Humanized antibody and application thereof
A technology of humanized antibodies and anti-tumor drugs, applied in the direction of antibodies, applications, anti-tumor drugs, etc., to achieve the effect of inhibiting the growth of tumor cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Preparation and Screening of Humanized Antibody
[0033] 1. Obtained anti-SIG monoclonal antibody
[0034] Construct a recombinant IgG plasmid with an amino acid sequence as shown in SEQ ID NO.5 of the coding gene of the IgG epitope peptide, and transfer it into the host cell for cultivation. The expression product is verified to have a correct amino acid sequence. N-glycosyl sialic acid modification, which is identified as SIG, is collected and preserved.
[0035] SIG was used as antigen to immunize BALB / c mice. After multiple immunizations, mouse spleen cells were collected, fused with myeloma cells, and hybridoma cells were cultured. Positive hybridoma cells capable of producing anti-SIG antibodies were screened out and cloned and expanded. Then, the anti-SIG monoclonal antibody secreted by it will be identified and sequenced for immunoglobulin type, subclass, specificity, affinity, epitope and molecular weight of the recognized antigen, and hybridoma cell...
Embodiment 2
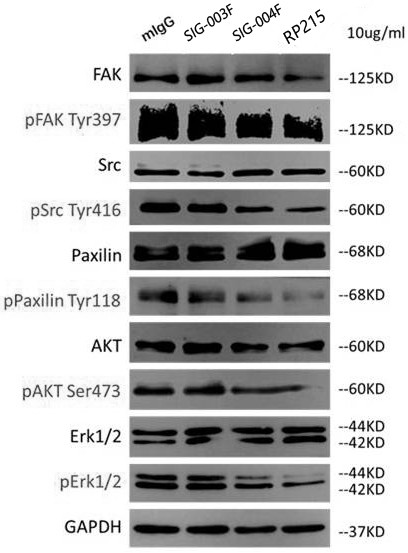
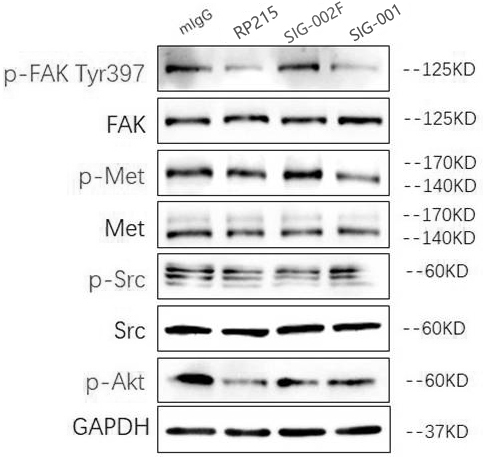
[0060] Example 2 Functional verification of humanized antibodies
[0061] 2.1 Inhibit cancer cell proliferation and invasion
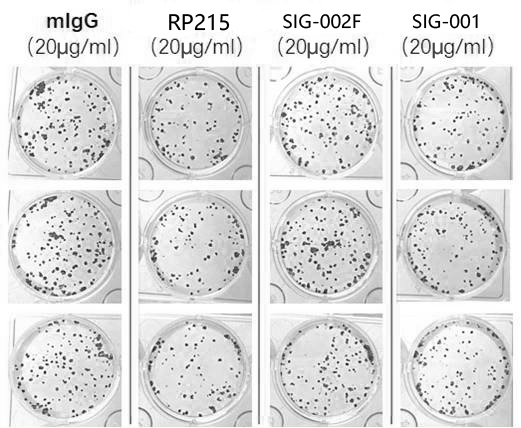
[0062] Taking lung squamous cell carcinoma cells-NCI-H520 as the experimental object, it was divided into three groups, and 10,000 cancer cells were added to a single well in each group, and each group was replicated three times. The first group was added with mlgG as a negative control, the second group was added with SIG-001, and the third group was added with RP215 as a positive control. Changes in the number of cancer cells in each group. In addition, the invasion ability of the three groups of antibodies was detected by Tran-swell invasion assay.
[0063] The result is as Figure 5 As shown in the above figure, it can be seen that the number of cancer cells in the wells of the culture plate added with antibody SIG-001 is equivalent to that added with RP215, which is significantly less than that of the negative control group added with mlgG, ind...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com