Use of anti-PD-1 antibody in treatment of tumors
A technology of PD-1 and antibody, which is applied in the field of melanoma and solid tumor treatment, and can solve problems such as poor efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0191] Example 1: Clinical research on anti-PD-1 antibody monotherapy for tumor treatment
[0192] Inclusion Criteria: Eligible subjects must (1) be between the ages of 18 and 70, (2) have metastatic melanoma or urological cancer, (3) be refractory to standard systemic therapy, (4) ) ECOG score of 0 or 1, (5) no history of autoimmune disease or persistent infection, (6) not received any immunotherapy before.
[0193] Subjects must have evaluable lesions according to RECIST v 1.1 standards, and are not allowed to use anti-tumor drug therapy, systemic steroid drug therapy or have not used anti-CTLA4, anti-PD-1, anti-PD-L1 antibody therapy. The demographic data of the enrolled subjects are shown in Table 1.
[0194] Table 1: Demographic data of enrolled subjects
[0195]
[0196] Test drug: anti-PD-1 antibody toripalimab (WO2014206107).
[0197] There are three anti-PD-1 antibody dose groups in this trial, namely: 1mg / kg (n=15), 3mg / kg (n=15) and 10mg / kg (n=6), administered...
Embodiment 2
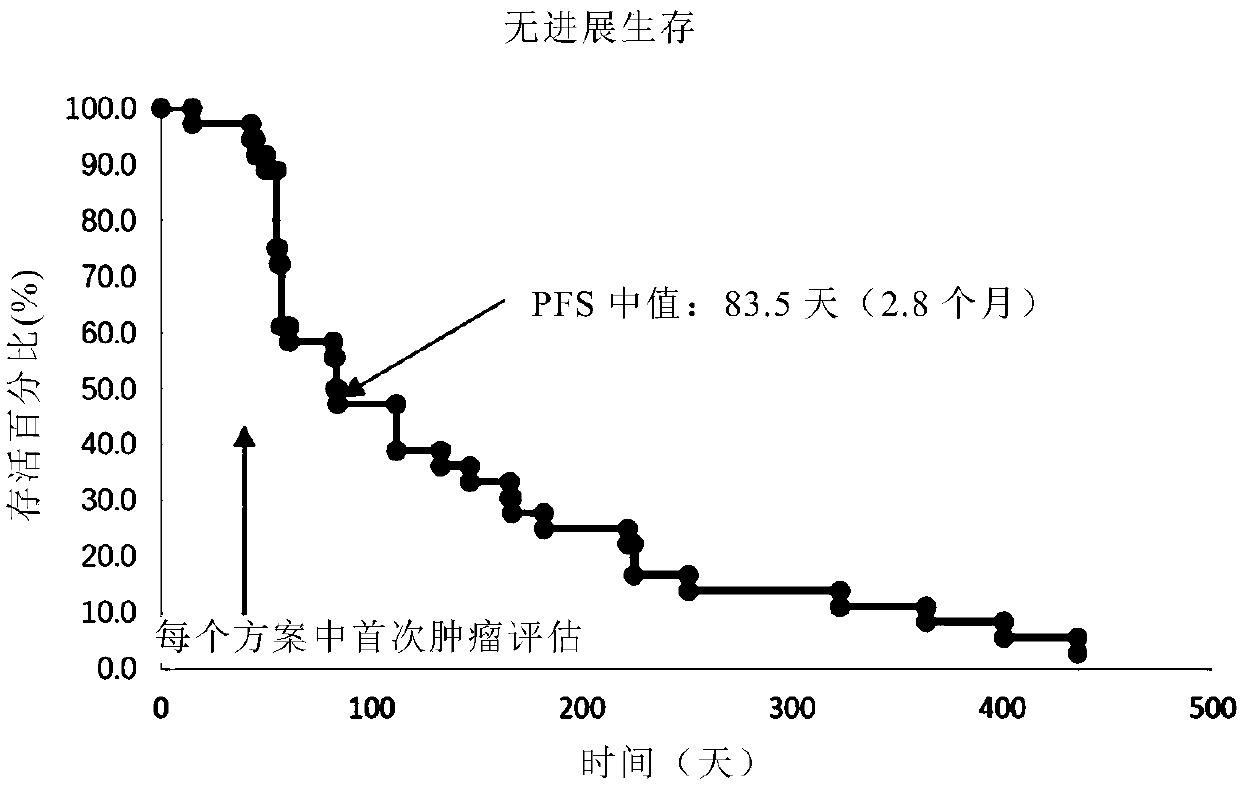
[0237] Example 2: Research on the correspondence between natural killer cells (NK) and anti-PD-1 antibody therapeutic effect on tumors
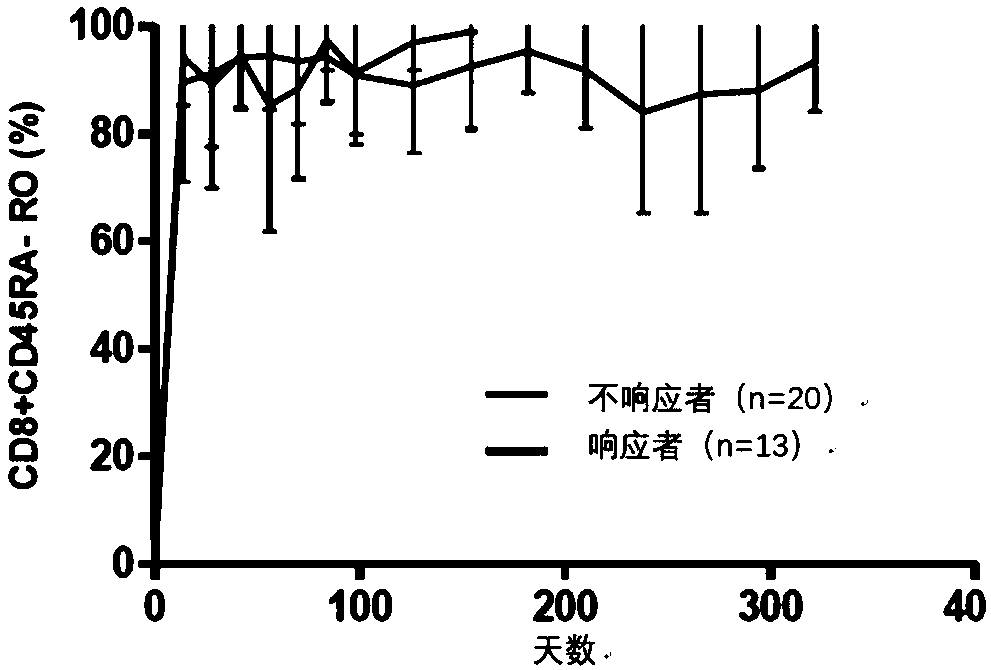
[0238] During the course of the clinical trial described in Example 1, the frequency and activation status (by CD45RA staining) of T lymphocytes in whole blood were assessed. Anti-PD-1 antibody against activated CD8 in the peripheral blood of a single subject + The frequency of T lymphocytes had little effect. Such as Figure 5 As shown, in the group with PFS greater than 250 days, CD8 + Subjects with high cell activation rates were enriched. Before toripalimab treatment, all subjects activated CD8 + The average percentage is 65%. 4 / 5 durable responders (PFS >300 days) were found to have more than 80% activated CD8 in peripheral blood + (total CD8 + cells) and more than 26% CD3 - CD16 + CD54 + NK cells (total monocytes); in contrast activated CD8 in all subjects + The average percentages of T cells and NK cells were 65% and 20%, re...
Embodiment 3
[0239] Example 3: Research on the correlation between biomarkers and clinical efficacy
[0240] By performing PD-L1CD8 double IHC staining on tumor biopsy specimens of 28 patients, the correlation between tumor histology and anti-PD-1 antibody clinical efficacy was analyzed. Rabbit anti-human PD-L1 antibody SP142 from Roche and anti-CD8 clone 4B11 were used. PD-L1 positive status was defined as the presence of ≥1% membrane staining intensity of tumor cells.
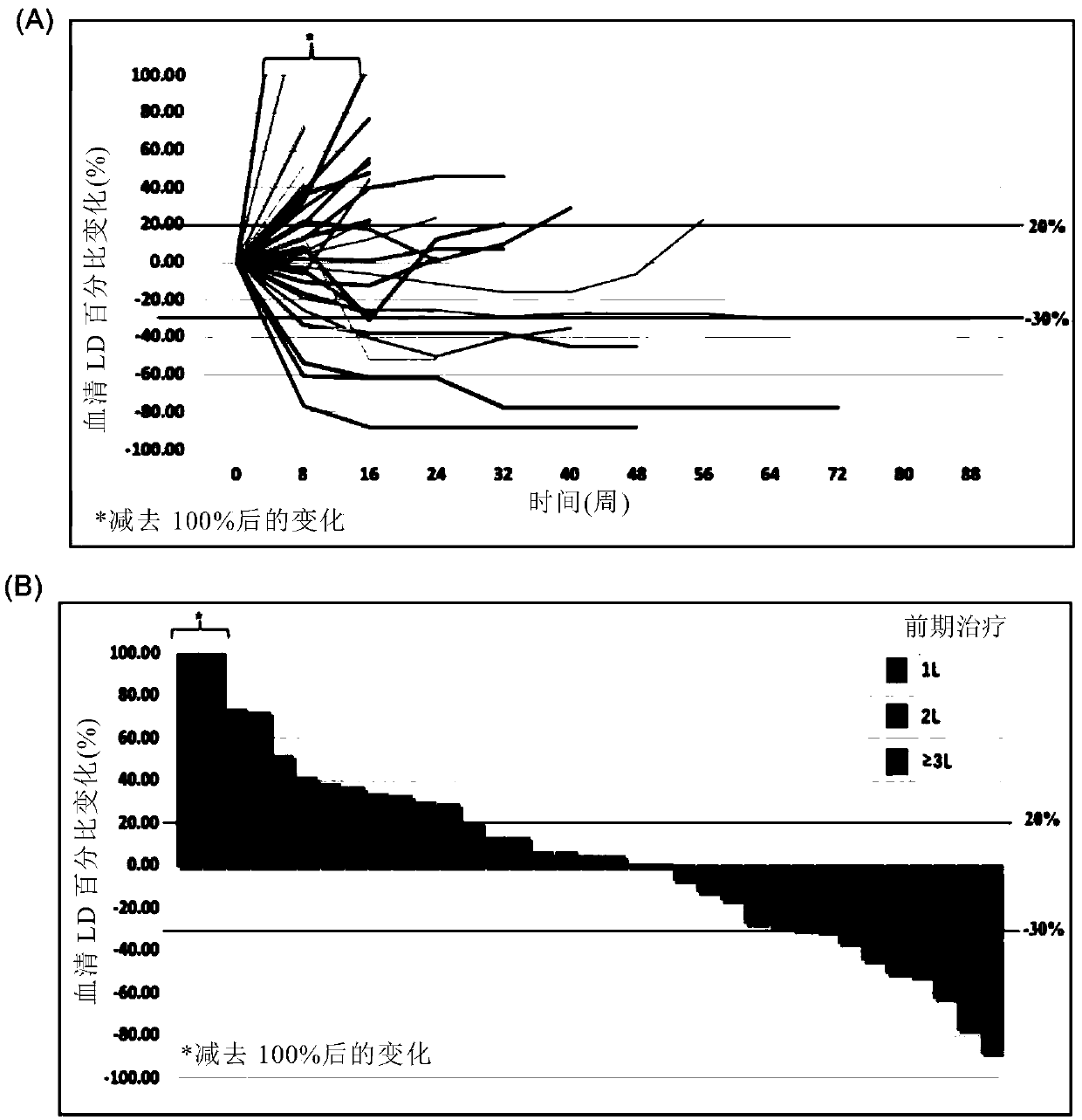
[0241] Such as Figure 6 and Figure 7 As shown, subjects with positive PD-L1 expression in tumor biopsies benefited more from TORIPALIMAB treatment than subjects with negative PD-L1 expression (43.8% ORR, 62.5 DCR vs. 0% ORR, 50% DCR) . The subpopulation with high PD-1 expression (>50%) responded best to TORIPALIMAB treatment, with an ORR of 57.1% and a DCR of 71.4%. Notably, all ORR (CR+PR) subjects were dual positive for PD-L1 and TIL. Co-localization of TILs and PD-L1+ cells was found in tumor biopsies, and 93.8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com