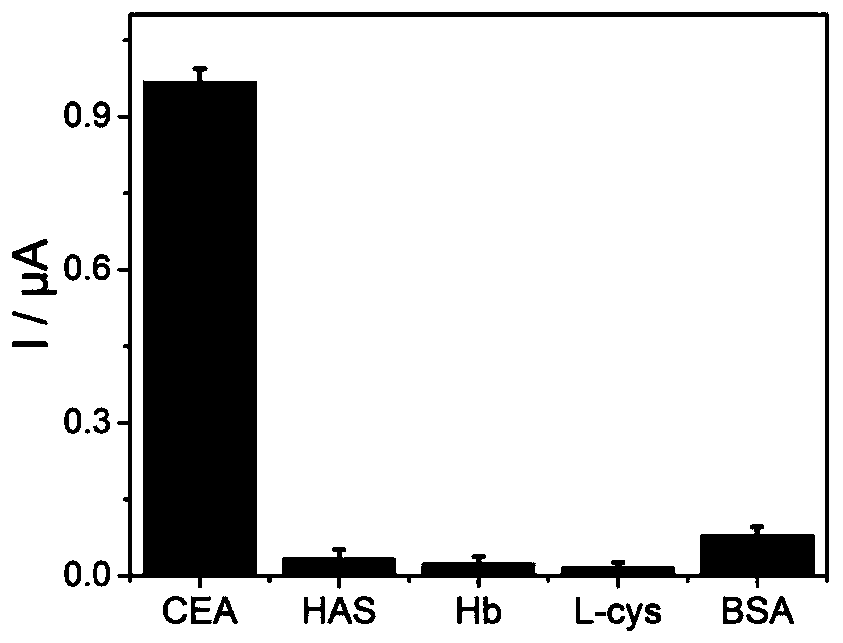
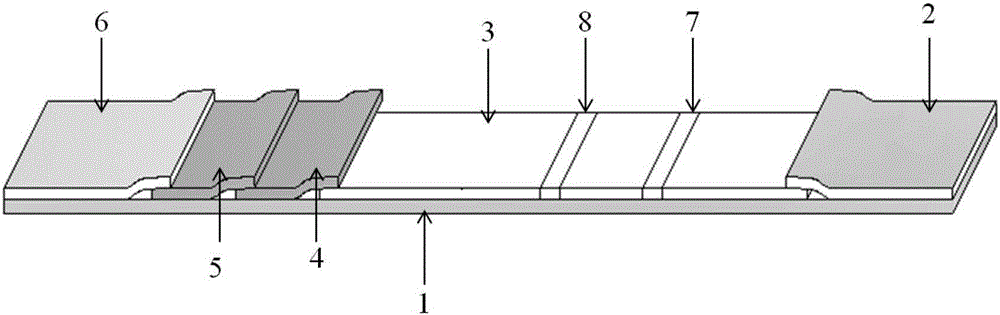
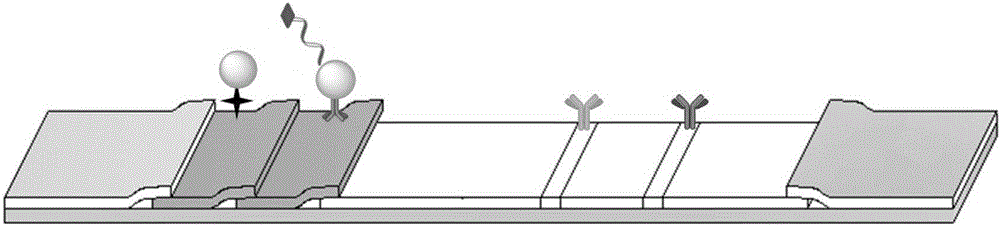
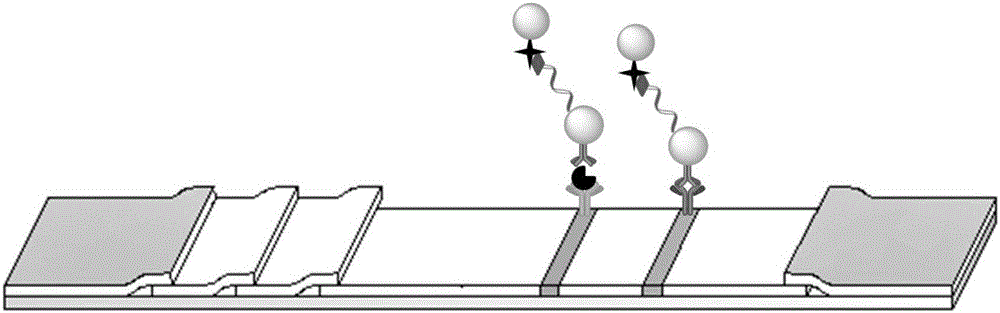
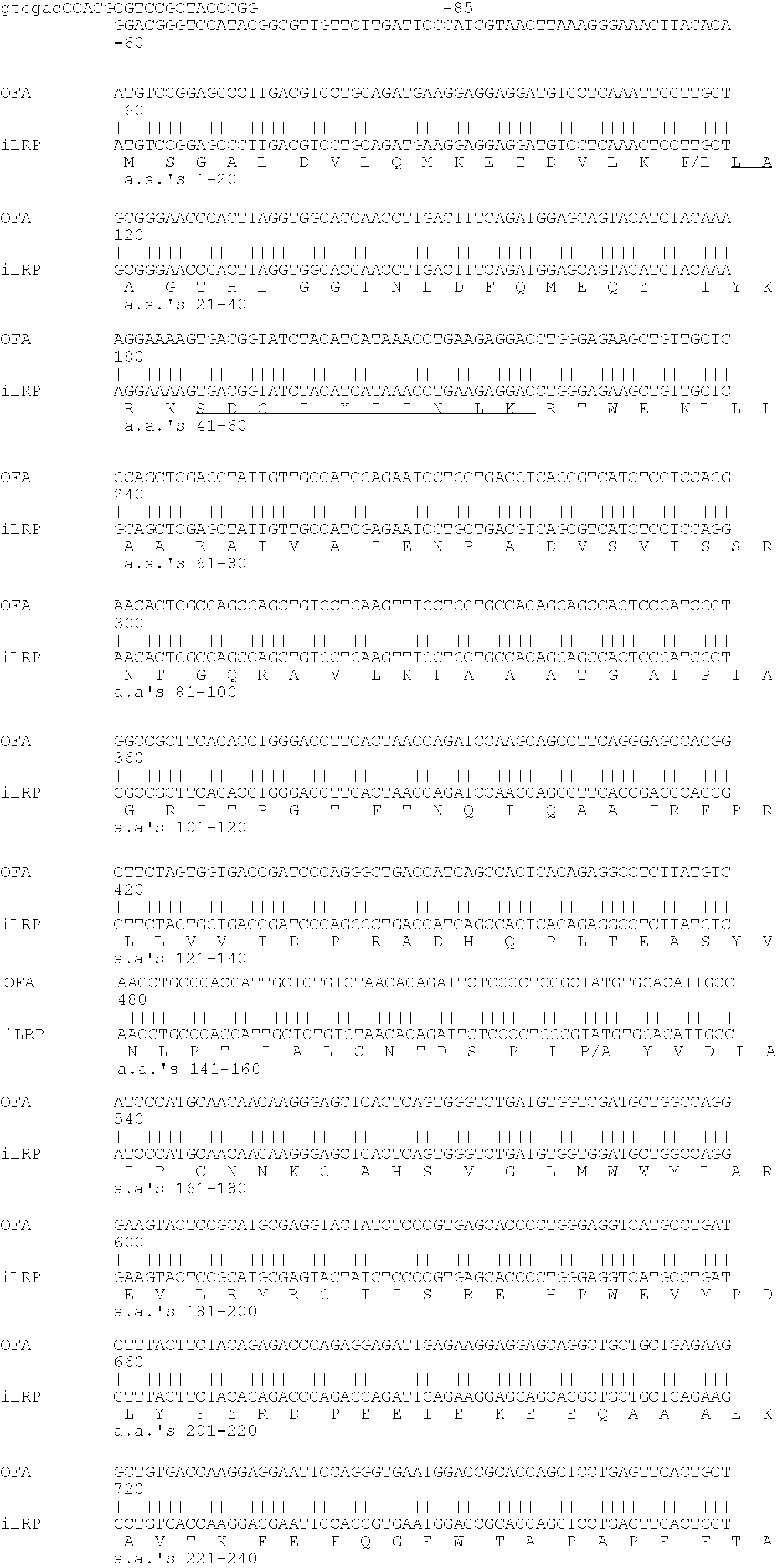Patents
Literature
81 results about "Carcinoembryonal antigen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cancer specific antigen of colon carcinoma, also present in adenocarinomas of endodermal origin and in human embryonic gastrointestinal tissue.
Preparation and application of alpha fetoprotein and carcino-embryonic antigen electrochemiluminescence sensor
InactiveCN103245656AAchieving Simultaneous DetectionHigh sensitivityChemiluminescene/bioluminescenceGraphene nanocompositesElectrochemiluminescence
The invention provides preparation and application of an alpha fetoprotein and carcino-embryonic antigen electrochemiluminescence sensor, and belongs to the technical fields of nano function material, clinical analysis, a bioseneor technology and electrochemistry. The characteristics that platinum nanoparticle @meso-porous silicon @ graphene nanocomposite (PtNPs@m-Si@GS) is strong in conductivity, good in stability, large in specific surface area, good in biocompatibility, strong in catalytic activity and the like are utilized in preparation; an alpha fetoprotein second antibody (anti-alpha fetoprotein (AFP)) and a carcino-embryonic antigen secondary antibody (anti-carcino-embryonicantigen (CEA)) are marked, so as to prepare the marked second antibodies Ru-PtNPs@M-Si@GS / anti-AFP and luminol-PtNPs@M-Si@GS / anti-CEA; and the sensitivity of the sensor is obviously improved. Compared with other single-channel electrode sensors, alpha fetoprotein and carcino-embryonic antigen can be simultaneously detected on a same electrode at one time; the detection efficiency is obviously improved; and the alpha fetoprotein and carcino-embryonic antigen electrochemiluminescence sensor has important scientific significance and application value on clinical early diagnosis of hepatic carcinoma.
Owner:UNIV OF JINAN
Preparation method of liquid phase protein chip
InactiveCN101144815AEarly detectionEarly treatmentMaterial analysisHigh risk populationsFluorescence
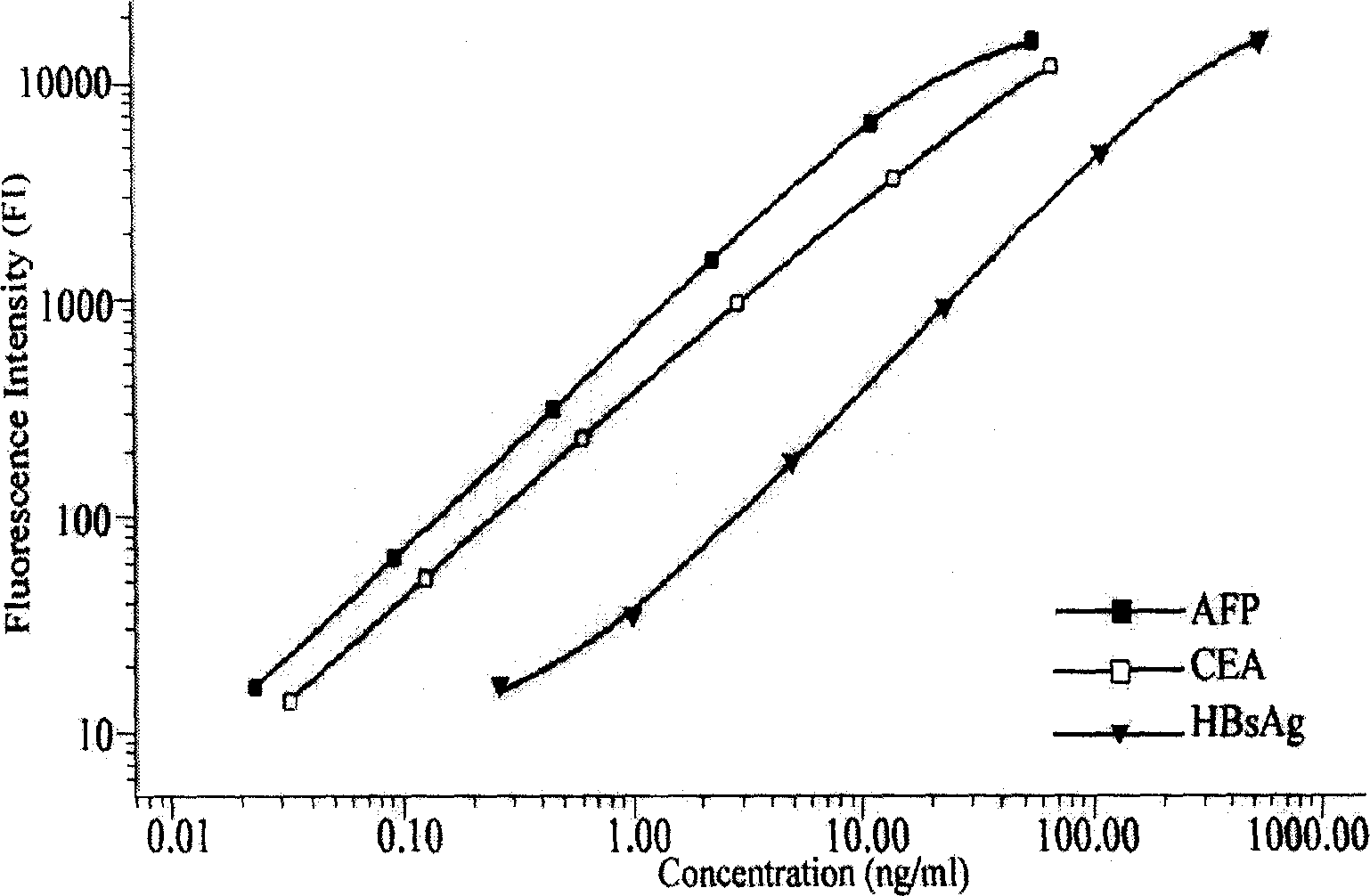
The present invention relates to the biologic technology field, and discloses a liquid phase albumen chip and the preparation and the usage method thereof which can simultaneously test human serum carcinoma embryonic antigen (CEA), Alpha fetoprotein (AFP), and hepatitis B surface antigen (HBsAg). The present invention couples the specificity antibody of CEA, AFP, and HBsAg on different fluorescence micro-spheres, and uses the test antibody marked by biotin or phycoerythrin to determine the nature quickly and quantitatively analyze the above three indexes with the double antibody sandwich method. The present invention uses the filtering membrane board when testing, and washes the board 3 times after each reaction is finished to increase the signal and improve the sensitivity. The present invention has high sensitivity, strong specificity, stable result, excellent repeatability, and simple operation; 1 micro liter serum sample can test three indexed simultaneously. The present invention is applicable to the health test and the general examination as well as the clinic test of the high risk population, and can facilitate the early diagnosis and the early treatment of the knub.
Owner:GUANGZHOU DARUI BIOTECH
Carcinoembryonic antigen fusions and uses thereof
ActiveUS8188244B2Prevent and inhibit developmentEnhance immune responseAntibody mimetics/scaffoldsWhole-cell/virus/DNA/RNA ingredientsPolynucleotideTGE VACCINE
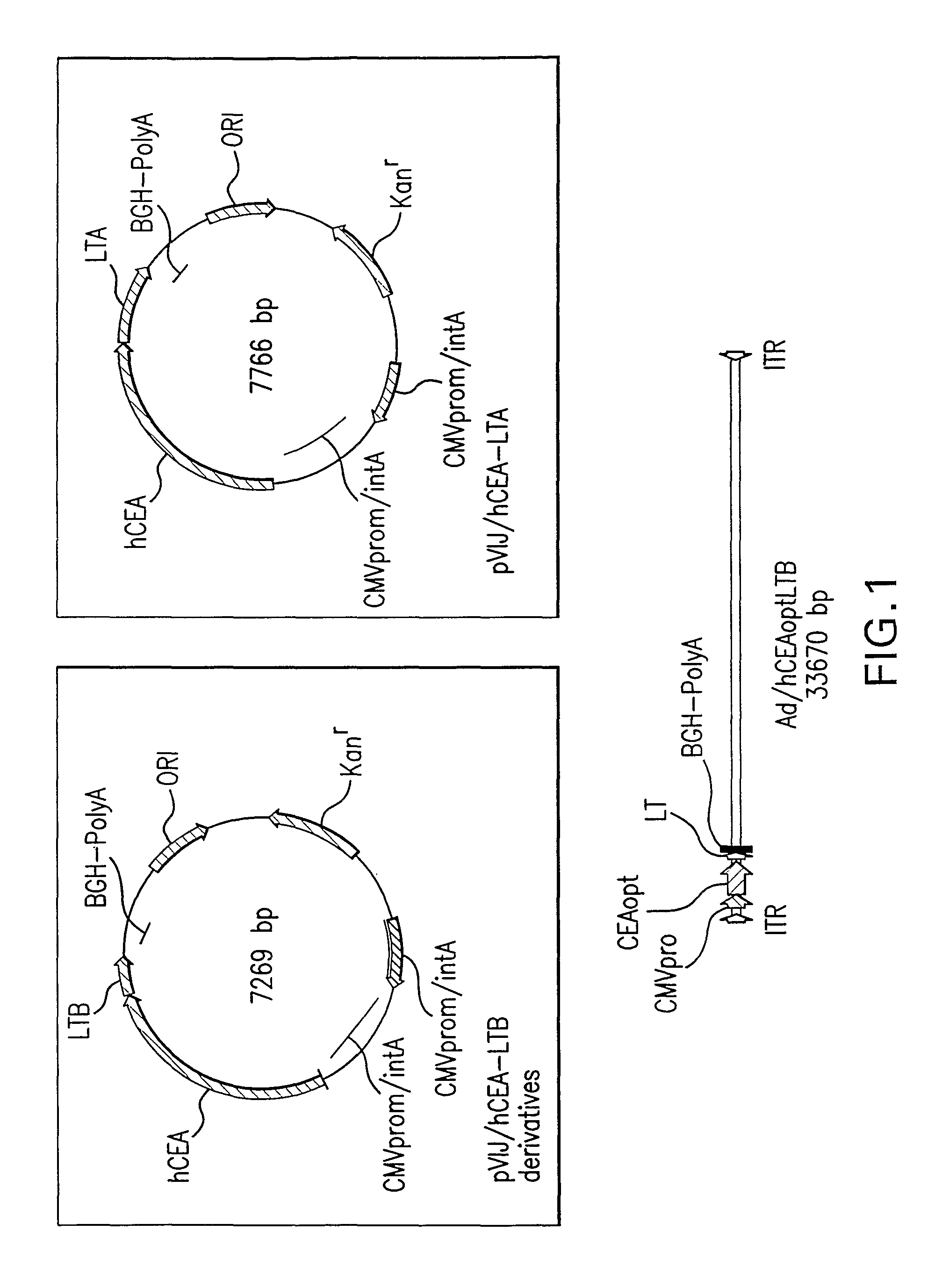
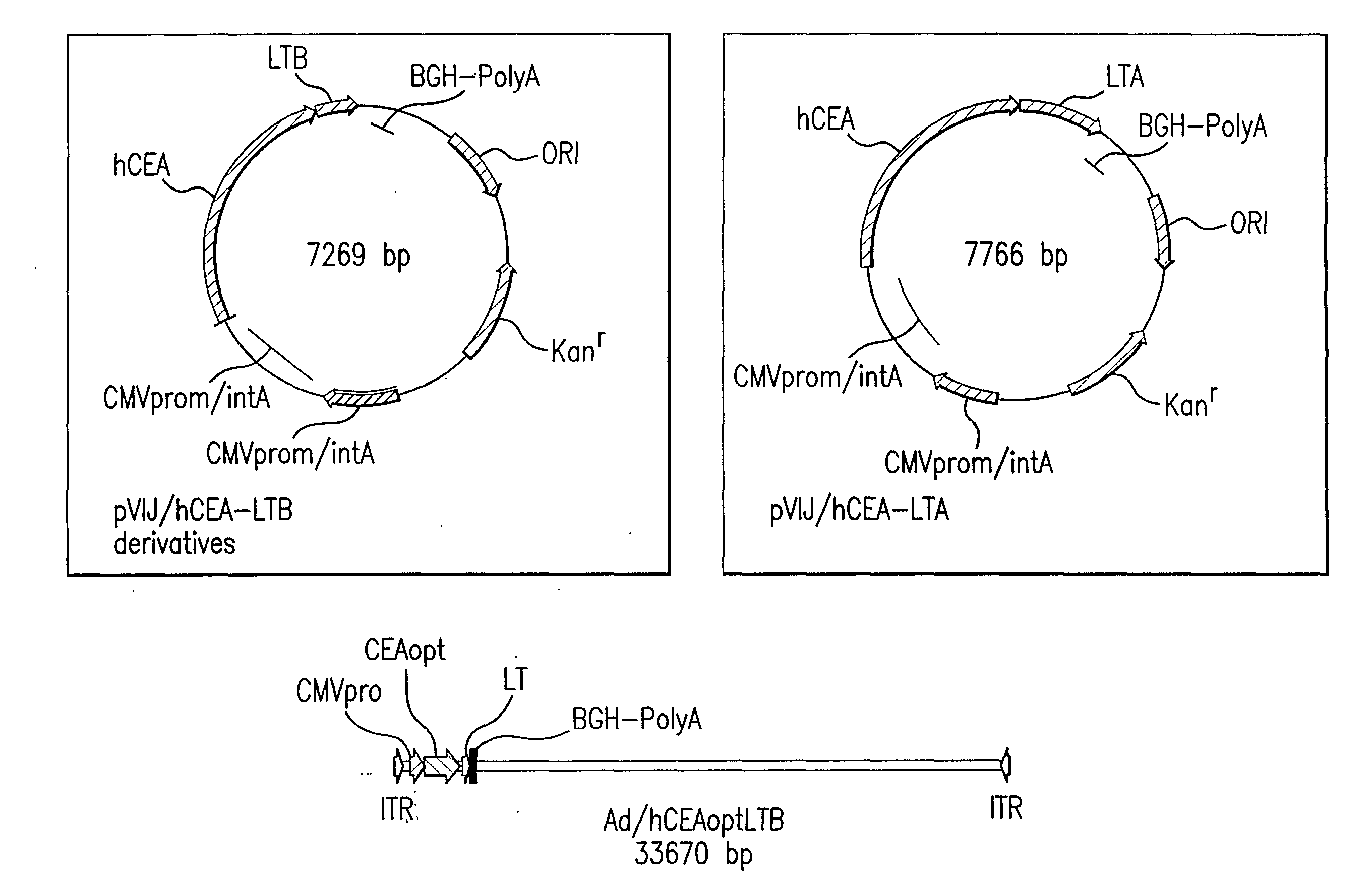
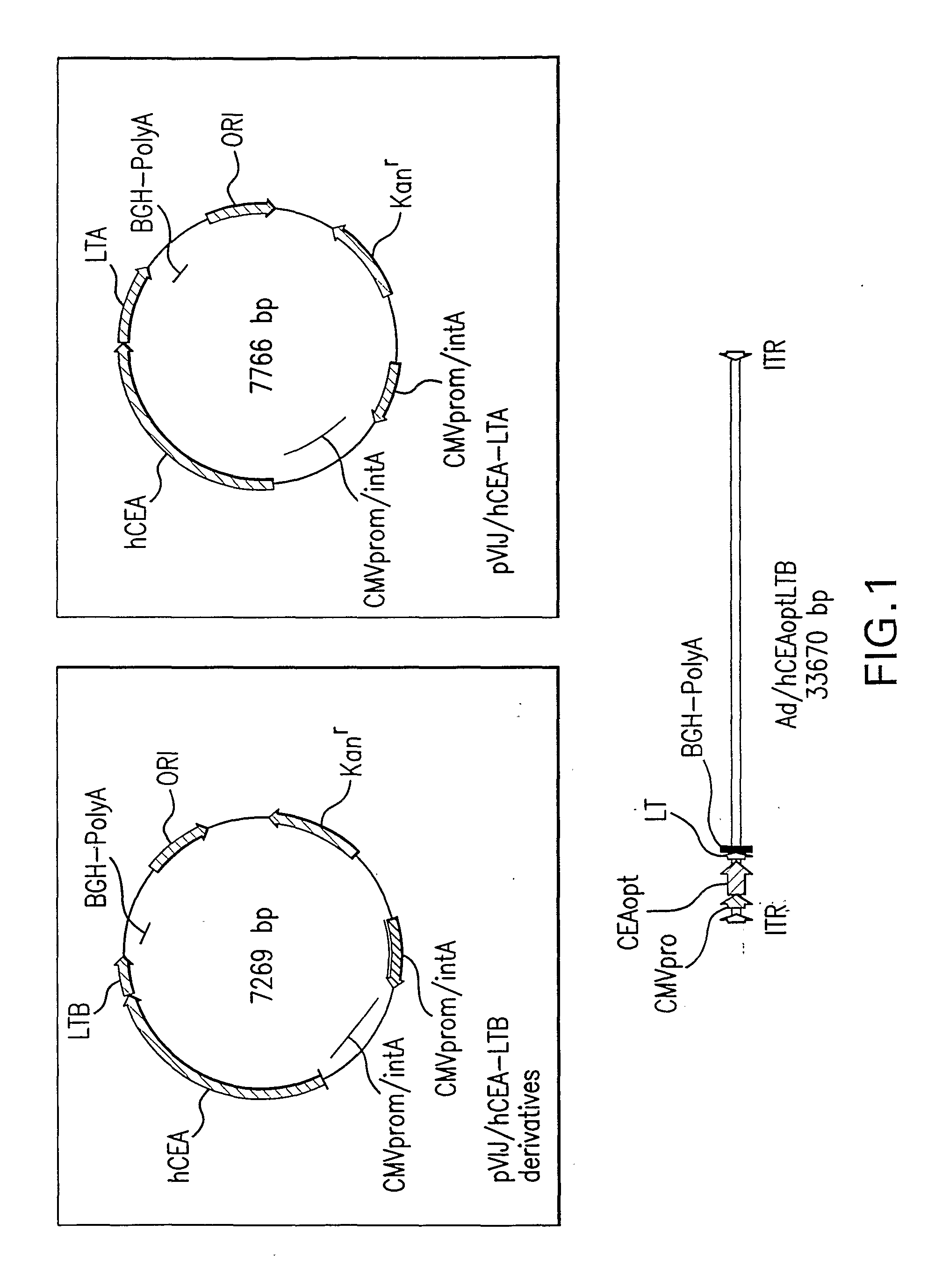
Polynucleotides encoding carcinoembryonic antigen (CEA) fusion proteins are provided, the CEA fusion proteins comprising a CEA protein, or functional variant thereof, fused to a substantial portion of an immunoenhancing element. The polynucleotides of the present invention can elicit an immune response in a mammal, which, in preferred embodiments, is stronger than the immune response elicited by a wild-type CEA. The gene encoding CEA is commonly associated with the development of human carcinomas. The present invention provides compositions and methods to elicit or enhance immunity to the protein product expressed by the CEA tumor-associated antigen, wherein aberrant CEA expression is associated with a carcinoma or its development. This invention specifically provides adenoviral vector and plasmid constructs carrying polynucleotides encoding CEA fusion proteins and discloses their use in vaccines and pharmaceutical compositions for preventing and treating cancer.
Owner:MSD ITAL
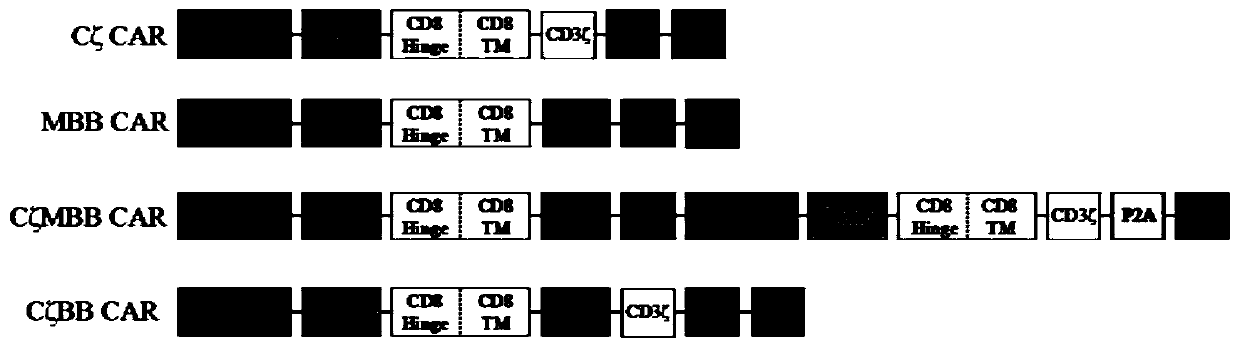
Chimeric antigen receptor for identifying carcino-embryonic antigens and application of chimeric antigen receptor
ActiveCN107033248ANo mismatchImprove efficiencyMammal material medical ingredientsNucleic acid vectorSide effectSingle-Chain Antibodies
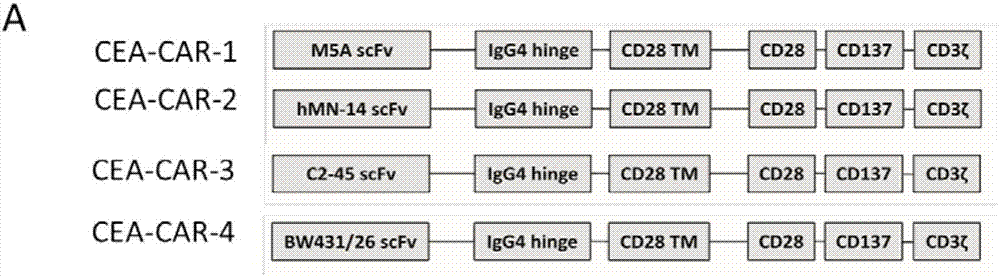
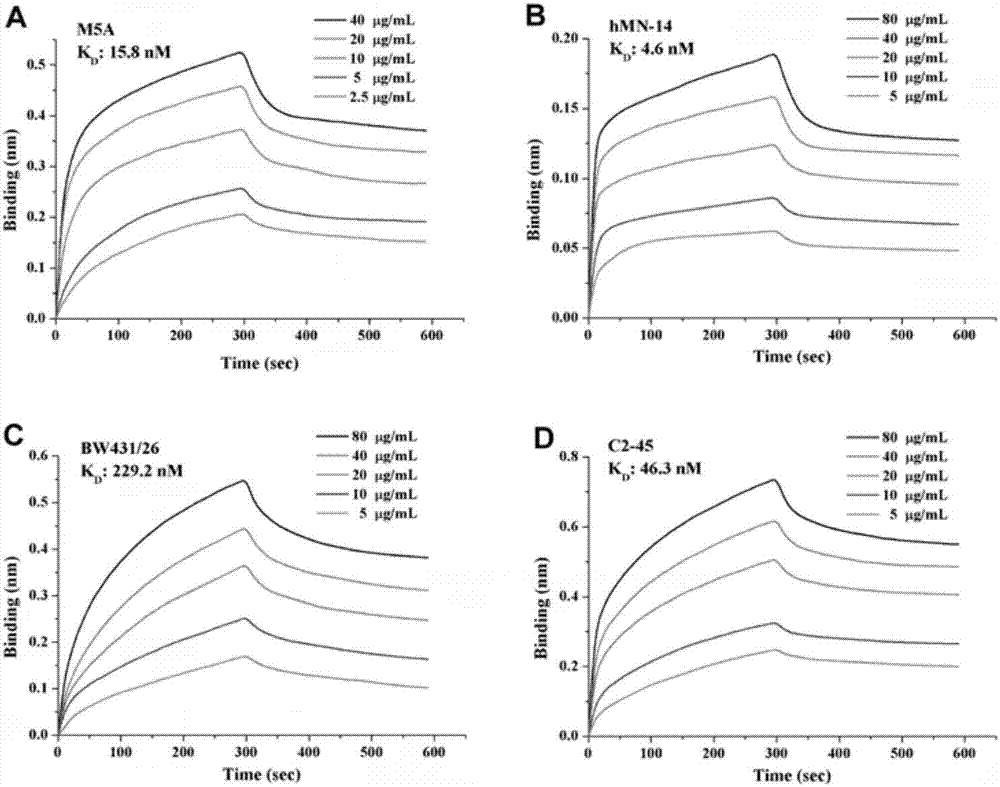
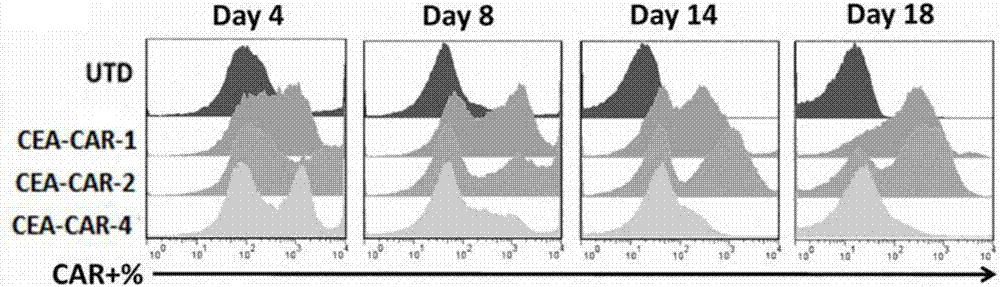
The invention belongs to the field of gene engineering, and particularly relates to a chimeric antigen receptor for identifying carcino-embryonic antigens and an application of the chimeric antigen receptor. A single-chain fragment variable (scFV) for identifying the carcino-embryonic antigens, a hinge region, a transmembrane region and an intracellular signal domain are sequentially connected to form the chimeric antigen receptor, and the amino acid sequence of the single-chain fragment variable (scFV) for identifying the carcino-embryonic antigens includes M5A-scFV amino acid sequence or amino acid sequence acquired by performing random mutation on M5A-scFV polypeptide. When the chimeric antigen receptor identifies the carcino-embryonic antigens, T lymphocytes can be more stably expressed, the positive rate of a chimeric antigen receptor of a target CEA (carcino-embryonic antigen) can be maintained in the culture process of patient cells, proliferation capacity and tumor killing capacity of CAR-T (chimeric antigen receptor T lymphocytes) can be improved, the chimeric antigen receptor does not have toxic and side effects on confrontation of original negative cells, can be used for targeted treatment of tumors and high in humanization degree, immunogenicity of the CAR can be effectively reduced, and continuity and safety of the CAR-T in human bodies are improved.
Owner:CHONGQING PRECISION BIOTECH CO LTD
Synthetic gene encoding human carcinoembryonic antigen and uses thereof
InactiveUS20070104685A1Easy to insertEasy to removeVirusesGenetic material ingredientsNucleotideCarcinoembryonic antigen
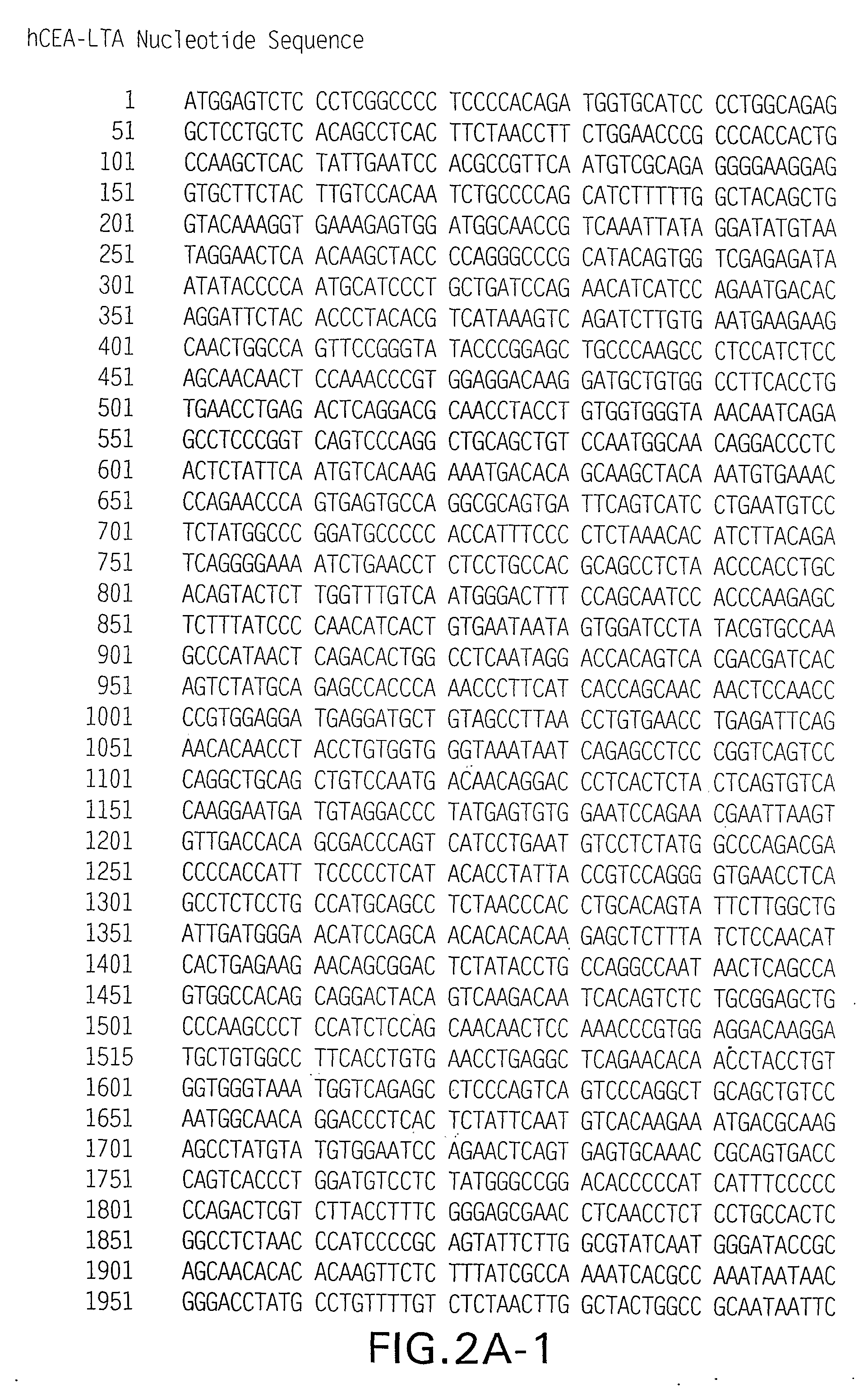
Synthetic polynucleotides encoding human carcinoembryonic antigen (CEA) are provided, the synthetic polynucleotides being codon-optimized for expression in a human cellular environment. The gene encoding CEA is commonly associated with the development of human carcinomas. The present invention provides compositions and methods to elicit or enhance immunity to the protein product expressed by the CEA tumor-associated antigen, wherein aberrant CEA expression is associated with a carcinoma or its development. This invention specifically provides adenoviral vector and plasmid constructs carrying codon-optimed human CEA and discloses their use in vaccines and pharmaceutical compositions for preventing and treating cancer.
Owner:LA MONICA NICOLA +3
Preparation method and application of immunosensor established based on manganese dioxide loaded silver nanoparticle multiwalled carbon nanotube
InactiveCN104569427ALarge specific surface areaImprove conductivityBiological testingImmune profilingMultiwalled carbon
The invention belongs to the technical field of nano functional materials, immunoassay and biosensing, and provides a preparation method and application of an immunosensor established based on a manganese dioxide loaded silver nanoparticle multiwalled carbon nanotube. The electrochemical immunosensor which is prepared by taking the manganese dioxide loaded silver nanoparticle multiwalled carbon nanotube AgNPS@MnO2@MWCNTs as a detection antibody marker has the advantages of high specificity, high sensitivity, low detection limit and the like, and has significant scientific meanings and application values in detection on carcino-embryonic antigen CEA and alpha fetoprotein AFP.
Owner:SHANDONG UNIV OF TECH
Preparation method of oncofetal antigen electrochemical immunosensor based on AuNPs-PDDA-GR composite material and application thereof
InactiveCN105158479AImprove conductivityIncrease the effective areaBiological material analysisBiological testingCarcinoembryonal antigenBiology
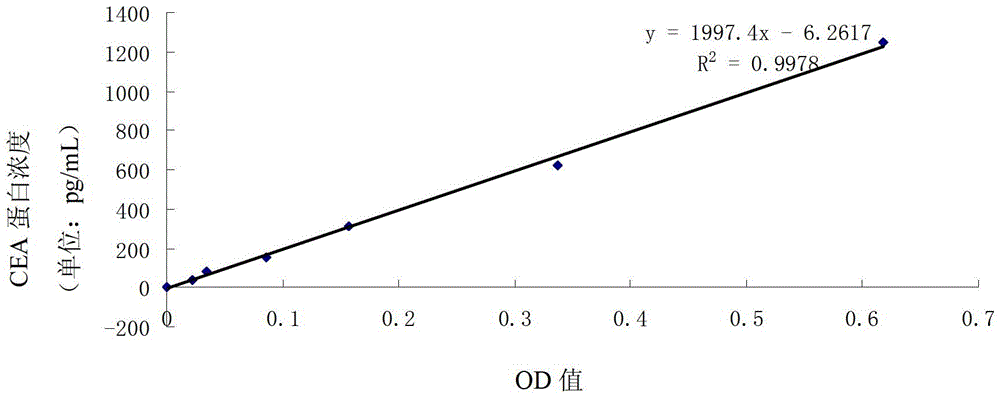
The invention relates to a preparation method of an oncofetal antigen electrochemical immunosensor based on AuNPs-PDDA-GR composite material and an application thereof, and belongs to the field of electrochemical immunization sensing technology. K3Fe(CN)6 / K4Fe(CN)6 is used as an electrochemical probe, the AuNPs-PDDA-GR composite material is used as a substrate material with good biological compatibility and stability, large specific surface area, excellent conductivity and other advantages, thereby substantially improving stability and sensitivity of the immunosensor. The sensor is used for detection of oncofetal antigens, and the detection linear scope is 0.1pg / mL-10ng / mL, and the detection limit is 0.05pg / mL.
Owner:UNIV OF JINAN
Preparation method and application of magnetic electrochemical immunosensor for simultaneously detecting two tumor markers based on metal substrate sign
InactiveCN105738457ALarge active surface areaElectrochemical signal amplificationBiological testingMaterial electrochemical variablesCarcinoembryonal antigenNanoparticle
The invention discloses a preparation method and application of a magnetic electrochemical immunosensor for simultaneously detecting two tumor markers based on a metal substrate sign, relates to the field of preparation of immunosensors for rapidly and sensitively detecting two tumor markers of alpha fetoprotein (AFP) and carcino-embryonic antigen (CEA) simultaneously, and in particular to a magnetic electrochemical immunosensor for simultaneously detecting two tumor markers on the basis of ionic compound signs. The advantages that magnetic dopamine nanoparticles are large in active surface and are easy to separate are taken, an appropriate end-capping reagent is selected to increase the load amount of metal ions, then an electromechancial signal can be amplified, and detection on tumor rkers can be rapid and sensitive. A modification electrode is simple and convenient to manufacture, good in stability, rapid to react, and relatively high in selectivity and sensitivity for alpha fetoprotein and carcino-embryonic antigen.
Owner:UNIV OF JINAN
Novel protein-DNA complex for detecting carcino-embryonic antigen as well as synthetic method and application thereof
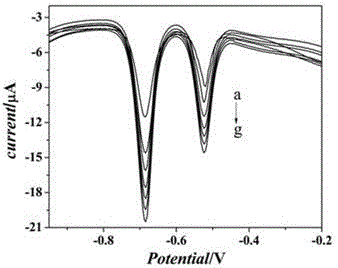
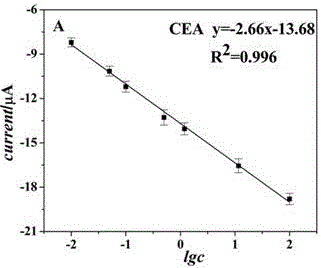
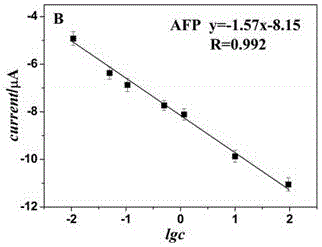
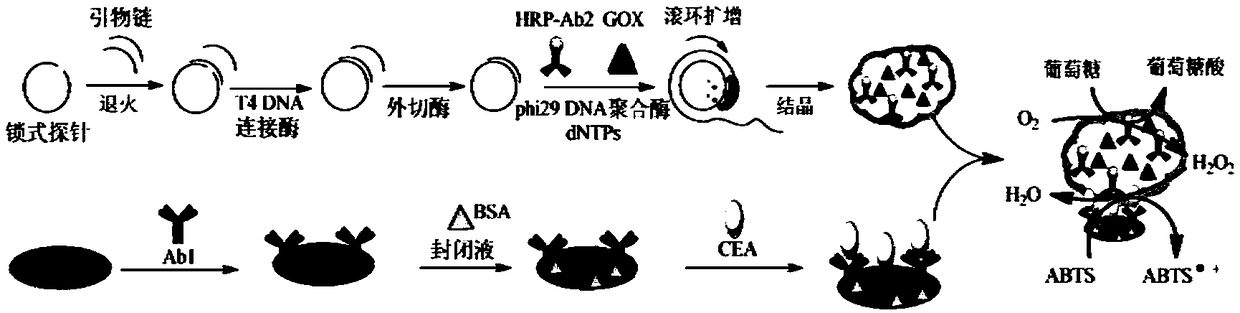
The invention belongs to the technical field of biological detection, and particularly relates to a novel protein-DNA complex for detecting a carcino-embryonic antigen as well as a synthetic method and application thereof. The novel protein-DNA complex comprises a horseradish peroxidase-marked carcino-embryonic antigen secondary antibody, glucose oxidase and a DNA structure. The novel protein-DNAcomplex structure encapsulated with a great amount of enzyme-labeled secondary antibodies and glucose oxidase is established on the basis of a rolling circle replication technology and is used as enzyme cascade signal amplification probe. A 96-pore plate is used for fixing carcino-embryonic antigen monoclonal antibody molecules, after the to-be-detected carcino-embryonic antigen is added, a sandwiched structure is formed. The encapsulated glucose oxidase is used for catalyzing the glucose to be oxidized to generate H2O2, then the H2O2 is catalyzed by virtue of the horseradish peroxidase to oxidize ABTS, so that the high-sensitivity and high-specificity colorimetry detection of the carcino-embryonic antigen can be realized, and the novel protein-DNA complex has important significance for the early diagnosis and clinical research of cancers.
Owner:QINGDAO UNIV
Construction method of nanoprobe C60 based electrochemical immunosensor for carcino-embryonic antigens
InactiveCN105675697AFacilitates electron transferHigh sensitivityMaterial electrochemical variablesCyclic voltametryCarcinoembryonal antigen
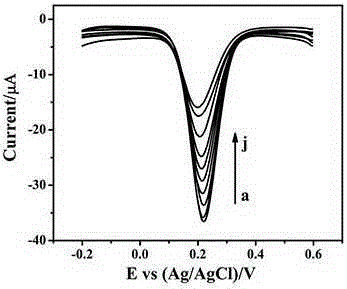
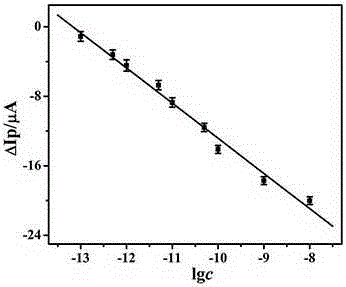
The invention relates to the technical field of electrochemical immunosensors, in particular to a construction method of a nanoprobe C60 based electrochemical immunosensor for carcino-embryonic antigens.The method includes: modifying gold nanoclusters on the surface of a glassy carbon electrode by means of cyclic voltammetry to obtain an antibody capture substrate; sequentially immobilizing first antibodies and antigens to the surface of the electrode; immobilizing AuNPs@L-cys-C60 marked second antibodies by specific binding of the antigens and the antibodies.The gold nanoclusters have excellent biocompatibility and electrical conductivity and large surface areas and are capable of promoting electron transferring between proteins and the electrode.The AuNPs@L-cys-C60 serves as an oxidation-reduction nanoprobe, the immunosensor is used for detection of the carcino-embryonic antigens on the basis of excellent specificity between the antigens and the antibodies, and detection of the carcino-embryonic antigens is realized according to different electrochemical signals of the carcino-embryonic antigens with different concentrations.
Owner:UNIV OF JINAN
Preparation and application of carcino-embryonic antigen electrochemical immunosensor based on Au@Ag@Au marker
InactiveCN106442675AHeavy loadFacilitates electron transferMaterial analysis by electric/magnetic meansCarcinoembryonic antigenHydroquinone Compound
The invention relates to the technical field of electrochemical immunosense, in particular to preparation and application of a carcino-embryonic antigen electrochemical immunosensor based on an Au@Ag@Au core-shell nano-composite (Au@Ag@Au NPs) marker. Specifically, the surface of a glassy carbon electrode is modified by nitrogen-doped graphene (NG) to prepare an antibody capture substrate, Au@Ag@Au NPs tracks and marks a carcino-embryonic antigen second antibody, the second antibody marked by Au@Ag@Au NPs is captured on the surface of the sensor through sandwich immune response, and detection of a carcino-embryonic antigen is achieved by detecting electrochemical signals of hydrogen peroxide and hydroquinone in a PBS solution.
Owner:UNIV OF JINAN
Antibodies and cyclic peptides which bind CEA (carcinoembryonic antigens) and their use as cancer therapeutics
InactiveUS20060024314A1Inhibition effectReduces CEA-dependent adhesion of cellCell receptors/surface-antigens/surface-determinantsAntibody ingredientsCyclic peptideHuman cancer
The present invention relates to differentiation and tumorigenicity. The present invention more particularly relates to ligands which target CEA and CEACAM6 such that the adhesion, differentiation-Inhibitory activities and tumorigenic effects of Ig superfamily members, CEA and CEACAM6, can be reduced or blocked. More particularly, the present invention relates to CEA-binding agents which reverse CEA-mediated tumorigenic effects by declustering CEA and CEACAM6. In one embodiment the invention relates to methods of reducing, preventing or reversing a CEA-mediated tumorigenic effect comprising a use of a CEA-mediated tumorigenic effect reducing CEA-declustering agent. In one embodiment, the invention relates to compositions and use thereof for reversing CEA-mediated tumorigenic effects on human cancer cells and uses thereof. In particular, the application relates to a monovalent CEA binding agent which interferes with a CEA interaction responsible for a CEA-mediated tumorigenic effects, thereby minimizing or reversing same.
Owner:STANNERS CLIFFORD +6
Identification of Hla-A2-Presented T-Cell Epitopes Derived from the Oncofoetal Antigen-Immature Laminin Receptor Protein and Uses Thereof
ActiveUS20090041794A1The relationship is accurateTumor rejection antigen precursorsPeptide/protein ingredientsImmunotherapeutic agentLaminin receptor protein
The present invention relates to the immunotherapB of cancer, in particular several tumor entities including hematological malignancies. The present invention relates to tumor-associated T-helper cell peptide epitopes, alone or in combination with other tumor-associated peptides, that serve as active pharmaceutical ingredients of vaccine compositions that stimulate anti-tumor immune responses. In particular, the present invention relates to two novel peptide sequences derived from HLA class I or II molecules of human oncofoetal antigen immature laminin receptor (OFA / iLR), which can be used in vaccine compositions for eliciting anti-tumor immune responses.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
Carcinoembryonic Antigen Fusions and Uses Thereof
ActiveUS20080311137A1Stimulate immune responseEnhance immune responseAntibody mimetics/scaffoldsTissue cultureMammalADAMTS Proteins
Polynucleotides encoding carcinoembryonic antigen (CEA) fusion proteins are provided, the CEA fusion proteins comprising a CEA protein, or functional variant thereof, fused to a substantial portion of an immunoenhancing element. The polynucleotides of the present invention can elicit an immune response in a mammal, which, in preferred embodiments, is stronger than the immune response elicited by a wild-type CEA. The gene encoding CEA is commonly associated with the development of human carcinomas. The present invention provides compositions and methods to elicit or enhance immunity to the protein product expressed by the CEA tumor-associated antigen, wherein aberrant CEA expression is associated with a carcinoma or its development. This invention specifically provides adenoviral vector and plasmid constructs carrying polynucleotides encoding CEA fusion proteins and discloses their use in vaccines and pharmaceutical compositions for preventing and treating cancer.
Owner:MSD ITAL
Nano-antibody combination for detecting carcino-embryonic antigen by double-antibody sandwich method
PendingCN111004328AImprove matchMeet detectionChemiluminescene/bioluminescenceImmunoglobulins against cell receptors/antigens/surface-determinantsCarcinoembryonal antigenAntigen testing
The invention discloses a nano-antibody combination for detecting carcino-embryonic antigen by a double-antibody sandwich method. The nano antibody combination comprises an anti-carcino-embryonic antigen bivalent nano antibody serving as a capture antibody and an anti-carcino-embryonic antigen monovalent nano antibody serving as a detection antibody. The invention further discloses an applicationof the nano antibody combination in preparation of a carcino-embryonic antigen detection kit. The nano antibody combination of the anti-cancer embryo antigen bivalent nano antibody and the anti-cancerembryo antigen monovalent nano antibody provided by the invention has good matching degree, shows excellent P / N value, lowest detection limit and accuracy in CEA antigen detection, and can meet detection of CEA in clinical samples.
Owner:深圳市国创纳米抗体技术有限公司
Sensor based on hybridization chain reaction and ribozyme and carcino-embryonic antigen detection method
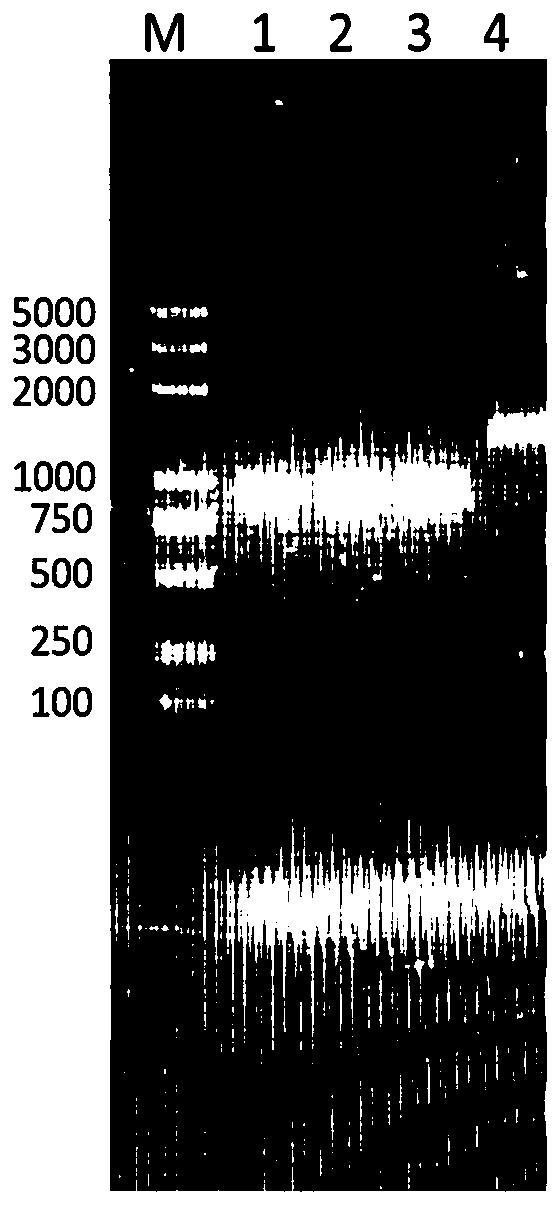
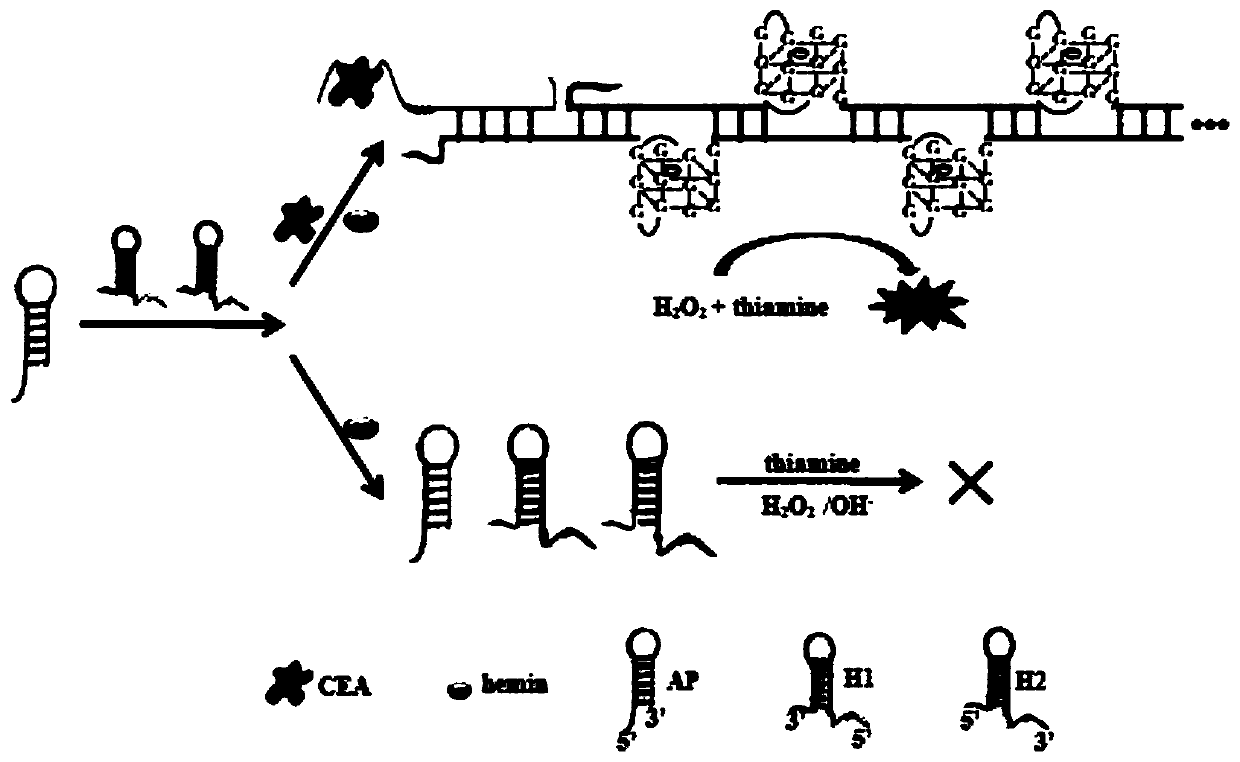
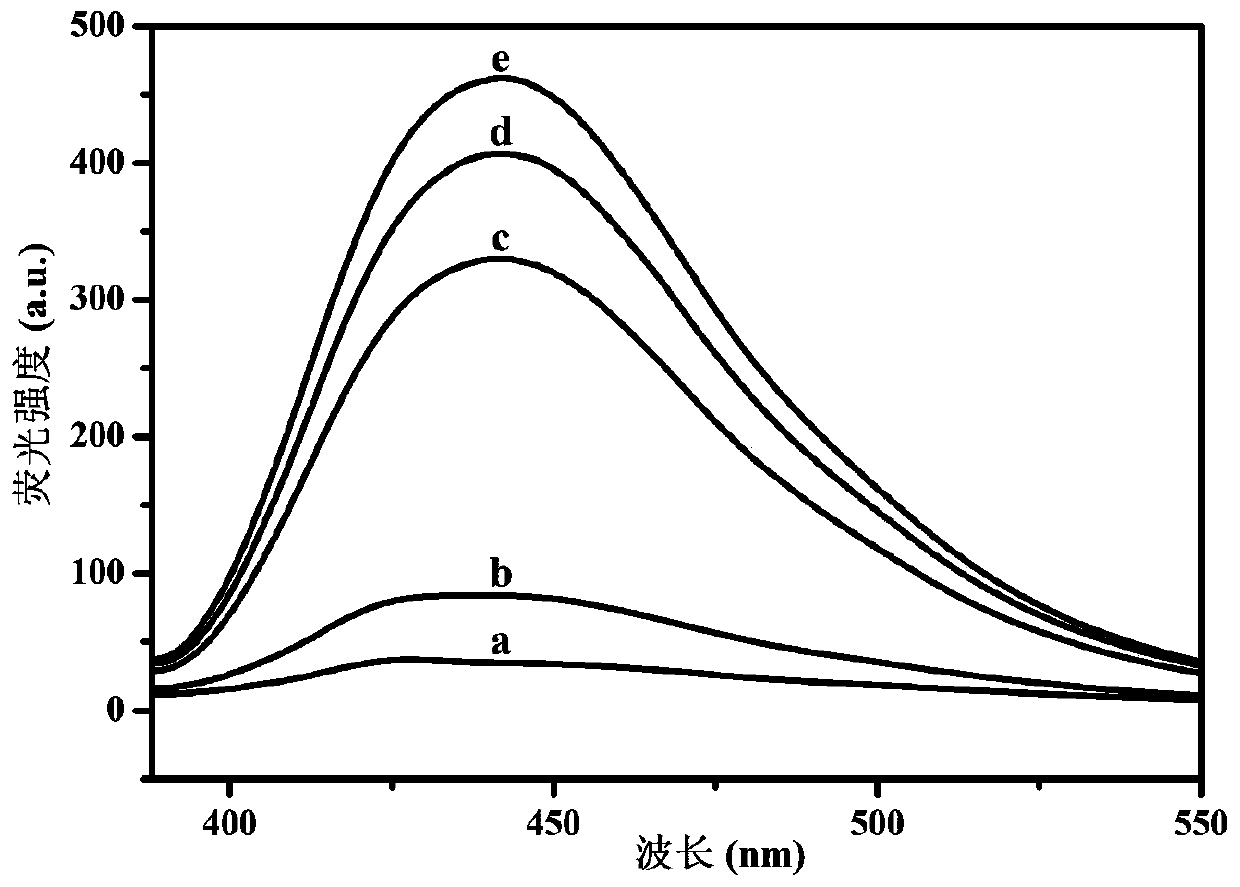
ActiveCN111175506AHigh selectivityHigh sensitivityMicrobiological testing/measurementMaterial analysisCarcinoembryonal antigenHemin
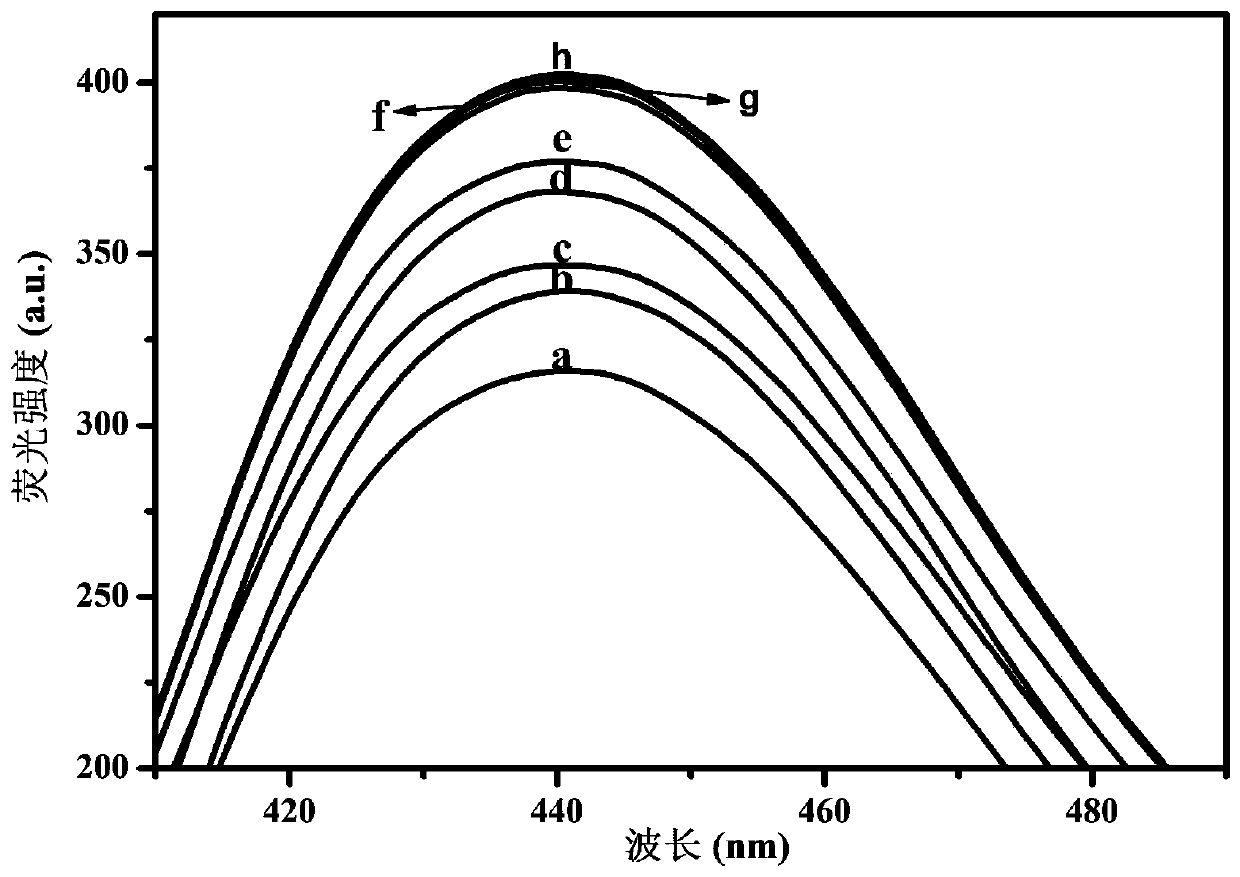
The invention relates to the technical field of chemical and biological sensing, and particularly a sensor based on hybridization chain reaction and ribozyme and a carcino-embryonic antigen detectionmethod. The sensor based on the hybridization chain reaction and ribozyme comprises a nucleic acid aptamer probe and a hairpin probe, wherein the aptamer probe comprises an aptamer sequence, an initiation chain sequence and a complementary sequence, the hairpin probes comprise a first hairpin probe and a second hairpin probe, the first hairpin probe and the second hairpin probe comprise G-quadruplex ribozyme base sequences, the nucleic acid aptamer probe specifically recognizes a carcino-embryonic antigen, the aptamer probe is subjected to conformational transformation to make the first hairpin probe and the second hairpin probe form double-stranded DNA, moreover, in the presence of hemin, the first hairpin probe and the second hairpin probe are self-assembled to form hemin / G-quadruplex ribozyme, and the hemin / G-quadruplex ribozyme catalyzes and oxidizes thiamine mediated by hydrogen peroxide and emits fluorescence, so the carcino-embryonic antigen can be quantitatively detected.
Owner:SHANXI DATONG UNIV
Cancer vaccines containing epitopes of oncofetal antigen
InactiveUS20060165709A1Enhance cytotoxic anti-tumor cell activityReduce immuno-regulationBiocideTumor rejection antigen precursorsEpitopeCarcinoembryonal antigen
Disclosed are fragments of oncofetal antigen, otherwise known as immature laminin receptor protein that specifically stimulate one T cell subclass. The fragments may be formulated into compositions for potentiating T cell-mediated response in mammalian cancer patients. They also have therapeutic uses in vitro.
Owner:SOUTH ALABAMA MEDICAL SCI FOUND
Cancer vaccines containing epitopes of oncofetal antigen
InactiveUS7718762B2Improve cell activityReduce immuno-regulationBiocideTumor rejection antigen precursorsEpitopeLaminin receptor protein
Disclosed are fragments of oncofetal antigen, otherwise known as immature laminin receptor protein that specifically stimulate one T cell subclass. The fragments may be formulated into compositions for potentiating T cell-mediated responses in mammalian cancer patients. They also have therapeutic uses in vitro.
Owner:SOUTH ALABAMA MEDICAL SCI FOUND
Double-chimeric antigen receptor, T cell and construction method and application thereof
PendingCN110669138AToxicityImprove securityImmunoglobulins against cell receptors/antigens/surface-determinantsBlood/immune system cellsTumor antigenSialyl tn
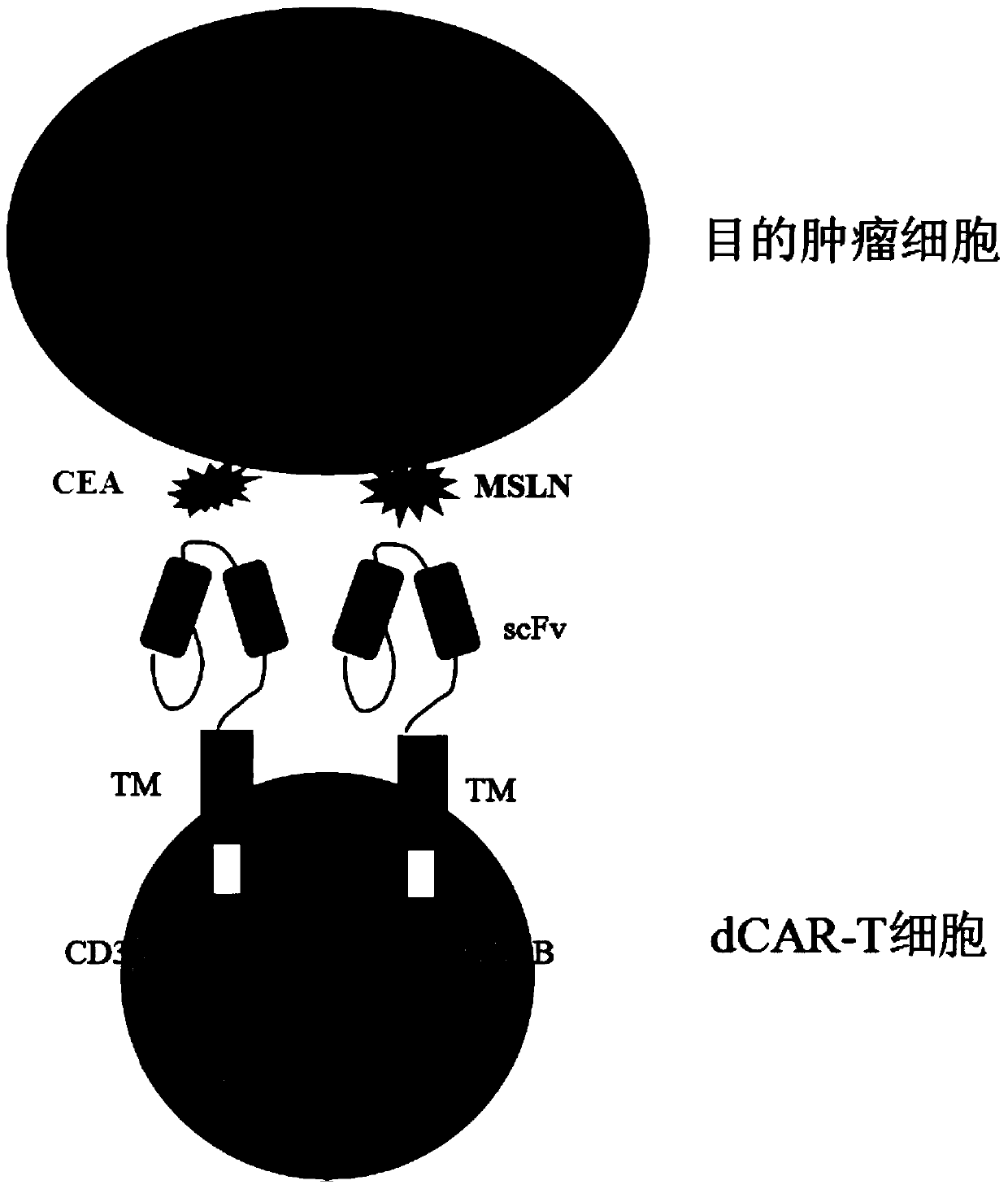
The invention discloses a double-chimeric antigen receptor, a T cell and a construction method and an application thereof, which belong to the field of cellular immunotherapy of tumors. The inventionspecifically relates to a specific structure and a construction method of the double chimeric antigen receptor T cell (dCAR-T cell), and preliminarily discusses the in-vivo and in-vitro activity of the dCAR-T cell. The selected tumor-associated antigens are mesothelin and carcino-embryonic antigens, and researches show that the two tumor antigens can be simultaneously expressed on the surface of asolid tumor, such as pancreatic cancer. The invention discloses an antigen receptor. The in-vitro and in-vivo tests prove that the constructed dCAR-T cell can be permanently and effectively activatedonly under the condition that two antigens are simultaneously recognized, and has efficient anti-tumor activity, so that a specific tumor killing function can be exerted, and the application of CAR-Tcell immunotherapy is improved.
Owner:CHINA PHARM UNIV
Anticancer embryo antigen antibody as well as preparation method and application thereof
ActiveCN110713539AIncrease lethalityImmunoglobulins against cell receptors/antigens/surface-determinantsBlood/immune system cellsComplementarity determining regionAntiendomysial antibodies
The invention provides an anti-CEA (carcinoembrynio antigen) antibody which comprises a heavy chain variable region and a light chain variable region, wherein the complementary determining region of the heavy chain variable region comprises CDR (complementary determining region)-H1 of which the amino acid sequences are shown in SEQ ID NO.1 in the description, CDR-H2 of which the amino acid sequences are shown in SEQ ID NO.2 in the description and CDR-H3 of which the amino acid sequences are shown in SEQ ID NO.3 in the description; and the complementary determining region of the light chain variable region comprises CDR-L1 of which the amino acid sequences are shown in SEQ ID NO.4 in the description, CDR-L2 of which the amino acid sequences are shown in SEQ ID NO.5 in the description and CDR-L3 of which the amino acid sequences are shown in SEQ ID NO.6 in the description. The inventor of the invention screens CEA scFv by using a phage display technology, and thus obtains a high-affinityanti-CEA single-chain antibody. In addition, an anti-CEA chimeric antigen receptor gene is introduced into T cells by using a genetic engineering method to prepare CEA CAR-T, so that CEA CAR-T cellsare capable of specifically recognizing and killing digestive tract tumor cells expressing CEA, and thus the anti-tumor effects of the CEA CAR-T cells can be achieved.
Owner:HUADAO SHANGHAI BIOPHARMA CO LTD
Bispecific antibody for anti-CD16 and CEA antigens
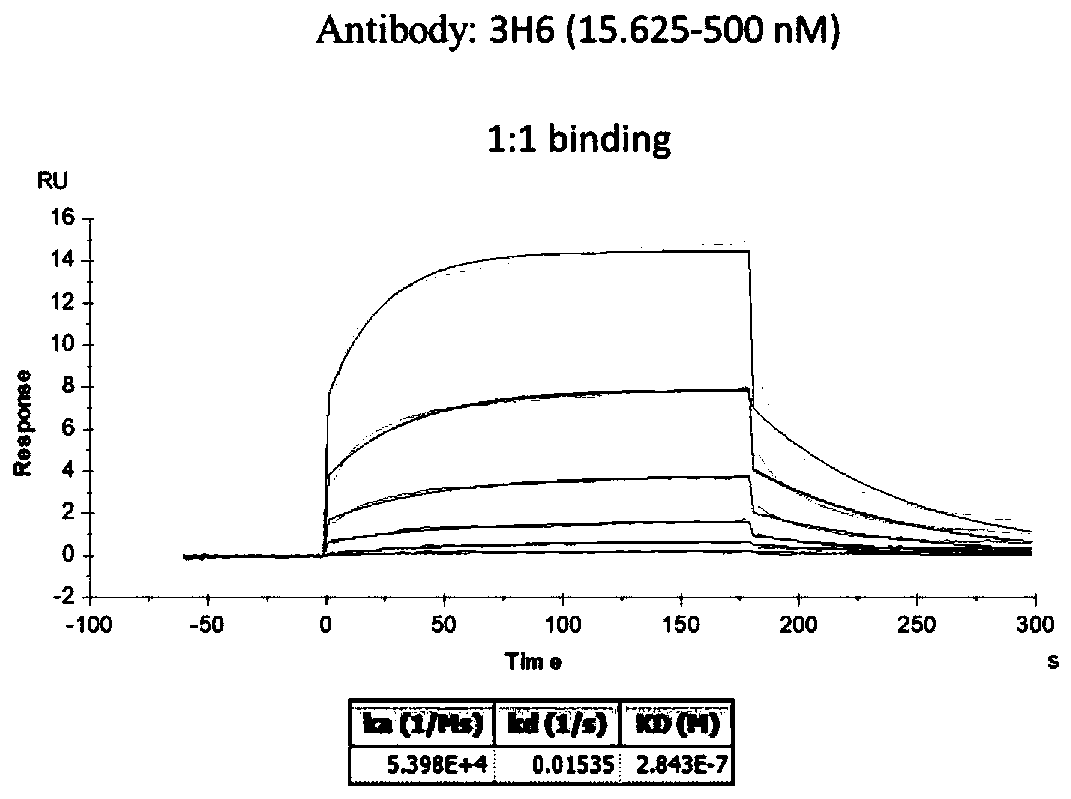
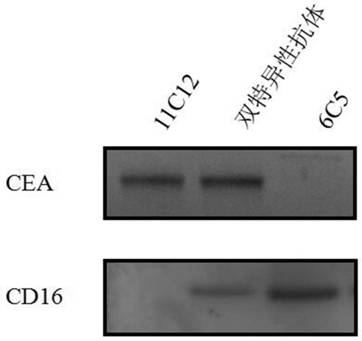
ActiveCN113912726AHigh affinityStrong cytotoxicityHybrid immunoglobulinsBacteriaTumor targetAntiendomysial antibodies
The invention discloses a nano antibody for resisting a CD16 antigen, and a bispecific antibody containing two VHH structural domains. The nano antibody of the anti-CD16 antigen has three unique complementary determining regions (CDR1, CDR2 and CDR3). The bispecific antibody is formed by fusing two heavy chains with different sequences and is used for respectively recognizing a CD16 antigen and a carcino-embryonic antigen CEA on the surface of an NK cell. The bispecific antibody can significantly enhance the activity of antibody-mediated NK cells in killing tumor target cells.
Owner:深圳市国创纳米抗体技术有限公司
Test paper for fast detecting carcinoma of urinary bladder
InactiveCN105891500AOvercoming Insufficient Inspection SensitivityOvercoming the invasiveness of cystoscopyBiological testingBladder tumor AntigenCell adhesion
The invention relates to test paper for fast detecting carcinoma of urinary bladder. The test paper is combined test paper capable of detecting three kinds of carcinoma of urinary bladder markers, namely, nuclear matrix protein 22 (NMP22), bladder tumor antigens (BTA stat) and carcino-embryonic antigen relevant cell adhesion molecules (CEACAM1) at the same time. The test paper comprises a three-channel bottom plate, a sample adding pad, a gold standard pad, a nitrocellulose membrane and an absorbent paper layer. The three-channel bottom plate is sequentially coated with the sampling pad, the gold standard pad, the nitrocellulose membrane and the absorbent paper layer, and NMP22, BTA, CEACAM1 resisting protein monoclonal gold-labeled antibodies are contained in the gold-labeled pad. The three-channel bottom plate achieves specimen flow division through three fine tubes, and detection of the three kinds of markers is independent from one another. The test paper is fast in detecting, high in sensitivity, high in specificity, good in stability, easy and convenient to operate, visual and reliable in result judgment and easy to master. The test paper can be used for screening of high-risk groups, can also be used for follow-up visiting of carcinoma of urinary bladder postoperative patients, and is very wide in application range.
Owner:刘晓强 +4
Preparation method and application of immunosensor based on Au-GQD@PtPd
InactiveCN105928997AHigh sensitivityWide detection rangeMaterial electrochemical variablesImmune profilingCarcinoembryonal antigen
The invention belongs to the technical field of nano functional materials, immunoassay and biosensing, and provides a preparation method and application of an immunosensor based on Au-GQD@PtPd. A sandwich type electrochemical immunosensor for carcino-embryonic antigens is prepared by taking Au-GQD@PtPd as a detection antibody marker; due to excellent biocompatibility and high catalysis property of Au-GQD@PtPd, the prepared sensor has the advantages of high specificity, high sensitivity, low detection limit and the like.
Owner:SHANDONG UNIV OF TECH
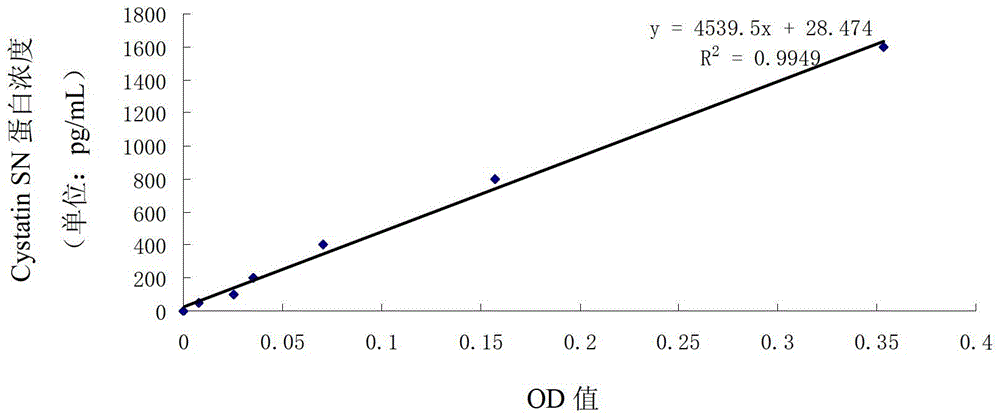
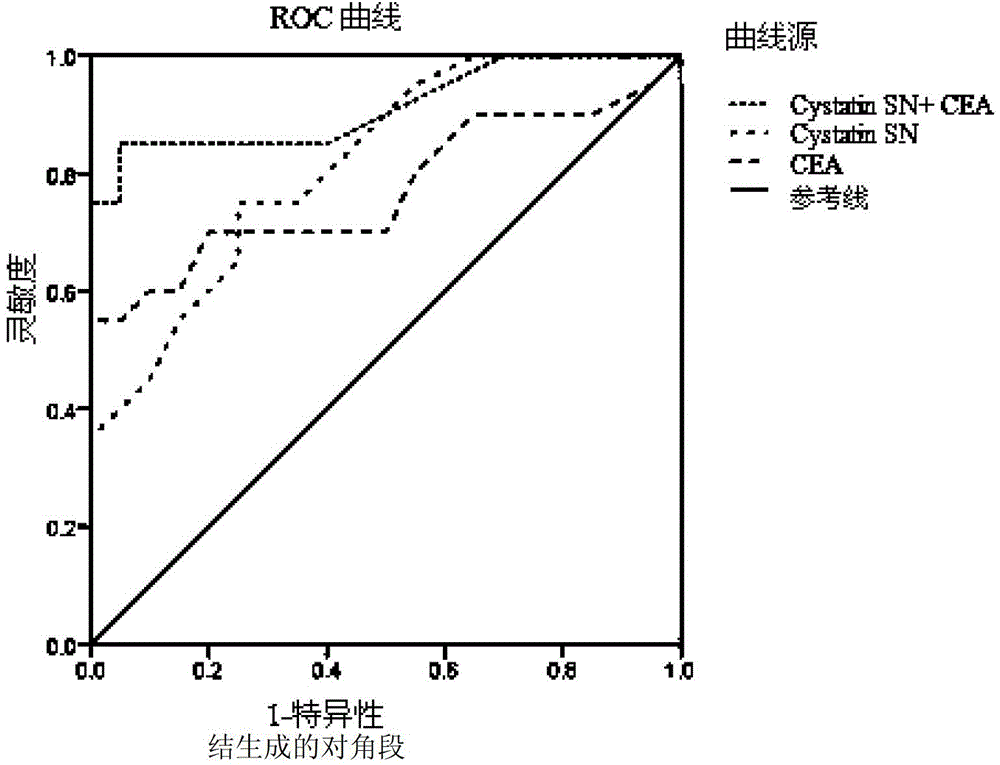
Combined application of cystatin SN and carcino-embryonic antigen
ActiveCN103941016AHigh sensitivityEasy to useImmunoglobulins against animals/humansDisease diagnosisStromal tumorLymphatic Spread
The invention discloses a combined application of a cystatin SN and a carcino-embryonic antigen (CEA), and particularly discloses the application of the cystatin SN and the CEA in preparation of a marker for detection of gastric cancer or gastrointestinal stromal tumor. The invention also discloses a capture agent of the gastric cancer or gastrointestinal stromal tumor marker and a kit containing the capture agent. The prepared kit has the advantages of good specificity, high sensitivity and the like, can be used for early diagnosis of gastric cancer or gastrointestinal stromal tumor, therapeutic effect evaluation in a therapeutic process and monitoring of metastasis and recurrence after therapy, and has test results sooner than clinical symptoms.
Owner:SHANGHAI LIANGRUN BIOMEDICINE TECH CO LTD
Oncofetal antigen determination kit
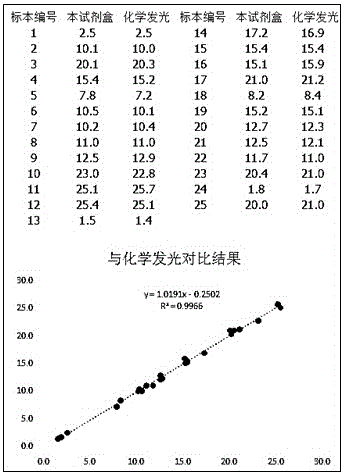
The invention relates to the technical fields of medical science and biochemistry, and concretely relates to an oncofetal antigen determination kit. The purpose of the invention is to solve the problems of complex operation and low determination accuracy of the oncofetal antigen detection process in the prior art. A technical scheme provided by the invention to solve the problems is characterized in that the oncofetal antigen determination kit comprises a reagent R1 and a reagent R2 which are liquid components, wherein the R1 reagent comprises 15-185 mmol / L of an MOPS buffer solution, 150-400 mmol / L of sodium chloride, 5-25 g / L of polyethylene glycol, 18-42 g / L of bovine serum albumins, 0.3-0.9 g / L of sodium azide, and a solvent being purified water, and the reagent R2 comprises 50-150 mmol / L of the MOPS buffer solution, 100-300 mmol / L of sodium chloride, 4-18 g / L of the bovine serum albumins, 5-35 g / L of trehalose, 0.3-0.9 g / L of sodium azide, 0.1-2.0% of a latex coated anti-human oncofetal antigen monoclonal antibody, and the solvent being purified water. The kit has the advantages of high accuracy and convenience in operation.
Owner:ANHUI IPROCOM BIOTECH CO LTD
Preparation method and application of amperometric immunosensor capable of realizing mutual verification by using dual detection method
InactiveCN108982629AImprove conductivityIncrease surface areaMaterial nanotechnologyMaterial analysis by electric/magnetic meansPolypyrroleEngineering
The invention belongs to the technical field of novel nano materials, immunoassay and bio-sensing, and provides a preparation method and application of an amperometric immunosensor capable of realizing mutual verification by using a dual detection method. According to the preparation method, the sandwich type amperometric immunosensor is constructed by taking gold nanoparticle-loaded aminated graphene (Au NPs / NH2-GS) as a substrate platform and a gold-palladium core-shell nano dendrite-loaded Fe<2+>-absorbed chitosan functional polypyrrole nanotube composite material (Au@Pd NDs / Fe<2+>-CS / PPy NTs) as a marker. The constructed immunosensor detects carcino-embryonic antigens with different concentrations by using a time-current curve method and a square-wave pulse voltammetry method, the coincident linear parts of the two test results are mutually verified, so that the accuracy of the sensor can be improved, and a reliable detection means is provided for accurately detecting the carcino-embryonic antigens.
Owner:SHANDONG UNIV OF TECH
Flexible label-free nano bulge metasurface structure and manufacturing and sensing methods thereof
InactiveCN110836872ASimple preparation processLow costMaterial nanotechnologyNanomedicineBovine serum albuminGold film
The invention belongs to the technical field of biomedical detection, and provides a flexible label-free nano bulge metasurface structure. The flexible label-free nano bulge metasurface structure comprises a metasurface structure chip, a biological reagent and an integrated optical fiber probe; the metasurface structure chip is sequentially composited by a substrate, a chromium film and a gold film from bottom to top, wherein the substrate is covered with a periodic nanocolumn array imprinted by a nickel die; the biological reagent comprises a 11-mercaptoundecanoic acid (MUA) solution, a phosphate buffer solution (PBS), ethyl-dimethyl-amino-propyl carbodiimide (EDC), N-hydroxysuccinimide (NHS), bovine serum albumin (BSA), a carcino-embryonic antigen (CEA) and a carcino-embryonic protein antibody (Anti-CEA); and the integrated optical fiber probe is used for measuring a reflection spectrum of the metasurface structure. The invention further provides manufacturing and sensing methods ofthe flexible label-free nano bulge metasurface structure. The metasurface structure provided by the invention is low in cost, and the biomedical function of the metasurface structure is applied to high-performance biosensors.
Owner:XIAMEN UNIV
Preparation method and application of immunosensor capable of simultaneously detecting human nuclear matrix protein and carcino-embryonic antigen with three antibodies and double channels
ActiveCN105445475ALarge specific surface areaFacilitates electron transferDisease diagnosisBiological testingBladder cancerCarcinoembryonal antigen
The invention relates to a preparation method and an application of an immunosensor capable of simultaneously detecting human nuclear matrix protein and carcino-embryonic antigen with three antibodies and double channels, and belongs to the technical field of building of novel sensors. On the basis of good specificity between the antigen and the antibodies, the detection ranges of the human nuclear matrix protein and the carcino-embryonic antigen are increased by the sensor by virtue of a sandwich structure of a human immunoglobulin immunization pair; the sensitivity of the sensor is significantly improved; the detection limit of the sensor is reduced; and the preparation method is of great significance in early diagnosis of bladder cancer.
Owner:UNIV OF JINAN
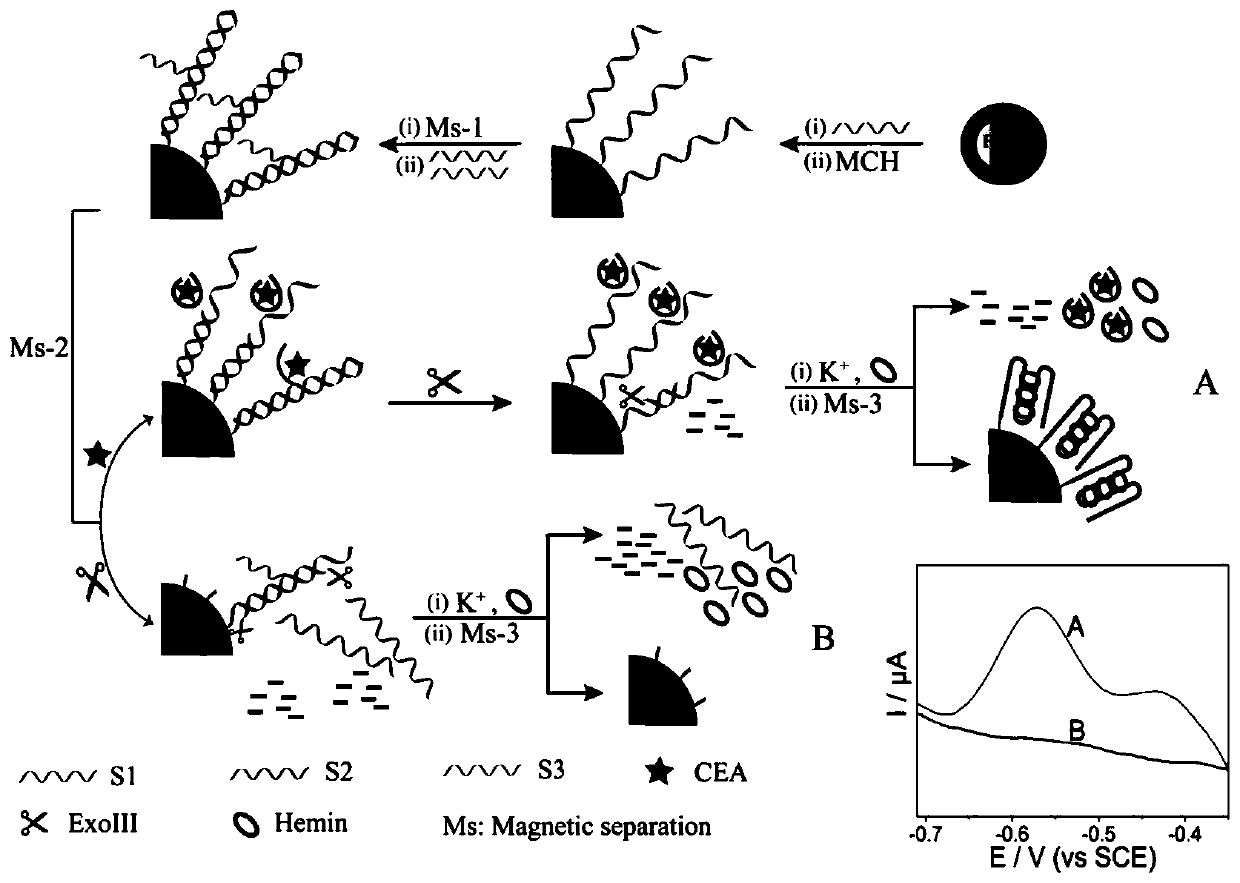
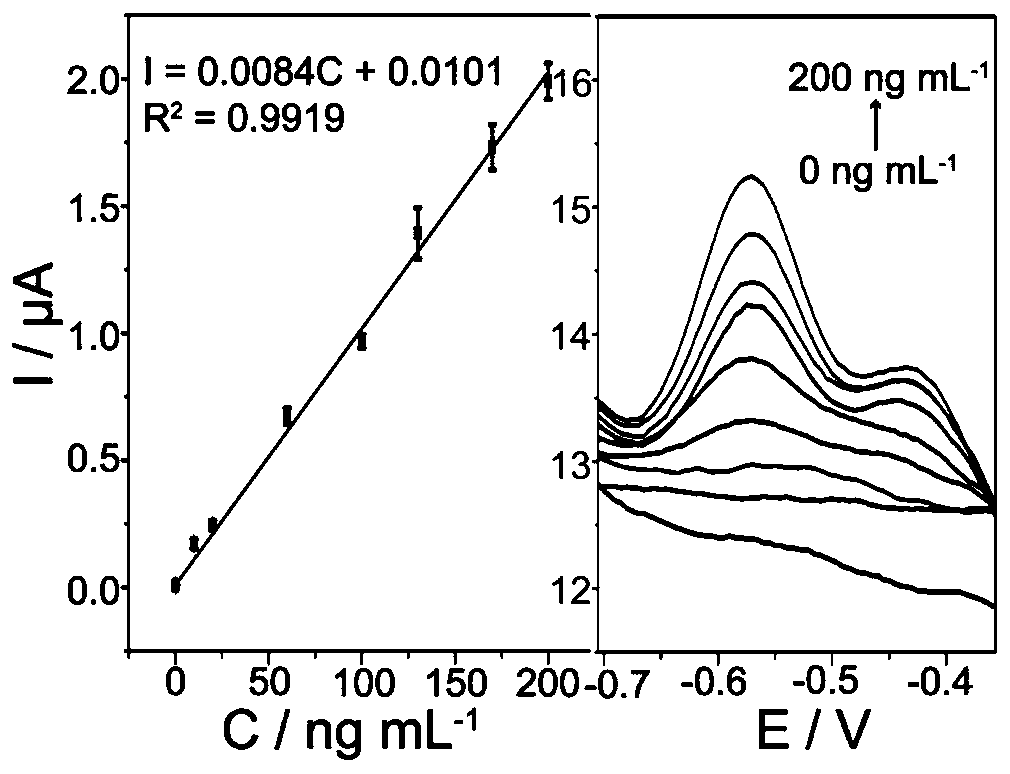
Carcino-embryonic antigen electrochemical sensor constructed from magnetic material and exonuclease III
PendingCN111500686AQuick checkAccurate detectionMicrobiological testing/measurementMaterial electrochemical variablesAptamerCarcinoembryonal antigen
The invention discloses a carcino-embryonic antigen electrochemical sensor constructed from a magnetic material and exonuclease III. The electrochemical sensor is constructed from a magnetic biological composite material Fe3O4@Au NPs-S1-S2-S3 under the assistance of the exonuclease III, wherein S1 is a nucleic acid sequence, S2 is a carcino-embryonic antigen aptamer, the nucleic acid sequence S1 comprises a G-rich sequence and a complementary pairing sequence of the carcino-embryonic antigen aptamer S2, and S3 is a complementary sequence of the G-rich sequence. The electrochemical sensor for detecting the carcino-embryonic antigen does not need electrode modification, can rapidly and accurately detect the carcino-embryonic antigen, has high selectivity on the carcino-embryonic antigen, andsolves the technical problems that an existing electrochemical detection method needs complex electrode modification, the process is tedious, the cost is high and electrode regeneration is difficult.
Owner:NANJING MEDICAL UNIV
Immunochromatographic assay test paper strip for quantitatively detecting carcino-embryonic antigen (CEA) and preparation method thereof
InactiveCN105891485AAchieving High Sensitivity Quantitative Immunochromatographic AssaysDetection Sensitivity AmplificationMaterial analysisCelluloseSmall sample
The invention relates to an immunochromatographic assay test paper strip for quantitatively detecting a carcino-embryonic antigen (CEA) and a preparation method thereof. The immunochromatographic assay test paper strip mainly comprises a sample pad, a first combining pad, a second combining pad, a cellulose nitrate membrane, a bottom plate and an absorbent paper, wherein the sample pad is treated, the first combining pad is coated with detection magnetic beads, and the second combining pad is coated with signal magnetic beads; an IgG antibody, i.e., a quality control line and a CEA coated antibody, i.e., a detection line are fixed on the cellulose nitrate membrane. The preparation method comprises the step of sequentially stacking and combining the absorbent paper, the cellulose nitrate membrane, the combining pads and the sample pad. According to the immunochromatographic assay test paper strip for quantitatively detecting the CEA and the preparation method thereof, characteristics of the magnetic beads and characteristics of an amplifying system are integrated, the aims of specific sensitivity and quantitative detection are achieved, and the cost is low; the immunochromatographic assay test paper strip simultaneously supports detection on small samples and large samples, is simple in operation and convenient and rapid in detection and is suitable for being subjected to large-scale popularized production.
Owner:SHANGHAI INST OF MICROSYSTEM & INFORMATION TECH CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com