Humanized monoclonal antibody against hIL-33 and application of monoclonal antibody
A technology of hil-33 and antibody, applied in the field of humanized monoclonal antibody, can solve the problems of degree of humanization, difference in affinity strength effect, no therapeutic effect, high adverse reaction rate of fully human antibody, etc., and achieve enhanced recruitment , enhance the effect of acute allergic reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] Example 1: Determination of the amino acid sequence of the light and heavy chain variable regions of the mouse-derived anti-hIL-33 monoclonal antibody
[0093] 1.1 Animal immunity
[0094] Using human IL33 (NP_254274.1) (Ser112-Thr270) expressed in Escherichia coli and fused with 6 histidine tags at the C-terminus, 6 BALB / c mice were immunized according to the routine Freund's adjuvant immunization procedure, and immunized in two batches. Three animals were immunized in each batch, with two weeks between batches. Each batch of animals was immunized 4 times, and Human IL33-his was used for ELISA detection. If the serum titer of the animal was >1:100,000, the animal was finally immunized, and the spleen was collected 3-4 days later.
[0095] 1.2 B cell panning and culture
[0096] 2 days before the formal experiment, spread the culture medium for feeder cells into four 10cm culture dishes (corning, cat.430167), and 1 day before the formal experiment, use 25µg / mL MMC to ...
Embodiment 2
[0106] Example 2: Recombinant expression and activity detection of murine anti-hIL-33 monoclonal antibody
[0107] 2.1 Recombinant expression of mouse anti-hIL-33 monoclonal antibody
[0108] The light and heavy chains from the same clone were combined to transfect CHO-K1 cells. After 24 hours of transfection, 10 μg / mL MSX was added for pressure selection. After the cell density and viability recovered, inoculated for Feed-batch expression, and the supernatant after the expression was completed was centrifuged. And purified by protein A, the antibody concentration was quantified by BCA method and used for quantitative screening.
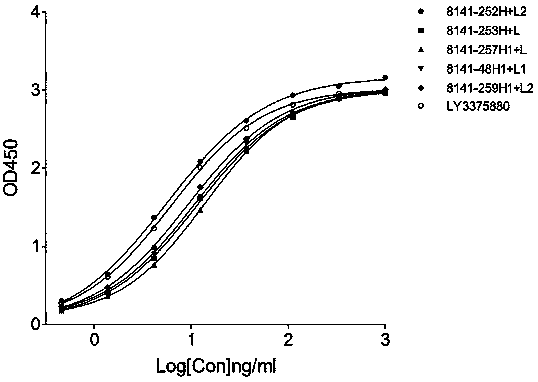
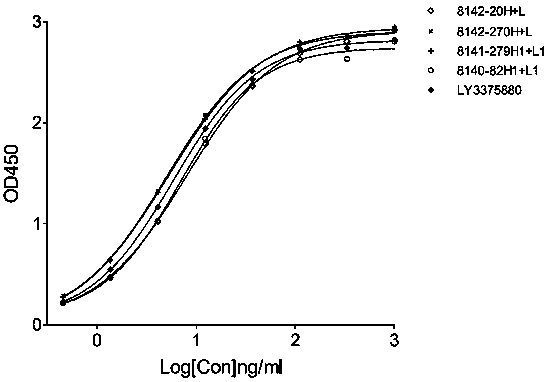
[0109] The name of the mouse antibody is indicated by the number of the cell clone from which the light and heavy chain (H+L) is contained, for example, the mouse antibody "8140-82H1+L1" means the mouse antibody from the 8140-82 cell clone, and its heavy chain is 8140-82H1 , the light chain is 8140-82L1.
[0110]2.2 Binding activity of mouse anti-h...
Embodiment 3
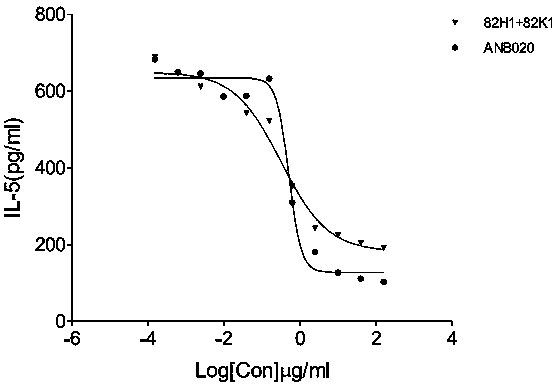
[0128] Example 3: Humanization of murine anti-hIL-33 monoclonal antibody 8140-82H1+L1
[0129] The heavy and light chain variable region sequences of the murine antibody 8140-82H1+L1 were compared to the human germline sequence using a blast search of the IMGT database. Redundant genes as well as those with unpaired cysteines were removed from the set of human germline genes. The remaining closest matching human germline genes were selected as recipient human frameworks in both framework and CDR regions. FR-4 was selected based on sequence similarity to the IGHJ / IGJK germline genes. The anti-hIL-33 humanized antibody 8140-82H2L1 was obtained through humanized design and screening, and the amino acid sequences of the variable regions of the heavy and light chains are shown in Table 8.
[0130] Table 8 Humanized anti-hIL-33 antibody 8140-82H2L1
[0131]
[0132] Note: The amino acid sequence underlined in bold is the CDR region in the heavy and light chain variable region,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com