Innate targeting of adoptive cellular therapies
A cell and immune cell technology, applied in animal cells, allergic diseases, vertebrate cells, etc., can solve the problems of poor clinical results and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0218] Example 1: SSI enhances the anti-tumor effectiveness of adoptively transferred tumor antigen-specific (TCR-tg) T cells
[0219] This example illustrates that site-specific immunotherapy (QBKPN SSI) of microbial PRR ligand preparations enhances adoptively transferred tumor antigen-specific CD8 + Antitumor efficacy of T cells (Pmel).
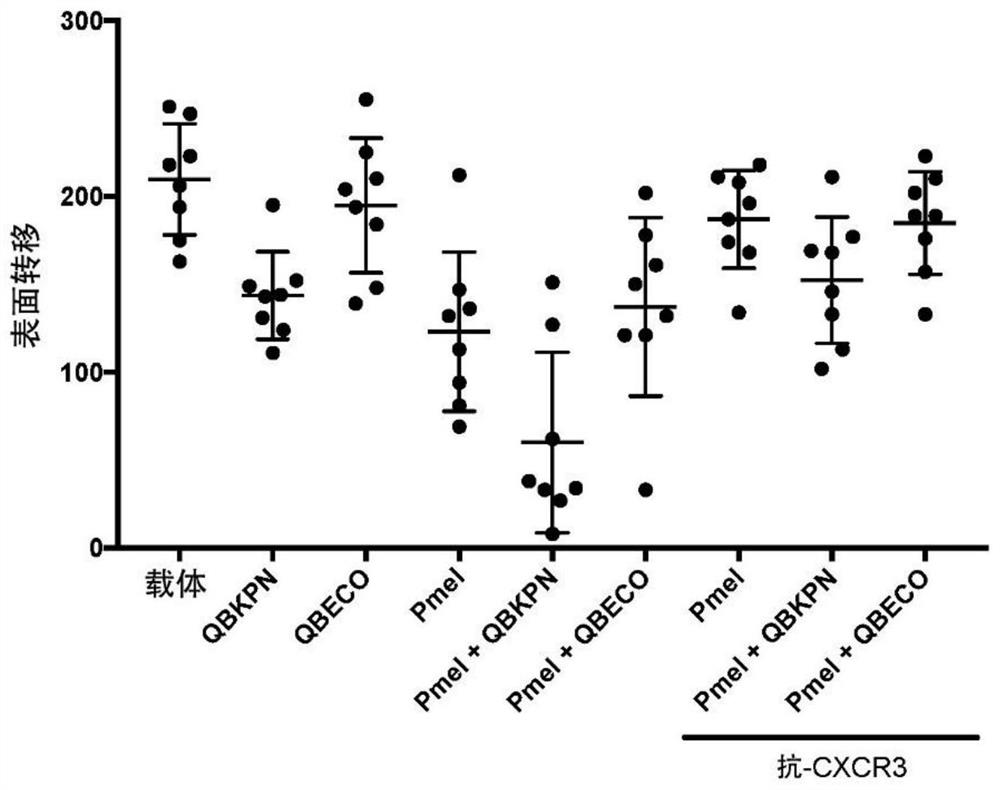
[0220] in order to achieve figure 1 Data shown, B6 mice received SSI or vehicle (30 mL SC) every other day from day -10 to day +18. On day 0, by IV injection with 3 x 10 5 Mice were challenged with B16-F10 (B16) melanoma cells. On days +2 and +6, some mice received an IP infusion of 1 mg anti-CXCR3 (clone CXCR3-173) mAb to block CXCR3-mediated chemotaxis / extravasation. On day +3, some mice were infused intravenously with 1 x 10 6 In vitro activation of CD8+Pmel(TCR-tg) cells. These activated T cells were obtained from an immunologically well-characterized mouse strain (JAX strain 005023-B6 genetic background) carrying a melanocyte / mel...
example 2
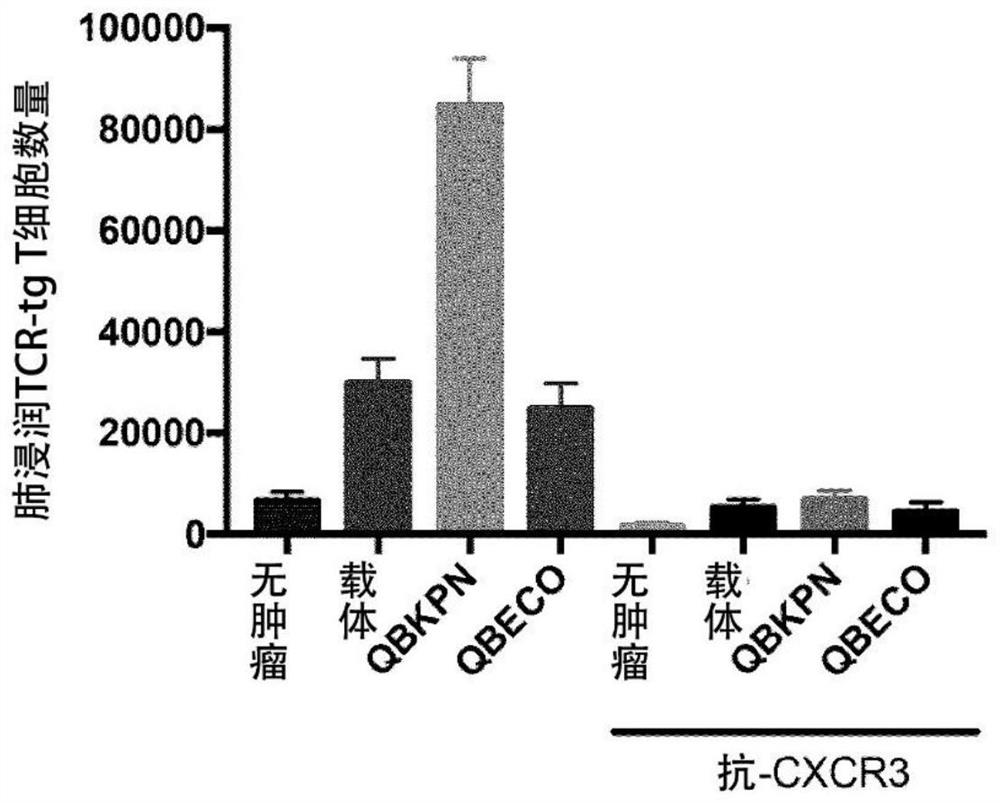
[0222] Example 2: SSI enhances antigen-specific (TCR-tg) T cells in infiltrating tumor-bearing lungs of metastatic tumors
[0223] This example illustrates site-specific immunotherapy (QBKPN SSI) of microbial PRR ligand preparations through activation of tumor antigen-specific (TCR-tg) CD8 + T cells enhanced chemoattraction and infiltration of tumor-bearing lungs.
[0224] in order to achieve figure 2 Data shown, B6 mice received SSI or vehicle (30 mL SC) every other day from day -10 to day +4. On day 0, by IV injection with 3 x 10 5 Mice were challenged with B16-F10 (B16) melanoma cells. On day +2, some mice received an IP infusion of 1 mg anti-CXCR3 (clone CXCR3-173) mAb to block CXCR3-mediated chemotaxis / extravasation. On day +3, some mice were infused intravenously with 1 x 10 6 In vitro activation of CD8+Pmel(TCR-tg) cells. On day +4, mice were sacrificed and surviving infiltrating TCR-tg T cells were counted by flow cytometry (based on Thy-mismatch labeling). and...
example 3
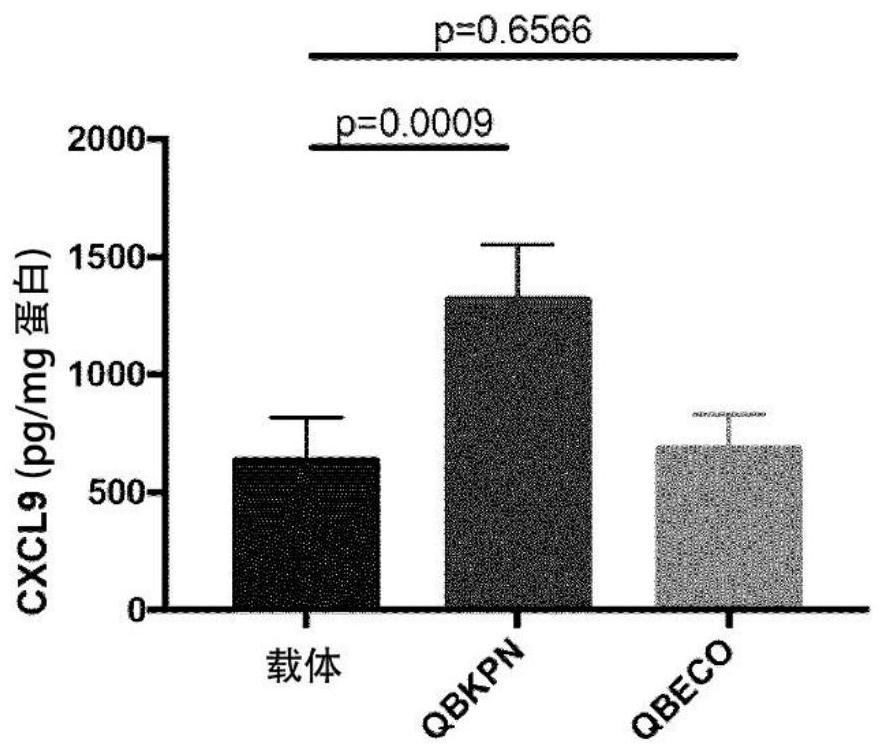
[0226] Example 3: SSI enhances T-cell chemokine production in tumor-bearing lungs
[0227] This example demonstrates that site-specific immunotherapy with tissue-specific microbial PRR ligand preparations can enhance chemokine production in tumor-bearing lungs late in tumor growth, when chemokines are otherwise suppressed by the tumor. This suggests that the chemokine production signature is associated with enhanced TCR-tg CD8 in SSI-treated animals + associated with T cell infiltration.
[0228] in order to achieve image 3 and Figure 4 Data shown, B6 mice received SSI (QBKPN or QBECO) or vehicle (30 mL SC) from day -10 to day +18, dosed every other day. On day 0, by IV injection with 3 x 10 5 Mice were challenged with B16-F10 (B16) melanoma cells. On day +18, mice were sacrificed and lung and serum were analyzed for chemokines by specific ELISA (not shown). As shown, QBKPN enhanced chemokine production in tumor-bearing lungs compared to vehicle and QBECO (SSI formulat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com