Target interference suppressed anti-drug antibody assay
An anti-drug antibody, antibody technology, applied in biological testing, measuring devices, biological material analysis, etc., can solve problems such as the inability to analyze human samples
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
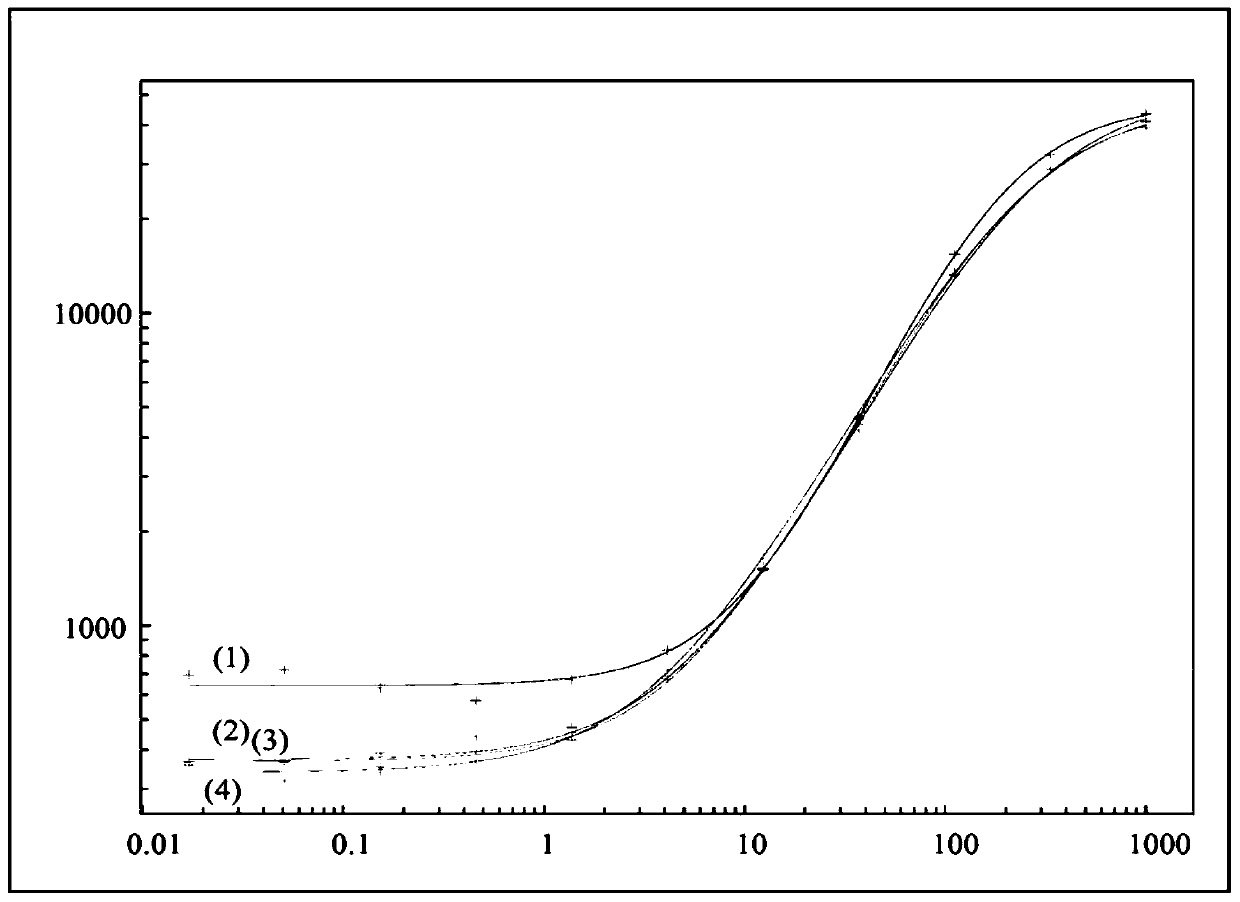
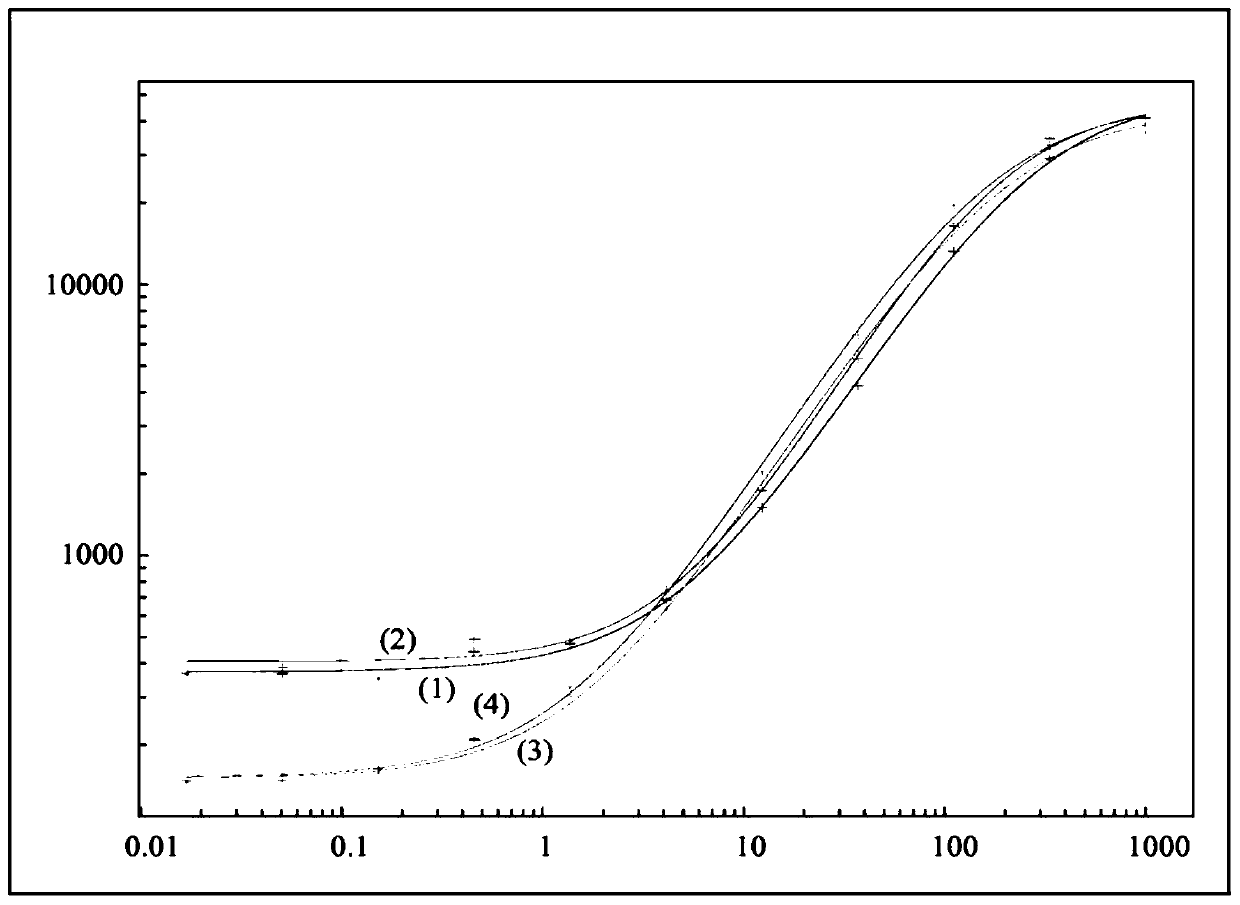
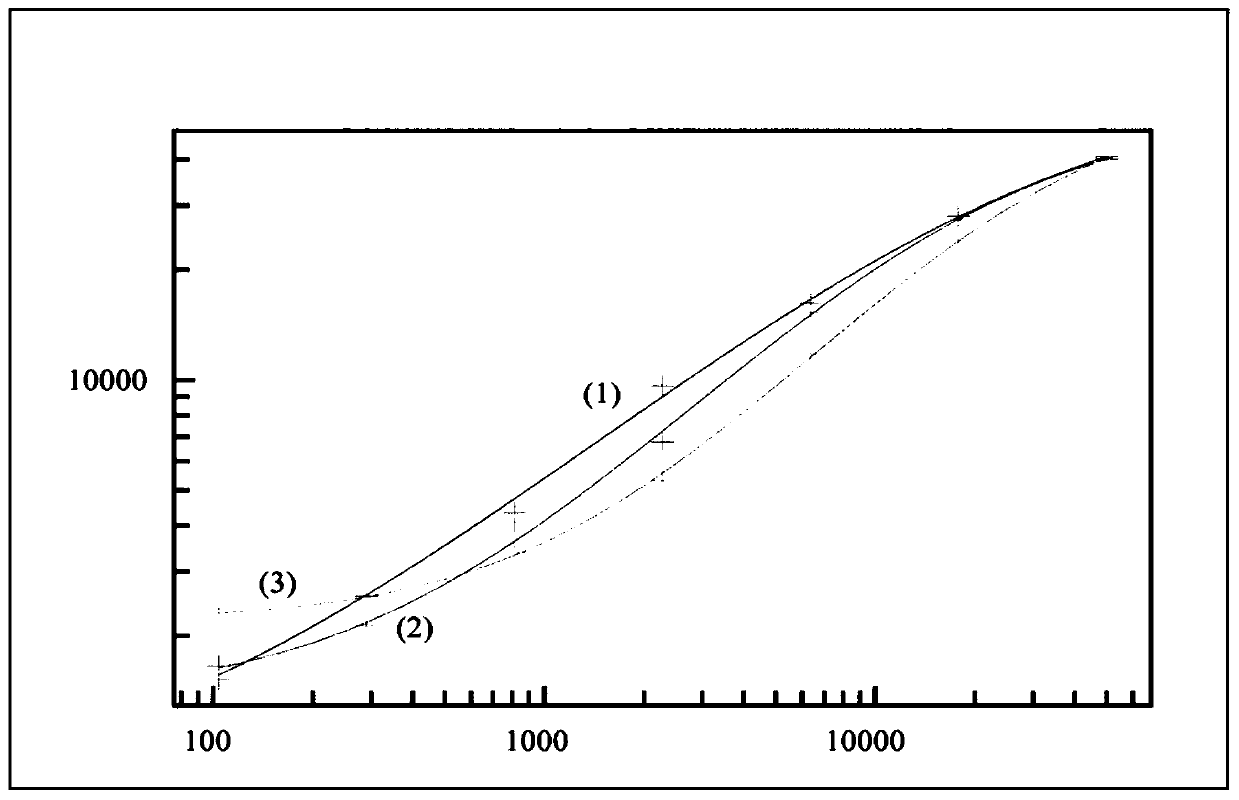
[0109] Drug interference in ADA assays is a well-known phenomenon, but interference with the target of a drug in an ADA assay is not.
[0110] Typically, after acid dissociation, a neutralization step is followed by acid or base dissociation to allow rebridging of the binding partners, causing interfering factors (if any) in solution to also recombine, which remains a problem.
[0111] A number of protocols have been used to alleviate this problem, such as acid or base dissociation, competitive inhibition with interfering effects of specific antibodies, removal of interfering factors, solid phase extraction with acid dissociation (SPEAD), affinity capture elution ( ACE) and many others. The use of acid dissociation in bridge assays has been shown to somewhat improve drug tolerance to ADA detection (see, e.g., Moxness, M. et al., Clin. Chem. 51(10), 1983; Patton, A et al., J. Immunol. Methods 304 (2005) 189).
[0112] PEG precipitation of target molecules or immune complexes ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com