Double-chimeric antigen receptor, T cell and construction method and application thereof
A chimeric antigen receptor and cell technology, applied in the direction of receptor/cell surface antigen/cell surface determinant, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, animal cells, etc., can solve normal Tissue damage, safety issues, cytokine release syndrome and other issues, to achieve the effect of improving safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Determination of the whole gene sequence of double CAR
[0075] 1.1 Obtain the gene sequence of hMSLNscFv and hCEAscFv, analyze and optimize their gene sequences to ensure that they are more suitable for high-efficiency expression in human T lymphocytes under the condition that the encoded amino acid sequence remains unchanged. For the sequence information of the hMSLNscFv gene, see SEQUENCE LISTING (SEQ ID NO.3); for the sequence information of the hCEAscFv gene, see SEQUENCE LISTING (SEQ ID NO.5).
[0076] 1.2 Obtain the gene sequences of human CD8α signal peptide gene, human CD8α hinge region gene, human CD8 transmembrane region and intracellular region gene, and human CD3ζ and 4 / 1BB signal domain.
[0077] 1.3 The above gene sequence was sequenced according to human CD8α signal peptide, hMSLNscFv, human CD8α hinge region, human CD8 transmembrane region and intracellular region, human 4 / 1BB co-stimulatory signal domain, IRES sequence, human CD8α signal peptide, hCEAs...
Embodiment 2
[0079] Construction of expression plasmids
[0080] 2.1 The optimized and complete double chimeric receptor gene sequence was subjected to whole gene synthesis, and cloned into The overexpression vector pFC-MCS of the site-specific recombinase system, and finally the recombinant plasmid pFC-dCAR was constructed, and the GFP fluorescent tag was introduced to facilitate the detection and tracking of the constructed dCAR-T cells.
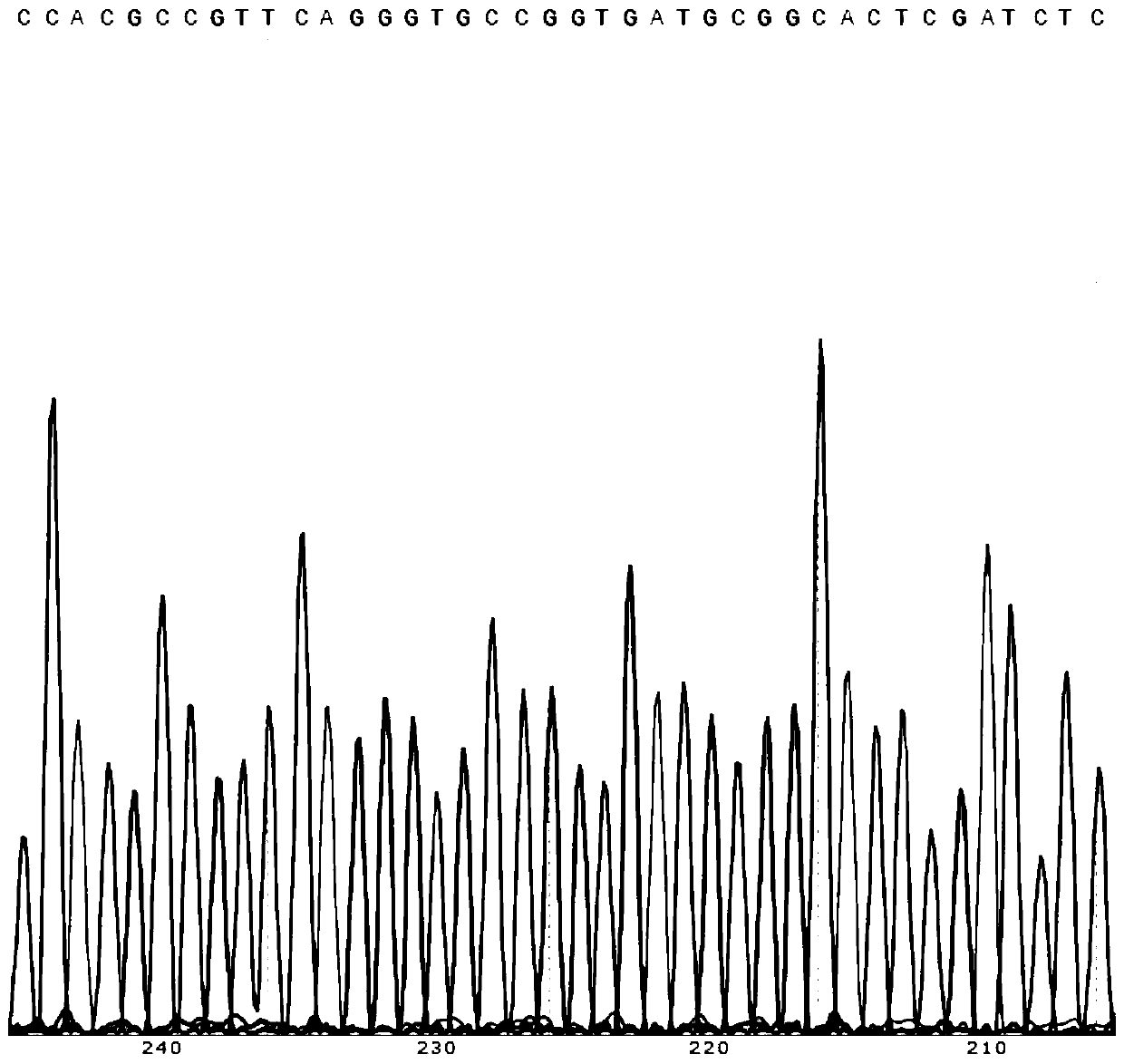
[0081] 2.2 Sequence the recombinant plasmid, and compare the sequencing result with the designed CAR gene sequence. The results confirm that the synthetic sequence obtained is correct and that the target gene fragment has been connected to the corresponding overexpression vector.
[0082] See attached for some sequencing results figure 2 .
Embodiment 3
[0084] control group genetic design
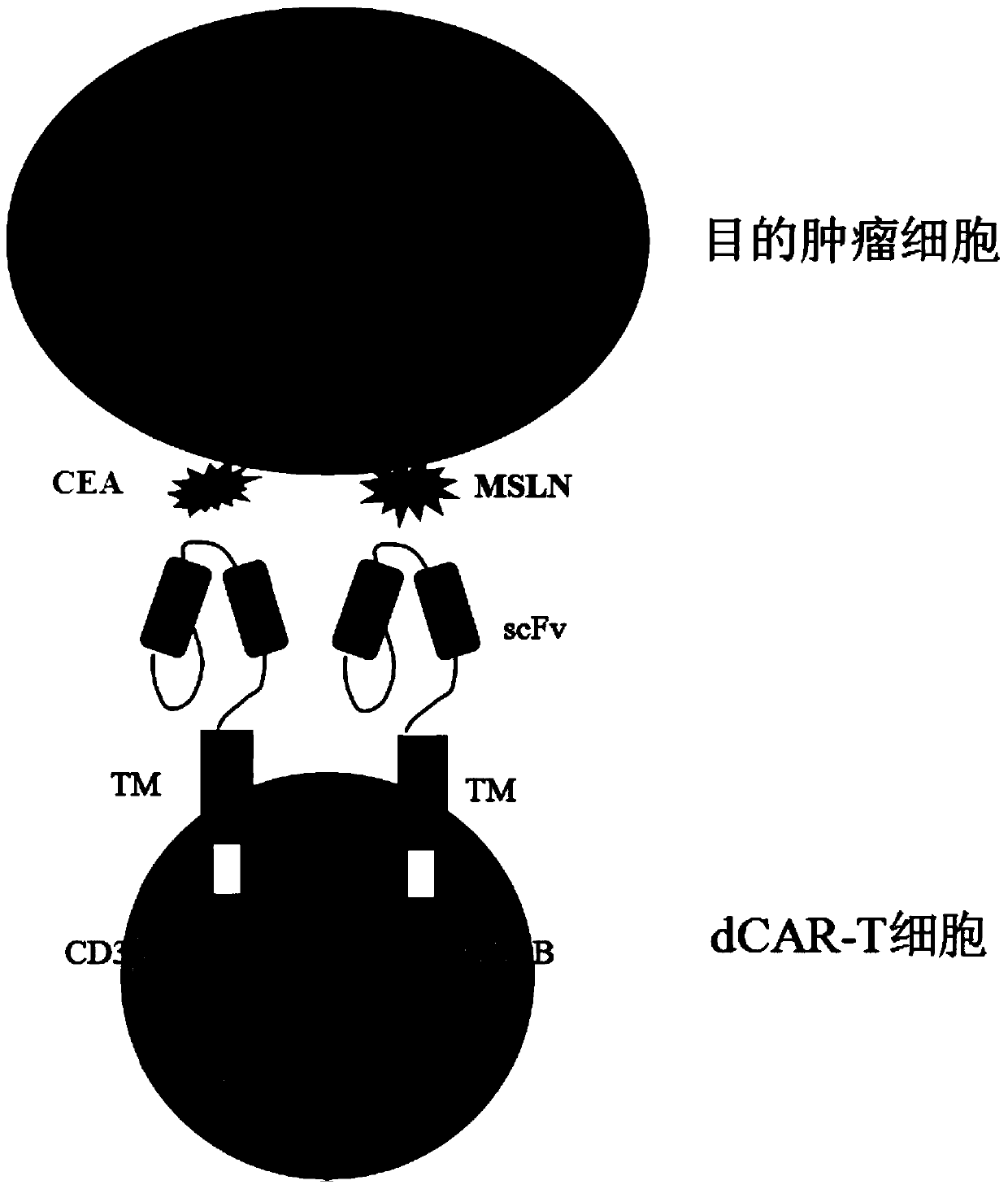
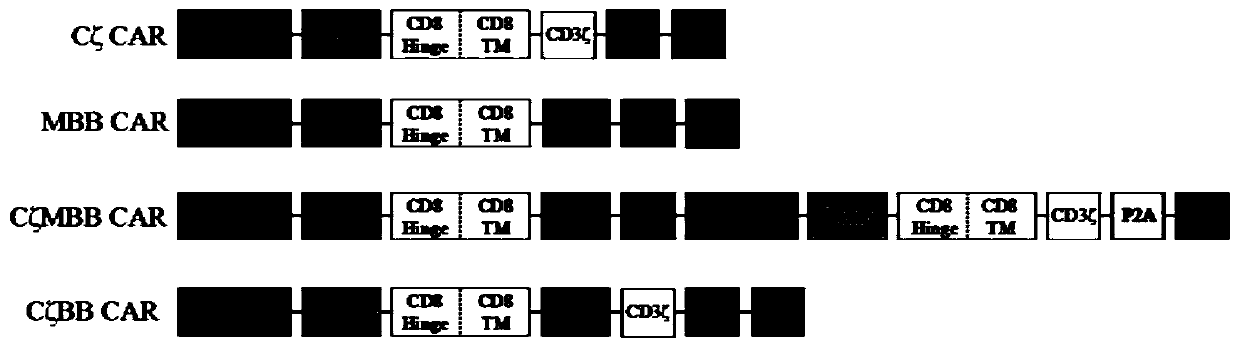
[0085] In order to fully verify the effectiveness and safety of the designed dual-receptor CAR-T cells, multiple control groups were designed in the experiment. That is, two negative control structures: CAR1, Signal peptide-hCEAscFv-CD8α-CD8TM-CD3ζ-IRES-GFP and CAR2, Signal peptide-hMSLNscFv-CD8α-CD8TM-4 / 1BB-IRES-GFP; one positive control structure: CAR3, Signal peptide-hCEAscFv-CD8α-CD8TM-4 / 1BB-CD3ζ-IRES-GFP. The sequence information of each element is detailed in Example 1, and the schematic diagrams of the gene sequence elements of the three control groups are detailed in image 3 , the schematic diagram of various types of CAR-T cells constructed is detailed in Figure 4 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com