Vaccine compositions
A technology of composition and medicine, which is applied in the field of tumor treatment and can solve problems such as missing parts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0110] Example 1: Hyper-IL-6 (H6) Enhances T Cell Proliferative Response in Allogeneic Mixed Tumor-Lymphocyte Reaction (AMTLR)
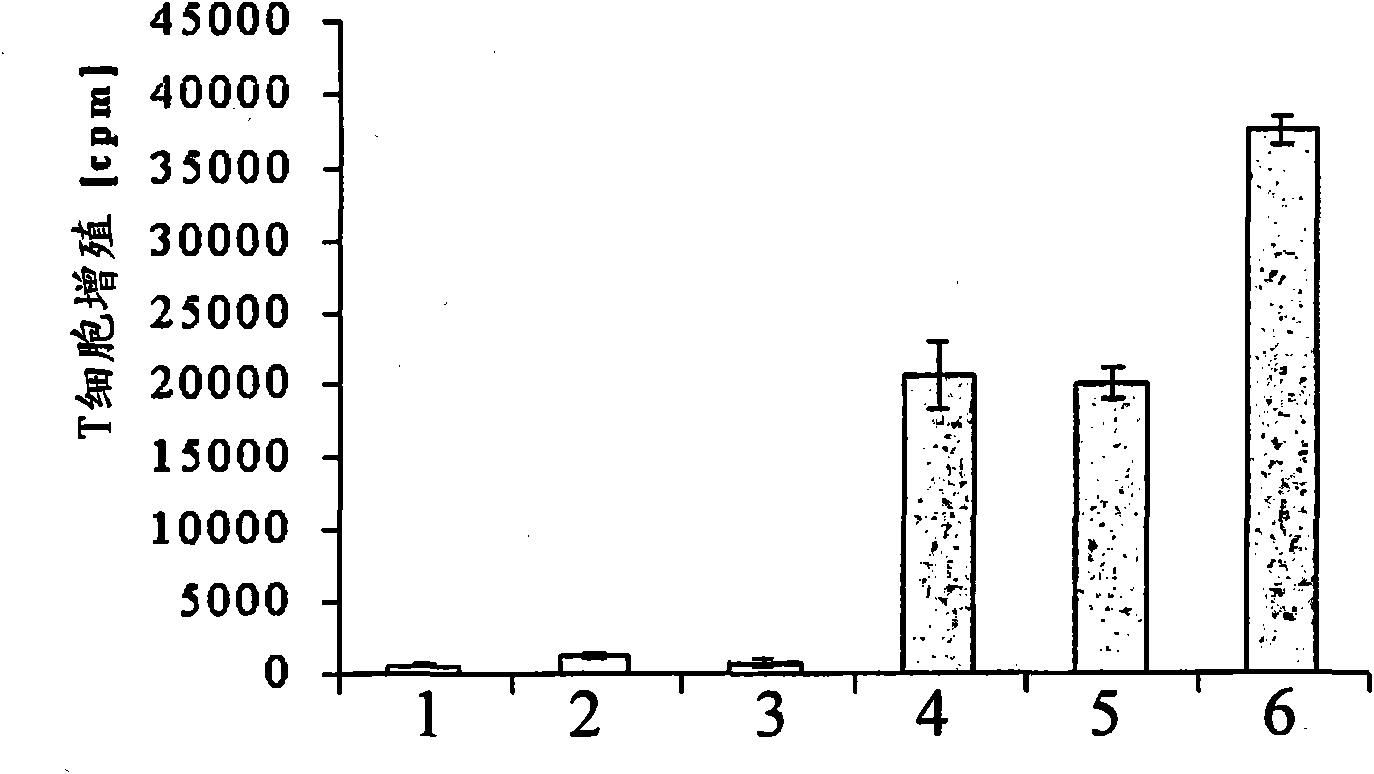
[0111] Irradiated tumor cells were mixed with unprimed allogeneic lymphocytes in the presence or absence of IL-6 (1 ng / ml) or purified H6 (1 ng / ml). three days later, with 3 T cell proliferation was measured by H-thymidine incorporation and expressed as counts per minute (cpm). For the results of this experiment, see figure 1 .
[0112] Columns 1-3 show spontaneous proliferation of T cells, ie proliferation in the absence of tumor cell stimulation. Clearly, spontaneous proliferation of T cells was not significantly increased (columns 1-3), regardless of whether IL-6 (column 2) or H6 (column 3) was added to the mixture.
[0113] Columns 4-6 show the results of T cell proliferation in response to allogeneic tumor cells. T cells showed very strong proliferation in the presence of allogeneic tumor cells (column 4). The addition of IL-6 had no sign...
Embodiment 2
[0114] Example 2: T cell proliferation in an allogeneic mixed tumor-lymphocyte response is dependent on IL-2
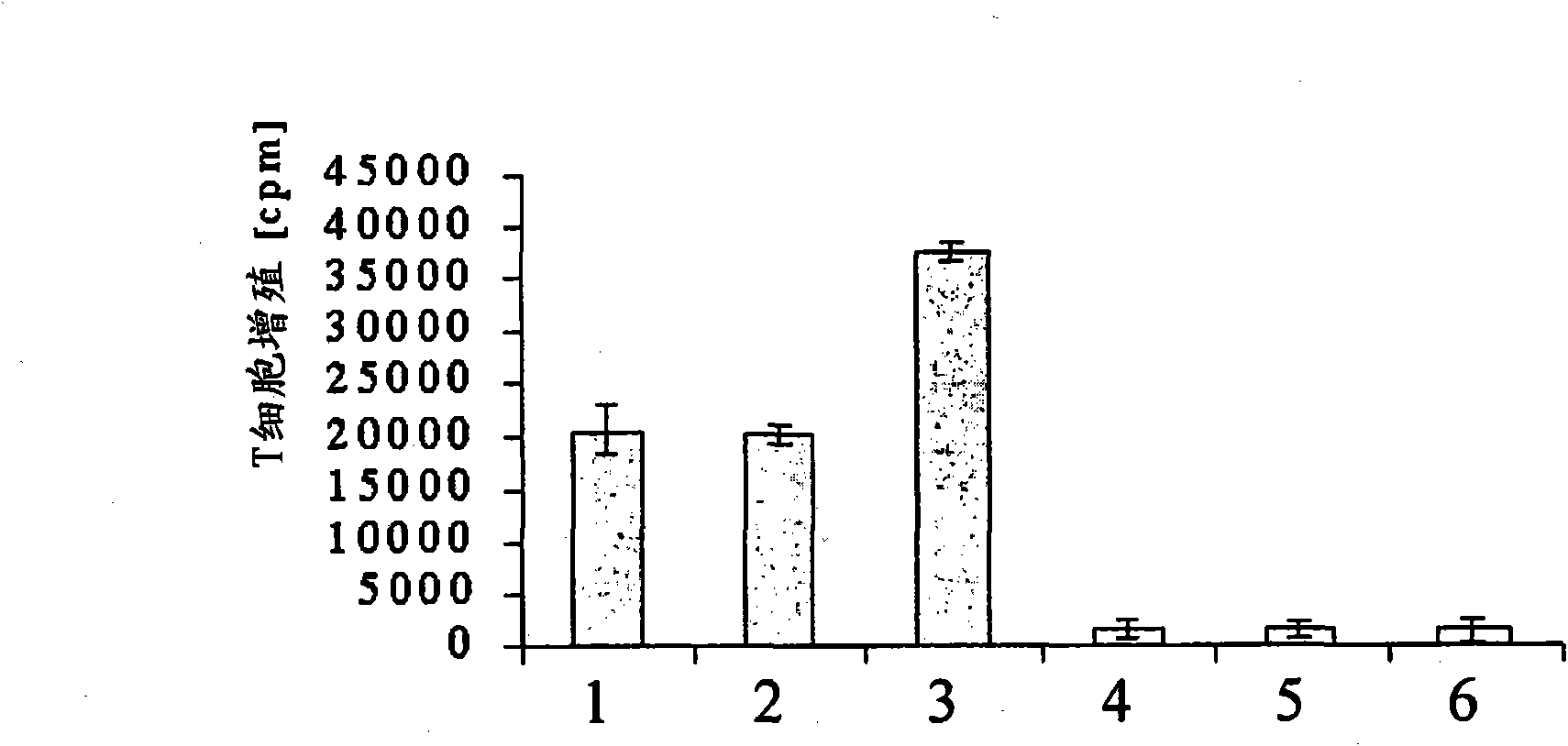
[0115] Irradiated tumor cells were mixed with unprimed allogeneic lymphocytes in the presence or absence of IL-6 (1 ng / ml) or purified H6 (1 ng / ml) and anti-IL-2 antibody (1 μg / ml). Cells are mixed. three days later, with 3 T cell proliferation was measured by the H-thymidine incorporation method and expressed as cpm. For the results of this experiment, see figure 2 .
[0116] figure 2 Middle columns 1-3 show the results of T cell proliferation in response to allogeneic tumor cells in the absence of anti-IL-2 antibodies. The results of this experiment and figure 1 The results in columns 4-6 are almost the same. As shown in Example 1, T cells showed very strong proliferation in the presence of allogeneic tumor cells ( figure 2 column 1). The addition of IL-6 had no significant effect on the proliferation of T cells ( figure 2 column 2). The addition of ...
Embodiment 3
[0118] Example 3: Hyper-IL-6 Enhances IL-2 and IFN-γ Production by T Cells in Allogeneic Mixed Tumor-Lymphocyte Reaction (AMTLR)
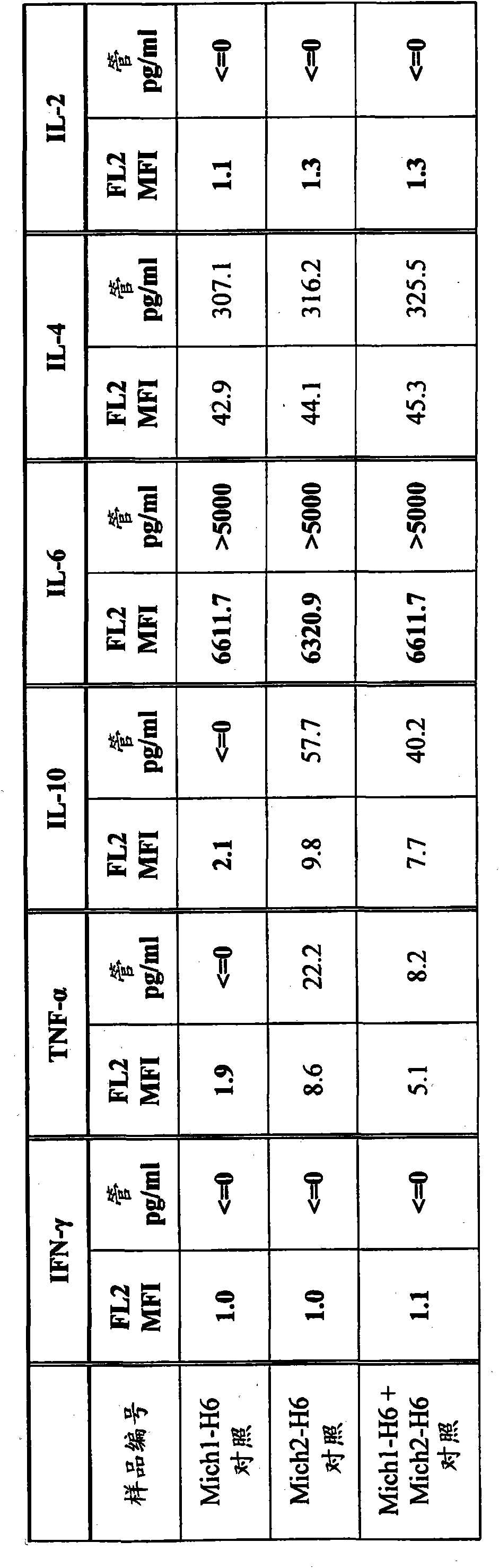
[0119] This example describes the evaluation of the immunostimulatory potential of hyper IL-6 (H6) in an allogeneic mixed tumor-lymphocyte response. The obtained results indicate that super IL-6 enhances the immunostimulatory potential of allogeneic melanoma cells. Moreover, ultra-IL-6 is not only more potent than IL-6, but also exhibits qualitatively different biological activities. Compared with native IL-6, a known Th2 inducer, hyper-IL-6 appears to reduce the expression of IL-10 and increase the amount of IFN-γ and IL-2 produced by peripheral blood lymphocytes (PBLC), which is a Th1 characteristics of the response.
[0120] Test sample : A375 melanoma cells and their derivatives A375-H6 cells; PBLC isolated from healthy volunteers.
[0121] Media, Reagents and Equipment : FBS (GIBCO / Invitrogen), PBS (GIBCO / Invitrogen), DMEM (GIBCO / Invitr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com