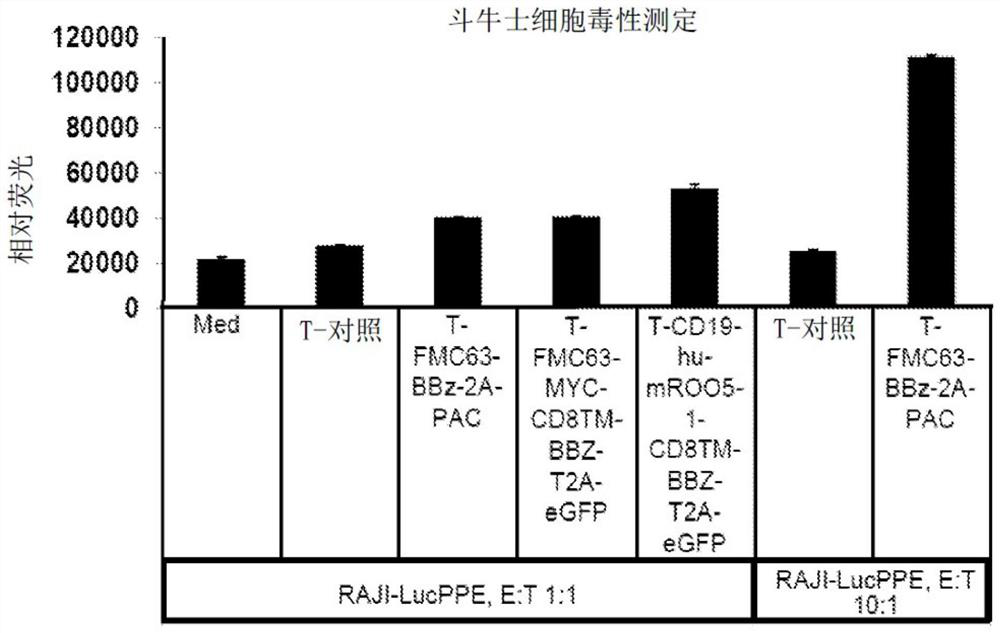
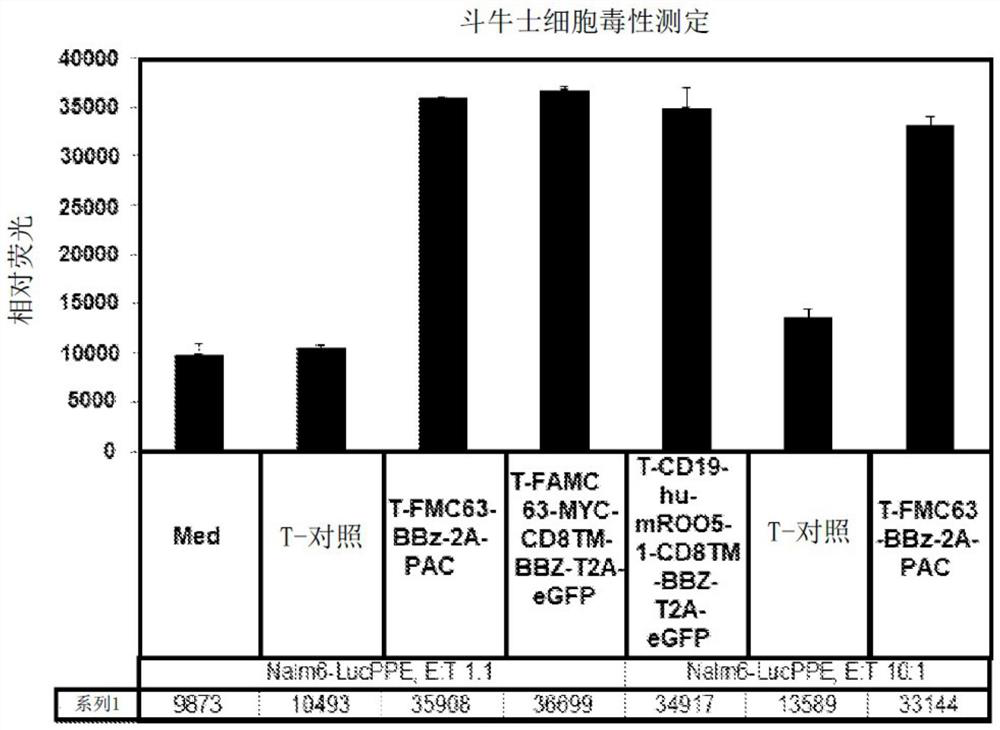
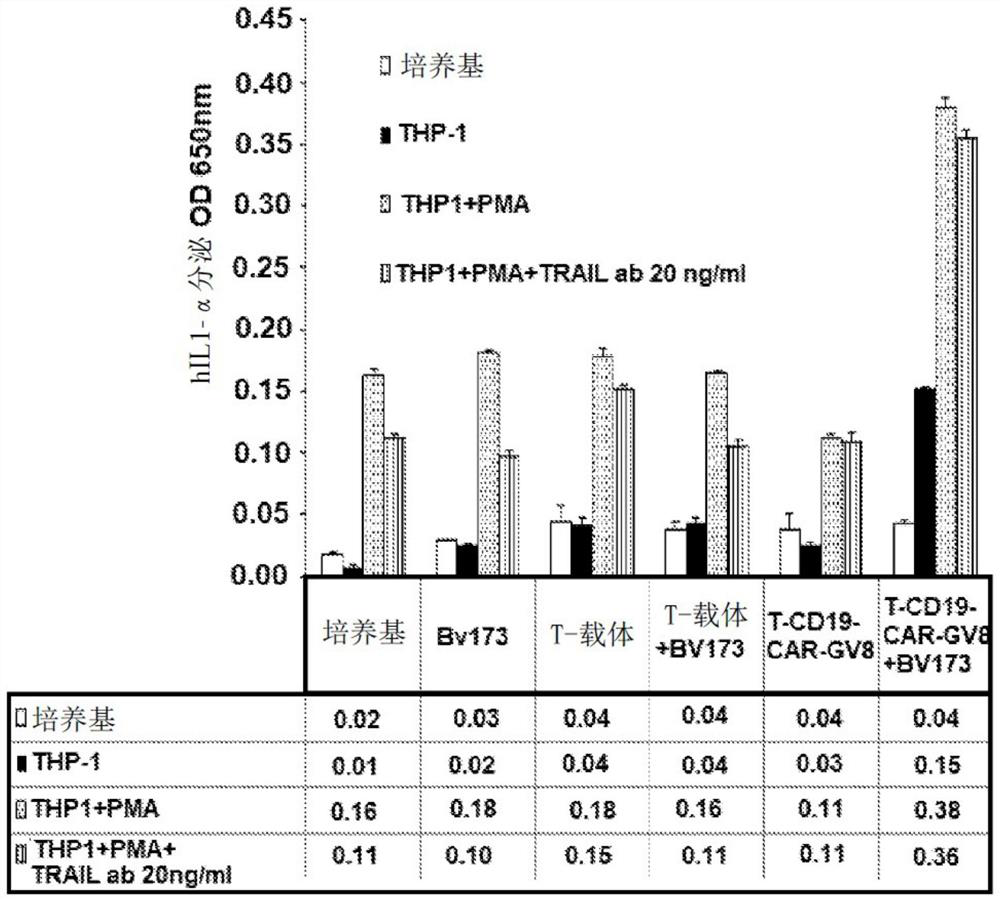
Improving the efficacy and safety of adoptive cellular therapies
A cell and cytokine technology, used in cell culture active agents, animal cells, vertebrate cells, etc., can solve problems such as excessive stimulation and proliferation, failure of T cell reproduction, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0732] The present disclosure is described in further detail with reference to the following experimental examples. These examples are provided for illustrative purposes only and are not intended to be limiting unless otherwise indicated. Accordingly, the present disclosure should in no way be construed as limited to the following examples, but rather should be construed to cover any and all modifications that become apparent as a result of the teachings presented herein.
[0733] Methods for generating and characterizing CAR-T cells, including lentivirus and retrovirus production, infection of T cells and PBMCs, culture and propagation of T cells, in vitro assays of T cell function, such as ELISA, flow cytometry, Cell death assays (e.g. Matador assay), antigen detection assays (e.g. Topanga assay) and in vivo assays are known in the literature and have been described in WO 2018 / 102795 (which is incorporated herein by reference in its entirety) .
[0734] Production of Lenti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com