Patents
Literature
88results about "Colon cancer vaccine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Composite superimmunogen for bi-functional vaccine use for the treatment of illnesses associated with a stromal tissue disorder
The invention is relative to novel means of systemic or mucosal vaccinial therapy against some cancers, viral infections and allergy which are provided by the invention under the form of a family of composite superimmunogenic compounds for bifunctional vaccinial use able to induce an immune response raised towards two distinct targets, respectively, the causal pathogenic antigenic structure, on the one hand, and locally produced factors responsible for a subsequent immunotoxic or neoangiogenic stroma disorder, on the other hand.
Owner:NEOVACS SA
Compositions and methods for selected tumour treatment
InactiveUS20050063995A1Reduce decreaseShorten the progressEnergy modified materialsColon cancer vaccineAbnormal tissue growthMelanoma
Disclosed are novel compositions, methods and vaccines, which upon administration to a patient suffering from a melanoma, colon carcinoma tumor or breast cancer, postpone and / or reduce the need for chemotherapy treatment, slow the progression of or eliminate the tumor and / or alleviate the symptoms of the tumor. The compositions comprise stressed colon carcinoma, melanoma or breast cancer cells, preferably autologous such cells.
Owner:VASOGEN IRELAND LTD
Compositions and methods for the induction of cd8+ t-cells
ActiveUS20190343944A1Strong immune responseApproach is limitedAntibacterial agentsOrganic active ingredientsDiseaseMedicine
Owner:KEIO UNIV +1
A Composition, A Treatment Method and An Application Thereof
InactiveUS20190015458A1Good effectImprove efficiencyColon cancer vaccineProtozoa material medical ingredientsMelanomaTreatment field
The present invention relates to the field of treatment of tumor, and especially to a composition comprising a plasmodium, a treatment method and an application thereof. The composition of the present invention has therapeutic effects on colorectal carcinoma, lung carcinoma, breast carcinoma, gastric carcinoma and hepatic carcinoma etc., can inhibit the growth of tumor and prolong the life of the tumor patients, whereas has no therapeutic effect on melanoma and lymphoma; meanwhile, the present invention describes that the long-term plasmodium infection has better therapeutic effect on tumors, and the plasmodium immunotherapy of the present invention does not take the fever time as a course standard when treating tumors, but should be used to extend the duration of plasmodium infection as much as possible until the progression of tumors can be controlled under the premise of protecting the organ functions and life safety of the patients.
Owner:BLUE ELEGANT BIOTECH CO LTD
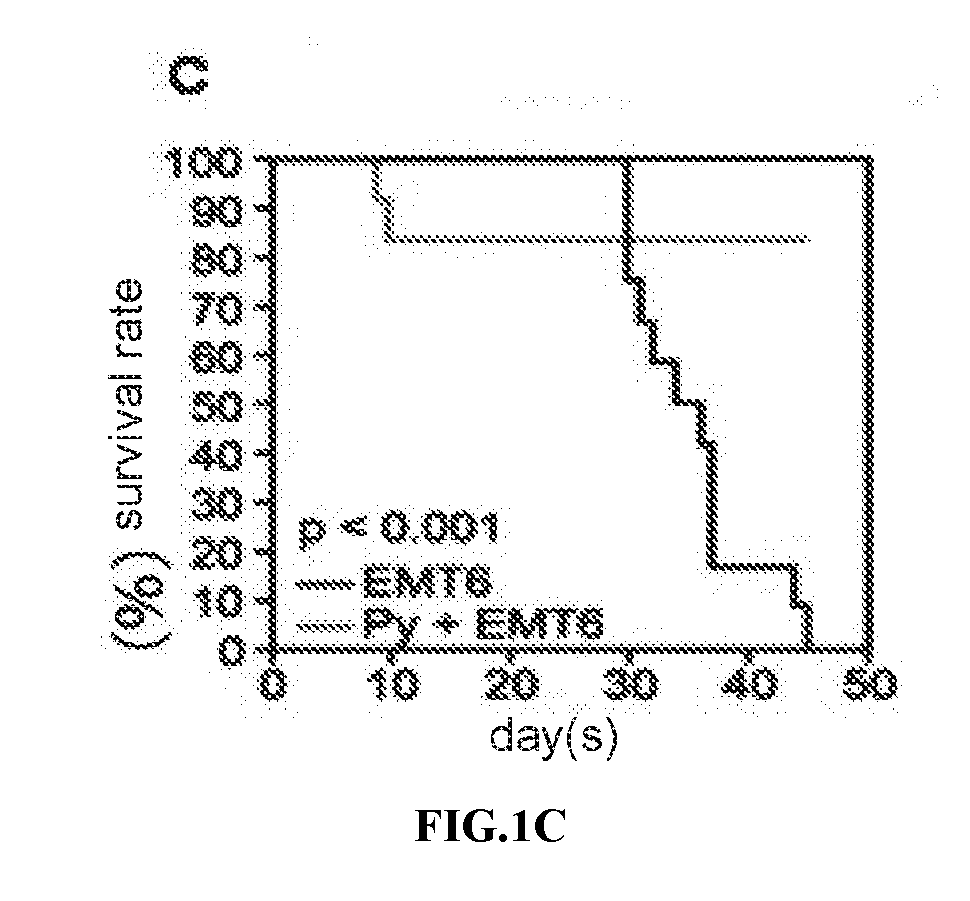
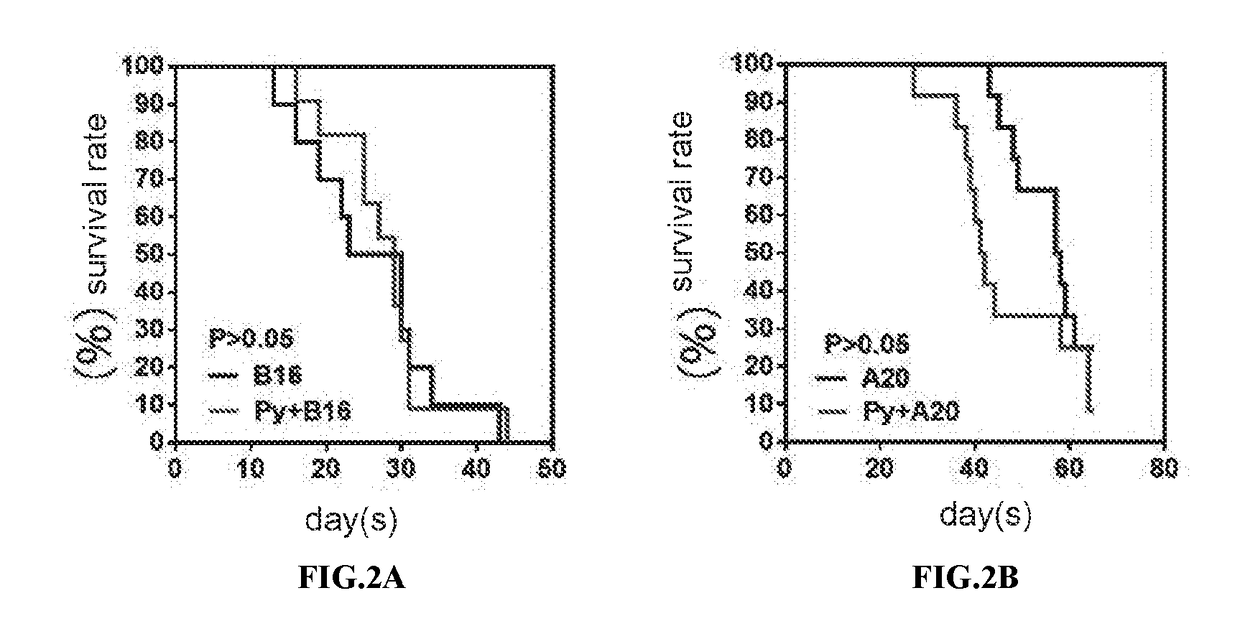
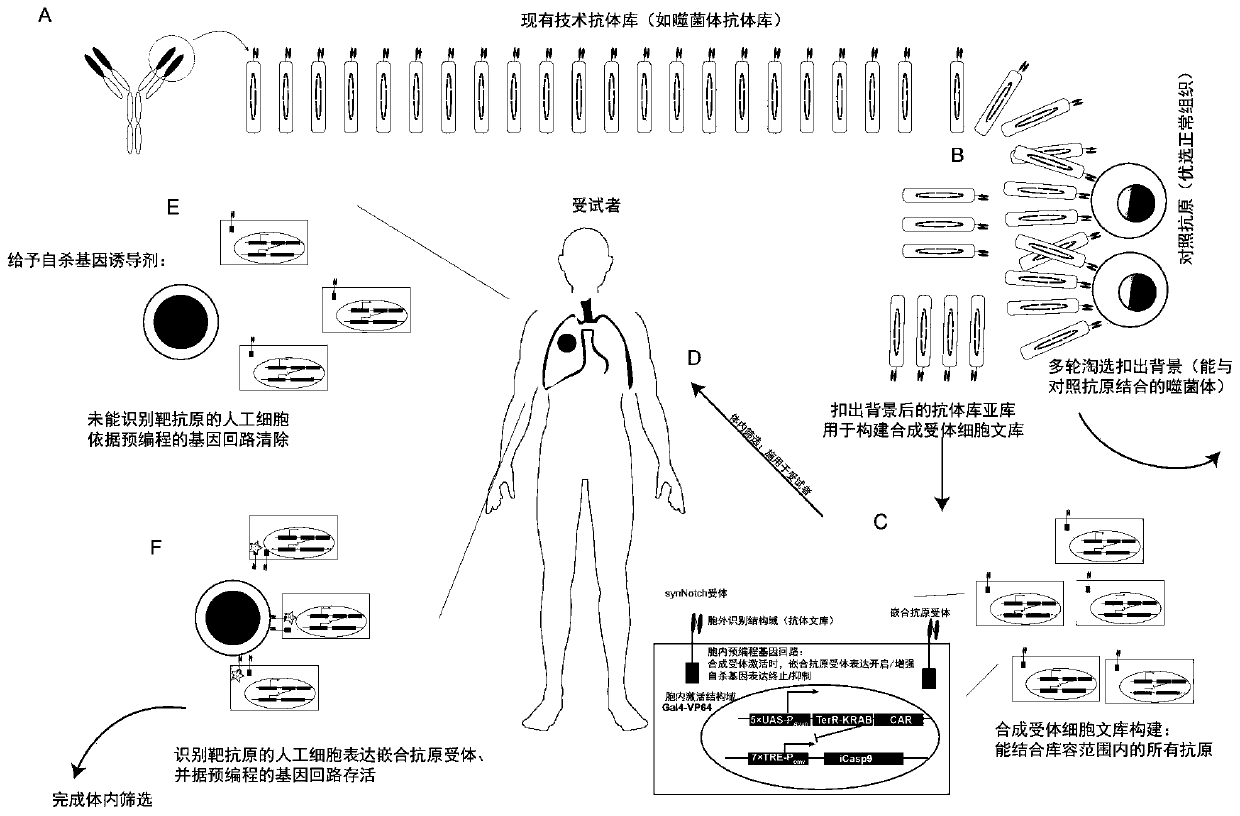
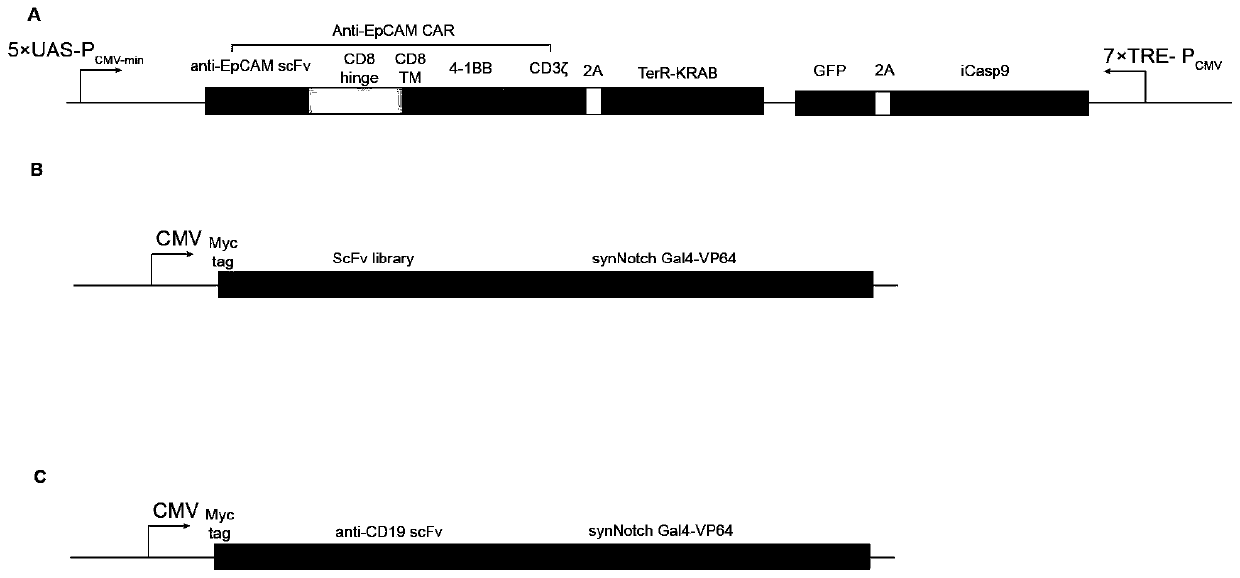
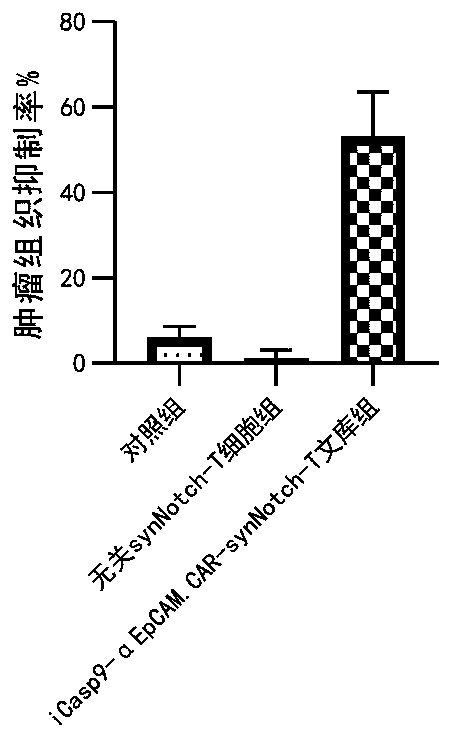
Carrier assembly carrying gene element combination, recipient cell library, preparation and screening methods and application
The invention provides a carrier assembly carrying a gene element combination, a recipient cell library, a preparation and screening method and an application, the recipient cell library is formed byfusing cells and the carrier assembly, and the carrier assembly at least carries three gene elements, respectively a plurality of first gene elements encoding one or more idiotypic synNotch receptors,respectively; a second gene element carrying one or more gene loops; a third gene element encoding one or more idiotypic chimeric antigen receptors. Wherein, when the first genetic element encodes one idiotypic synNotch receptor, the third genetic element must encode at least three idiotypic chimeric antigen receptors, and when the third genetic element encodes one idiotypic chimeric antigen receptor, the first genetic element must encode three idiotypic synNotch receptors. The gene loop is pre-programmed, a regulatory homeopathic factor is combined with a transcription factor, upon activation of the synNotch receptor encoded by the first gene element, the chimeric antigen receptor encoded by the third gene element is controllably expressed.
Owner:PHARCHOICE THERAPEUTICS INC
USE OF IL-1beta BINDING ANTIBODIES
InactiveUS20190048072A1Improve treatmentOrganic active ingredientsIntestine cancer vaccineAntigen bindingGevokizumab
Owner:NOVARTIS AG +3
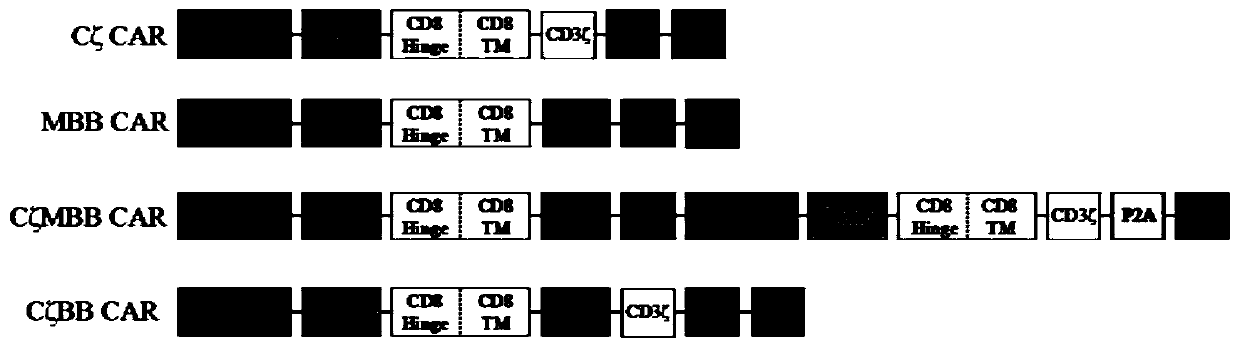
Double-chimeric antigen receptor, T cell and construction method and application thereof
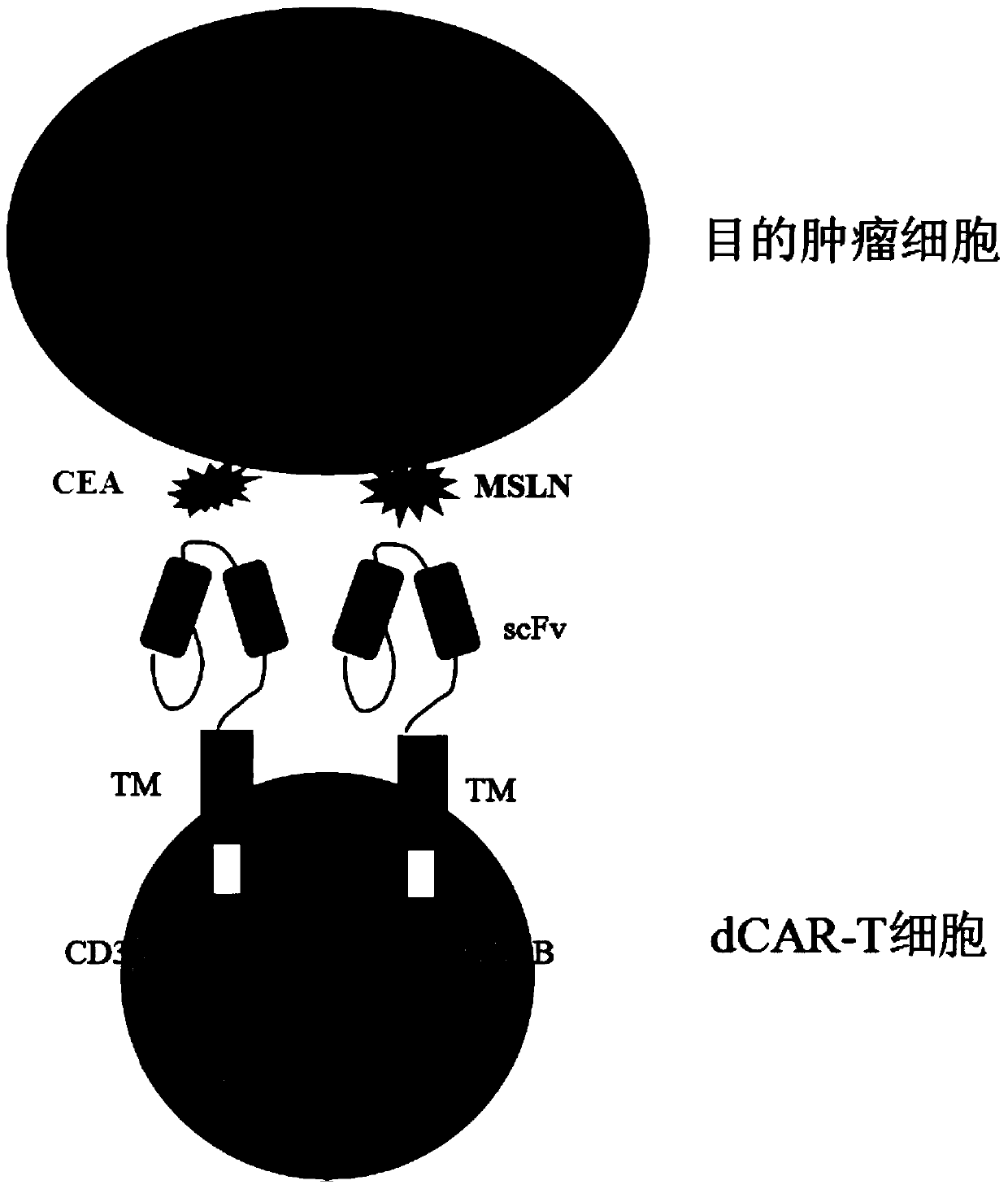
PendingCN110669138AToxicityImprove securityImmunoglobulins against cell receptors/antigens/surface-determinantsBlood/immune system cellsTumor antigenSialyl tn
The invention discloses a double-chimeric antigen receptor, a T cell and a construction method and an application thereof, which belong to the field of cellular immunotherapy of tumors. The inventionspecifically relates to a specific structure and a construction method of the double chimeric antigen receptor T cell (dCAR-T cell), and preliminarily discusses the in-vivo and in-vitro activity of the dCAR-T cell. The selected tumor-associated antigens are mesothelin and carcino-embryonic antigens, and researches show that the two tumor antigens can be simultaneously expressed on the surface of asolid tumor, such as pancreatic cancer. The invention discloses an antigen receptor. The in-vitro and in-vivo tests prove that the constructed dCAR-T cell can be permanently and effectively activatedonly under the condition that two antigens are simultaneously recognized, and has efficient anti-tumor activity, so that a specific tumor killing function can be exerted, and the application of CAR-Tcell immunotherapy is improved.
Owner:CHINA PHARM UNIV
ROBO1 CAR-NK cell carrying suicide gene as well as preparation method and application of ROBO1 CAR-NK cell
PendingCN111269925ASmall side effectsGood killing effectVirusesHydrolasesNatural Killer Cell Inhibitory ReceptorsROBO1
The invention discloses an ROBO1 CAR-NK cell carrying a suicide gene as well as a preparation method and application of the ROBO1 CAR-NK cell. In order to improve the safety and controllability of theCAR-NK therapy, a suicide gene switch element is integrated into a genome through a lentiviral transfection technology on the basis of the current ROBO1 CAR-NK cell to form the CAR-NK with the suicide gene. By adding the suicide gene, the CAR-NK cells can be better controlled, and the clinical safety is further improved.
Owner:ASCLEPIUS SUZHOU TECH CO GRP CO LTD
Humanized antibody and application thereof
ActiveCN114031688AGrowth inhibitionPrevent invasionColon cancer vaccineAntibody ingredientsHumanized antibodySignal Pathways
The invention discloses a humanized antibody and application thereof, the humanized antibody comprises a heavy chain variable region and a light chain variable region, and can specifically target a CH1 structural domain 162 site sialylated epitope of IgG, the amino acid sequence of the heavy chain variable region is as shown in SEQ ID NO. 1, and the amino acid sequence of the light chain variable region is as shown in SEQ ID NO. 2. The humanized antibody can effectively inhibit the growth, invasion and suspension growth ability of tumor cells, has an obvious anti-tumor effect, and also can inhibit the activation of a c-Met / Wnt signal channel and an FAK signal channel of the tumor cells.
Owner:PEKING UNIV +1
Vaccine
The disclosure relates to polypeptides and pharmaceutical compositions comprising polypeptides that find use in the prevention or treatment of cancer, in particular breast cancer, ovarian cancer and colorectal cancer. The disclosure also relates to methods of inducing a cytotoxic T cell response in a subject or treating cancer by administering pharmaceutical compositions comprising the peptides, and companion diagnostic methods of identifying subjects for treatment. The peptides comprise T cell epitopes that are immunogenic in a high percentage of patients.
Owner:TREOS BIO LTD
Monocyte and macrophage for expressing chemokine receptor with solid tumor directional chemotactic ability, and preparation and application thereof
ActiveCN113322238ADownregulation of expression levelReduce non-specific immune attackPeptidesBlood/immune system cellsPluripotential stem cellLentivirus
The invention provides a monocyte and a macrophage for expressing a chemokine receptor with solid tumor directional chemotactic ability, and preparation and application thereof, and relates to the field of biotechnology. The invention provides a macrophage, the macrophage overexpresses the chemokine receptor, the macrophage has the ability of chemotactic migration to a solid tumor and remarkable effects of chemotactic migration to the tumor and infiltration, the specific killing efficiency and utilization rate of adoptively transplanted macrophage can be improved, and non-specific immune attack to normal tissues is reduced. The invention provides a preparation method of macrophages, which comprises the following steps: constructing a lentivirus expression system containing a chemokine receptor gene, integrating the chemokine receptor gene into pluripotent stem cells or mononuclear macrophages by using the lentivirus expression system, performing induced differentiation to obtain the macrophages, and the macrophages can stably overexpress the chemokine receptor for a long time.
Owner:ZHEJIANG UNIV
Compositions and methods for the induction of cd8+ t-cells
ActiveUS20190381111A1Increase the number ofIncrease volumeAntibacterial agentsOrganic active ingredientsDiseaseMedicine
Owner:THE UNIV OF TOKYO +1
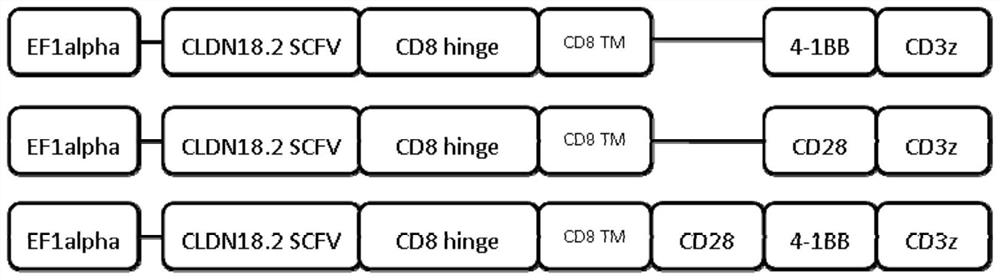
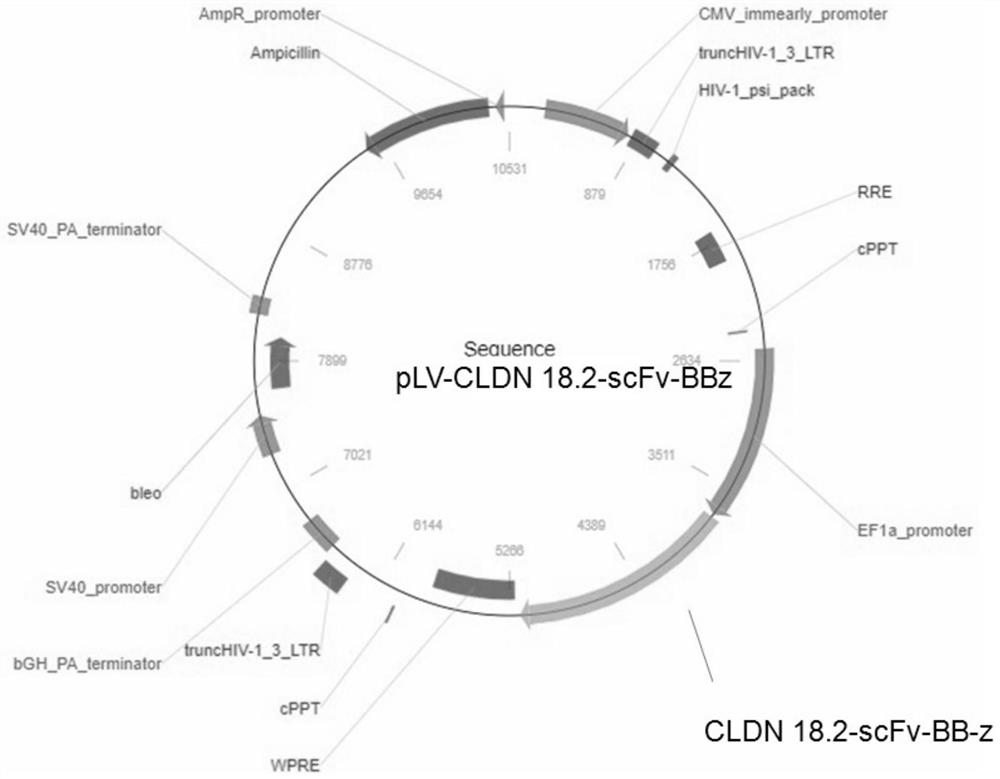
Chimeric antigen receptor of cell for targeted expression of Claudin 18.2 and application of chimeric antigen receptor
ActiveCN113354739AEfficient killingStrong killing and cytokine release functionVirusesAntibody mimetics/scaffoldsAntigenSingle-Chain Antibodies
The invention discloses a chimeric antigen receptor of a cell for targeted expression of Claudin 18.2 (CLDN 18.2), and particularly discloses a chimeric antigen receptor with an amino acid sequence as shown in SEQ ID NO.14. The chimeric antigen receptor comprises a Claudin 18.2-targeted single-chain antibody, a hinge region, a transmembrane structural domain and an intracellular signal structural domain. The Claudin 18.2-targeted chimeric antigen receptor disclosed by the invention can achieve effective and specific targeted expression of malignant cells (such as tumor cells) of the Claudin 18.2 surface antigen, so that a more efficient method with fewer side effects and adverse reactions is provided for treating some tumors expressing the Claudin 18.2 surface antigen.
Owner:SHANGHAI LIFE SCI & TECH CO LTD
Chorionic gonadotropin DNA vaccines and methods
InactiveUS20060121010A1Elicit immune responseInhibit progressOrganic active ingredientsBiocideEpitopeChorionic gonadotropins
The invention relates to immunotherapy of a mammalian subject by exposing the immune response cells of the subject to a nucleic acid construct encoding at least one hCG immunogenic epitope or precursor thereof such that the nucleic acid construct is taken up and processed by the immune response cells. The invention further relates to compositions comprising such hCG-encoding nucleic acid constructs.
Owner:AVI BIOPHARMA
Peptides, combination of peptides, and cell based medicaments for use in immunotherapy against urinary bladder cancer and other cancers
InactiveUS20190330274A1Enhance stability and solubilityAid in diagnosisPeptide/protein ingredientsAntibody mimetics/scaffoldsWilms' tumorEx vivo
The present invention relates to peptides, proteins, nucleic acids and cells for use in immunotherapeutic methods. In particular, the present invention relates to the immunotherapy of cancer. The present invention furthermore relates to tumor-associated T-cell peptide epitopes, alone or in combination with other tumor-associated peptides that can for example serve as active pharmaceutical ingredients of vaccine compositions that stimulate anti-tumor immune responses, or to stimulate T cells ex vivo and transfer into patients. Peptides bound to molecules of the major histocompatibility complex (MHC), or peptides as such, can also be targets of antibodies, soluble T-cell receptors, and other binding molecules.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
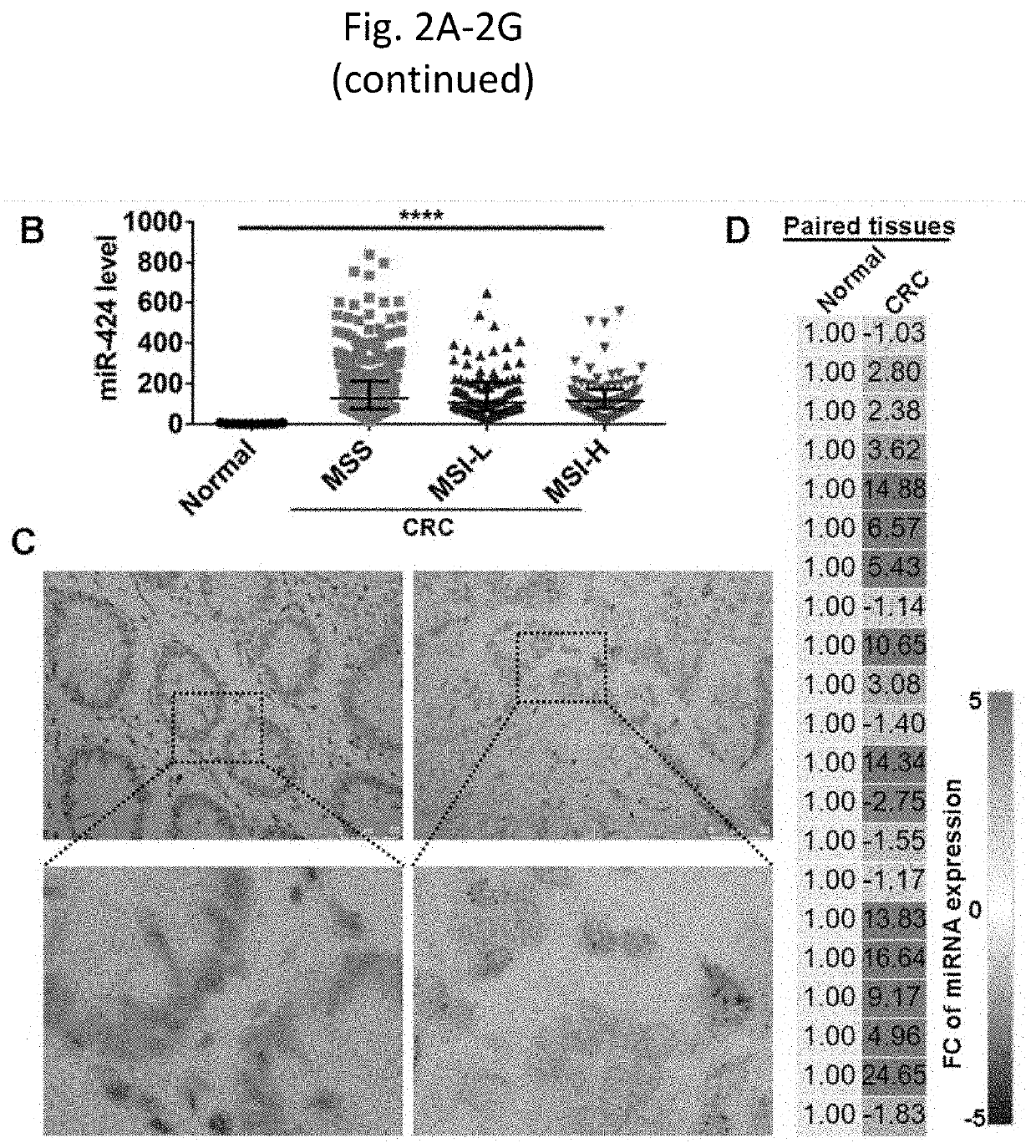
Tumor cell-derived exosomes and method of treating colorectal cancer
PendingUS20210220456A1Enhance immune responseReduce decreaseOrganic active ingredientsHydrolasesExtracellular vesicleImmuno suppression
The present invention provides tumor-derived extracellular vesicles (EVs) lacking an immune suppressive factor, for example, miR-424, methods of making and methods of use for treating cancer. Further the present invention provide vaccine compositions comprising modified tumor-derived EVs for use in treating secondary tumors.
Owner:RGT UNIV OF MINNESOTA
Immunoenhancer, immunotherapy medicine composition, preparation method of composition and application of immunoenhancer and composition
ActiveCN108567977AStrong immune responseImprove immunityVirus peptidesAntiviralsDiseaseGranular cell
The invention discloses an immunoenhancer, an immunotherapy medicine composition, a preparation method of the immunotherapy medicine composition and an application of the immunoenhancer and the immunotherapy medicine composition. The immunoenhancer at least comprises interferon and granular cell-macrophage colony stimulating factors, and the immunotherapy medicine composition at least comprises antigen and the immunoenhancer. The immunoenhancer can remarkably enhance the immunity of a body and improve the antigen presenting efficiency of the body, so that the body can establish effective immune activation and response. Strong antibody and cellular immune protection reaction and pathogen removing capacity can be generated, and the immunoenhancer can be applied to therapy of diseases and tumors caused by microorganisms such as viruses and bacteria.
Owner:FUDAN UNIV
Immunomagnetic composition, preparation method and use thereof, and kit for treating cancer
ActiveCN108785668AReduce dosageImprove microenvironmentPowder deliveryOrganic active ingredientsSuperparamagnetic iron oxide nanoparticlesFucoidan
An immunomagnetic composition, a preparation method and a use thereof, and a kit for treating a cancer are provided. The immunomagnetic composition comprises a core layer, a shell layer, and an outerlayer. The shell layer is composed of a composite and encapsulates the core layer. The composite is formed by the combination of fucoidan, oxidized dextran, and a plurality of superparamagnetic iron oxide nanoparticles by hydrophobic interaction. The outer layer comprises at least one antibody that is grated on the shell layer so as to form the outer layer. The immunomagnetic composition is applied to preparation of anti-cancer drugs.
Owner:洪明奇
Oncolytic virus therapy
PendingUS20200000862A1Peptide/protein ingredientsAntibody mimetics/scaffoldsPharmaceutical drugT cell
The presently disclosed subject matter relates to tumor infiltrated T cells induced by oncolytic virus (“OV-induced T cells”), methods of making and using said OV-induced T cells for an adoptive T-cell therapy. The presently disclosed subject matter further relates to oncolytic viruses and armed oncolytic viruses, methods of making and using said oncolytic viruses, as well as pharmaceutical compositions and kits comprising said oncolytic viruses.
Owner:UNIVERSITY OF PITTSBURGH
Peptides, combination of peptides, and cell based medicaments for use in immunotherapy against urinary bladder cancer and other cancers
ActiveUS20200055898A1Enhance stability and solubilityAid in diagnosisPeptide/protein ingredientsAntibody mimetics/scaffoldsAntiendomysial antibodiesReceptor
The present invention relates to peptides, proteins, nucleic acids and cells for use in immunotherapeutic methods. In particular, the present invention relates to the immunotherapy of cancer. The present invention furthermore relates to tumor-associated T-cell peptide epitopes, alone or in combination with other tumor-associated peptides that can for example serve as active pharmaceutical ingredients of vaccine compositions that stimulate anti-tumor immune responses, or to stimulate T cells ex vivo and transfer into patients. Peptides bound to molecules of the major histocompatibility complex (MHC), or peptides as such, can also be targets of antibodies, soluble T-cell receptors, and other binding molecules.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
Chimeric antigen receptor (CAR) and application thereof
ActiveCN110981960AImprove packaging efficiencyHigh titerVirusesAntibody mimetics/scaffoldsTumor targetingCD8
The invention discloses a PD-L1 CAR structure, a specific structure of a CAR sequence of the PD-L1 CAR structure is as follows: a CD8 alpha leading peptide-PD-L1 scFv-CD8 alpha transmembrane region, aCD137 intracellular costimulatory domain, a CD247 intracellular activation domain. Tumor targeting and T cell double-activation pathway molecules are integrated into a single PD-L1 chimeric receptorgene, so that the feasibility of treating solid tumors by genetically modified T cells is greatly improved, the preparation cost is reduced, and the product can be applied to treatment of PD-L1 high-expression solid tumors, such as lung cancer, liver cancer, colon cancer and the like.
Owner:GUANGZHOU YIYANG BIO TECH CO LTD
Combination therapy using C-C chemokine receptor 4 (CCR4) antagonists and one or more immune checkpoint inhibitors
ActiveUS11446289B2Reduce tumor weightOrganic active ingredientsColon cancer vaccineChemokine Receptor AntagonistCancer therapy
The present disclosure is drawn to the combination therapy of a C—C Chemokine Receptor 4 (CCR4) antagonist and one or more immune checkpoint inhibitors in the treatment of cancer.
Owner:CHEMOCENTRYX INC
Innate targeting of adoptive cellular therapies
PendingCN111867619AMammal material medical ingredientsBlood/immune system cellsAdoptive cellular therapyDisease
Therapeutic modalities are provided for targeting adoptive cellular therapies to specific sites of disease, involving the use of specific repertoirs of PRR ligands. In effect, innate immune system signaling is provoked so as to facilitate the homing of adoptive immune cells to sites of disease, for example to the site of a solid tumor.
Owner:QU BIOLOGICS INC
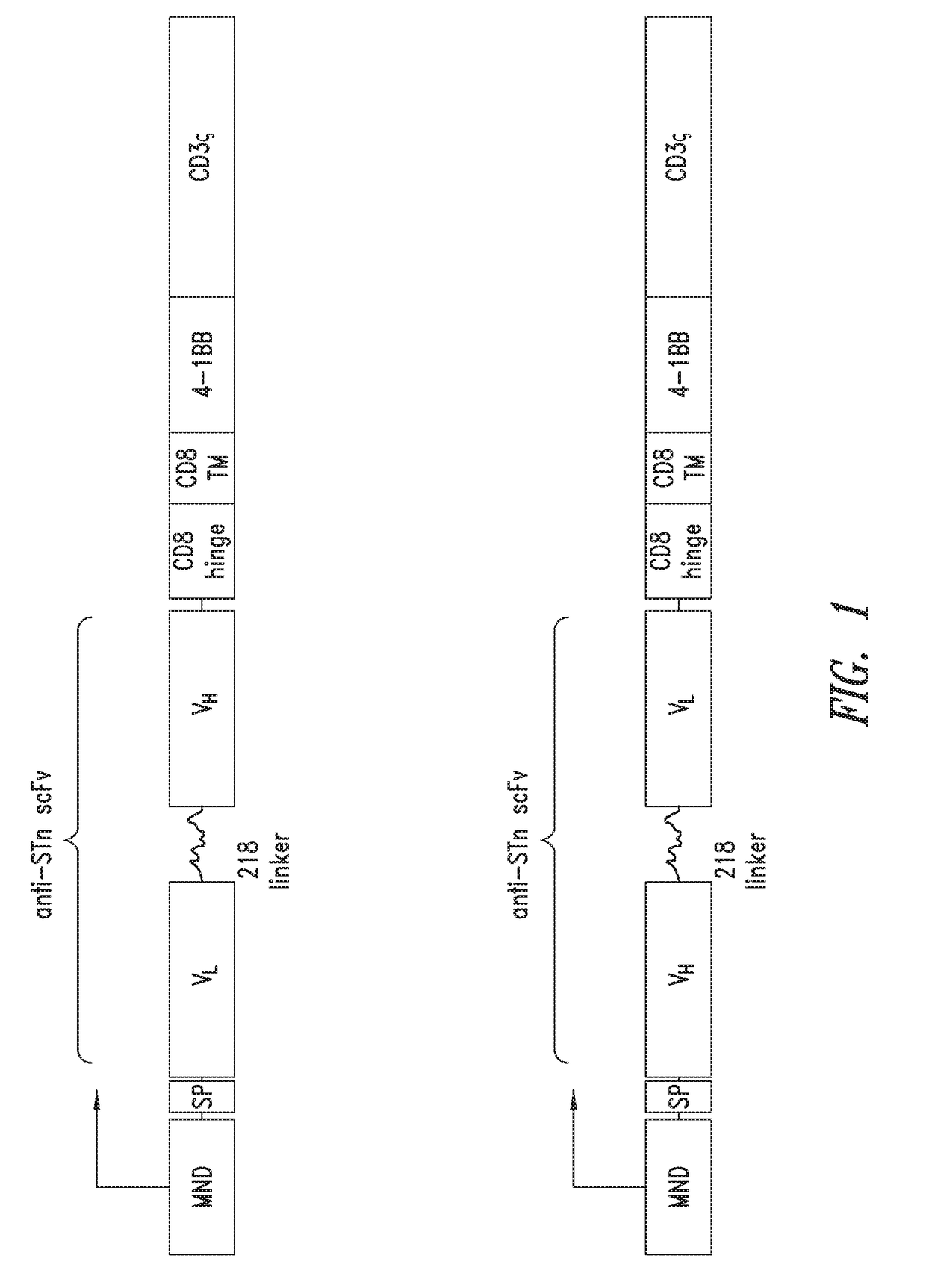
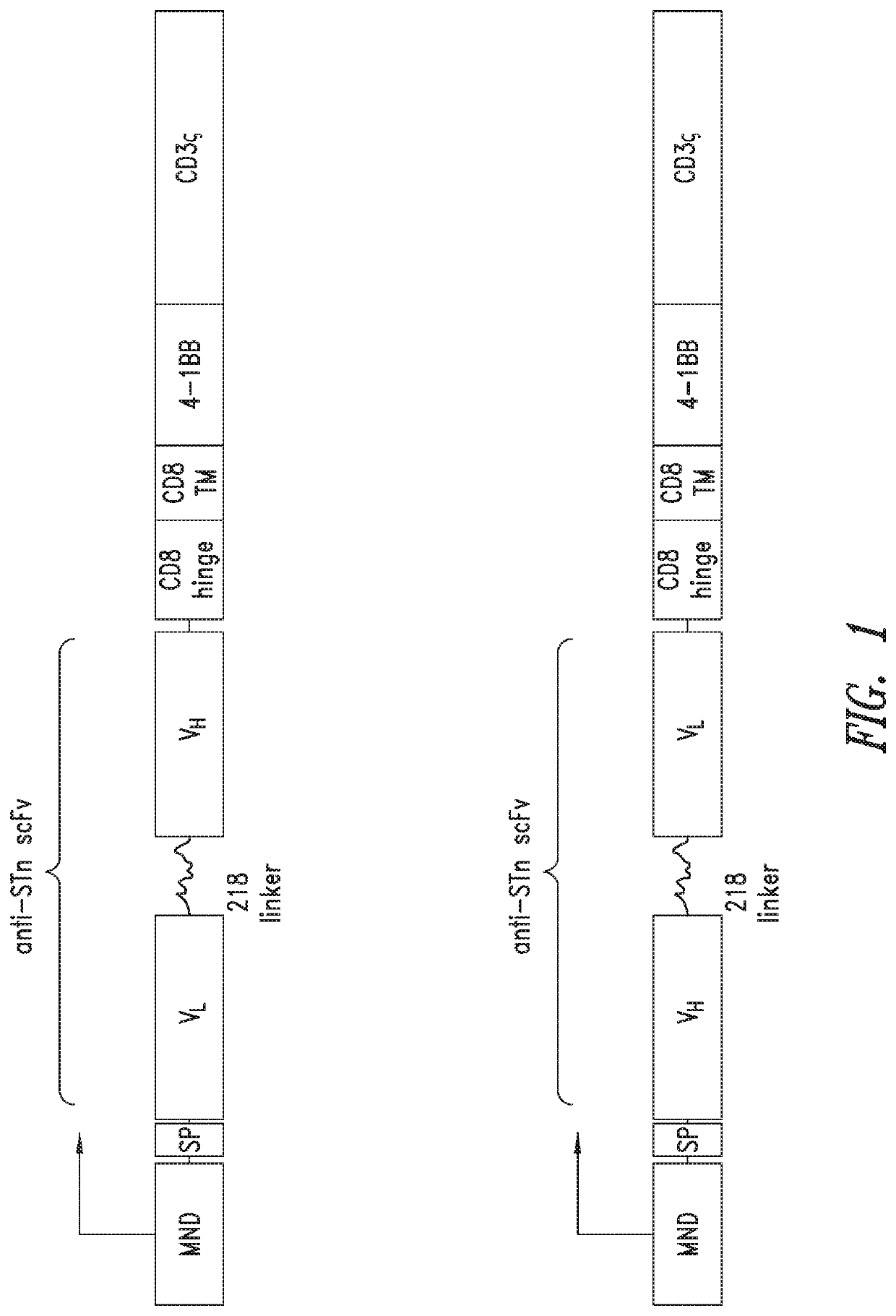
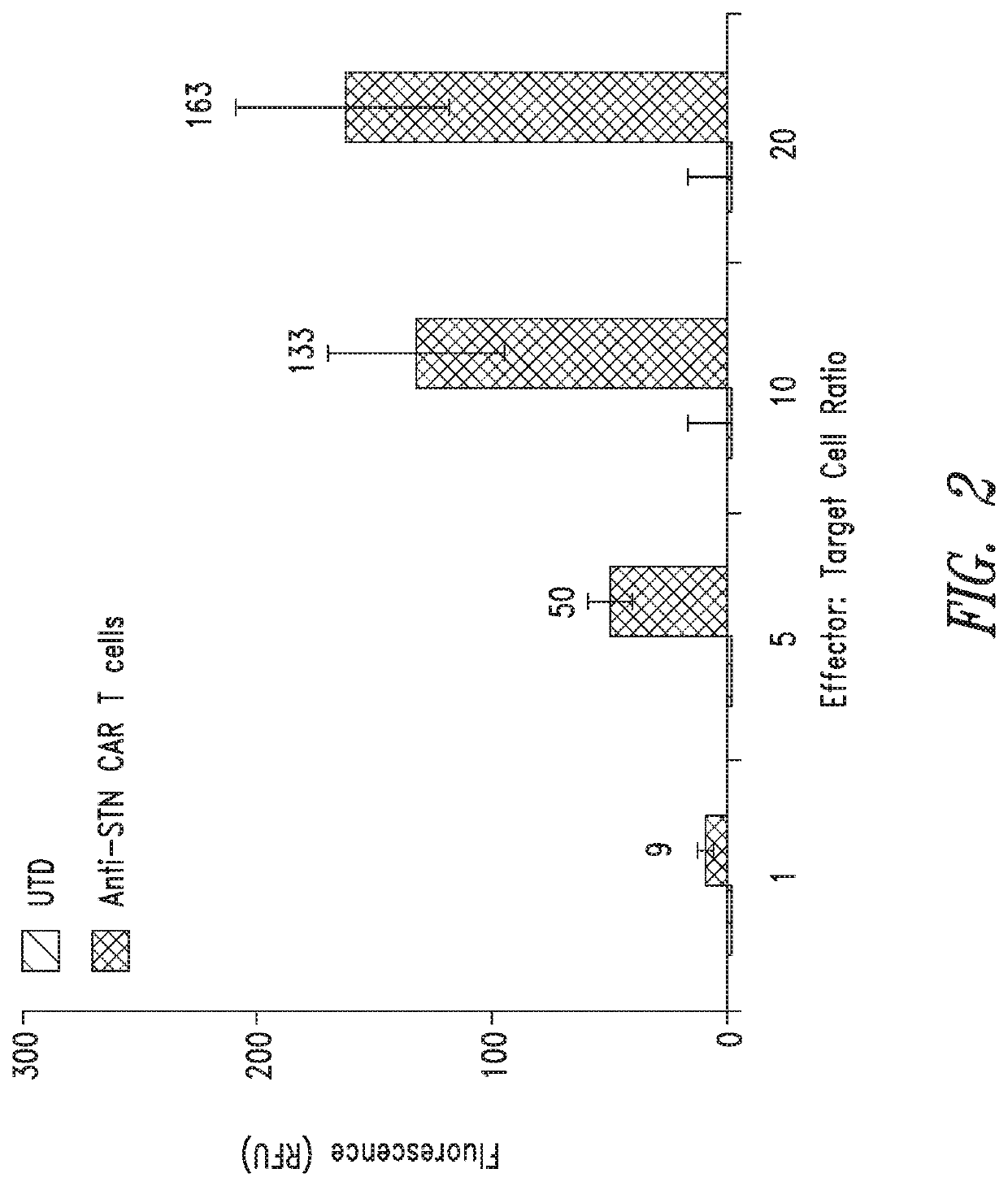
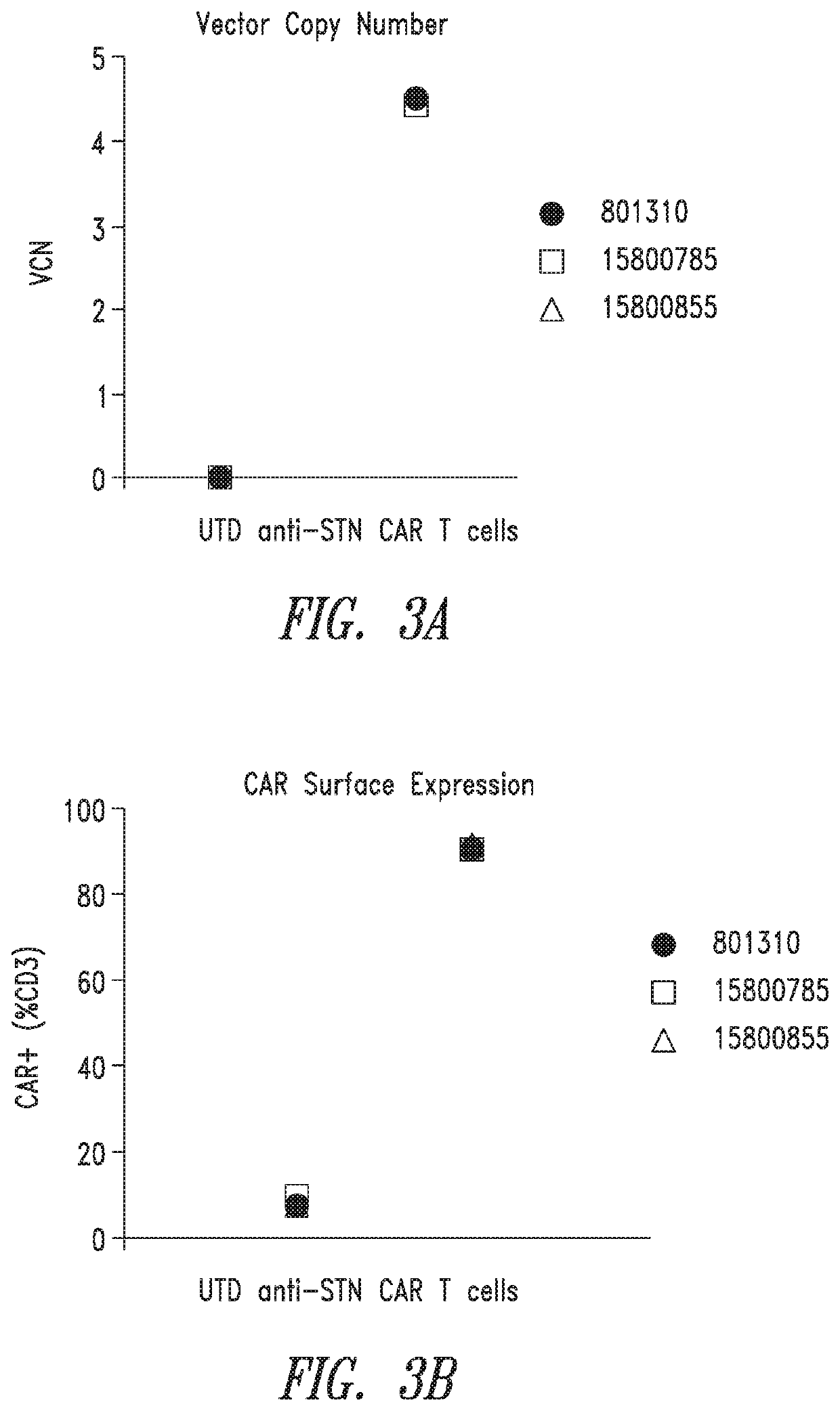
Anti-Sialyl Tn chimeric antigen receptors
ActiveUS11279769B2Maintaining proliferationReduce in quantityImmunoglobulins against cell receptors/antigens/surface-determinantsNGF-receptor/TNF-receptor superfamilySialyl LeAAntigen receptors
The invention provides improved compositions for adoptive cell therapies for cancers that express the glycoepitope STn on TAG-72.
Owner:HELIXMITH CO LTD
Compositions and methods for cancer therapy
PendingCN113474048AOrganic active ingredientsPeptide/protein ingredientsChemical compoundPharmaceutical medicine
One aspect of this disclosure is directed to a method for treating a cancer in a subject in need thereof by administering to the subject at least a first compound and a second compound in any order together or separately. The first compound is an effective amount of a checkpoint inhibitor optionally with at least one pharmaceutically acceptable carrier. The second compound is an effective amount of an Therapeutic Double Stranded RNA (tdsRNA) optionally with at least one pharmaceutically acceptable carrier. The compounds can be administered together or separately. Compositions for the practice of the method are also described.
Owner:AIM IMMUNOTECH INC
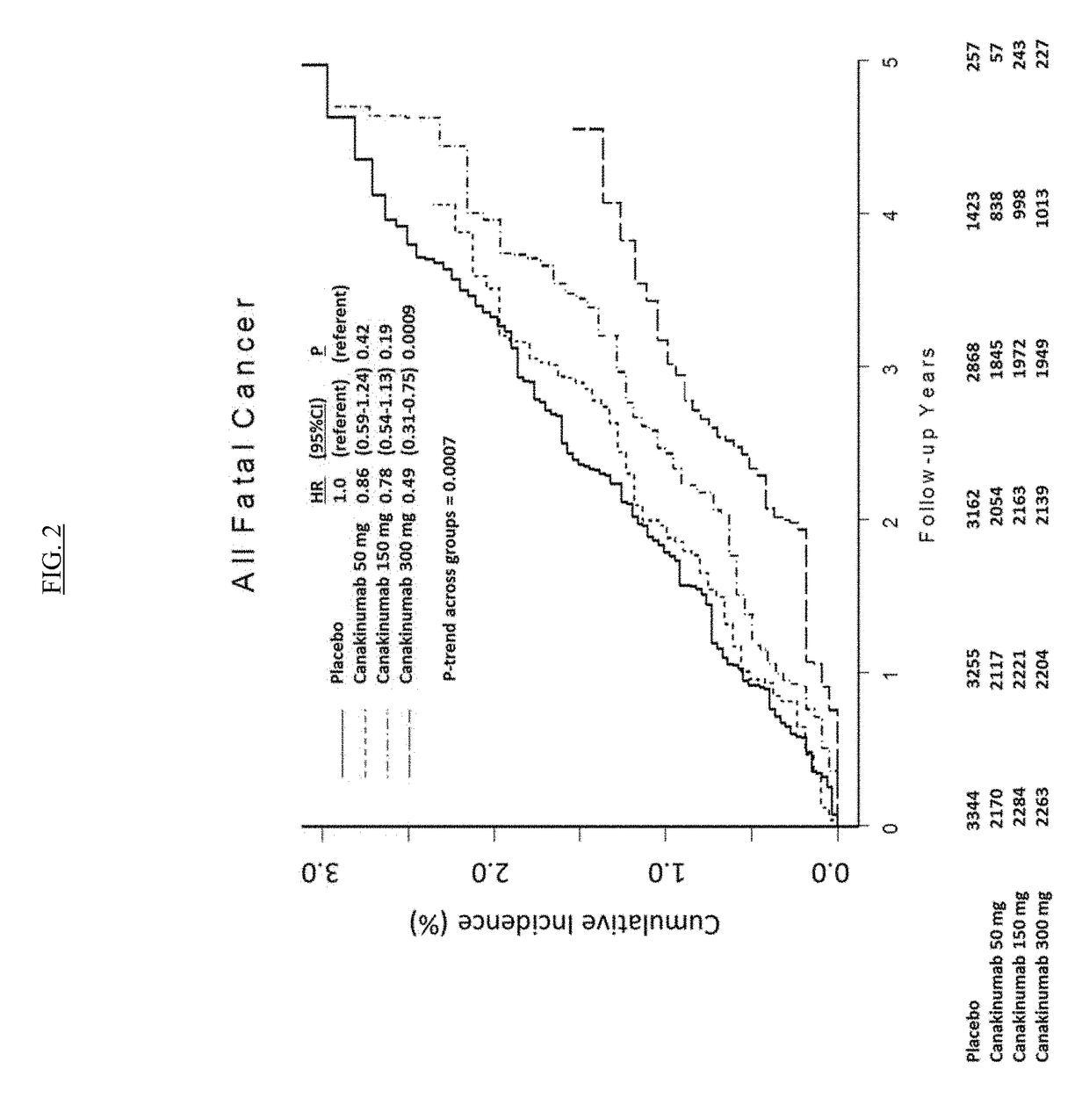
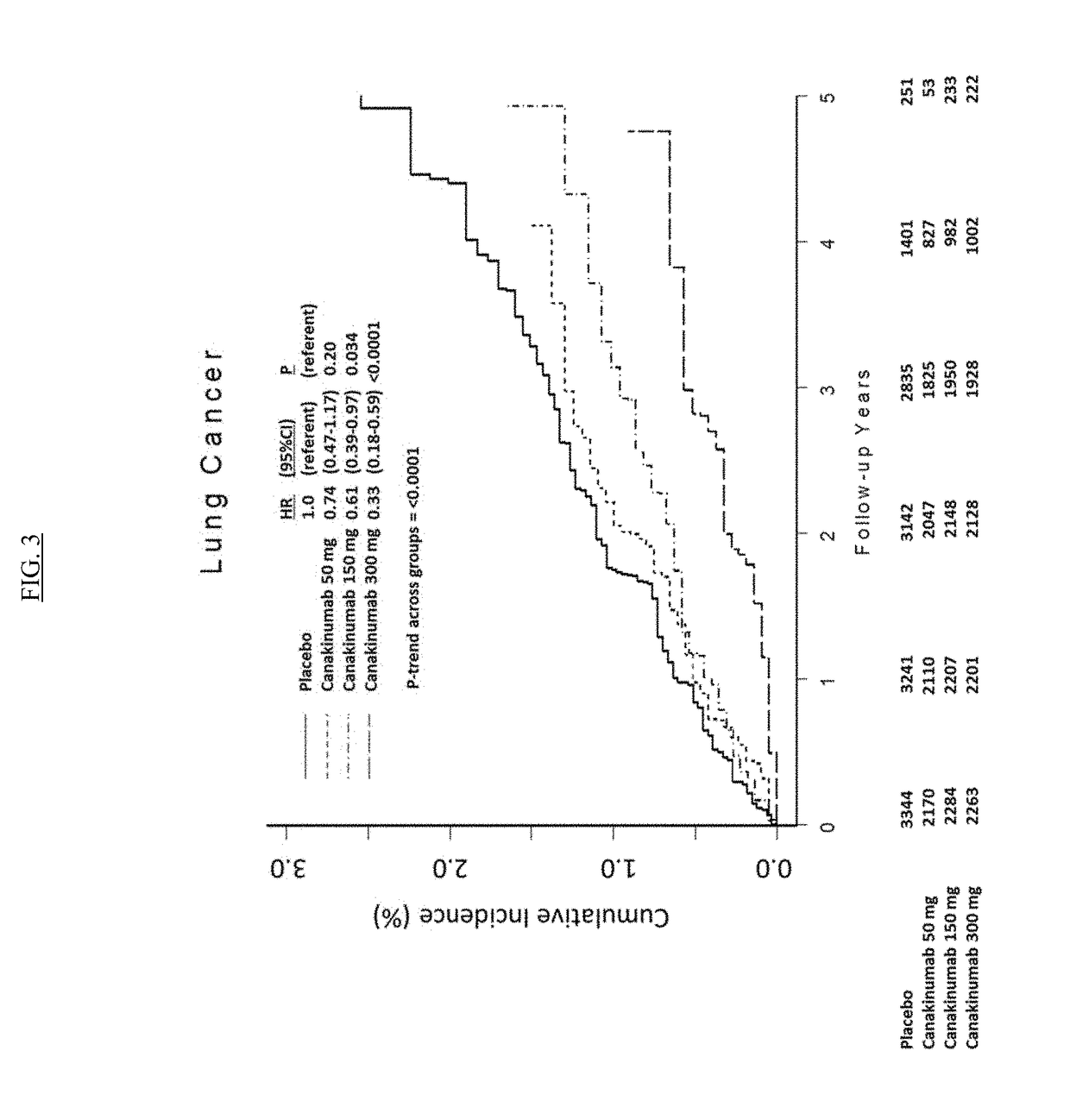
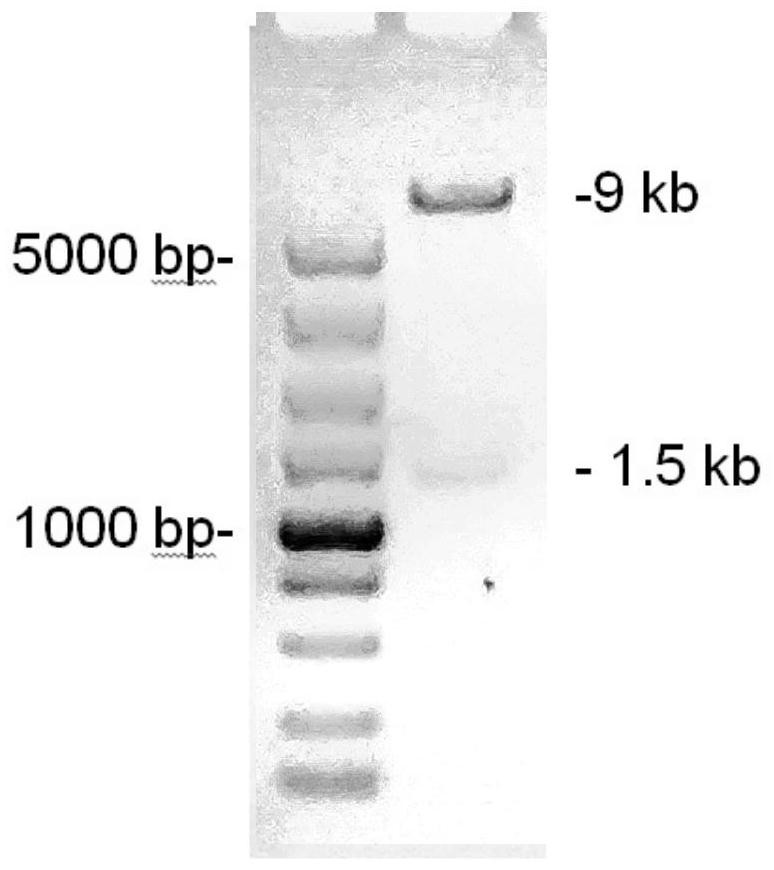
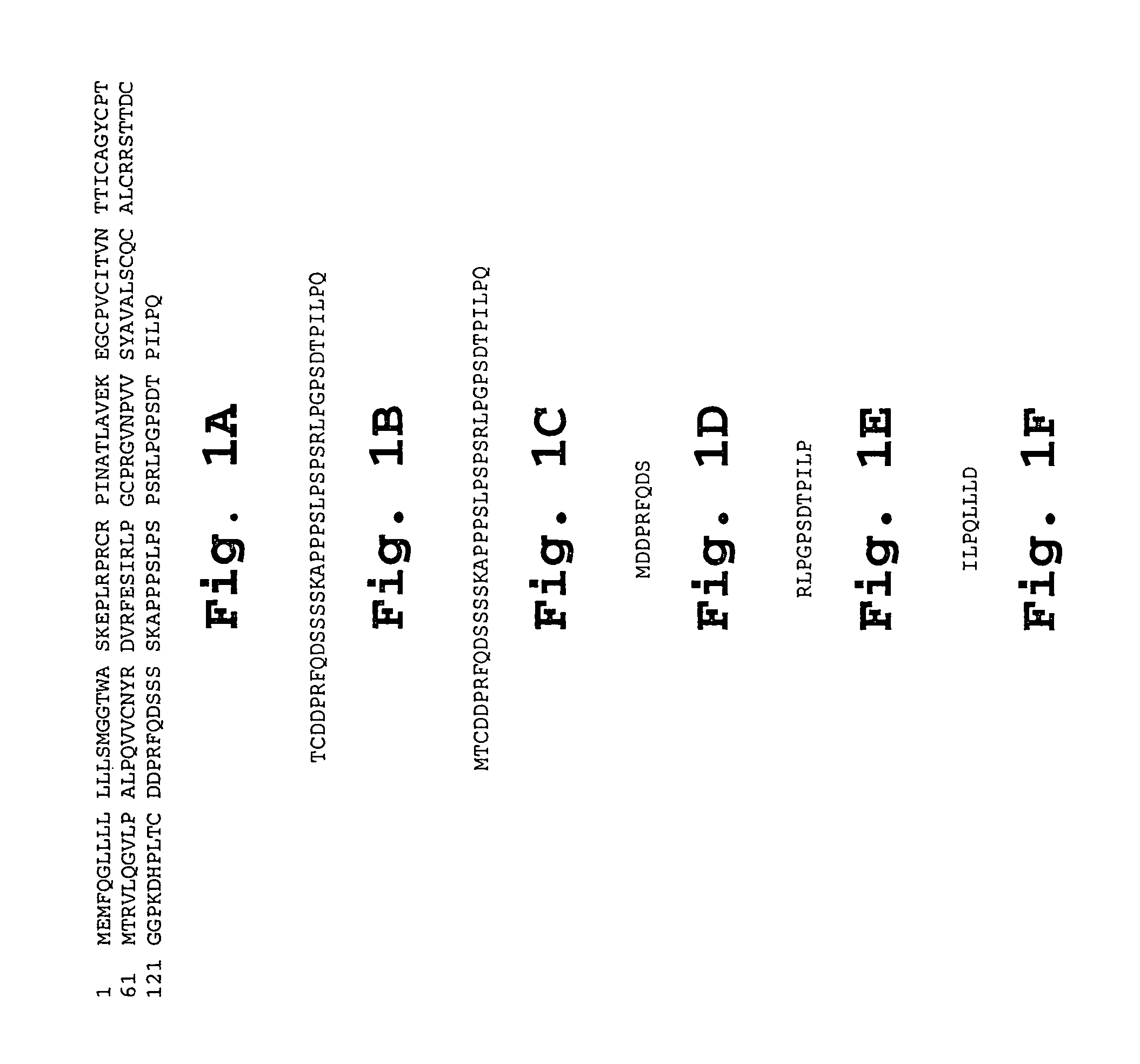
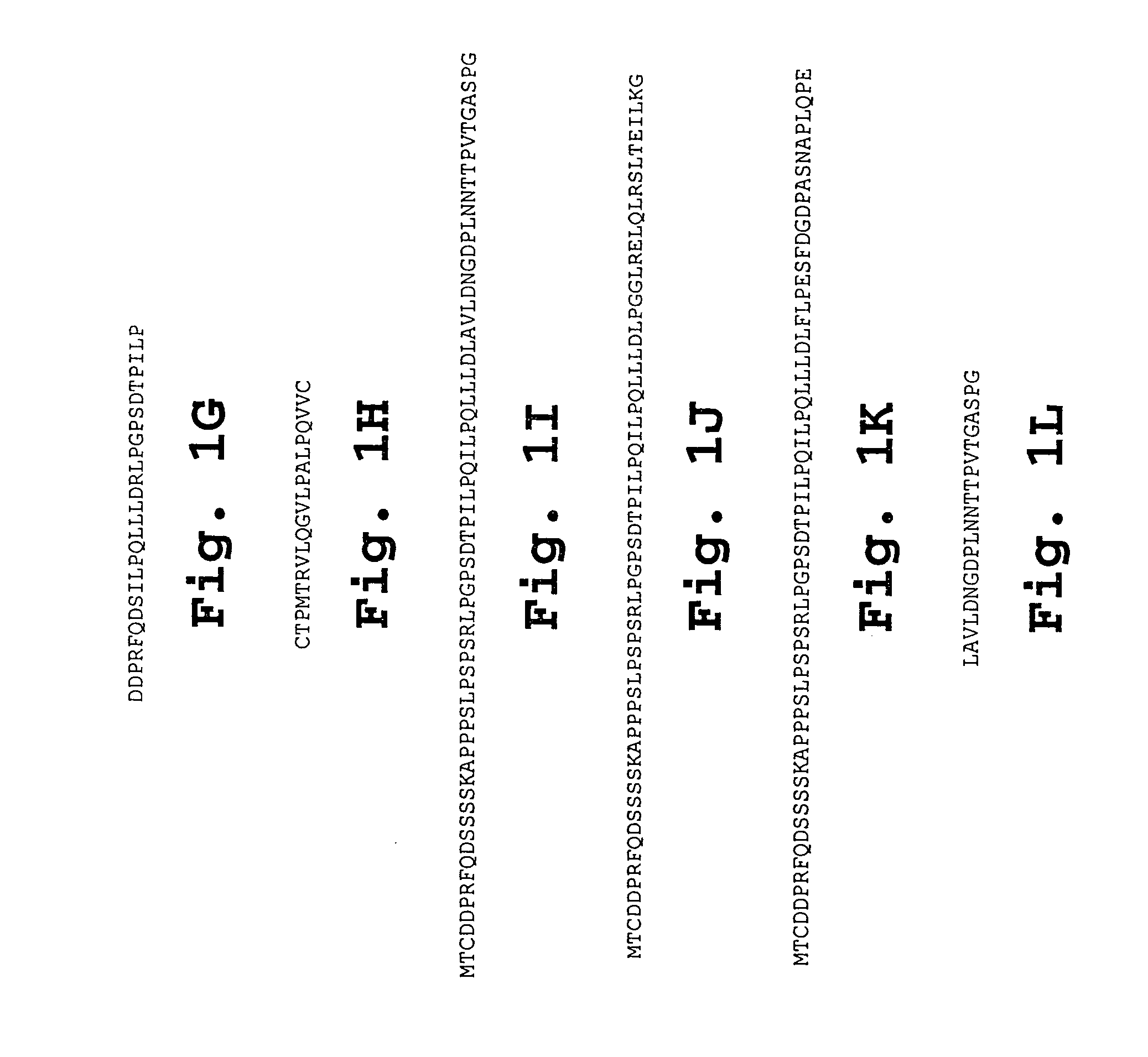
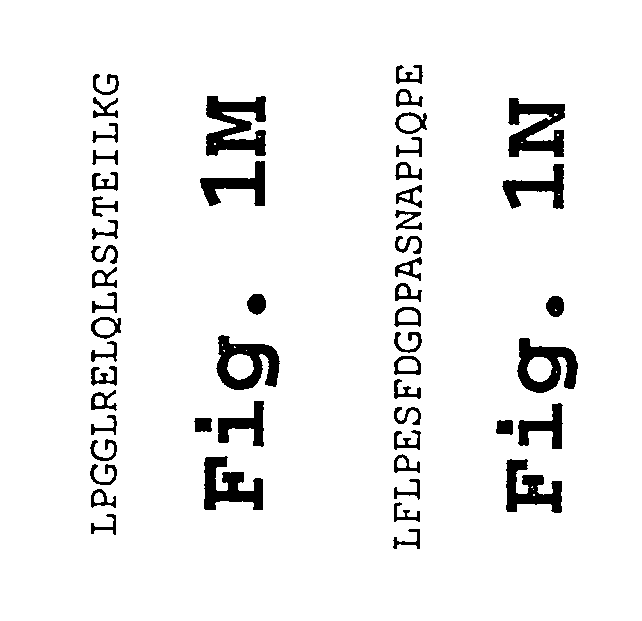
IL-1[beta] binding antibodies for use in treating cancer
PendingCN110831967AConvenient treatmentOrganic active ingredientsIntestine cancer vaccineAntiendomysial antibodiesAntigen binding
Owner:NOVARTIS AG
WT1 polypeptide tumor inhibitor
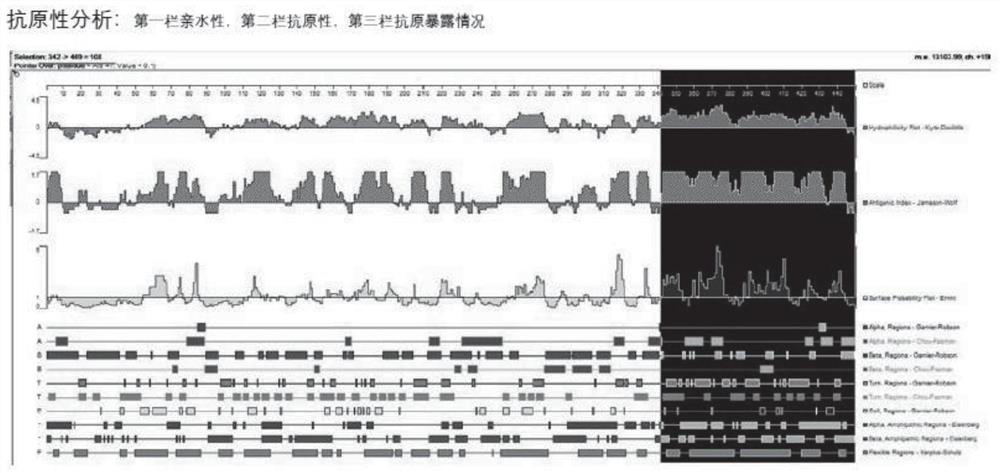
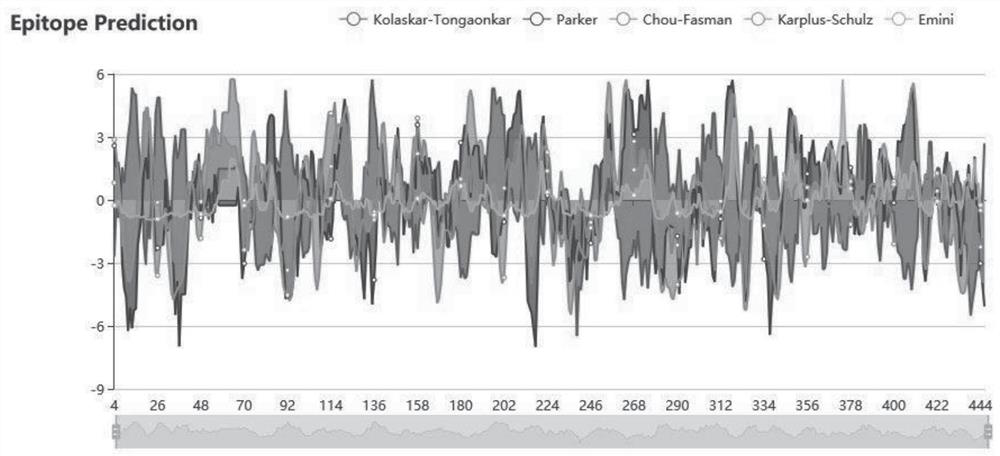
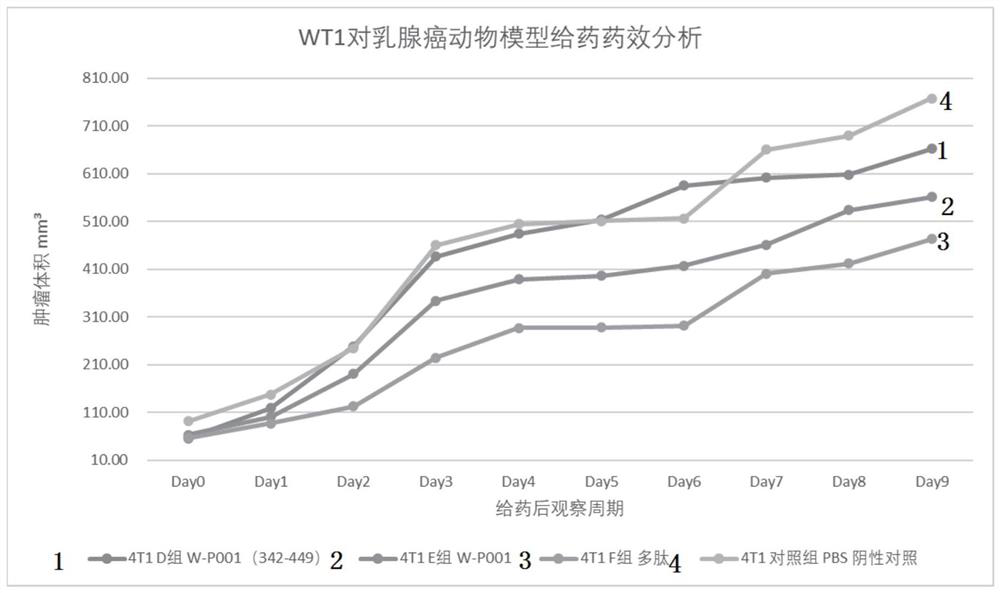
ActiveCN111647066AImprove anti-tumor effectWide pedigreeTumor rejection antigen precursorsColon cancer vaccineProtein s antigenGeneologies
The invention discloses and provides an antigen composed of nine polypeptides from a WT1 protein sequence. According to the antigen, a WT1 target is analyzed through computer software, sequence splitting is carried out on the part, with the best antigenicity, of a protein, and split polypeptides are combined into nine long peptides as antigen sequences. The polypeptide combination disclosed by theinvention has the advantages of better antitumor effect and wider lineage.
Owner:WINLEIN BIOTECH CO LTD
Anti-sialyl tn chimeric antigen receptors
ActiveUS20190085092A1Maintaining proliferationReduce in quantityImmunoglobulins against cell receptors/antigens/surface-determinantsNGF-receptor/TNF-receptor superfamilyAdoptive cellular therapyAntigen receptors
The invention provides improved compositions for adoptive cell therapies for cancers that express the glycoepitope STn on TAG-72.
Owner:HELIXMITH CO LTD
Bacteria-derived vesicles and uses thereof
Non-naturally occurring vesicles derived from bacteria, e.g., pathogenic bacteria, methods for making the vesicles, and methods for using compositions of these vesicles are disclosed. Methods of using the vesicles include prevention and / or treatment of bacterial infections. Also provided herein are compositions that include vesicles derived front bacteria and tumor vesicles, methods for making the tumor vesicles, and methods for using the compositions of bacterial vesicles and tumor vesicles. Methods of using the compositions of bacterial vesicles and tumor vesicles include treatment of cancer in a subject. Tumor vesicles may be derived from cancer cells present in the subject to be treated or from a cancer cell line expressing at least one neoantigen. The neoantigen may be specific to the subject and may have been identified by sequencing of the cancer cells from the subject. The neoantigen may be a neoantigen known to be commonly expressed in a particular type of cancer.
Owner:EXOCURE BIOSCIENCES INC
Compound for treating colon cancer and application thereof
PendingCN112957470AImplement federated applicationsImprove anti-tumor effectEnergy modified materialsColon cancer vaccineLiposomeNiosome
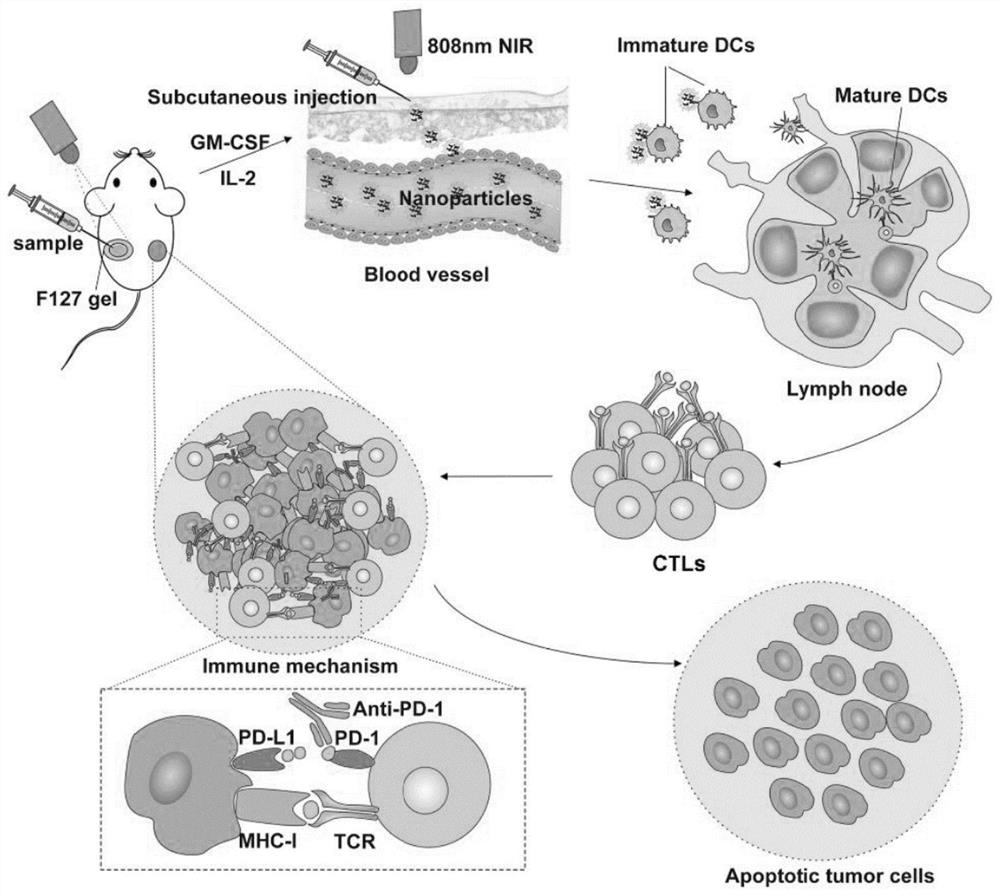
The invention discloses a compound for treating colon cancer and application thereof. The compound comprises black phosphorus quantum dots, liposomes, Adpgk peptides and F127 gel; the Adpgk peptides and the black phosphorus quantum dots are wrapped by the liposomes to form Adpgk peptide black phosphorus quantum dot liposomes; the Adpgk peptide black phosphorus quantum dot liposomes are embedded in the F127 gel. According to the invention, the black phosphorus quantum dots with good photothermal effect and Adpgk polypeptides are effectively loaded by the liposomes, so that the combined application of a photothermal method and an immune method is realized; meanwhile, it is found that application of the black phosphorus quantum dots loaded Adpgk polypeptide liposome (Adpgk polypeptide / BPQDs / Liposome) has more advantages in the anti-tumor effect than application of the black phosphorus quantum dots loaded composite liposome, and it is indicated that the anti-tumor effect can be effectively enhanced through combined application of thermal therapy and immunological therapy.
Owner:SUN YAT SEN UNIV SHENZHEN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
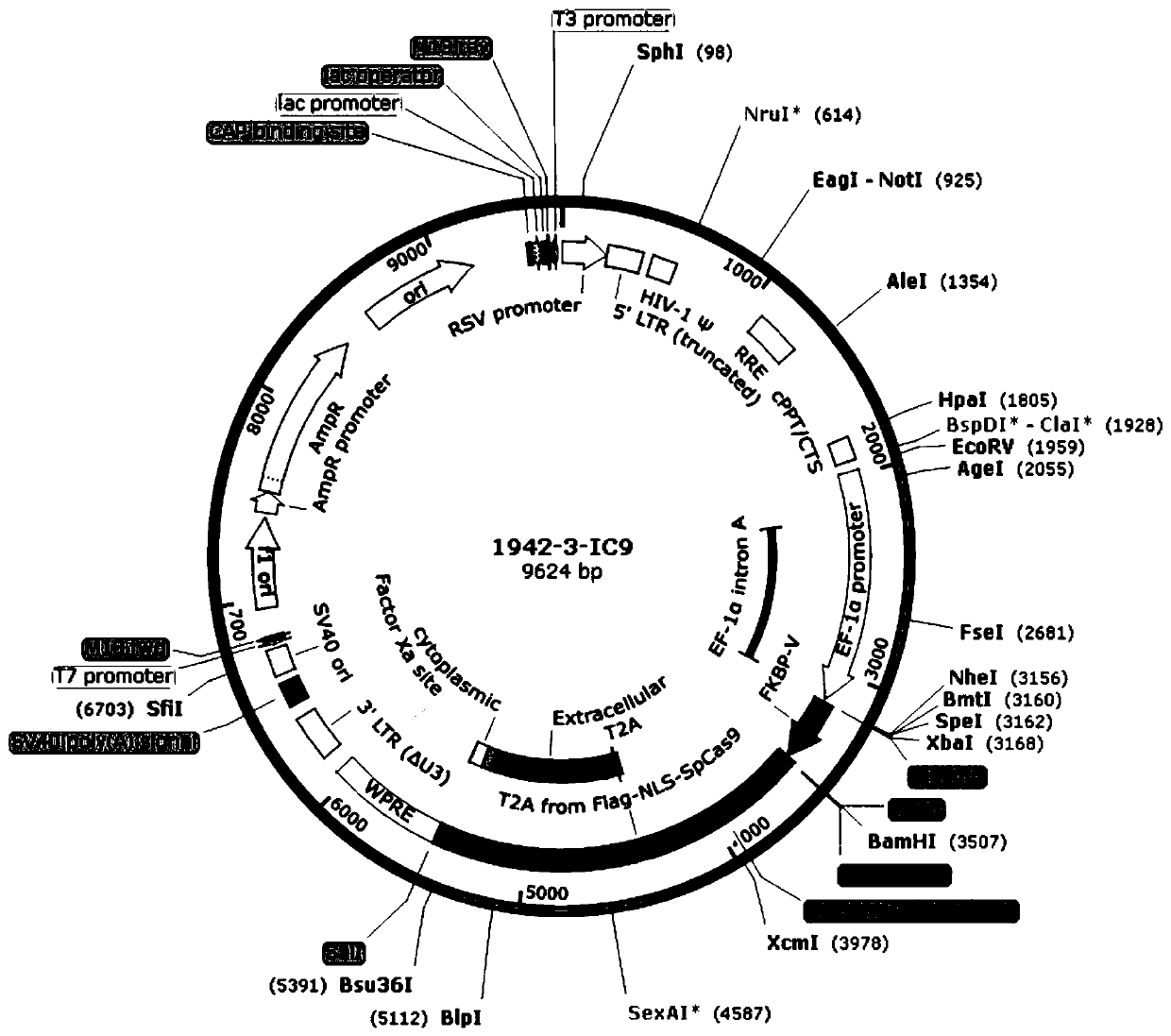
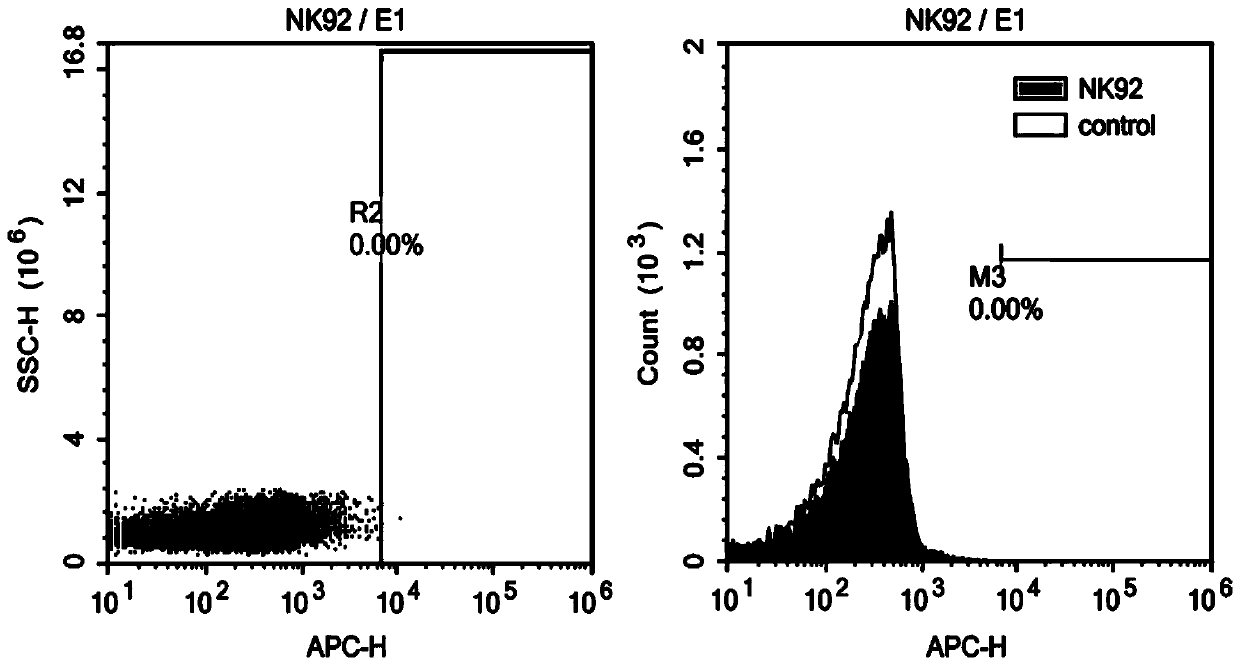
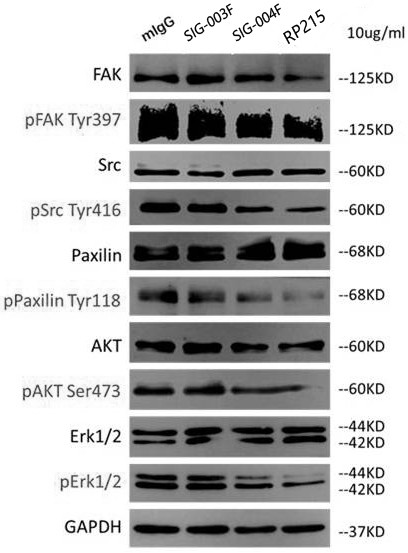
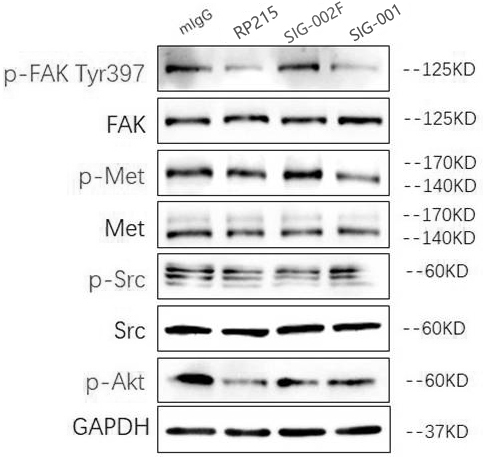



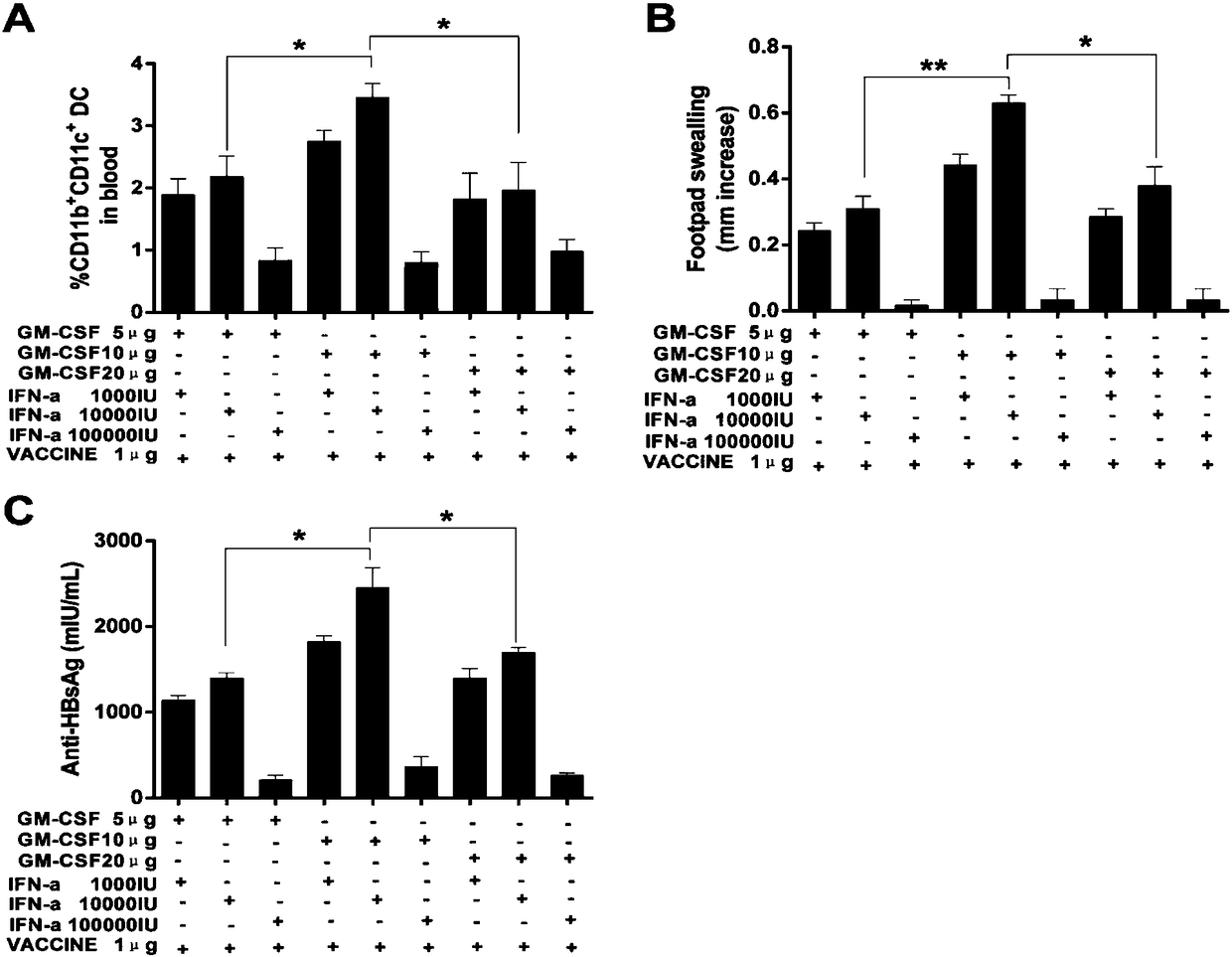
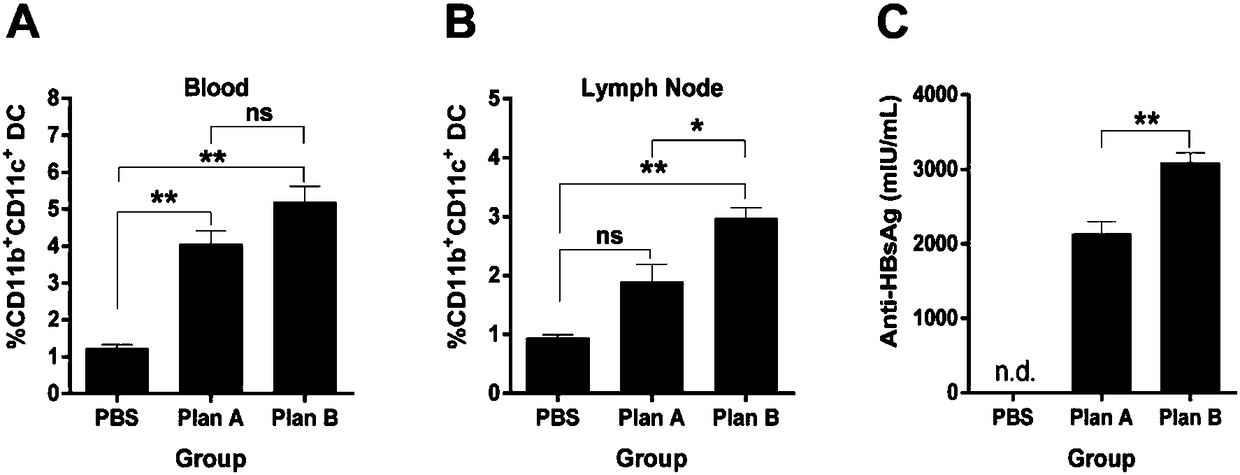
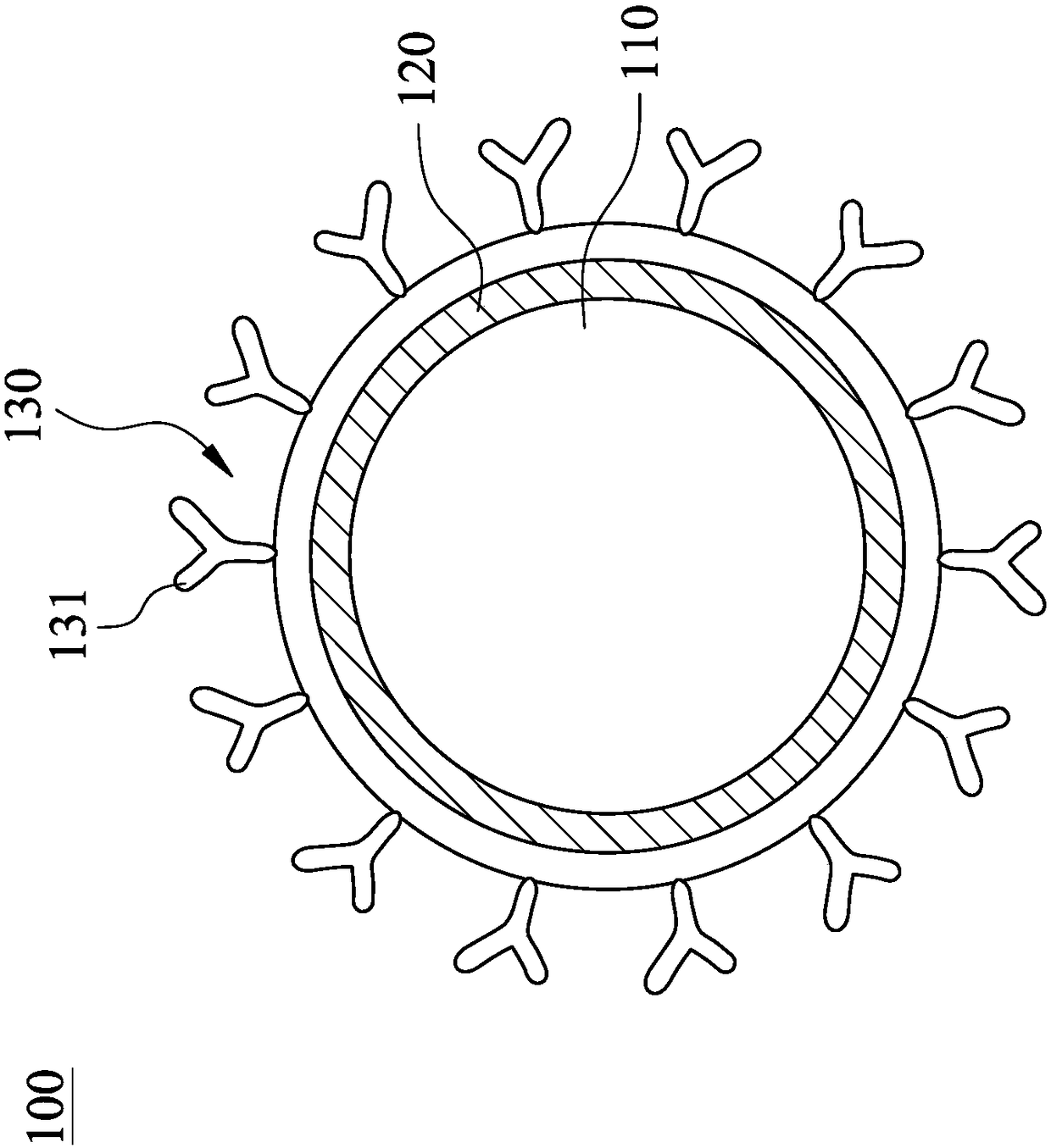
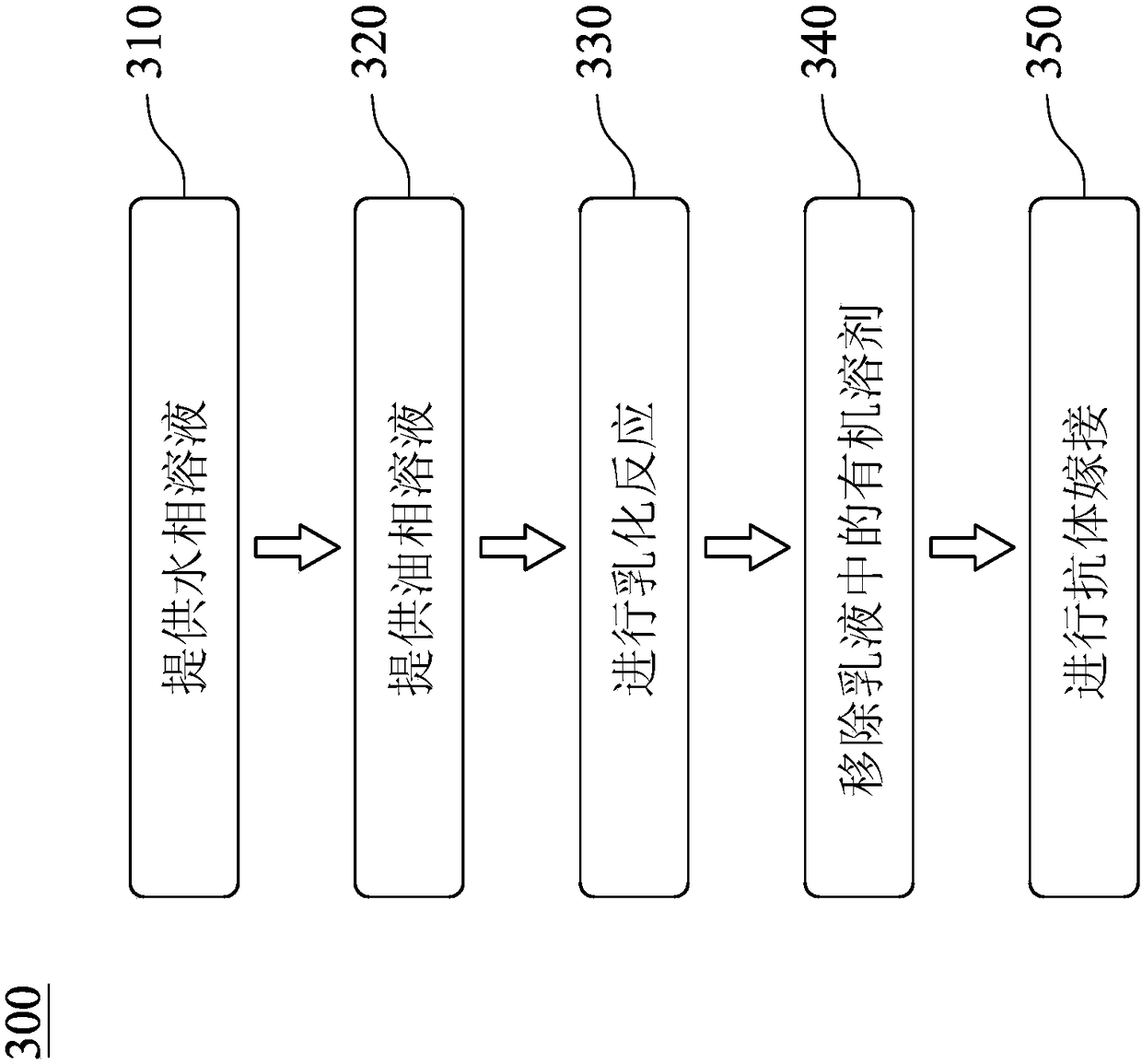





![IL-1[beta] binding antibodies for use in treating cancer IL-1[beta] binding antibodies for use in treating cancer](https://images-eureka.patsnap.com/patent_img/6d284657-23c7-4a72-af64-d4f0cdde0f9e/HDA0002328181950000011.png)
![IL-1[beta] binding antibodies for use in treating cancer IL-1[beta] binding antibodies for use in treating cancer](https://images-eureka.patsnap.com/patent_img/6d284657-23c7-4a72-af64-d4f0cdde0f9e/HDA0002328181950000021.png)
![IL-1[beta] binding antibodies for use in treating cancer IL-1[beta] binding antibodies for use in treating cancer](https://images-eureka.patsnap.com/patent_img/6d284657-23c7-4a72-af64-d4f0cdde0f9e/HDA0002328181950000031.png)