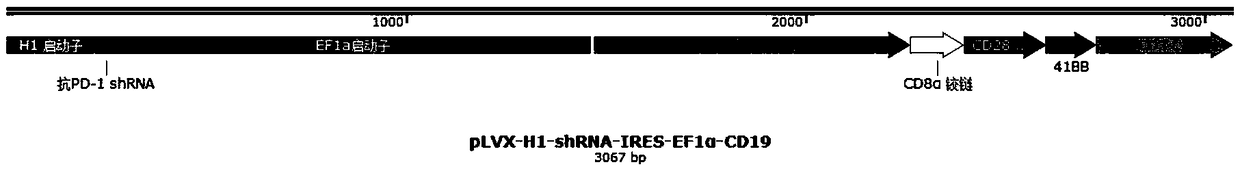
Plasmid structure for expressing CD19CAR and knocking down T cell surface PD-1 expression simultaneously and construction method thereof
A cell surface, PD-1 technology, applied in the biological field, can solve the problems of unsatisfactory CART cell effect, decreased CART cell function, impaired tumor clearance ability, etc., to achieve the effect of less apoptosis and better cell proliferation ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
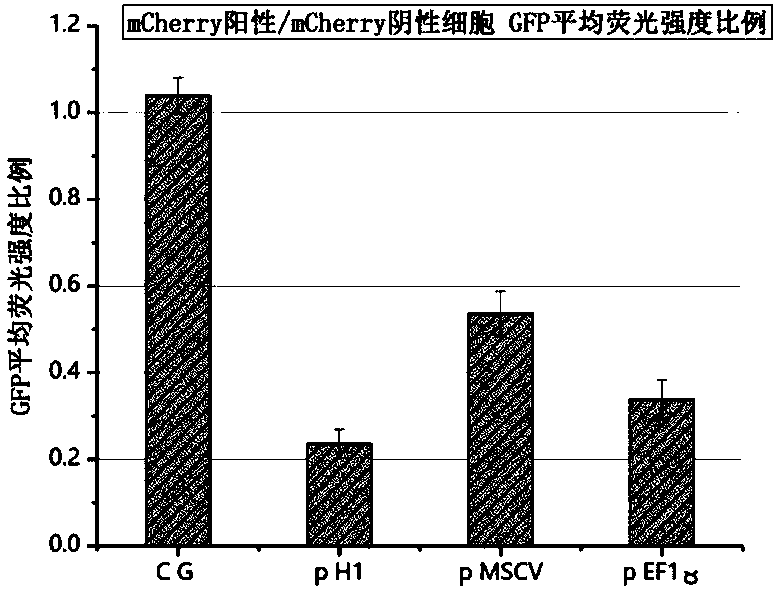
[0047] Example 1 Screening of backbone plasmids capable of co-expressing CAR and shRNA, GFP expression in K-562 human chronic myelogenous leukemia cells can be effectively knocked down by pH1 constructs
[0048] In order to simplify the experiment, we first constructed a GFP+K562 stable cell line by lentiviral transduction and puromycin screening, then replaced the CAR sequence with the mCherry fluorescent sequence, and constructed three backbone plasmids p H1 and p MSCV using different promoters and plasmid structures and pEF1α. The three plasmids were transduced into the GFP+K-562 human chronic myelogenous leukemia cell line by lentivirus, and the fluorescence expression intensity of mCherry and GFP was detected by flow cytometry. Experimental results such as figure 2 As shown, p H1+K562 showed a more obvious down-regulation of GFP expression than the other two, indicating that this backbone can allow the simultaneous high-efficiency expression of shRNA and the target CAR ...
Embodiment 2
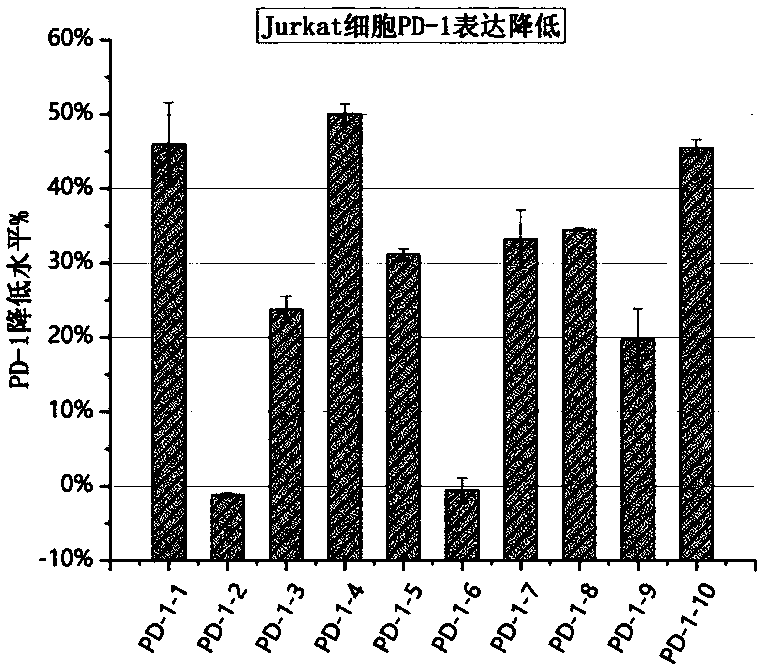
[0049] Example 2 Screening and verification of the most efficient anti-PD-1 shRNA sequence in jurkat cell lines and human CD3+ T cells
[0050] Preliminary experiments proved that Jurkat cell line and human CD3+ T cells can also be induced to express PD-1 by human T cell activation immunomagnetic beads CD3 / CD28 magnetic beads. We used ten shRNA sequences targeting PD-1 mRNA to knock down its expression, as shown in Table 1, to construct expression plasmids, using GFP fluorescence as a marker. Then ten plasmids were transduced into the Jurkat cell line by lentivirus, stimulated with CD3 / CD28 magnetic beads, and flow cytometry was used to detect the GFP fluorescence and PD-1 expression on the surface of Jurkat cells. The experimental results showed that in activated jurkat cells, PD1-1, PD1-4, PD1-8 and PD1-10 all showed better PD-1 knockdown effects, such as image 3 shown.
[0051] In order to verify the role in human T cells, we concentrated and purified the lentiviruses of...
Embodiment 3
[0055] Example 3 Reduced expression of PD-1 on the surface of CD19-CAR T cells carrying anti-PD-1 shRNA
[0056] The two shRNA sequences PD1-1 and PD1-8 obtained by screening were subcloned into the plasmid structure of the pLVX-IRES-puro standard vector using the structure of pH1 and the third-generation anti-CD1941BB+CD28CAR. Using the basic CD19-CAR as a negative control group, through lentiviral packaging, the concentrated and purified high and low levels of virus were used to transduce CD3+ T cells stimulated by CD3 / CD28 magnetic beads in healthy volunteers. 72 hours after transduction, flow cytometry was used to detect the conversion effect. Three groups of samples (CD19-control; CD19-PD1-1; CD19-PD1-8) were collected at 24 hours and 72 hours after the transduction to extract total RNA, and the relative quantitative q-PCR analysis was performed for T PD-1 mRNA expression level in cells (GAPDH as internal reference). The experimental results showed that compared with the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com