Anti-drug antibody assay
A technology of drugs and antibodies, applied in the field of determination of anti-drug antibodies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
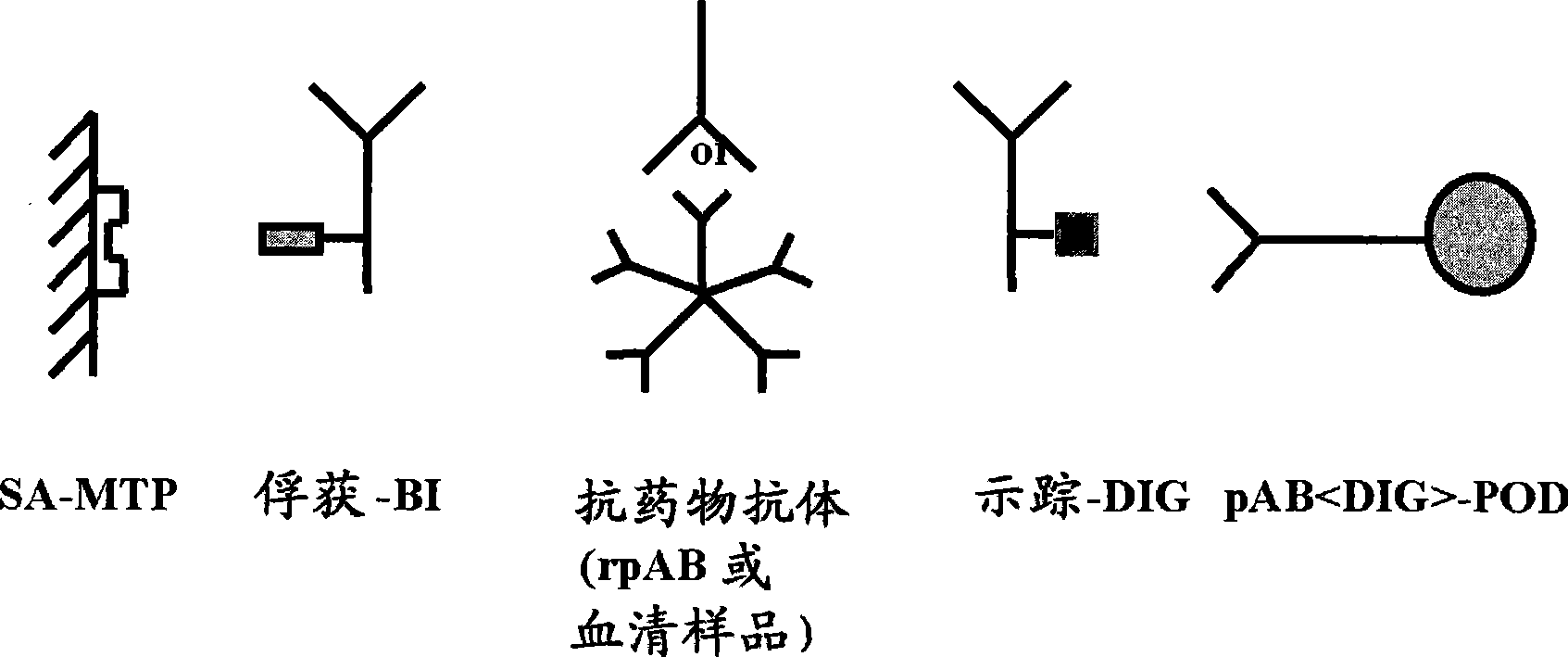
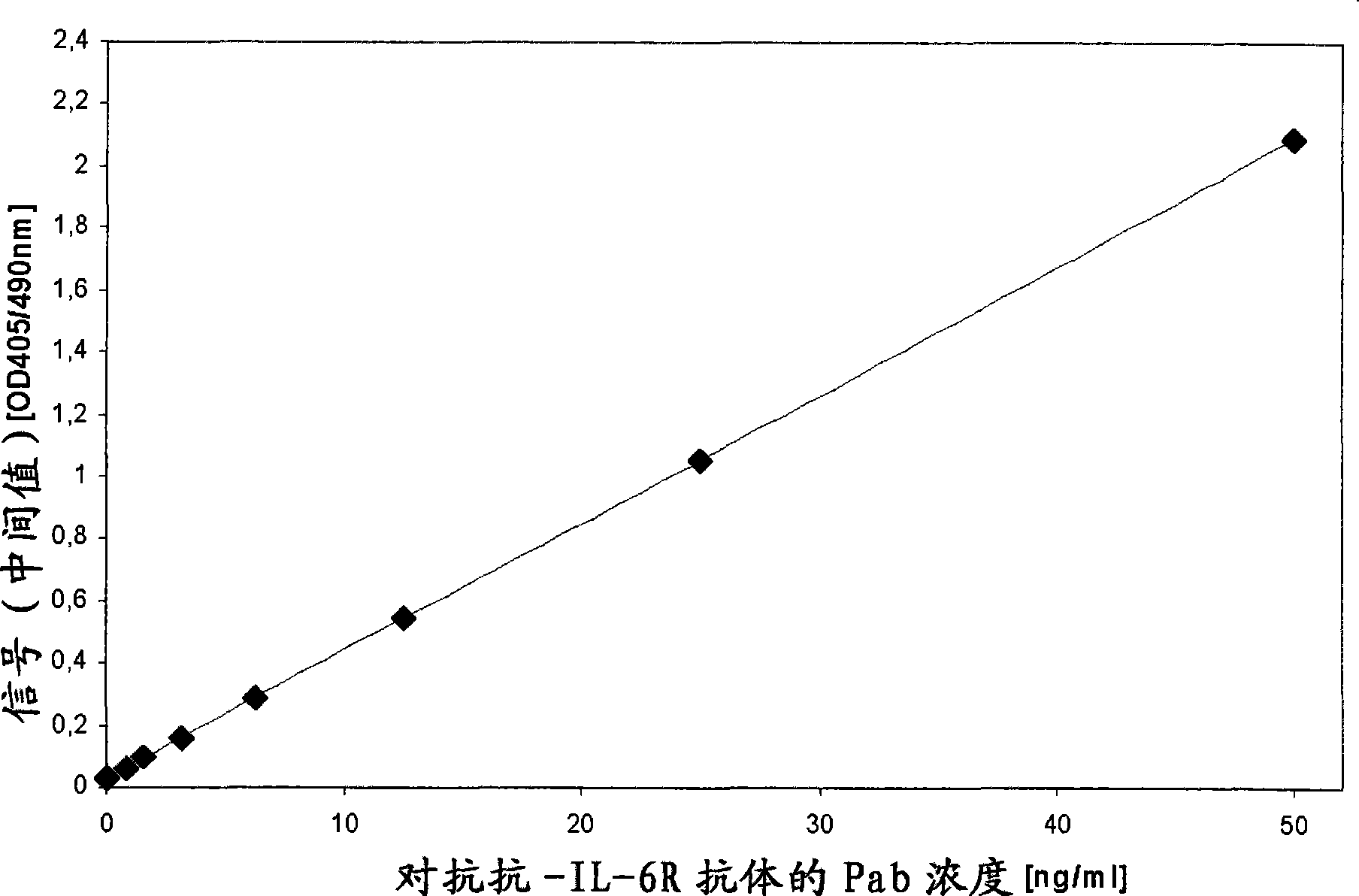
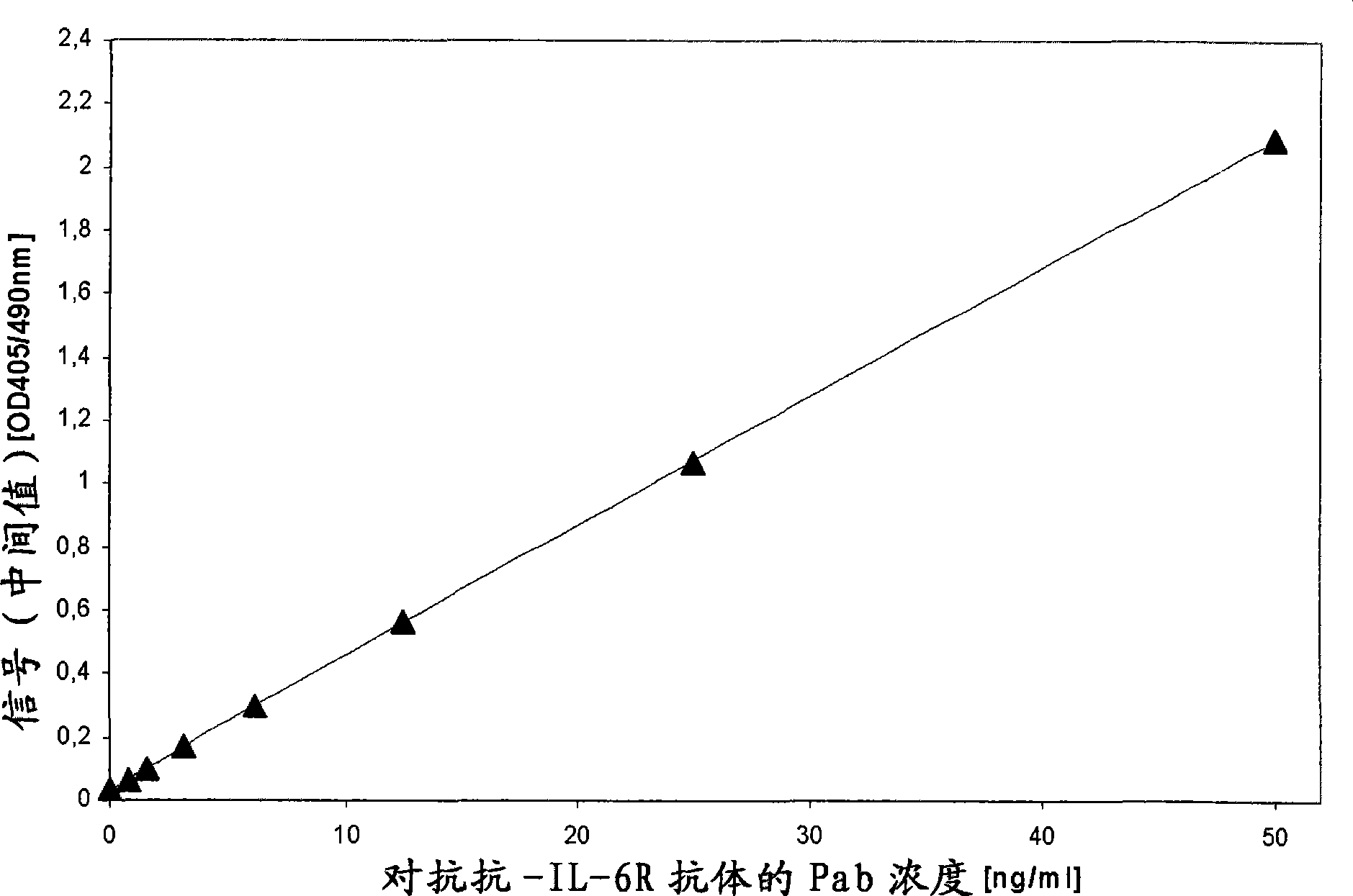
[0015] According to the present invention, the term "drug antibody" means an antibody that can be administered to an individual, so it is presumed that the sample of said individual contains said drug antibody after administration. In an assay performed according to the invention, the drug antibody, capture drug antibody and tracer drug antibody comprise the "identical" antibody molecule, eg recombinantly produced using the same expression vector and comprising the same amino acid sequence. Pharmaceutical antibodies (therapeutic monoclonal antibodies) are widely used in the treatment of various diseases such as neoplastic diseases (eg, hematological and solid malignancies including non-Hodgkin's lymphoma, breast cancer and colorectal cancer). Such antibodies have been described by Levene, A.P. et al. in Journal of the Royal Society of Medicine 98 (2005) 146-152. Such antibodies are, for example, against CD20, CD22, HLA-DR, CD33, CD52, EGFR, G250, GD3, HER2, PSMA, CD56, VEGF, V...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com