Improved competitive ligand binding assays
An antibody and specific technology, applied in the field of determination of anti-drug antibodies, can solve the problem of undetectable neutralizing antibody reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0074] Example 1: Competitive Ligand Binding NAb Assay: Format and Drug Resistance
[0075] Materials and methods
[0076] Materials and Reagents
[0077] All solutions were prepared in assay buffer (1% BSA in 1XPBS) unless otherwise stated. Read buffer T (4X) was from Meso Scale Discovery (MSD, Gaithersburg, MD). Glacial acetic acid (17.4M) was from Thermo Fisher Scientific (Waltham, MA). Human and monkey sera were from BioIVT (Westbury, NY). Fully human monoclonal antibody drug, fully human competitive anti-target A antibody, recombinant human target, monoclonal neutralizing anti-drug antibody used as a positive control, and anti-human monoclonal antibody were purchased from Regeneron (Barretta Village) , New York State) production. Antibodies and targets were labeled with biotin using EZ-link Sulfo-NHS-LC-Biotin (Thermo Fisher Scientific) and ruthenium NHS ester (MSD) according to the manufacturer's instructions. DyNAbeads TM Antibody conjugation kits were from The...
example II
[0097] Example II: Residue of bead-coupled proteins in the NAb assay step
[0098] Materials and methods
[0099] See Example 1.
[0100] result
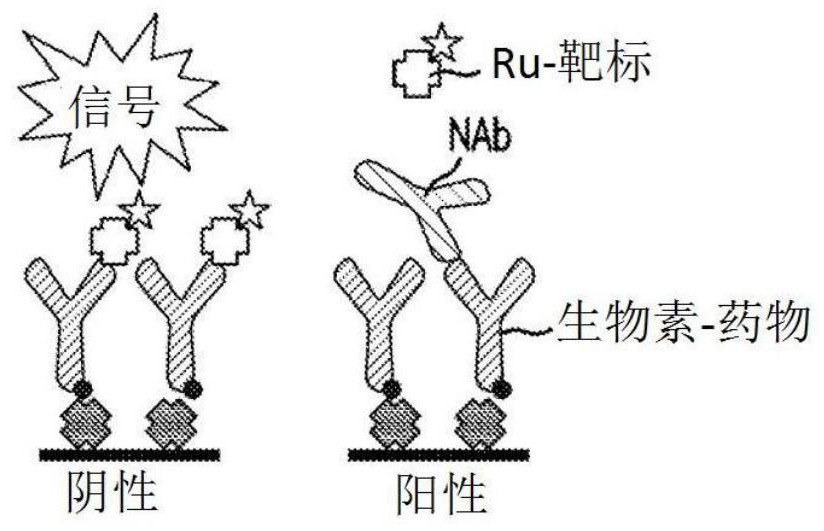
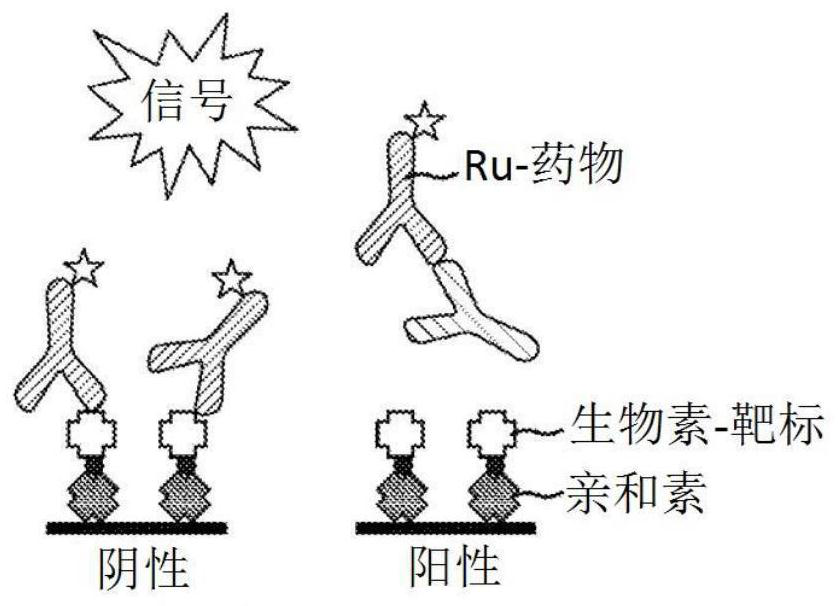
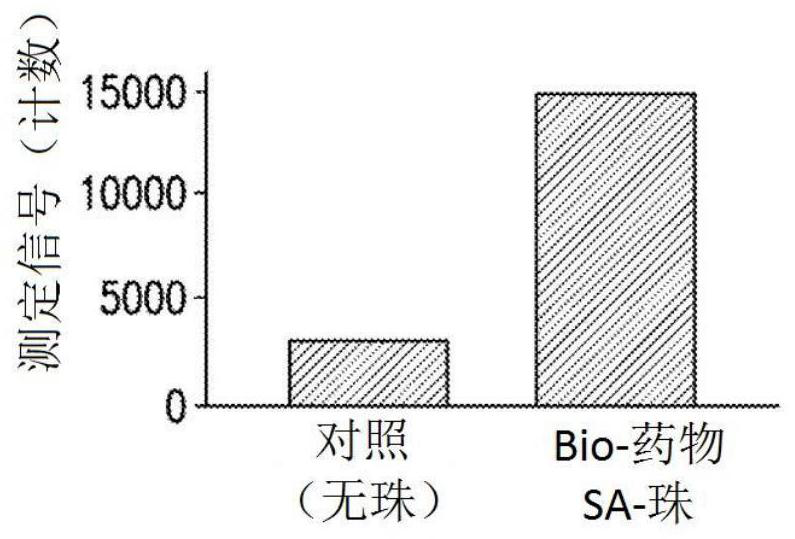
[0101] Initial experiments used biotin-drug bound to streptavidin beads to extract ADA (and NAb) from samples. However, in the CLB NAb assay, low labeled drug concentrations are necessary to achieve maximum sensitivity (Hu, J. et al., Journal of Immunological Methods 419:1-8 (2015); Wu, B.W., et al., " A Competitive Ligand-Binding Assay for the Detection of Neutralizing Antibodies," Detection and Quantification of Antibodies and Biopharmaceuticals: Practical and Applied Considerations, Michael G. Tovey (ed.), John Wiley & Sons, Hoboken, NJ , USA (2011)). Therefore, any biotin-drug transferred from the enrichment step to the assay step may interfere. Figure 2A The data in showed that the biotinylated drug used in the NAb enrichment step would carry over to the NAb assay step, resulting in an approximately 5-fold increase in...
example III
[0103] Example III: Minimizing Interference from Drug-Trapping Proteins
[0104] Materials and methods
[0105] See Example 1.
[0106] result
[0107] Attempts to reduce the amount of coupled protein or increase wash steps did not adequately minimize interference from these proteins after their transfer to the NAb assay. To mitigate residual interferences, different methods were developed for each NAb assay.
[0108] When the target is used as a capture reagent in the drug removal step, the protein remains and inhibits the NAb assay signal ( Figure 2B , 2C and 3A). However, in a drug capture assay format, the addition of an anti-target antibody resolves the problem caused by protein carryover. In the presence of anti-target mAb, positive and negative control samples were subjected to a drug removal step, and target-conjugated beads produced assay signals that were nearly identical to control samples without a drug removal step ( Figure 3A ).
[0109] In target c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com