Anticancer embryo antigen antibody as well as preparation method and application thereof
An antibody and amino acid technology, applied in the biological field, can solve the problems of low survival rate and no particularly effective treatment for patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Panning and ELISA preliminary screening of natural human phage antibody library
[0085] Panning of phage antibody library
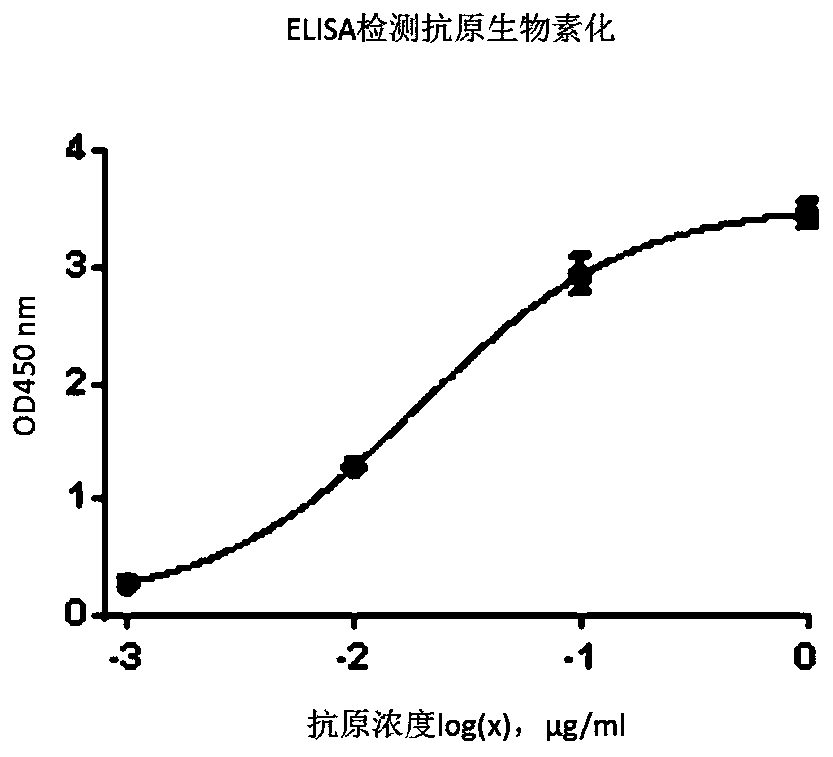
[0086] Prepare 3 EP tubes for magnetic bead blocking and wall blocking (A: 1ml PBS, 2% Blotting-GradeBlocker, 50μl streptavidin magnetic beads, 100μl phage library; B: 1ml PBS, 2%Blotting-GradeBlocker, 100 μl streptavidin magnetic beads; C: 1 ml PBS, 2% Blotting-Grade Blocker) at room temperature for 1-2 h. Discard the solution in tube C and the magnetic beads in tube A, collect the phage in tube A and add them to tube C, and at the same time add biotin-labeled antigen CEA (final concentration 20 μg / ml), incubate at room temperature for 2 h. Collect the magnetic beads in tube B and discard the solution, add the mixture in tube C and incubate on a rotary shaker for 15 minutes. Put the EP tube on the magnetic stand and let it stand for 30s, so that the magnetic beads are adsorbed to the tube wall. Discard the supernatant, and add MPBST (PBS+2%Blo...
Embodiment 2
[0094] FACS screening of candidate clones
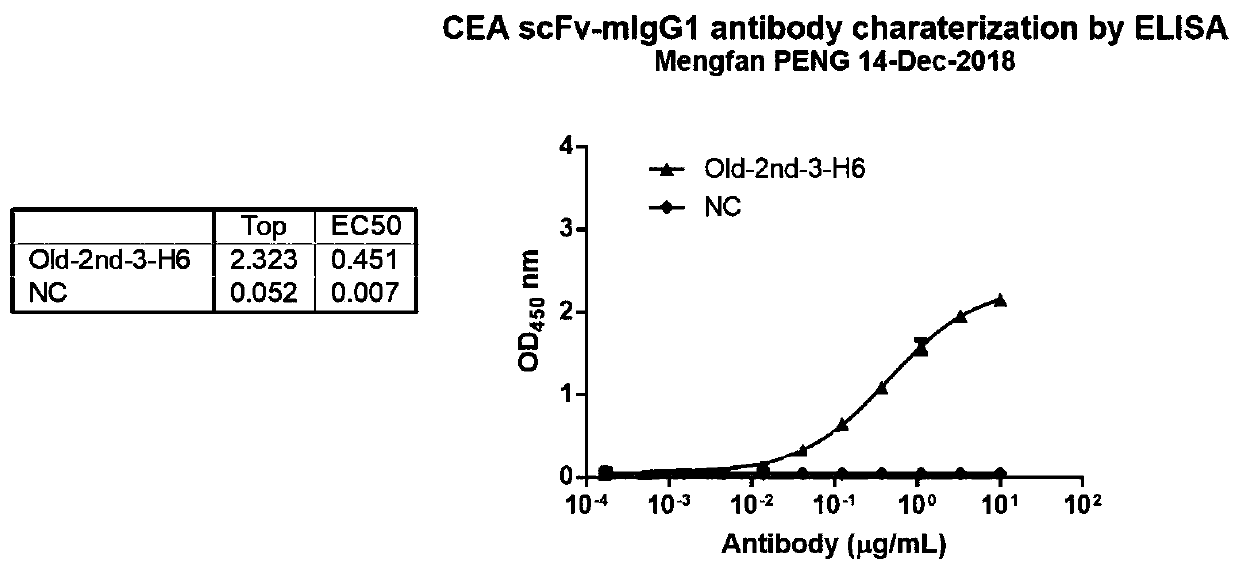
[0095] Cell culture was performed according to standard cell culture protocols, and single-cell suspensions were prepared by trypsinizing the cells. Centrifuge (300g, 5min) to remove the culture medium and resuspend the cells to 2*10 6 cell / ml. Add 2*10 to each well of the round bottom 96-well plate 5 cell suspension of cells. After centrifugation at 300 g for 5 min, the supernatant was removed, and the cells were resuspended with 100 μl of periplasmic protein extract, and incubated at 4°C for 1 h. After centrifugation at 300g for 5min, the supernatant was removed, the cells were resuspended in Flow solution, and repeated 3 times to remove periplasmic protein extracts. Dilute anti-myc antibody to 2 μg / ml with blocking solution, resuspend cells in 100 μl per well, and incubate at 4°C for 1 hour. After washing the cells with Flow Buffer for 3 times, add 100 μl of Alexa-488 anti-mouse antibody to resuspend the cells, and incubate a...
Embodiment 3
[0103] Off-rate ranking candidate clones
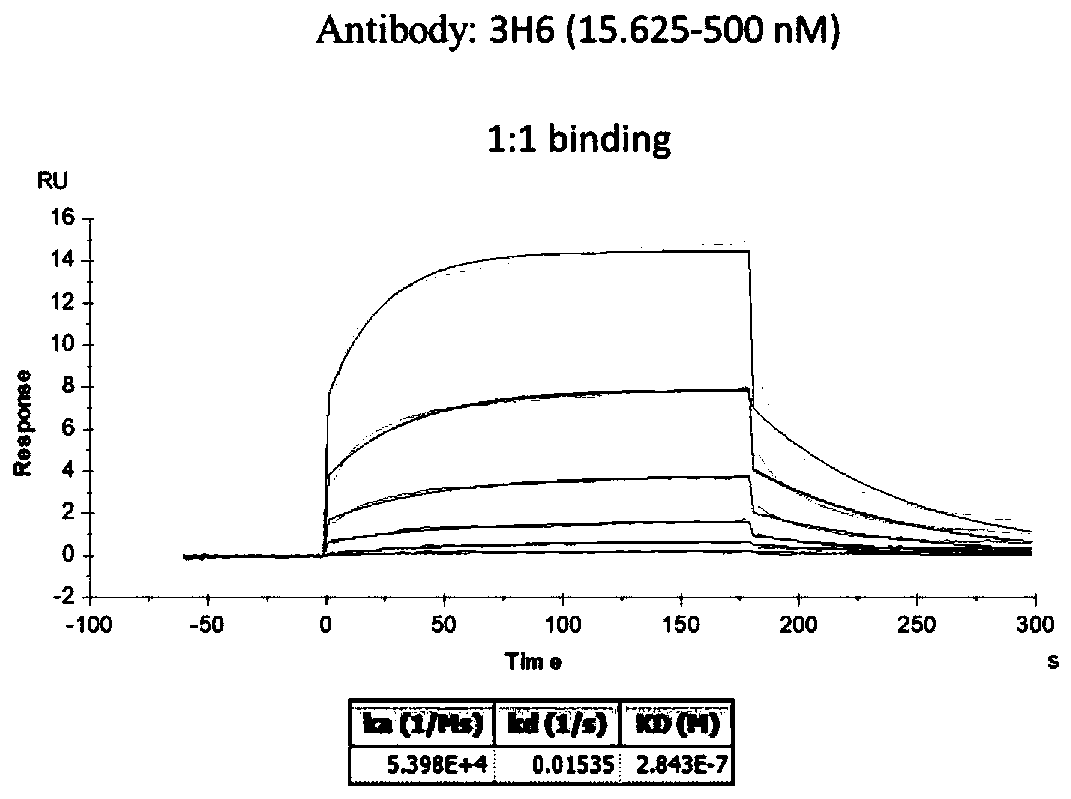
[0104] Antibody affinity depends on the degree of fit between the variable region of the antibody and the antigen, and is generally assessed by affinity. The dissociation constant (Koff) is generally the main kinetic mechanism affecting antibody affinity, so by measuring the dissociation rate, the level of antibody affinity can be initially compared. At the same time, Koff is concentration-independent, so Koff can be measured without purification of antibodies. Determining the Koff of candidate clones by Octet Red and sorting will help to further select clones for subsequent analysis. Bacterial periplasmic extracts were prepared according to standard operating procedures. The biotin-labeled antigen (5 μg / ml) was immobilized on the streptavidin SA biosensor, the periplasmic extract was diluted 2-fold with 1×kinetic buffer, the binding time of antigen and scFv antibody was 300s, and the dissociation time was 300s. SA biosensors were ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com