Use of an anti-PD-1 antibody combined with a vegfr inhibitor in the preparation of a drug for treating cancer
A PD-1, inhibitor technology, applied in the direction of antibody medical components, antibodies, drug combinations, etc., can solve problems such as poor inhibitory effect, burden on mental health and quality of life, and no inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
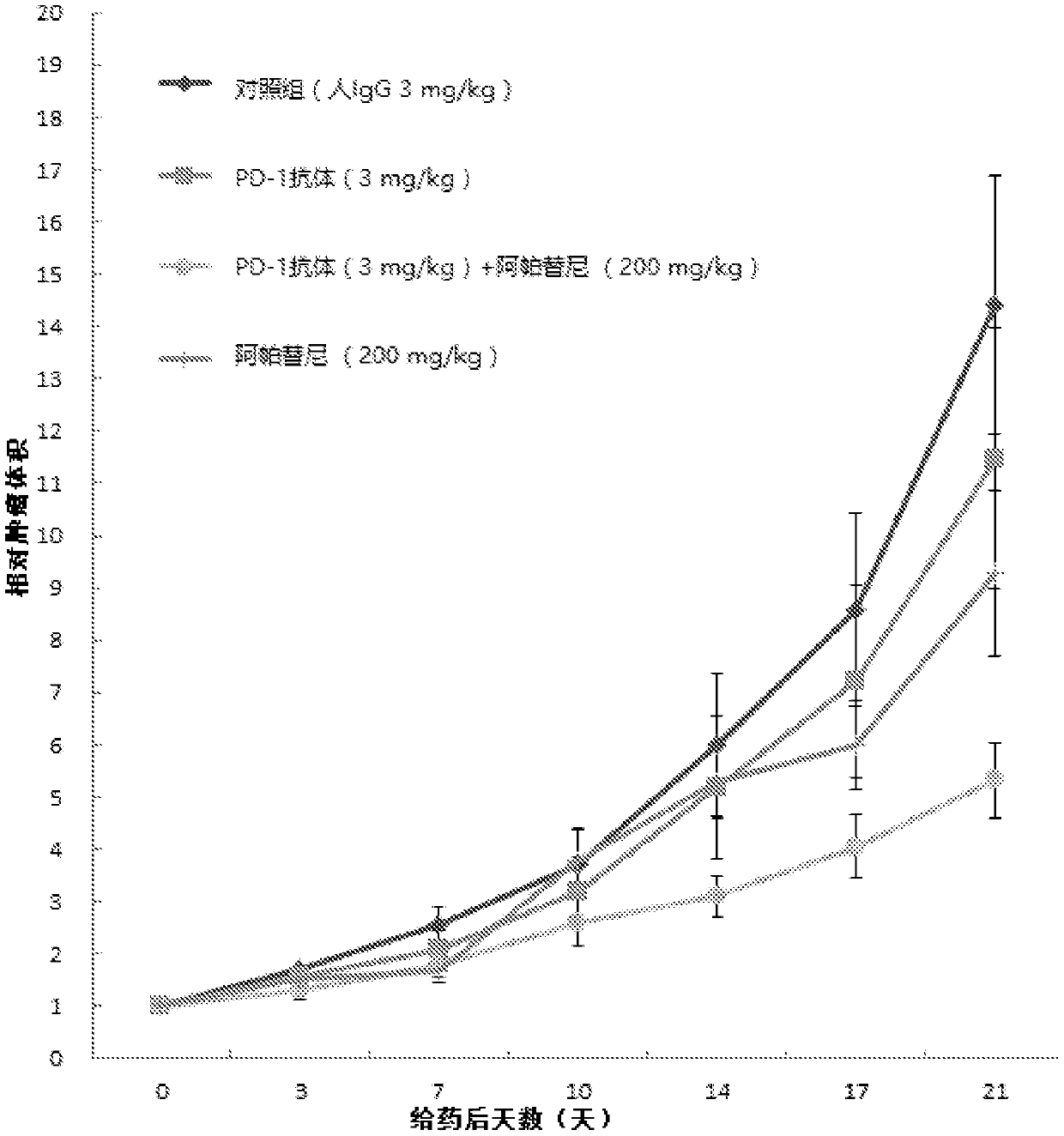
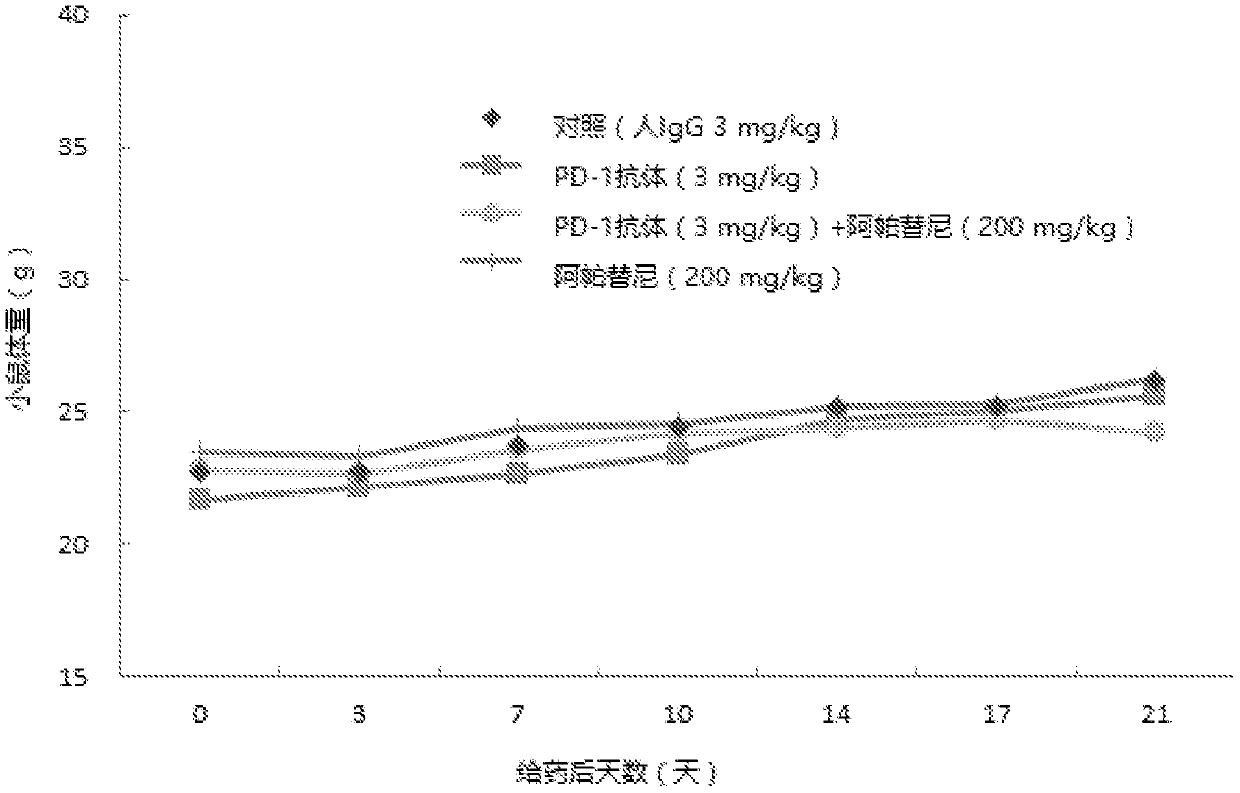
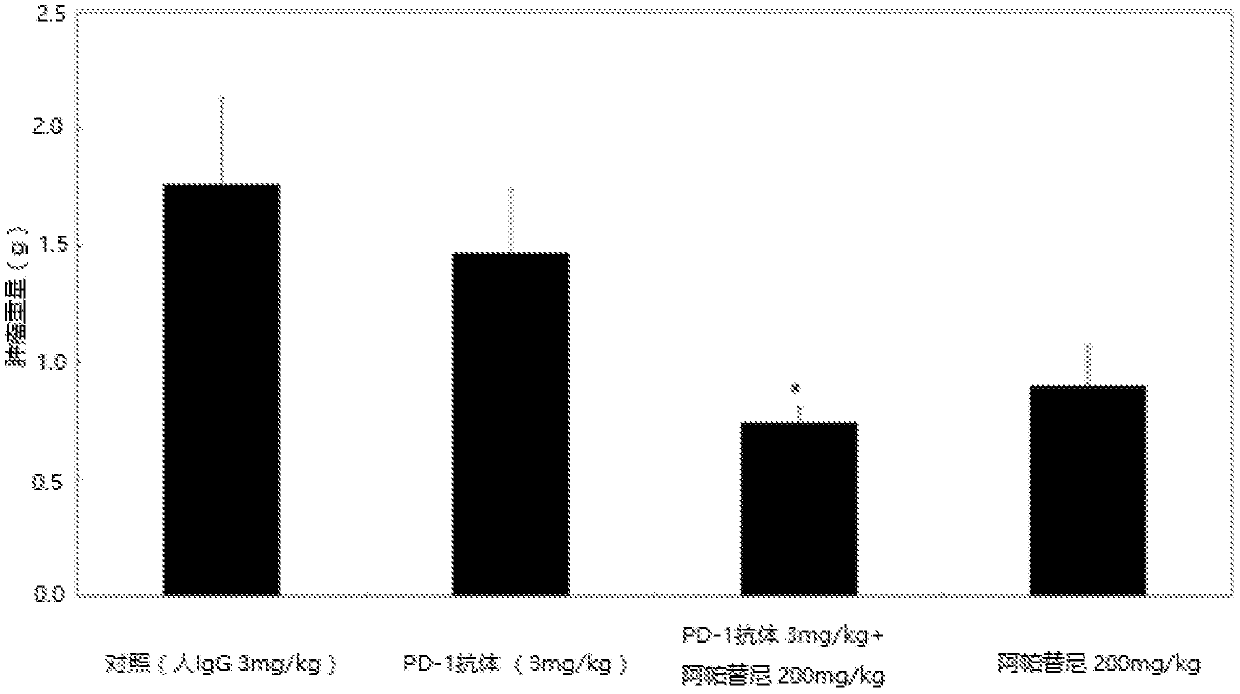
[0043] Example 1: PD-1 antibody, apatinib mesylate alone or in combination with PD-L1 gene transferred mouse colon cancer cell MC-38 (PD-L1) transplanted tumor C57 human PD- 1 Efficacy of transgenic mice
[0044] 1. Research purpose
[0045] Human PD-1 transgenic mice were used as experimental animals to evaluate the effect of PD-1 antibody combined with apatinib on the transplanted tumor C57 of mouse colon cancer cell MC-38 (PD-L1) with PD-L1 gene Efficacy of Human PD-1 Transgenic Mice.
[0046] 2. Antibodies and compounds to be tested
[0047] The PD-1 antibody is prepared according to the method disclosed in WO2015085847, corresponding to its code name H005-1, and the sequences of its heavy and light chains are as SEQ ID NO: 7 and SEQ ID NO: 8 in the present invention. Batch number: P1512, 200mg / bottle, prepared as 20mg / ml for later use.
[0048] Apatinib mesylate, prepared according to the method disclosed in CN101676267A, batch number: 668160401; molecular weight: 493...
Embodiment 2
[0071] Example 2: Clinical study of anti-PD-1 antibody combined with apatinib mesylate in the treatment of advanced malignant tumors
[0072] Inclusion criteria: (1) Advanced malignant tumors; (2) Failure of first-line or second-line chemotherapy; (3) Measurable lesions; (4) ECOG score 0-1.
[0073] Test drug: commercially available apatinib mesylate tablets; PD-1 antibody in Example 1.
[0074] Administration method: As of September 20, 2017, a total of 31 subjects have been screened, and 30 subjects have been enrolled (14 subjects have withdrawn from treatment, and 16 subjects are still regrouped for administration) middle).
[0075]The administration method of 001-005 cases was intravenous infusion of PD-1 antibody, 3 mg / kg, every 2 weeks; oral administration of apatinib, 500 mg, once a day; the administration method of 006-010 cases was intravenous infusion of PD-1 antibody Infusion, 200mg, 2 weeks / time; Oral apatinib, 125mg, once a day; 011-031 cases, PD-1 antibody intr...
Embodiment 3
[0077] Example 3: Phase II clinical study of anti-PD-1 antibody combined with apatinib mesylate in the treatment of advanced non-small cell lung cancer
[0078] Inclusion criteria: (1) Advanced non-small cell lung cancer; (2) Failure of first-line or second-line chemotherapy; (3) Measurable lesions; (4) ECOG score 0-1.
[0079] Test drug: commercially available apatinib mesylate tablets; PD-1 antibody in Example 1.
[0080] Administration method: PD-1 antibody, once every 2 weeks, intravenous infusion, 200 mg each time; apatinib mesylate was taken orally once a day, 250 mg or 375 mg or 500 mg each time.
[0081] Clinical results: As of July 28, a total of 15 subjects have been screened, of which 12 have been enrolled. A total of 12 subjects completed at least one cycle of medication observation, 10 patients (10 / 12) had stable disease, and 1 patient had partial remission. See Table 5 for details. In addition, in the process of combining apatinib mesylate and PD-1 antibody, w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com