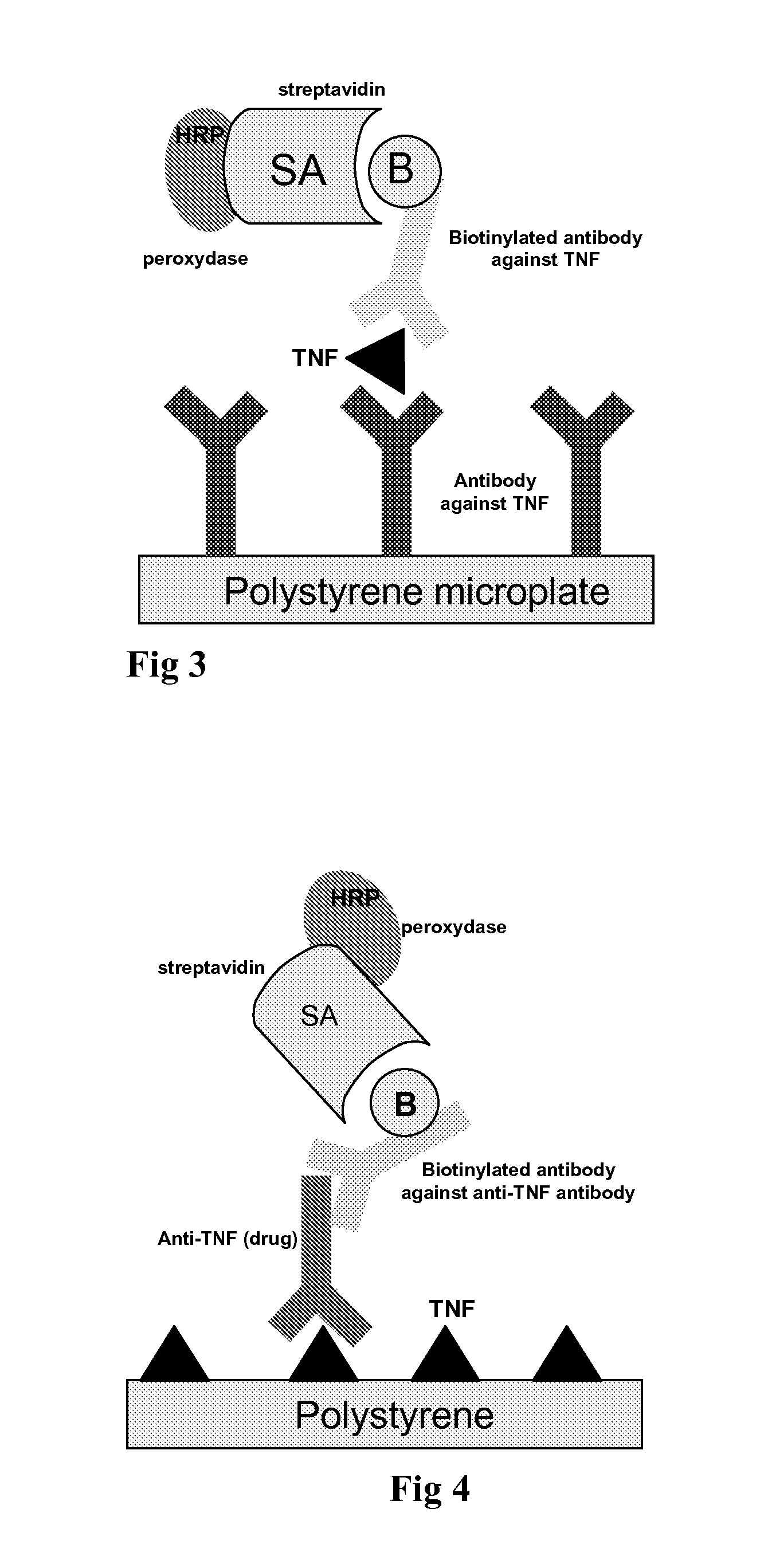
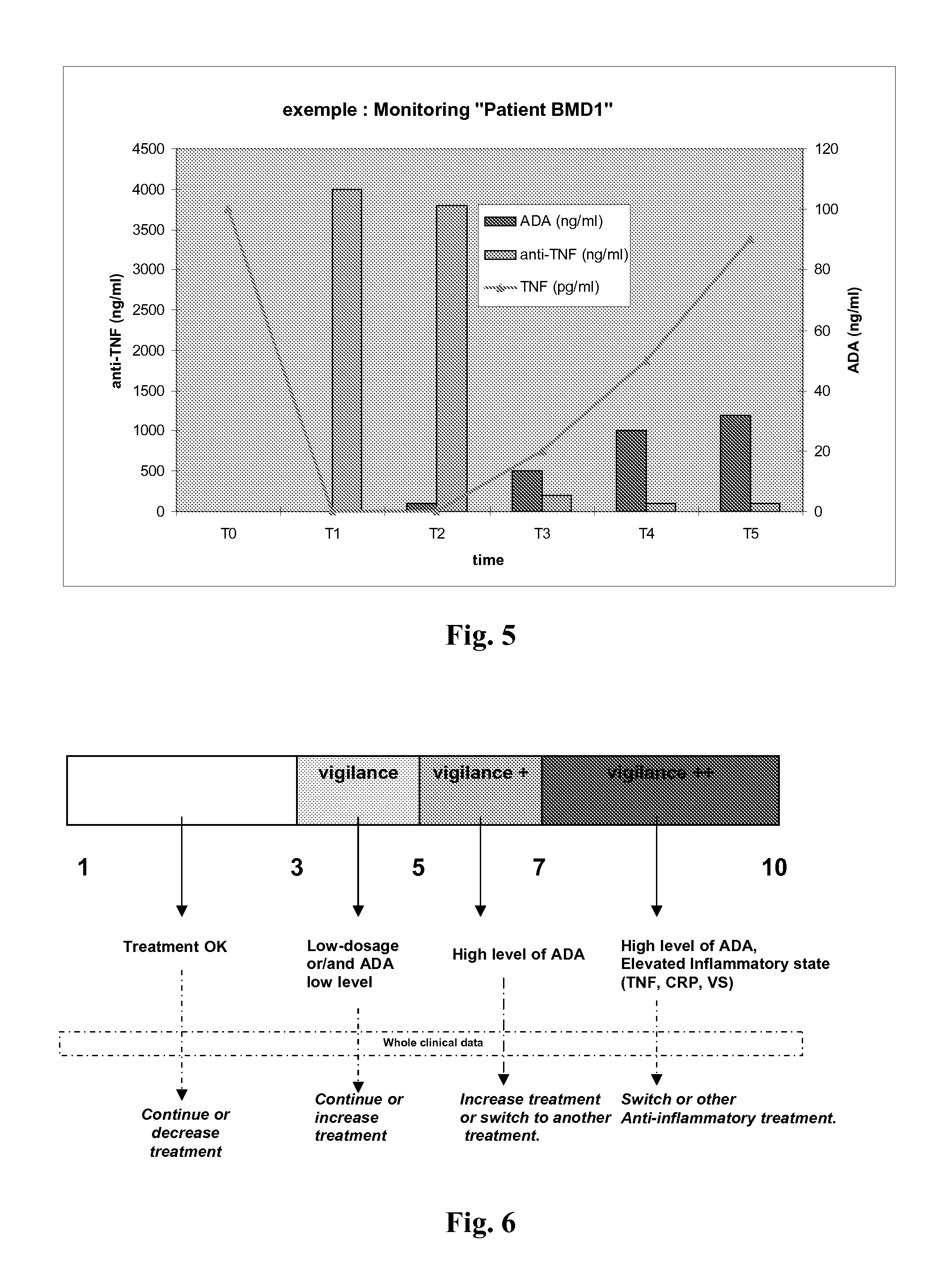
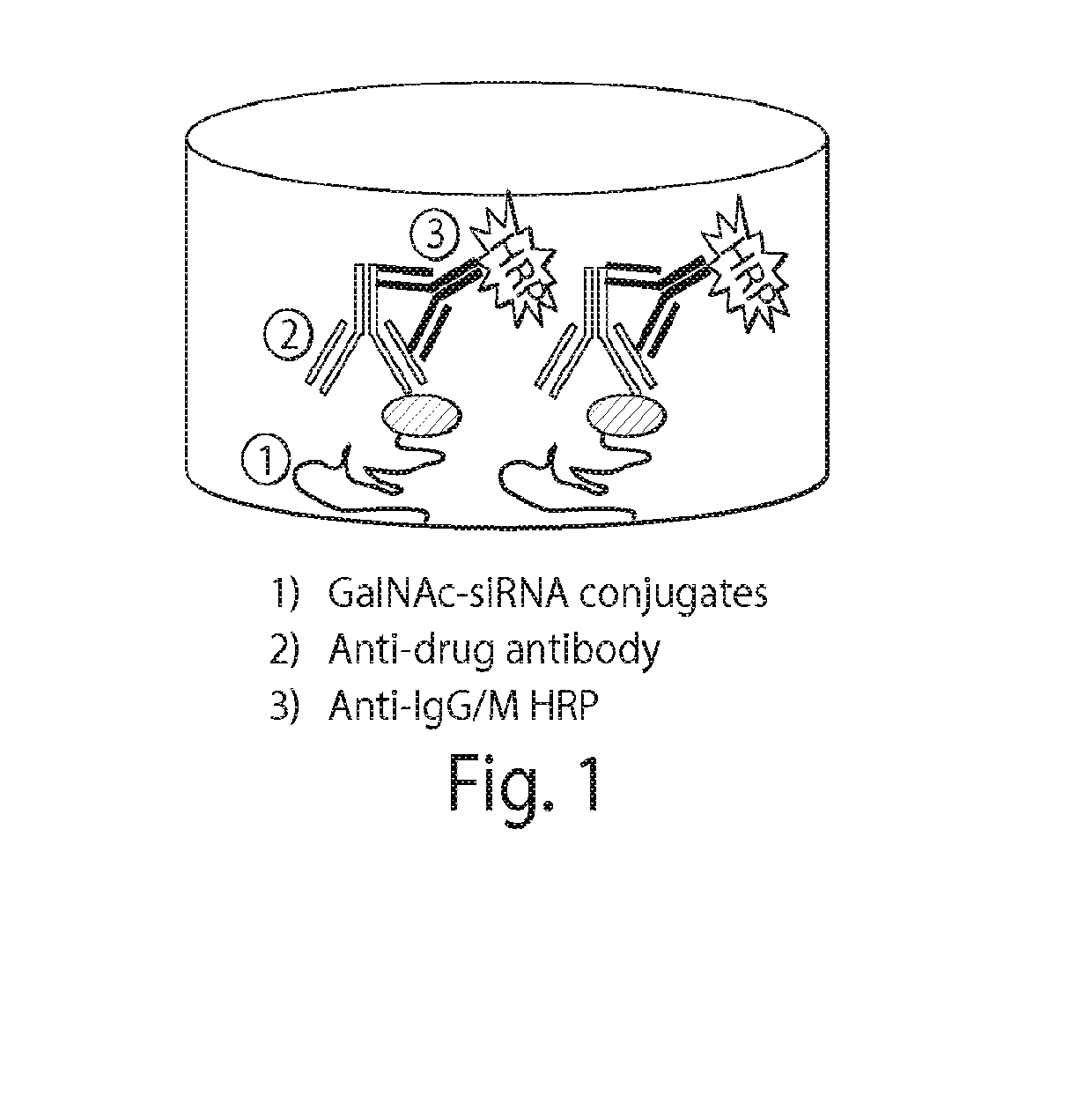
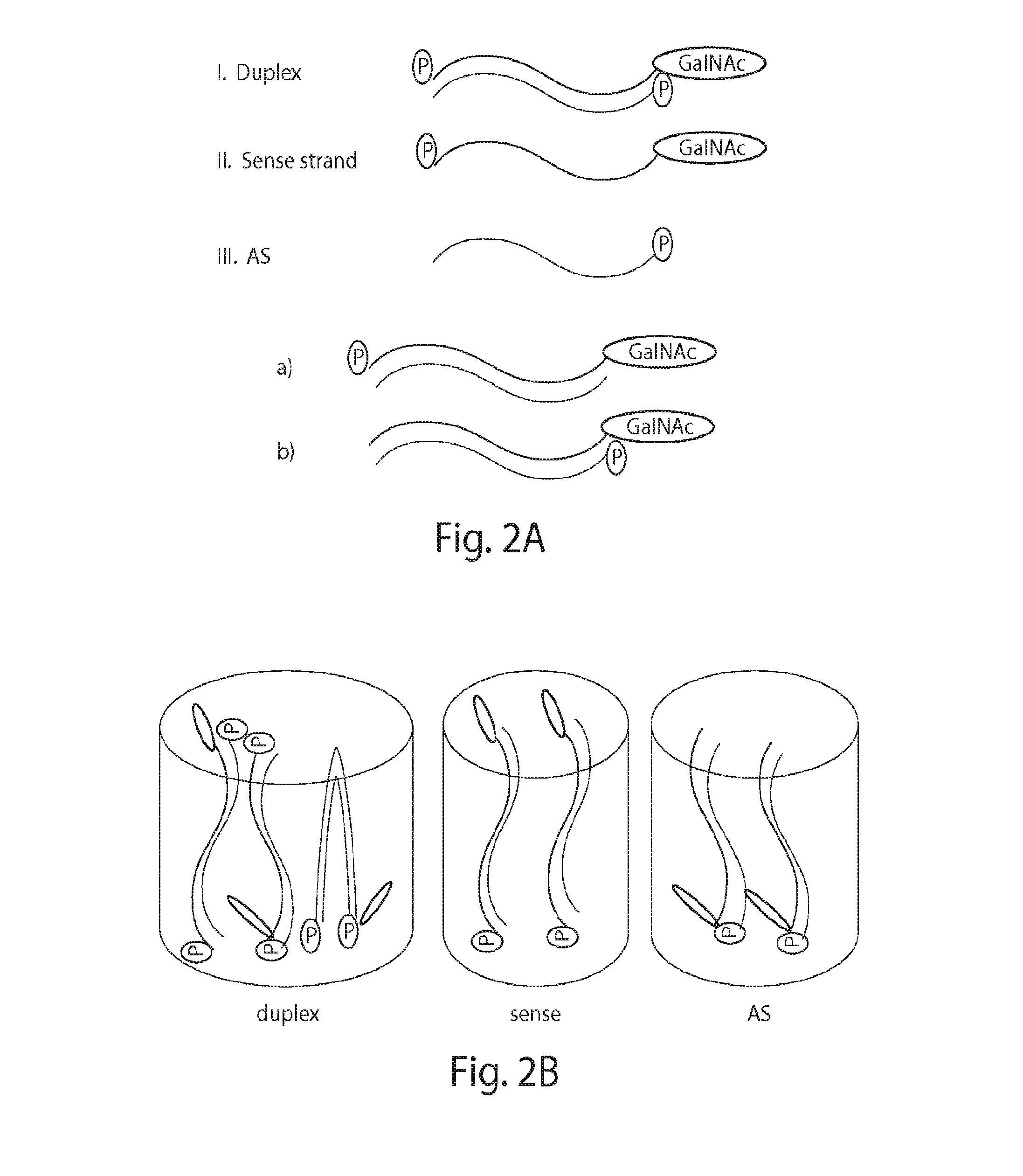
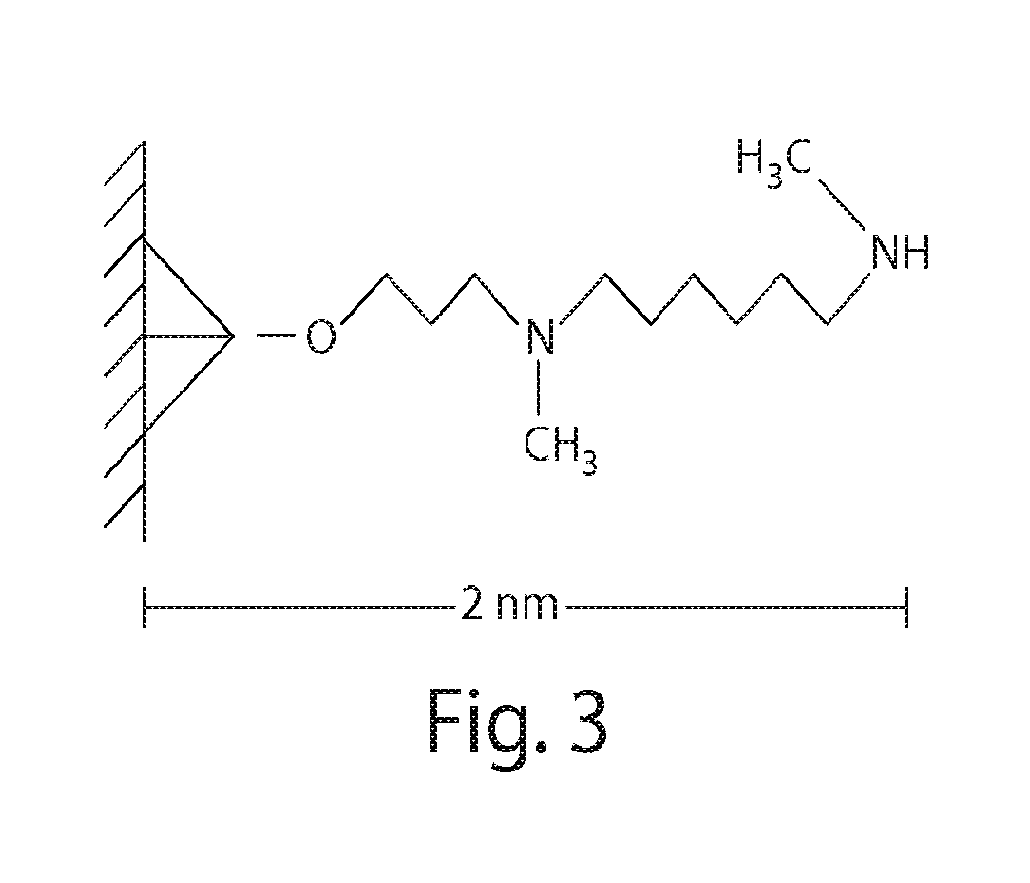
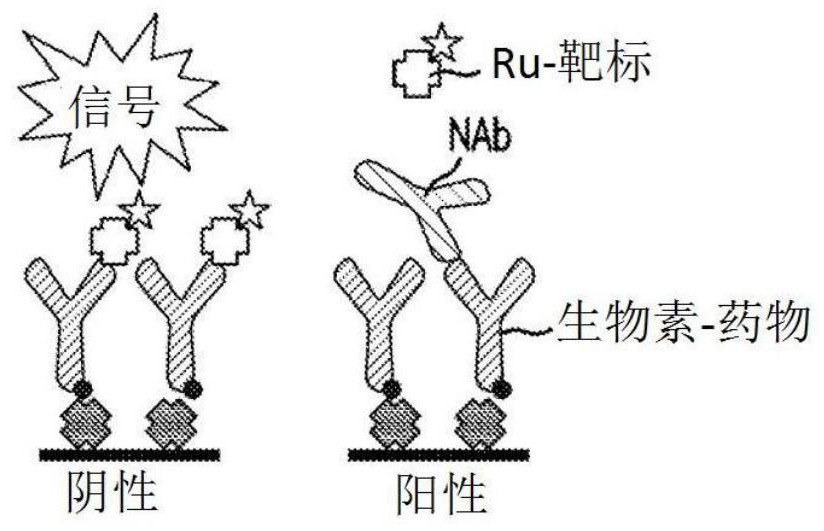
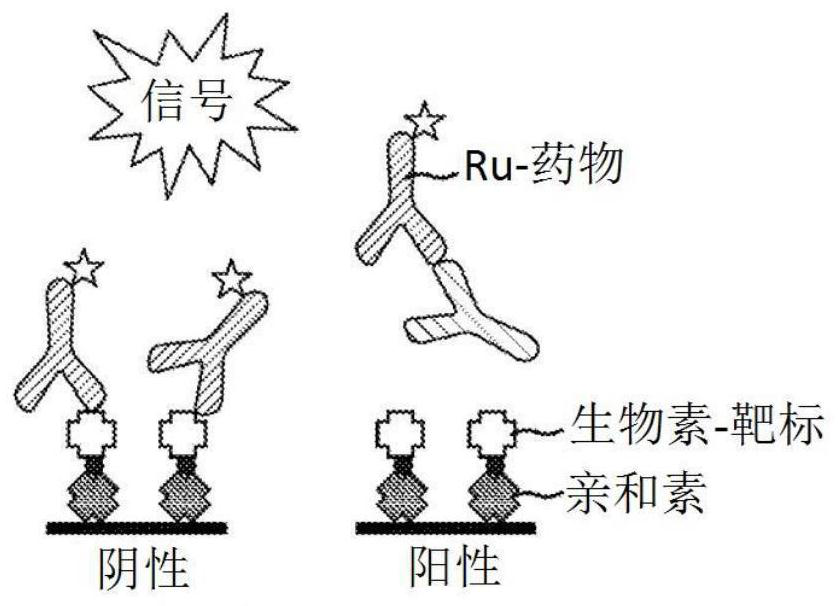
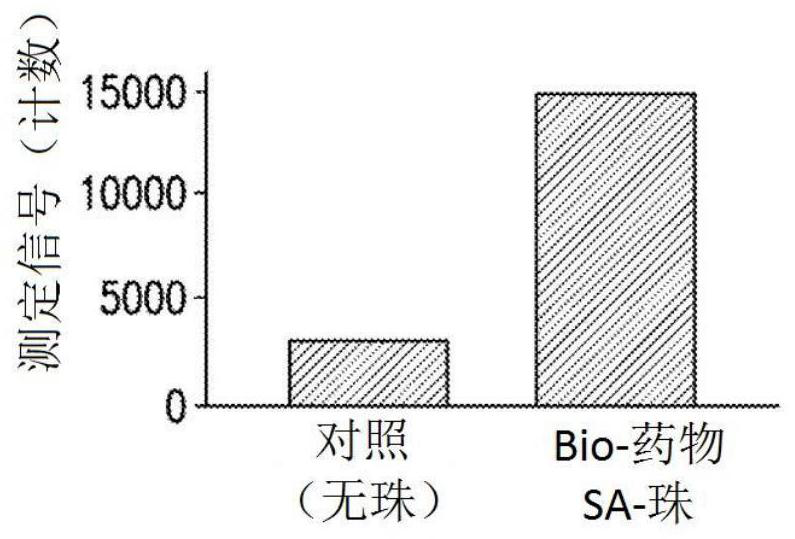
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
57 results about "Anti-Drug Antibody" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
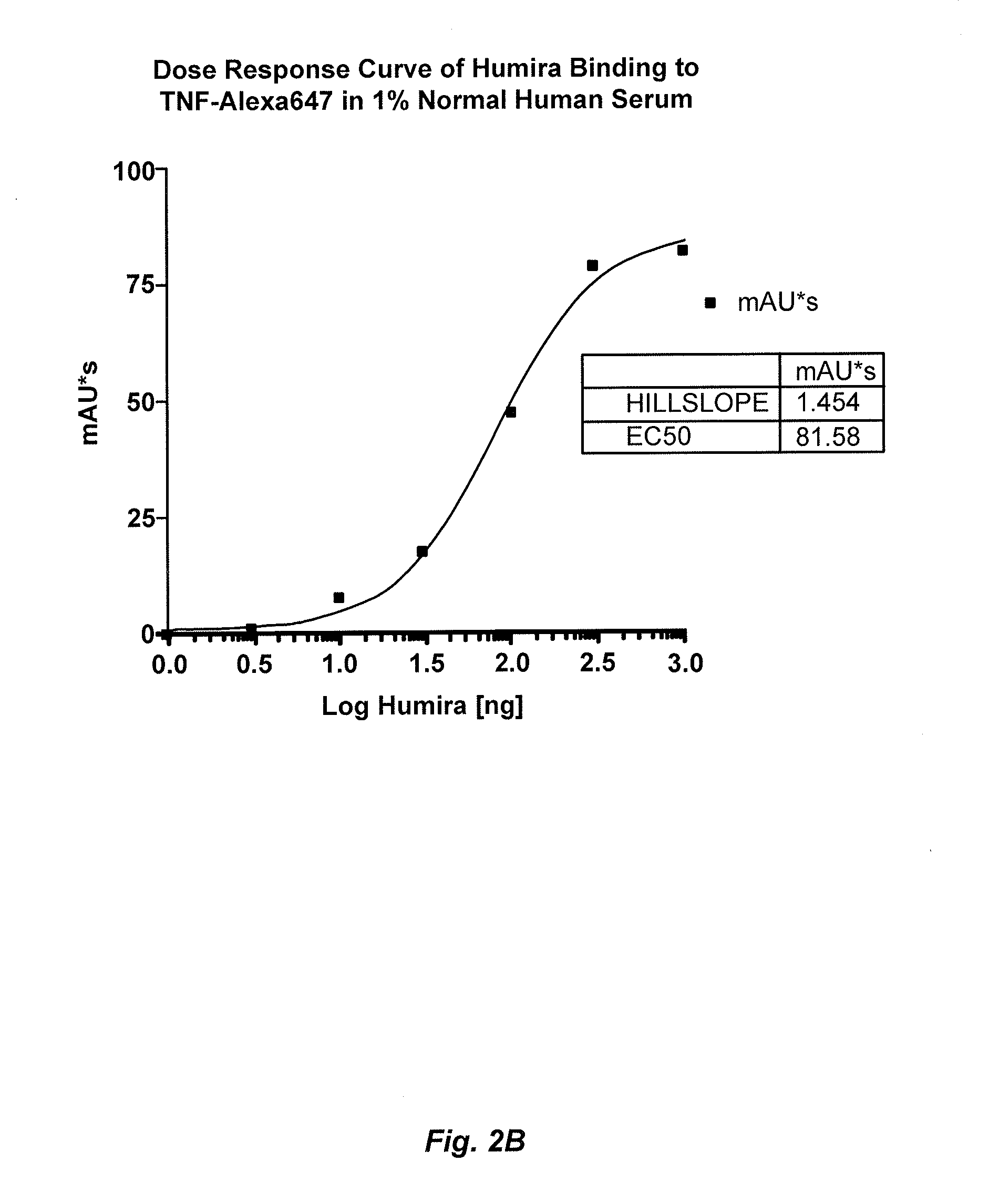
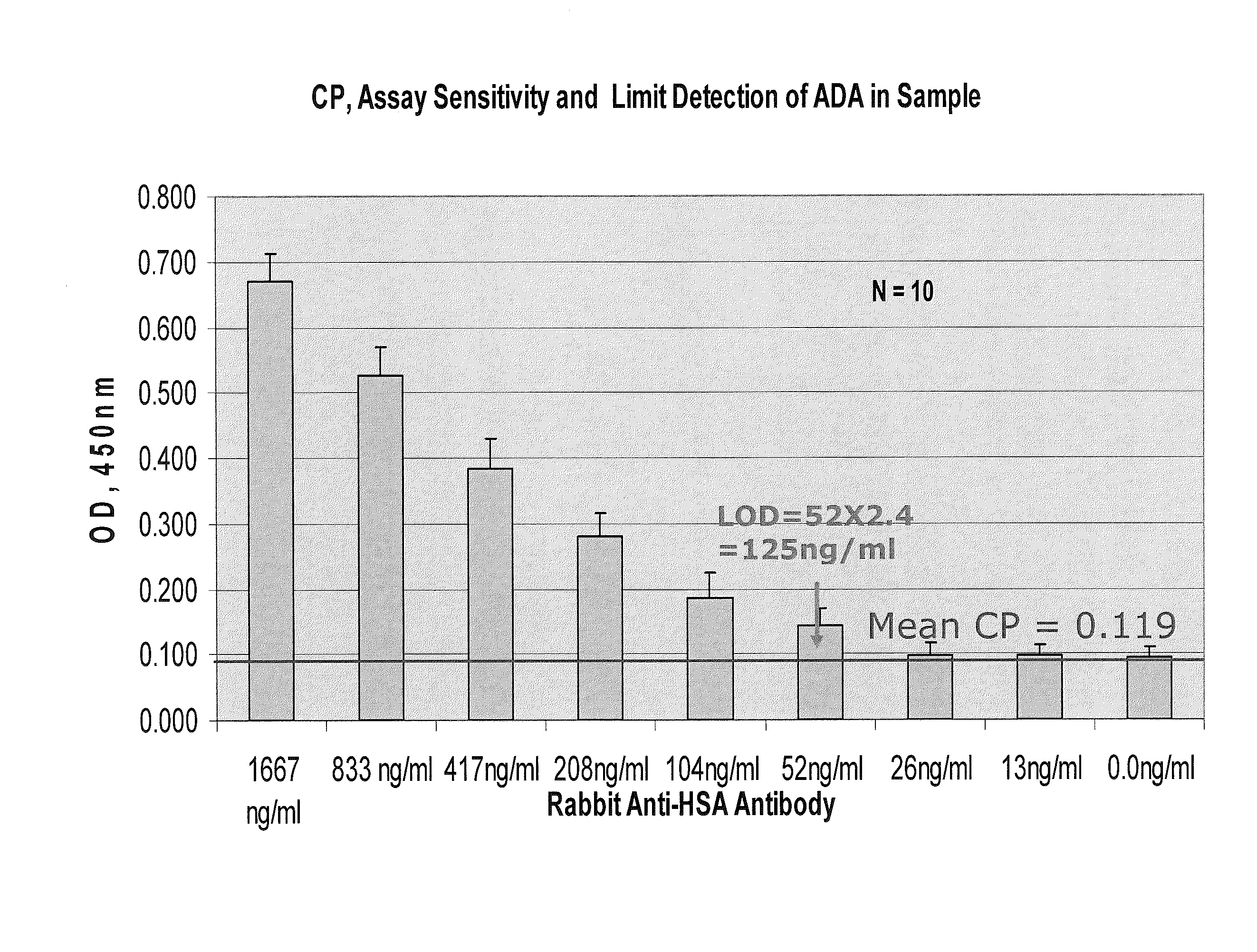
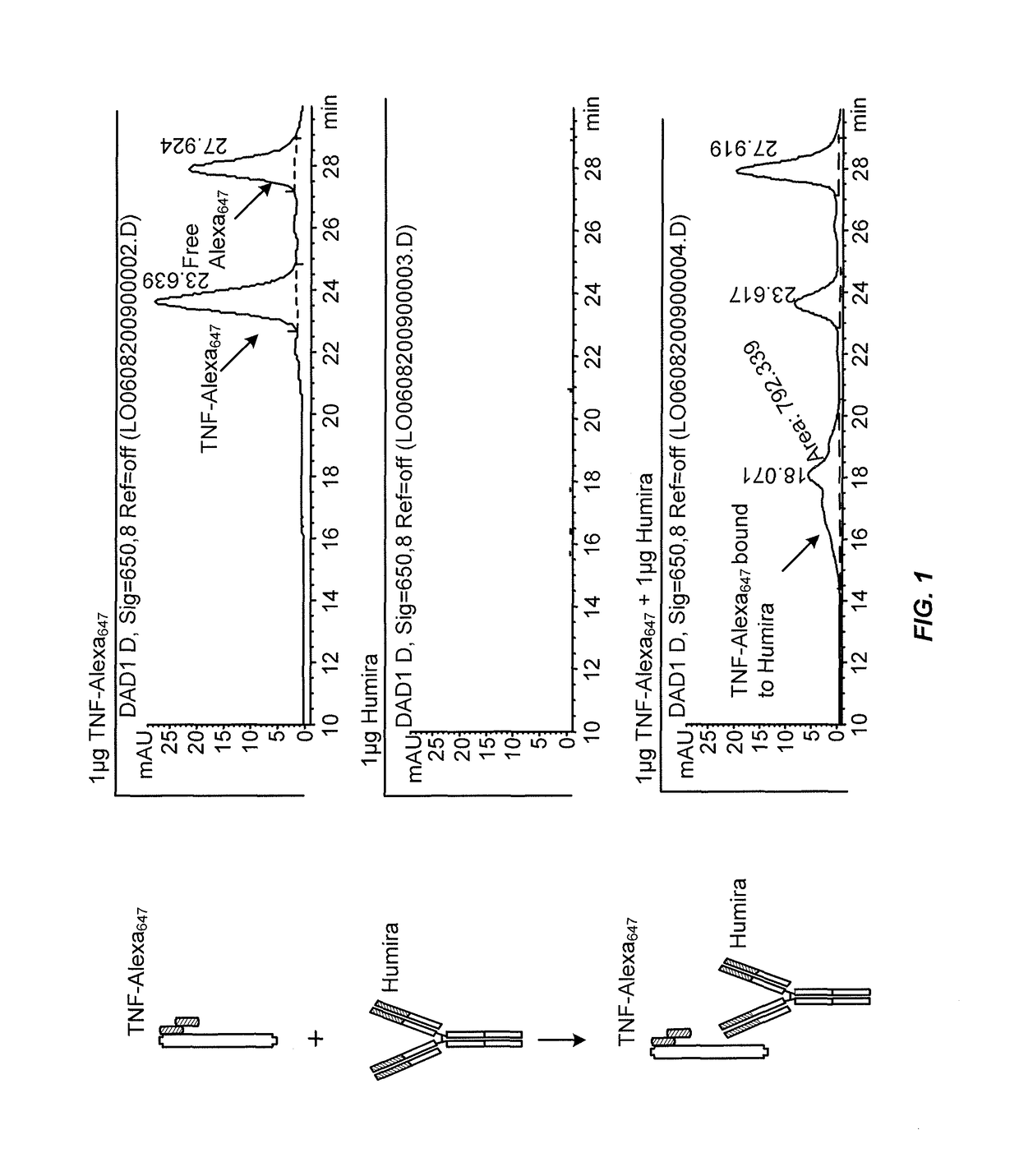
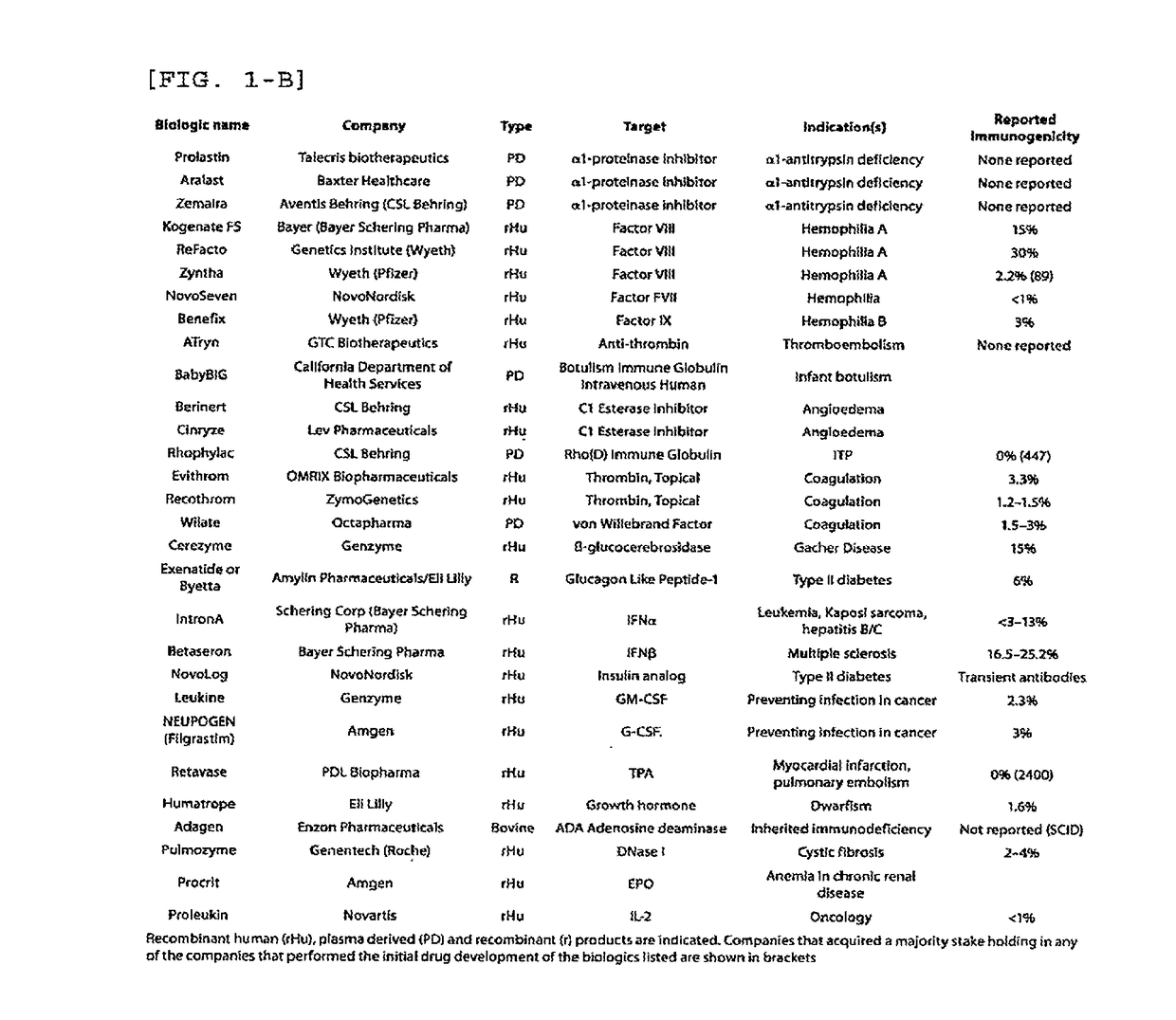
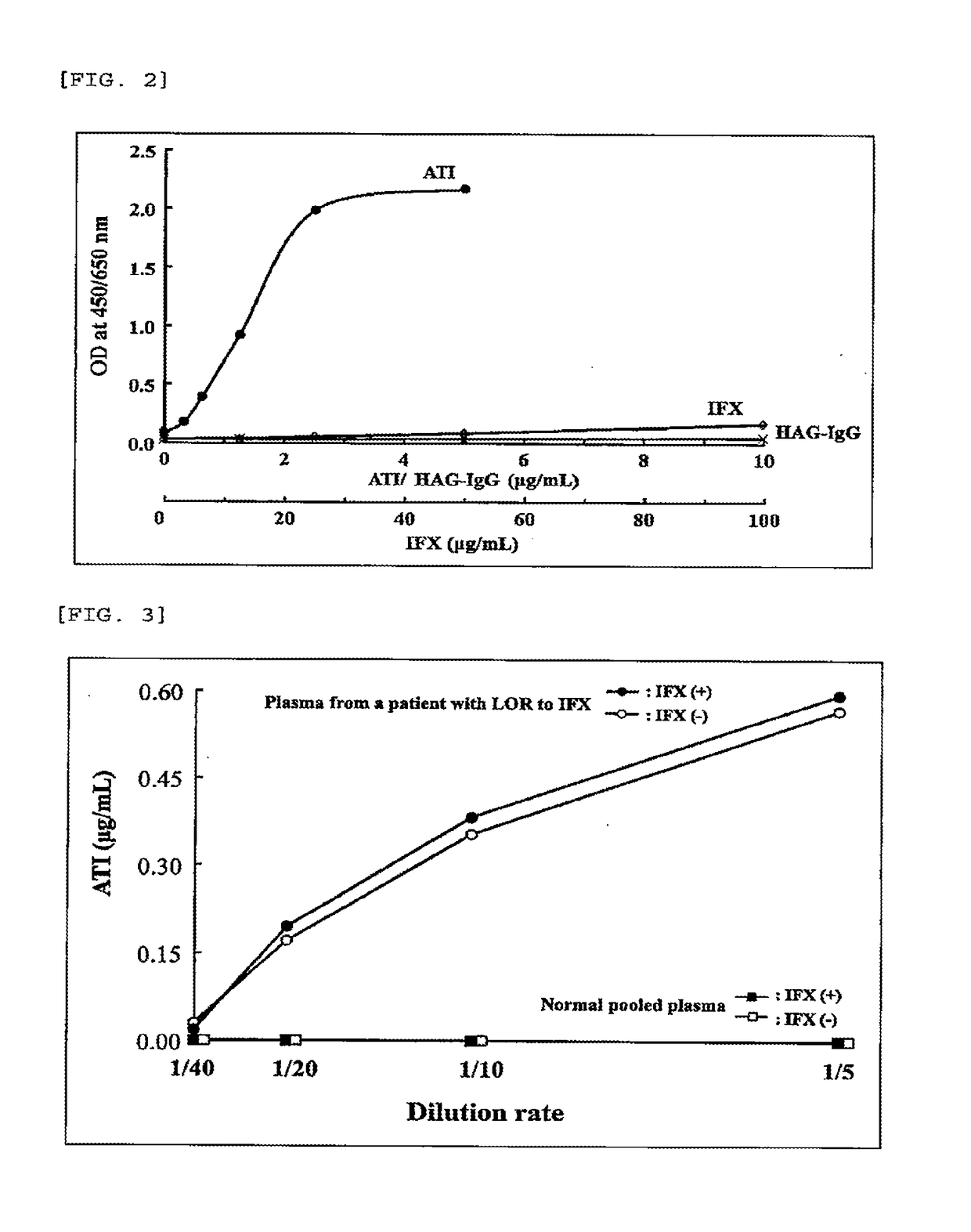
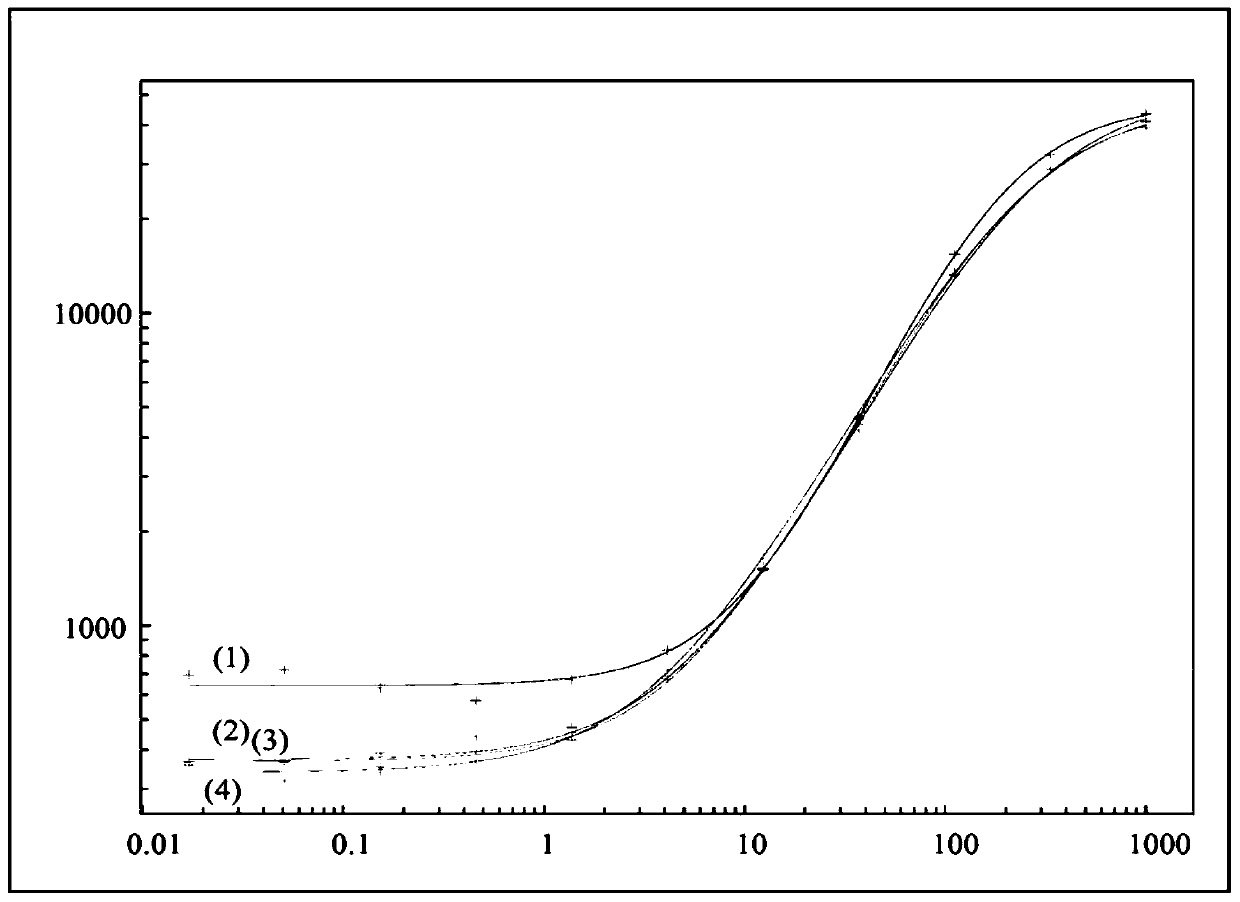
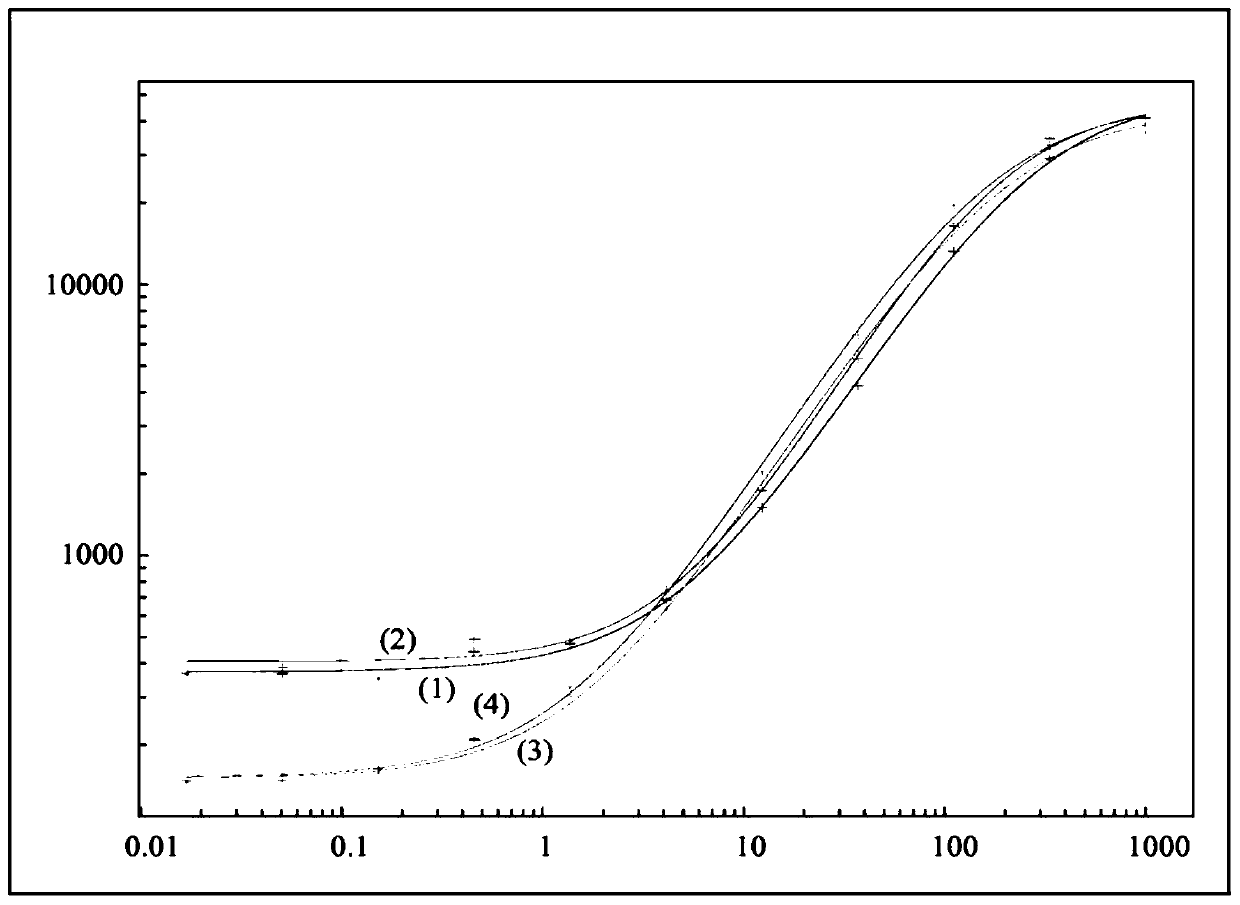
Immunogenicity Assays. Polyclonal anti-IDs are commonly used for immunogenicity assays as reference controls. Immunogenicity is the ability of a therapeutic, such as an antibody drug, to induce a humoral and/or cell-mediated immune response which leads to development of anti-drug- antibodies (ADAs).
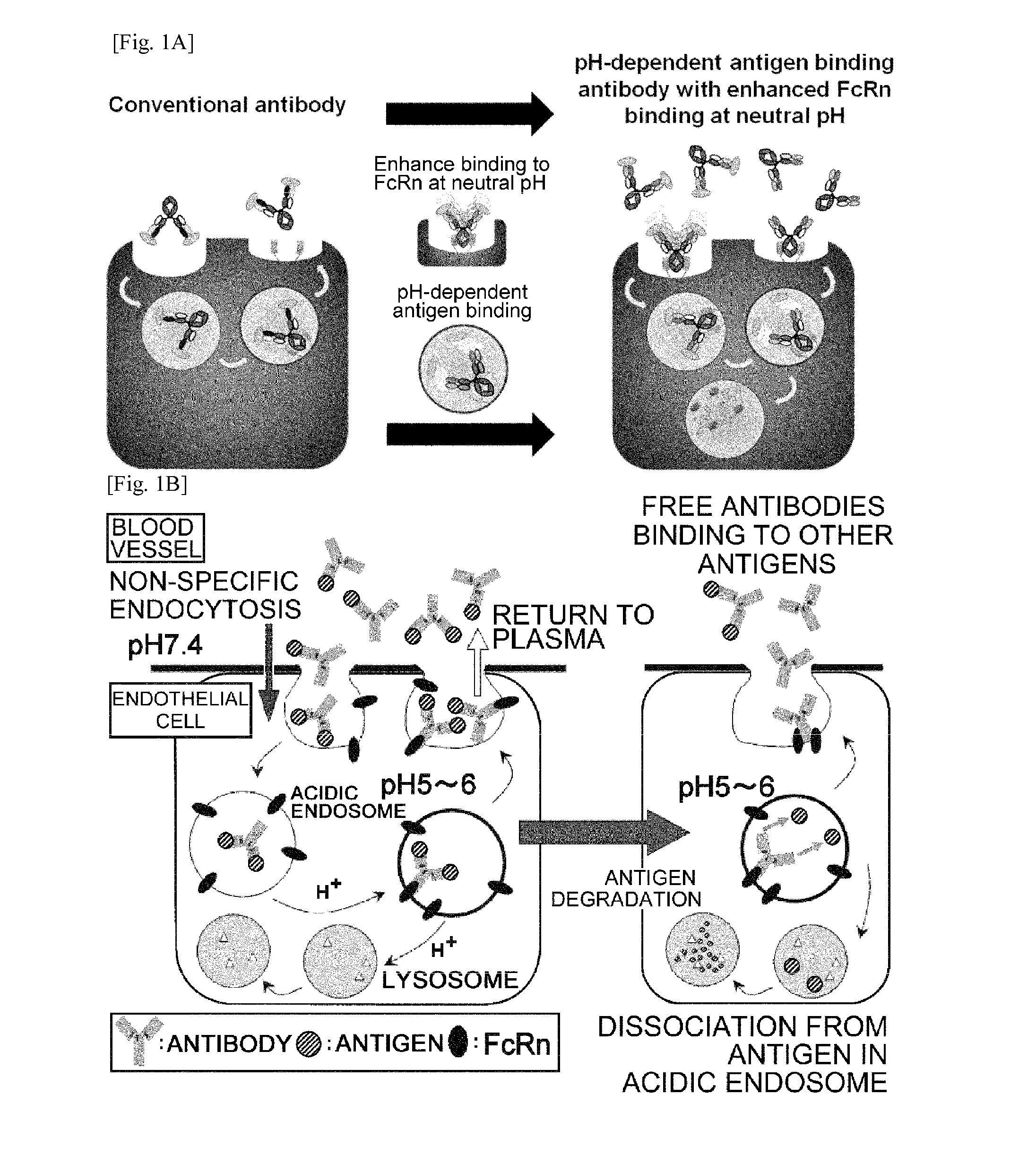
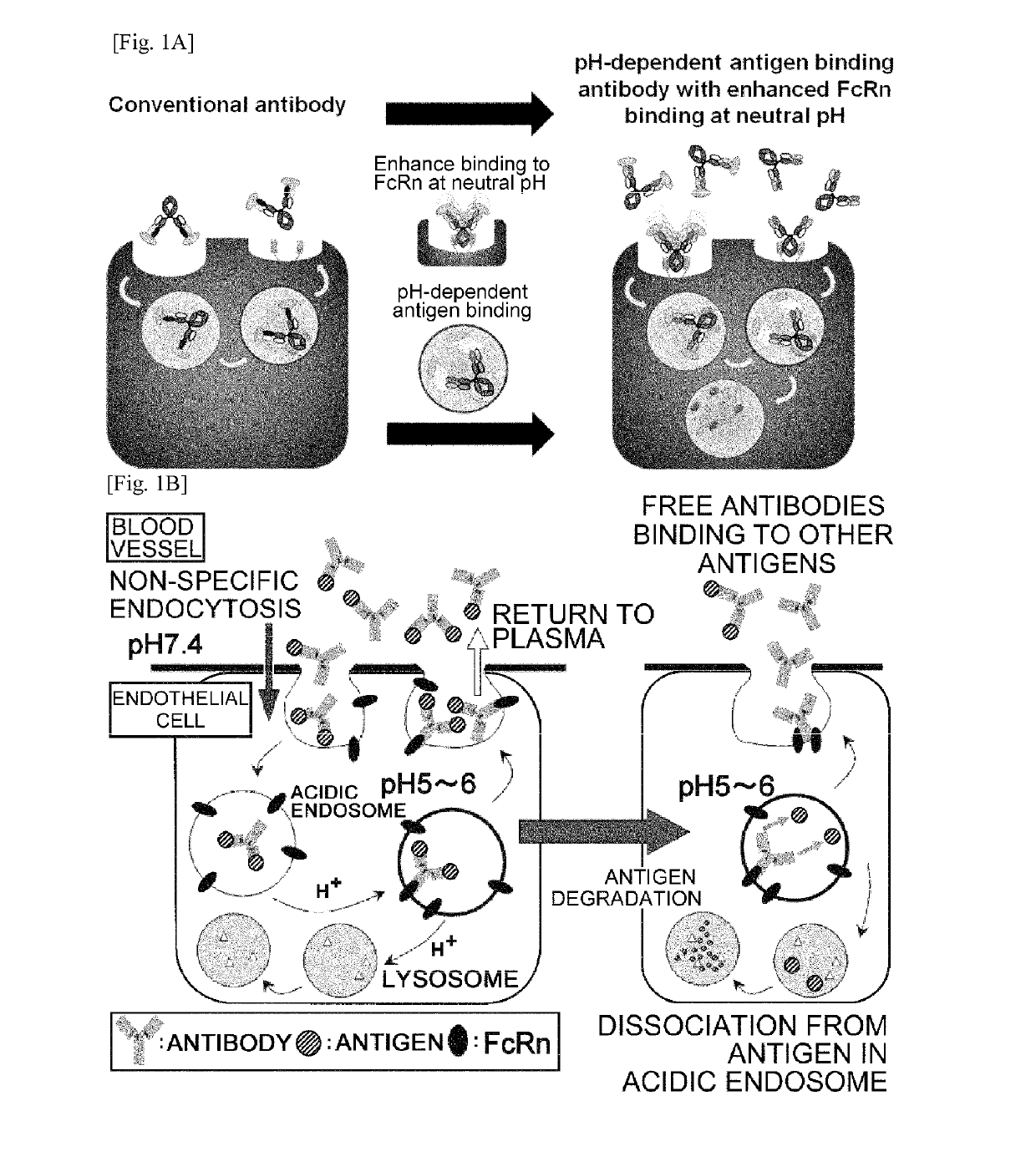
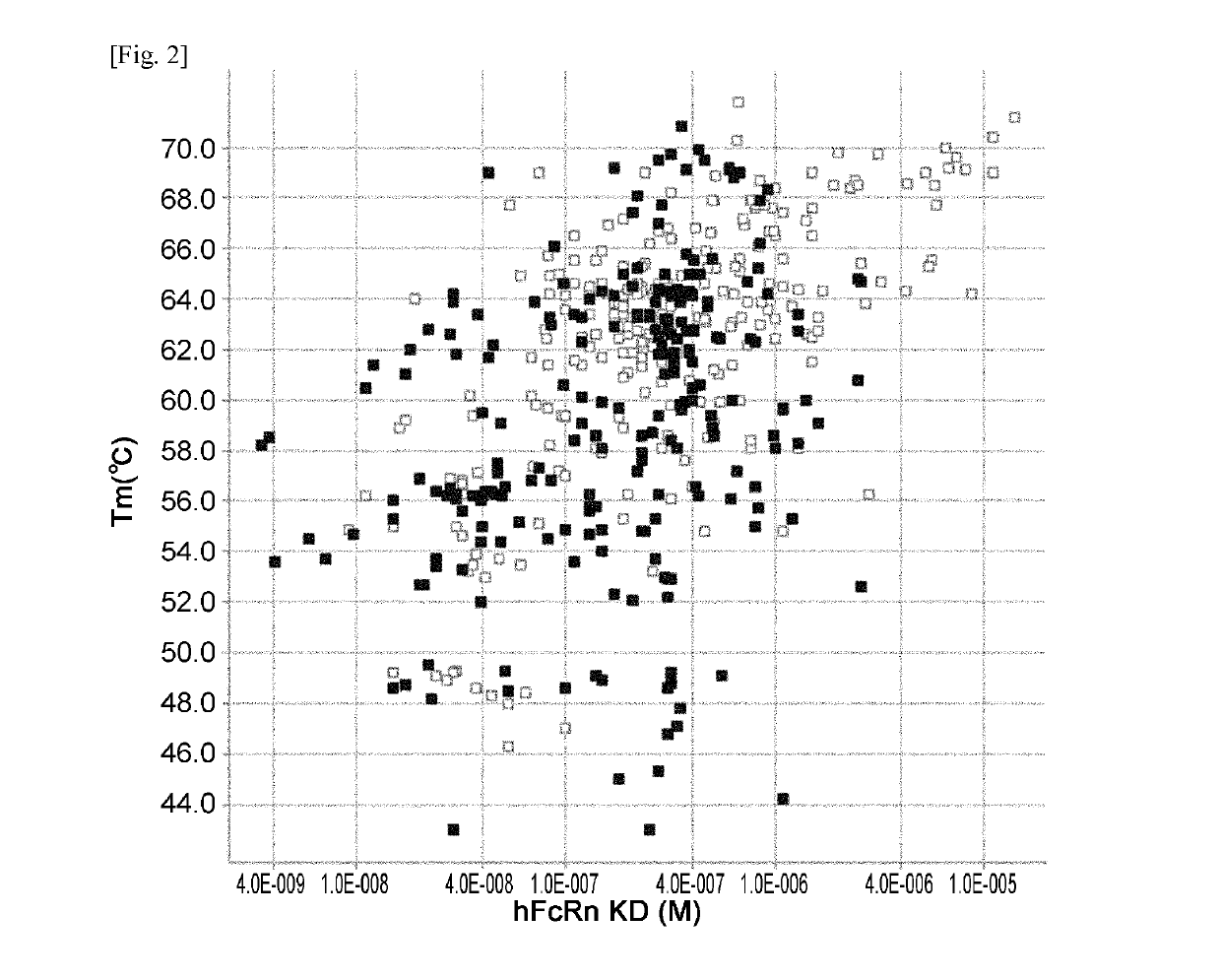
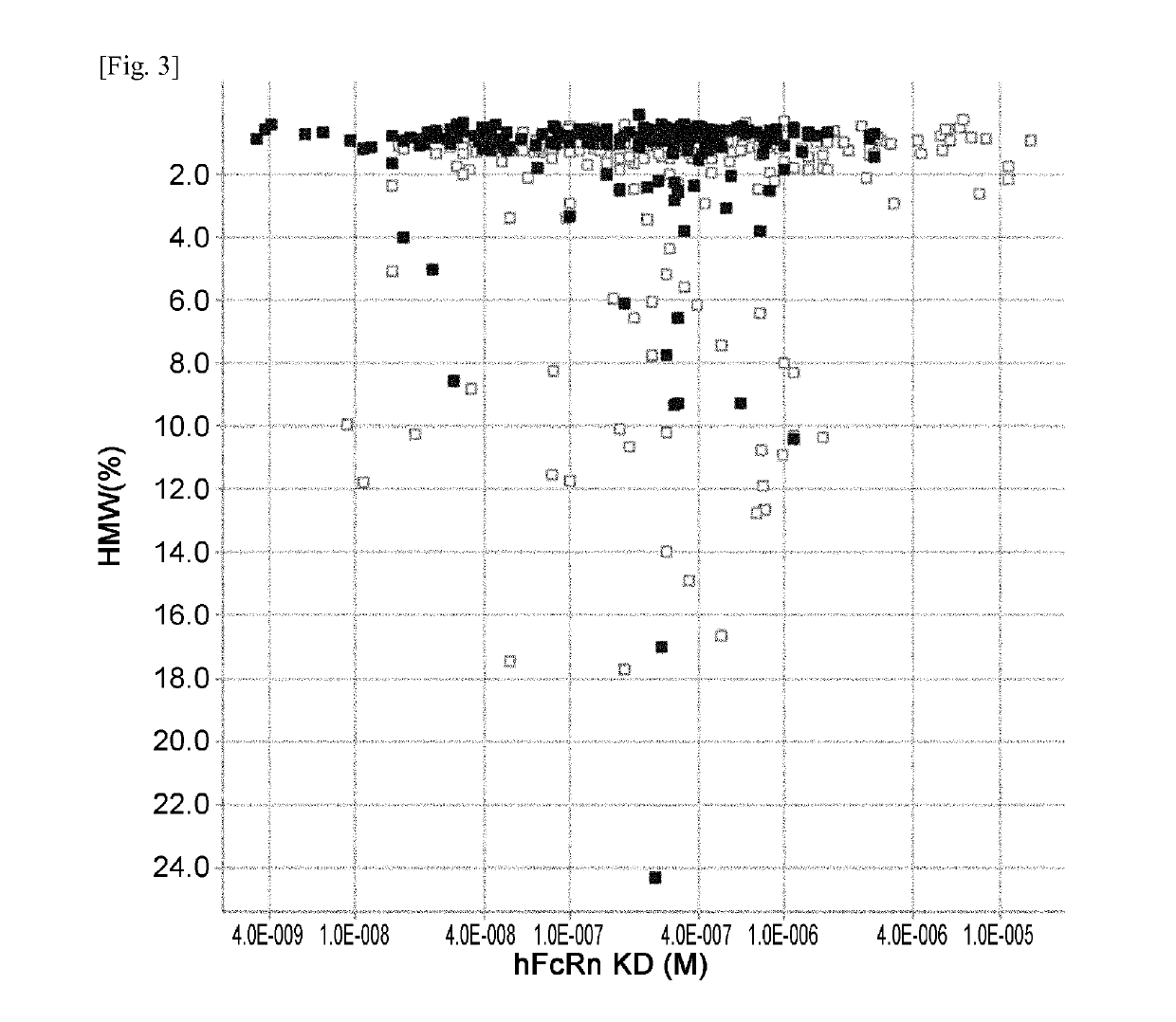
THERAPEUTIC ANTIGEN-BINDING MOLECULE WITH A FcRn-BINDING DOMAIN THAT PROMOTES ANTIGEN CLEARANCE
ActiveUS20140363428A1Low immunogenicityImprove stabilityAntipyreticAnalgesicsFc(alpha) receptorNeutral ph
The present invention provides: a modified FcRn-binding domain having an enhanced affinity for the Fc Receptor neonatal (FcRn) at neutral pH; an antigen-binding molecule comprising said FcRn-binding domain, which has low immunogenicity, high stability and form only a few aggregates; a modified antigen-binding molecule having an increased FcRn-binding activity at neutral or acidic pH without an increased binding activity at neutral pH for a pre-existing anti-drug antibody; use of the antigen-binding molecules for improving antigen-binding molecule-mediated antigen uptake into cells; use of the antigen-binding molecules for reducing the plasma concentration of a specific antigen; use of the modified FcRn-binding domain for increasing the total number of antigens to which a single antigen-binding molecule can bind before its degradation; use of the modified FcRn-binding domain for improving pharmacokinetics of an antigen-binding molecule; methods for decreasing the binding activity for a pre-existing anti-drug antibody; and methods for producing said antigen-binding molecules.
Owner:CHUGAI PHARMA CO LTD
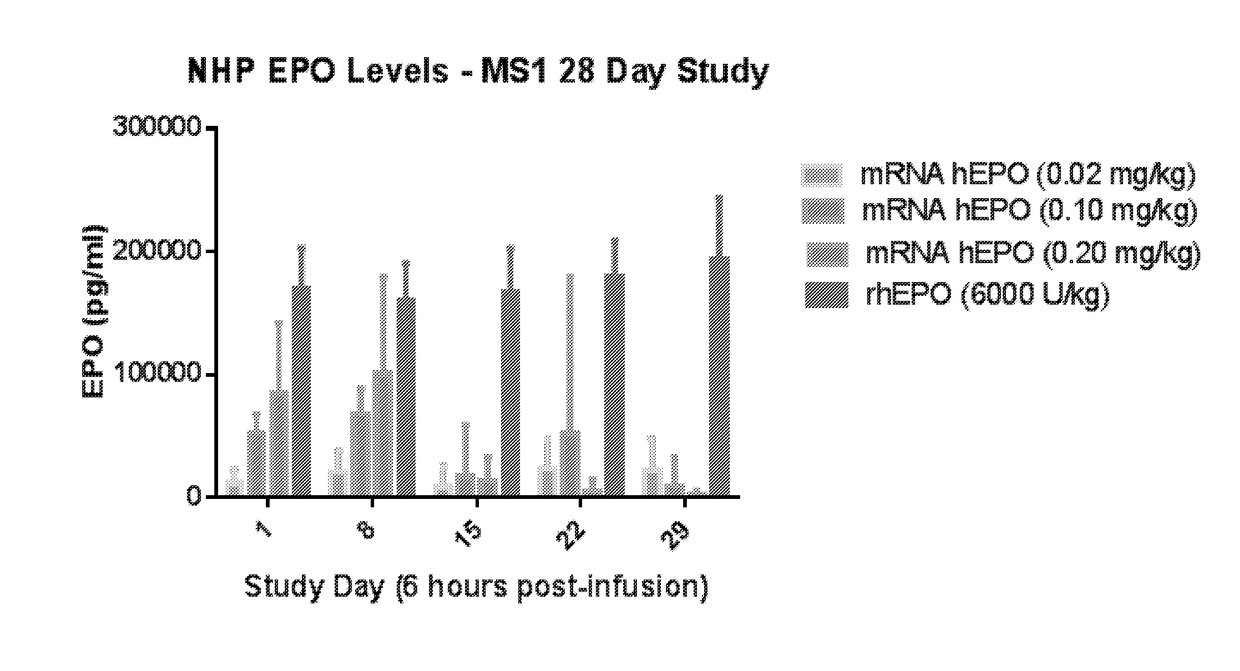
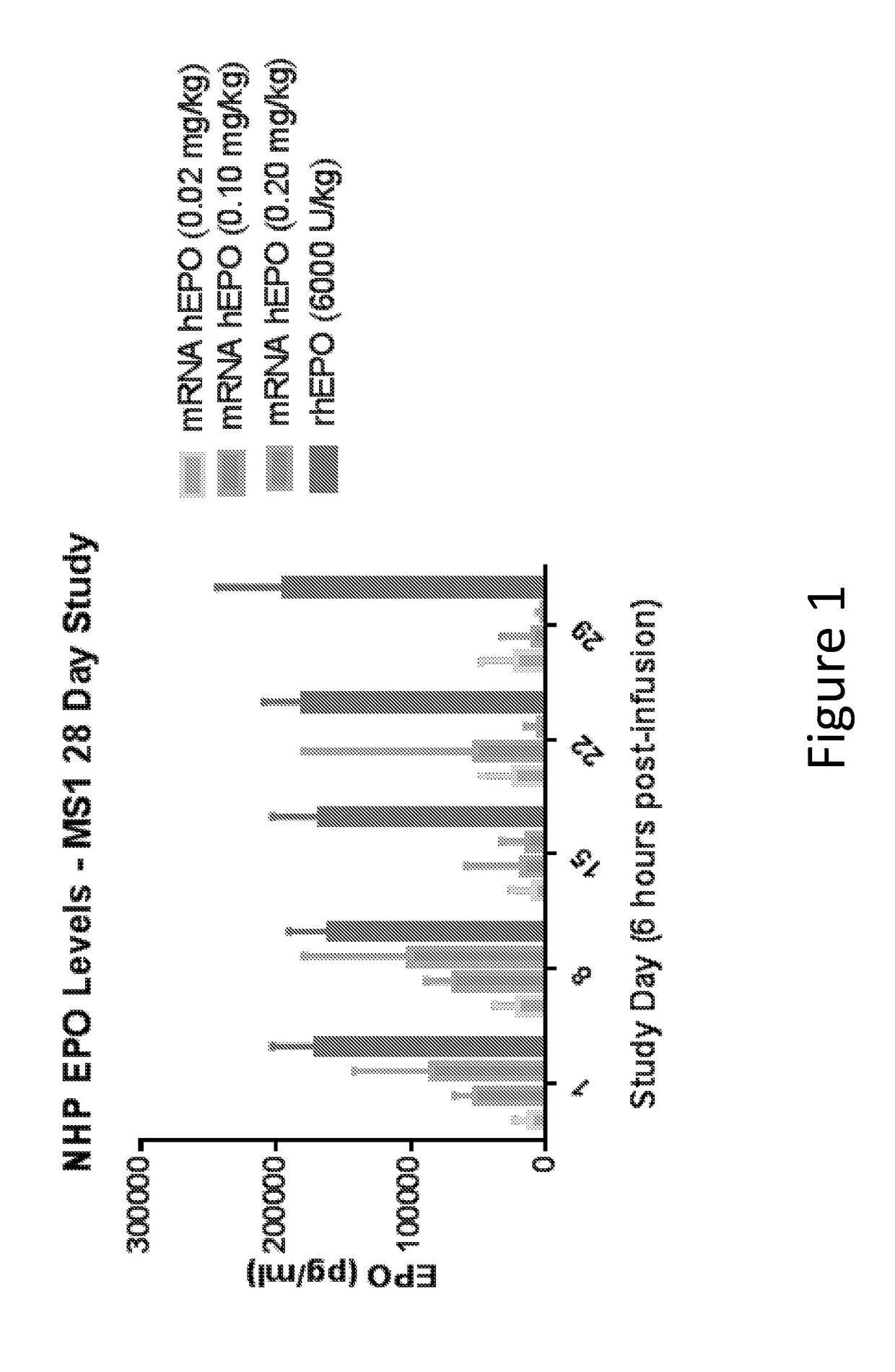
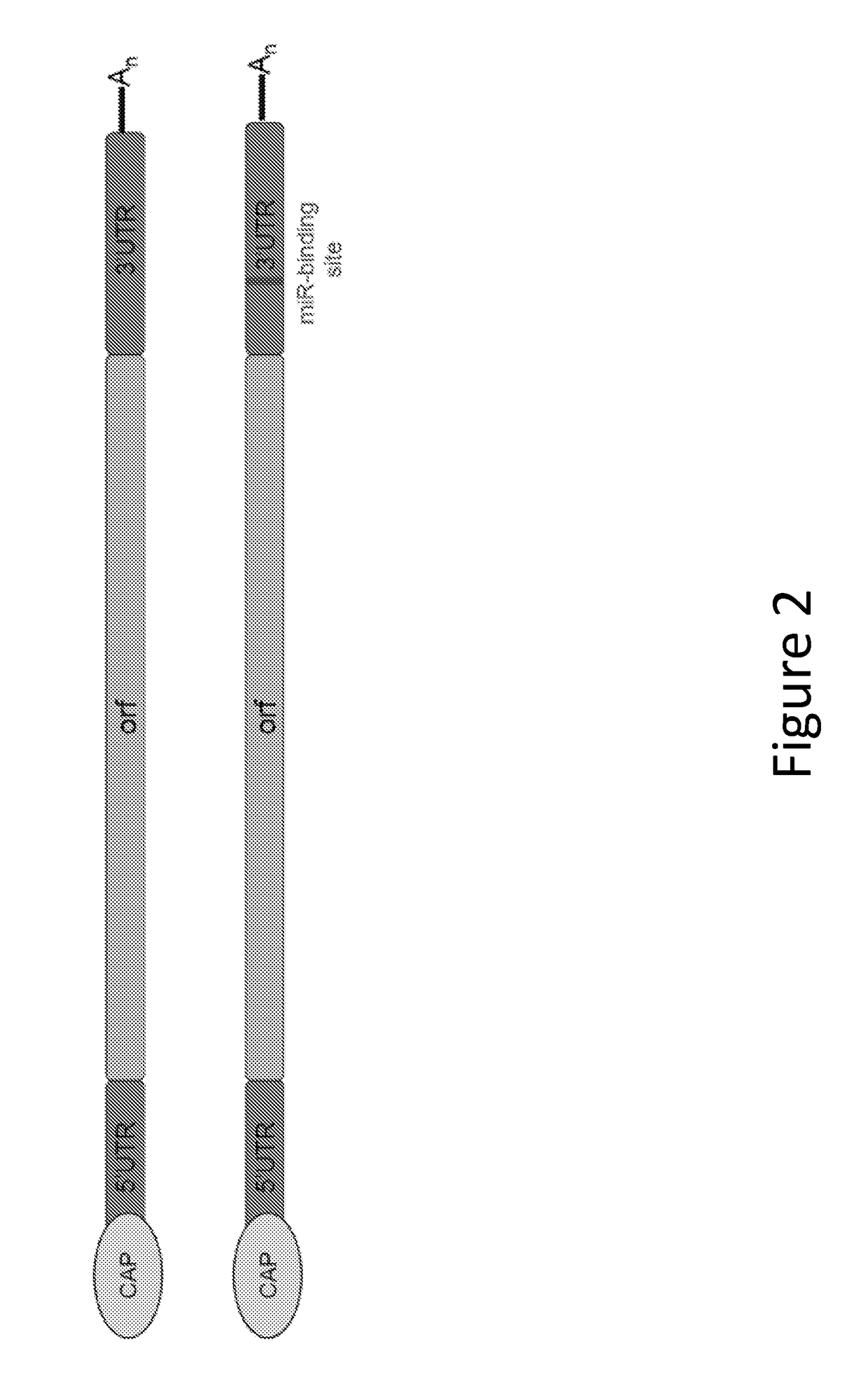
Methods for therapeutic administration of messenger ribonucleic acid drugs
ActiveUS20180256628A1Reducing and inhibiting accelerated blood clearanceBlood clearance is reduced and inhibitedOrganic active ingredientsNucleic acid vectorAntiendomysial antibodiesBinding site
The disclosure features methods of reducing or inhibiting an anti-drug antibody response in a subject, as well as methods of reducing or inhibiting unwanted immune cell activation in a subject to be treated with a messenger RNA (mRNA), comprising administering to the subject a mRNA, e.g., a chemically modified messenger RNA (mmRNA), encoding a polypeptide of interest, wherein the mRNA comprises at least one microRNA (miR) binding site for a miR expressed in immune cells, such as miR-126 binding site and / or miR-142 binding site, such that an anti-drug antibody response to the polypeptide or interest, or unwanted immune cell activation (e.g., B cell activation, cytokine secretion), is reduced or inhibited in the subject. The disclosure further provides therapeutic treatment regimens designed to reduce or inhibit AD A or unwanted immune cell activation (e.g., B cell activation, cytokine secretion) in a subject being treated with mRNA-based therapeutics.
Owner:MODERNATX INC
Methods for determining Anti-drug antibody isotypes
ActiveUS20130344621A1Convenient treatmentLow toxicityComponent separationBiological testingAntiendomysial antibodiesAssay
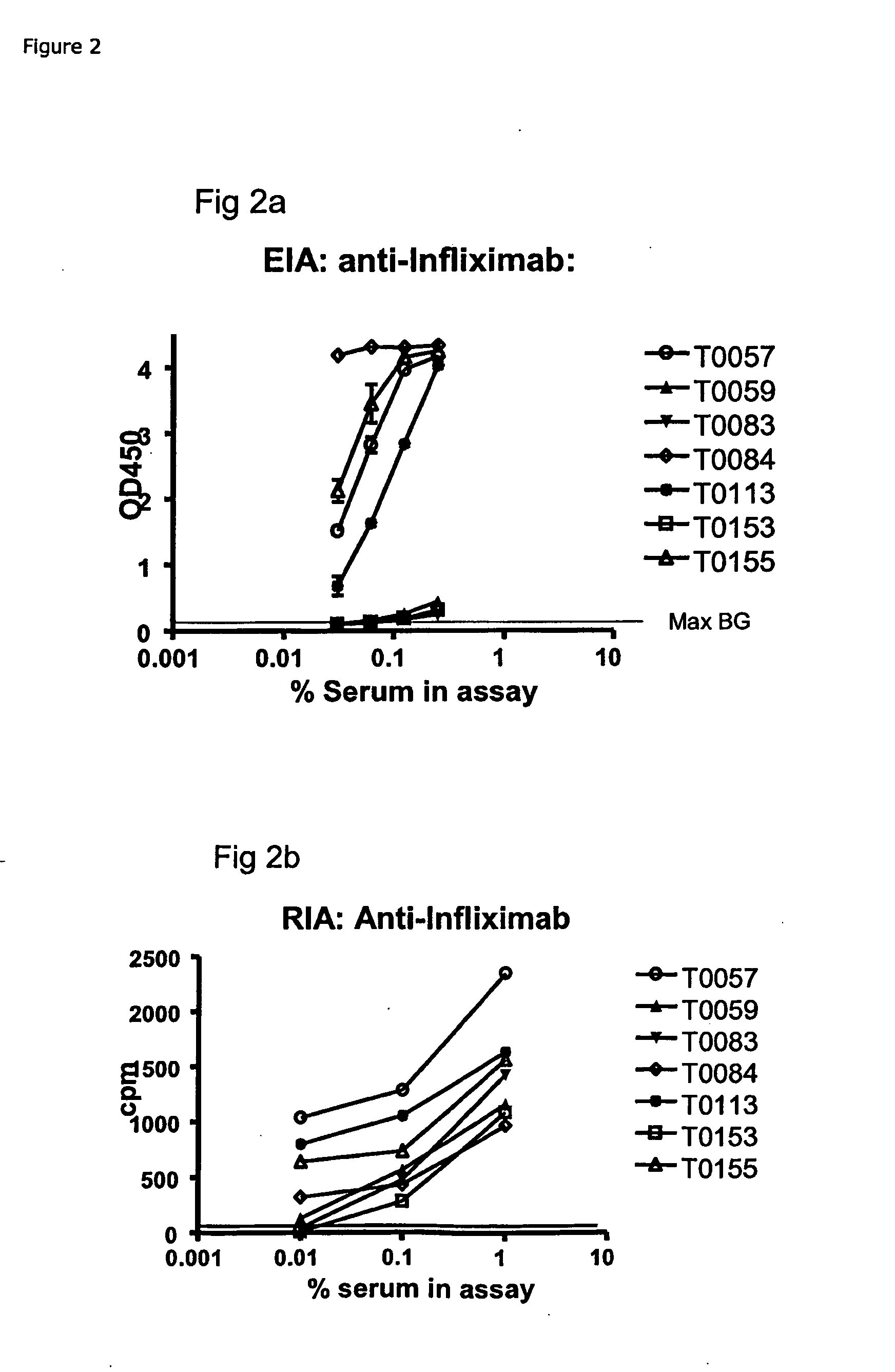
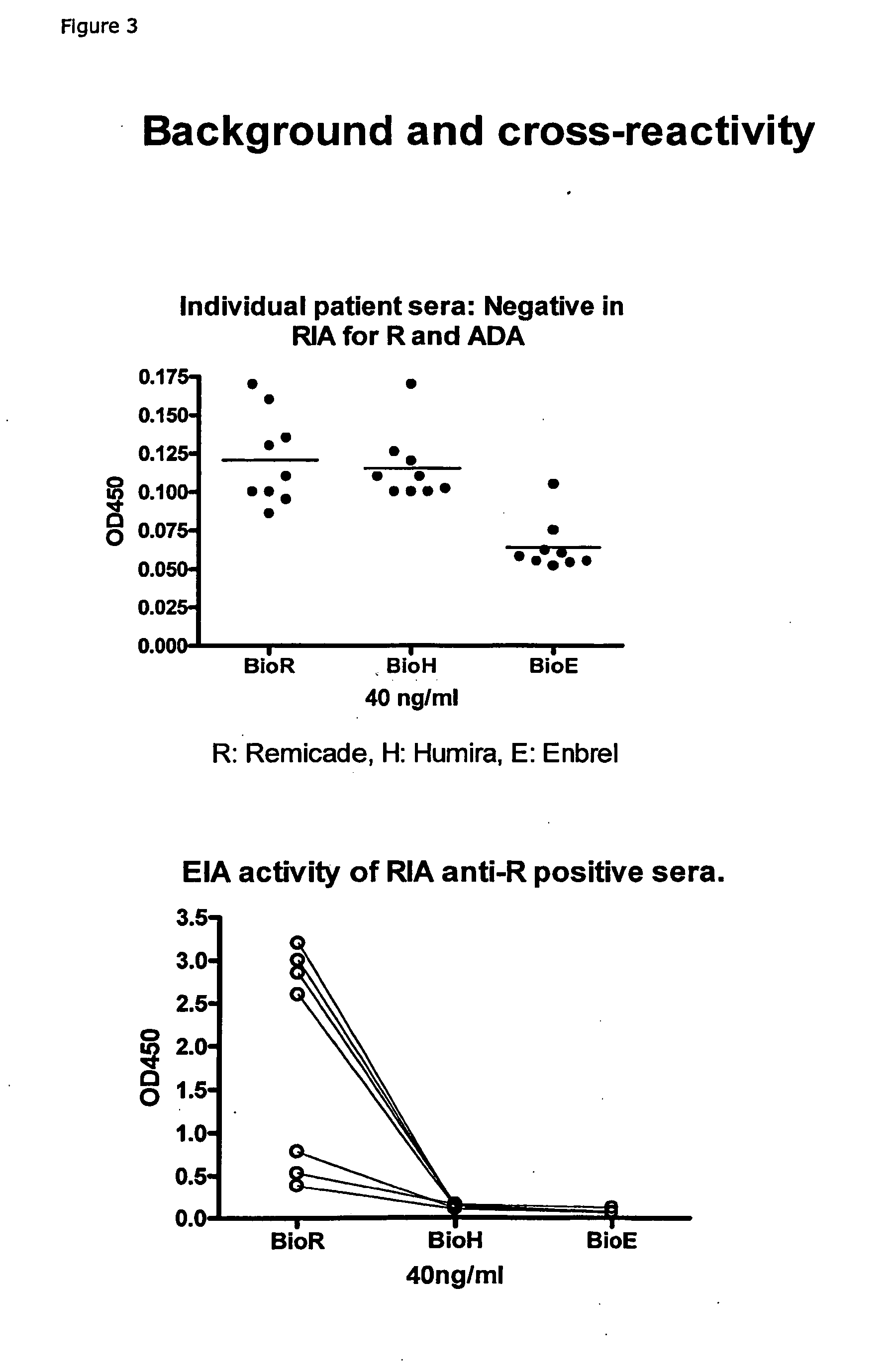
The present invention provides assay methods for the determination of one or more anti-drug antibody (ADA) isotypes in a sample. As a non-limiting example, the assays of the present invention are particularly useful for determining different ADA isotypes in samples from ADA-positive patients receiving an anti-TNFα drug such as REMICADE™ (infliximab) or HUMIRA™ (adalimumab). The present invention also provides methods for optimizing therapy and / or reducing toxicity in subjects receiving TNFα inhibitors for the treatment of TNFα-mediated disease or disorders.
Owner:PROMETHEUS LAB
Method for detecting Anti-drug antibodies
InactiveUS20130203075A1Highly effectiveSensitive highDisease diagnosisBiological testingPoor antibody responseAnti-Drug Antibody
Owner:BIOMONITOR
Therapeutic antigen-binding molecule with a FcRn-binding domain that promotes antigen clearance
The present invention provides: a modified FcRn-binding domain having an enhanced affinity for the Fc Receptor neonatal (FcRn) at neutral pH; an antigen-binding molecule comprising said FcRn-binding domain, which has low immunogenicity, high stability and form only a few aggregates; a modified antigen-binding molecule having an increased FcRn-binding activity at neutral or acidic pH without an increased binding activity at neutral pH for a pre-existing anti-drug antibody; use of the antigen-binding molecules for improving antigen-binding molecule-mediated antigen uptake into cells; use of the antigen-binding molecules for reducing the plasma concentration of a specific antigen; use of the modified FcRn-binding domain for increasing the total number of antigens to which a single antigen-binding molecule can bind before its degradation; use of the modified FcRn-binding domain for improving pharmacokinetics of an antigen-binding molecule; methods for decreasing the binding activity for a pre-existing anti-drug antibody; and methods for producing said antigen-binding molecules.
Owner:CHUGAI PHARMA CO LTD
Cloned enzyme-donor immunoassay (CEDIA) ImmunoChip drug detecting kit
Rapid drug detection is a regular detection item for public security departments, drug control organizations, sports events, enlistment and entry departments. At present, colloidal gold test paper is a basic choice for rapid drug detection, but the sensitivity of the colloidal gold test paper is low, the possibility of fake positive and fake negative results is high, the repeatability is low and the quantification is impossible. The invention discloses a cloned enzyme-donor immunoassay (CEDIA) ImmunoChip drug detecting kit. The kit comprises an immuno chip on which the combination of an enzyme donor and an enzyme receptor is immobilized, wherein the enzyme donor can be coupled with a drug micromolecule by a chemical bond to form an enzyme-donor-labeled exogenous antigen so as to be used for detecting whether a specific anti-drug antibody which can be competitively combined with the enzyme-donor-labeled exogenous antigen exists in a sample to be detected by a CEDIA ImmunoChip drug detecting method and calibration is carried out by using a novel fluorogenic substrate. The kit has the advantages of sensitive reaction, high accuracy, no cross reaction and high repeatability, and is simple and convenient to operate.
Owner:GUANGZHOU YIHANG BIOTECH
Assays for detecting antibodies specific to therapeutic Anti-ige antibodies and their use in anaphylaxis
ActiveUS20110183363A1Low affinityImmunoglobulins against animals/humansDisease diagnosisAnaphylactic reactionAntibody
The invention provides methods and reagents useful for detecting anti-drug antibodies of IgE isotype to therapeutic anti-IgE antibodies, and methods for assessing risk of anaphylaxis to administration of a therapeutic anti-IgE antibody.
Owner:GENENTECH INC
Anti-drug antibody assay
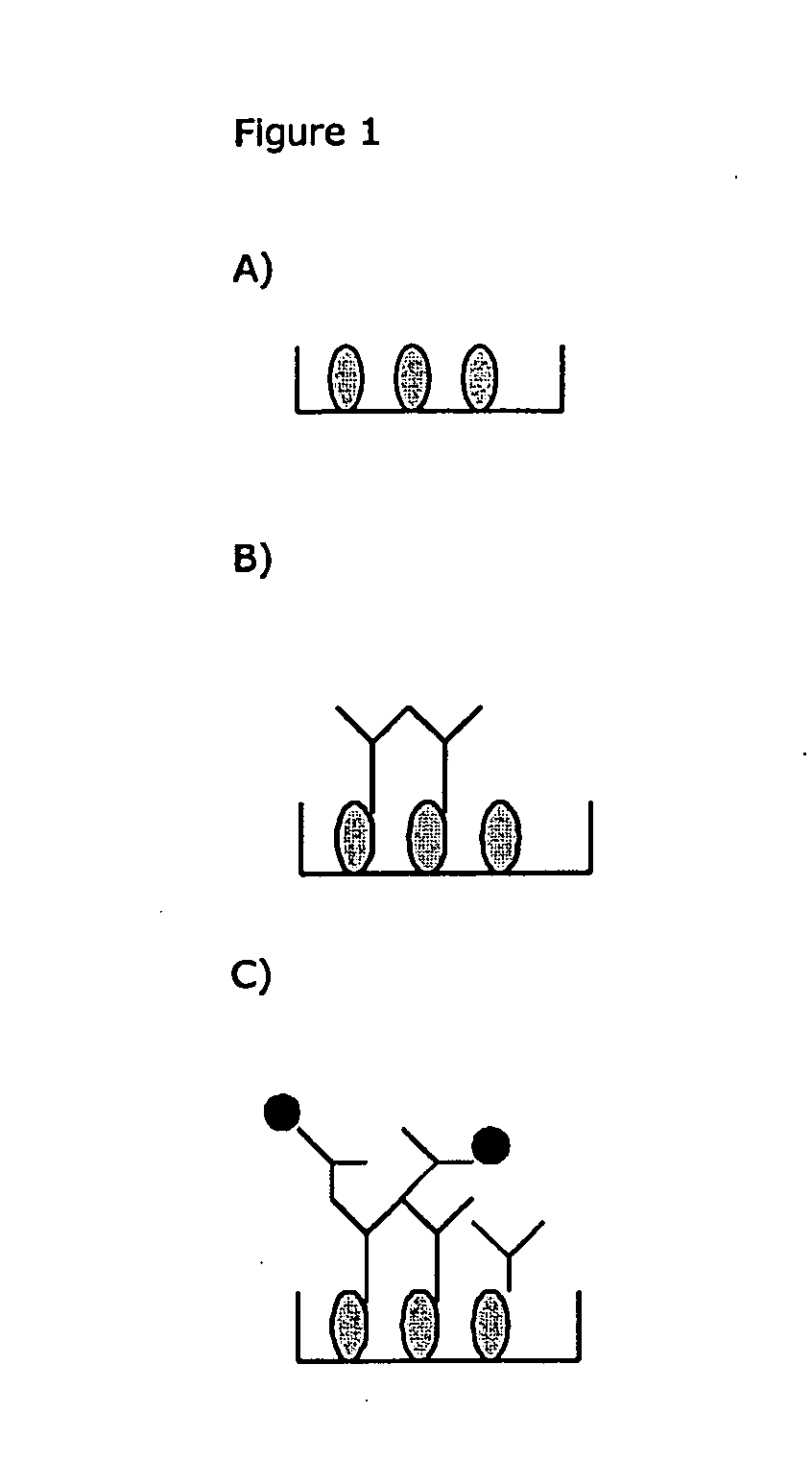
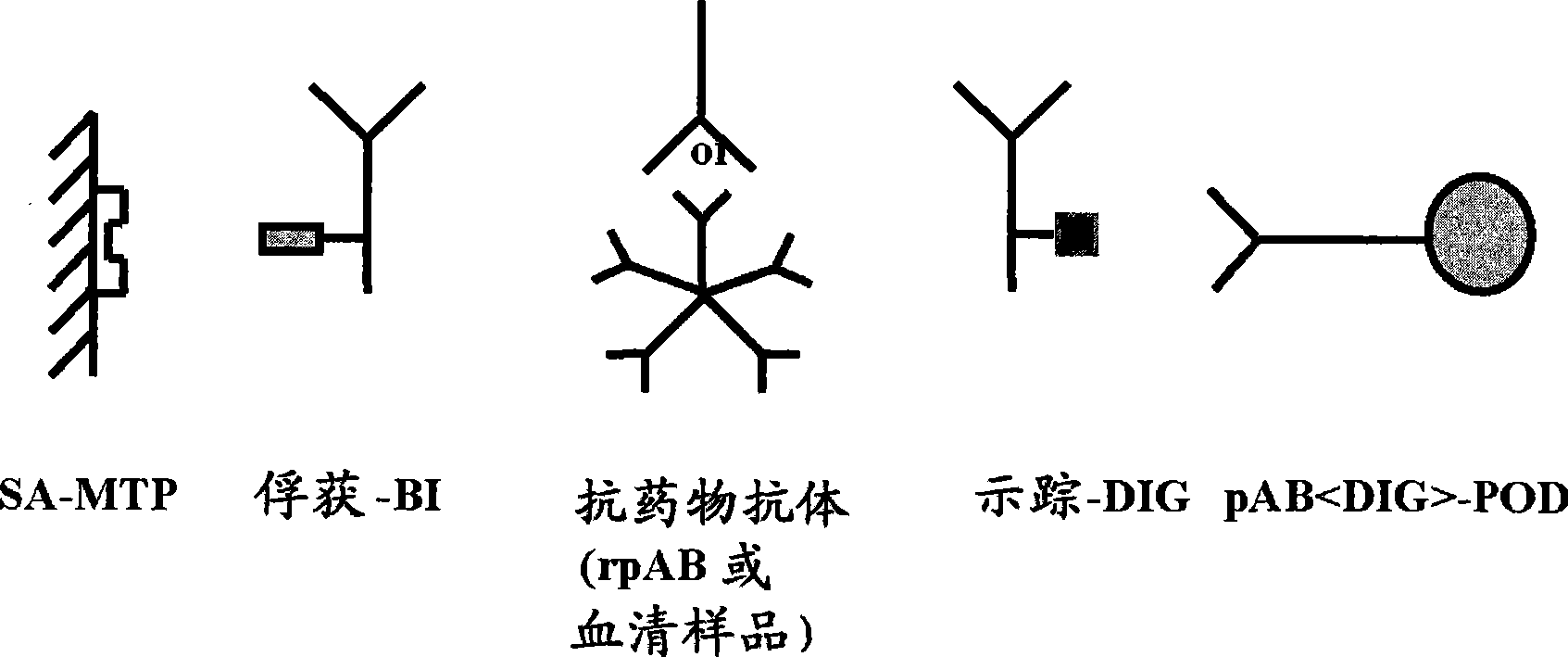
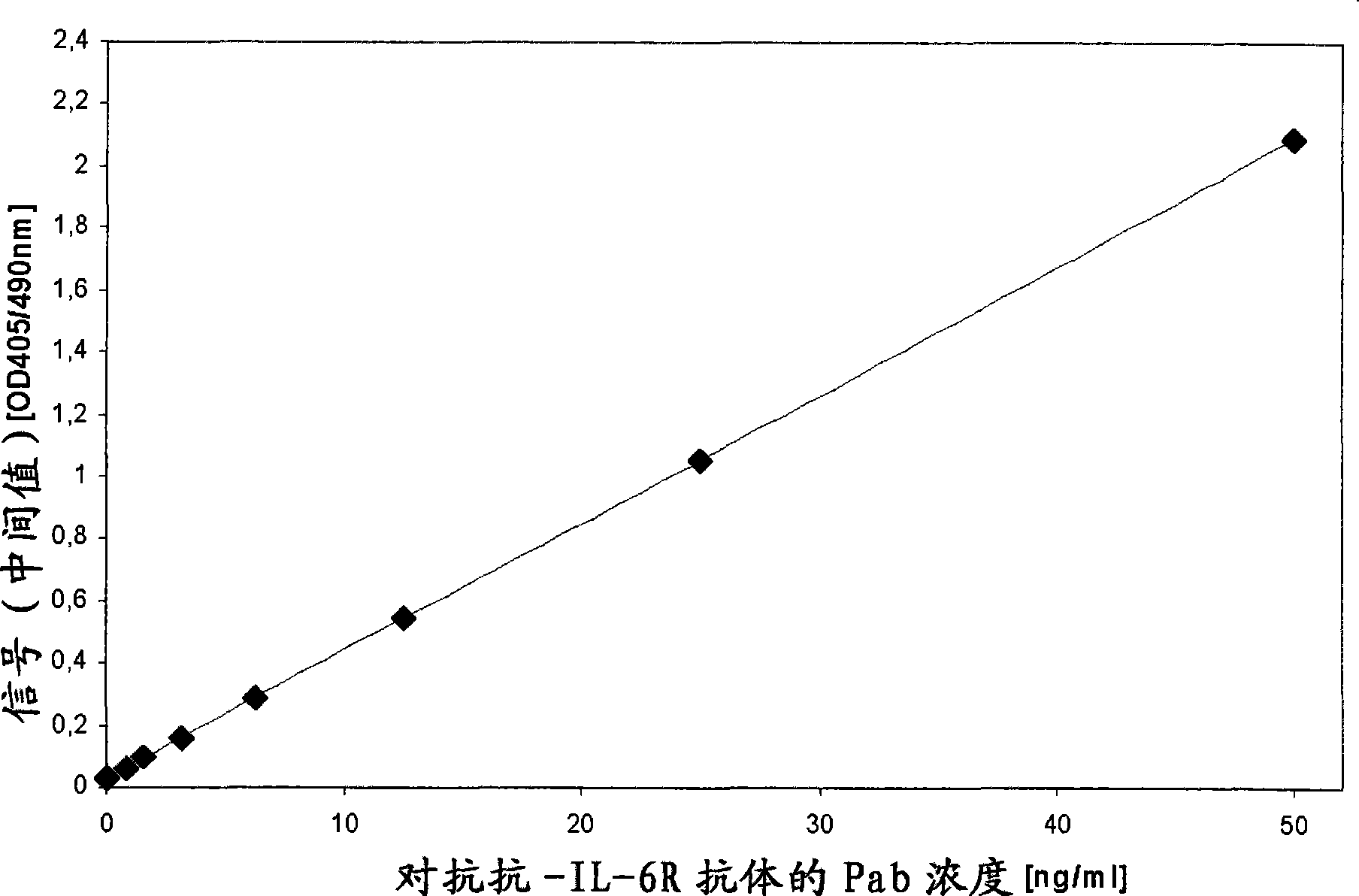
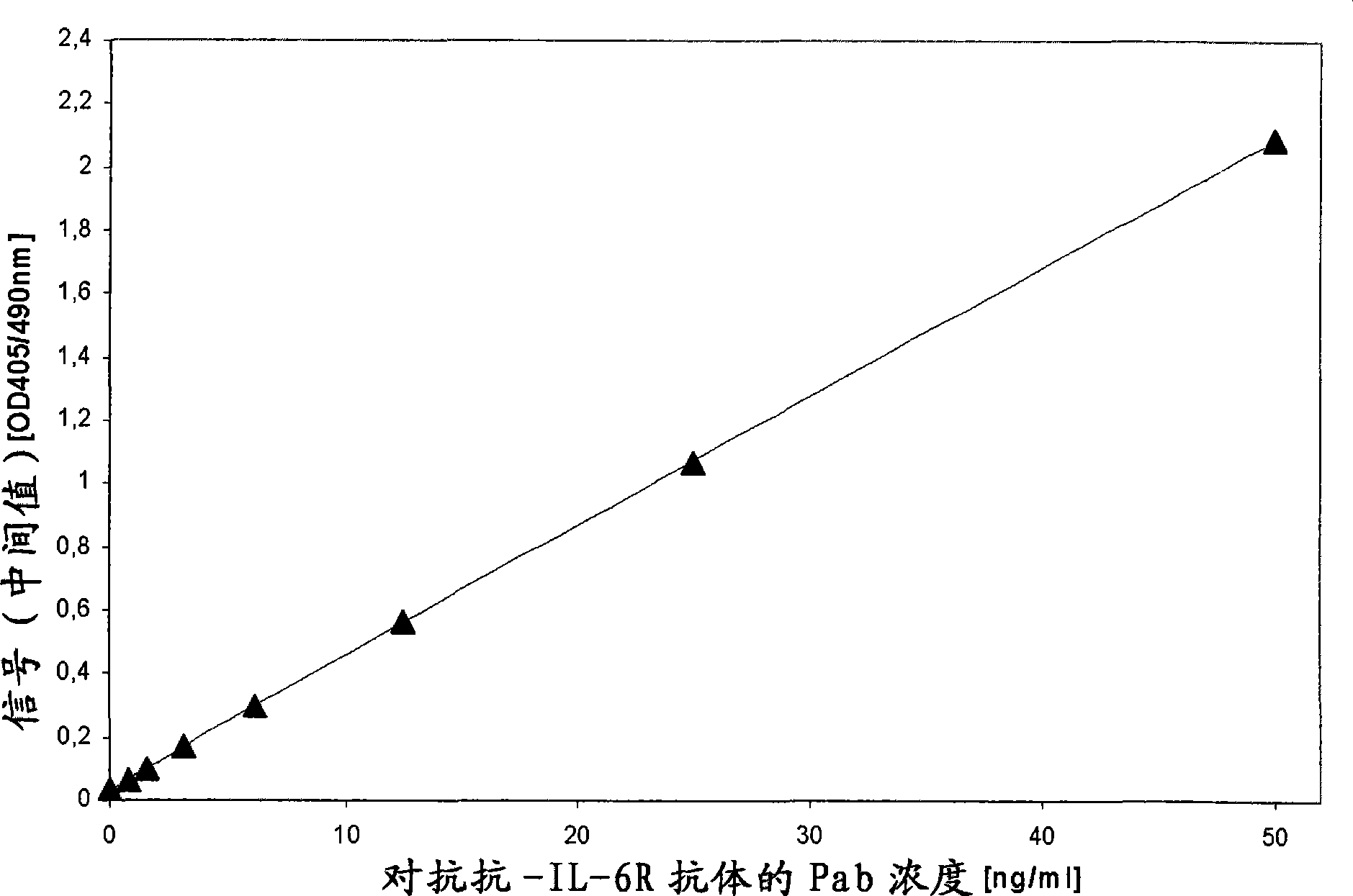
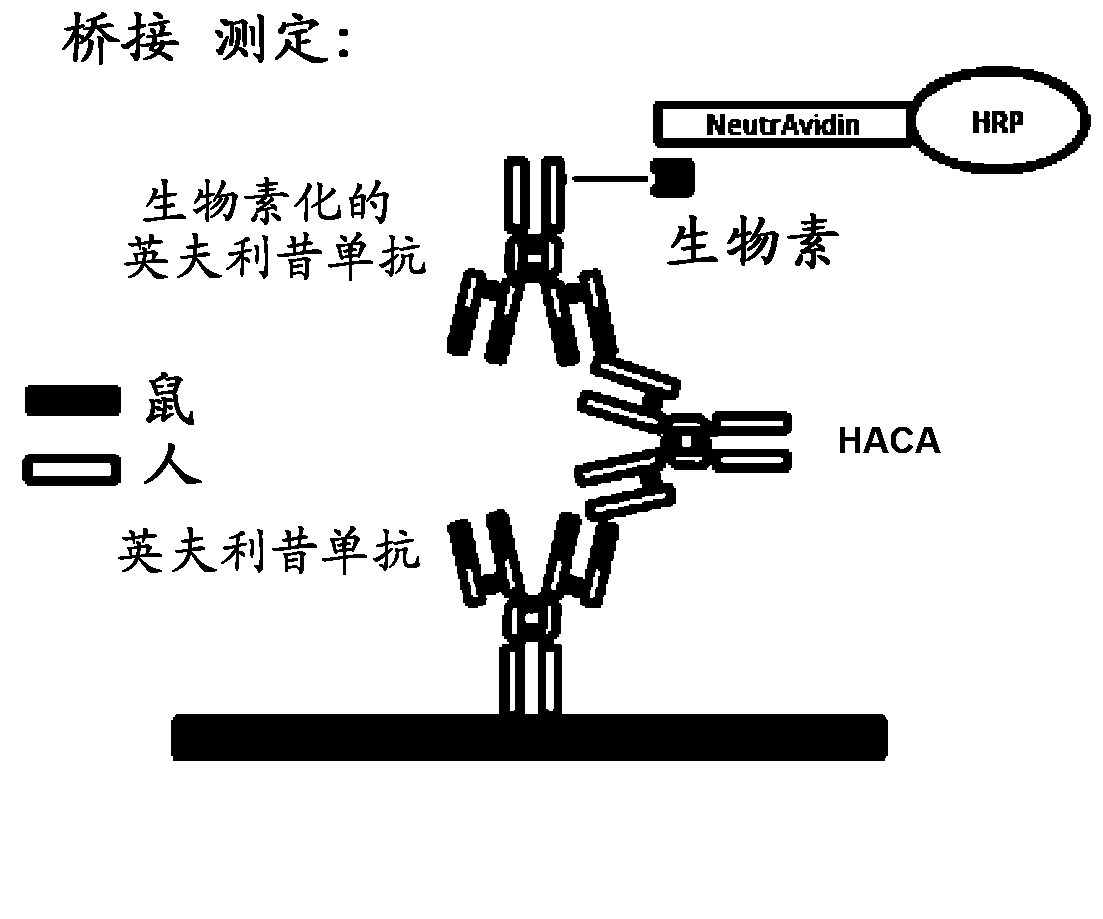
The invention provides a method for the immunological determination of an antibody against a drug antibody in a sample using a double antigen bridging immunoassay comprising a capture drug antibody and a tracer drug antibody, characterized in that the capture drug antibody is a mixture of said drug antibody conjugated to the solid phase at at least two different antibody sites and the tracer drug antibody is a mixture of said drug antibody conjugated to the detectable label at at least two different antibody sites.
Owner:F HOFFMANN LA ROCHE & CO AG
Compositions and methods to mitigate or prevent an immune response to an immunogenic therapeutic molecule in non-human primates
ActiveUS20190022186A1Reduce probabilityReduce intensityOrganic active ingredientsPeptide/protein ingredientsAntibody fragmentsSignalling pathways
Owner:JOMOCO CORP
Assays for anti-drug antibodies in the presence of abundant endogenous protein counterpart of the drug
InactiveUS8877687B2High detection sensitivityHigh sensitivityLibrary screeningBiological material analysisSerum igeBody fluid
Methods and kits for detecting antibodies (e.g., anti-drug antibodies) specific for abundant body fluid components are provided. Such methods and kits permit the detection of, for example, human serum albumin in human body fluids, such as blood, plasma and serum.
Owner:MERRIMACK PHARMACEUTICALS INC
Antibodies to polyethylene glycol
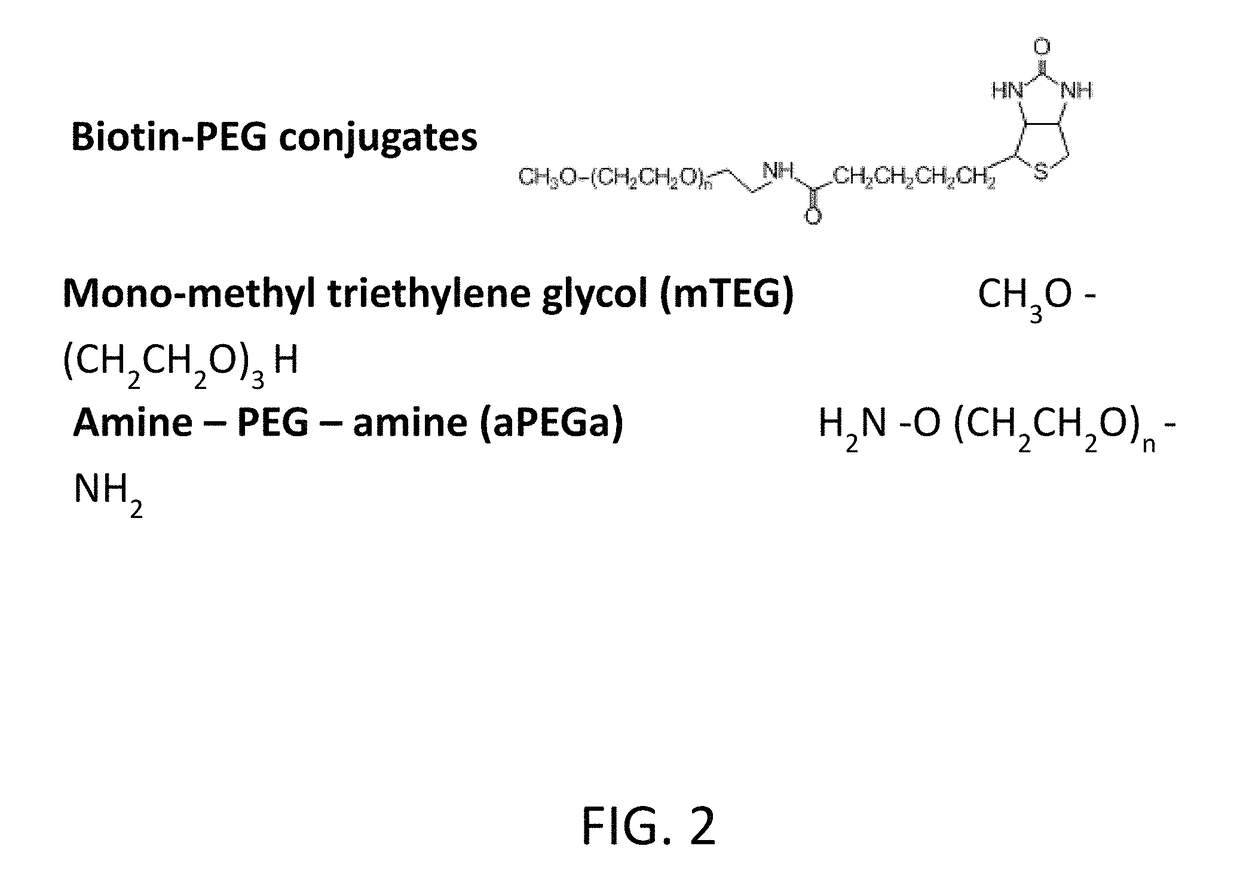
Polyethylene glycol (PEG) is often conjugated with therapeutic proteins to enhance their PK properties. PEG may, however, be immunogenic, and the presence of PEG in food and cosmetics is believed to result in pre-existing anti-PEG antibodies in humans. Polyclonal and monoclonal antibodies reactive to PEG are provided for use in immunogenicity assay development to detect such anti-drug antibodies. Such antibodies exhibit preferential binding based on the size of PEG with molecular weight ranging from 350 daltons to 40 kD. Anti-PEG antibodies of the invention are engineered to comprise human Fc regions to enable non-bridging immunoassay formats.
Owner:BRISTOL MYERS SQUIBB CO
Detection method of anti-drug antibody and detection kit
ActiveCN108318680AImprove bindingEliminate distractionsBiological material analysisBiological testingHigh concentrationMagnetic bead
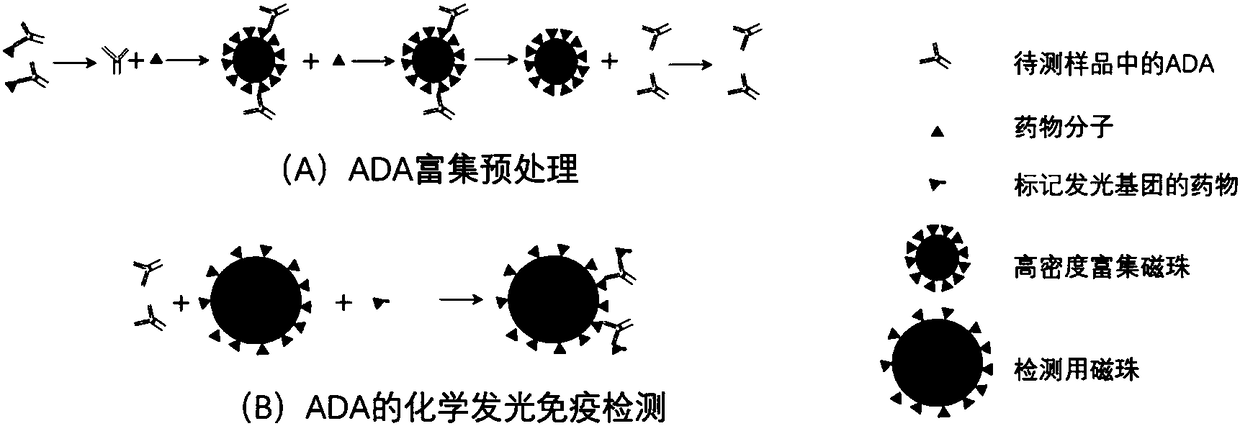
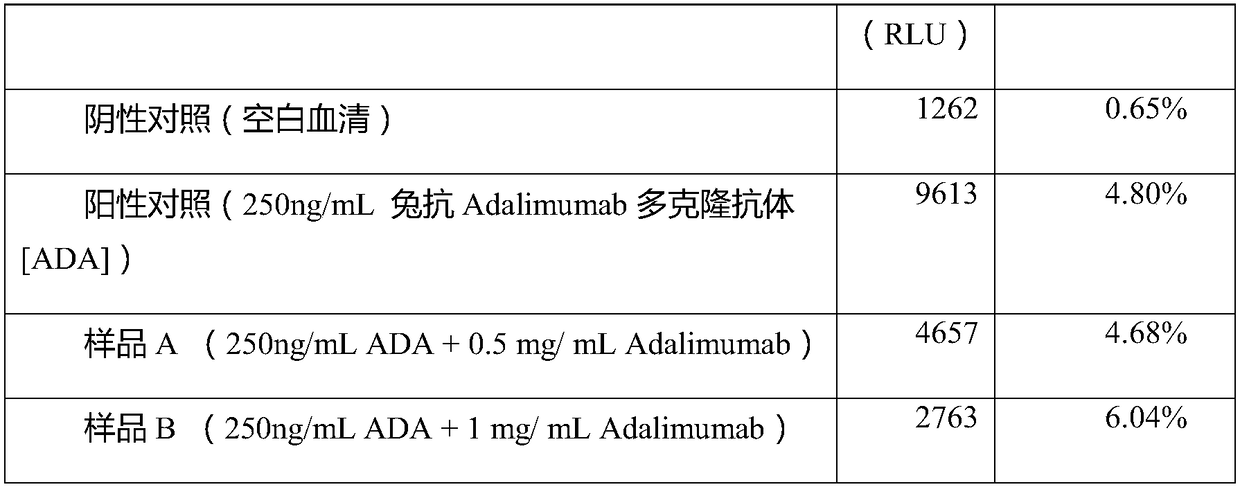
The invention discloses a detection method of an anti-drug antibody and a detection kit. The detection method comprises the following steps of: pretreating a sample to be detected and detecting the pretreated sample, and after high-density enriching magnetic beads are adopted for enriching the sample to be detected, chemiluminesent immunoassay of magnetic particles is carried out on the sample. The detection method and the detection kit disclosed by the invention have the beneficial effects that by pretreatment from the high-density enriching magnetic beads, the detection for the total ADA (Adenosine Deaminase) existing in the form of drug-ADA complex in the sample can be realized, so that the influence of high-concentration drug protein in the sample can be overcome, and the drug resistance of the ADA detection method can be improved; simultaneously the detection method also has the advantages of fast and automatic analysis of a chemiluminesent immunoassay method. The detection kit comprises a high-density enriching magnetic bead reagent, a magnetic bead reagent for detection, a drug for labeling luminophores, sample diluted liquid, acidifying liquid, neutralizing liquid, a quality control product and exciting liquid. By adoption of the kit, the anti-drug antibodies can be rapidly and accurately detected.
Owner:北京新艾进生物科技有限公司
Methods for determining anti-drug antibody isotypes
ActiveUS9784748B2Convenient treatmentLow toxicityComponent separationBiological testingAntiendomysial antibodiesAssay
The present invention provides assay methods for the determination of one or more anti-drug antibody (ADA) isotypes in a sample. As a non-limiting example, the assays of the present invention are particularly useful for determining different ADA isotypes in samples from ADA-positive patients receiving an anti-TNFα drug such as REMICADE™ (infliximab) or HUMIRA™ (adalimumab). The present invention also provides methods for optimizing therapy and / or reducing toxicity in subjects receiving TNFα inhibitors for the treatment of TNFα-mediated disease or disorders.
Owner:PROMETHEUS LAB
Methods for determining anti-drug antibody isotypes
The present invention provides assay methods for the determination of one or more anti-drug antibody (ADA) isotypes in a sample. As a non-limiting example, the assays of the present invention are particularly useful for determining different ADA isotypes in samples from ADA-positive patients receiving an anti-TNFa drug such as REMICADETM (infliximab) or HUMIRATM (adalimumab). The present invention also provides methods for optimizing therapy and / or reducing toxicity in subjects receiving TNFa inhibitors for the treatment of TNFa-mediated disease or disorders.
Owner:SOC DES PROD NESTLE SA
Assays for detecting neutralizing autoantibodies to biologic therapy
ActiveUS9465027B2Improve accuracyLow toxicityBiological material analysisSolid sorbent liquid separationTherapeutic treatmentAutoantibody production
The present invention provides assays for detecting and measuring the presence or level of neutralizing and non-neutralizing autoantibodies to biologics such as anti-TNFα drug therapeutics in a sample. The present invention is useful for monitoring the formation of neutralizing and / or non-neutralizing anti-drug antibodies over time while a subject is on biologic therapy. The present invention is also useful for predicting and / or determining the cross-reactivity of neutralizing anti-drug antibodies in a subject's sample with alternative biologic therapies. As such, the present invention provides information for guiding treatment decisions for those subjects receiving therapy with a biologic agent and improves the accuracy of optimizing therapy, reducing toxicity, and / or monitoring the efficacy of therapeutic treatment to biologic therapy.
Owner:PROMETHEUS BIOSCI INC
Method of characterizing antibodies
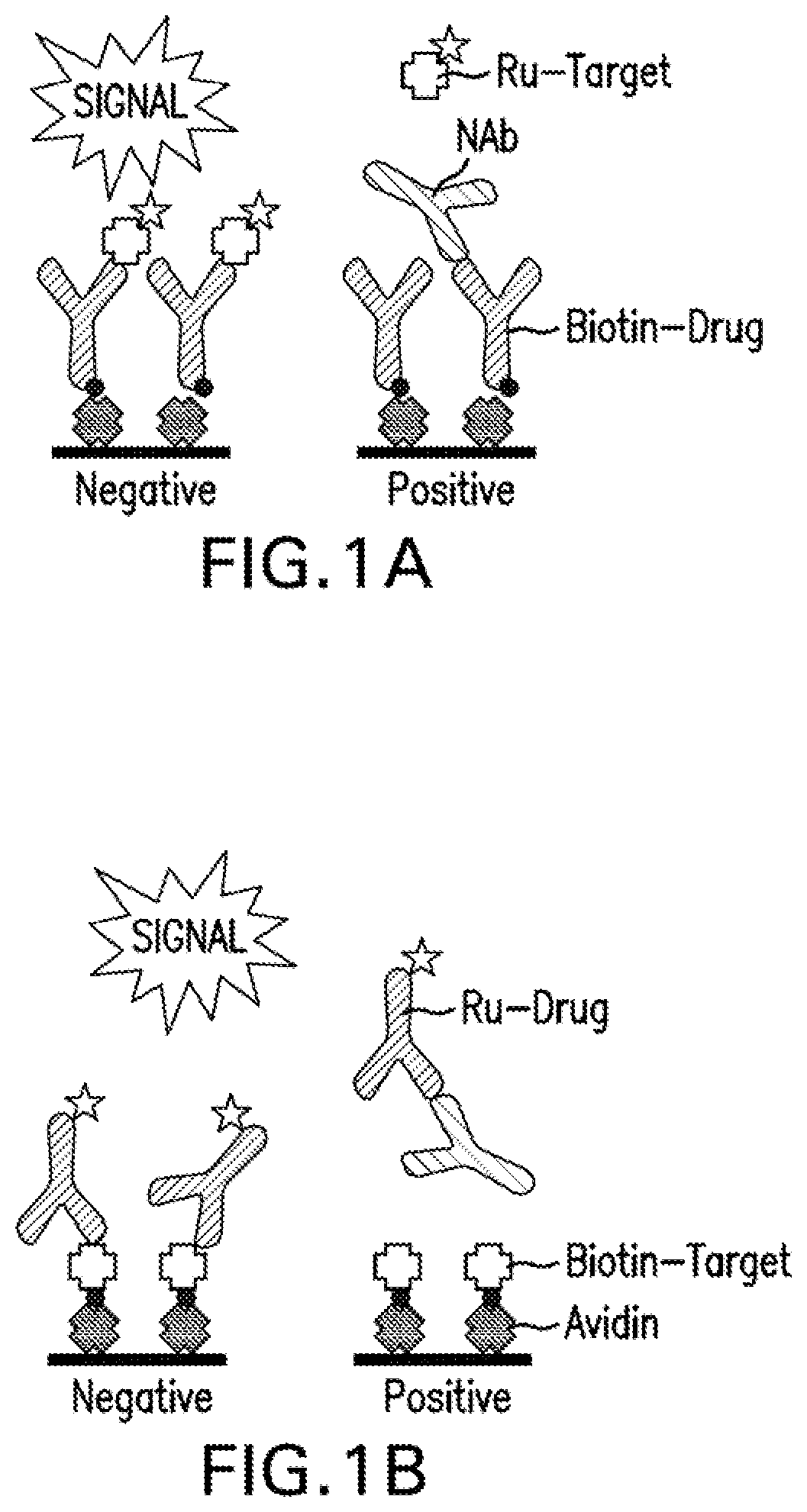
A method of characterizing a population of antibodies to an antigen comprises contacting a sensor surface having the antigen immobilized thereto with a sample containing the antibody population to bind antibodies to the surface, and detecting dissociation of antibodies from the sensor surface during a dissociation phase when sample no longer contacts the surface. Based on the dissociation behaviour, the antibody population is characterized in terms of subpopulations of more and less stable antibodies, respectively. The method may be used to determine the immunogenicity of drug by monitoring the appearance of anti-drug antibodies in a patient over time, and determining any shift in proportions between more stable and less stable antibodies.
Owner:CYTIVA SWEDEN AB
Reagent for detecting monoclonal antibody drugs and anti monoclonal antibody drug antibodies and application of reagent
ActiveCN111024958AImprove non-specificityStrong specificityBiological testingSodium acetateAntiendomysial antibodies
The invention relates to blood concentration detection, in particular to a reagent for detecting monoclonal antibody drugs and anti monoclonal antibody drug antibodies and application of the reagent.The reagent comprises a dissociation solution, the dissociation solution is a glycine buffer solution or an acetic acid-sodium acetate buffer solution with the pH value of 2-3 and 8-10 mM, and the dissociation solution contains Tween-20 with the volume fraction of 0.05%-0.1%, 10-15 mug / ml of mouse IgG and / or 10-15 mug / ml of human IgG. The reagent provided by the invention can effectively reduce non-specific reaction, and solves the problems of strong background signal and low signal-to-noise ratio of the existing acid dissociation reagent.
Owner:同昕生物技术(北京)有限公司
Anti-pembrolizumab antibodies
ActiveUS9995753B2Immunoglobulins against cell receptors/antigens/surface-determinantsDisease diagnosisPositive controlAntigen Binding Fragment
Owner:MERCK SHARP & DOHME LLC
Immunological method
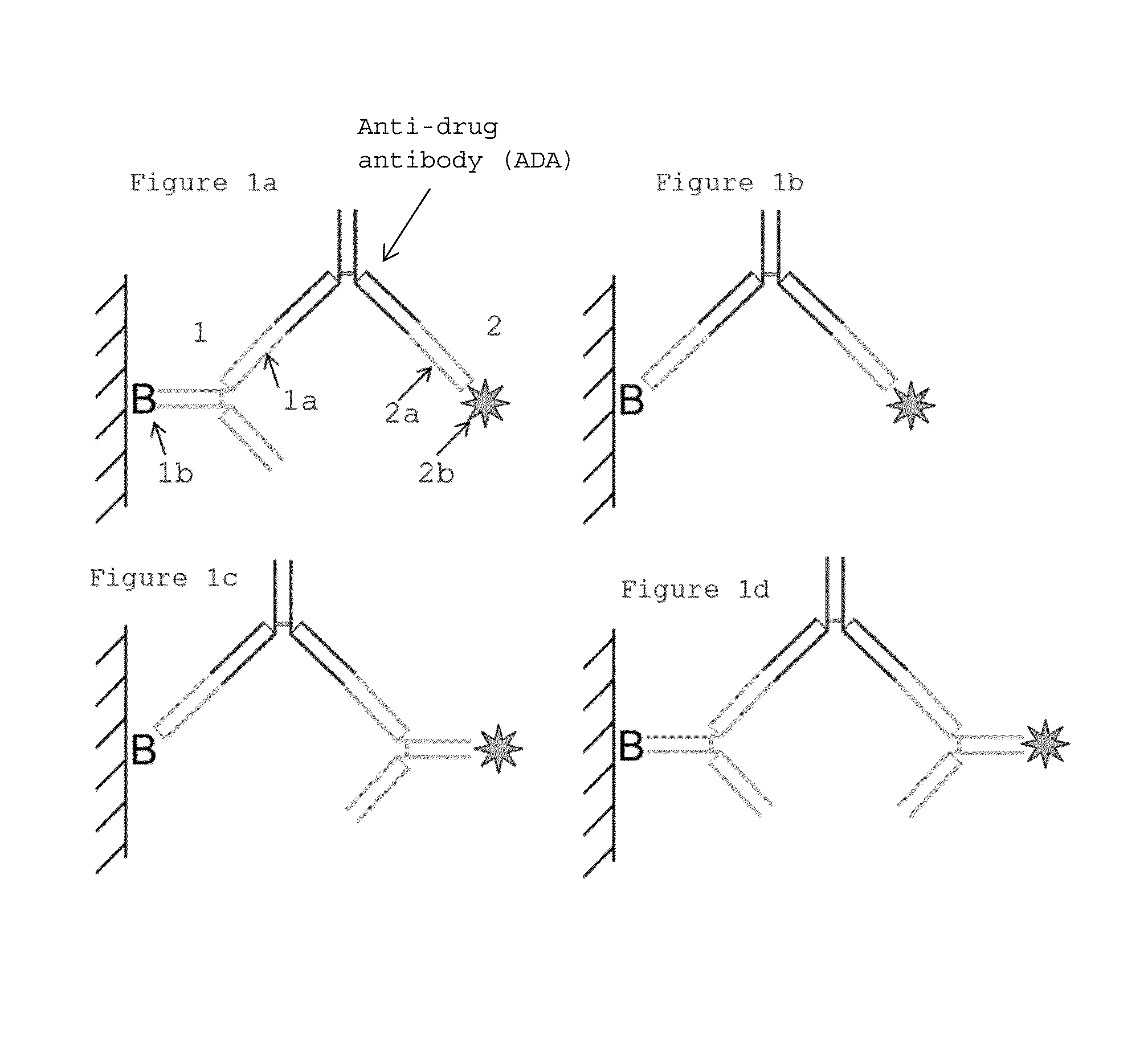
The present invention relates to an in vitro method for the detection of an anti-drug antibody (ADA) against a therapeutic drug antibody (TDA) in a biological sample. The in vitro method comprises detecting and capturing said ADA using affinity moieties obtained from an intact therapeutic drug antibody, or a Fab fragment thereof, in a certain manner. Avoiding using only intact therapeutic drug antibodies or only Fab fragments thereof as reagents in such a method has been proven as an efficient way of increasing sensitivity, decreasing unspecific binding and the formation of large protein complexes. Such a method has great utility in therapeutic drug development or in the evaluation of a particular treatment of a patient.
Owner:GYROS
Assays for Anti-drug antibodies in the presence of abundant endogenous protein counterpart of the drug
InactiveUS20130203616A1High detection sensitivityHigh sensitivityLibrary screeningBiological material analysisSerum igeBody fluid
Owner:MERRIMACK PHARMACEUTICALS INC
Method for measuring Anti-drug antibody
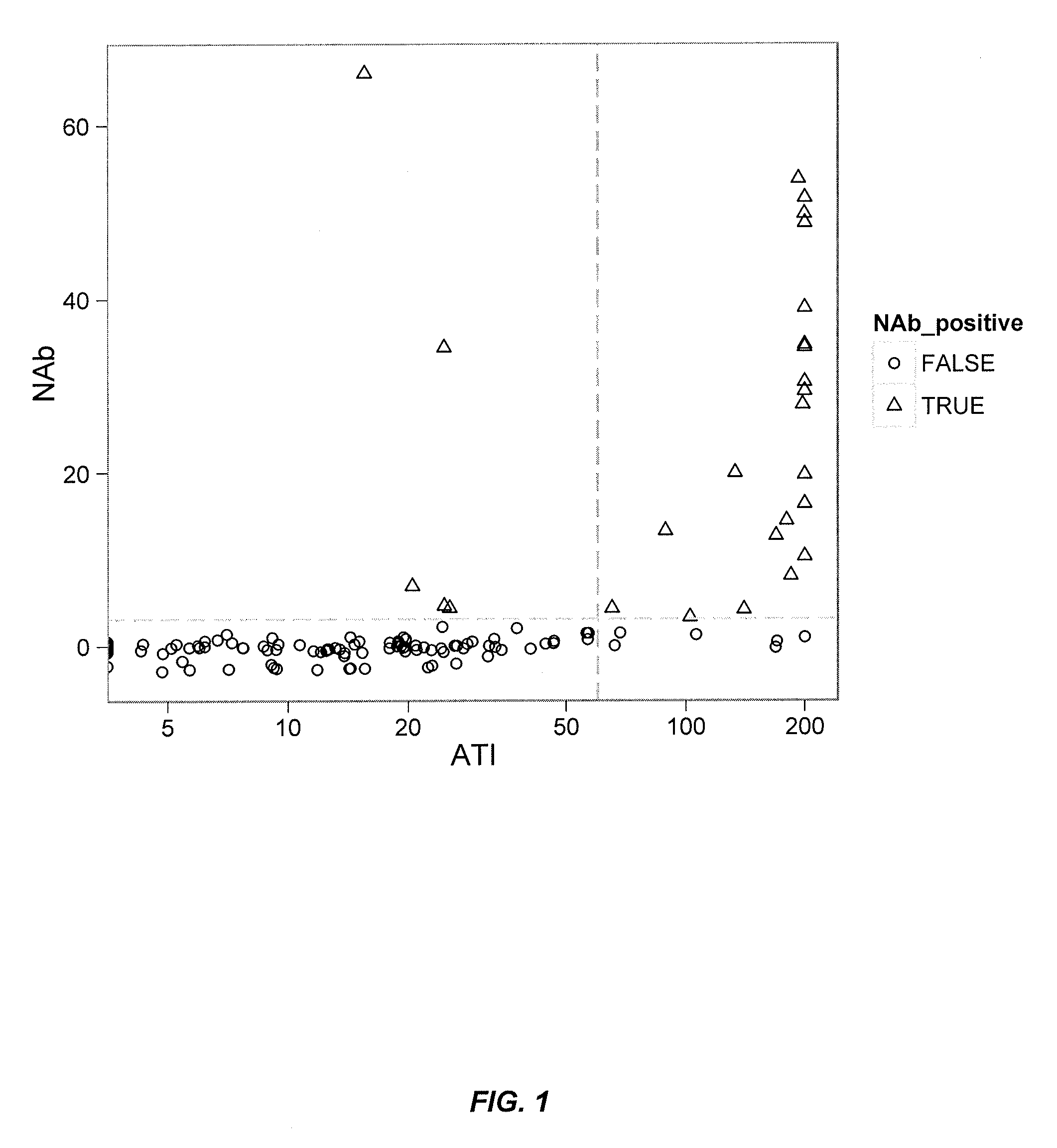
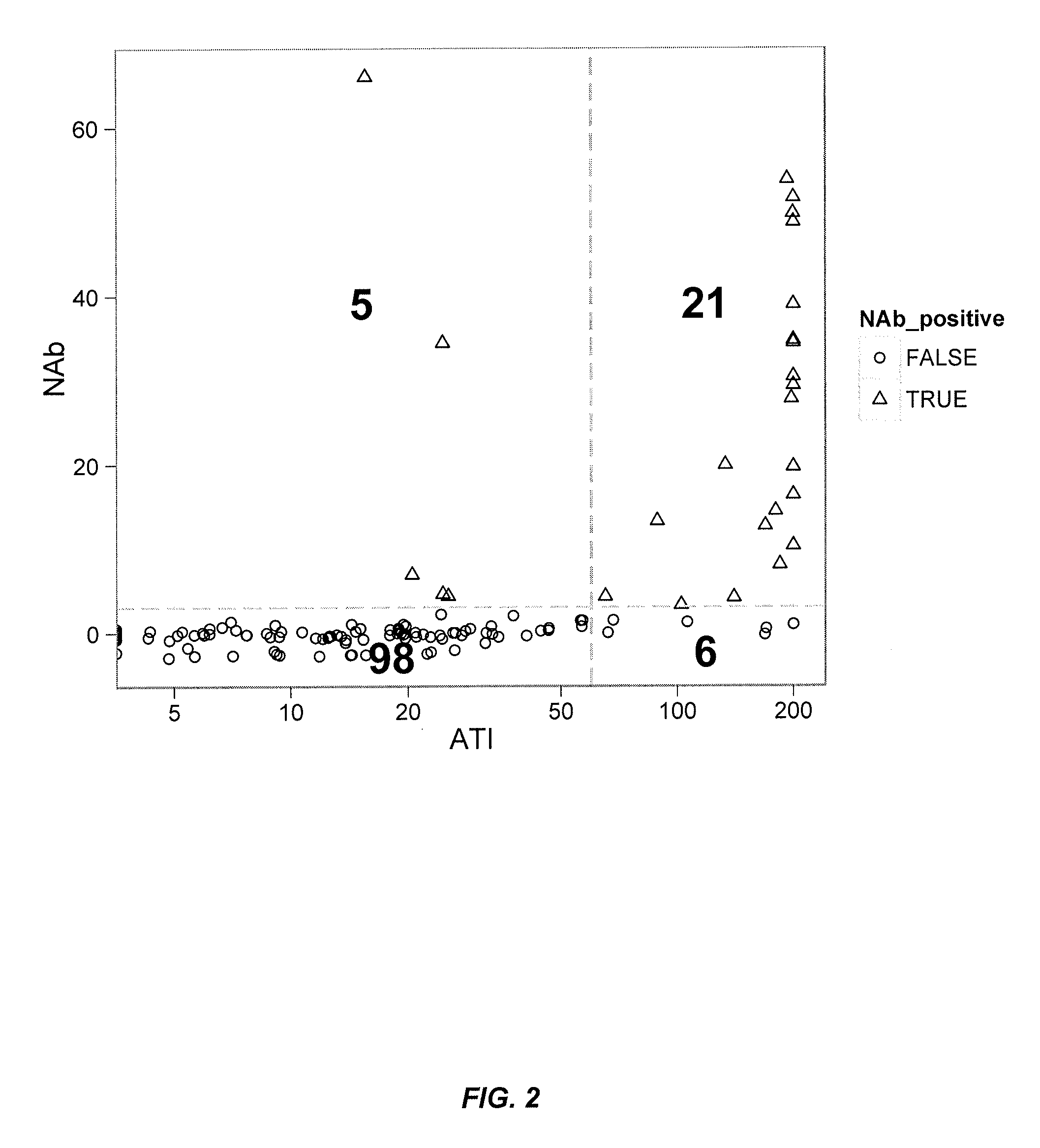
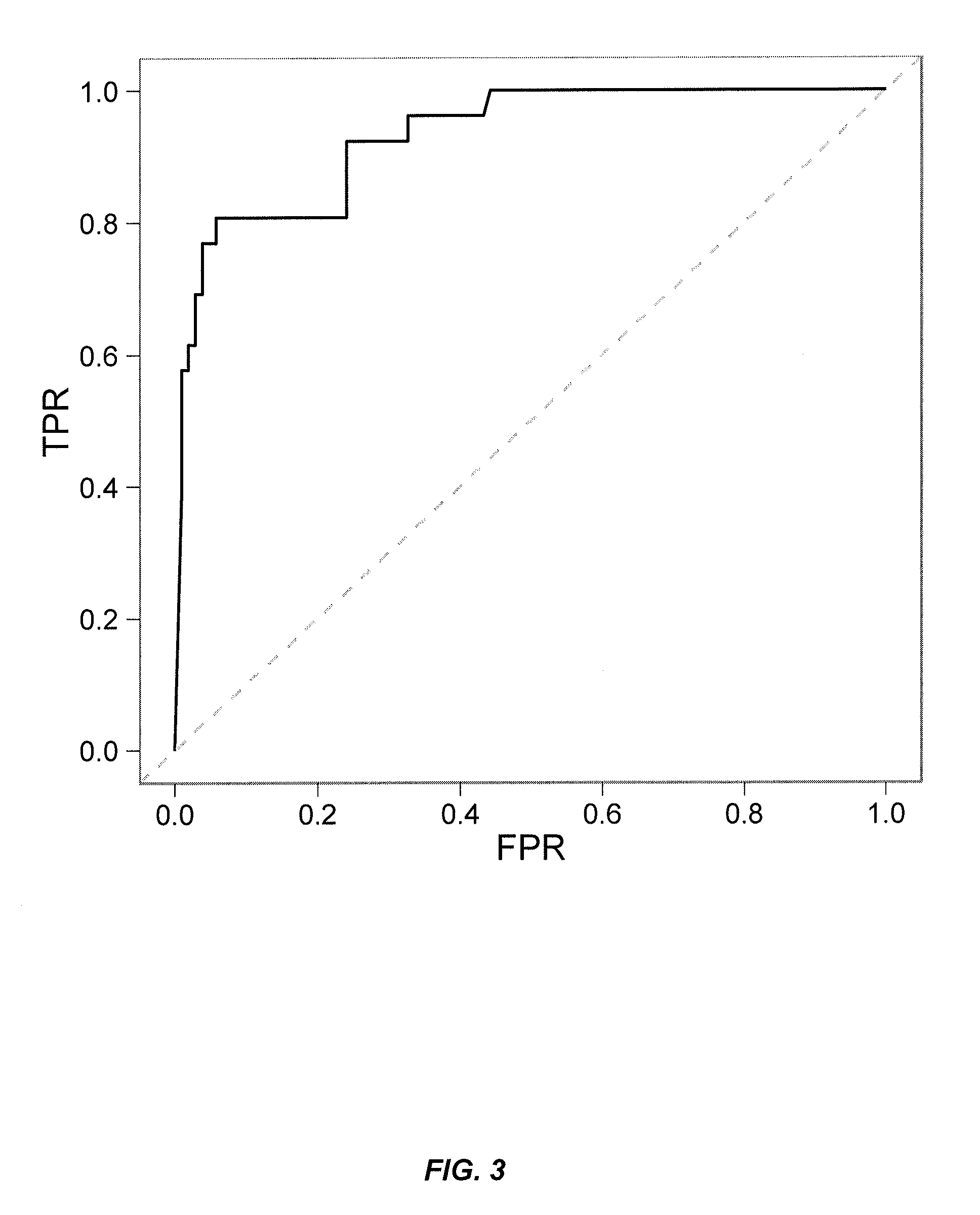
Provided is a method for measuring ADAs appearing in a patient receiving molecular-targeted therapy with a drug or the like in a simpler and more accurate manner.A method for measuring anti-drug antibodies (ADAs) in an analyte to be measured, the method comprising:(1) a step of providing a sample comprising an analyte to be measured for ADAs, the step comprising adding a corresponding drug to an analyte to be measured for ADAs to prepare a sample with a drug-ADAs immune complex formed;(2) a step of contacting the sample prepared in step (1) with a carrier coated with complement Clq, anti-C3d antibody, or anti-Clq antibody;(3) a step of contacting a labeled or non-labeled antibody to a drug corresponding to the ADAs with the coated carrier prepared in step (2); and(4) a step of quantifying the drug-ADAs immune complex binding to the complement Clq, anti-C3d antibody, or anti-Clq antibody.
Owner:JIMRO
Target interference suppressed anti-drug antibody assay
Herein is reported an immunoassay for quantifying the amount of anti-drug antibody, which anti-drug antibody can specifically bind to a drug antibody, which drug antibody can specifically bind to a therapeutic target, in a serum or plasma sample comprising the steps of a) incubating the serum or plasma sample at a pH value that is about the pI value of the target, and optionally removing formed precipitate after the incubation, b) incubating the serum or plasma sample obtained in step a) at a pH value of about 2, and optionally centrifuging the incubated sample to remove formed precipitate, c)adjusting the pH value to about 7.4, adding capture antibody conjugated to a first member of a binding pair and tracer antibody conjugated to a detectable label to the serum or plasma sample obtainedin step b) and incubating the mixture to form a capture antibody-anti-drug antibody-tracer antibody-complex, d) quantifying the complex formed in step c) and thereby quantifying the amount of anti-drug antibody in the serum or plasma sample.
Owner:F HOFFMANN LA ROCHE & CO AG
Human fc-bearing igg antibodies to polyethylene glycol
Polyethylene glycol (PEG) is often conjugated with therapeutic proteins to enhance their PK properties. PEG may, however, be immunogenic, and the presence of PEG in food and cosmetics is believed to result in pre-existing anti-PEG antibodies in humans. Polyclonal and monoclonal antibodies reactive to PEG are provided for use in immunogenicity assay development to detect such anti-drug antibodies. Such antibodies exhibit preferential binding based on the size of PEG with molecular weight ranging from 350 daltons to 40 kD. Anti-PEG antibodies of the invention are engineered to comprise human Fc regions to enable non-bridging immunoassay formats.
Owner:BRISTOL MYERS SQUIBB CO
Device and method for removing free drug in anti-drug antibody detecting sample, preparation method and application thereof
The invention provides a device and method for removing free drug in an anti-drug antibody detecting sample, a preparation method and application thereof. Specifically, a substance that specifically binds to a drug is coupled to a solid phase carrier, and does not react with other components in the sample other than the free drug in the device. The interference of the antibody drug on the anti-drug antibody detection can be effectively reduced, and the sensitivity of anti-drug antibody detection in the sample is improved by using the device of the invention to pretreat the anti-drug antibody detecting sample and detecting the anti-drug antibody in the sample in combination with the anti-drug antibody detecting method (such as a bridging method).
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Methods for detecting antibodies
The present invention relates to methods of detecting Anti-Drug Antibodies. The present invention also relates to methods of monitoring patients undergoing therapeutic antibody treatment. The invention further relates to kits suitable for the implementation of the above methods.
Owner:THERADIAG
Methods and compositions for detecting Anti-drug antibodies
InactiveUS20170044591A1Microbiological testing/measurementImmunoglobulinsDouble strandedOligonucleotide
Assays, methods, reagents and kits for evaluating the level of an antibody against a nucleic acid molecule, e.g., a double-stranded oligonucleotide or RNA molecule (e.g., dsRNA), are disclosed herein.
Owner:ALNYLAM PHARM INC
Improved competitive ligand binding assays
Improved assays for the detection and optionally the quantification of anti-drug antibodies (ADAs) in a sample are provided. The disclosed assays include a protein drug capture assay format and a protein drug target capture assay format, each of which have certain advantages over existing assays. In some embodiments the assays are designed so that drug:anti-drug complexes are washed away before adding to the target coated plate. Target interference can potentially be eliminated or minimized with a competing target blocking reagent in the sample incubation step. In an exemplary target capture assay format, a mild acid approach is used to minimize free target interference.
Owner:REGENERON PHARM INC
Analysis of antibodies
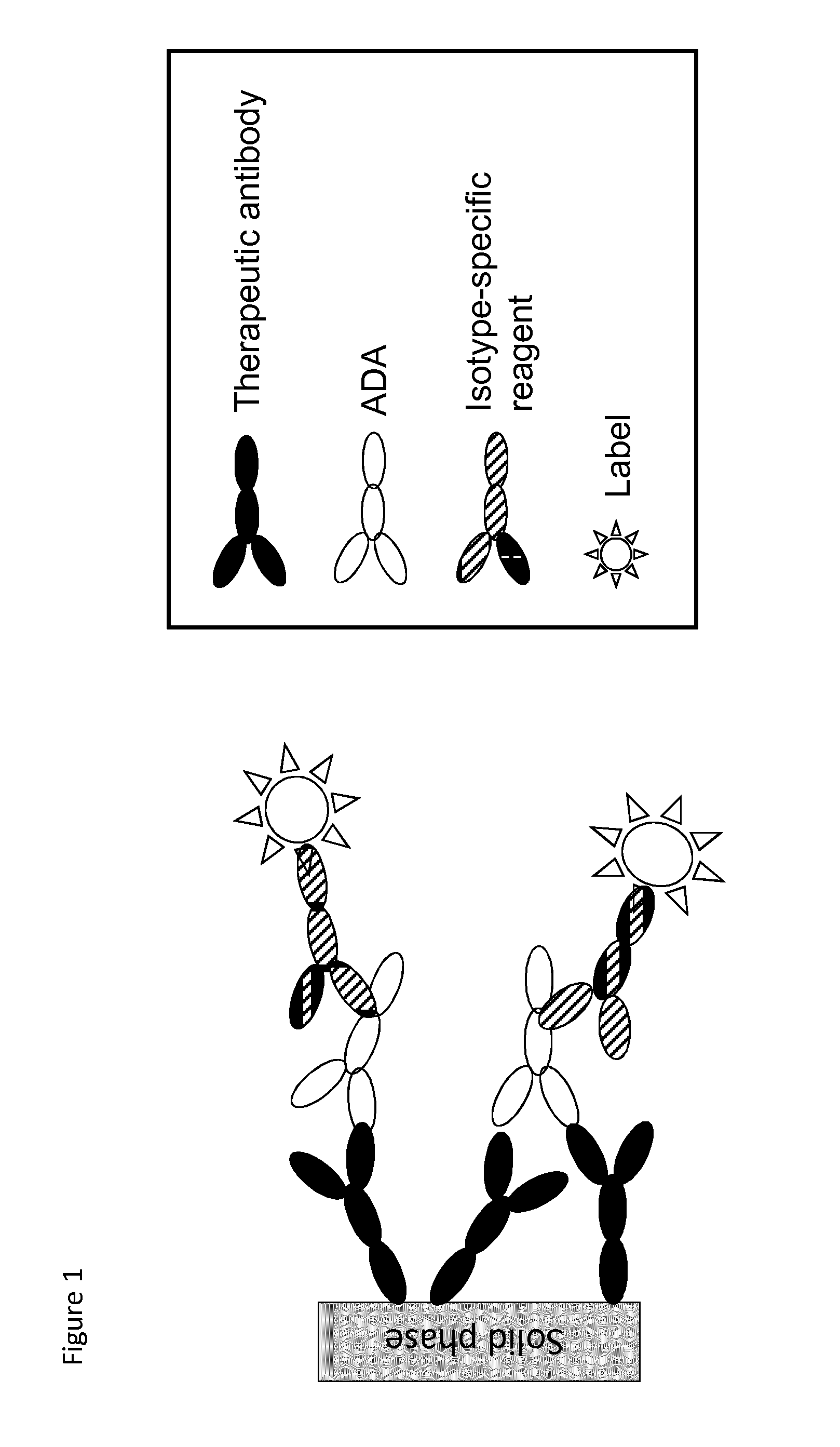
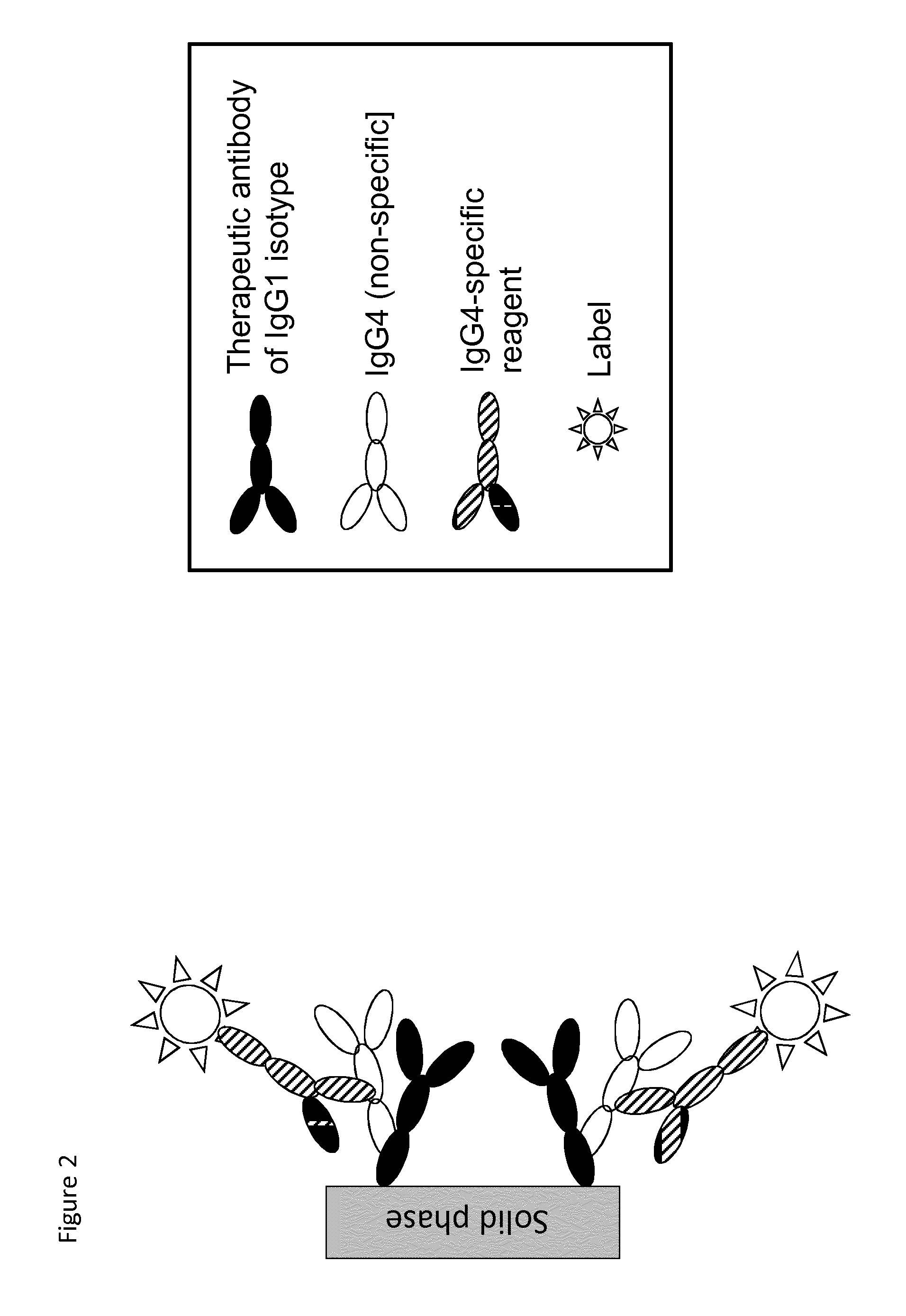
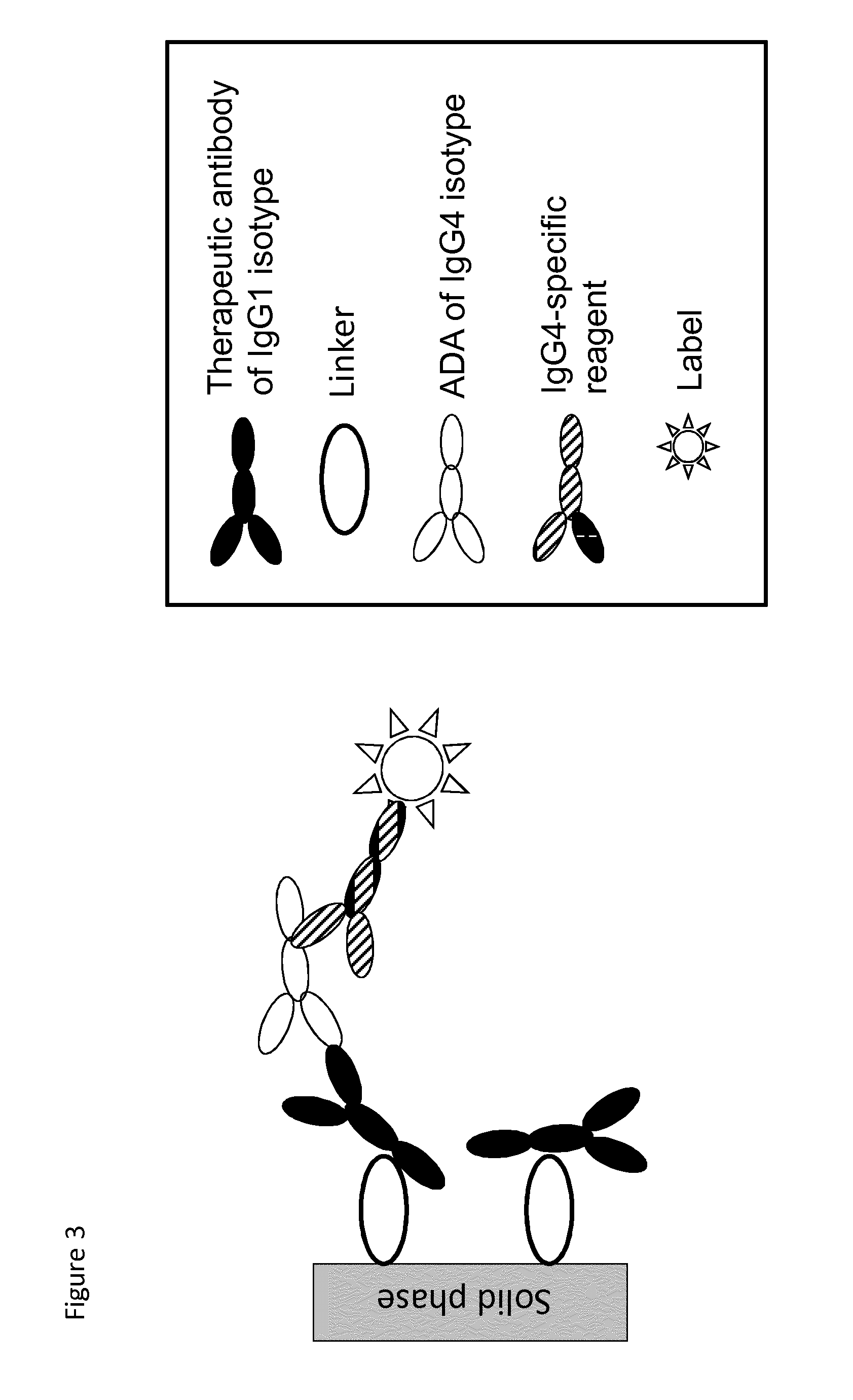
The present invention relates to a method which may be used for the analysis of endogenously formed antibodies such as anti-drug antibodies (ADAs) or rheumatoid factor (RF) in a solution, which method comprises (a) contacting the solution with a solid phase to which e.g. IgG molecule(s) have been attached; (b) allowing the ADAs or RF to bind specifically to the attached molecule(s); (c) adding a labeled isotype-specific reagent capable of binding ADAs or RF; (d) removing any excess of reagent; and (f) detecting the label bound or unbound to determine directly or indirectly the presence or concentration of ADAs in the solution. The molecule(s) have been attached to the solid phase via a linker, which may be an organic molecule, an amino acid, a peptide, a protein a molecule of protein origin, a monosaccharide, an oligosaccharide or a polysaccharide. The method according to the invention may be used e.g. as an immunoassay for making clinical decisions in patient care, as well as in the development of new drugs.
Owner:PHADIA AB
Compositions and methods to mitigate or prevent an immune response to an immunogenic therapeutic molecule in non-human primates
InactiveUS20170157215A1Reduce probabilityReduce formationOrganic active ingredientsPeptide/protein ingredientsAntibody fragmentsIL-2 receptor
Methods and compositions for preventing an immune response to an immunogenic therapeutic agent [i.e. anti-drug antibody (ADA), a.k.a. anti-therapeutic antibody (ATA)] are disclosed. One of the disclosed methods comprises administering an effective amount of an immunosuppressant such as an IL-2 signaling pathway inhibitor, including an antagonist, super agonist or partial agonist to the cytokine IL-2, to the IL-2 receptor (IL-2R), or to IL-2R signal transduction molecules. Inhibitors can be in the form of antibodies or antibody fragments, peptide inhibitors, fusion molecule, small molecules, antibody / small molecule conjugates. Administration of a given inhibitor can decrease the incidence and / or magnitude of an immune response or prevent an immune response, including an antibody response, to a potentially immunogenic therapeutic agent in a non-human primate (NHP). Some of the disclosed methods produce a tolerizing effect in NHPs.
Owner:JOMOCO CORP
Competitive Ligand Binding Assays
PendingUS20200363400A1Reduced effectivenessMitigates and reduces and eliminates carryover problemBiological testingAssay labelsAntiendomysial antibodiesAssay
Owner:REGENERON PHARM INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com