Methods for determining anti-drug antibody isotypes
一种同种型、药物的技术,应用在生物测试、材料检验品、测量装置等方向,能够解决有害副作用等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 29
[0127] Example 29 describes an exemplary proximity-based isotyping assay of the invention for determining at least one, two, three, four, five or more ATI isotypes using FRET, For example, the presence (or absence) or level of IgA ATI, IgD ATI, IgE ATI, IgG ATI and / or IgM ATI isotypes. As a non-limiting example, combining IFX labeled with a first fluorophore ("F1") and an anti-IgA antibody labeled with a second fluorophore ("F2") with one or more ATI isotypes (e.g., IgA ATI) samples (eg, serum samples) were incubated. In some embodiments, F2 is excited by F1 only when the two fluorophores are in close proximity, and the presence and / or level of a ternary complex of F1-IFX, F2-anti-IgA and IgA ATI is indicative of a sample The presence and / or levels of IgA ATI isotypes present in
[0128] In other embodiments, the signal generated by the autoantibody isotyping proximity assay is a fluorescent signal, which can be detected by electrophoretic techniques such as capillary electr...
Embodiment 1
[0225] Example 1 New Mobility Shift Assay for Measuring Levels of Anti-TNFα Biologics
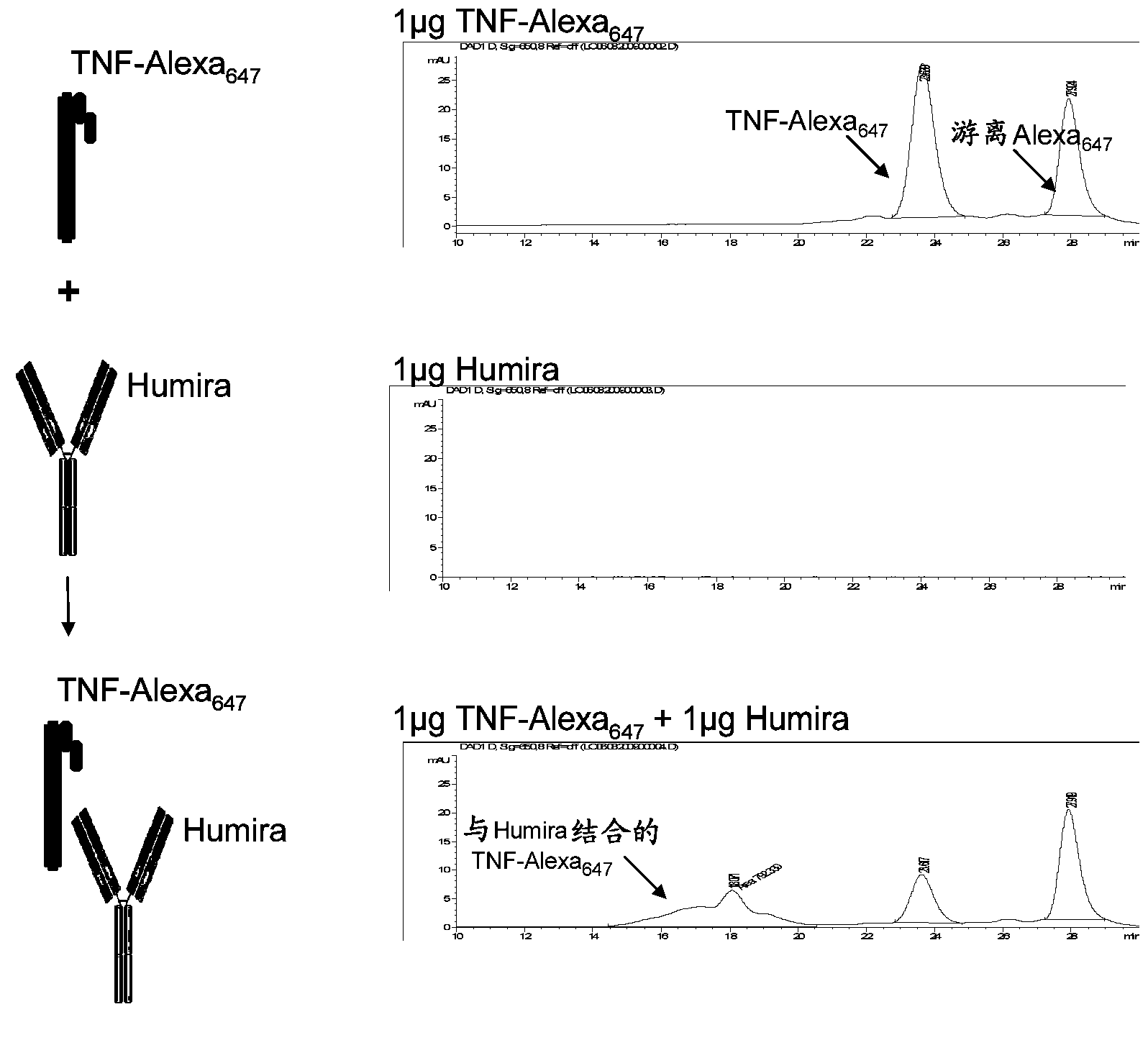
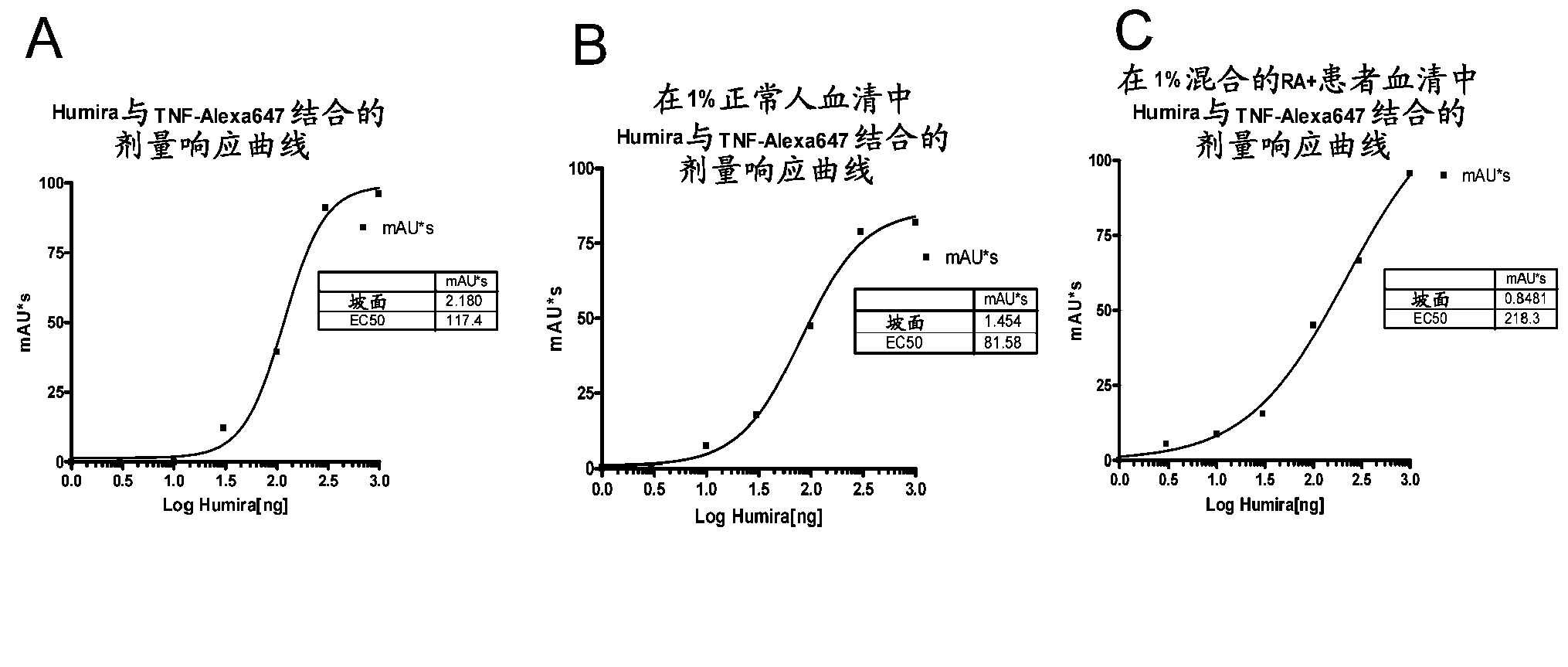
[0226] This example illustrates a new homogeneous assay for measuring anti-TNFα drug concentrations in patient samples (eg, serum) that uses size exclusion chromatography to detect binding of anti-TNFα drugs to fluorescently labeled TNFα. The assay is advantageous because it avoids the need for washing steps, uses fluorophores that allow detection in the visible and / or IR spectrum (which reduces background and serum interference issues), increases detection due to high sensitivity of fluorescent label detection The ability to have low titers of anti-TNF[alpha] drugs in patients, as well as proceed as a liquid phase reaction, reduces the chance of any epitope alteration due to attachment to a solid surface such as an ELISA plate.
[0227] In an exemplary embodiment, TNFα is treated with a fluorophore (such as Alexa 647 ) label, wherein the fluorophore can be detected by visible or infrared ...
Embodiment 2
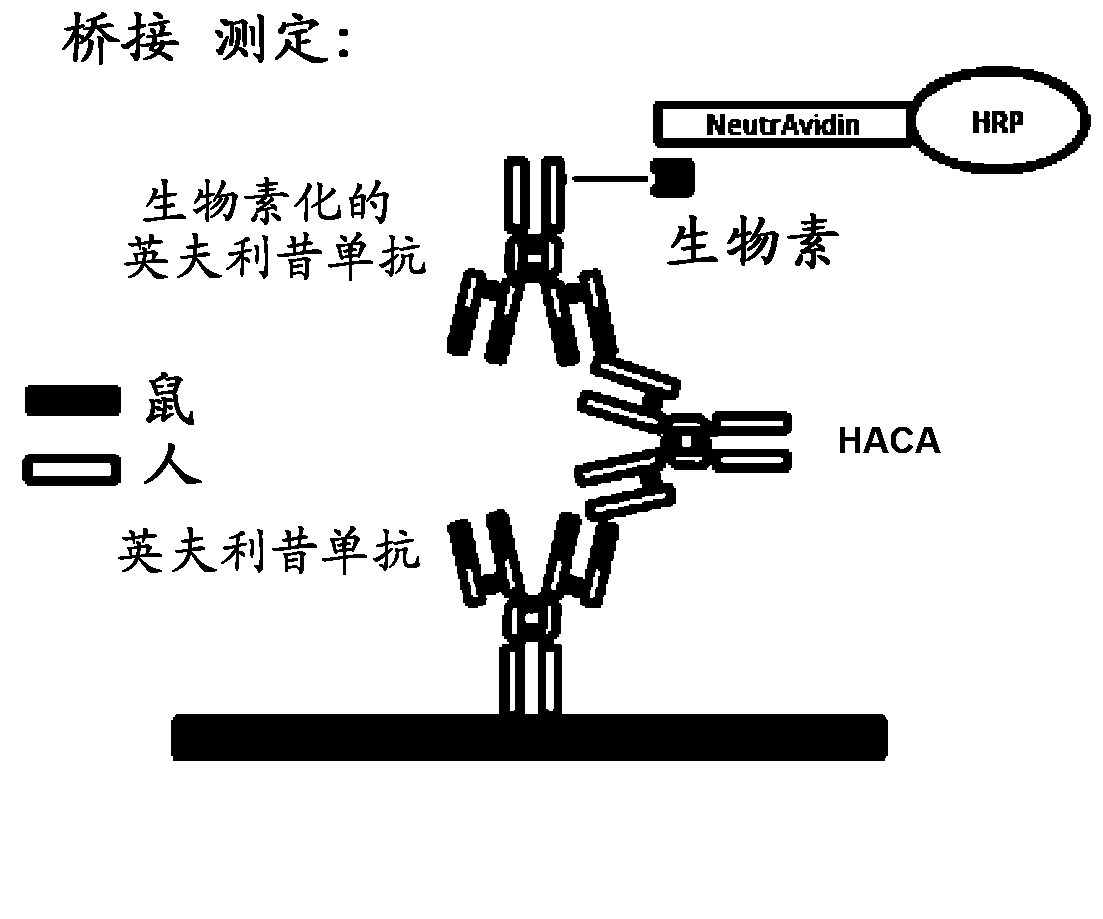
[0231] Example 2 New Mobility Shift Assay for Measuring HACA and HAHA Levels
[0232] This example illustrates a novel homogeneous assay for measuring the concentration of autoantibodies (e.g. HACA and / or HAHA) in patient samples (e.g. serum) using size exclusion chromatography to detect these autoantibodies in combination with fluorescently labeled anti-TNFα Combination of drugs. The assay is advantageous because it avoids the need for washing steps to remove low-affinity HACA and HAHA, uses fluorophores that allow detection in the visible and / or IR spectrum (which reduces background and serum interference issues), due to The high sensitivity of fluorescent label detection increases the ability to detect low titers of HACA and HAHA in patients, as well as proceeding as a liquid phase reaction, thereby reducing the chance of any epitope alteration due to attachment to a solid surface such as an ELISA plate.
[0233] The fact that HACA was detected in 53%, 21%, and 7% of rheum...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com