Chimeric antigen receptor of cell for targeted expression of Claudin 18.2 and application of chimeric antigen receptor
A technology of chimeric antigen receptors and expression vectors, applied in the field of cellular immunotherapy, can solve the problem of no significant improvement in patient survival
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0148] Construction of chimeric antigen receptor expression plasmid containing scFv targeting CLDN 18.2
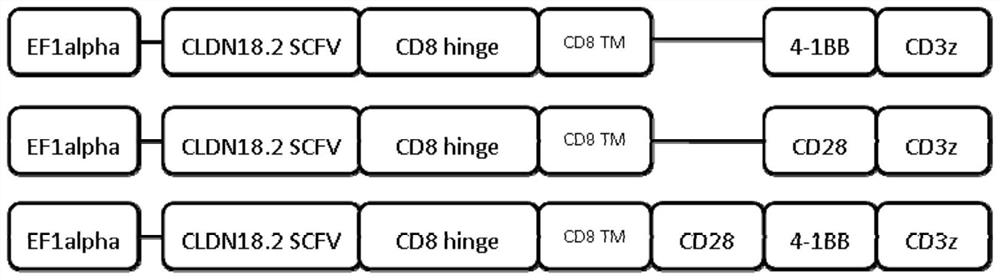
[0149] By artificially synthesizing a DNA fragment with a length of 1461bp shown in SEQ ID NO.:13, the nucleotides at positions 1-63 encode a leader, and the nucleotides at positions 64-789 encode CLDN 18.2scFv (targeting cancer scFv of embryonic antigen), the nucleotides at 790-924 encode the CD8 hinge region, the nucleotides at 925-996 encode the CD8 transmembrane region, the nucleotides at 997-1122 encode 4-1BB, and the nucleotides at 997-1122 encode 4-1BB. Nucleotides 1123-1458 encode CD3ζ.
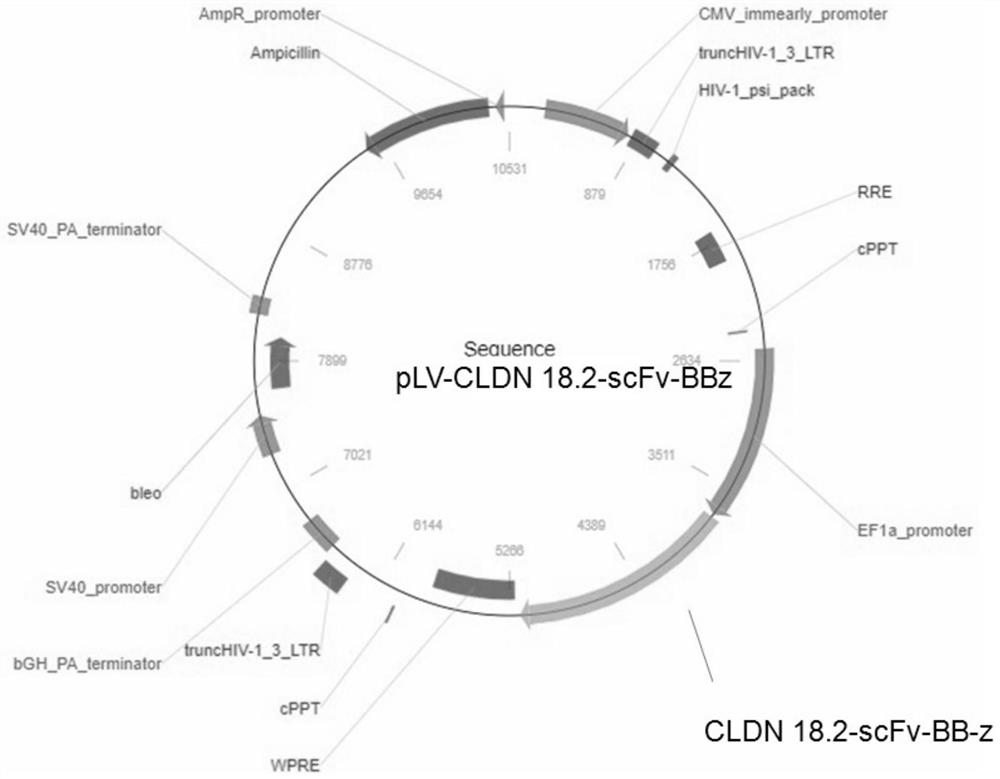
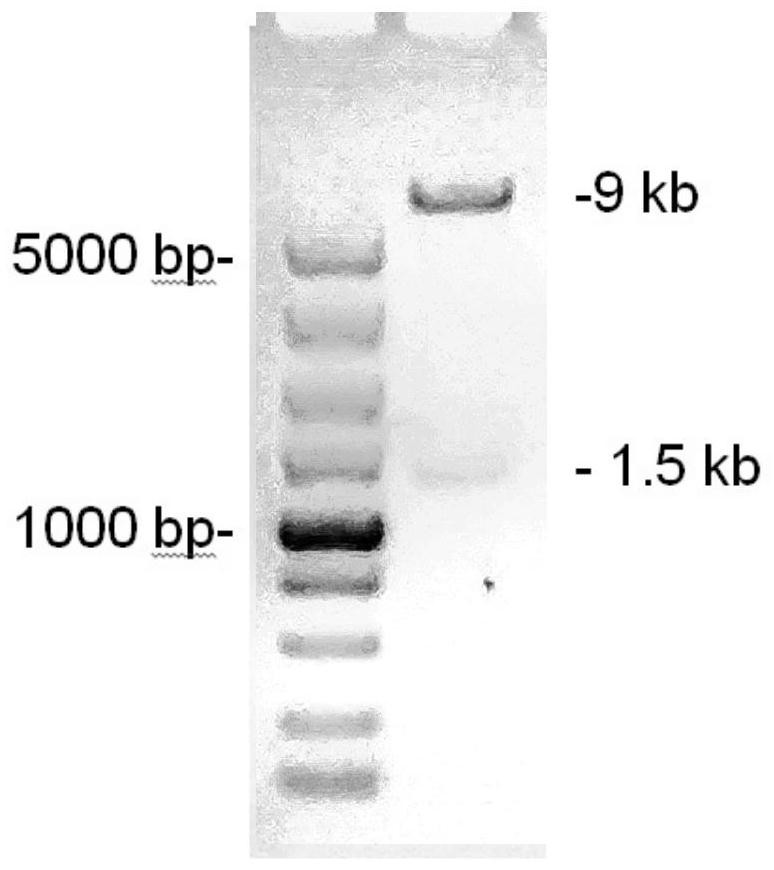
[0150] The above DNA fragment was inserted into the downstream of the EF1alpha promoter of the lentiviral expression vector to obtain a chimeric antigen receptor expression plasmid (pLV-CLDN 18.2-scFv-BBz) targeting CLDN18.2. image 3 It is an electrophoresis identification diagram of the restriction endonuclease Xba I restriction endonuclease Xba I inserted into the lentiviral e...
Embodiment 2
[0152] Transfection of T cells with a chimeric antigen receptor expression plasmid targeting CLDN 18.2
[0153] (1) Packaging preparation of lentivirus
[0154] The chimeric antigen receptor expression plasmid, structural plasmid, and envelope plasmid were transfected into 293T / 17 cells at a ratio of 3:2:1 by calcium phosphate transfection method. After 12 hours of transfection, the medium was replaced with fresh DMEM medium containing 10% FBS, and sodium butyrate was added at a final concentration of 5 mM at the same time. After 48 hours of transfection, the cell culture supernatant containing the virus was aspirated into a centrifuge tube, centrifuged at 1500 rpm at 4 °C for 10 min, transferred the supernatant to a new centrifuge tube, filtered through a 0.45 μm filter and stored at -80 °C.
[0155] (2) Preparation of T cells
[0156] Take 10ml of fresh blood from a healthy person, and use lymphocyte separation medium (Yunfei Biology) to separate peripheral blood mononucle...
Embodiment 3
[0161] The specific killing activity of CAR-T cells against CLDN18.2-positive malignant cells
[0162] Take 5×10 4 K562 cells highly expressing CLDN18.2 (CLDN18.2 positive, represented by CLDN18.2-K562) and control K562 cells highly expressing CLDN18.1 (CLDN18.2 negative, represented by CLDN18.1-K562) were inoculated at 96 Orifice plate, add CAR-T cells (CLDN18.2-CART) into the two kinds of cell culture wells according to the three ratios of effector cells (Effector): target cells (Target) = 10:1, 3:1, 1:1 , CAR-T positive control cells (CLDN18.2-CART positive control) and unmodified T cells (T). After incubation for 18 hours, the specific killing activity of CAR-T cells on K562 cells highly expressing CLDN18.2 was detected according to the instructions of the LDH Cytotoxicity Analysis Kit (Beiyuntian). The results showed that the CAR-T cell group (group 1) had a stronger effect on K562 cells highly expressing CLDN18.2 compared with the CAR-T positive control cell group (gro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com