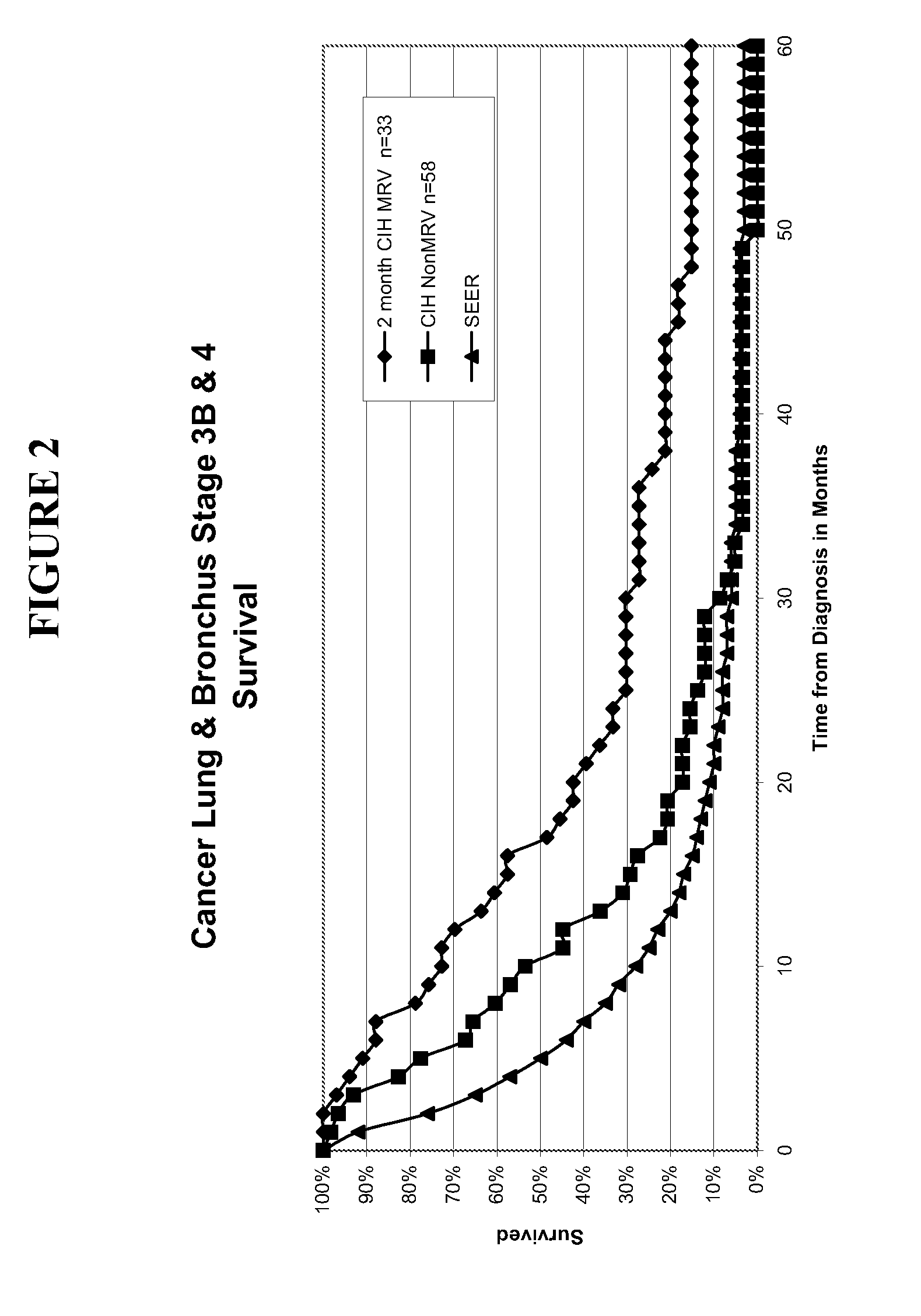
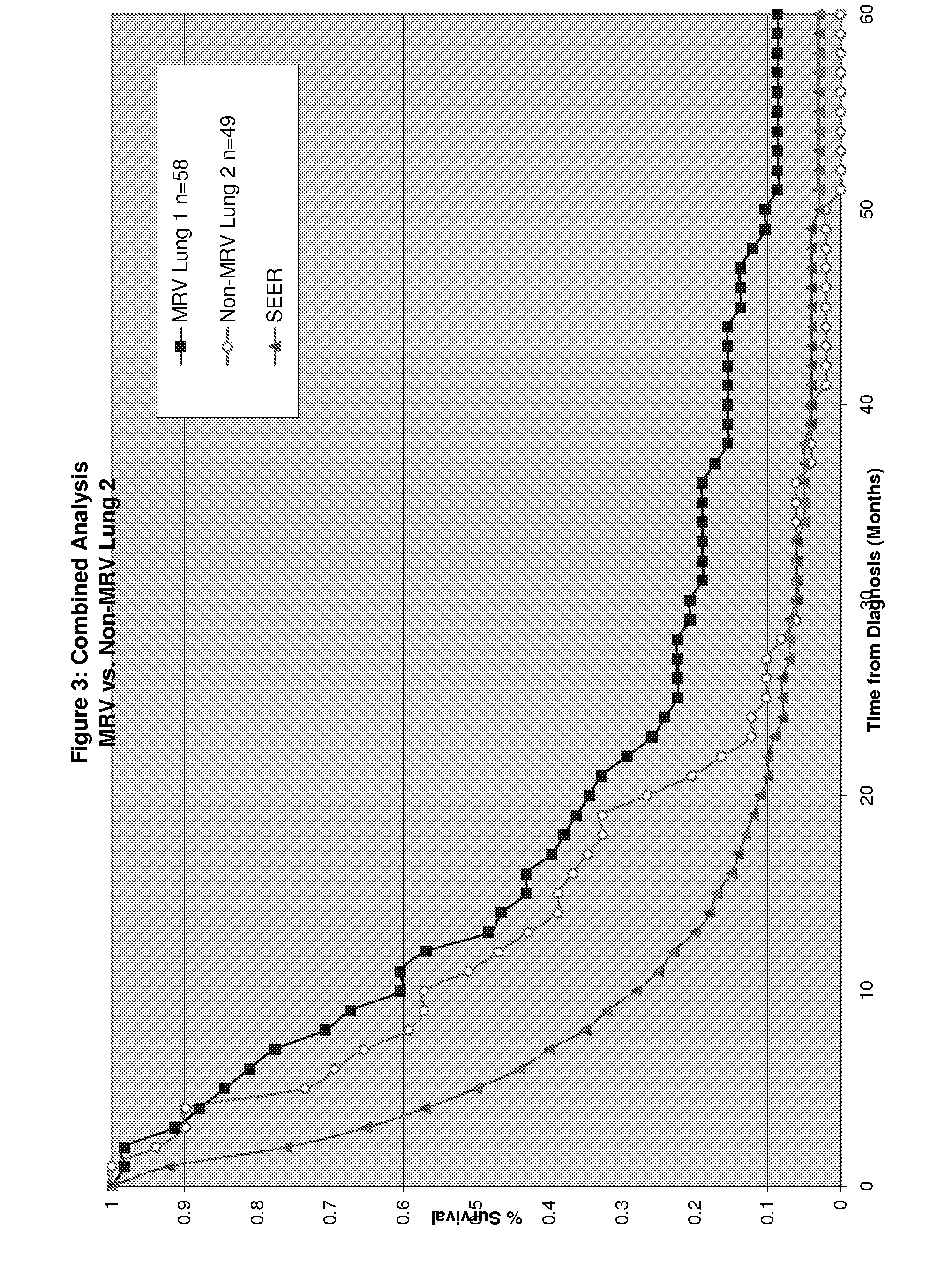
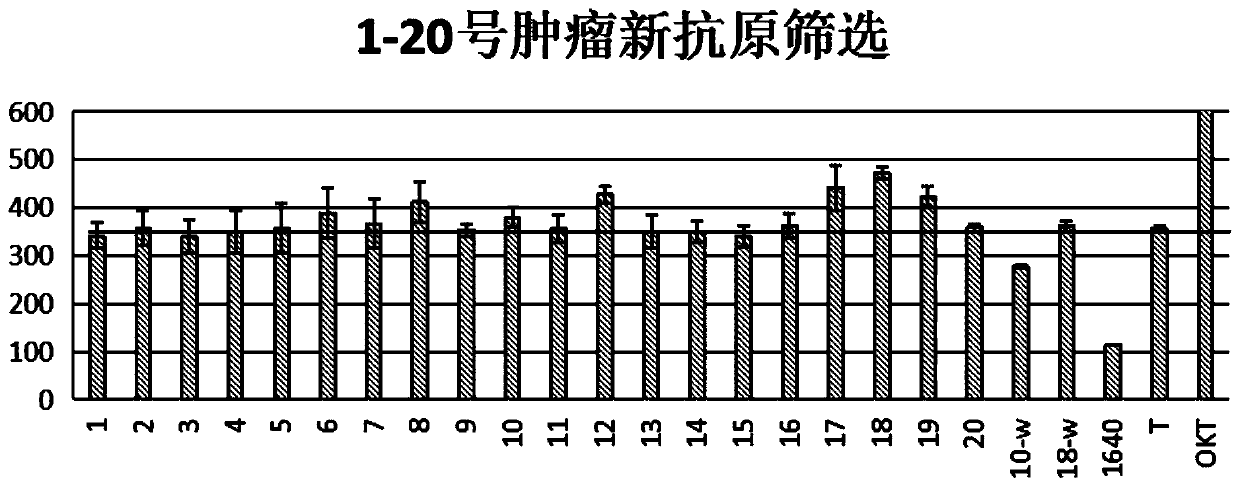
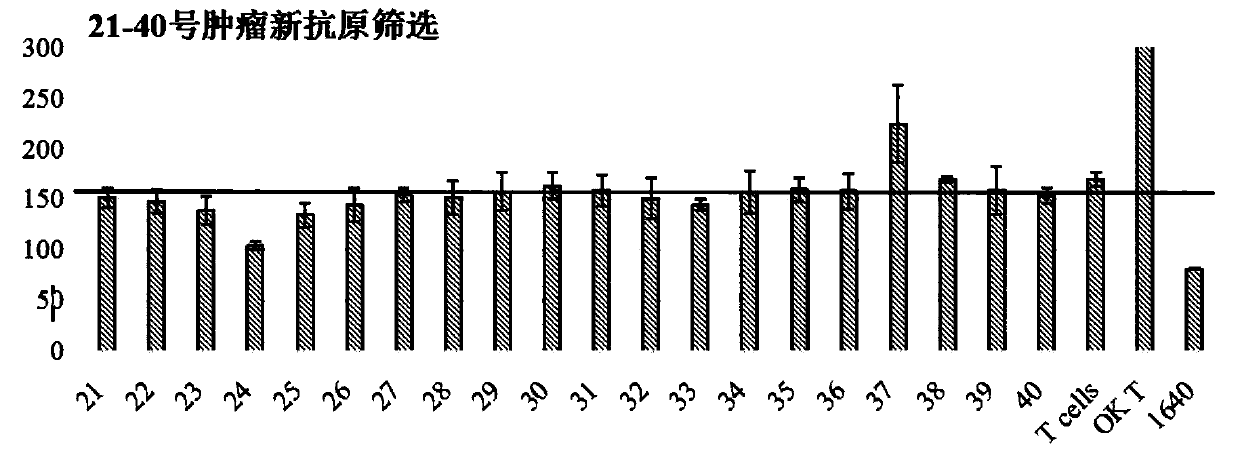
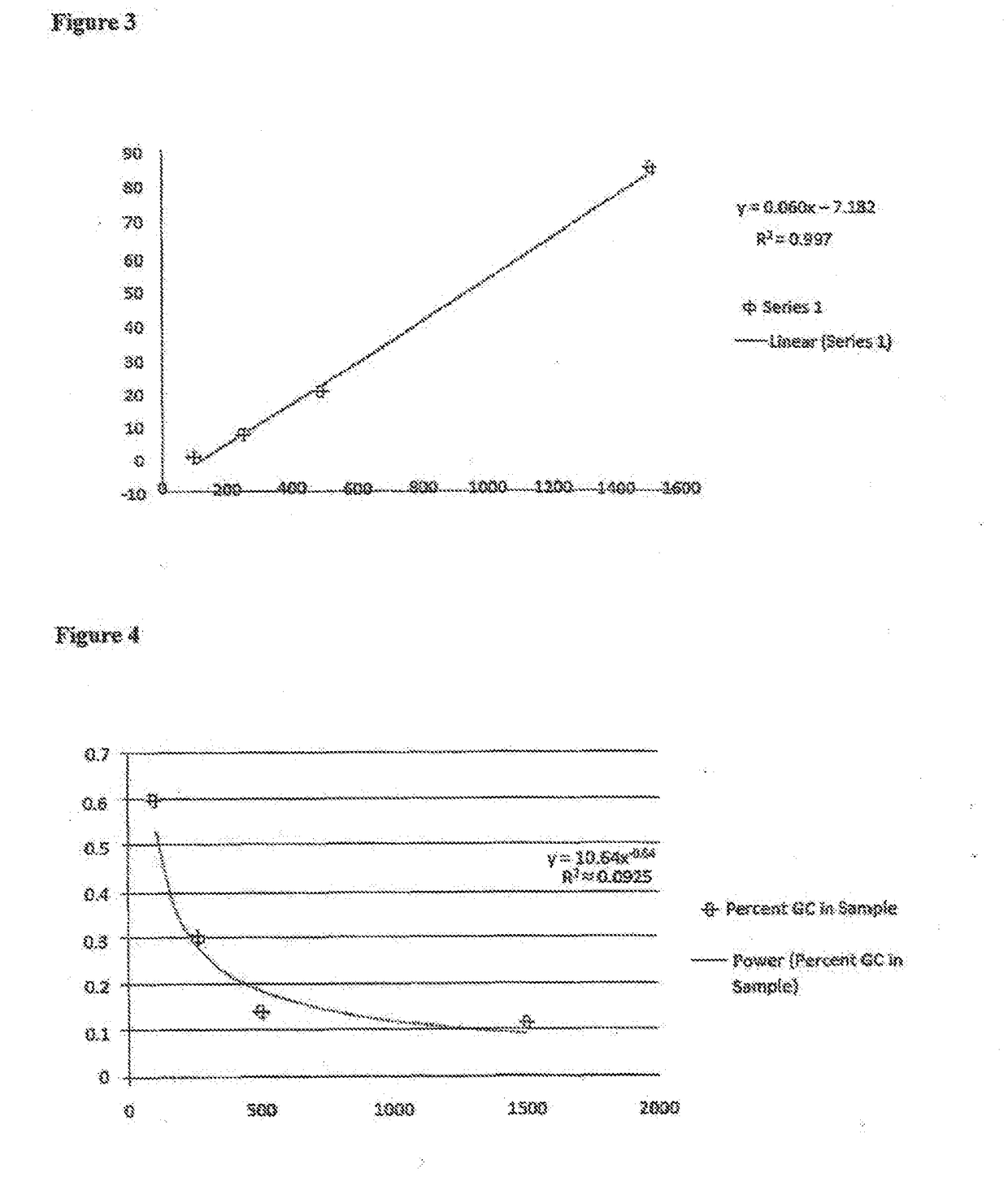
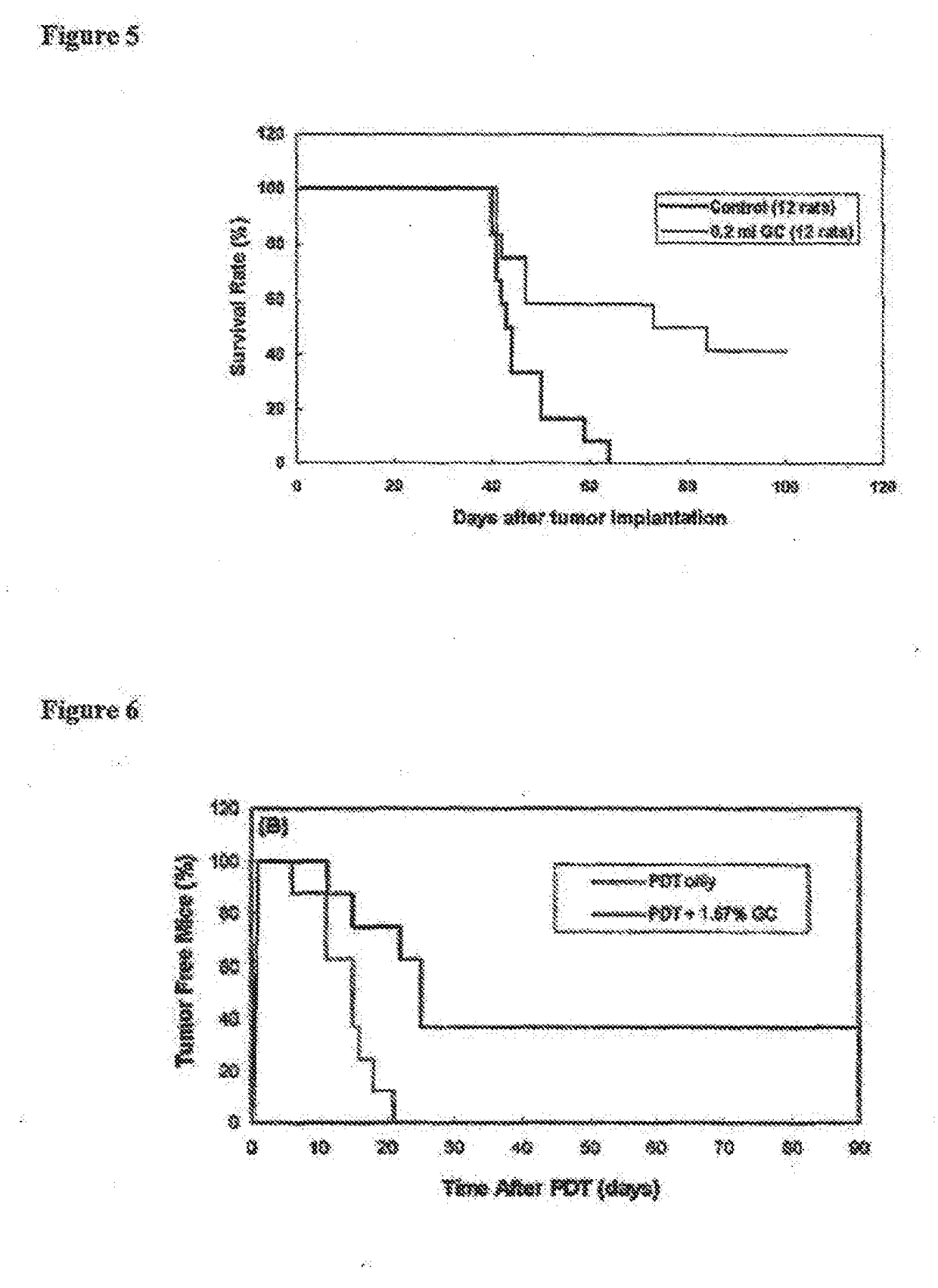
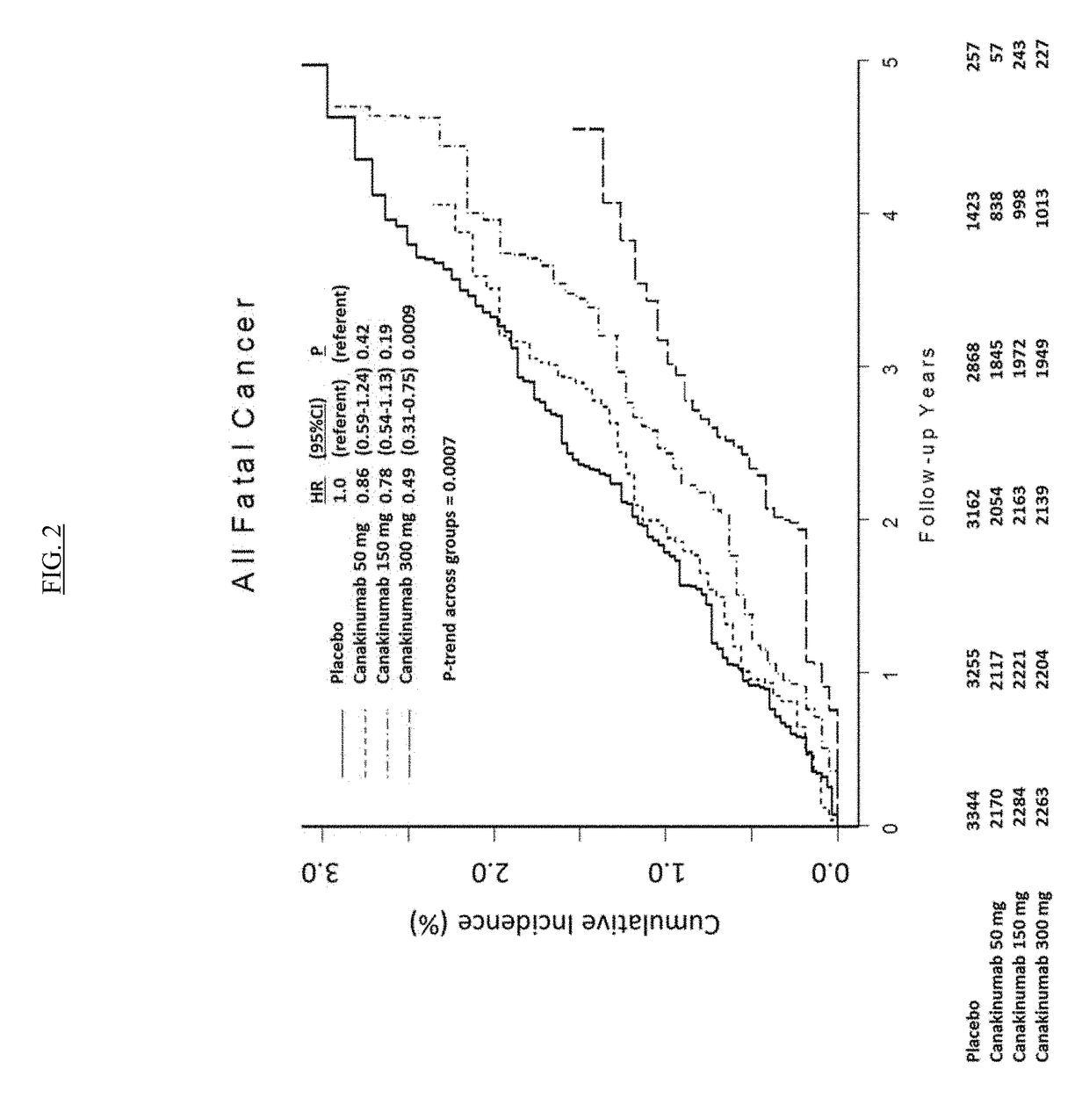
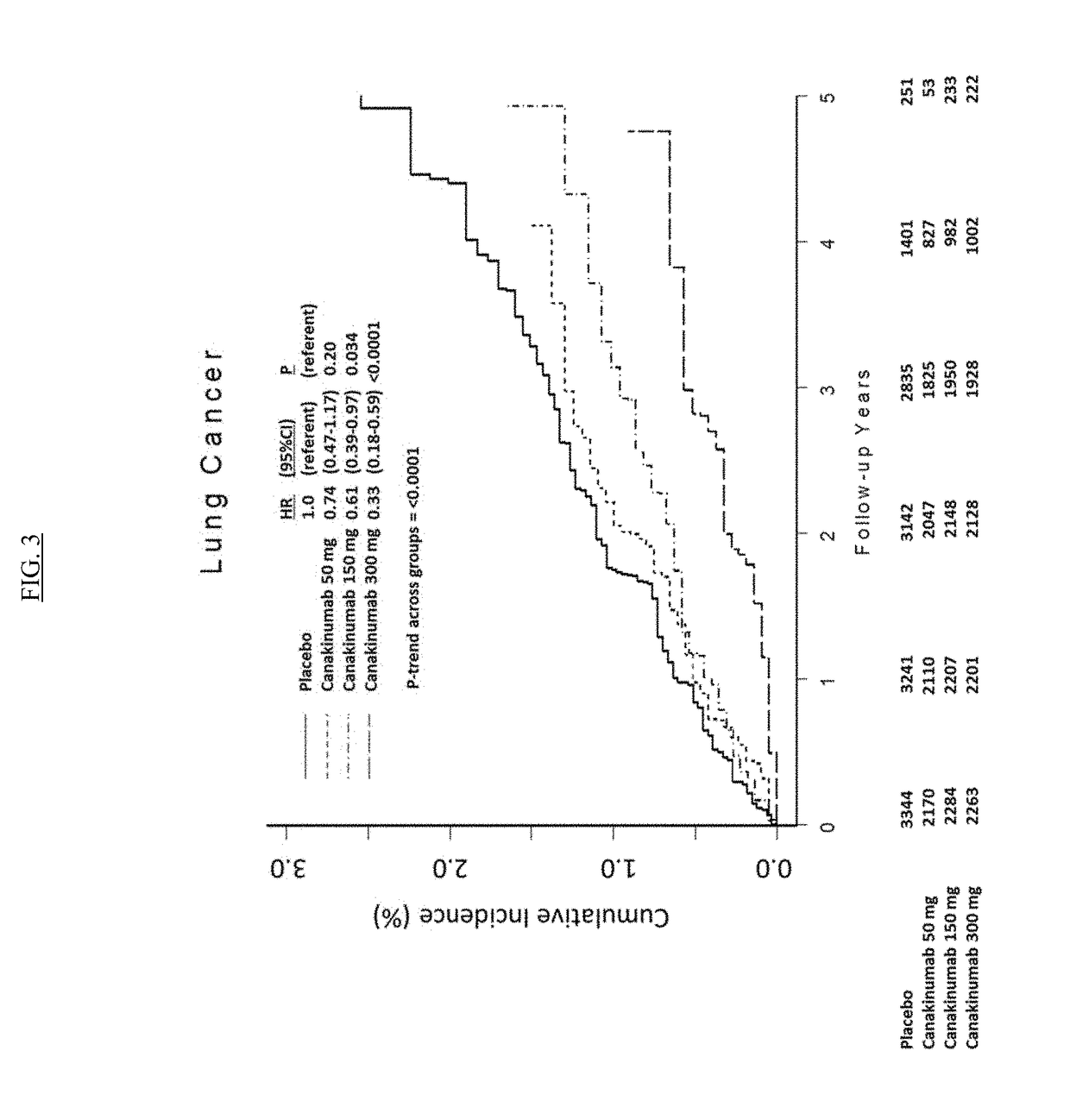
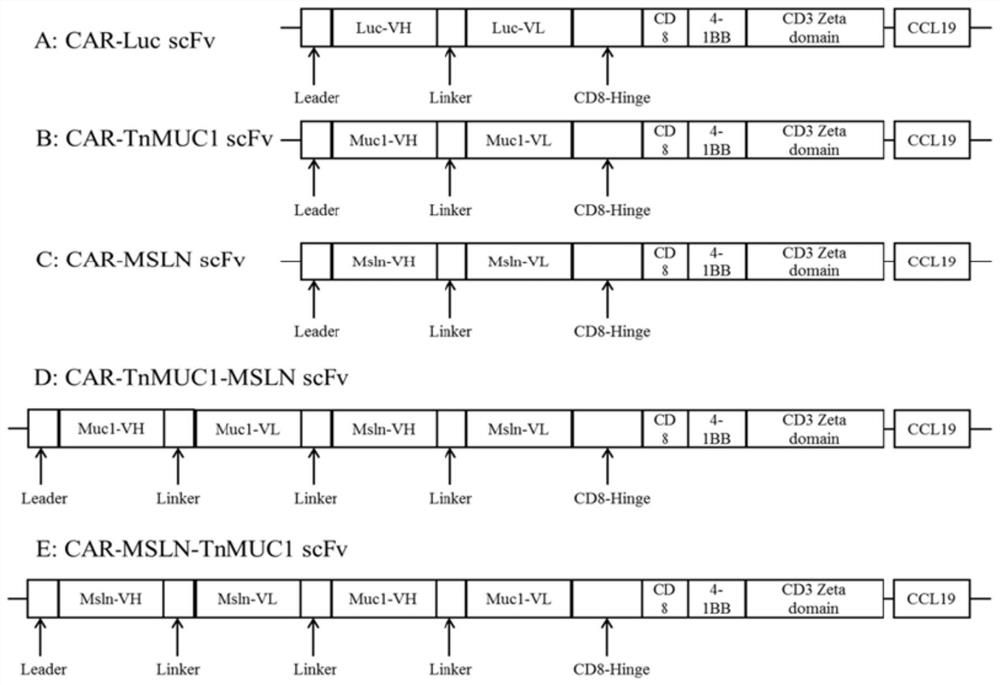
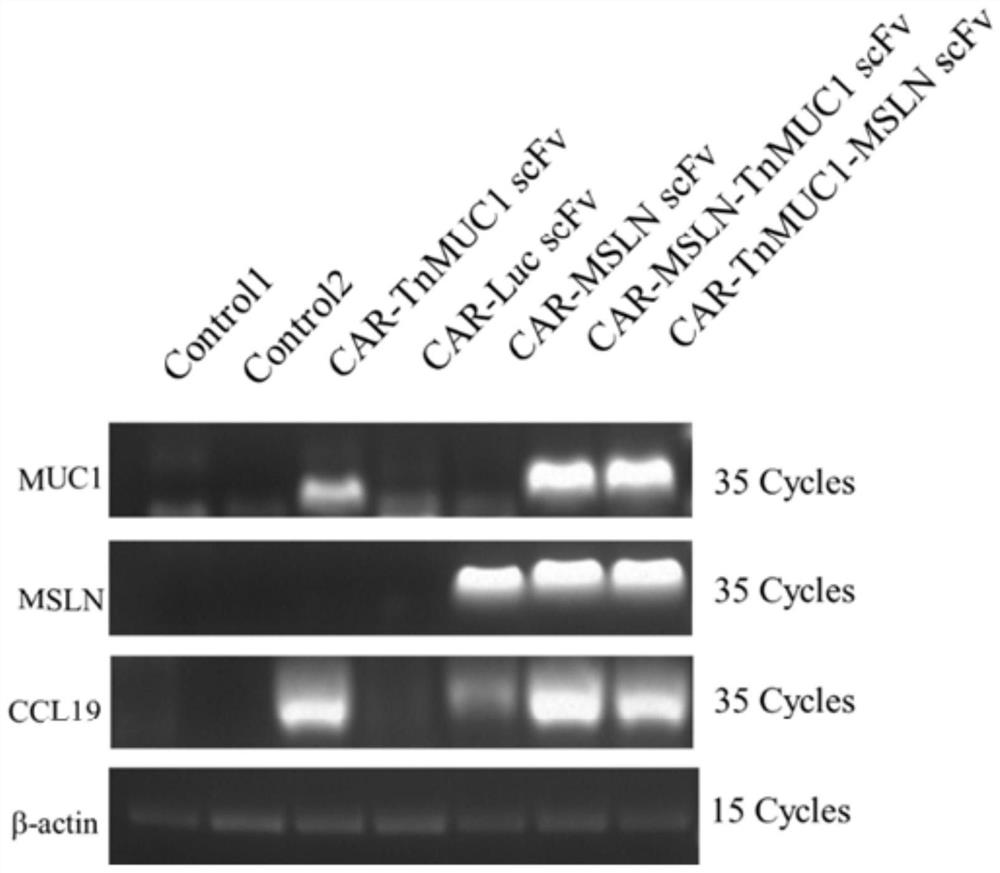
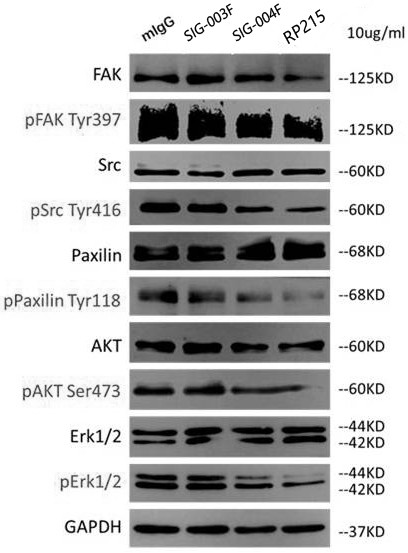
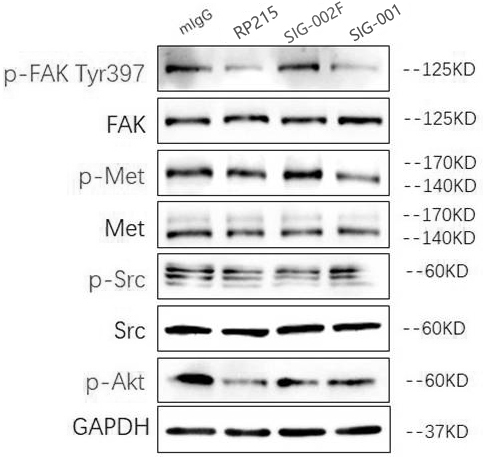
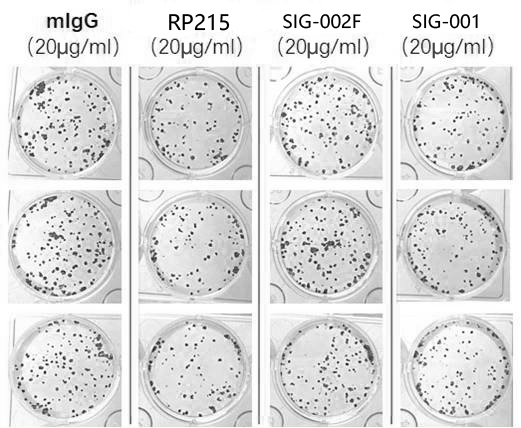
Patents
Literature
116results about "Lung cancer vaccine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
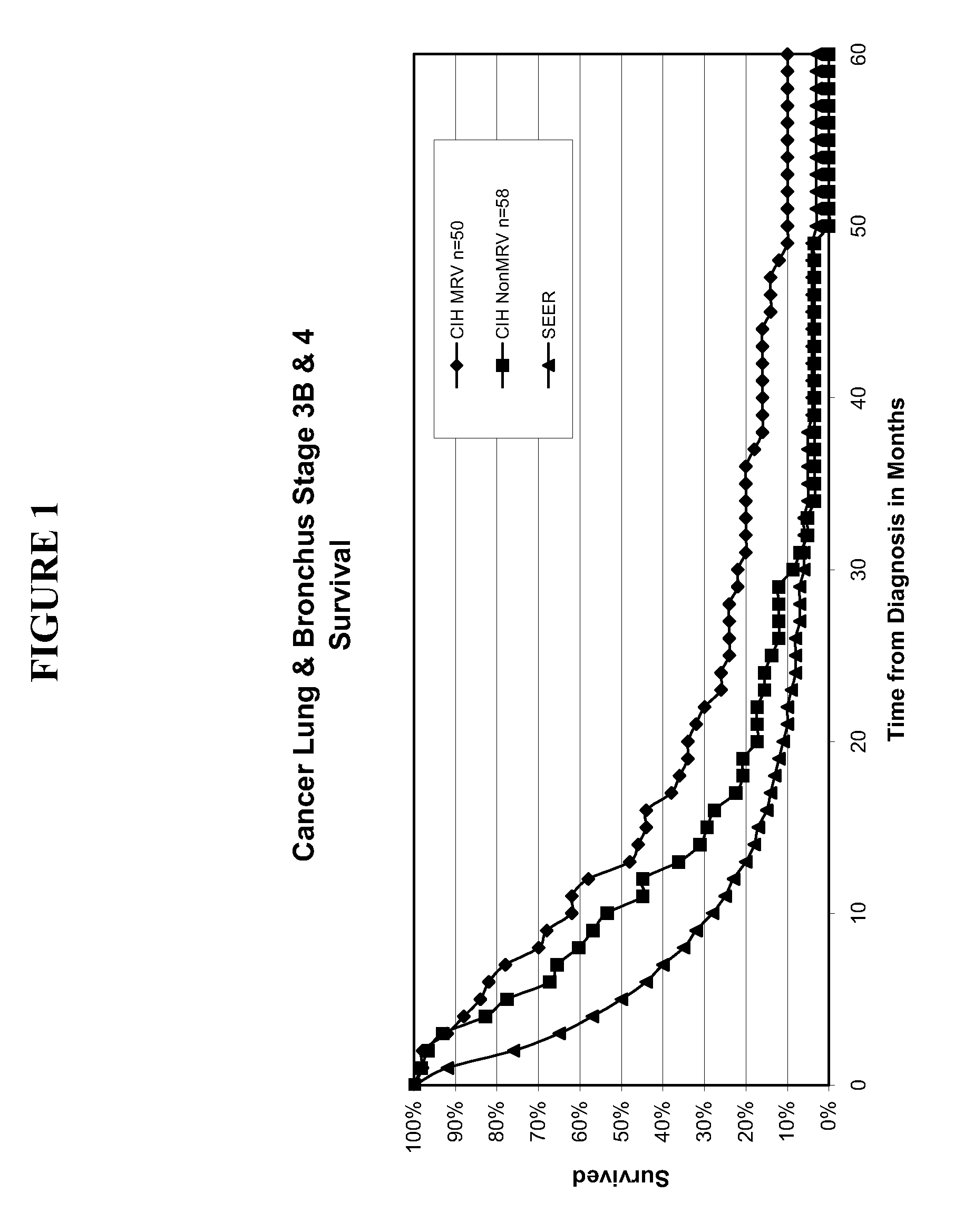
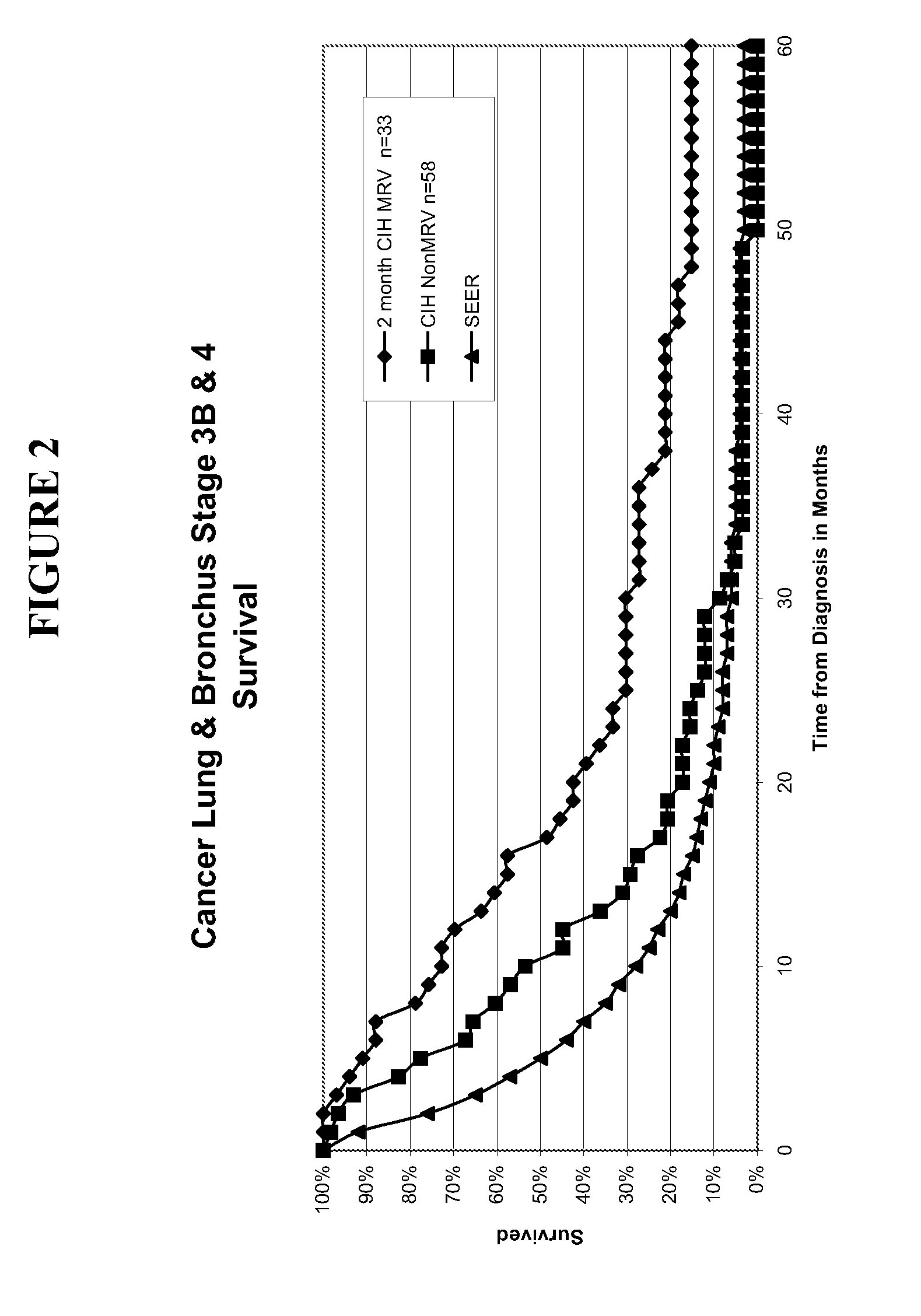
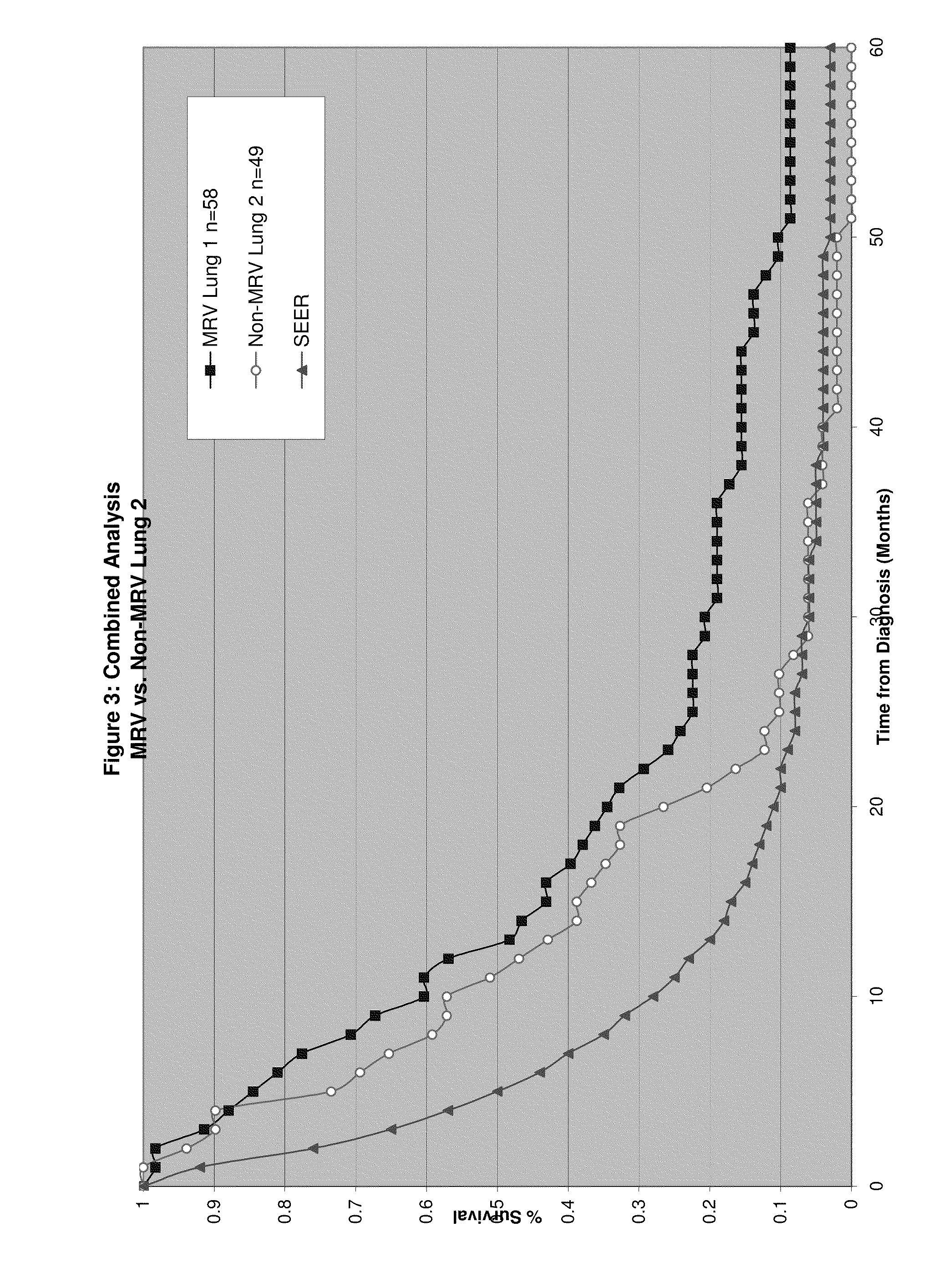
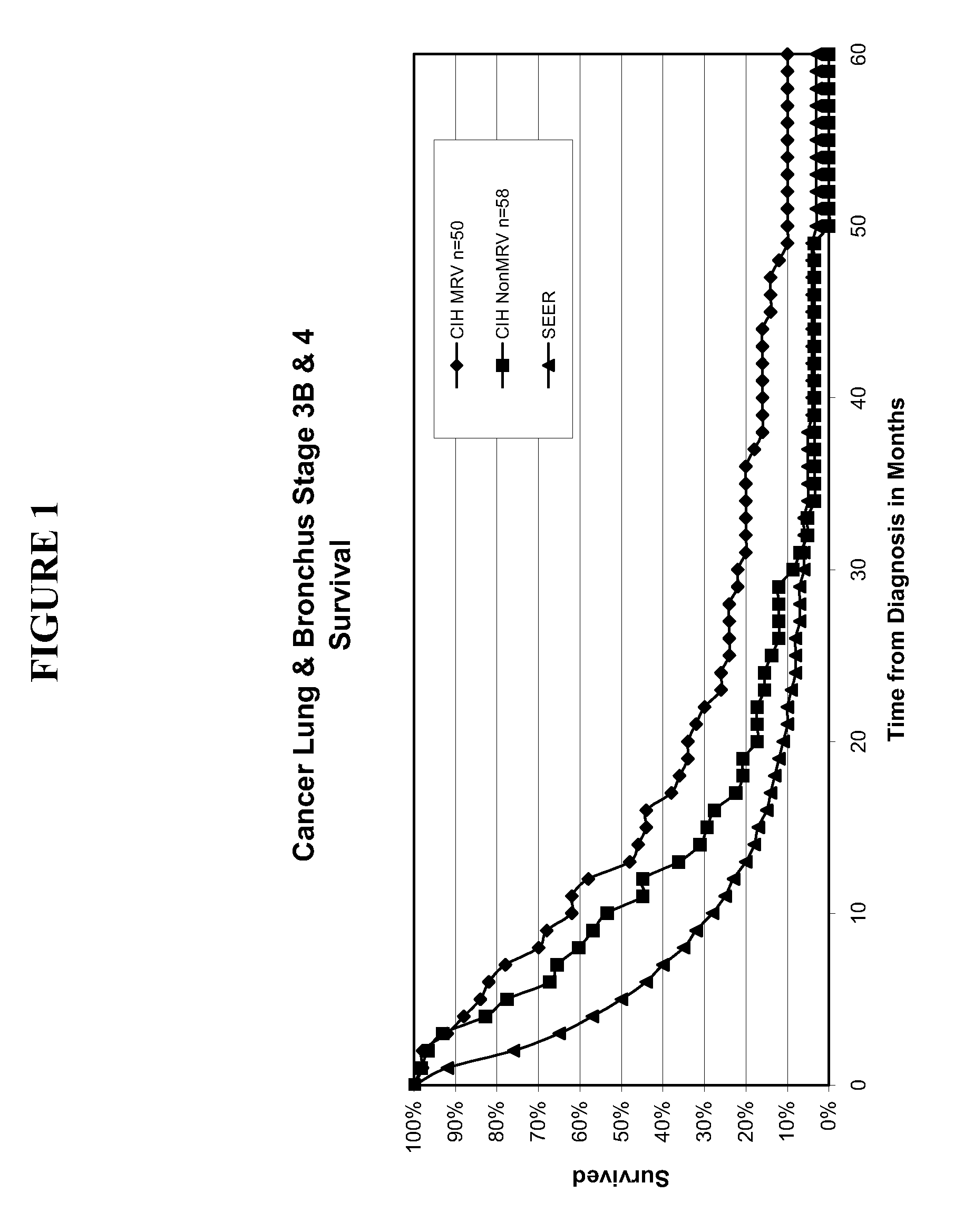
Composition and vaccine for treating lung cancer
InactiveUS20160168227A1High in proteinEffectively stimulating the (adaptive) immune systemOrganic active ingredientsTumor rejection antigen precursorsAntigenDisease
The present invention relates to a composition comprising at least one mRNA encoding a combination of antigens capable of eliciting an (adaptive) immune response in a mammal, wherein the antigens are selected from the group consisting of 5T4 (Trophoblast glycoprotein, TPBG), Survivin (Baculoviral TAP repeat-containing protein 5; BIRC5), NY-ESO-1 (New York esophageal squamous cell carcinoma 1, CTAG1B), MAGE-C1 (Melanoma antigen family C1), MAGE-C2 (Melanoma antigen family C2), and MUC1 (Mucin 1). The invention furthermore relates to a vaccine comprising at least one mRNA encoding such a combination of antigens, and to the use of said composition (for the preparation of a vaccine) and / or of the vaccine for eliciting an (adaptive) immune response for the treatment of lung cancer, preferably of non-small cell lung cancer (NSCLC), and diseases or disorders related thereto. Finally, the invention relates to kits, particularly to kits of parts, containing the composition and / or the vaccine.
Owner:CUREVAC AG
Tissue targeted antigenic activation of the immune response to cancers
ActiveUS8034359B2Inhibit growthPrevent proliferationBacterial antigen ingredientsViral antigen ingredientsTissue targetingMicroorganism
The invention provides in part methods of treating cancers of a specific organ or tissue by administering a composition that is antigenically specific for one or more microbes that are pathogenic in the specific organ or tissue in which the cancer is situated. The formulations of the invention thereby facilitate activation of a treatment response to a cancer in a particular tissue or organ. The compositions may for example include killed or attenuated microbial pathogens, and may be administered at sites distant from the cancer, for example the skin. In some embodiments, microbial species of endogenous flora that are known to cause infection in the relevant organ or tissue may be used in the formulation of the antigenic compositions. In alternative embodiments, exogenous microbial pathogens that are known to cause infection in the relevant organ or tissue may be used in the formulation of the antigenic compositions. The administration of the immunogenic compositions may be repeated relatively frequently over a relatively long period of time. In embodiments for intradermal or subcutaneous injection, dosages may be adjusted so that injections reproduce a consistent visible delayed inflammatory immune reaction at the successive site or sites of administration.
Owner:QU BIOLOGICS INC
Tissue targeted antigenic activation of the immune response to cancers
ActiveUS20090074816A1Inhibit growthPrevent proliferationBacterial antigen ingredientsViral antigen ingredientsTissue targetingMicroorganism
The invention provides in part methods of treating cancers of a specific organ or tissue by administering a composition that is antigenically specific for one or more microbes that are pathogenic in the specific organ or tissue in which the cancer is situated. The formulations of the invention thereby facilitate activation of a treatment response to a cancer in a particular tissue or organ. The compositions may for example include killed or attenuated microbial pathogens, and may be administered at sites distant from the cancer, for example the skin. In some embodiments, microbial species of endogenous flora that are known to cause infection in the relevant organ or tissue may be used in the formulation of the antigenic compositions. In alternative embodiments, exogenous microbial pathogens that are known to cause infection in the relevant organ or tissue may be used in the formulation of the antigenic compositions. The administration of the immunogenic compositions may be repeated relatively frequently over a relatively long period of time. In embodiments for intradermal or subcutaneous injection, dosages may be adjusted so that injections reproduce a consistent visible delayed inflammatory immune reaction at the successive site or sites of administration.
Owner:QU BIOLOGICS INC
Lung cancer antigen combination and application thereof, and cytotoxic T lymphocytes
ActiveCN110551198AEfficient killingTumor rejection antigen precursorsMammal material medical ingredientsPeripheral blood mononuclear cellT lymphocyte
The invention provides a lung cancer antigen combination and an application thereof, and cytotoxic T lymphocytes, and belongs to the technical field of biological medicines. The amino acid sequences of lung cancer antigens are as shown in SEQ ID NO.1-10. The lung cancer antigen combination is used for co-culturing peripheral blood mononuclear cells; and the obtained cytotoxic T lymphocytes can effectively kill cells expressing lung cancer associated antigens.
Owner:BEIJING DCTY BIOTECH CO LTD
Antibodies, pharmaceutical compositions and methods
ActiveUS20180339061A1Effective preventionEffective treatmentOrganic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsSquamous CarcinomasProstate cancer
Pharmaceutical composition comprising antibodies or antigen binding fragments thereof that bind to stage-specific embryonic antigen 4 (SSEA-4) are disclosed herein, as well as methods of use thereof. Methods of use include, without limitation, cancer therapies and diagnostics. The antibodies of the disclosure can bind to certain cancer cell surfaces. Exemplary targets of the antibodies disclosed herein can include carcinomas, such as breast cancer, lung cancer, esophageal cancer, rectal cancer, biliary cancer, liver cancer, buccal cancer, gastric cancer, colon cancer, nasopharyngeal cancer, kidney cancer, prostate cancer, ovarian cancer, cervical cancer, endometrial cancer, pancreatic cancer, testicular cancer, bladder cancer, head and neck cancer, oral cancer, neuroendocrine cancer, adrenal cancer, thyroid cancer, bone cancer, skin cancer, basal cell carcinoma, squamous cell carcinoma, melanoma, and / or brain tumor.
Owner:OBI PHARMA
Peripheral blood TCR marker of lung cancer, and detection kit and application thereof
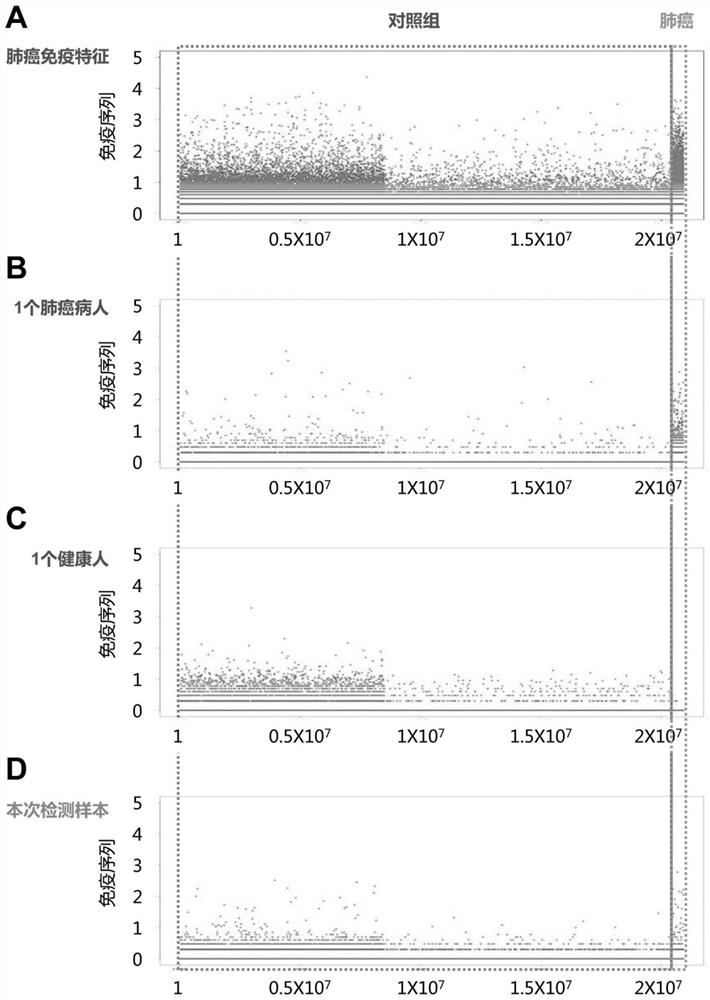
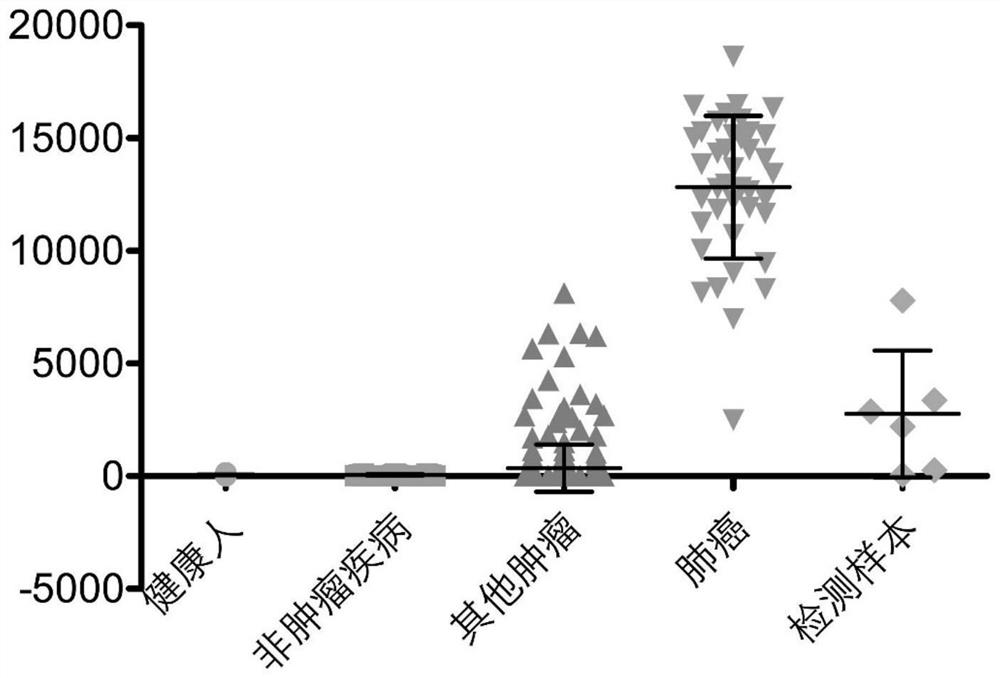
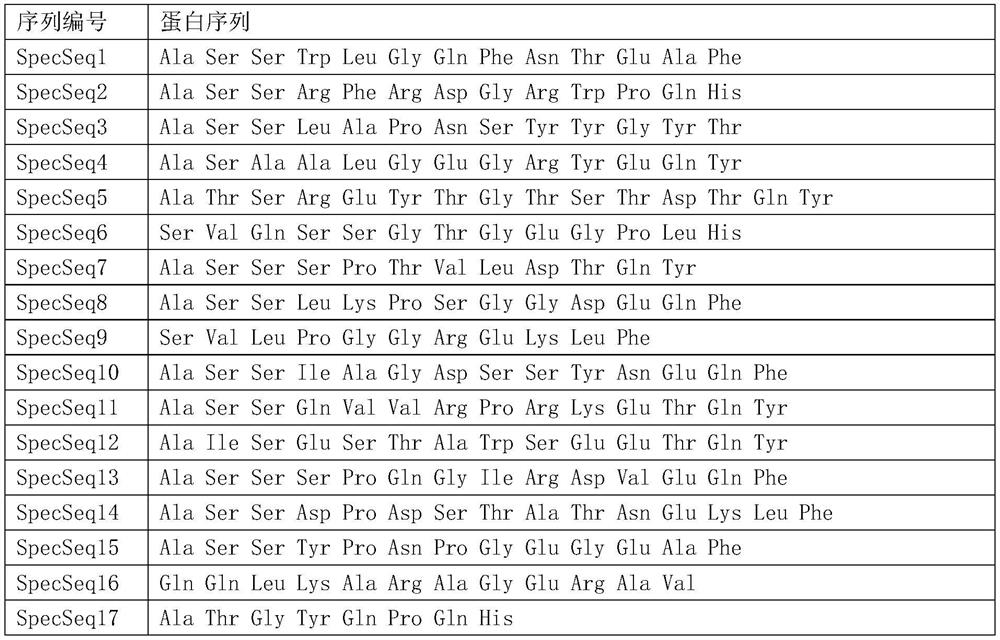
PendingCN111665359AImprove featuresImprove accuracyBiological material analysisLung cancer vaccineHigh throughput sequenceBioinformatics
The invention discloses a peripheral blood TCR marker of a lung cancer, and a detection kit and an application thereof. The marker comprises at least one of proteins as shown in a sequence SpecSeq1-100. Based on a high-throughput sequencing method, only a small amount of peripheral blood needs to be adopted, RNA needs to be extracted, an immune map library is established by processing a sample, acharacteristic TCR sequence in the lung cancer peripheral blood is determined firstly through high-throughput sequencing and TCR data analysis, and then a test result of a sample to be tested is compared with the characteristic TCR sequence so that whether a lung cancer exists or not is determined. The marker, the kit and the application can be used to simultaneously compare a huge number of lungcancer specific TCR sequences, have higher specificity and accuracy compared with single detection of one or more markers, and improve diagnosis efficiency.
Owner:CHENGDU EXAB BIOTECH CO LTD
Chitosan-Derived Compositions
InactiveUS20190002594A1Organic active ingredientsPhotodynamic therapyMedical disorderKidney Neoplasm
The present invention relates generally to therapeutic compositions comprising chitosan-derived compositions used in connection with methods for treating neoplasms, such as for instance, malignant lung, thyroid and kidney neoplasms, and other types of malignant neoplasms, and other medical disorders.
Owner:IMMUNOPHOTONICS INC
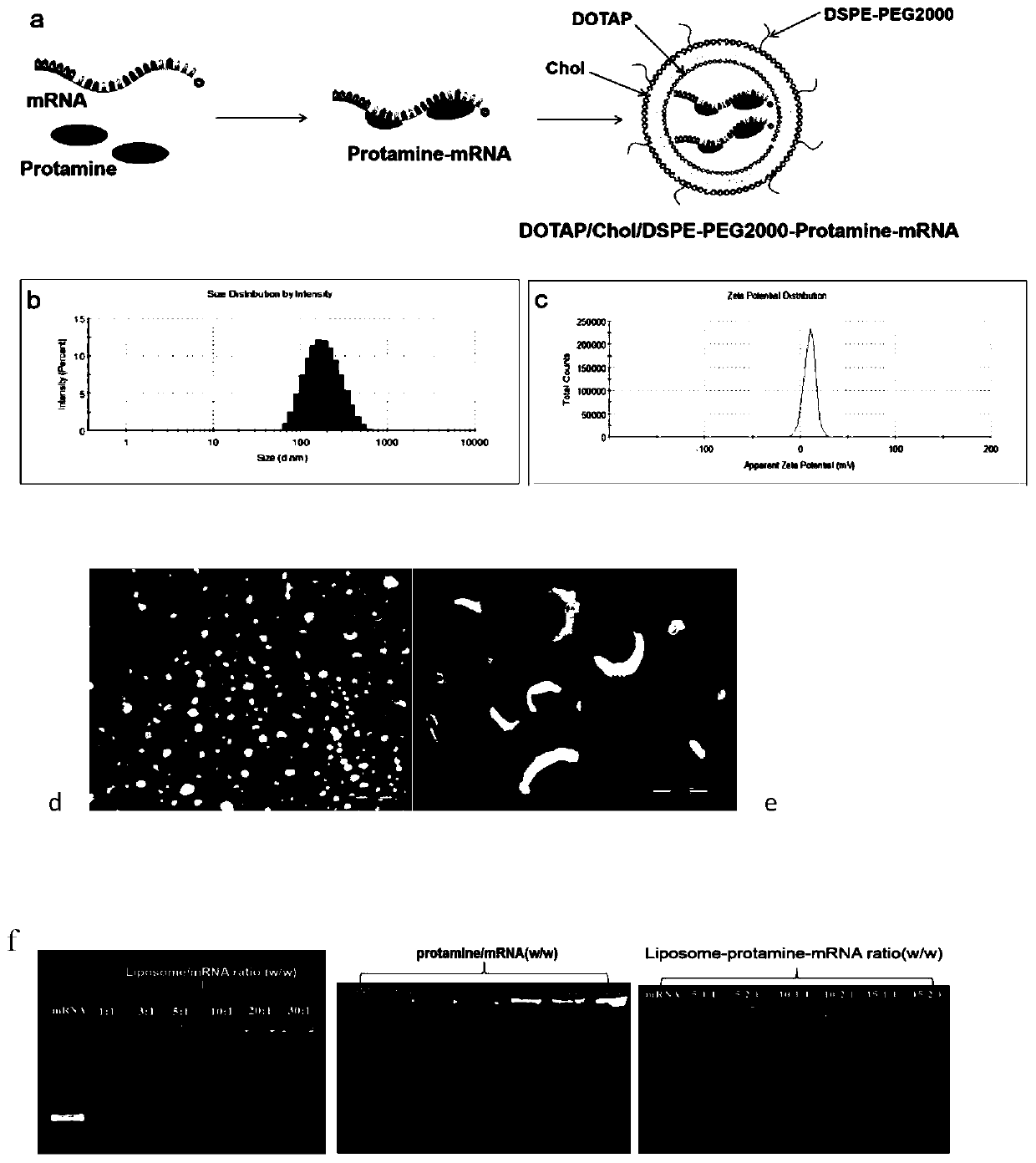
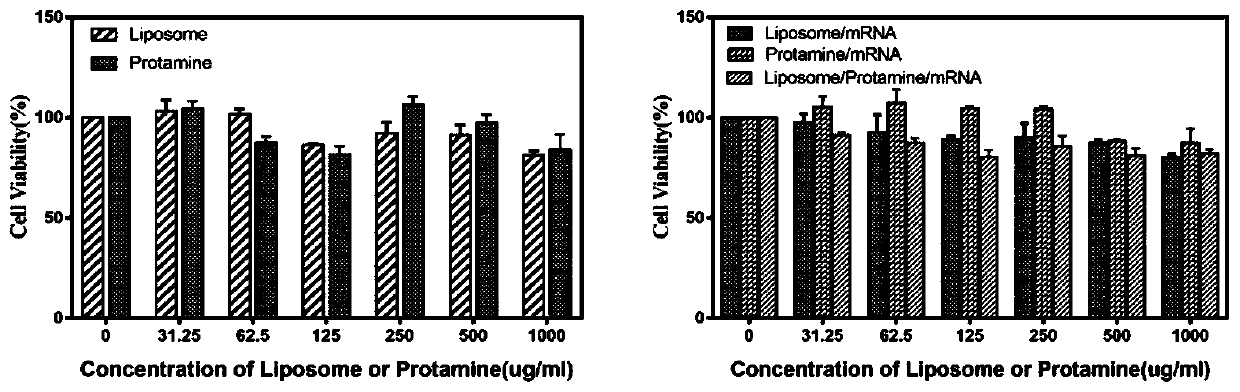
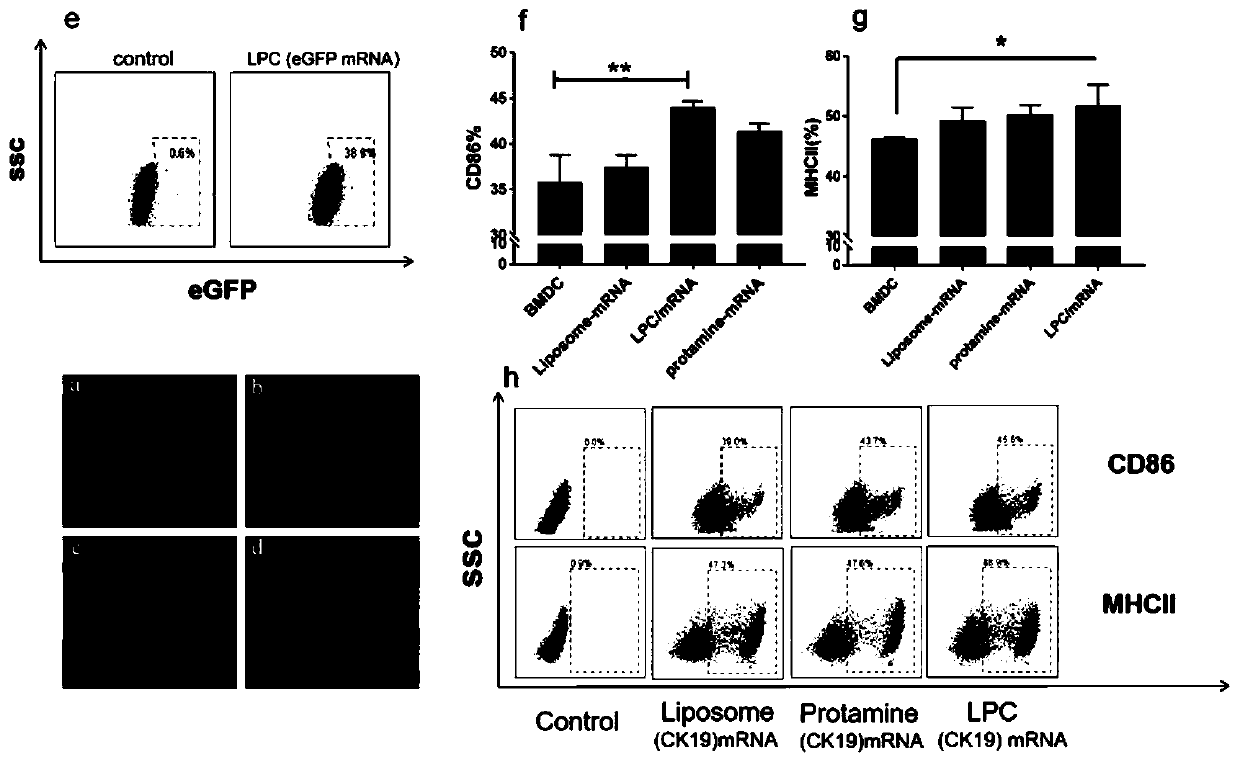
Cationic liposome-protamine-mRNA tumor vaccine and preparation method and application method thereof
PendingCN110882383ASynthesis fastRapid purificationSkin cancer vaccineCancer antigen ingredientsAdjuvantTumor therapy
The invention relates to a cationic liposome-protamine-mRNA tumor vaccine and a preparation method and an application method thereof. According to the tumor vaccine, a cationic liposome-protamine compound is used as a delivery carrier and adjuvant of mRNA, wherein the mass ratio of the cationic liposome to protamine to mRNA is 5:1: 1 to 15: 2:1, and the optimal mass ratio is preferably 10:1:1; theparticle size of the constructed cationic liposome-protamine-mRNA tumor vaccine is 50 to 800nm; the Zeta potential is 10 to 60mv; and the encapsulation efficiency is 70% to 95%. The cationic liposome-protamine-mRNA tumor vaccine prepared by the invention can effectively promote antigen uptake of APCs, induce DC stimulation and maturation, promotes secretion of cytokines and cause anti-tumor immune response. The tumor vaccine is used for immunotherapy through intramuscular and subcutaneous injection or nasal mucosa administration, wherein nasal mucosa administration can show a more excellent tumor treatment effect. The tumor vaccine has a wide application prospect in the aspect of tumor treatment.
Owner:NINGXIA MEDICAL UNIV
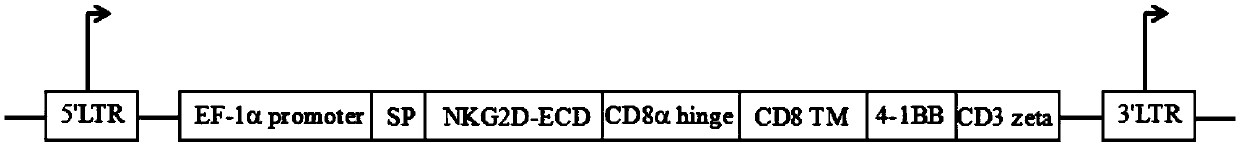
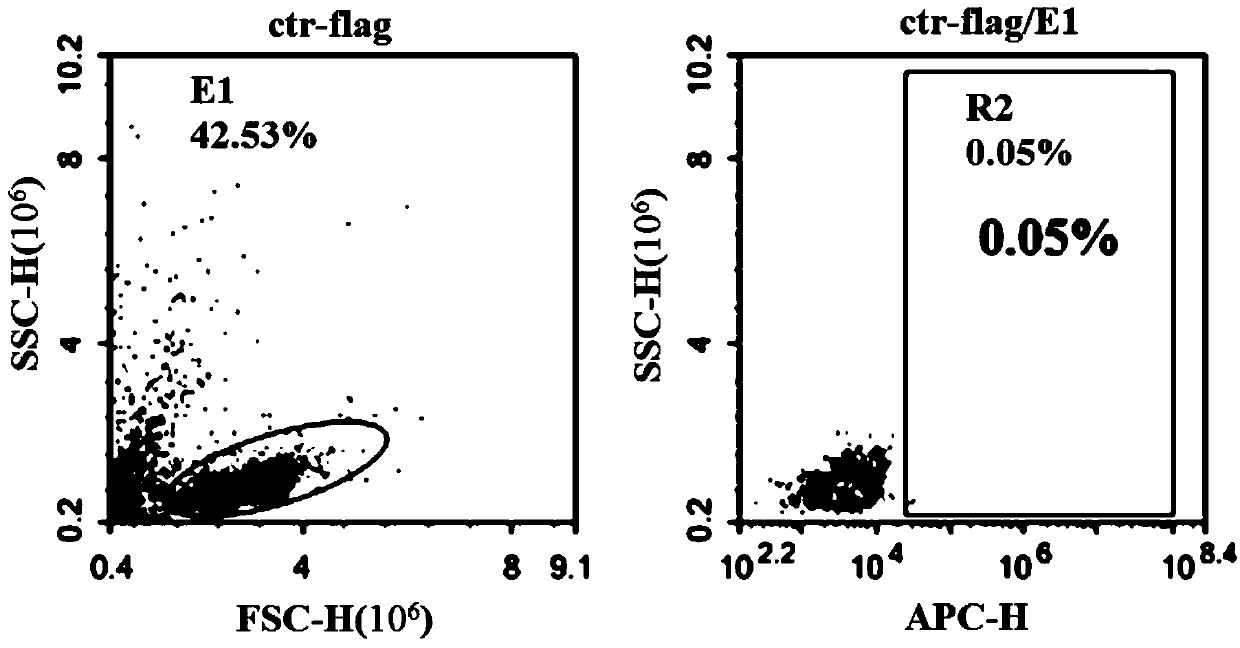
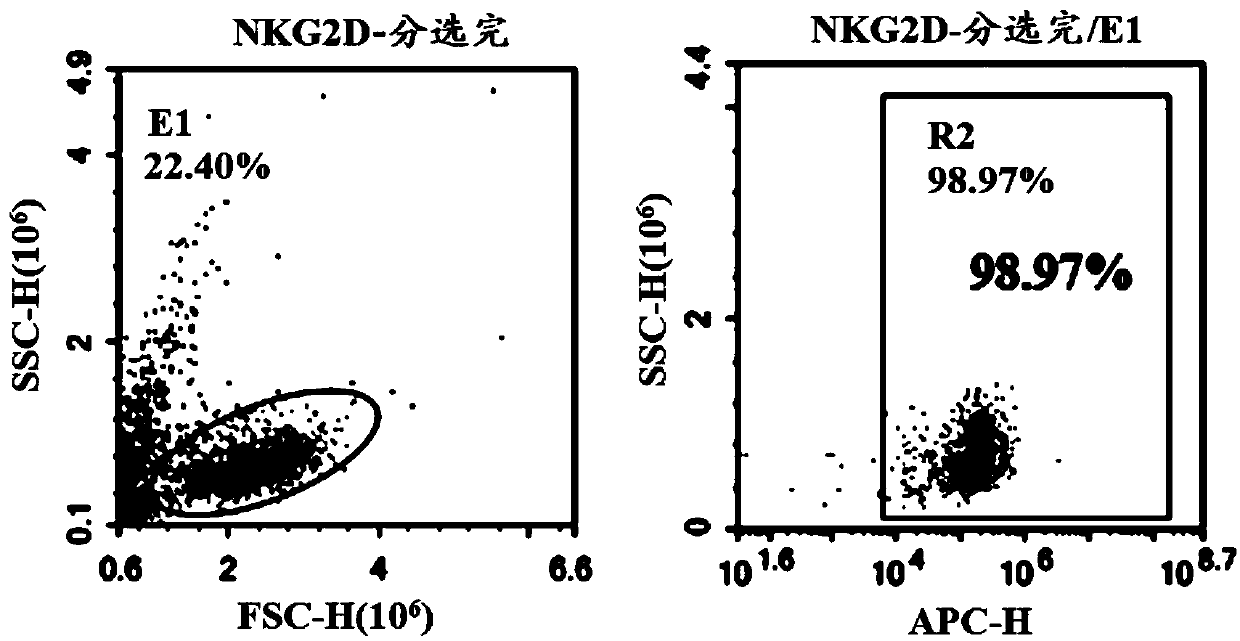
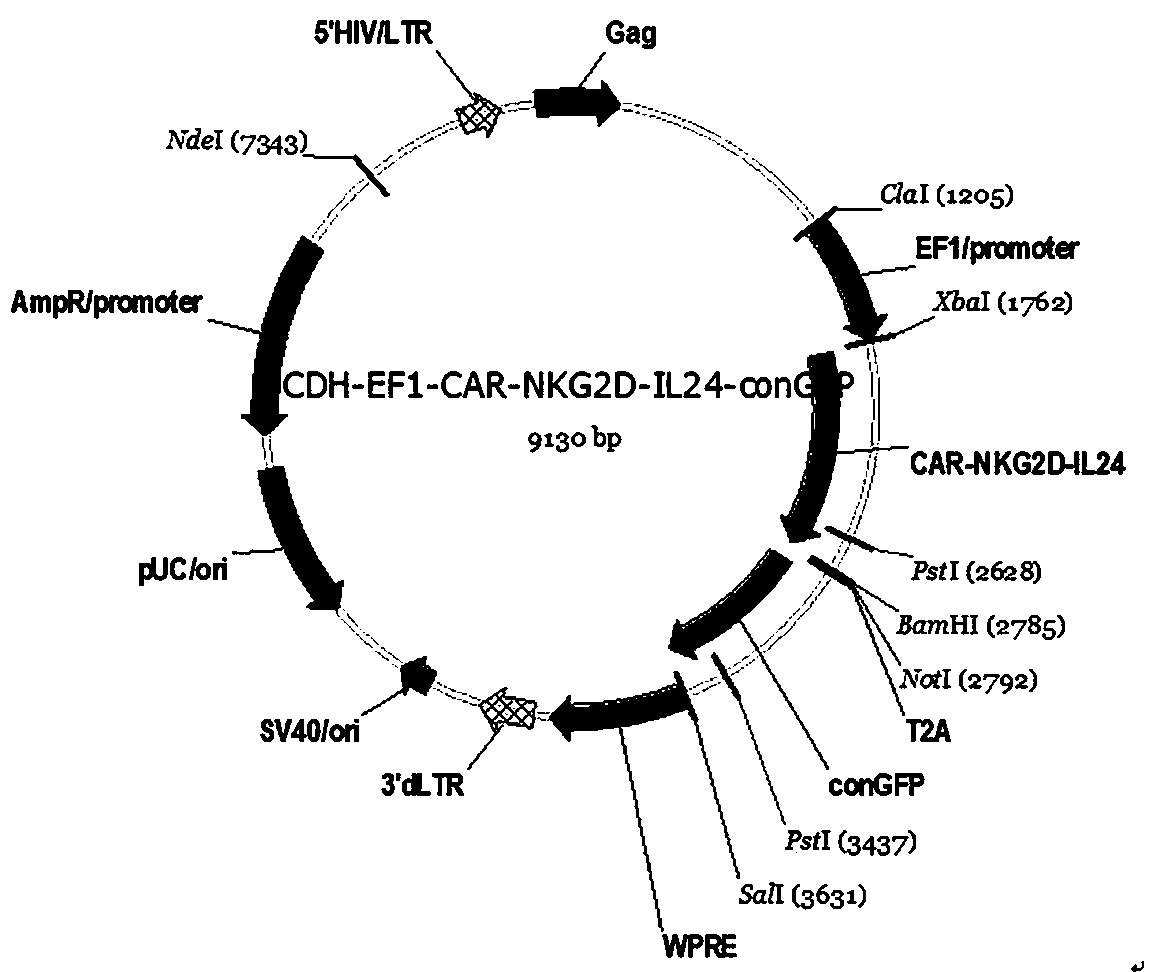
Chimeric antigen receptor, NKG2D CAR-NK cell expressing chimeric antigen receptor, preparation method and application thereof
ActiveCN110028589AImprove anti-tumor effectExcellent anti-tumor performanceVirusesAntibody mimetics/scaffoldsDiseaseNkg2d ligands
The invention provides a chimeric antigen receptor, a NKG2D CAR-NK cell expressing the chimeric antigen receptor, a preparation method and an application thereof. The chimeric antigen receptor comprises an antigen binding domain, a transmembrane domain, and a costimulatory signaling domain, and the antigen binding domain is capable of specifically binding to a tumor specific antigen NKG2D ligand and activating NK cells through the transmembrane domain and the costimulatory signaling domain. The CAR-NK cell uses the NKG2D ligand as a target antigen and specifically uses the NKG2D CAR-NK cells to kill tumor cells. The cell can be used as a therapeutic drug for tumor diseases for the treatment of tumors with high expression of the ligand of NKG2D molecules, and provides a novel method for tumor prevention and treatment.
Owner:ASCLEPIUS SUZHOU TECH CO GRP CO LTD
Microsphere lyophilized preparation coupled with proteins and preparation method and preservation mode thereof
ActiveCN111110638AImprove stabilityImprove versatilityPowder deliveryAntibody ingredientsMicrospherePyrrolidinones
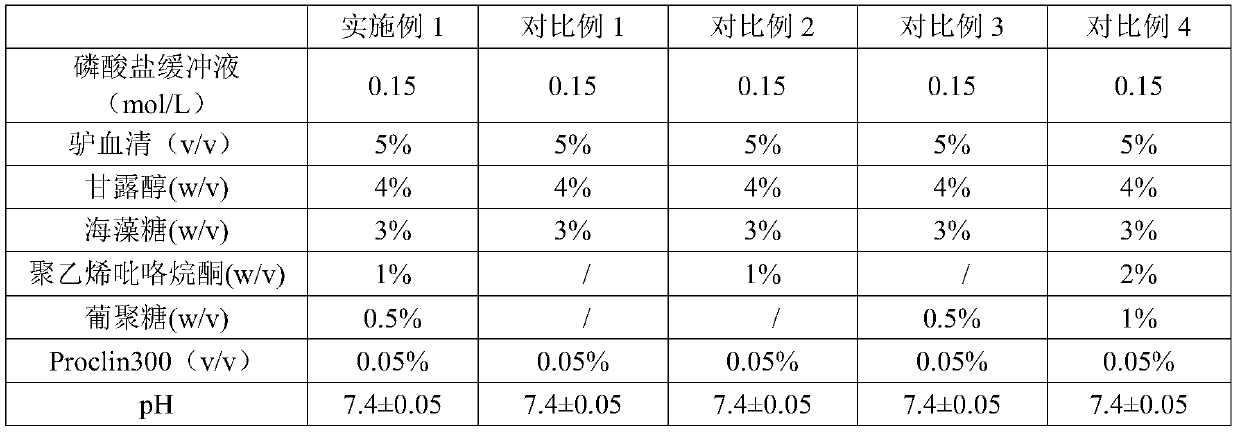
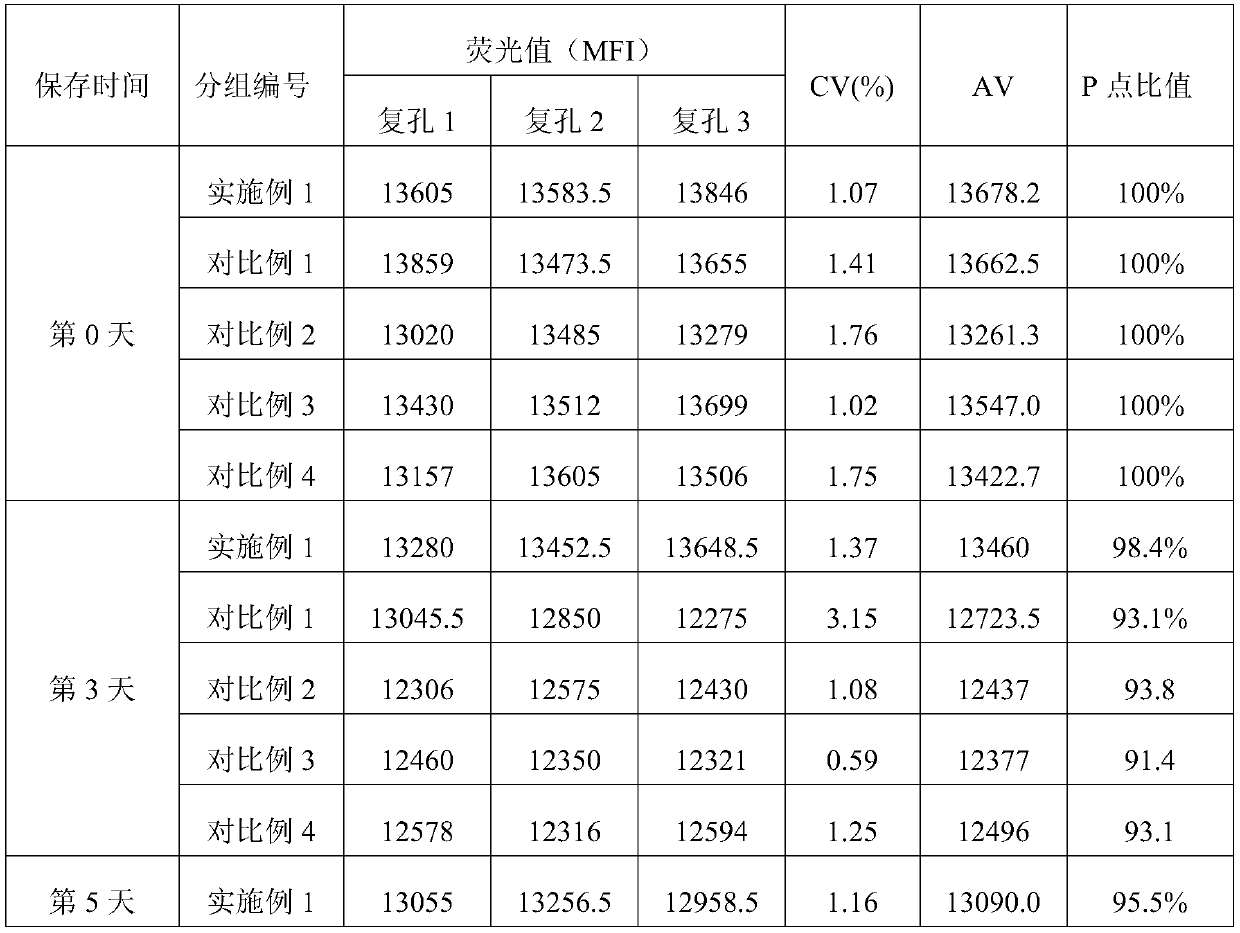
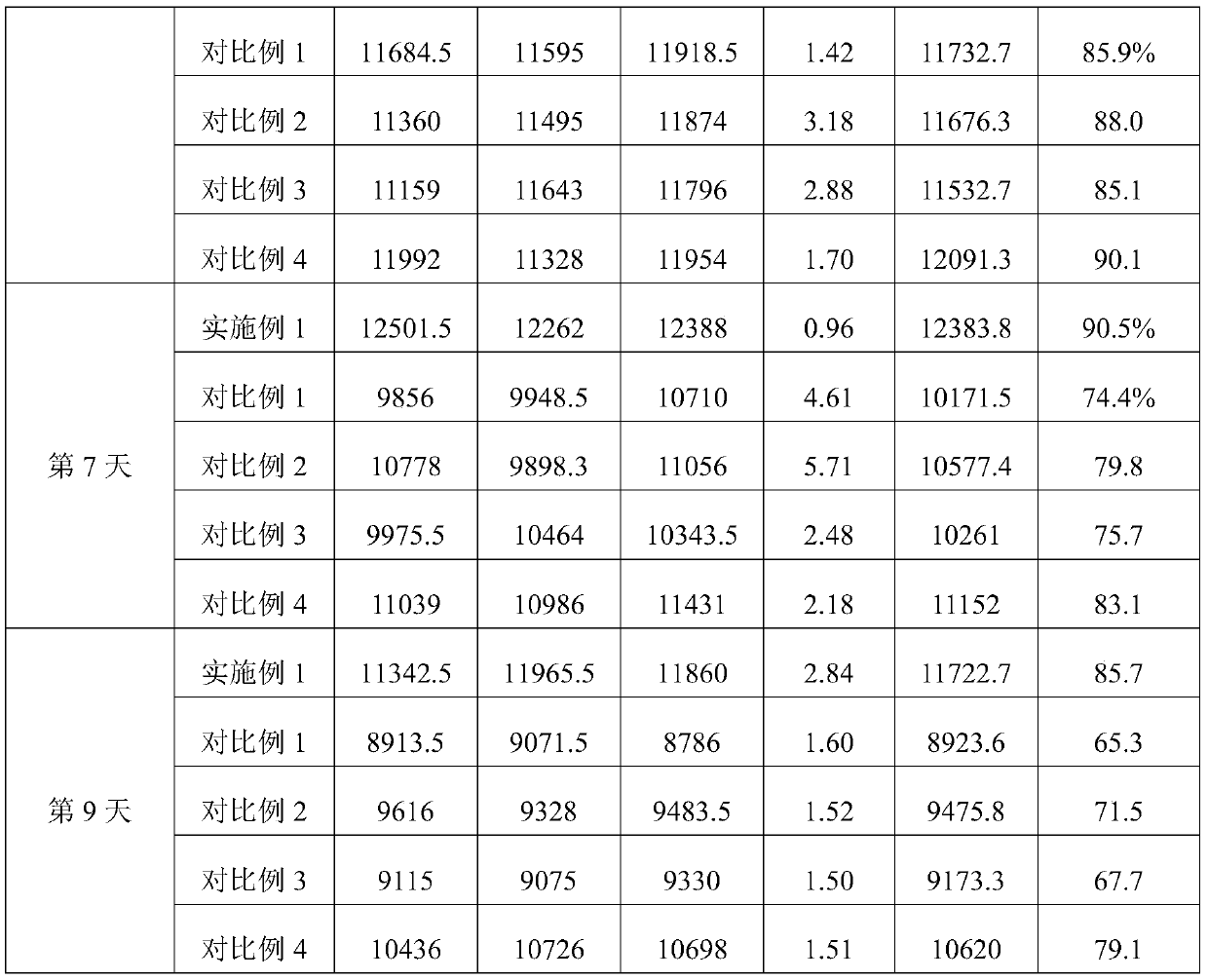
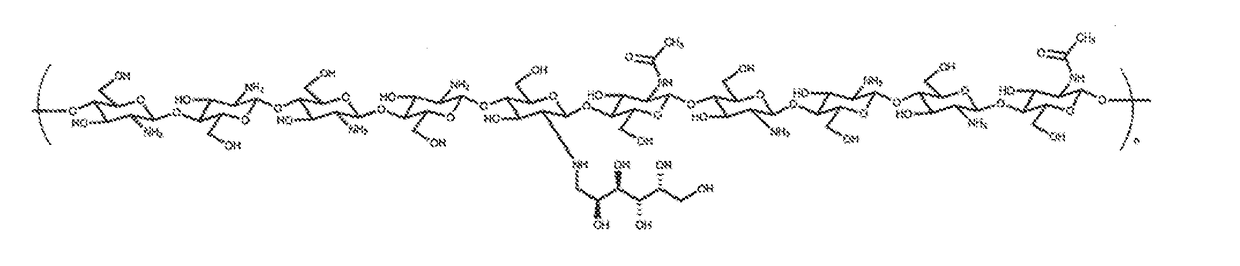
The invention discloses a microsphere lyophilized preparation coupled with proteins and a preparation method and a preservation mode thereof. The microsphere lyophilized preparation comprises a lyoprotectant and microspheres coupled with the proteins; the lyoprotectant comprises a solvent and a solute; the solvent is phosphate buffer solution with concentration of 0.1 to 0.2mol / L; by volume of thesolvent, the solute comprises 1 to 10% (v / v) of animal serum, 1 to 10% (w / v) of trehalose, 0.2 TO 5% (w / v) of polyvinylpyrrolidone, 0.2 to 5% (w / v) of glucan, 3% to 10% (w / v) of mannitol and 0.01 to0.1% (v / v) of Proclin300; a pH value of the lyoprotectant is 7.4 plus and minus 0.5; the microsphere lyophilized preparation coupled with the proteins is obtained by carrying out lyophilization aftermixing the lyoprotectant and the microspheres coupled with the proteins. By the lyophilized preparation disclosed by the invention, stability of the microspheres coupled with the proteins can be obviously improved.
Owner:GUANGZHOU BIO BLUE TECH CO LTD
Chitosan-Derived Compositions
The present invention relates generally to therapeutic compositions comprising chitosan-derived compositions used in connection with methods for treating neoplasms, such as for instance, malignant lung, thyroid and kidney neoplasms, and other types of malignant neoplasms, and other medical disorders.
Owner:IMMUNOPHOTONICS INC
A Composition, A Treatment Method and An Application Thereof
InactiveUS20190015458A1Good effectImprove efficiencyColon cancer vaccineProtozoa material medical ingredientsMelanomaTreatment field
The present invention relates to the field of treatment of tumor, and especially to a composition comprising a plasmodium, a treatment method and an application thereof. The composition of the present invention has therapeutic effects on colorectal carcinoma, lung carcinoma, breast carcinoma, gastric carcinoma and hepatic carcinoma etc., can inhibit the growth of tumor and prolong the life of the tumor patients, whereas has no therapeutic effect on melanoma and lymphoma; meanwhile, the present invention describes that the long-term plasmodium infection has better therapeutic effect on tumors, and the plasmodium immunotherapy of the present invention does not take the fever time as a course standard when treating tumors, but should be used to extend the duration of plasmodium infection as much as possible until the progression of tumors can be controlled under the premise of protecting the organ functions and life safety of the patients.
Owner:BLUE ELEGANT BIOTECH CO LTD
Specific chimeric antigen receptor T cell targeting nkg2dl, preparation method and application thereof
ActiveCN109803983BEffective targeted attackHigh kill rateVirusesAntibody mimetics/scaffoldsAbnormal tissue growthAntigen receptors
A specific chimeric antigen receptor targeting NKG2DL, its coding sequence, and modified immune response cells, as well as their preparation method and application. The modified immune response cells can effectively target and attack various tumor cells, especially positive tumor cells expressing NKG2DL, and can be used to prepare preparations for treating tumors.
Owner:NANJING KAEDI BIOTHERAPEUTICS LTD
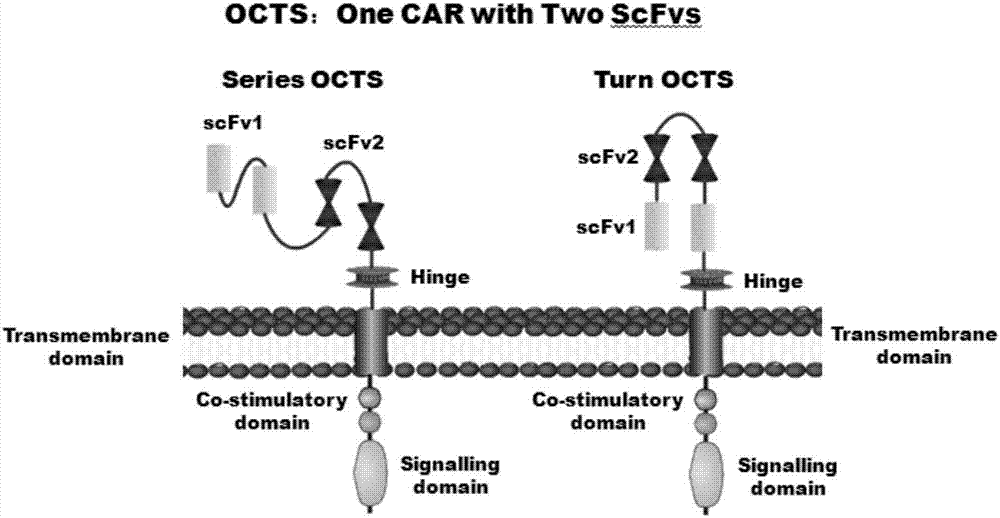
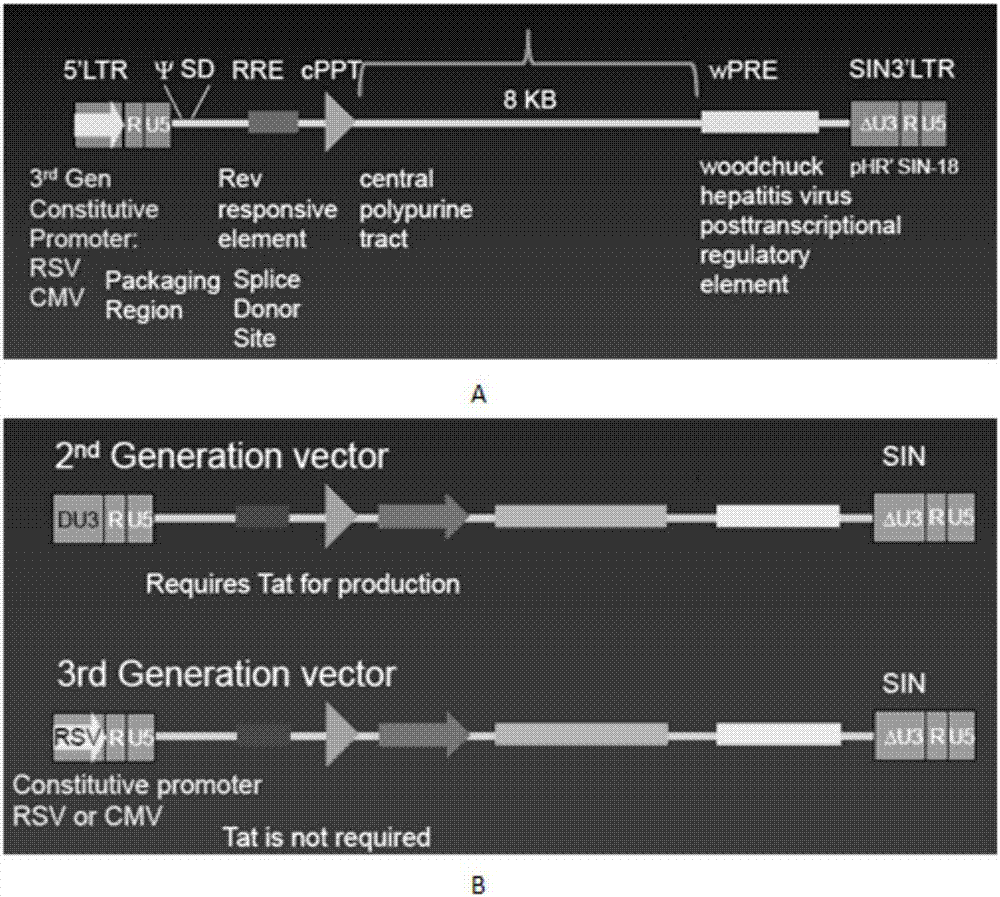
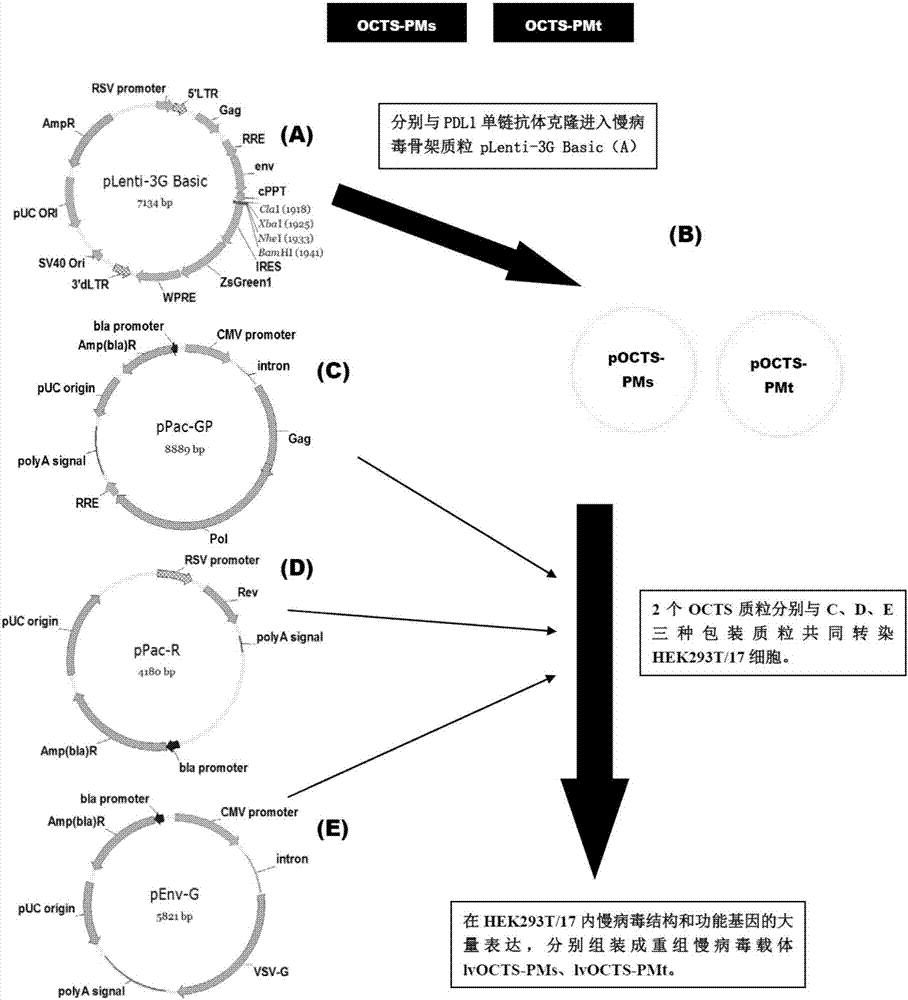
OCTS technology-based pancreatic cancer and malignant mesothelioma CAR-T therapy vector, as well as construction method and applicaitno thereof
ActiveCN107299110AExpand the scope of recognitionAvoid batch cultureVirusesPeptide/protein ingredientsSingle-Chain AntibodiesCD8
The invention discloses an OCTS technology-based pancreatic cancer and malignant mesothelioma CAR-T therapy vector. The vector comprises lentivirus framework plasmid, a human EF1alpha promoter (SEQ ID NO. 14), an OCTS chimeric receptor structural domain and a PDL1 single-chain antibody; the OCTS chimeric receptor structural domain comprises CD8 leader chimeric receptor signal peptide (SEQ ID NO. 15), a PDL1 single-chain antibody light chain VL (SEQ ID NO. 16), a PDL1 single-chain antibody heavy chain VH (SEQ ID NO. 17), an MESOTHELIN single-chain antibody light chain VL (SEQ ID NO. 18), an MESOTHELIN single-chain antibody heavy chain VH (SEQ ID NO. 19), an antibody inner linker Inner-Linker (SEQ ID NO. 20), a single-chain antibody inter-linker Inter-Linker (SEQ ID NO. 21), a CD8 Hinge chimeric receptor linker (SEQ ID NO. 22), a CD8Transmembrane chimeric receptor transmembrane domain (SEQ ID NO. 23), a TCR chimeric receptor T-cell activation domain (SEQ ID NO. 26), and a chimeric receptor inducible co-stimulater area. In addition, the invention also discloses a construction method of the vector as well as application of the vector to preparation of medicines for treating pancreatic cancer and malignant mesothelioma.
Owner:SHANGHAI UNICAR THERAPY BIOPHARM TECH CO LTD
USE OF IL-1beta BINDING ANTIBODIES
InactiveUS20190048072A1Improve treatmentOrganic active ingredientsIntestine cancer vaccineAntigen bindingGevokizumab
Owner:NOVARTIS AG +3
Double-targeting chimeric antigen receptor, encoding gene and recombinant expression vector
PendingCN113248619AHigh precisionGood treatment effectVirusesAntibody mimetics/scaffoldsSingle-Chain AntibodiesAntigen binding
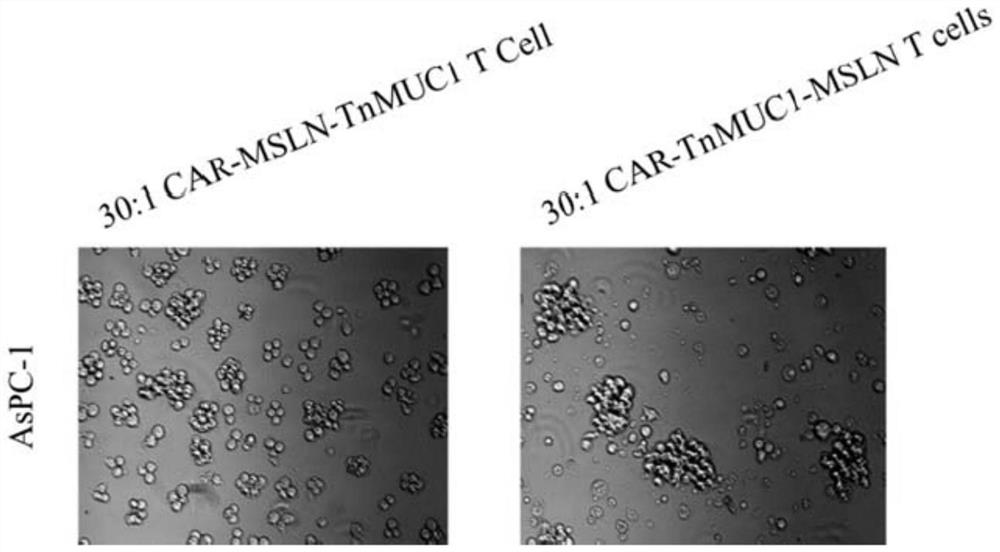
The invention discloses a double-targeting chimeric antigen receptor, which comprises a double-antigen binding region, a first hinge region, a chimeric antigen receptor T cell activation domain and a protein co-expression self-cleavage domain which are sequentially connected in series, and the double-antigen binding region comprises a heavy chain VH and a light chain VL of an MSLN and MUC1 single-chain antibody which are connected in series, and a second hinge region connected with the MSLN and the MUC1 single-chain antibody. The double-targeting CAR-T cell can kill tumor cells expressing double antigens, killing is more accurate and efficient, meanwhile, the off-target effect is reduced or avoided, the side effect of non-specific killing is reduced, and the anti-tumor effect is enhanced.
Owner:上海帝鹤思医疗科技有限公司
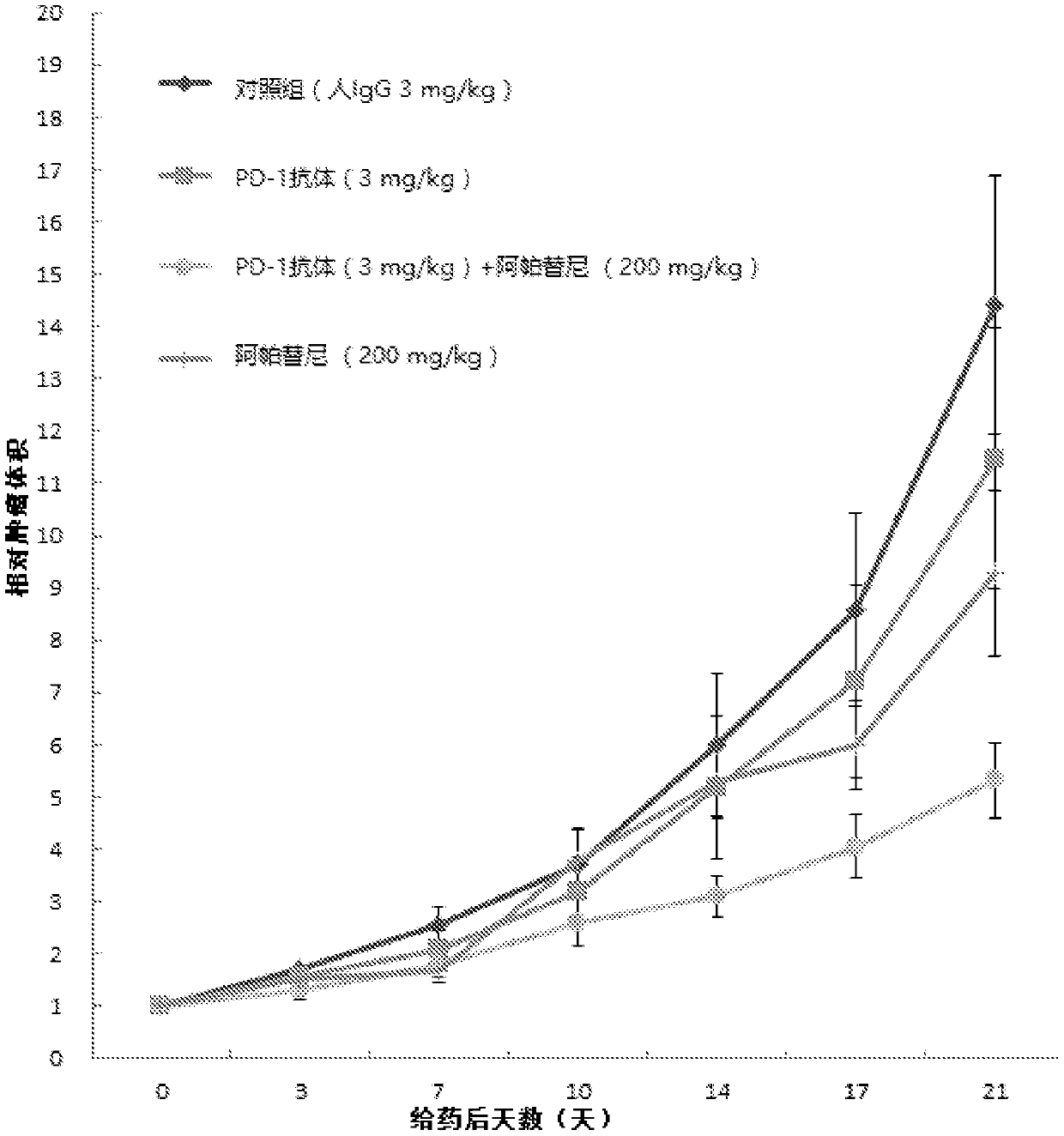
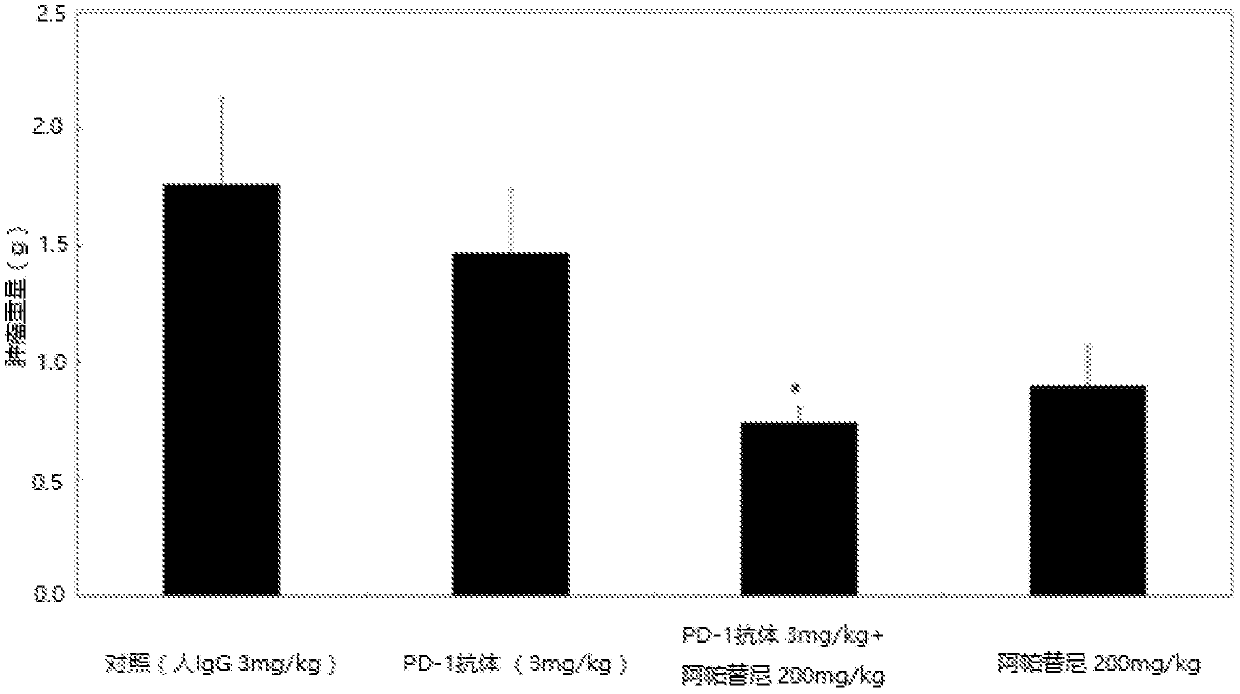
Use of an anti-PD-1 antibody combined with a vegfr inhibitor in the preparation of a drug for treating cancer
ActiveCN108601831BOrganic active ingredientsPharmaceutical delivery mechanismCancer researchVEGFR Inhibitor
The invention discloses the use of an anti-PD‑1 antibody combined with a VEGFR inhibitor in the preparation of a drug for treating cancer.
Owner:SUZHOU SUNCADIA BIOPHARM CO LTD +2
Tumor treatment drug
ActiveCN111467489AAdaptableHigh cure rateSsRNA viruses negative-senseVirus peptidesTumor therapyTherapeutic effect
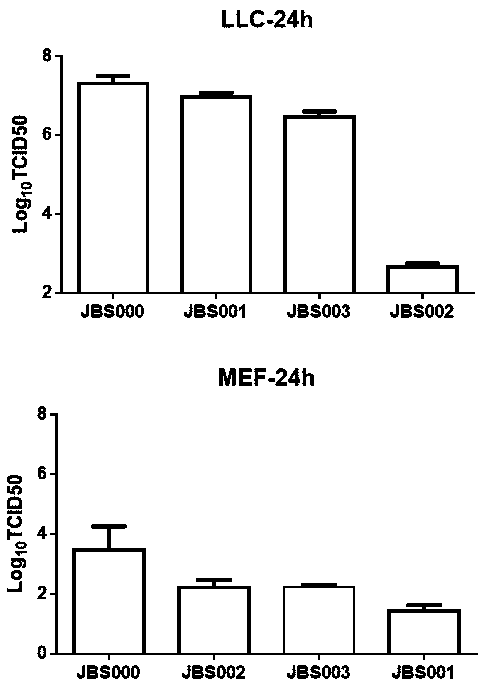
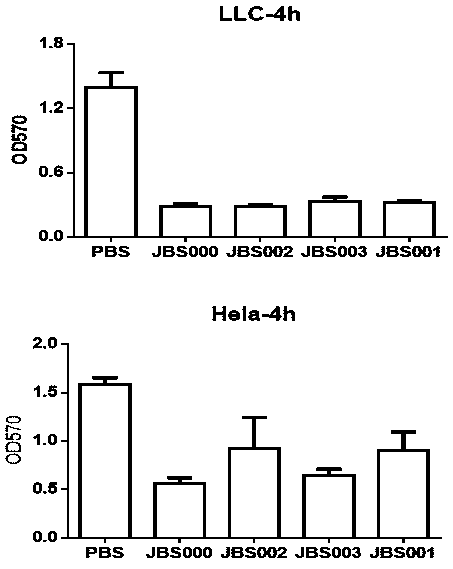
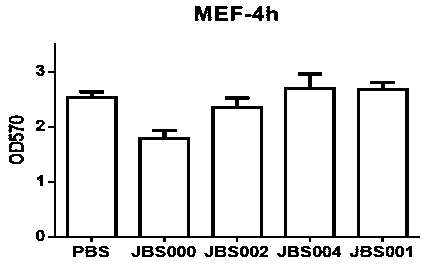
The invention belongs to the field of biotechnology, and particularly relates to an oncolytic virus vaccine and a tumor treatment drug combing oncolytic virus vaccine with an immune checkpoint inhibitor. A brand-new oncolytic virus attenuated strain is provided by site-directed mutagenesis of wild-type virus matrix protein M of VSV (vesicular stomatitis virus). The attenuated strain can be used asa drug alone to treat tumors, and has safety and cure rate higher than those of wild-type viruses and other known attenuated strains. On the basis of the oncolytic virus attenuated strain, a vaccinethat can be used in tumor treatment is also provided by inserting NY-ESO-1 into the attenuated strain. The vaccine has high cure rate and high biological safety. On the basis of the vaccine, the invention also combines the vaccine with the immune checkpoint inhibitor to provide the drug capable of efficiently treating various tumors. In mouse lung cancer models, the cure rate can be astonishing, up to 87.5%, and the treatment effect on large tumors is also good.
Owner:JOINT BIOSCIENCES (SH) LTD
Double-chimeric antigen receptor, T cell and construction method and application thereof
PendingCN110669138AToxicityImprove securityImmunoglobulins against cell receptors/antigens/surface-determinantsBlood/immune system cellsTumor antigenSialyl tn
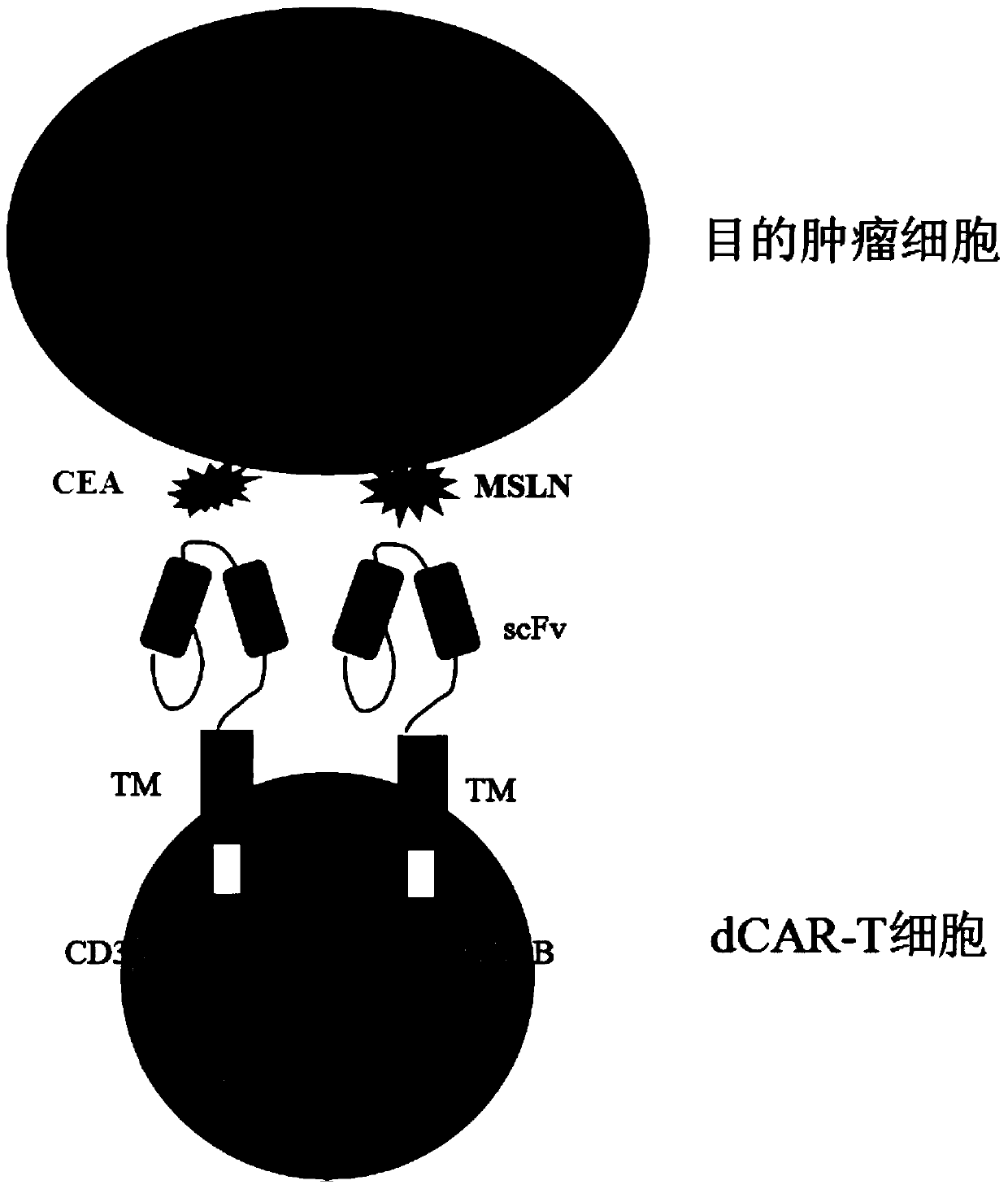
The invention discloses a double-chimeric antigen receptor, a T cell and a construction method and an application thereof, which belong to the field of cellular immunotherapy of tumors. The inventionspecifically relates to a specific structure and a construction method of the double chimeric antigen receptor T cell (dCAR-T cell), and preliminarily discusses the in-vivo and in-vitro activity of the dCAR-T cell. The selected tumor-associated antigens are mesothelin and carcino-embryonic antigens, and researches show that the two tumor antigens can be simultaneously expressed on the surface of asolid tumor, such as pancreatic cancer. The invention discloses an antigen receptor. The in-vitro and in-vivo tests prove that the constructed dCAR-T cell can be permanently and effectively activatedonly under the condition that two antigens are simultaneously recognized, and has efficient anti-tumor activity, so that a specific tumor killing function can be exerted, and the application of CAR-Tcell immunotherapy is improved.
Owner:CHINA PHARM UNIV
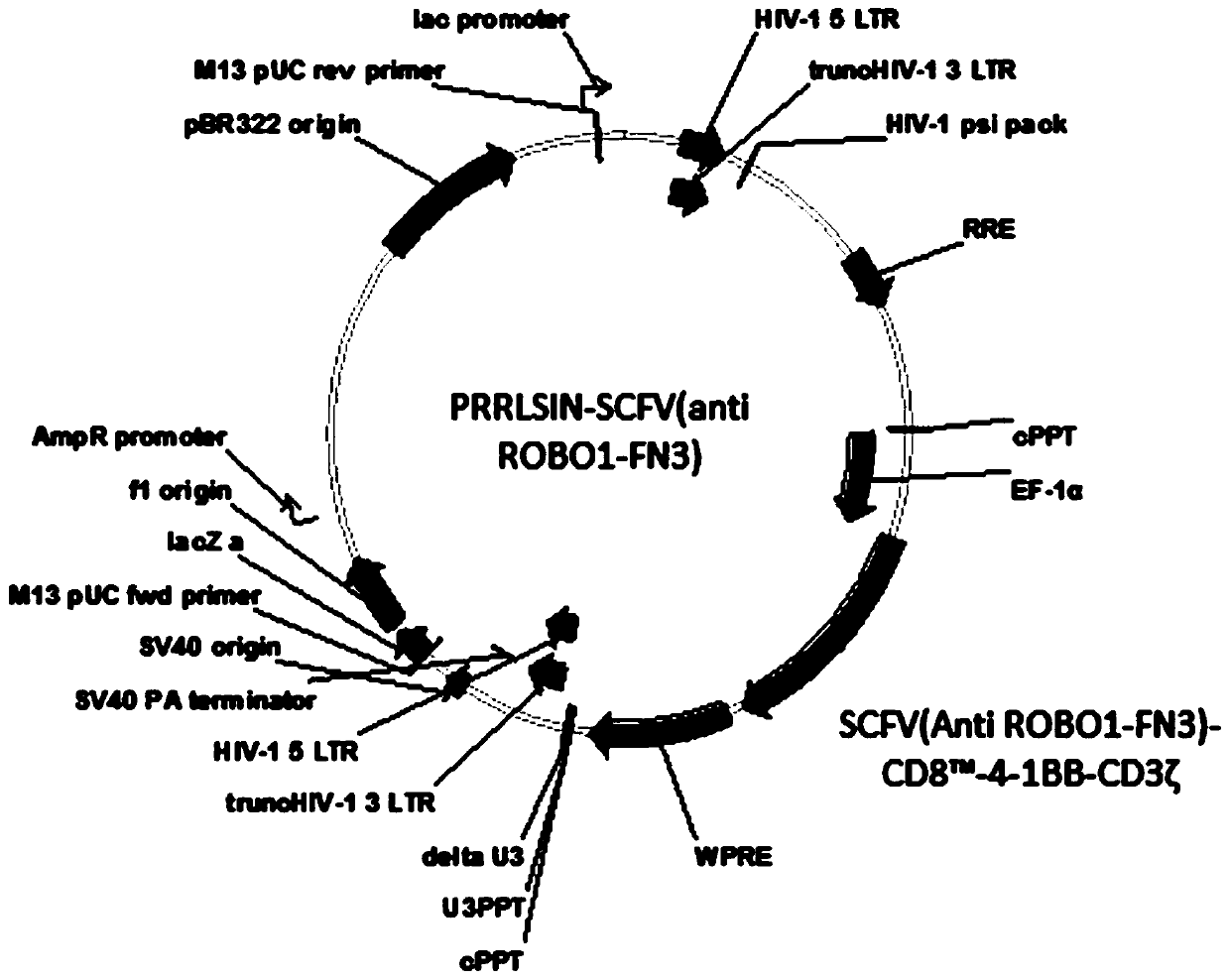
ROBO1 CAR-NK cell carrying suicide gene as well as preparation method and application of ROBO1 CAR-NK cell
PendingCN111269925ASmall side effectsGood killing effectVirusesHydrolasesNatural Killer Cell Inhibitory ReceptorsROBO1
The invention discloses an ROBO1 CAR-NK cell carrying a suicide gene as well as a preparation method and application of the ROBO1 CAR-NK cell. In order to improve the safety and controllability of theCAR-NK therapy, a suicide gene switch element is integrated into a genome through a lentiviral transfection technology on the basis of the current ROBO1 CAR-NK cell to form the CAR-NK with the suicide gene. By adding the suicide gene, the CAR-NK cells can be better controlled, and the clinical safety is further improved.
Owner:ASCLEPIUS SUZHOU TECH CO GRP CO LTD
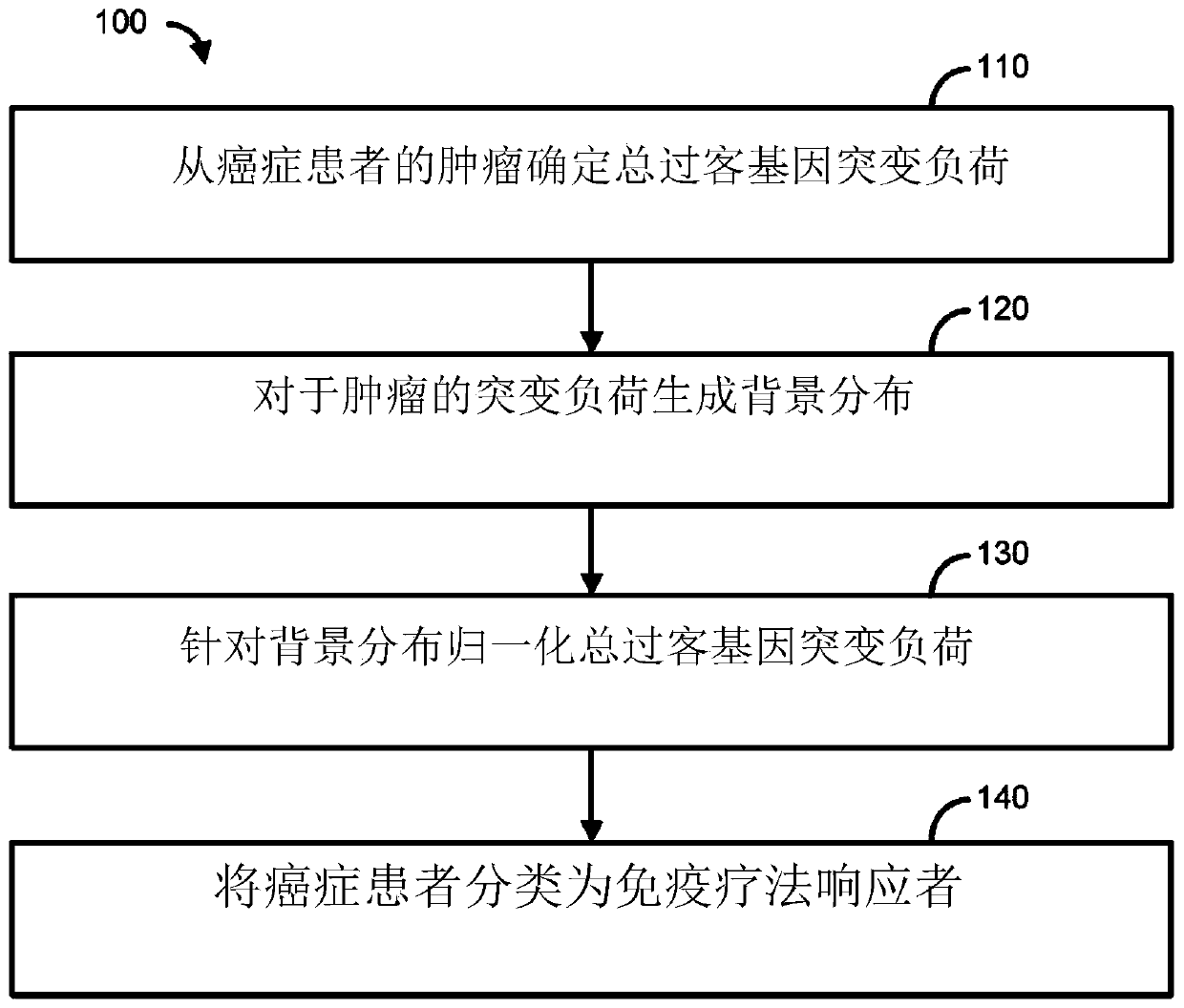
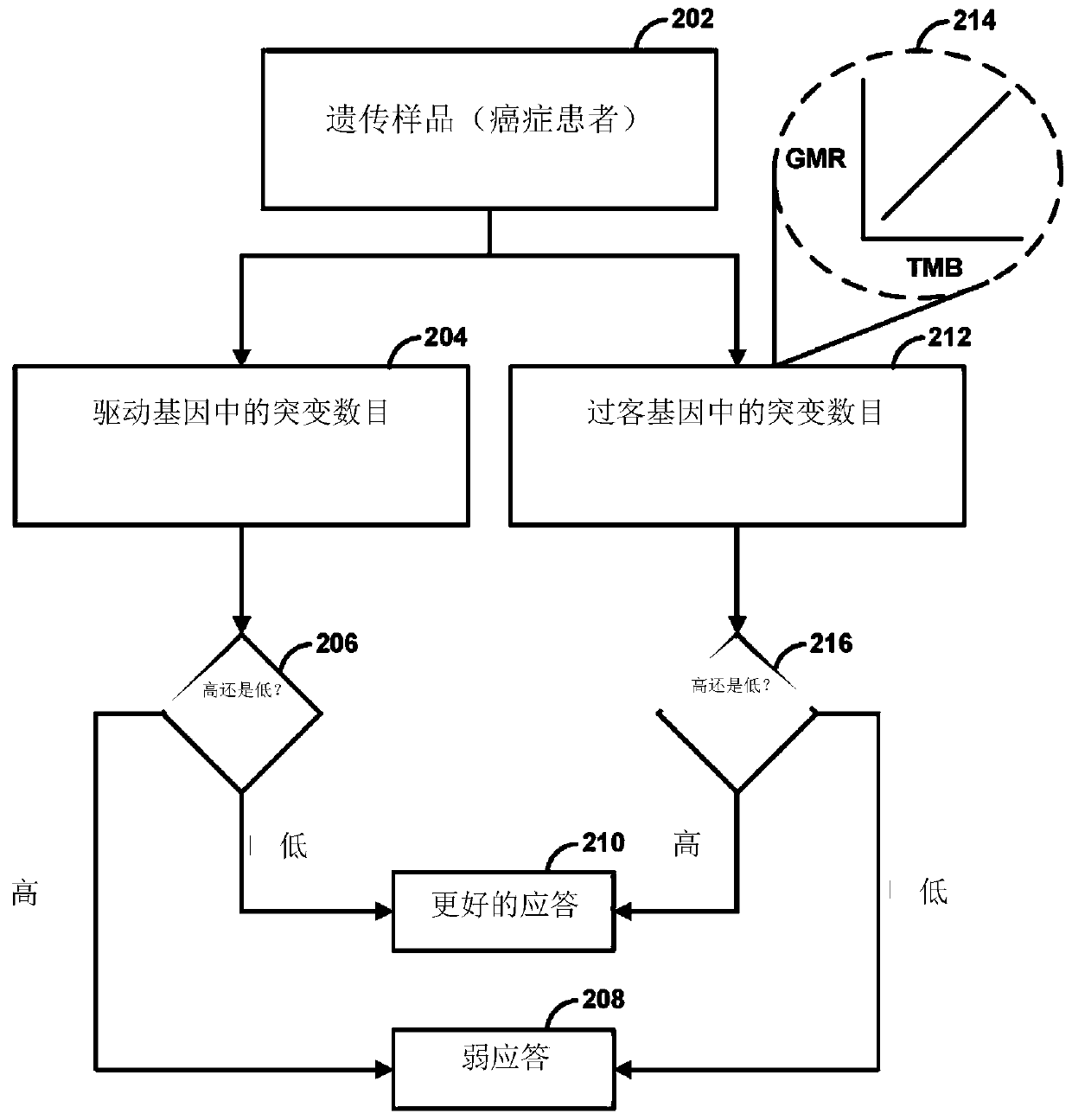
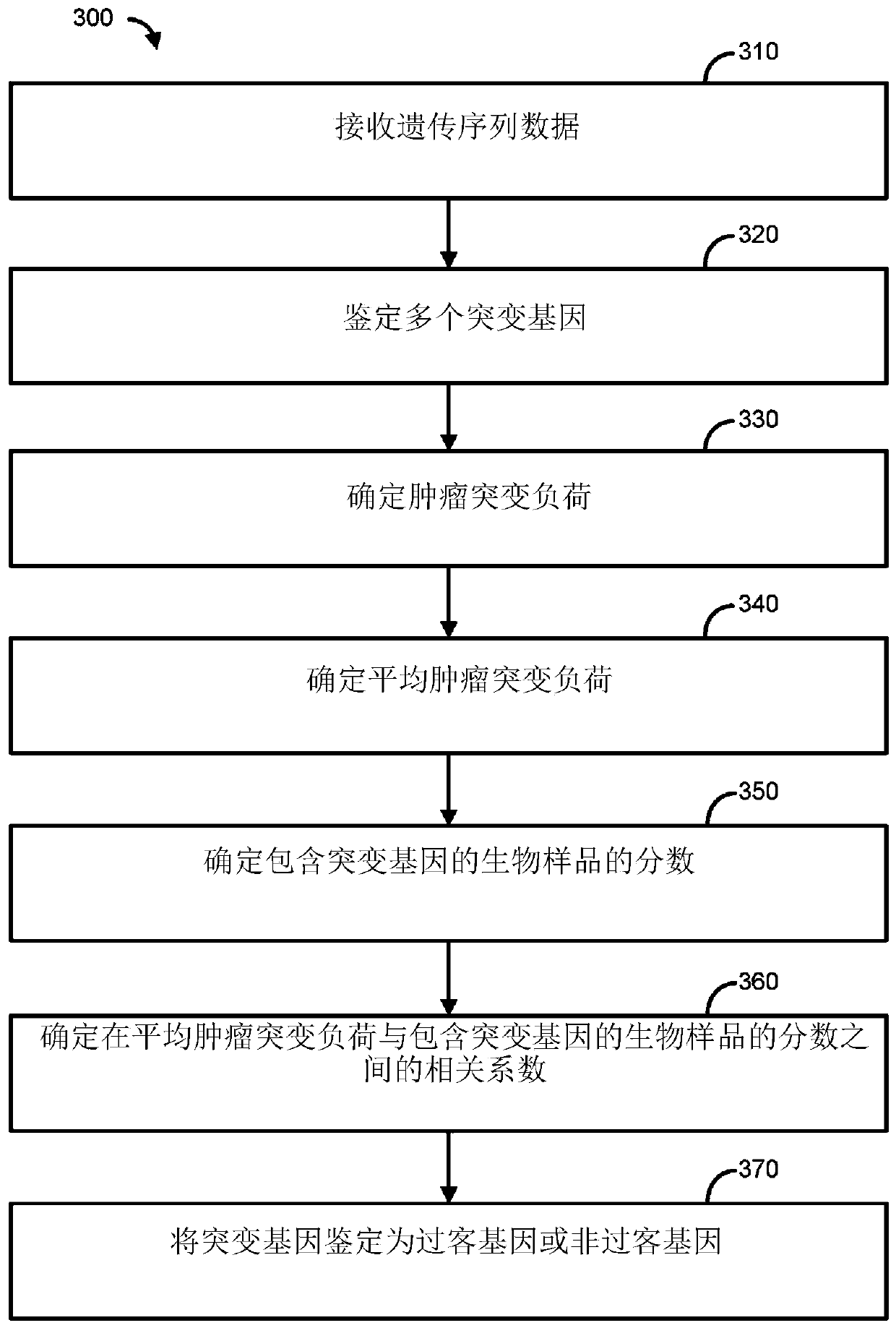
Immunotherapy methods for patients whose tumors carry a high passenger gene mutation burden
Methods for selecting a cancer patient for immunotherapy comprise establishing a total passenger gene mutation burden from a tumor of a cancer patient, generating a background distribution for the mutational burden of the tumor, normalizing the total passenger gene mutation burden against the background distribution, and categorizing the cancer patient as an immunotherapy responder when the totalpassenger gene mutation burden is greater than the mean of the background distribution. When the cancer patient is an immunotherapy responder, the patient may be administered an immunotherapy regimenthat comprises activation / inhibition of T cell receptors that promote T cell activation and / or prolong immune cytolytic activities.
Owner:REGENERON PHARM INC
Humanized antibody and application thereof
ActiveCN114031688AGrowth inhibitionPrevent invasionColon cancer vaccineAntibody ingredientsHumanized antibodySignal Pathways
The invention discloses a humanized antibody and application thereof, the humanized antibody comprises a heavy chain variable region and a light chain variable region, and can specifically target a CH1 structural domain 162 site sialylated epitope of IgG, the amino acid sequence of the heavy chain variable region is as shown in SEQ ID NO. 1, and the amino acid sequence of the light chain variable region is as shown in SEQ ID NO. 2. The humanized antibody can effectively inhibit the growth, invasion and suspension growth ability of tumor cells, has an obvious anti-tumor effect, and also can inhibit the activation of a c-Met / Wnt signal channel and an FAK signal channel of the tumor cells.
Owner:PEKING UNIV +1
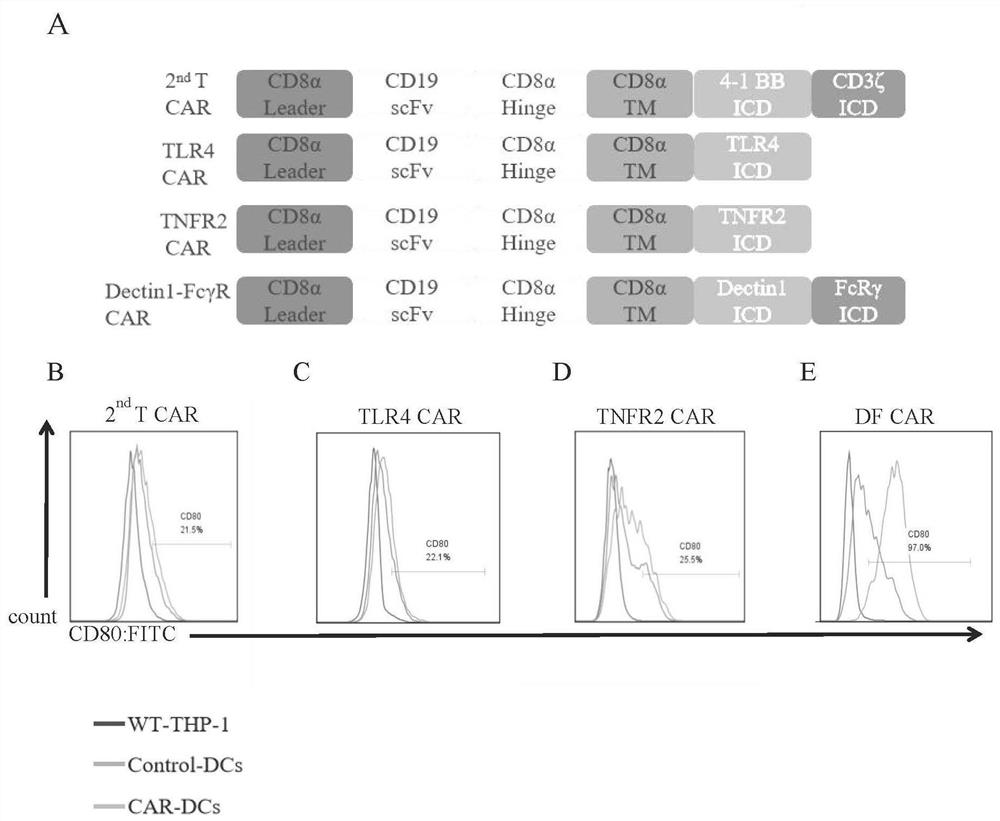
Chimeric antigen receptor, vector, human dendritic cell, cell line, solid tumor treatment medicine, preparation method and application
ActiveCN112830974AImprove cleanlinessSimple preparation processVirusesAntibody mimetics/scaffoldsTumor targetDendritic cell
The invention relates to a chimeric antigen receptor, a lentiviral vector, a human dendritic cell, a cell line, an immunosuppressive solid tumor treatment drug, and a preparation method and application thereof, and belongs to the technical field of chimeric antigen receptors. The intracellular signal domain of the chimeric antigen receptor is selected from at least one of TLR4, TNFR2, Dectin-1 and Fc receptor gamma chain intracellular domain structures. In the presence of tumor targets, the chimeric antigen receptor disclosed by the invention can effectively activate human dendritic cells in vivo and in vitro, and can resist the environment formed by immunosuppressive molecules CTLA4-Ig and PD-L1, so that the ability of traditional CAR-T cells to remove immunosuppressive solid tumors is improved. According to the invention, in a clinically related humanized mouse tumor model, the human dendritic cell modified by the chimeric antigen receptor can effectively reverse an immunosuppressive tumor microenvironment and reactivate in-vivo depleted CAR-T cells, so that the progress of solid tumors is inhibited.
Owner:SHENZHEN FRONTIERGATE BIOTECHNOLOGY CO LTD +1
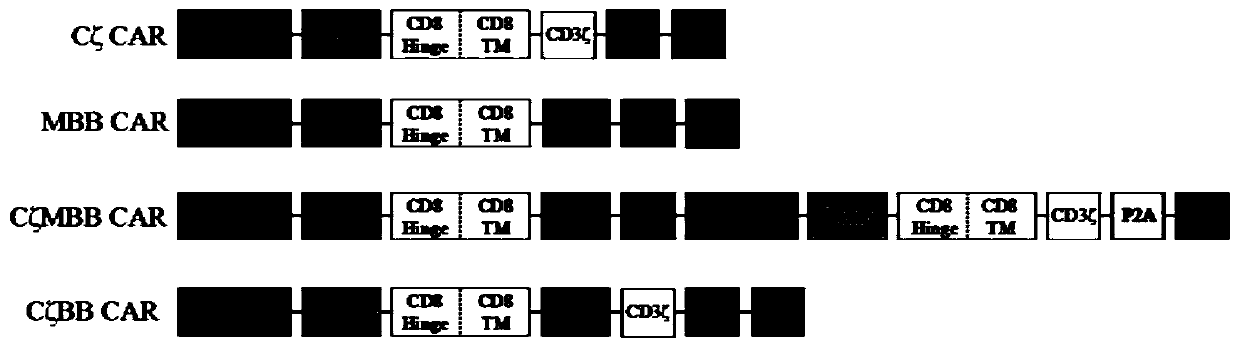
Embedded antigen acceptor and method for treating lung cancer through embedded antigen acceptor
The invention relates to a new embedded antigen acceptor, and provides an expression vector for expressing the embedded antigen acceptor and a host cell. The invention further provides an applicationof the embedded antigen acceptor to treatment of cancer or preparation of medicines for treating cancer. The embedded antigen acceptor and the medicines, provided by the invention, can effectively treat lung cancer.
Owner:DAREN BIOTECH LTD
Monocyte and macrophage for expressing chemokine receptor with solid tumor directional chemotactic ability, and preparation and application thereof
ActiveCN113322238ADownregulation of expression levelReduce non-specific immune attackPeptidesBlood/immune system cellsPluripotential stem cellLentivirus
The invention provides a monocyte and a macrophage for expressing a chemokine receptor with solid tumor directional chemotactic ability, and preparation and application thereof, and relates to the field of biotechnology. The invention provides a macrophage, the macrophage overexpresses the chemokine receptor, the macrophage has the ability of chemotactic migration to a solid tumor and remarkable effects of chemotactic migration to the tumor and infiltration, the specific killing efficiency and utilization rate of adoptively transplanted macrophage can be improved, and non-specific immune attack to normal tissues is reduced. The invention provides a preparation method of macrophages, which comprises the following steps: constructing a lentivirus expression system containing a chemokine receptor gene, integrating the chemokine receptor gene into pluripotent stem cells or mononuclear macrophages by using the lentivirus expression system, performing induced differentiation to obtain the macrophages, and the macrophages can stably overexpress the chemokine receptor for a long time.
Owner:ZHEJIANG UNIV
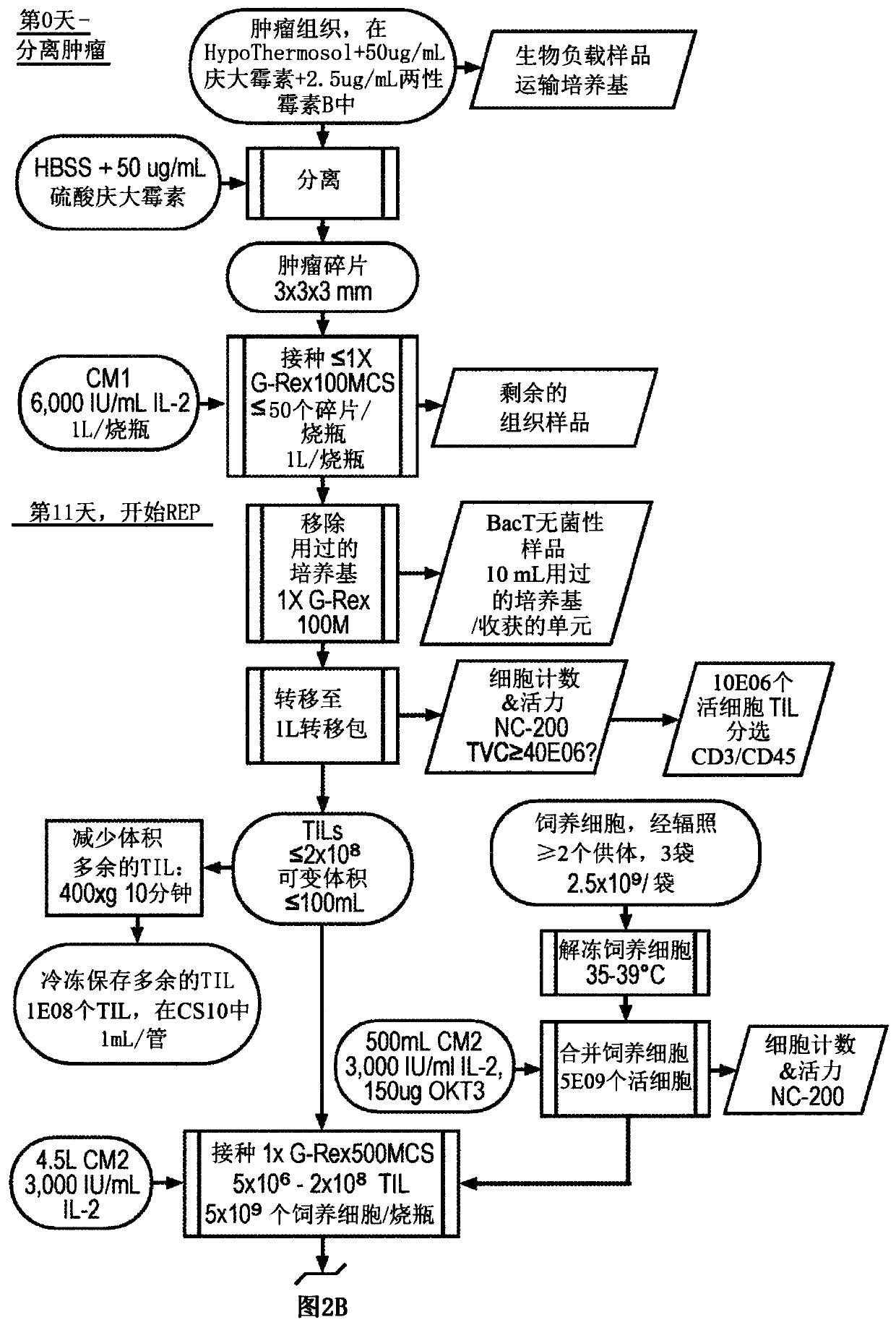
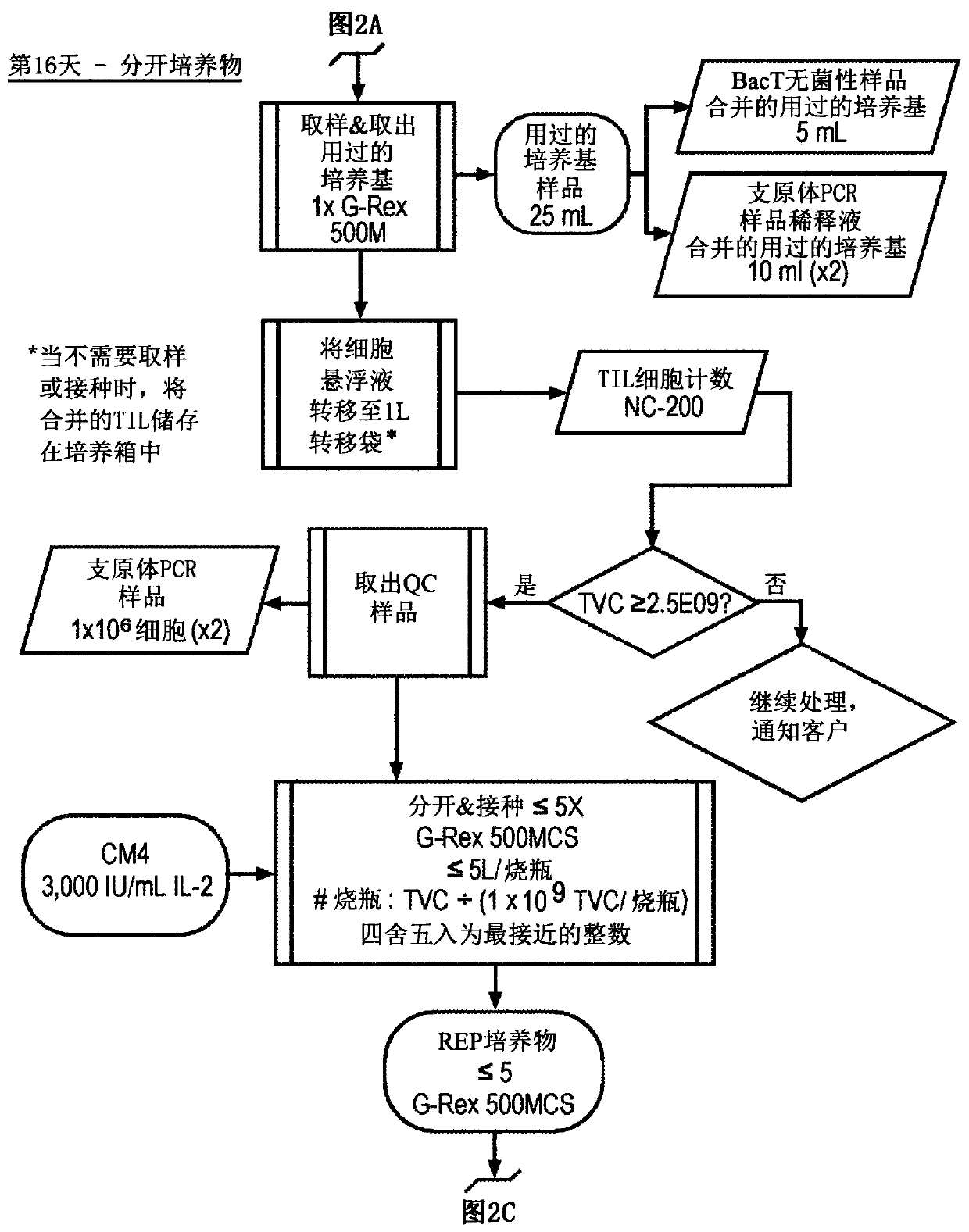
Til expansion from fine needle aspirates and small biopsies
The present disclosure provides methods for expanding TIL populations from fine needle aspirates (FN As) or small biopsies which contain low numbers of TILs, using the methods disclosed herein including in a closed system that leads to improved phenotype and increased metabolic health of the TILs in a shorter time period.
Owner:IOVANCE BIOTHERAPEUTICS INC
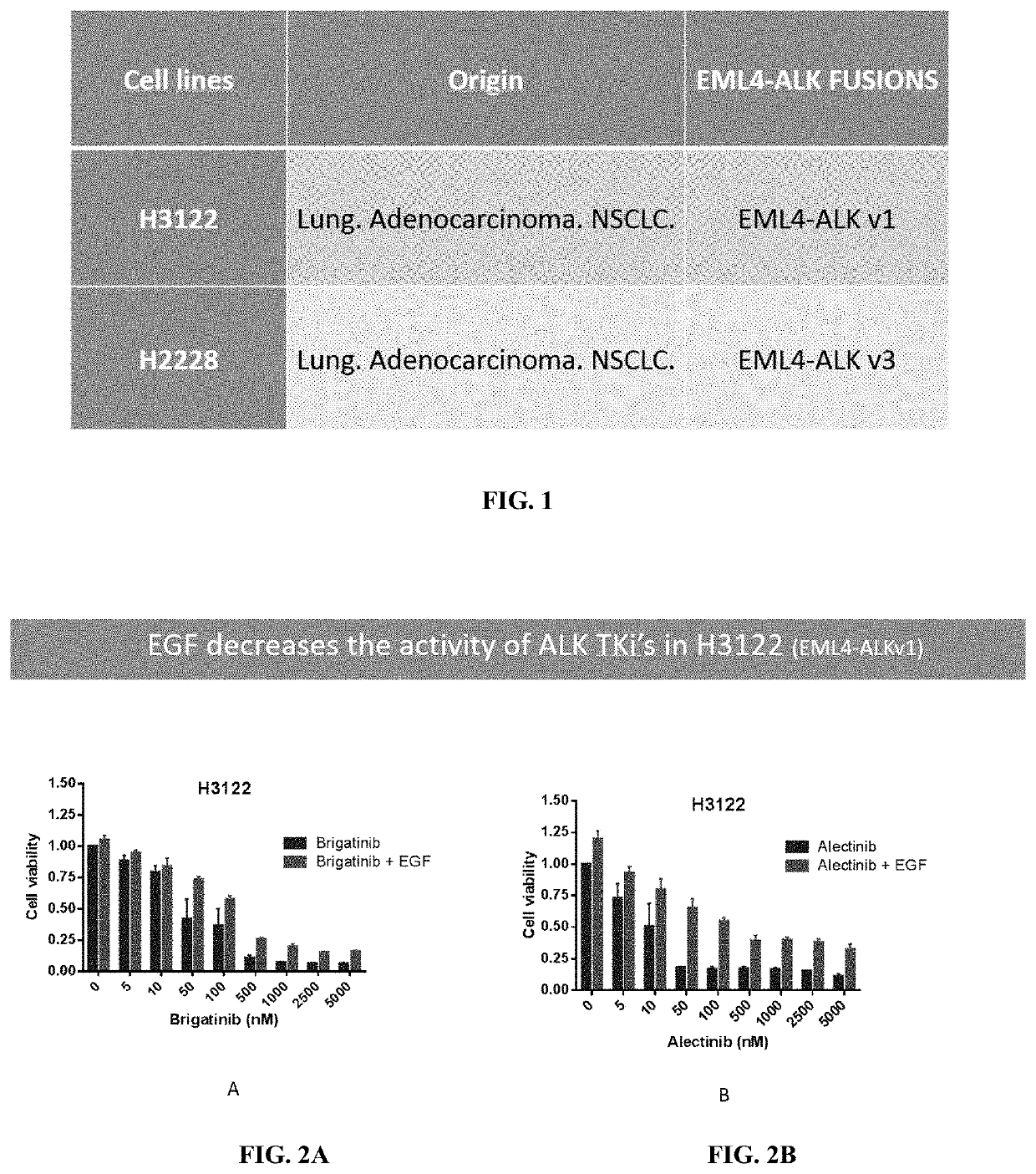
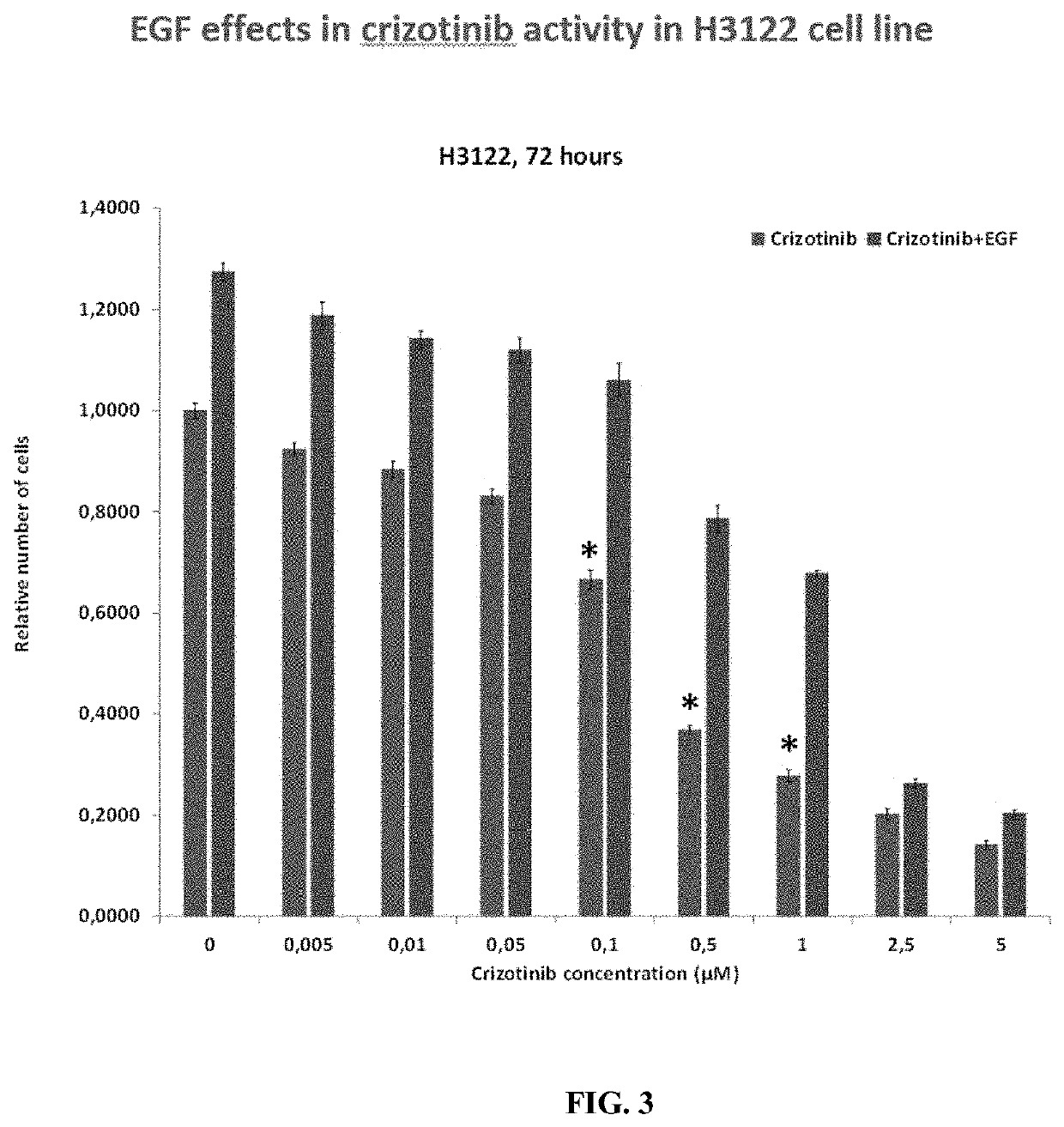
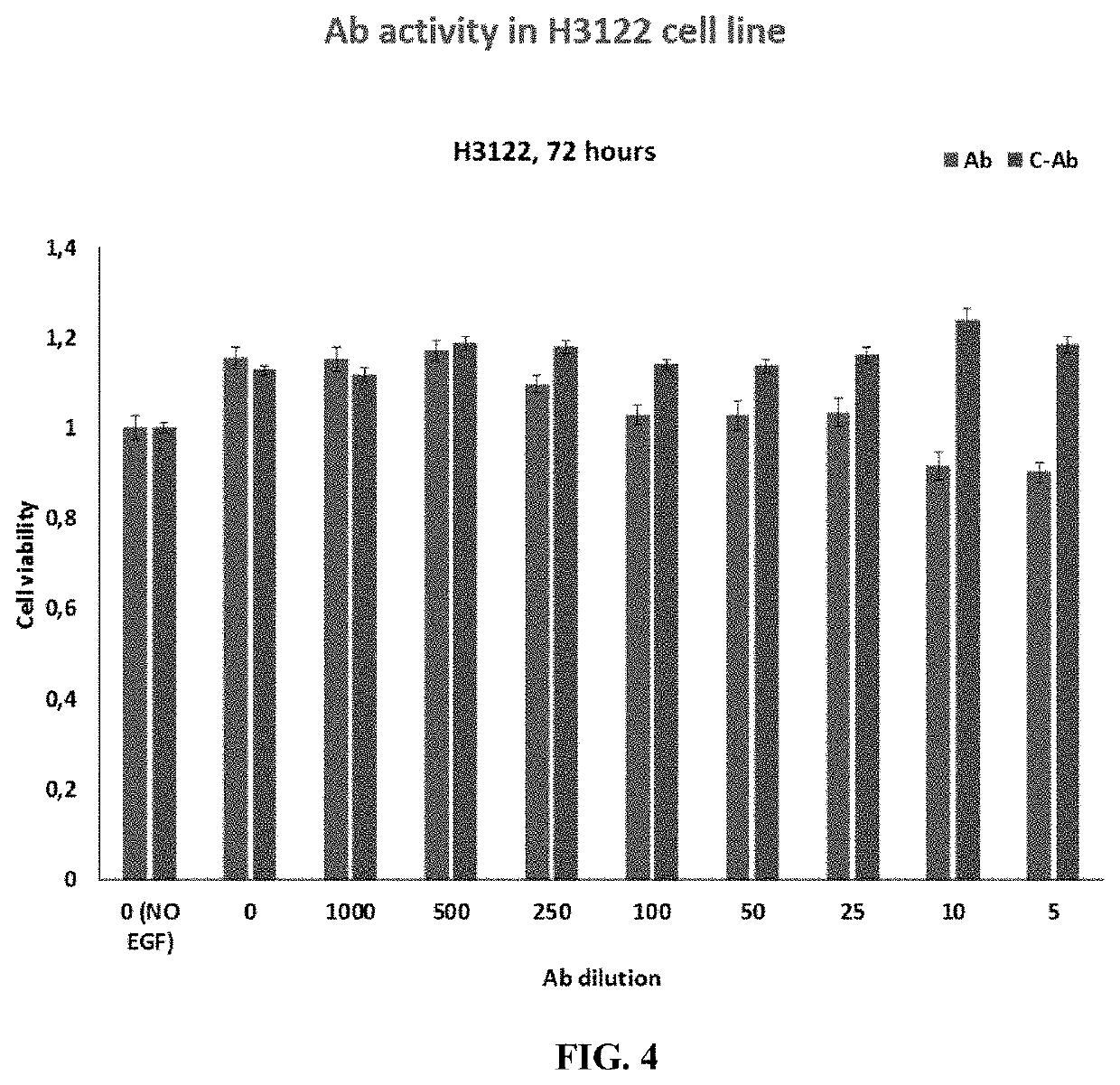
Methods and compositions for inhibition of egf/egfr pathway in combination with anaplastic lymphoma kinase inhibitors
PendingUS20200046690A1Avoid resistanceOrganic active ingredientsImmunoglobulins against growth factorsDosing regimenEgfr pathway
A method of treating patients suffering from cancers driven by deregulated Human Epidermal Growth Factor Receptor (HER1 / Human EGFR) comprising administering to a patient in need of such treatment a flexible and active regimen for combining Anaplastic Lymphoma Kinase Inhibitors (ALK Inhibitors) and anti-EGF antibodies for inhibition of the pathway activated by EGF-EGFR binding (mAb). The anti-EGF antibodies can be produced by active immunization or provided passively by the administration of antibodies that are anti-EGF. The method comprises ALK Inhibitors administered according to a continuous regimen based on an average daily dose in the range of 10 to 250 mg and the mAb is co-administered either actively or passively according to a dosing regimen achieving a therapeutic effective amount repeated thrice, twice or once a week, once in two weeks, once in three weeks or at least once monthly.
Owner:IN3BIO LTD
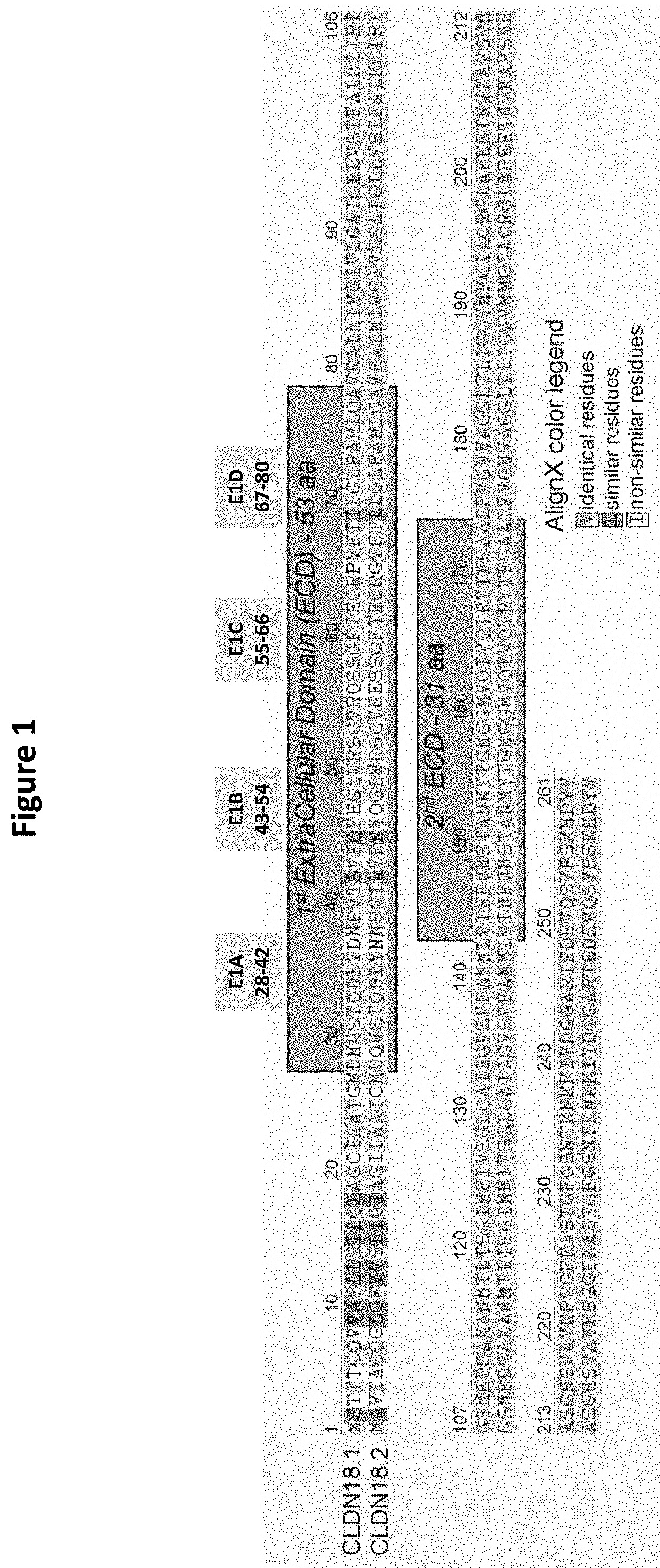
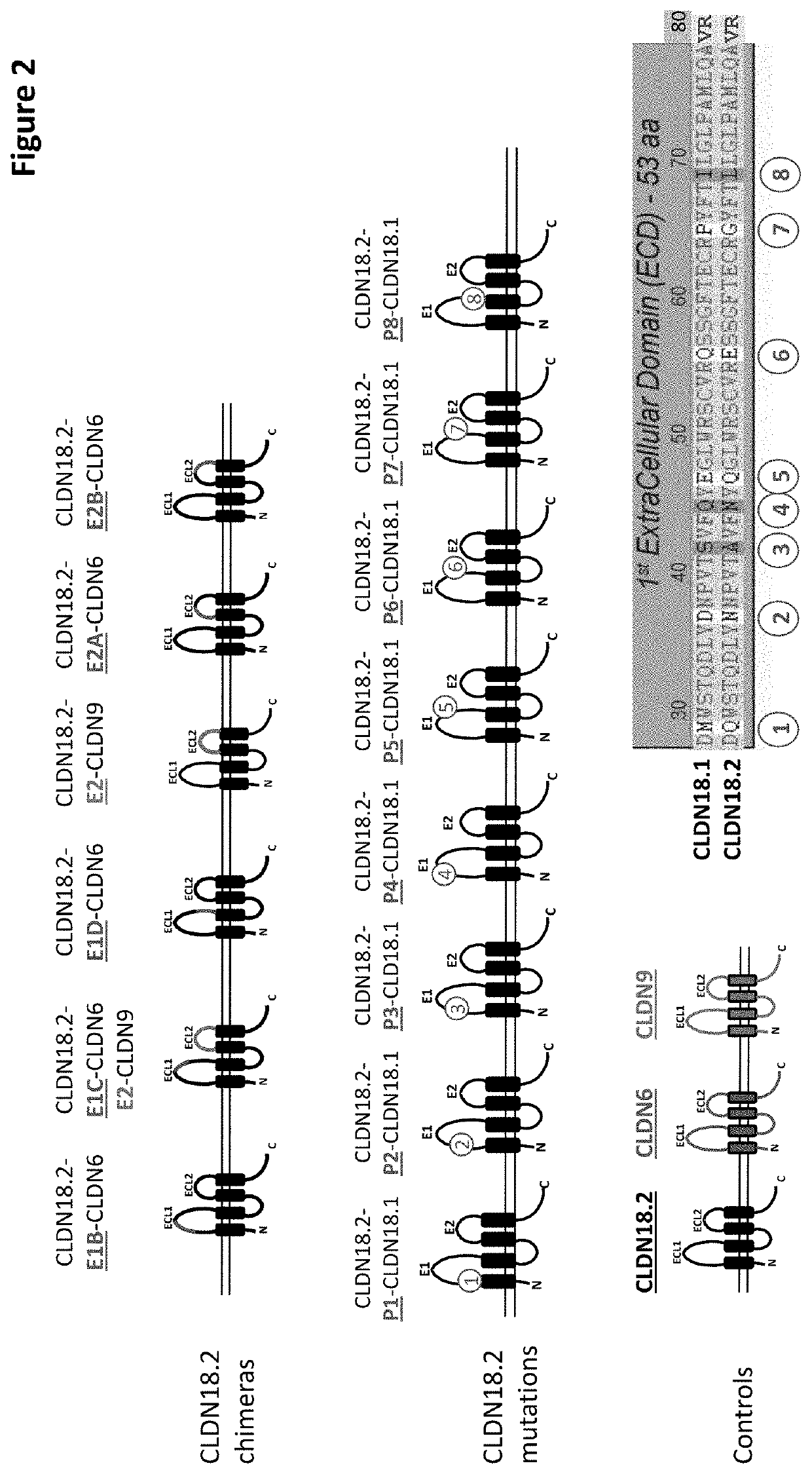
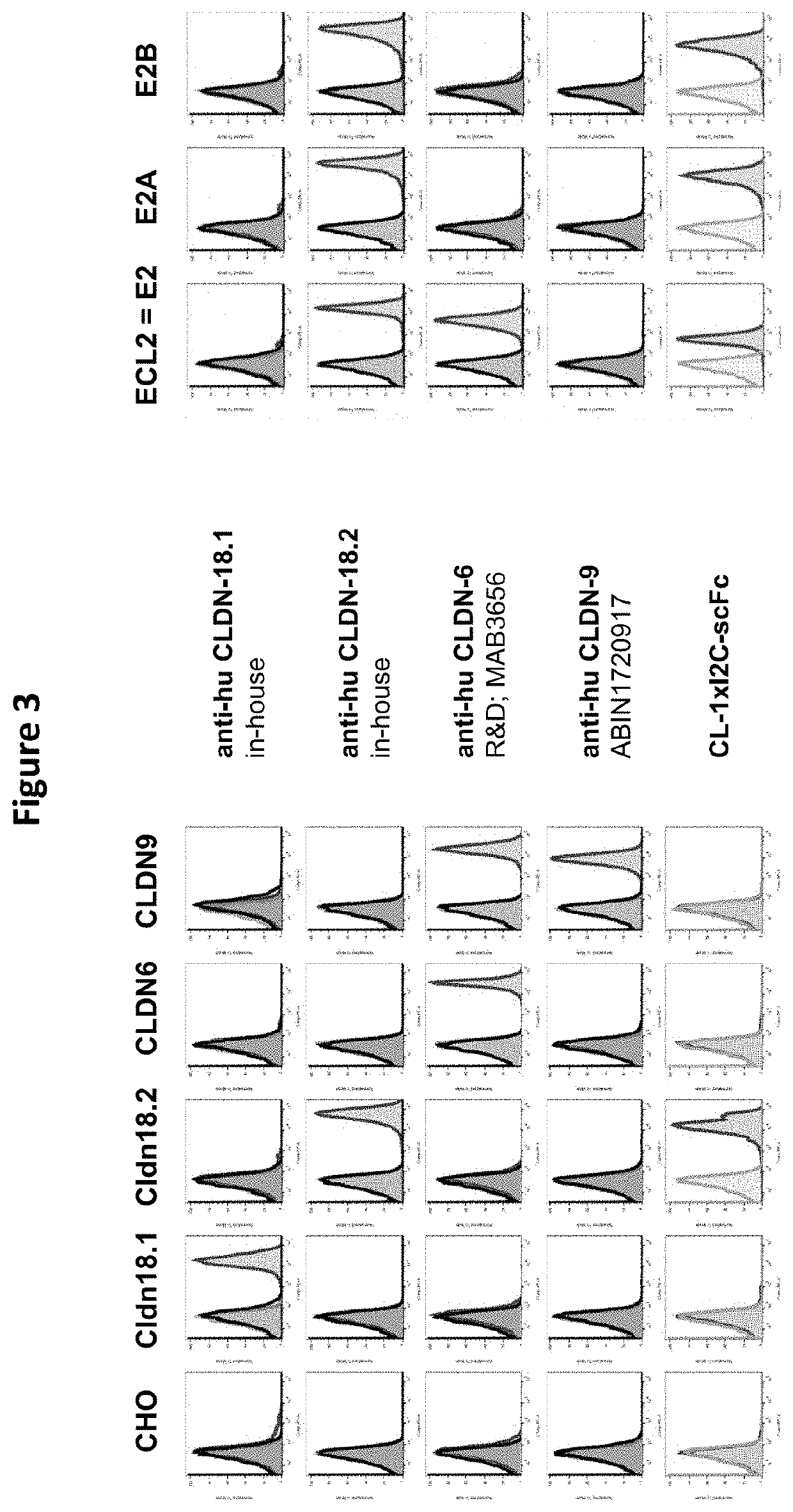
Antibody Constructs for CLDN18.2 and CD3
The present invention relates to an antibody construct comprising a domain which binds to Claudin 18.2 (CLDN18.2) and another domain which binds to CD3. Moreover, the invention provides a polynucleotide encoding the antibody construct, a vector comprising said polynucleotide and a host cell transformed or transfected with said polynucleotide or vector. Furthermore, the invention provides a process for producing the antibody construct of the invention, a medical use of said antibody construct and a kit comprising said antibody construct.
Owner:AMGEN INC +1
Tumor cell dryness restriction-type CAR and application thereof
ActiveCN111423517AGood application effectEfficient killingAntibody mimetics/scaffoldsNucleic acid vectorDiseaseTumor cells
The invention belongs to the technical field of tumor cell immunotherapy, and particularly relates to a restriction-type CAR capable of inhibiting the dryness of tumor cells and application of the CAR. The chimeric antigen receptor is an amino acid sequence obtained by connecting a plurality of protein fragments, and is obtained by sequentially connecting a human CD8a molecule signal peptide CD8a,a human NKG2D extracellular region, a human CD8 molecule transmembrane region and a 41BB molecule intracellular region 41BB, and a human CD3z molecule intracellular region CD3Zeta. In the preferred design, the chimeric antigen receptor is further obtained by connecting a connecting sequence to an IL24 CDS region sequence IL24. By further optimizing the structure of the CAR and simultaneously in combination with SFN application, the chimeric antigen receptor has good technical effects on effectively killing tumor cells and tumor stem cells, reducing the recurrence of diseases and improving theapplication effect of CAR-T cells, so that the has good practical value and popularization and application significance.
Owner:赛德特生物制药有限公司
Chimeric antigen receptor of cell for targeted expression of Claudin 18.2 and application of chimeric antigen receptor
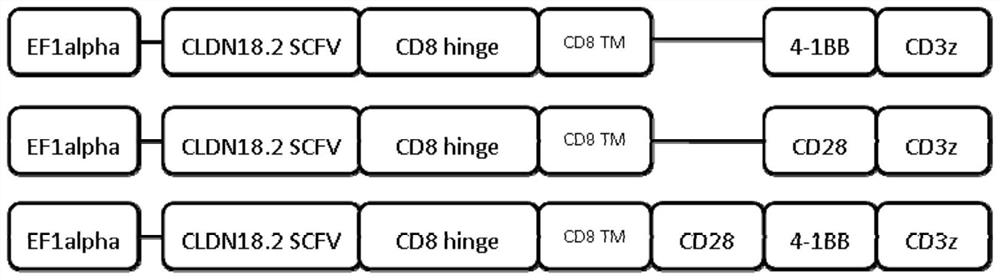
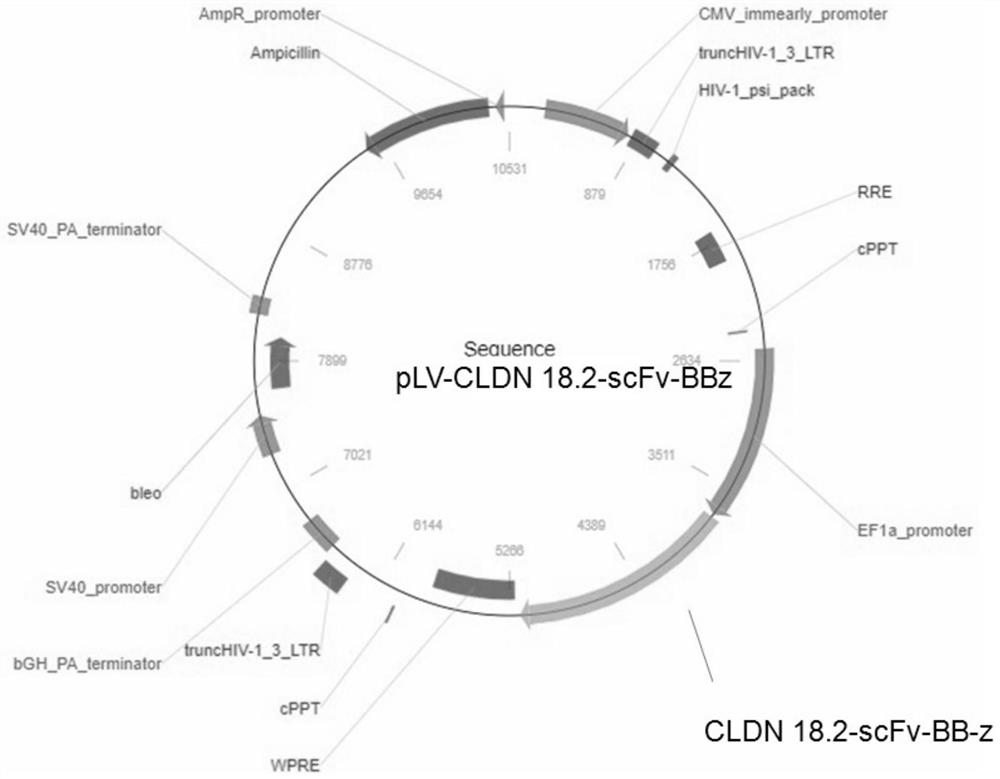
ActiveCN113354739AEfficient killingStrong killing and cytokine release functionVirusesAntibody mimetics/scaffoldsAntigenSingle-Chain Antibodies
The invention discloses a chimeric antigen receptor of a cell for targeted expression of Claudin 18.2 (CLDN 18.2), and particularly discloses a chimeric antigen receptor with an amino acid sequence as shown in SEQ ID NO.14. The chimeric antigen receptor comprises a Claudin 18.2-targeted single-chain antibody, a hinge region, a transmembrane structural domain and an intracellular signal structural domain. The Claudin 18.2-targeted chimeric antigen receptor disclosed by the invention can achieve effective and specific targeted expression of malignant cells (such as tumor cells) of the Claudin 18.2 surface antigen, so that a more efficient method with fewer side effects and adverse reactions is provided for treating some tumors expressing the Claudin 18.2 surface antigen.
Owner:SHANGHAI LIFE SCI & TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com