Chimeric antigen receptor, vector, human dendritic cell, cell line, solid tumor treatment medicine, preparation method and application
A chimeric antigen receptor, dendritic cell technology, applied in anti-tumor drugs, antibody medical components, antibody mimics/scaffolds, etc., can solve the problem of not yet obtained tumor-infiltrating DCs activity, and achieve the reversal of immunosuppressive effects. The effect of tumor microenvironment, optimization of preparation process, and enhancement of clearance capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
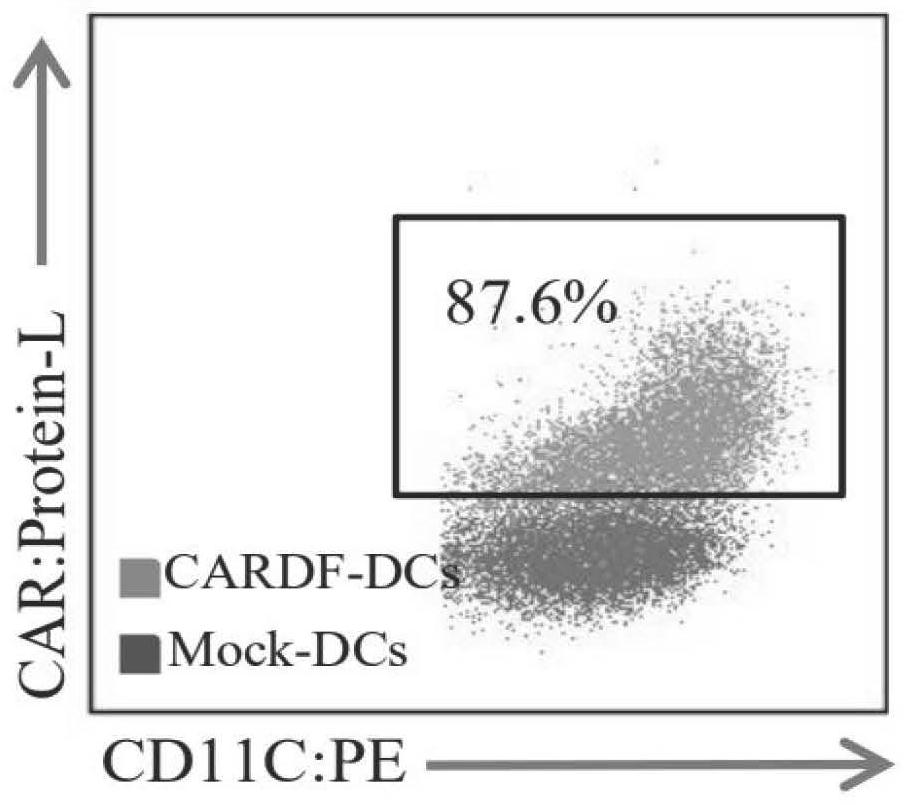
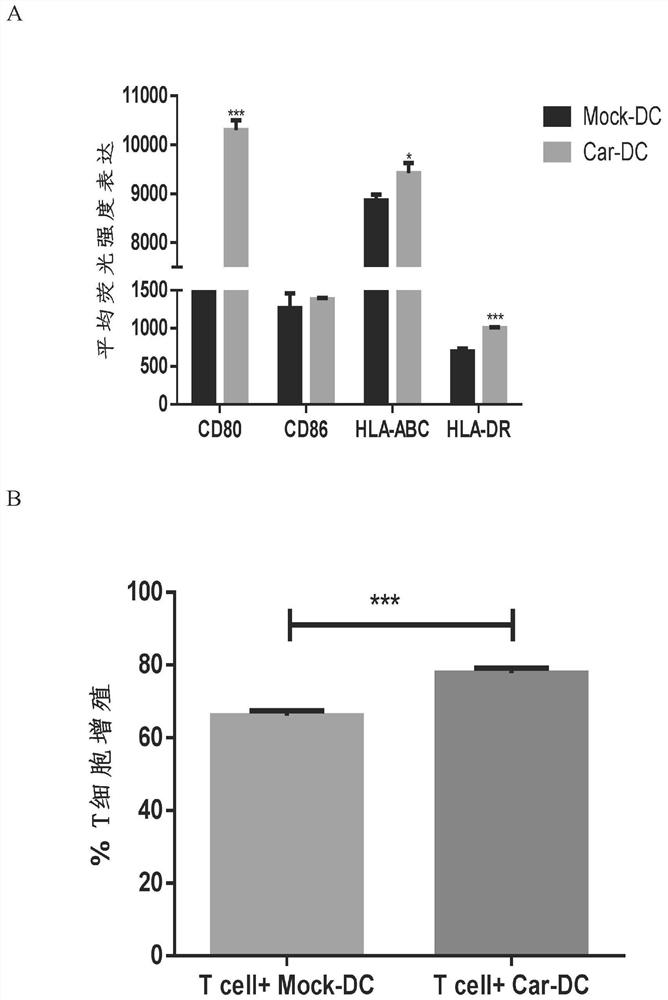
[0028] The present invention also provides a method for preparing human dendritic cells modified by the chimeric antigen receptor described in the above technical scheme, comprising the following steps: transducing the chimeric antigen receptor into the precursor cells of human dendritic cells, Induce differentiation to obtain chimeric antigen receptor-modified human dendritic cells. By adopting this method, the efficiency of expressing the CARDF structure on the surface of the dendritic cells is greatly improved, and the purity of the finally obtained CARDF-DCs reaches more than 85%. The chimeric antigen receptor in the present invention preferably infects precursor cells, and when the precursor cells are monocytes in human peripheral blood mononuclear cells, the virus multiplicity of infection in the process of infecting mononuclear cells in the present invention is preferably 100. In the present invention, the time for inducing differentiation of infected monocytes into DC ...
Embodiment 1
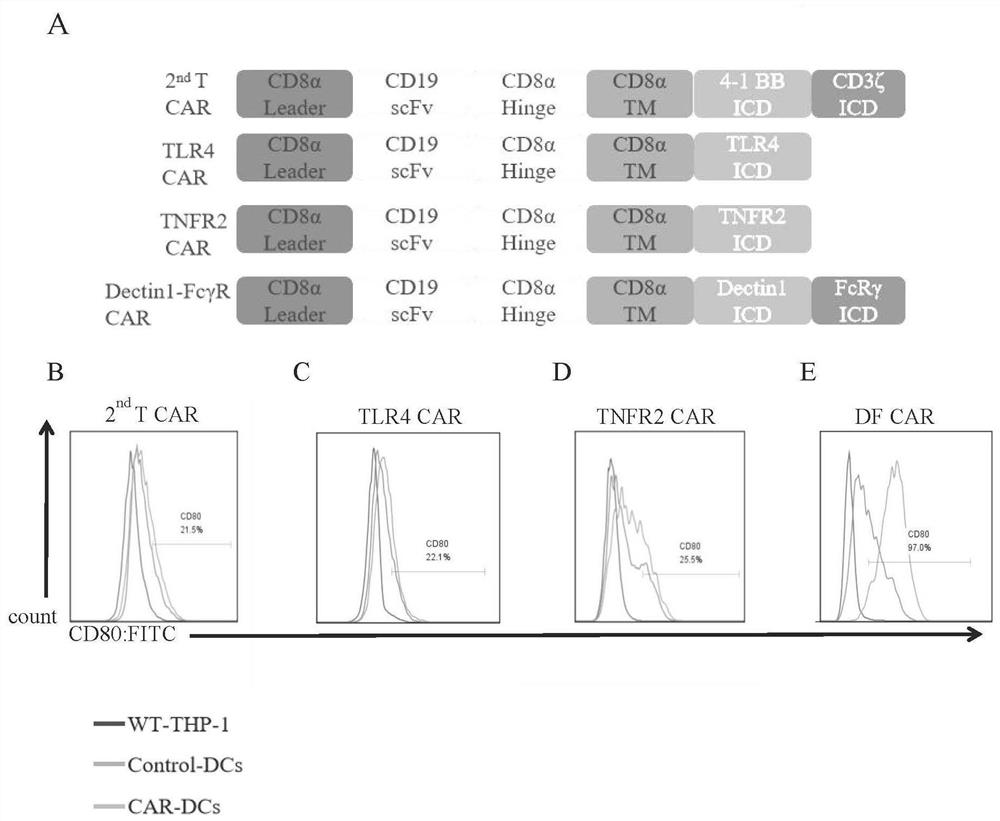
[0035] 1. Construction of lentiviral vector expressing CAR and the effect of different CAR structures on activating DCs derived from THP-1:
[0036] (1) Construction of lentiviral vector. The intracellular signaling domains of all DC-specific CARs were replaced by TLR4 (NM_138554.5), TNFR2 (NM_001066.3), Dectin1 (NM_197947) and FcRγ (NM_004106) by replacing 4-1BB and CD3ξ of the traditional second-generation T-CAR structure The intracellular domain part, all sequences were optimized and synthesized by the company (Guangzhou Aiji). The CAR was finally cloned into the lenti-Cas9 (Addgene) vector to replace Cas9. See the structure diagram figure 1 A in
[0037] The DF sequence is as follows:
[0038] nucleic acid sequence
[0039] CGCTGGCCTCCTTCTGCAGCTTGTTCGGGAAAAGAGTCAGTTGTT GCTATAAGGACCAATAGCCAATCTGACTTCCACTTACAAACTTATGGAG ATGAAGATTTGAATGAATTAGATCCTCATTATGAAATGCGACTGAAGATCCAAGTGCGAAAGGCAGCTATAACCAGCTATGAGAAATCAGATGGTGT TTACACGGGCCTGAGCACCAGGAACCAGGAGACTTACGAGACTCTGA AGCATG...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average volume | aaaaa | aaaaa |
| Average volume | aaaaa | aaaaa |
| Average volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com