Method for detecting Anti-drug antibodies
a technology of anti-drug antibodies and detection methods, which is applied in the direction of measurement devices, instruments, material testing goods, etc., can solve the problems of reducing the efficacy of biopharmaceuticals, wasting considerable expenditure on ineffective therapy, and losing time in the treatment of disorders, etc., and achieves high efficacy and sensitive
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Assay for ADA Against Human Monoclonal Ab Constructs
Principle:
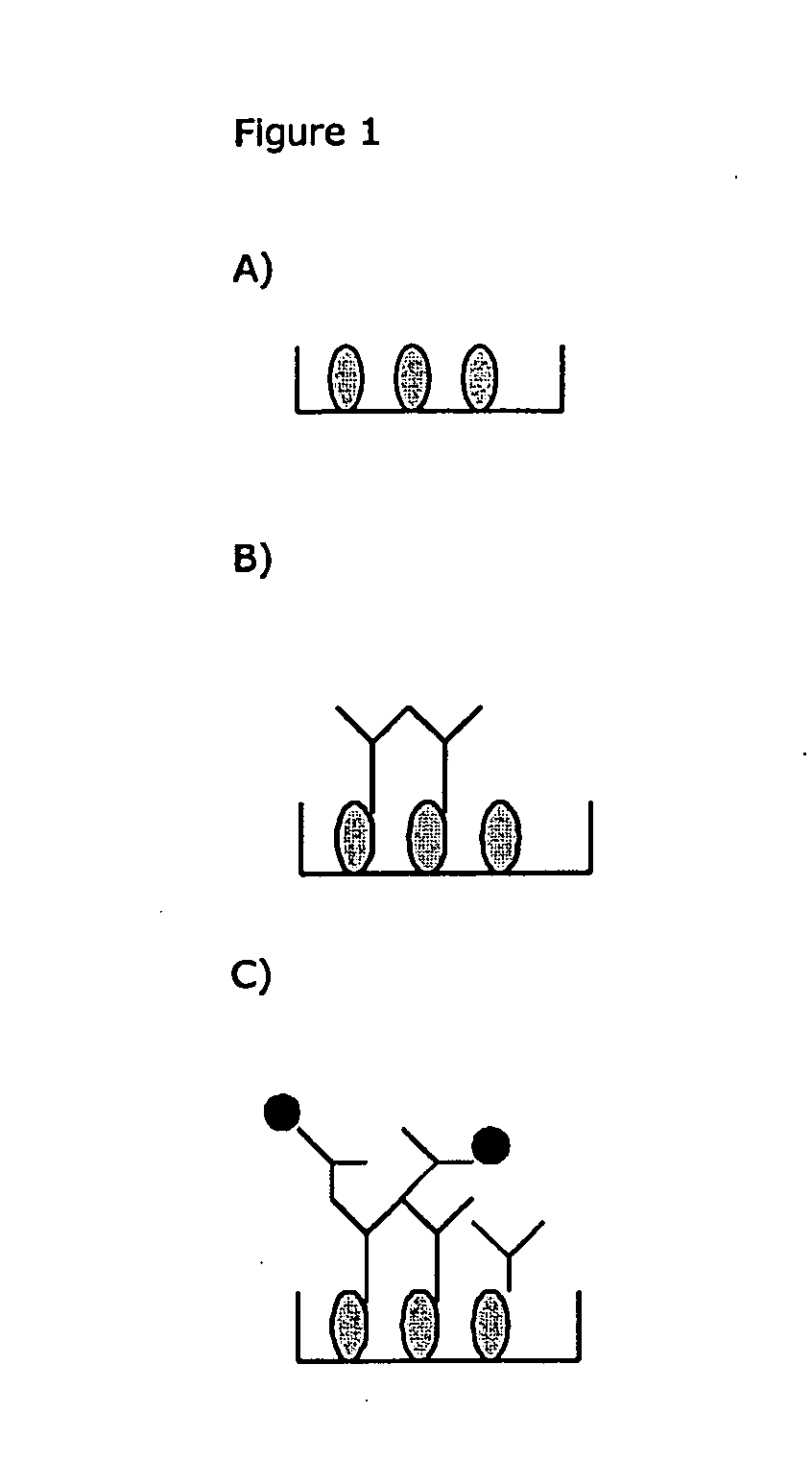
[0162]1) ELISA-plate or other solid phase for fixation of Ig-binding molecules (See FIG. 1A). The Ig-binding molecules can be ex. anti-Ig (Fc-specific or Fab specific), protein A, protein G, protein H or similar reagents. Washing is preferably made between each new manipulation. Fixation can be non- or covalent.
[0163]All binding sites are then blocked by an unspecific reagent not interfering with the Ig-binding capacity of the fixed Ig-binding reagent.
[0164]2) Binding of serum / plasma Ig (see FIG. 1B)
[0165]3) Blockade of Ig-binding sites on the fixed Ig-binding molecules, by adding non-labeled non-ADA Ig, before or together with the labelled human monoclonal Ig construct (see FIG. 1C).
[0166]4) Measurement of the bound label by standard methods.
example 2
Detailed Protocol for ADA Assay
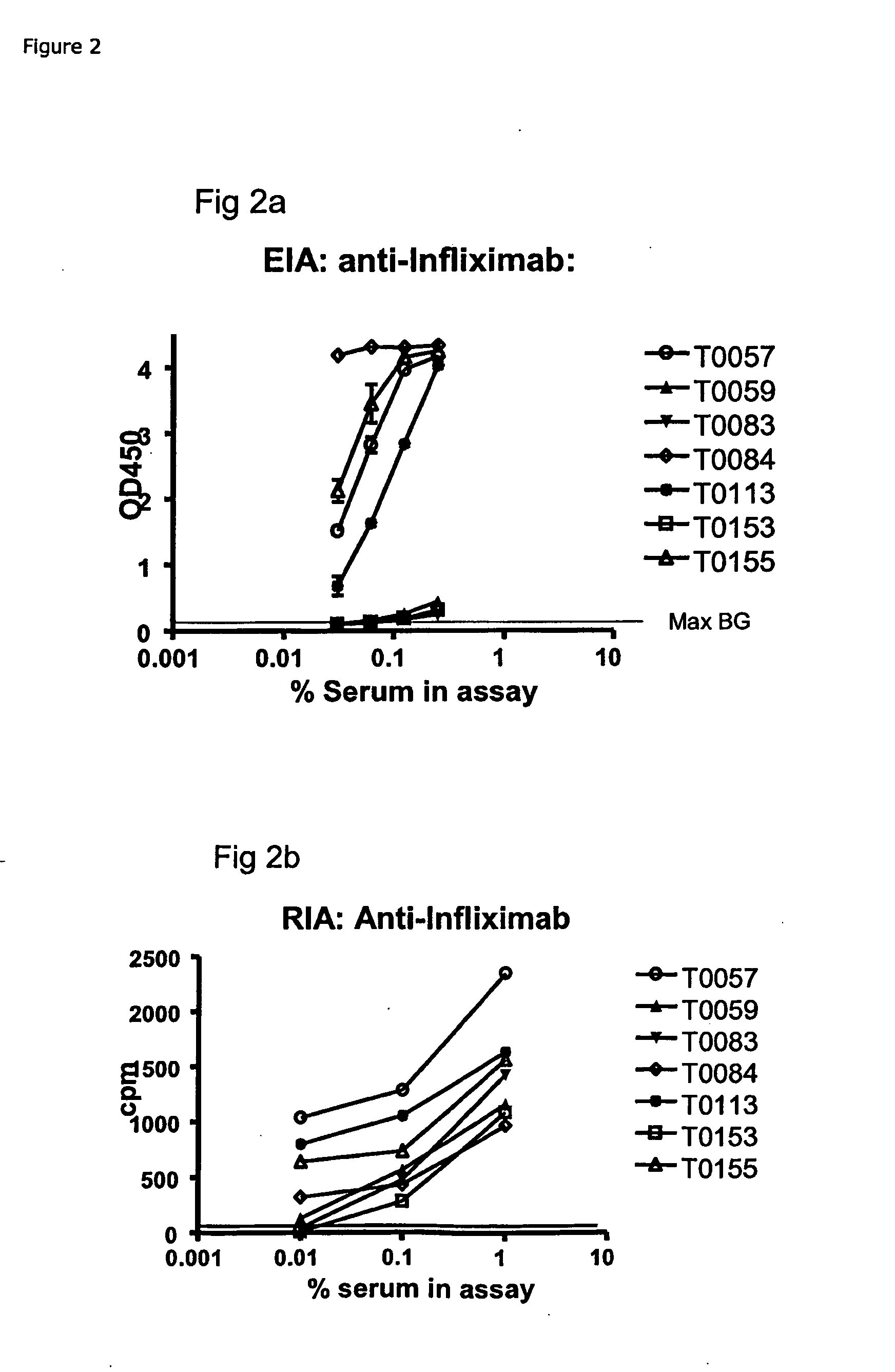
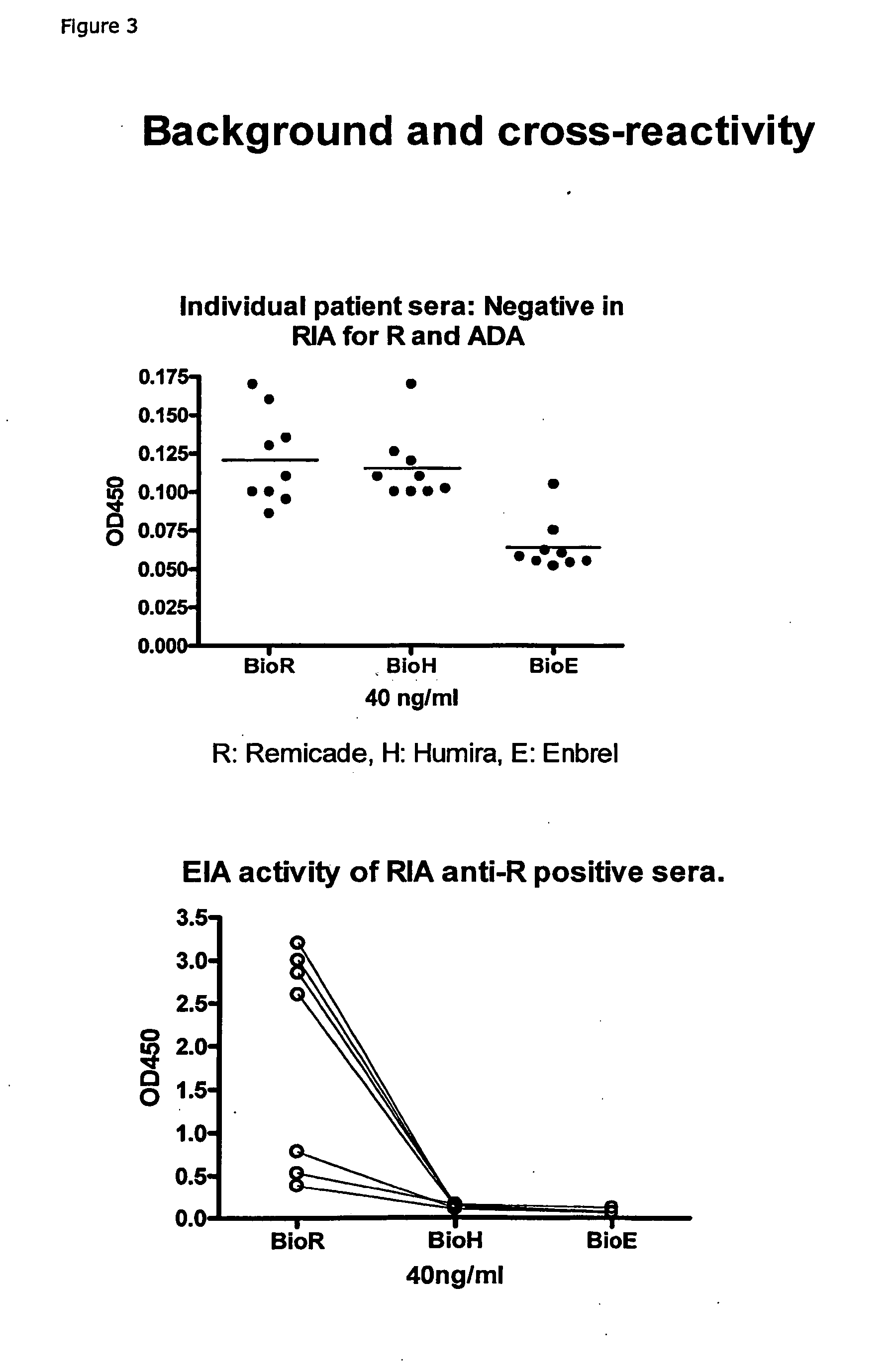
[0167]Example: EIA for ADA Against Infliximab (anti-TNFa IgG)[0168]1) Coat: 25 μg / ml of protein G in PBS, 100 μL / well on a 96-well flat bottom plastic plate, followed by 18-36 h incubation at 4-8 C.[0169]2) Wash the wells manually or by a plate washer with PBS+0.05% Tween 20[0170]3) Block of residual binding sites by PBS+2% BSA or Superblock (Pierce cat#37515) 2 h at room temperature or 18-24 at 4-8 C[0171]4) Wash the wells manually or by a plate washer with PBS+0.05% Tween 20[0172]5) Sample: Serum / plasma is added at 100 μl / well diluted to ex. 0.25% in PBS+5 mM EDTA+1% human serum albumin, or if further diluted, diluted in PBS+5 mM EDTA+1% human serum albumin with or without 0.25% pooled normal human serum. Incubate 18-24 h at 4-8 C.[0173]6) Wash the wells manually or by a plate washer with PBS+0.05% Tween 20.[0174]7) Ad 100 μl / well of Biotin labelled Infliximab, at ex. 40 ng / ml of PBS+1% human serum albumin+4% pooled normal human serum. Incubate 2-3 h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com