Therapeutic composition for treating B cell leukemia and B cell lymphoma
A lymphocyte and cell surface technology, applied in the field of biomedicine, can solve problems that need to be further developed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0160] Cell lines and basic experimental techniques used in the embodiments of the present invention are as follows:
[0161] cell line
[0162] Use following cell line in the embodiment: OCI-LY3 cell (CD19 + Diffuse large B-cell lymphoma cell line (DLBCL), Jurkat cells (CD19 - T lymphoma cell line), and K562 (target cell of natural killer cells). The above cells are all from ATCC (American cell bank) and DSMZ (German cell bank), and cultured in RPMI-1640 medium (purchased from Gibco-BRL, San Francisco, CA, USA).
[0163] Generation of lentivirus and transduction of human T lymphocytes
[0164] Replication-defective lentiviral vectors were generated and collected by centrifugation for transduction of human T lymphocytes. The following is a brief introduction to the production and collection of lentiviral vectors: 293T cells were placed in a cell culture dish with a bottom area of 150-cm2, and Express-In (purchased from Open Biosystems / Thermo Scientific, Waltham , MA) f...
Embodiment 2
[0173] Example 2 Construction of a vector co-expressing PD1-shRNA and anti-CD19 chimeric antigen receptor
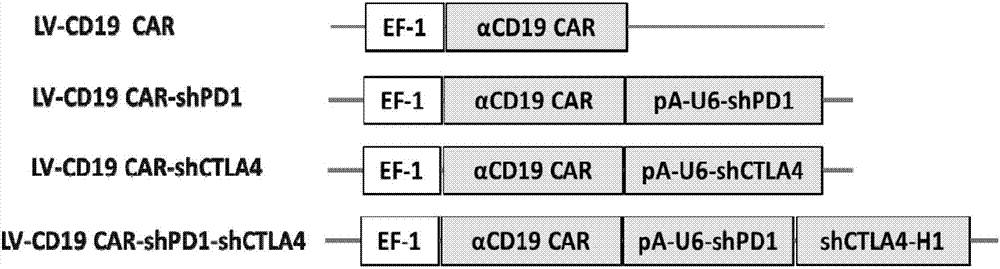
[0174] In this example, the inventor cloned the sequence encoding the single-chain antibody against human CD19, the ζ-chain sequence of the combination of the intracellular segment of CD28 and the T cell receptor into the LV lentiviral vector. During the cloning process, the selection restriction The specific restriction enzyme digestion is XbaI and NotI double enzyme digestion, and NotI and XhoI double enzyme digestion. Through enzyme digestion, ligation, screening and amplification of the target plasmid, a slow DNA sequence linked to the nucleotide sequence encoding the anti-CD19 chimeric antigen receptor is generated. Viral plasmid (LV-CD19 CAR); the sequence of U6 promoter and PD1-shRNA (shPD1) was cloned into the lentiviral plasmid of the above LV-CD19 CAR to generate the nuclear sequence linked with shPD1 sequence and encoding anti-CD19 chimeric antigen receptor Le...
Embodiment 3
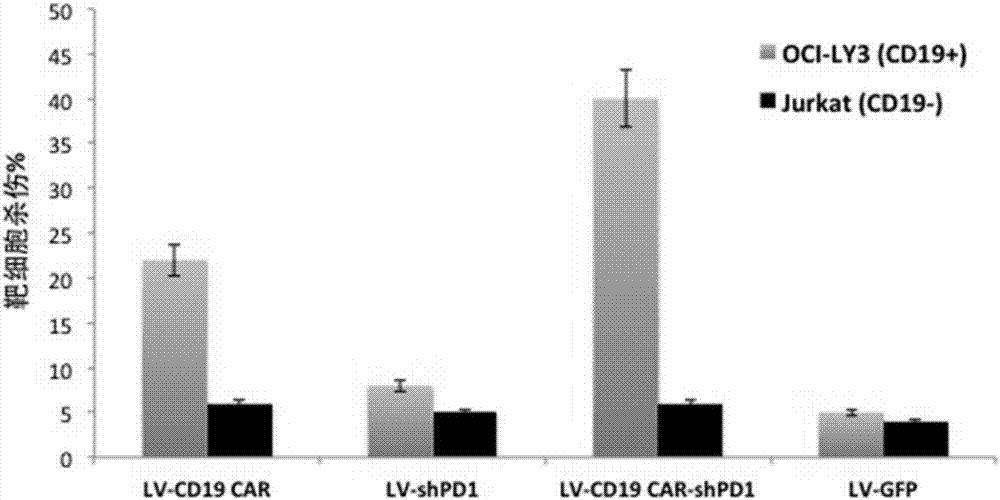
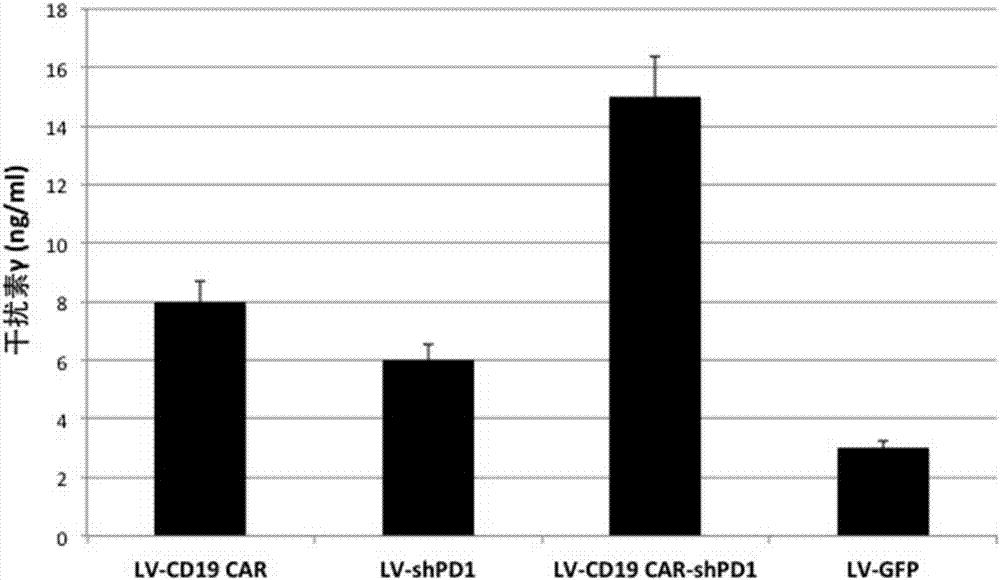
[0175] Example 3 Enhanced targeted killing ability of T cells co-expressing PD1-shRNA and anti-CD19 chimeric antigen receptor
[0176] In this example, peripheral blood lymphocytes were obtained from anonymous blood donors. Peripheral blood lymphocytes were separated by gradient centrifugation using Ficoll-Hypaque. T lymphocytes and T cell activator magnetic beads CD3 / CD28 (purchased from Invitrogen, Carlsbad, CA) in 5% CO 2 , Incubated at 37 degrees Celsius for 72 hours, the medium was added with 2mmol / L glutamine, 10% high temperature inactivated fetal calf serum (FCS) (purchased from Sigma-Aldrich Co.) and 100U / ml of penicillin / chain RPMI medium 1640 (purchased from Invitrogen Gibco Cat. no. 12633-012) with antimycin double antibody. After activating and culturing for 72 hours, the cells were rinsed with washing solution to wash away the magnetic beads. The T cells were planted on cell culture dishes covered with recombinant fibronectin fragments (FN ch-296; Retronectin)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com