Tumor cell dryness restriction-type CAR and application thereof
A tumor cell, restricted technology, applied in the field of tumor cell immunotherapy, which can solve problems such as unsatisfactory, residual tumor stem cells, recurrence, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
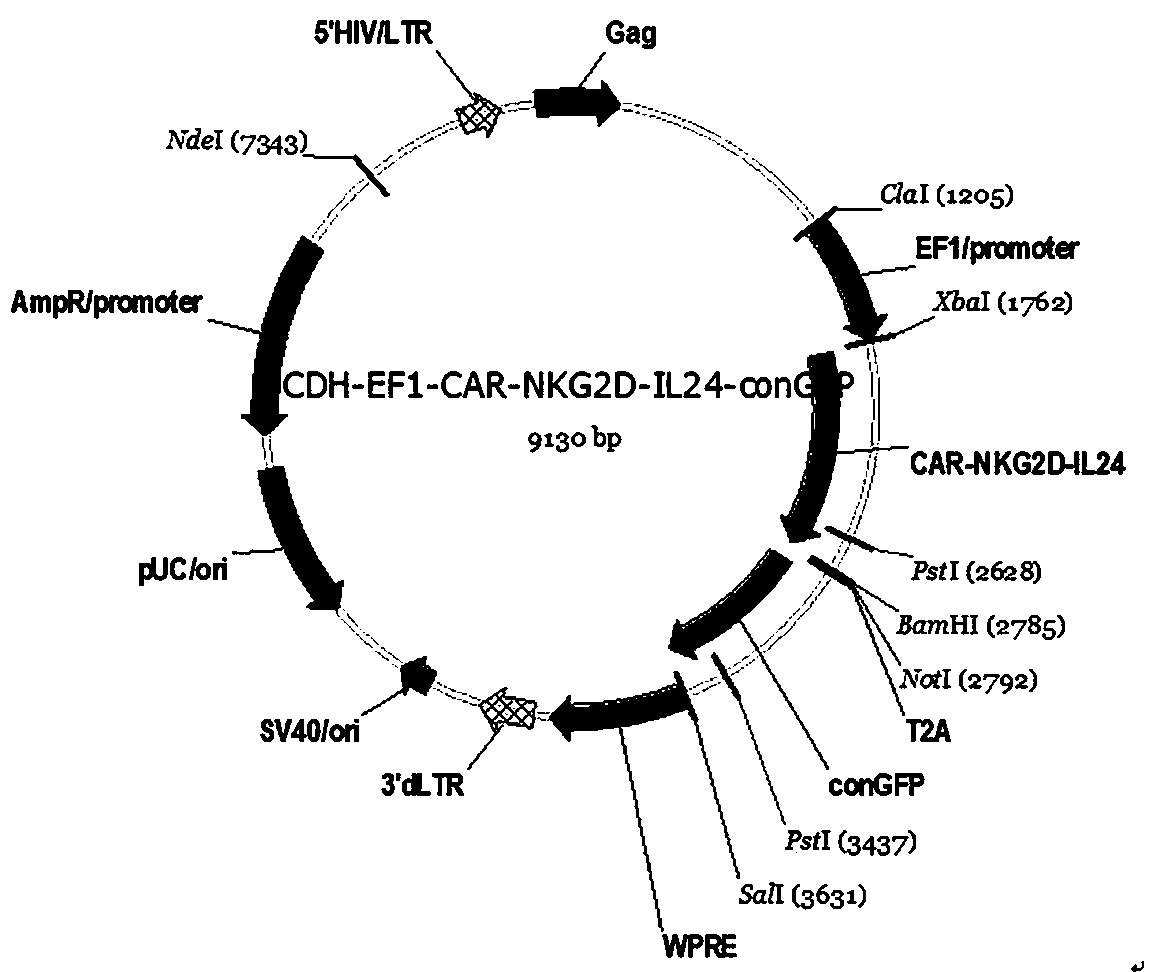
[0064] Based on the existing CAR technology research, in order to further improve the application effect of CAR-T in the treatment of solid tumors, the inventors designed a new CAR structure, the specific structure diagram is as follows figure 1 shown.
[0065] in particular:
[0066] NKG2D-41BB-CD3z is: human CD8a molecular signal peptide (CD8a), human NKG2D extracellular region, human CD8 molecular transmembrane region and 41BB molecular intracellular region (41BB), human CD3z molecular intracellular region (CD3Zeta) connected in sequence ), namely: CD8a-NKG2D-41BB-CD3Zeta (CD3ζ);
[0067] NKG2D -41BB-CD3z-IL24 is: human CD8a molecular signal peptide (CD8a), human NKG2D extracellular region, human CD8 molecular transmembrane region and 41BB molecular intracellular region (41BB), human CD3z molecular intracellular region connected in sequence (CD3Zeta), PA2 junction sequence (P2A), IL22 CDS region sequence (IL22); namely: CD8a-NKG2D-41BB-CD3Zeta (CD3ζ)-P2A-IL24.
[0068] T...
Embodiment 2
[0090] On the basis of Example 1, the inventors further constructed a lentiviral expression vector, so that it can be further used to infect T cells and prepare CAR-T. The specific construction process of the lentiviral expression vector is introduced as follows.
[0091] (1) According to the central dogma, using existing technology to obtain the coding sequence DNA of the CAR;
[0092] (2) Using the pCDH-EF1-conGFP plasmid as the expression vector, use the restriction endonuclease EcoR I (NEB) to digest it at 37°C, and then use In-Fusion HD Cloning Kits (Bao Biology) to convert the step (1 The coding sequence DNA in ) was integrated and recombined into the pCDH-EF1-conGFP plasmid;
[0093] (3) Transform the ligation product in step (2) into STABL3 competent cells, screen, expand and culture, and further extract the plasmid to obtain recombinant lentiviral expression that can express NKG2D-41BB-CD3z or NKG2D-41BB-CD3z-IL24 Plasmids (respectively named: pCDH-EF1-NKG2D-41BB-CD3...
Embodiment 3
[0096] On the basis of Example 2, the inventors further transfected the constructed lentiviral expression plasmid into CAR-T cells, and further conducted preliminary cell experiments. The specific experimental process is briefly introduced as follows.
[0097] (1) Lentiviral packaging
[0098] 293T cells are used as the primary target cells to be transfected, specifically:
[0099] First, six-well plates were plated and incubated for 24 hours to culture 293T cells (37°C, DMEM complete medium);
[0100] Subsequently, the medium was replaced with OPTI-MEM medium, and then 1.5 g of the lentiviral expression plasmid constructed in Example 2, 1.5 g of the packaging plasmid psPAX2, and 1 g of Pmd2.G, and 8 μl of liposome transfection reagent were carried out. Mix to transfect target cell 293T;
[0101] Replace with normal DMEM medium 12 hours after transfection;
[0102] Finally, after continuing to culture for 48 hours, the virus supernatant (that is, the packaged virus-like pa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com