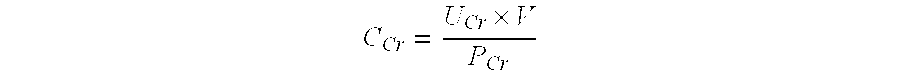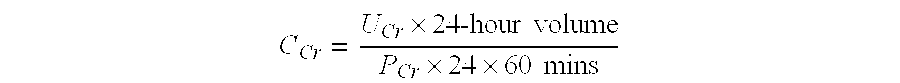Patents
Literature
77 results about "Ciliary neurotrophic factor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Ciliary neurotrophic factor is a protein that in humans is encoded by the CNTF gene. The protein encoded by this gene is a polypeptide hormone and neurotrophic factor whose actions have mainly been studied in the nervous system where it promotes neurotransmitter synthesis and neurite outgrowth in certain neural populations including astrocytes. It is a hypothalamic neuropeptide that is a potent survival factor for neurons and oligodendrocytes and may be relevant in reducing tissue destruction during inflammatory attacks. A mutation in this gene, which results in aberrant splicing, leads to ciliary neurotrophic factor deficiency, but this phenotype is not causally related to neurologic disease. In addition to the predominant monocistronic transcript originating from this locus, the gene is also cotranscribed with the upstream ZFP91 gene. Cotranscription from the two loci results in a transcript that contains a complete coding region for the zinc finger protein but lacks a complete coding region for ciliary neurotrophic factor.
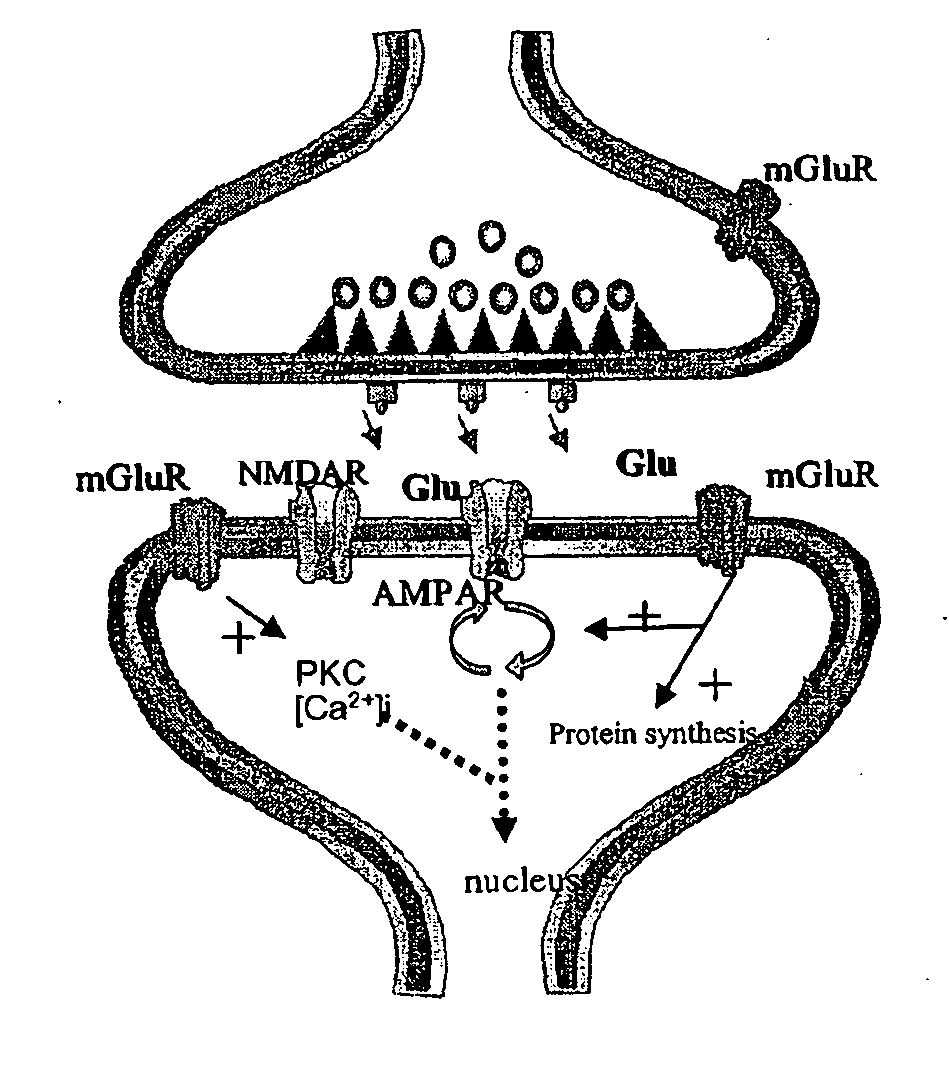
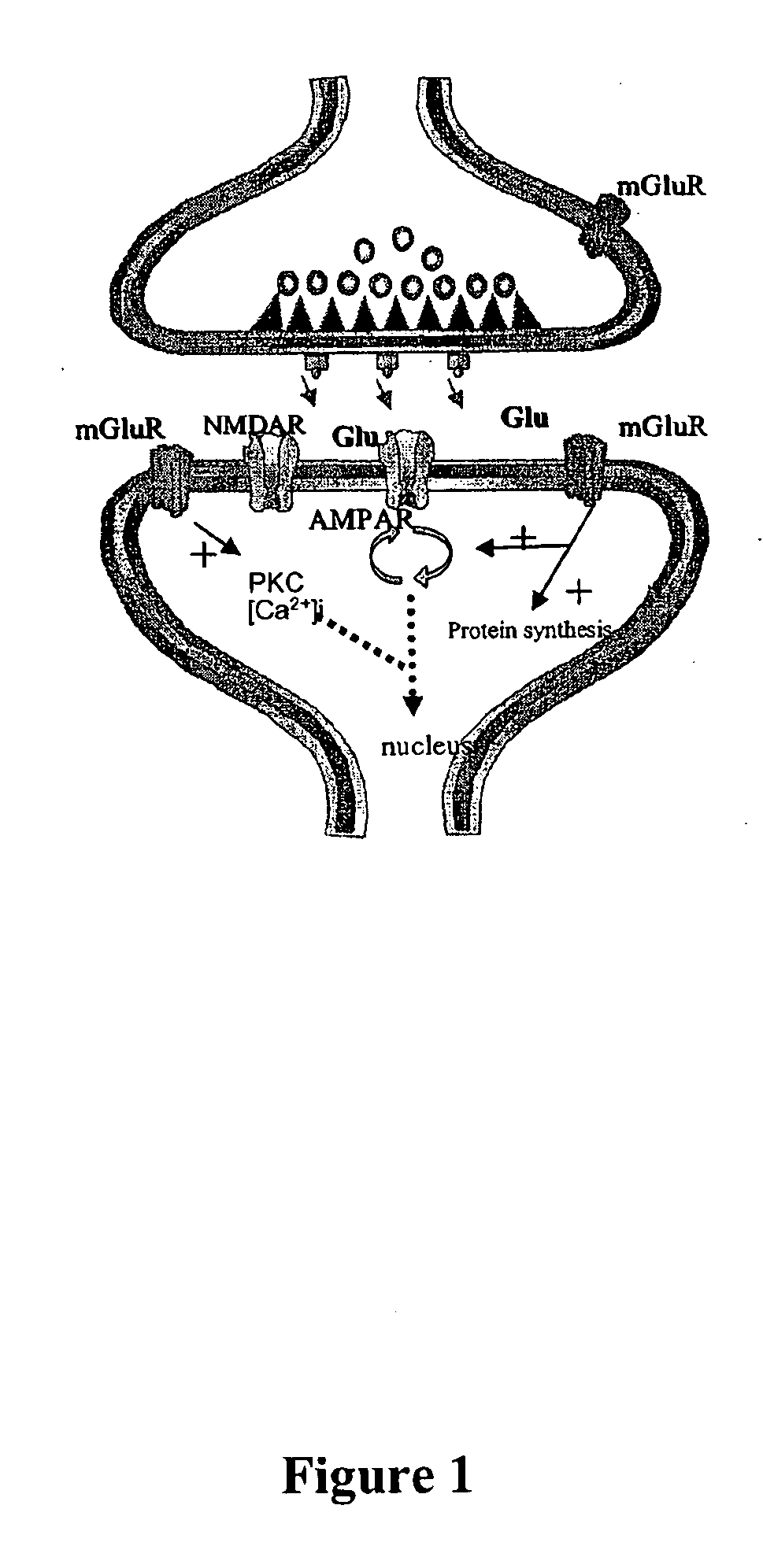
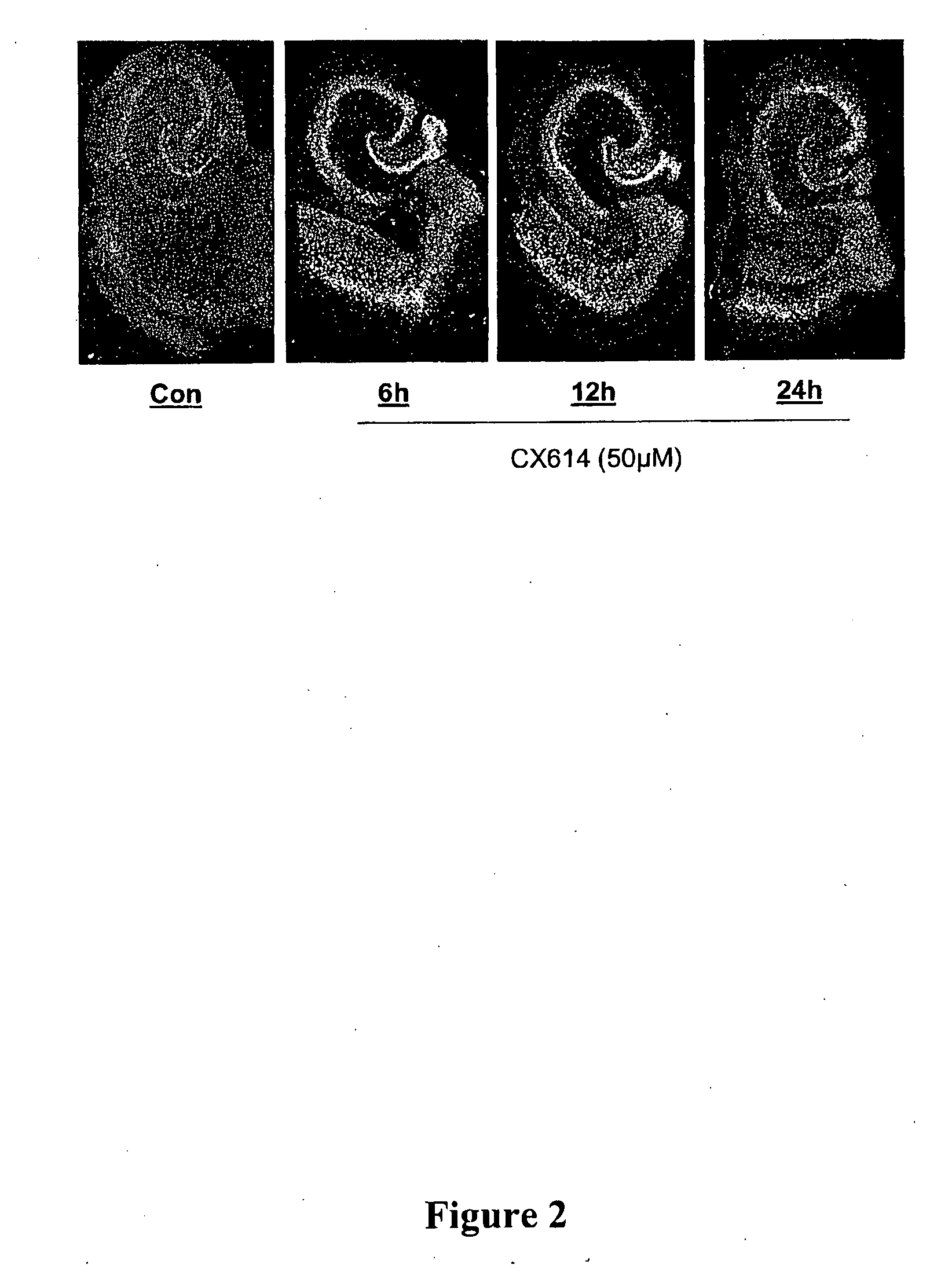
Positive AMPA receptor modulation to enhance brain neurotrophic factor expression
InactiveUS6030968AImprove the level ofHigh expressionHeterocyclic compound active ingredientsFactor iiCiliary neurotrophic factor
Methods for increasing the level of neurotrophic factors and neurotrophic factor receptors in the brain of a mammal afflicted with a pathology which produces neurodegeneration without significant loss of memory or learning comprising administering to a mammal an effective amount of an allosteric upmodulator of alpha -amino-3-hydroxy-5-methyl-isoxazole-4-proprionic acid (AMPA) receptors.
Owner:RGT UNIV OF CALIFORNIA
Method(s) of stabilizing and potentiating the actions and administration of brain-derived neurotrophic factor (BDNF)
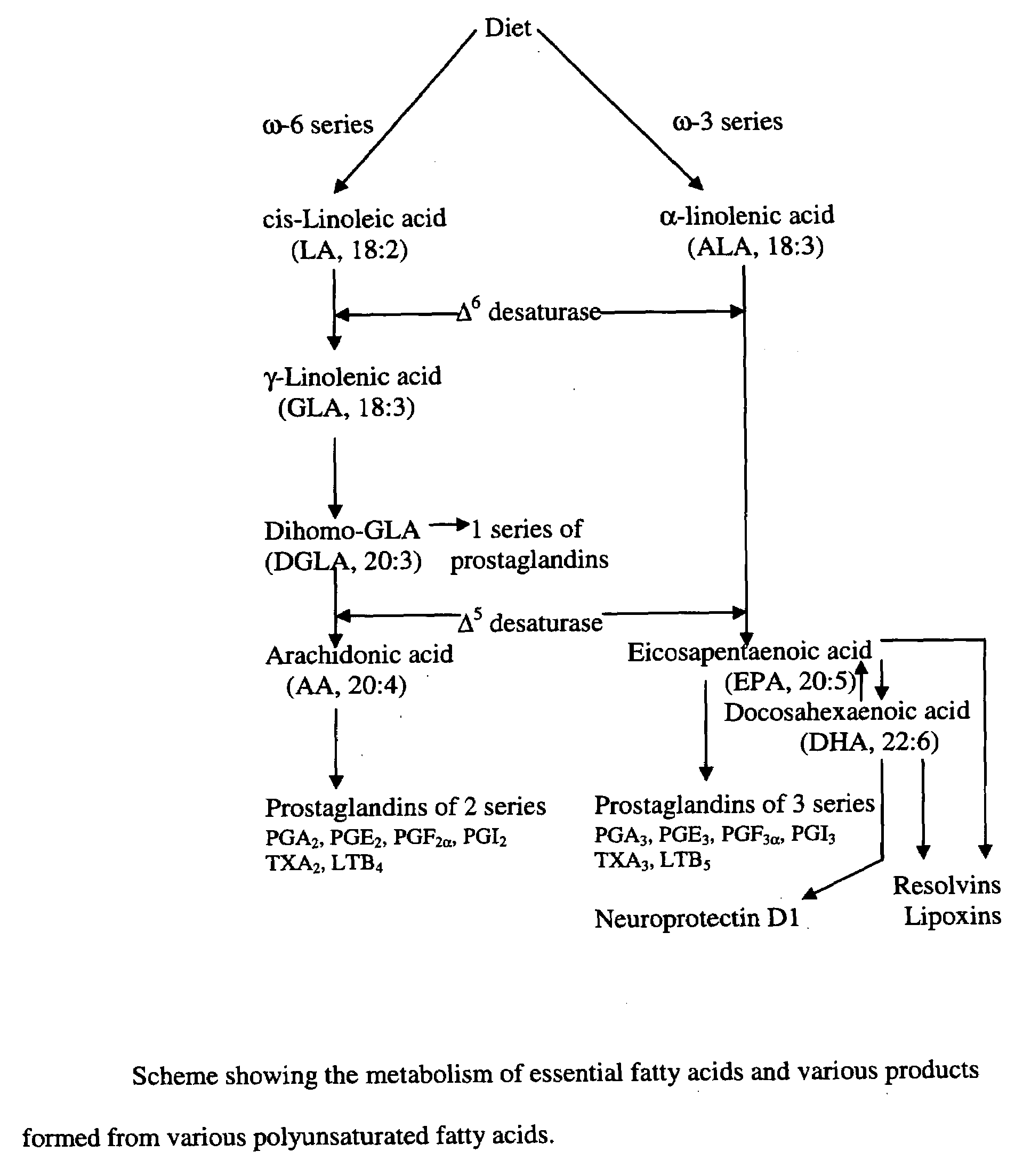
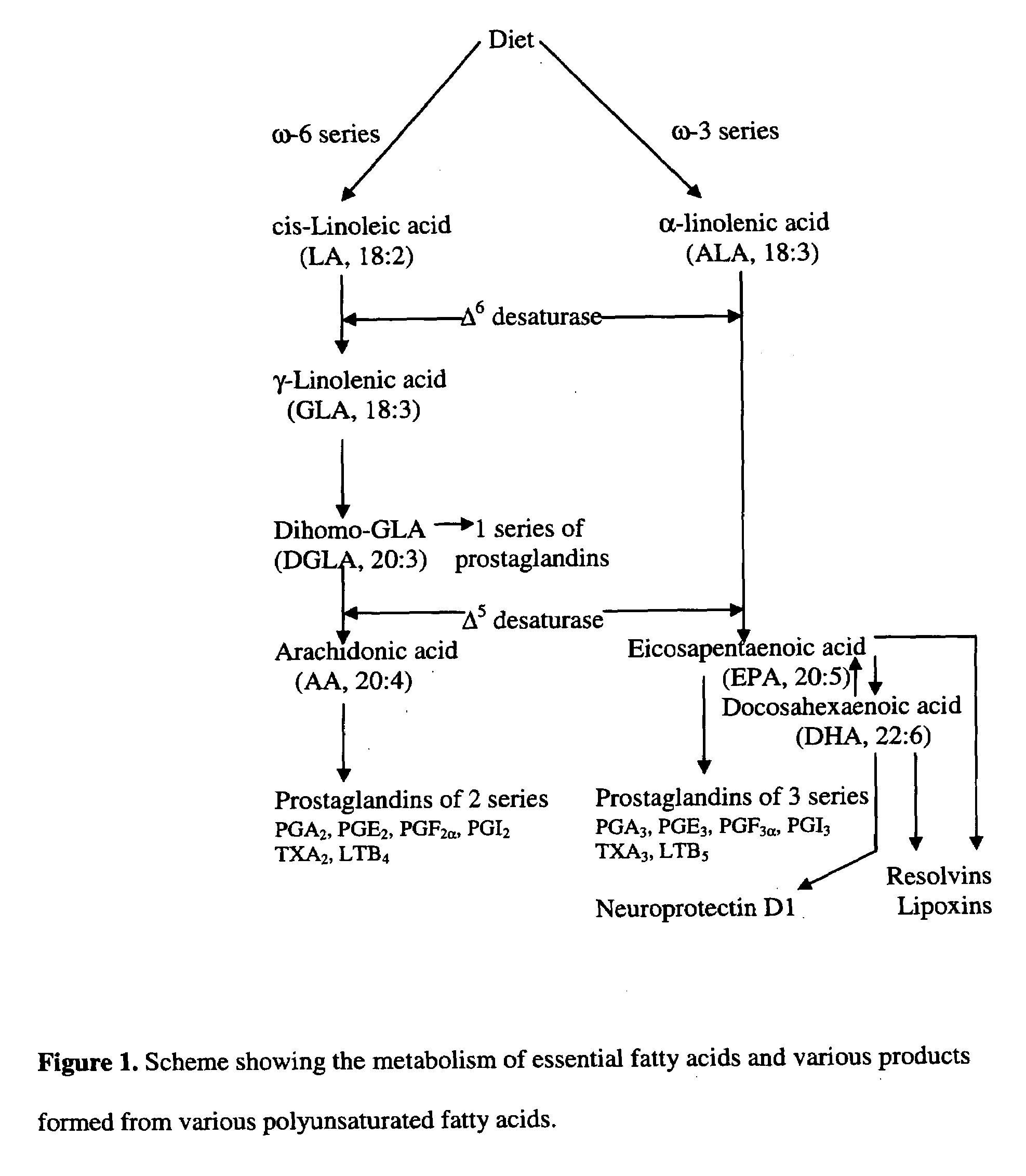
A method of stabilizing and potentiating actions and administration of neurotrophins such as brain-derived neurotrophic factor (BDNF), neurotrophin-3, neurotrophin-4, and nerve growth factor and their receptors by using in coupling conjugation with polyunsaturated fatty acids (PUFAs) in the prevention and / or treatment of obesity, type 2 diabetes mellitus, metabolic syndrome X, Alzheimer's disease, depression, Parkinson's disease, and schizophrenia is described. The invention is directed to the efficacious use of various neurotrophins by using them in combination with polyunsaturated fatty acids chosen from linoleic acid, gamma-linolenic acid, dihomo-gamma-linolenic acid, arachidonic acid, alpha-linolenic acid, eicosapentaenoic acid, docosahexaenoic acid, cis-parinaric acid, docosapentaenoic acid and conjugated linoleic acid in predetermined quantities. The invention also provides methods of efficiently delivering neurotrophins to the desired areas of the brain by complexing or conjugating with PUFAs, so that they are able to cross blood brain barrier efficiently and reach the desired regions of the brain in adequate amounts.
Owner:DAS UNDURTI N
Treatment of brain derived neurotrophic factor (BDNF) related diseases by inhibition of natural antisense transcript to bdnf
ActiveUS20110319475A1Modulate its functionOrganic active ingredientsSenses disorderDiseaseBrain-derived neurotrophic factor
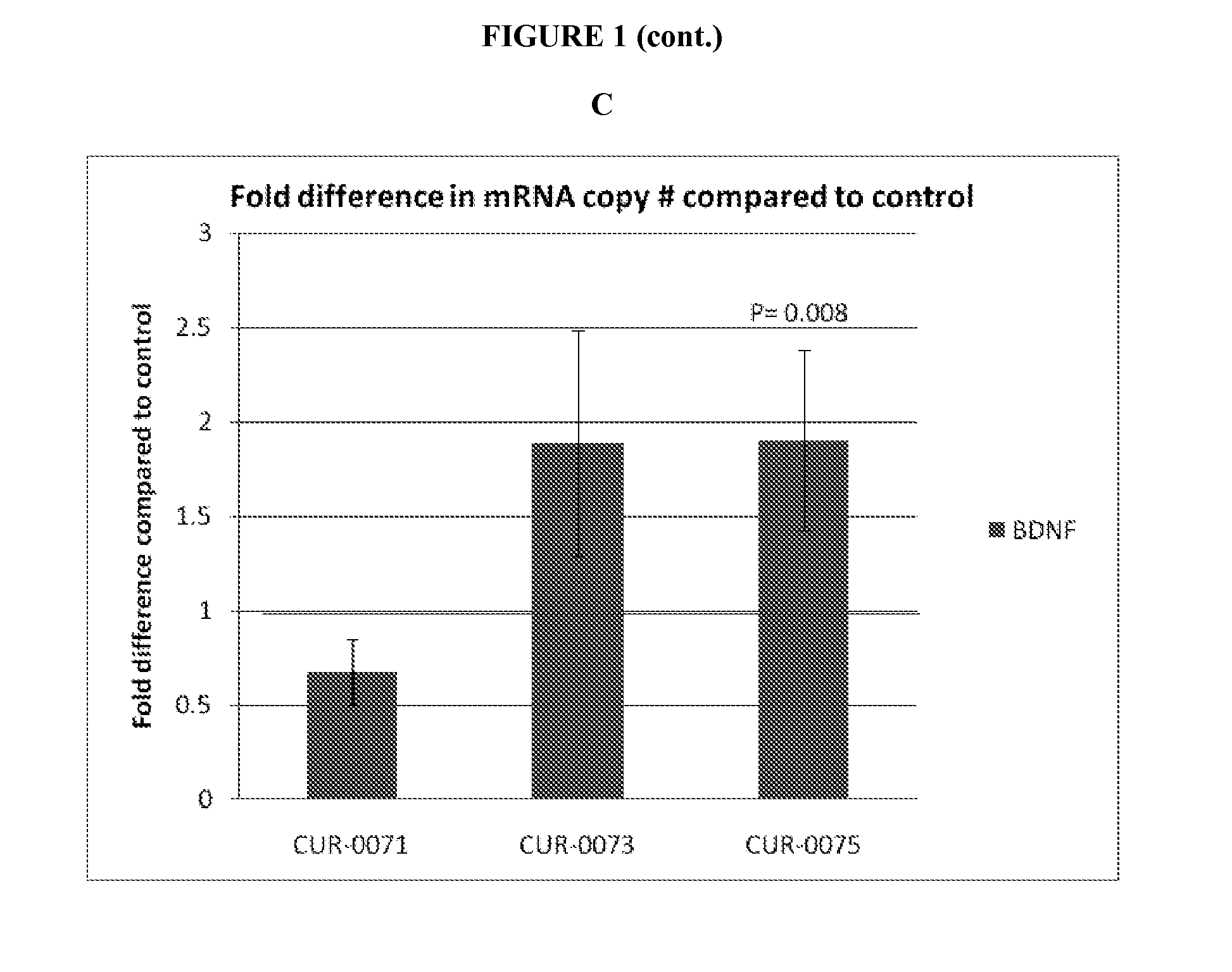
The present invention relates to antisense oligonucleotides that modulate the expression of and / or function of Brain derived neurotrophic factor (BDNF), in particular, by targeting natural antisense polynucleotides of Brain derived neurotrophic factor (BDNF). The invention also relates to the identification of these antisense oligonucleotides and their use in treating diseases and disorders associated with the expression of BDNF.
Owner:CURNA INC
Novel neurotrophic factor immunoassays
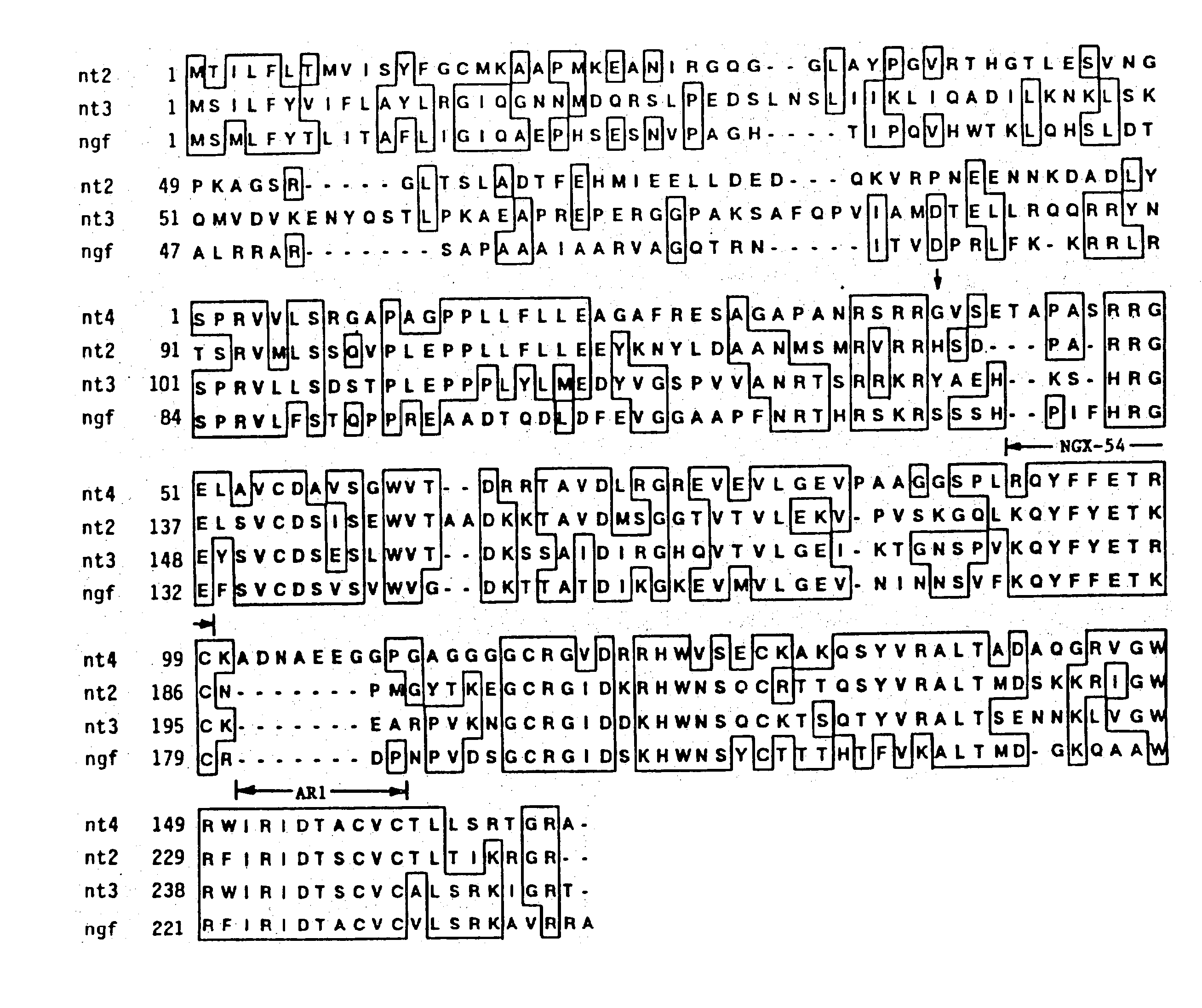
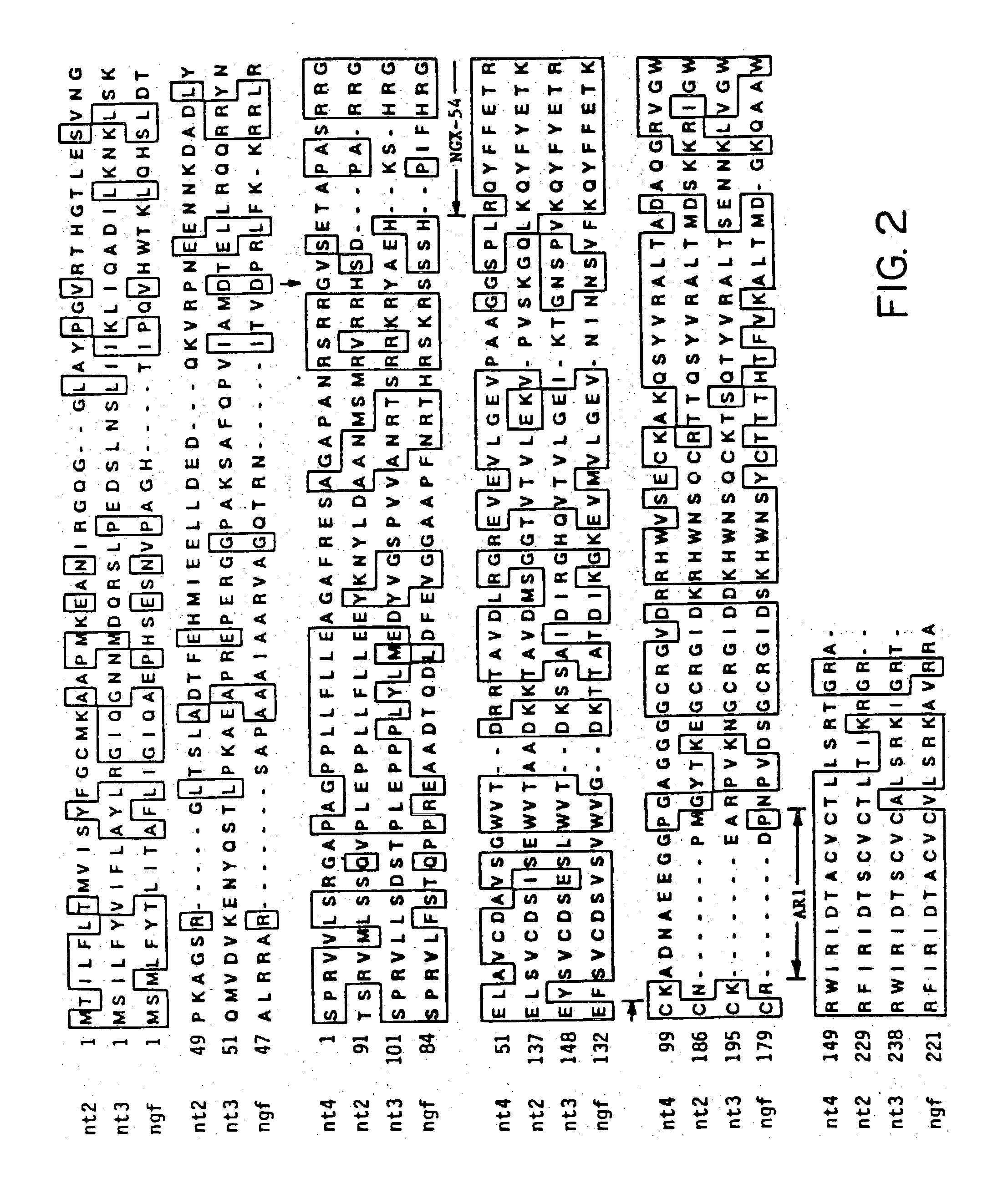
A novel polypeptide designated neurotrophic factory (NT-4), has been identified by PCR amplification of human genomic DNA. Provided herein is nucleic acid encoding NT-4 useful in diagnostics and in the recombinant preparation of NT-4. Also provided herein are nucleic acids encoding naturally occurring amino acid sequence variants of NT-4, designated NT-4beta, NT-4gamma, and NT-4Delta. The neurotrophic factors of the invention are useful in the treatment of nerve cells and in diagnostic assays.
Owner:GENENTECH INC
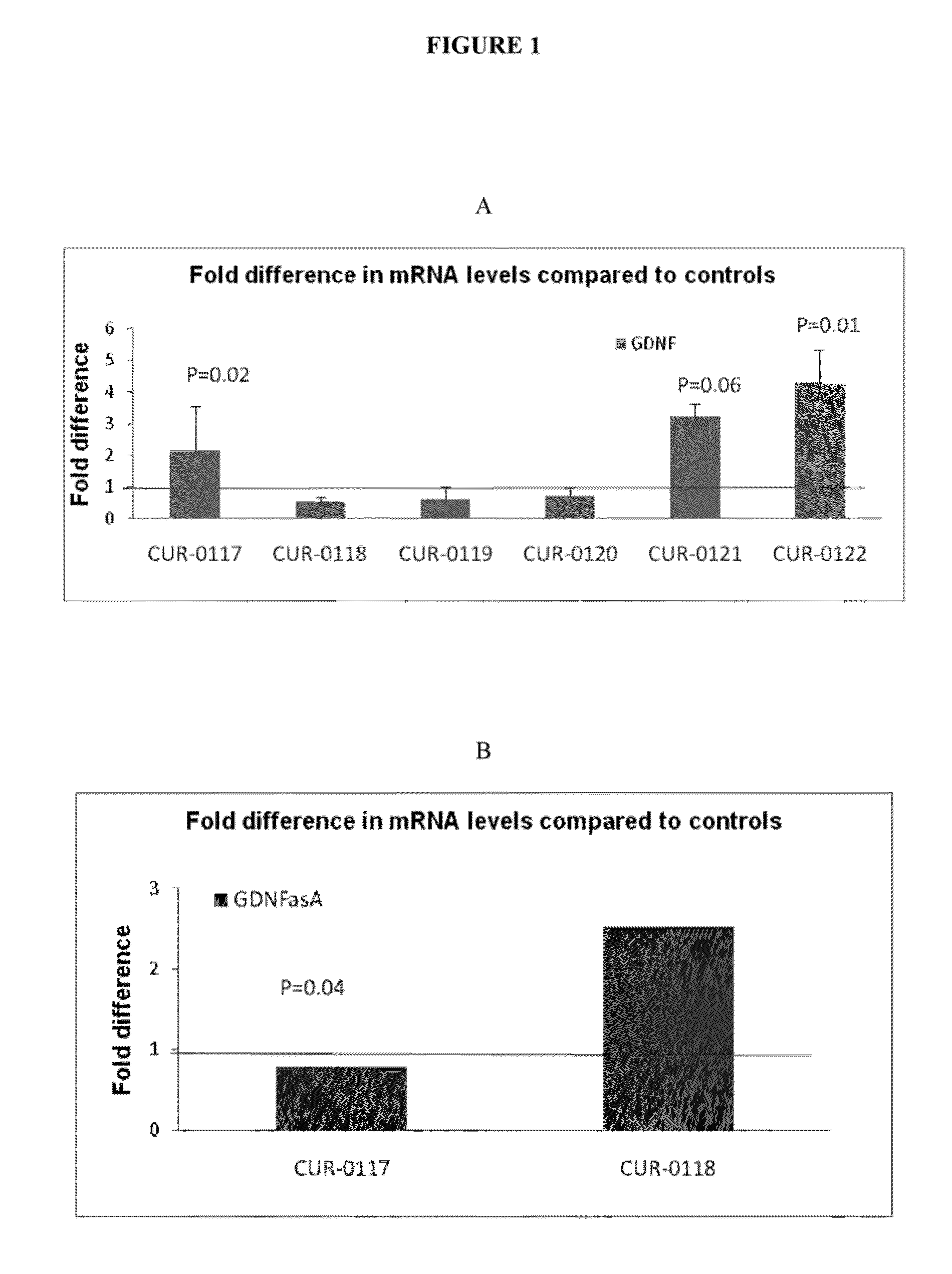
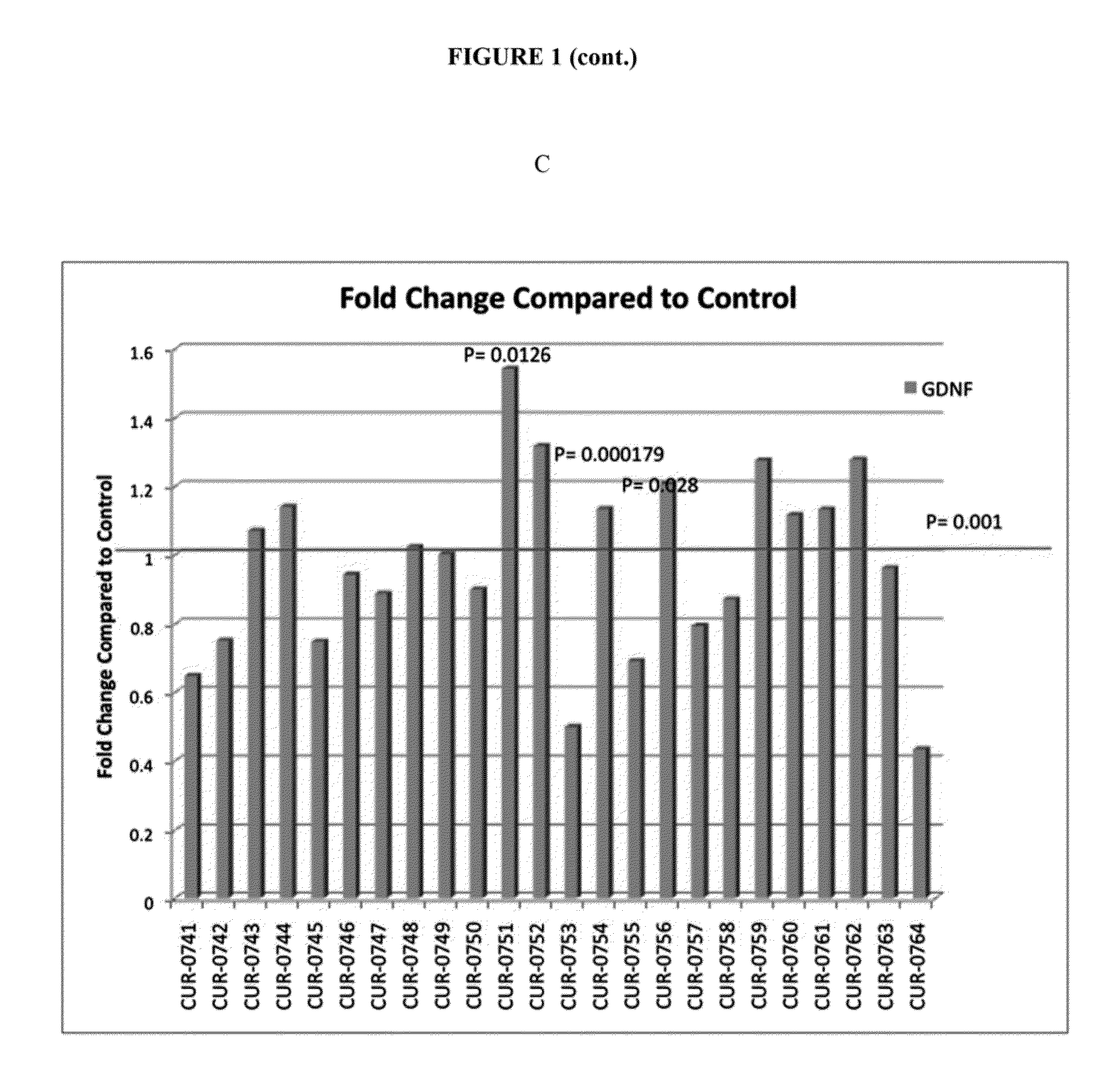
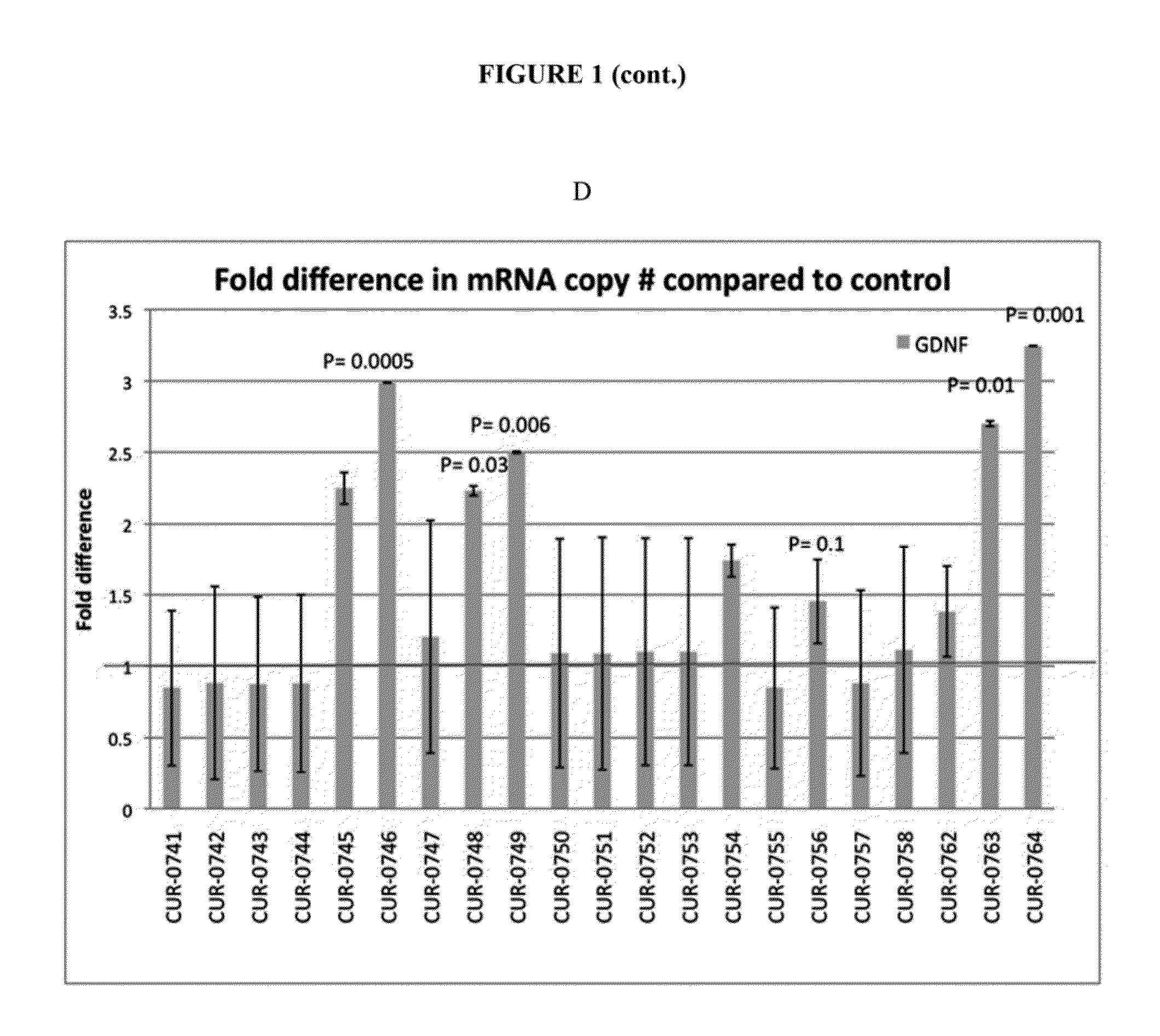
Treatment of glial cell derived neurotrophic factor (GDNF) related diseases by inhibition of natural antisense transcript to gdnf
InactiveUS20110319476A1Modulate its functionOrganic active ingredientsSenses disorderDiseaseCiliary neurotrophic factor
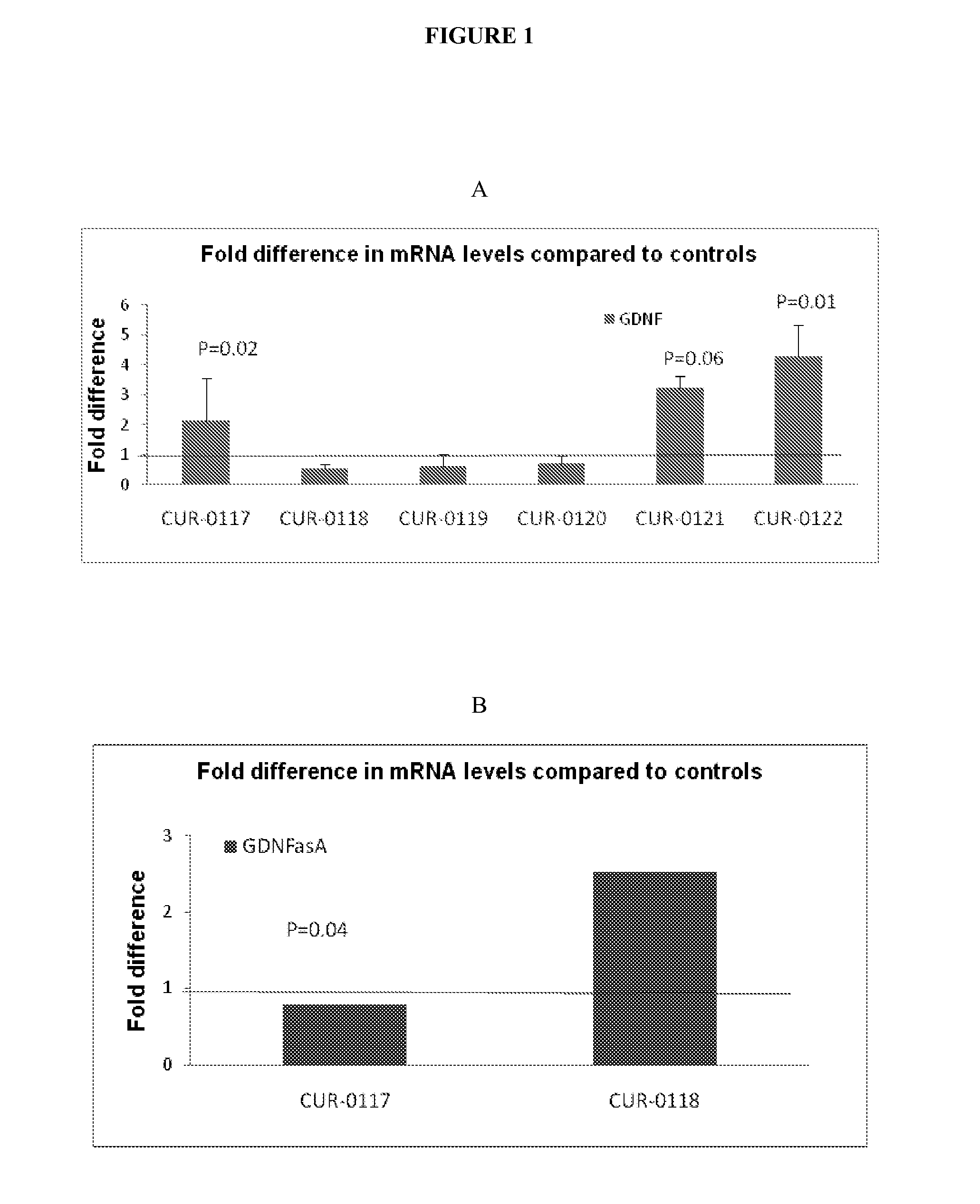
The present invention relates to antisense oligonucleotides that modulate the expression of and / or function of Glial cell derived neurotrophic factor (GDNF), in particular, by targeting natural antisense polynucleotides of Glial cell derived neurotrophic factor (GDNF). The invention also relates to the identification of these antisense oligonucleotides and their use in treating diseases and disorders associated with the expression of GDNF.
Owner:CURNA INC
Double-factor peripheral nerve injury repairing collagen material and preparation method thereof
ActiveCN104001212APromote regenerationPromotes electrophysiological recoveryProsthesisFacial nerveCollagen scaffold
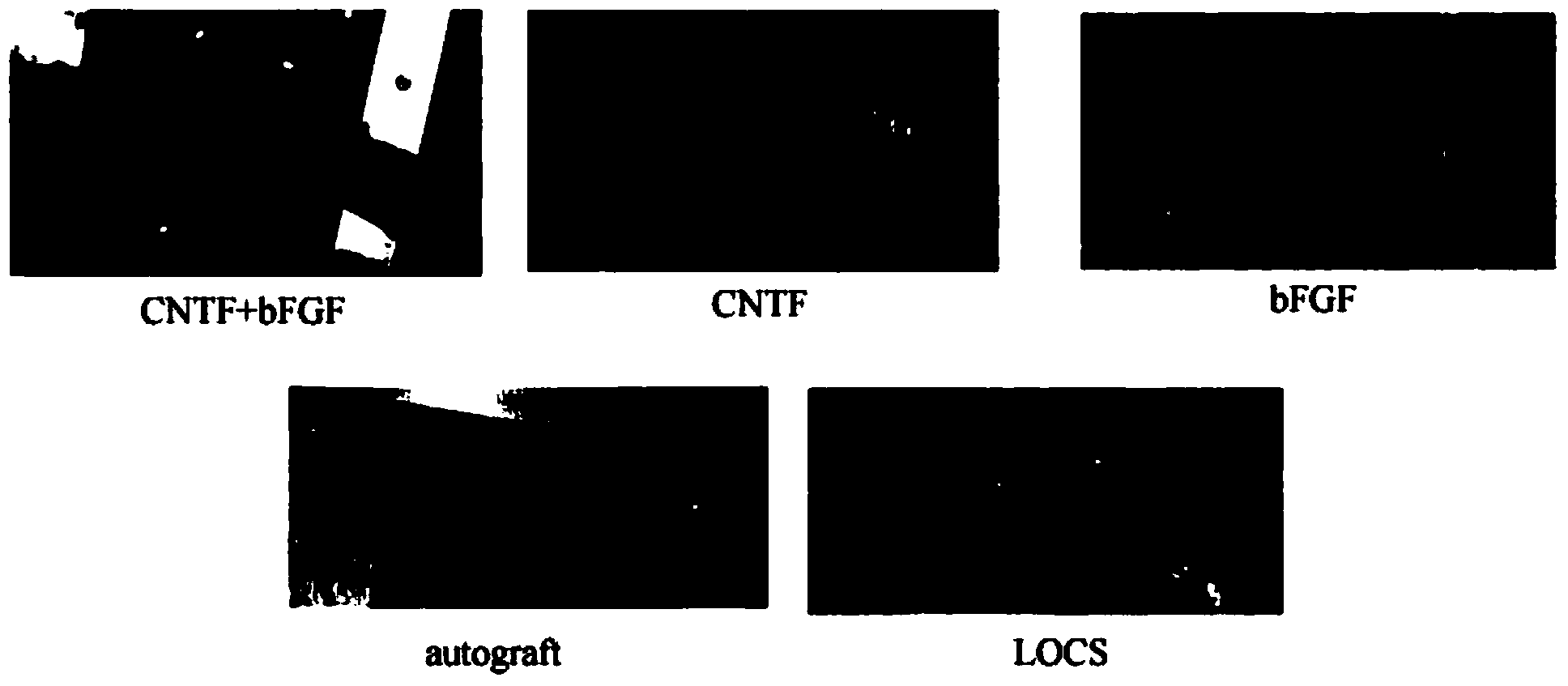
The invention discloses a method for preparing a collagen material for repairing peripheral nerve injury. The method comprises the following step: co-incubating a ciliary neurotrophic factor with a collagen binding domain (CBD-CNTF), a basic fibroblast growth factor with a collagen binding domain (CBD-bFGF) and an orderly collagen material to obtain a collagen material for repairing the peripheral nerve injury. According to the preparation method, two factors CBD-CNTF and CBD-bFGF are simultaneously crosslinked to the orderly collagen scaffold material, and the action of the double-factor peripheral nerve injury repairing collagen material in long-cross-section nerve injury repairing can be evaluated by establishing an animal peripheral nerve-facial nerve long cross-section injury model. Results prove that compared with single factor CBD-CNTF or CBD-bFGF functional collagen scaffold material, the double-factor peripheral nerve injury repairing collagen material can be used for better promoting regeneration of facial nerves and restoration of functions, and has a more important realistic guiding magnificence to application of clinical peripheral nerve injury repairing in the future.
Owner:INST OF GENETICS & DEVELOPMENTAL BIOLOGY CHINESE ACAD OF SCI
Agent having neurotrophic factor-like activity
InactiveUS20100204498A1Improve securityPrevention and improvementNervous disorderOrganic chemistryFactor iiEthyl ester
The present invention provides a pharmaceutical agent having high safety and a neurotrophic factor-like activity, which contains, as an active ingredient, any one compound included in fatty acids each having 8 carbon atoms (C8) or having 10 carbon atoms (C10) to 12 carbon atoms (C12) or fatty acid esters thereof, such as 3,7-dimethyloctanoic acid ethyl ester, geranic acid ethyl ester, and the like, each of which has 8 carbon atoms (C8), decanoic acid methyl ester, trans-2-decenoic acid, trans-2-decenoic acid methyl ester, trans-2-decenoic acid ethyl ester, trans-2-decenoic acid-2-decenyl ester, trans-2-decenoic acid cyclohexyl ester, trans-2-decenoic acid isopropyl ester, trans-3-decenoic acid methyl ester, trans-9-decenoic acid methyl ester, and the like, each of which has 10 carbon atoms (C10), trans-10-undecenoic acid methyl ester, trans-10-undecenoic acid ethyl ester, and the like, each of which has 11 carbon atoms (C11), and dodecanoic acid, and the like, each of which has 12 carbon atoms (C12), or salts thereof or prodrugs thereof.
Owner:NAGOYA INDUSTRIAL SCIENCE RESEARCH INST
Treatment of glial cell derived neurotrophic factor (GDNF) related diseases by inhibition of natural antisense transcript to gdnf
InactiveUS20130137751A1Modulate its functionSenses disorderNervous disorderDiseaseCiliary neurotrophic factor
The present invention relates to antisense oligonucleotides that modulate the expression of and / or function of Glial cell derived neurotrophic factor (GDNF), in particular, by targeting natural antisense polynucleotides of Glial cell derived neurotrophic factor (GDNF). The invention also relates to the identification of these antisense oligonucleotides and their use in treating diseases and disorders associated with the expression of GDNF.
Owner:CURNA INC
Methods of generating mesenchymal stem cells which secrete neurotrophic factors
A method of generating MSCs which secrete neurotrophic factors (NTFs) comprising incubating a population of undifferentiated mesenchymal stem cells (MSCs) in a differentiating medium comprising basic fibroblast growth factor (bFGF), platelet derived growth factor (PDGF), heregulin and cAMP.
Owner:BRAINSTORM CELL THERAPEUTICS LTD
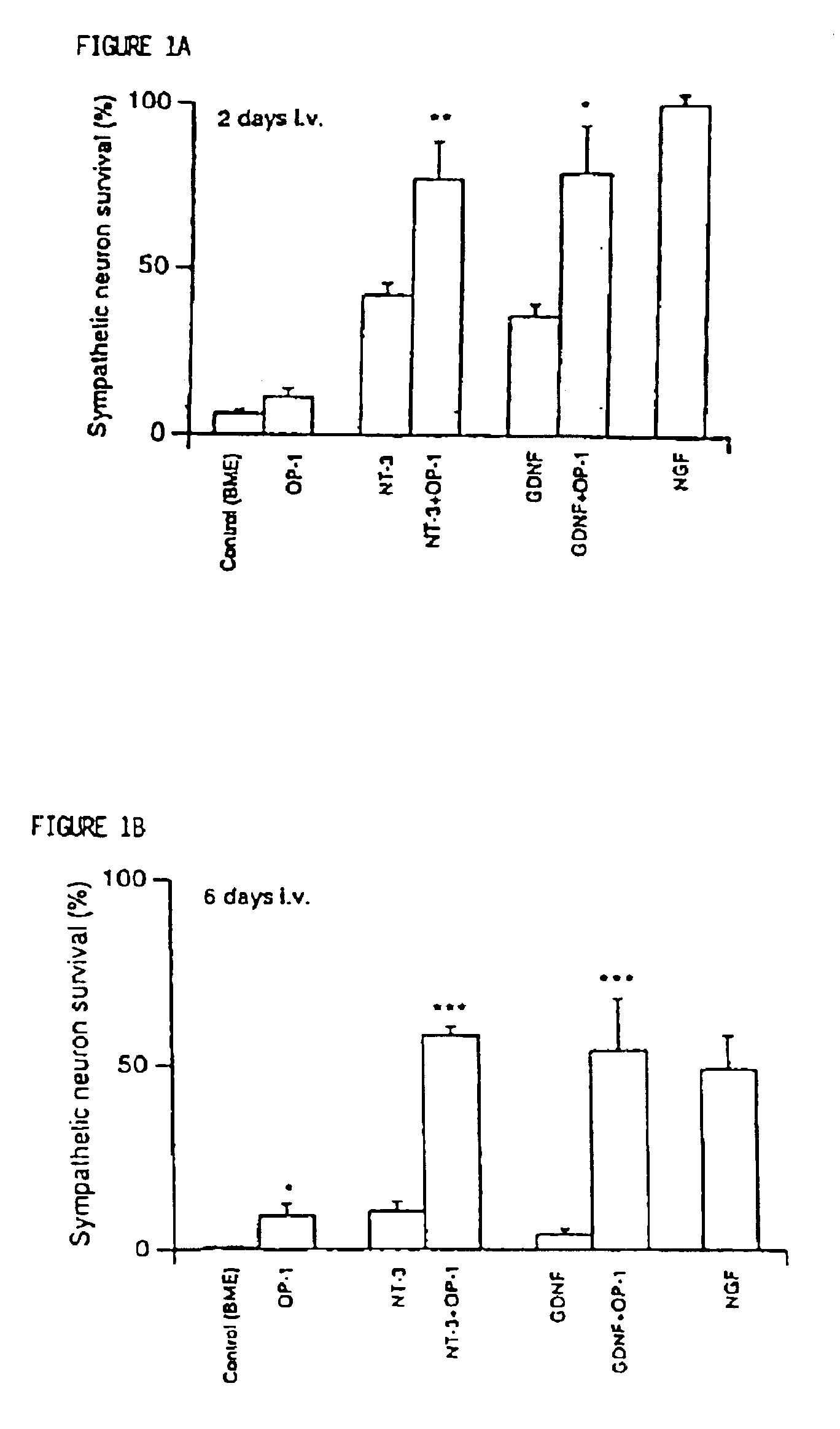
Synergistic effects of OP/BMP morphogens and GDNF/NGF neurotrophic factors
OP / BMP morphogen is combined with GDNF / NGF neurotrophic factors in promoting the survival or growth, or inhibiting the death or degeneration, of mammalian cells, particularly neural cells, which express OP / BMP-activated serine / threonine kinase receptors and GDNF / NGF-activated tyrosine kinase receptors. Also discosed are methods for the treatment of such cells, including treatments for mammals afflicted with, or at imminent risk of, damage or injury to, such cells, as well as new pharmaceutical preparations for such treatments.
Owner:MARIEL THERAPEUTICS
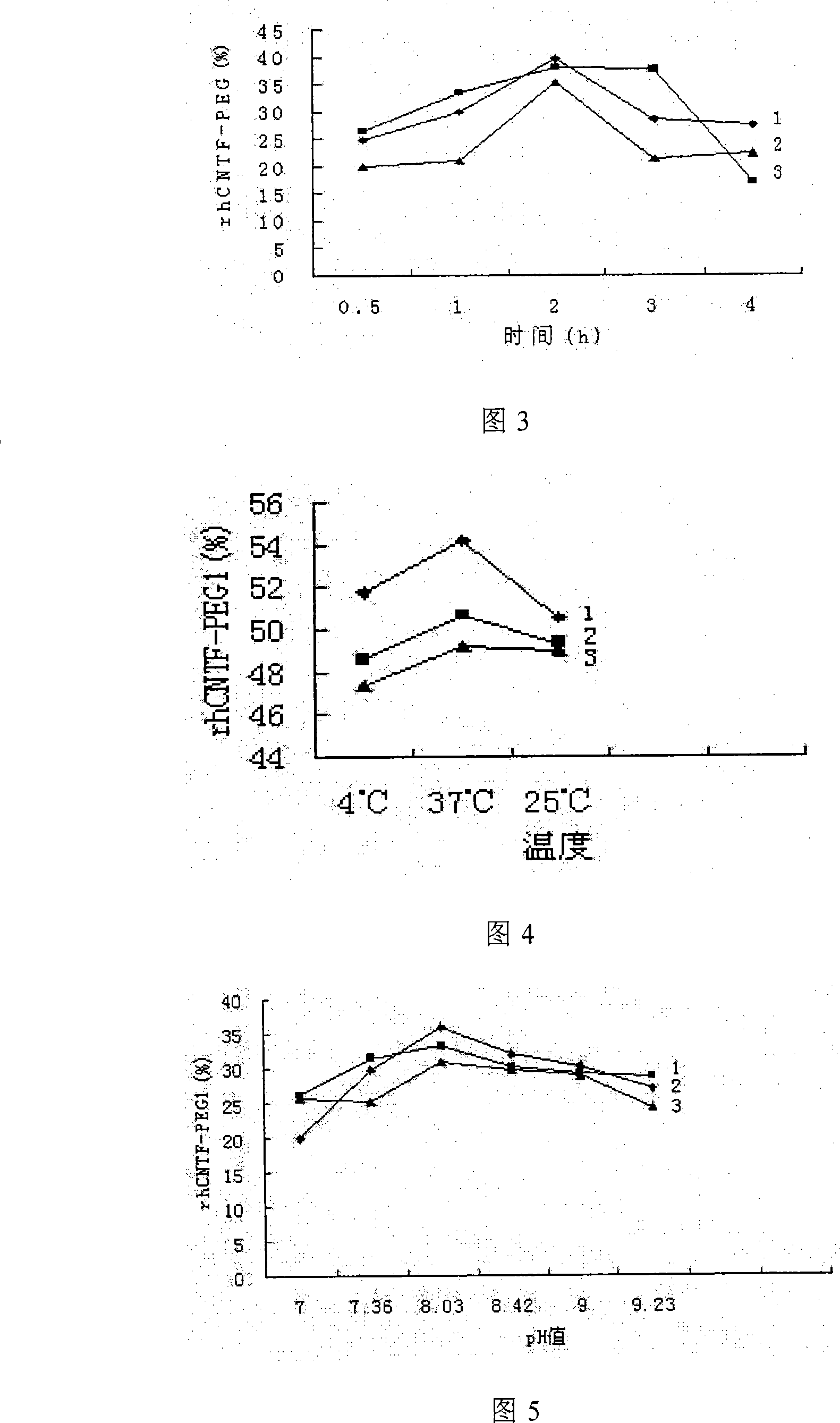
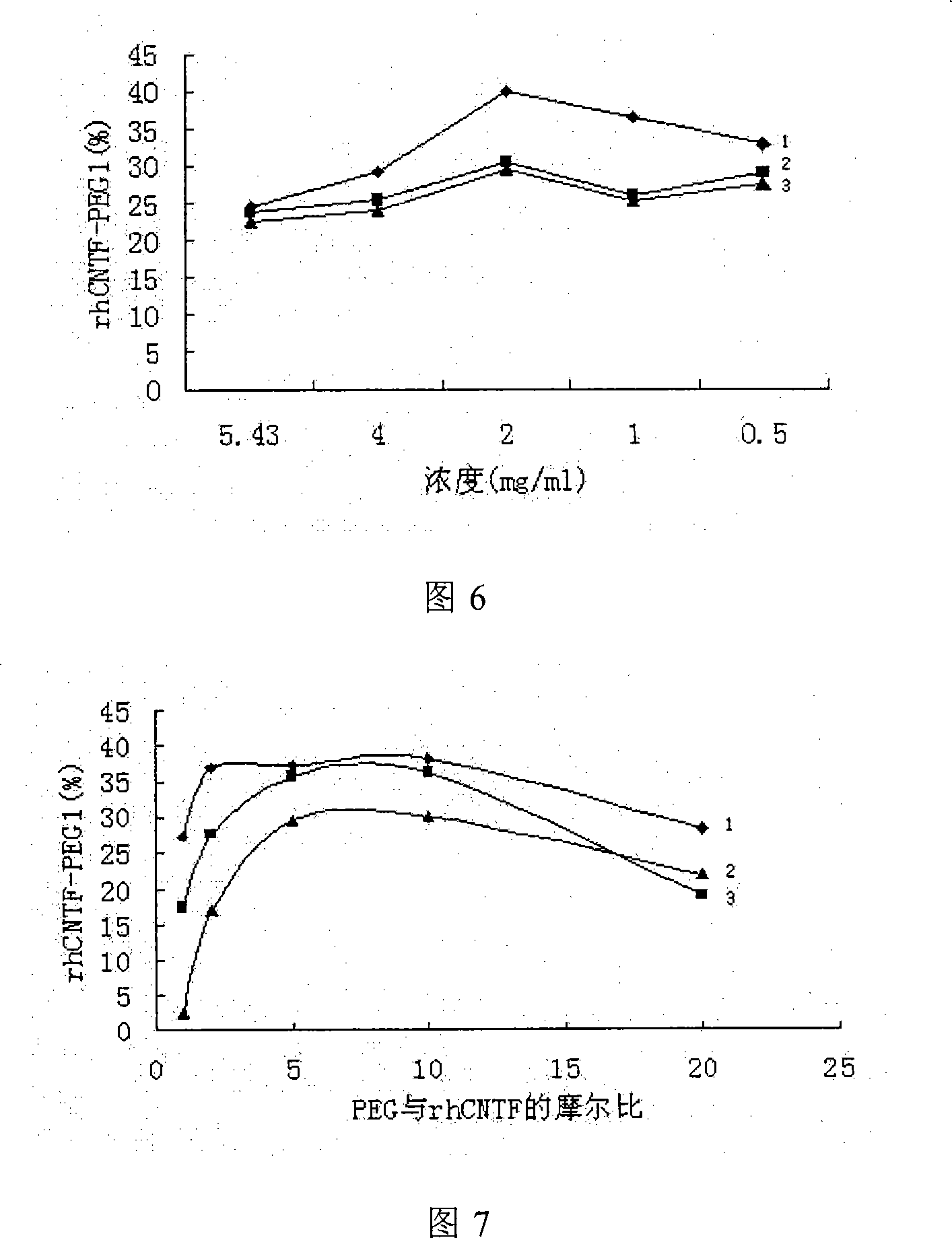
Ciliary neurotrophic factors decorated by polyglycol polymer and preparation method thereof
InactiveCN101185762AImprove stabilityLong circulating half-life in vivoPeptide/protein ingredientsMuscular disorderSide effectPolymer modified
The invention relates to a polyethylene glycol polymer-modified ciliary neurotrophic factor and a preparation method thereof. The method can just include the mixing reaction of polyethylene glycol polymer and ciliary neurotrophic factor. The polyethylene glycol polymer-modified ciliary neurotrophic factor related by the invention maintains the activity of the ciliary neurotrophic factor in the body, and the toxicity and side effects are significantly reduced, at the same time, the experimental results prove that the ciliary neurotrophic factor can obviously improve the pharmacokinetics property of the ciliary neurotrophic factor and greatly reduce the immunogenicity of the ciliary neurotrophic factor.
Owner:BEIJING INST OF BIOLOGICAL PROD
Nanoparticles loaded with neurotrophic factors, and preparation and applications thereof
InactiveCN103656623AStrong drug release functionGood biocompatibilityNervous disorderPeptide/protein ingredientsEpsilon-PolylysineMicrosphere
The invention discloses nanoparticles loaded with therapeutic factors or neurotrophic factors. The nanoparticles are made of a high molecular material; the high molecular material is composed of biocompatible positively charged epsilon-polylysine and negatively charged heparin; the mass ratio of epsilon-polylysine to heparin ranges from 1:20 to 1:1; and average particle size grain diameter of the nanoparticles ranges from 100 to 400nm. The epsilon-polylysine-heparin nanoparticles are taken as carriers for loading of neurotrophic factors, and are high in biocompatibility, and stable in biochemical properties. The nanoparticles loaded with neurotrophic factors are capable of promoting cell axon growth effectively via sustained release.
Owner:NANTONG UNIVERSITY
Neurotherapeutic Nanoparticle Compositions and Devices
There are provided compositions and methods for treatment of neurodegeneative diseases and CNS injury. The compositions a pharmaceutically acceptable carrier solution; and a plurality of biodegradable nanoparticles, wherein the nanoparticles comprise a targeting moiety that is able to bind selectively to the surface of a neural stem cell and wherein the nanoparticles further comprise factors such as leukaemia inhibitory factor (LIF); XAV939 and / or one or more of : brain-derived neurotrophic factor (BDNF) or an agonist thereof; epidermal growth factor (EGF) or an agonist thereof; glial cell-derived neurotrophic factor (GDNF) or an agonist thereof; retinoic acid and derivatives thereof; ciliary neurotrophic factor (CTNF) or an agonist thereof; and Wnt5A. The biodegradable nanoparticles may deliver via controlled time release.
Owner:YALE UNIV
T. cruzi-derived neurotrophic agents and methods of use therefor
InactiveUS7060676B2Provide supportNervous disorderPeptide/protein ingredientsNeuronal degenerationMammal
The invention relates to T. cruzi trans-sialidase (TS) and to the neurotrophic and IL-6 secretion-inducing activities of the protein. TS, neurotrophic variants and / or neurotrophic peptides based upon the sequence of TS can be administered to a mammal to directly or indirectly provide neurotrophic support for neurons. A mammalian neurotrophic factor (e.g., CNTF, LIF) can be co-administered with the TS, neurotrophic variant and / or neurotrophic peptide. TS, IL-6 secretion-inducing variants and / or IL-6 secretion-inducing peptides based upon the sequence of TS can be administered to a mammal to induce the secretion of IL-6. TS, active variants and / or active peptides can be administered to a mammal having an acquired or congenital condition characterized by neuronal degeneration or to a mammal that has experienced trauma to the brain, spinal cord or peripheral nerves. The invention also relates to neurotrophic and IL-6 secretion-inducing variants of TS and to neurotrophic and IL-6 secretion-inducing peptides. The invention also relates to compositions comprising TS, active variants thereof and / or active peptides and a physiologically acceptable carrier.
Owner:TRUSTEES OF TUFTS COLLEGE
Stabilizing medicinal agent containing ciliary nerve nutritive factor analogue
A high-stability medicine in the form of injection contains the ciliary neurotrophic factor (CNTF) and the substance chosen from medicinal surfactant, protein stabilizer and inorganic salt. Its advantages are high stability and long storage time.
Owner:HANGZHOU JIUYUAN GENE ENG
Ciliary neurotrophic factor variants
InactiveUS20050064555A1Retain weight loss and neuroprotective activityReduce and substantially eliminate immunogenicitySugar derivativesPeptide/protein ingredientsCiliary neurotrophic factorImmunogenicity
The present invention relates to variant ciliary neurotrophic factor (CNTF) proteins that possess neuroprotective and / or weight loss activity and reduced immunogenicty. In particular, variants of CNTF with reduced ability to bind one or more human class 11 MHC molecules are described.
Owner:XENCOR
Methods for therapeutic use of brain derived neurotrophic factor in the entorhinal cortex
InactiveUS20030124095A1Measured cognitive functionImprove cognitive functionBiocideSenses disorderFactor iiCortical tissue
A protocol for use of growth factors to stimulate neuronal cell growth and activity in trkB receptor containing cortical tissues, including the entorhinal and hippocampal cortices. The method introduces exogenous growth factor, such as BDNF, NT-4 / 5 and NT-3, into the EC. The method is useful in therapy of defective, diseased and damaged neurons in the mammalian brain, of particular usefulness for treatment of neurodegenerative conditions such as Alzheimer's disease or for normal aging.
Owner:RGT UNIV OF CALIFORNIA
Brain-targeting neurotrophic factor fusion polypeptide for preventing and treating Alzheimer's disease
ActiveCN101921314ARetain biological activityPlay a preventive roleNervous disorderTetrapeptide ingredientsDiseaseMedicine
The invention provides a brain-targeting neurotrophic factor fusion polypeptide for preventing and treating Alzheimer's disease, which is composed of a neurotrophic factor active peptide segment, 11 peptide and 6-aminohexanoyl (Ahx) in the middle of the neurotrophic factor active peptide segment and the 11 peptide. An amino acid sequence of the 11 peptide is YGRKKRRQRRR, and the neurotrophic factor active peptide segment comprises a brain-derived neurotrophic factor (BDNF), a nerve growth factor (NGF) or a ciliary neurotrophic factor (CNTF). The fusion polypeptide can penetrate a blood-brain barrier and can be used for preventing and treating Alzheimer's disease.
Owner:HUAZHONG UNIV OF SCI & TECH
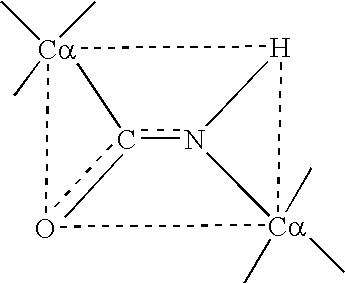
Modified ciliary neurotrophic factor (cntf ) with reduced immunogenicity
InactiveUS20040087503A1Modify characteristicImprove accuracyPeptide/protein ingredientsImmunoglobulinsFactor iiIn vivo
The present invention relates to polypeptides to be administered especially to humans and in particular for therapeutic use. The polypeptides are modified polypeptides whereby the modification results in a reduced propensity for the polypeptide to elicit an immune response upon administration to the human subject. The invention in particular relates to the modification of human ciliary neutrophic factor (CNTF) to result in CNTF proteins that are substantially non-immunogenic or less immunogenic than any non-modified counterpart when used in vivo.
Owner:MERCK PATENT GMBH
Neurotrophic factor secretion promoters
The present invention relates to a neurotrophic factor secretagogue, in particular, to a BDNF (brain-derived neurotrophic factor) secretagogue, which comprises as an active ingredient an NO donor. The medicament of the present invention promotes the secretion of neurotrophic factors from mammalian central neural cells. Thus, the medicament of the present invention, i.e., NO donor, is possibly applicable to the treatment of diseases caused by neutrotrophic factors, for example, neurodegenerative diseases, and is expected to exhibit the efficacious effects thereon. Also, the present invention provides a novel medication for neurodegenerative diseases.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
Neurogenerative or neurotrophic factors for mitigating a symptom of ischemia
InactiveUS20050222022A1Easy to addImprove stabilityCompound screeningNervous disorderHEPARIN-BINDING EGFCiliary neurotrophic factor
This invention pertains to the identification of neurogenerative and / or neurotrophic factors that can induce migration of stem cells to neural tissue and / or induce proliferation and / or differentiation of such cells into neurons. Such agents include, but are not limited to stem cell factor (SCF), heparin binding EGF (HB-EGF), and VEGF.
Owner:THE BUCK INST FOR RES ON AGING
Production of radial glial cells
InactiveUS7033995B2Increase percentageIncrease the number of cellsNervous disorderPeptide/protein ingredientsRadial glial cellEpendymal Cell
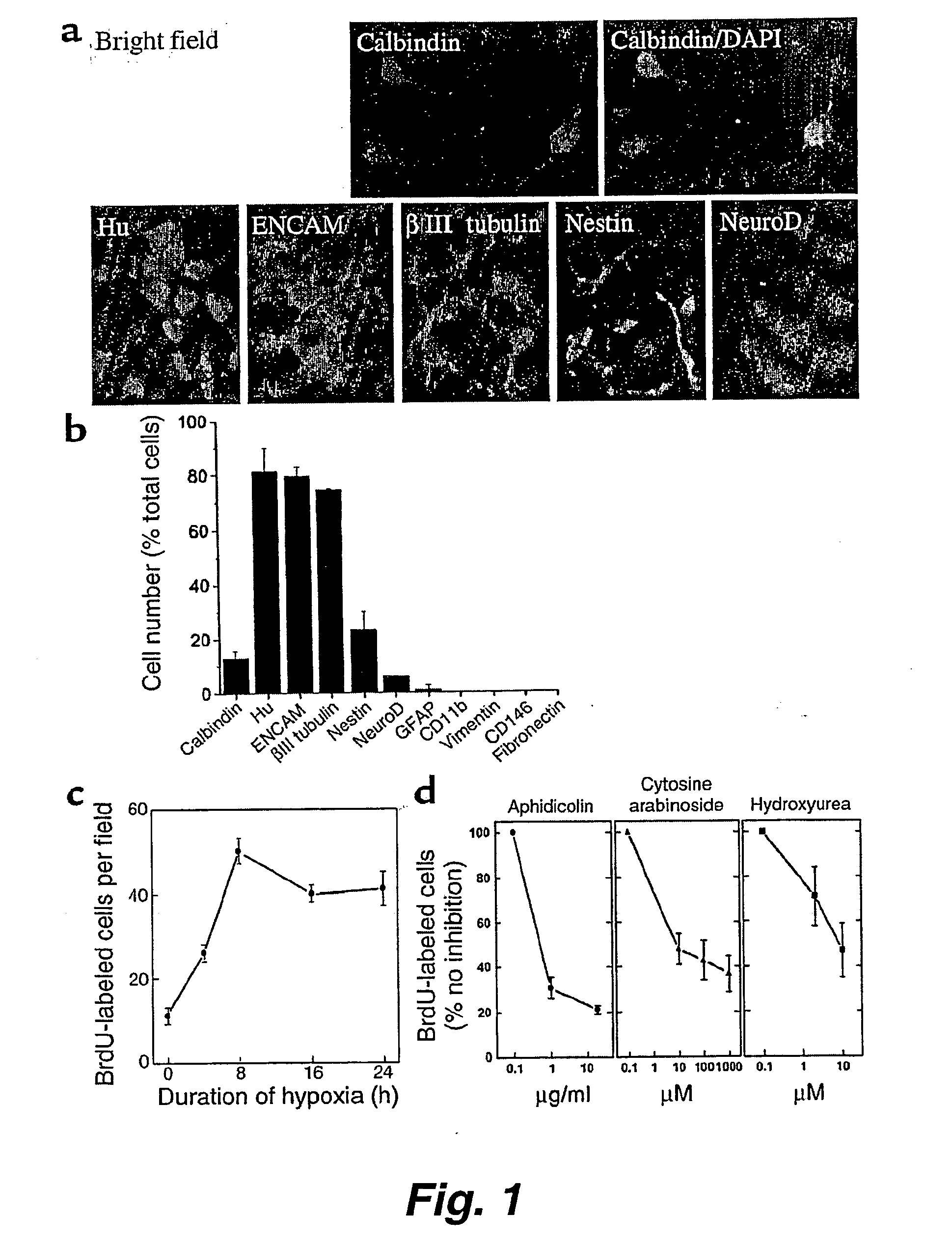
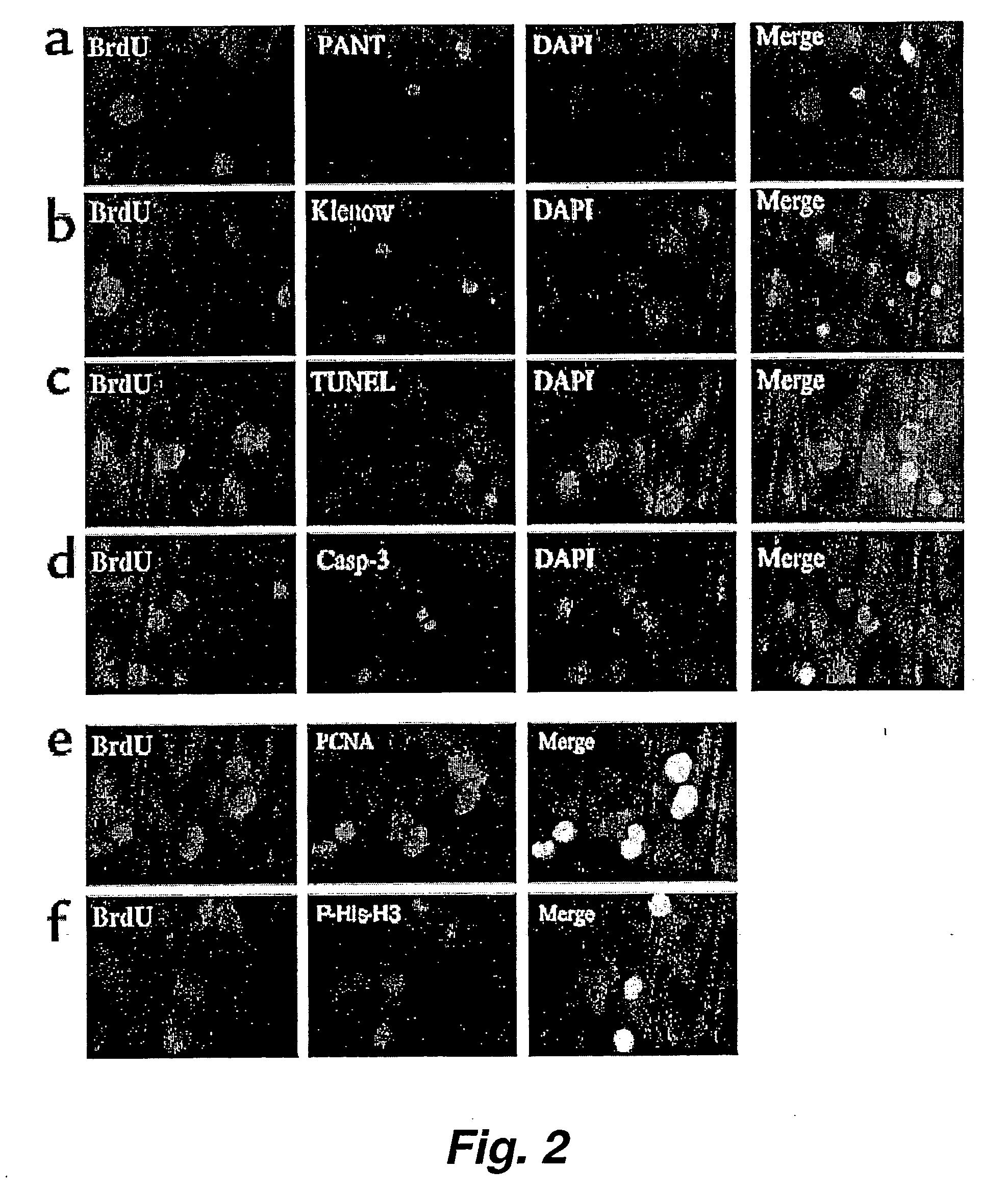
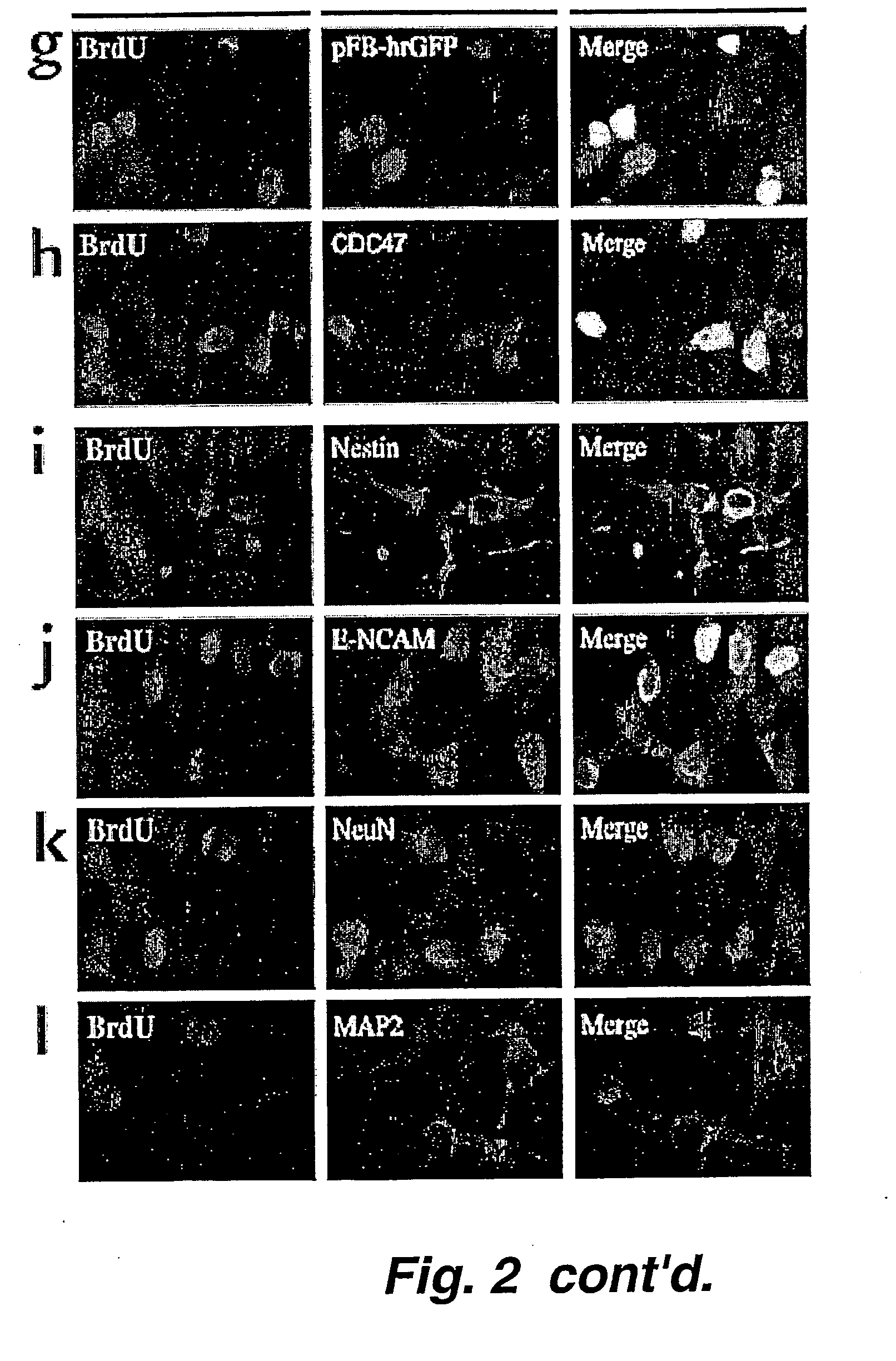
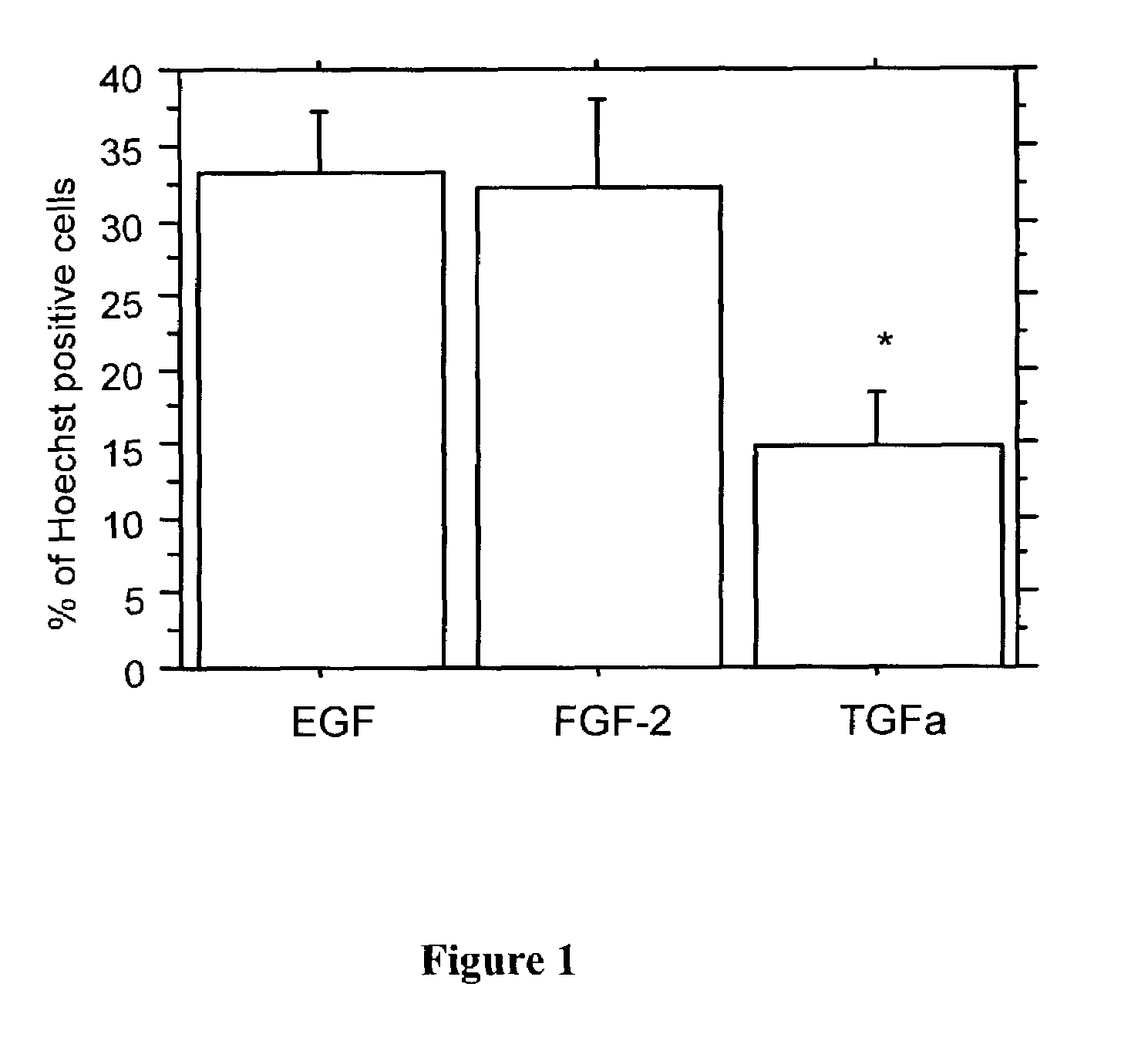
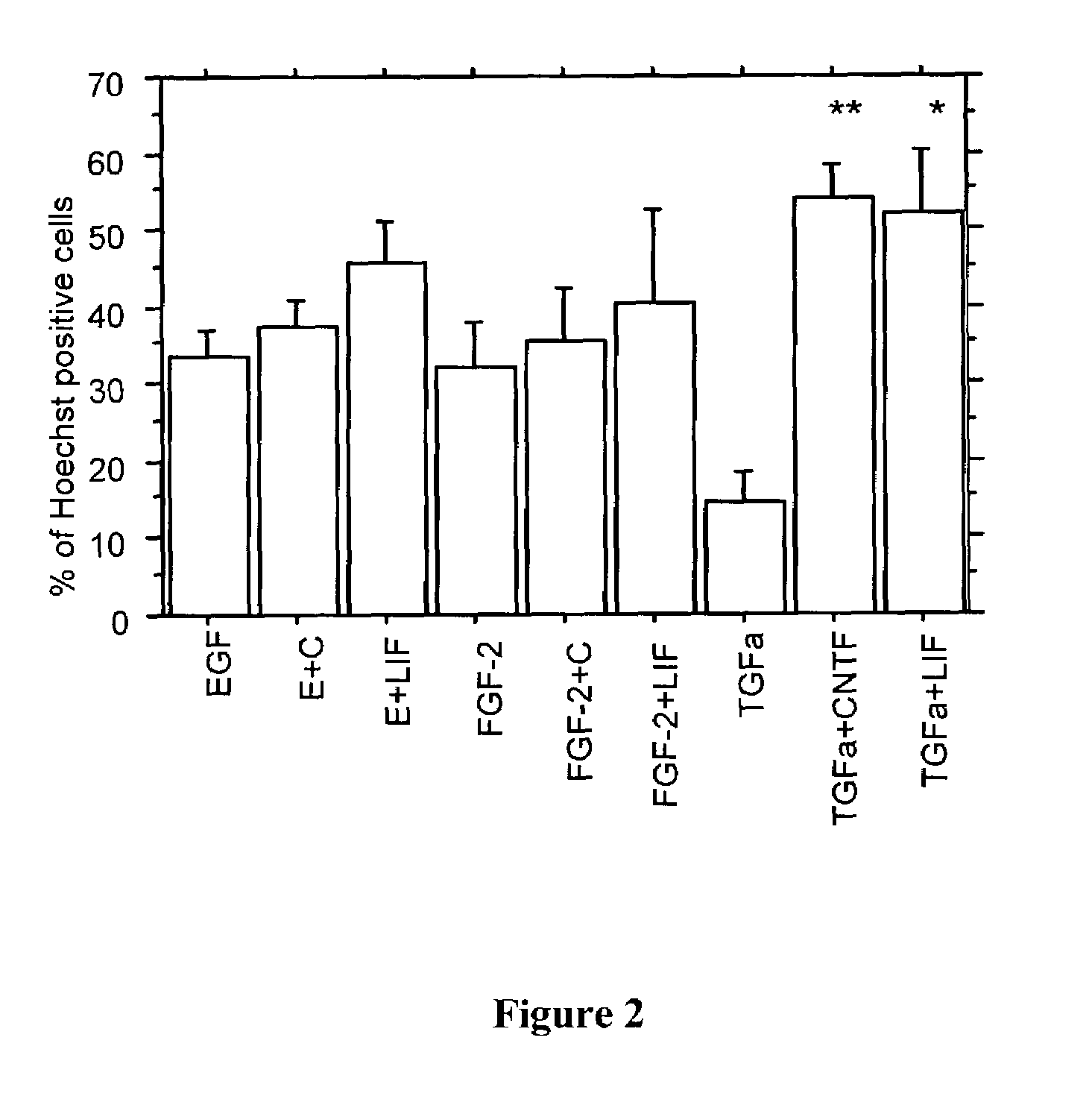
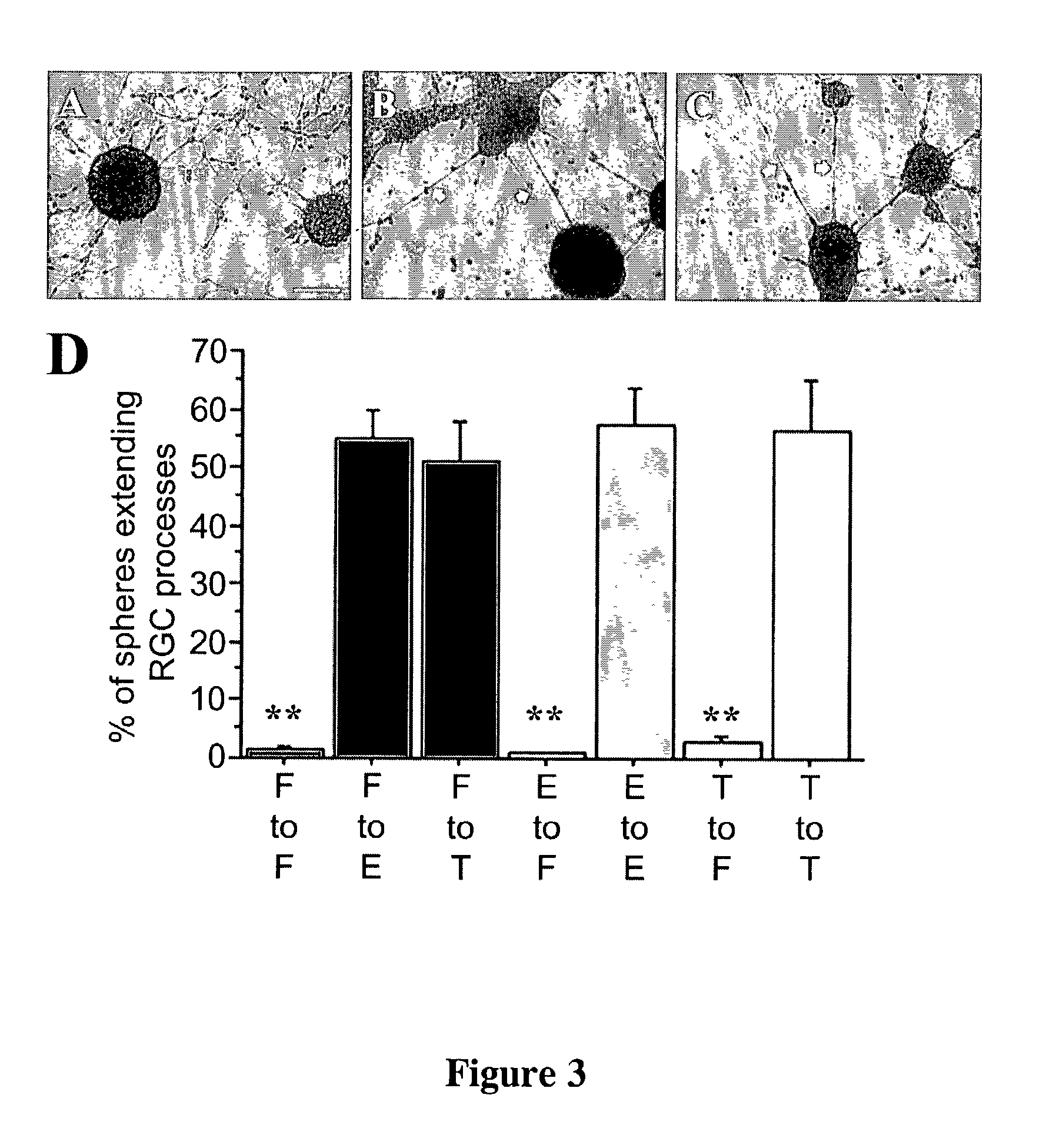
The present invention relates to a method of producing radial glial cells from neural stem cells, particularly by contacting neural stem cells with epidermal growth factor (EGF), fibroblast growth factor 2 (FGF-2) and / or TGFα. Leukemia inhibitory factor (LIF) and ciliary neurotrophic factor (CNTF) can optionally be added to enhance the effect of EGF, FGF-1 or TGFα. Also provided are methods of producing radial glial cells from ependymal cells, as well as methods of proliferating ependymal cells.
Owner:STEM CELL THERAPEUTICS
Method for separating isomerism protein from recombinant human ciliary neurotrophy factor and its mutant
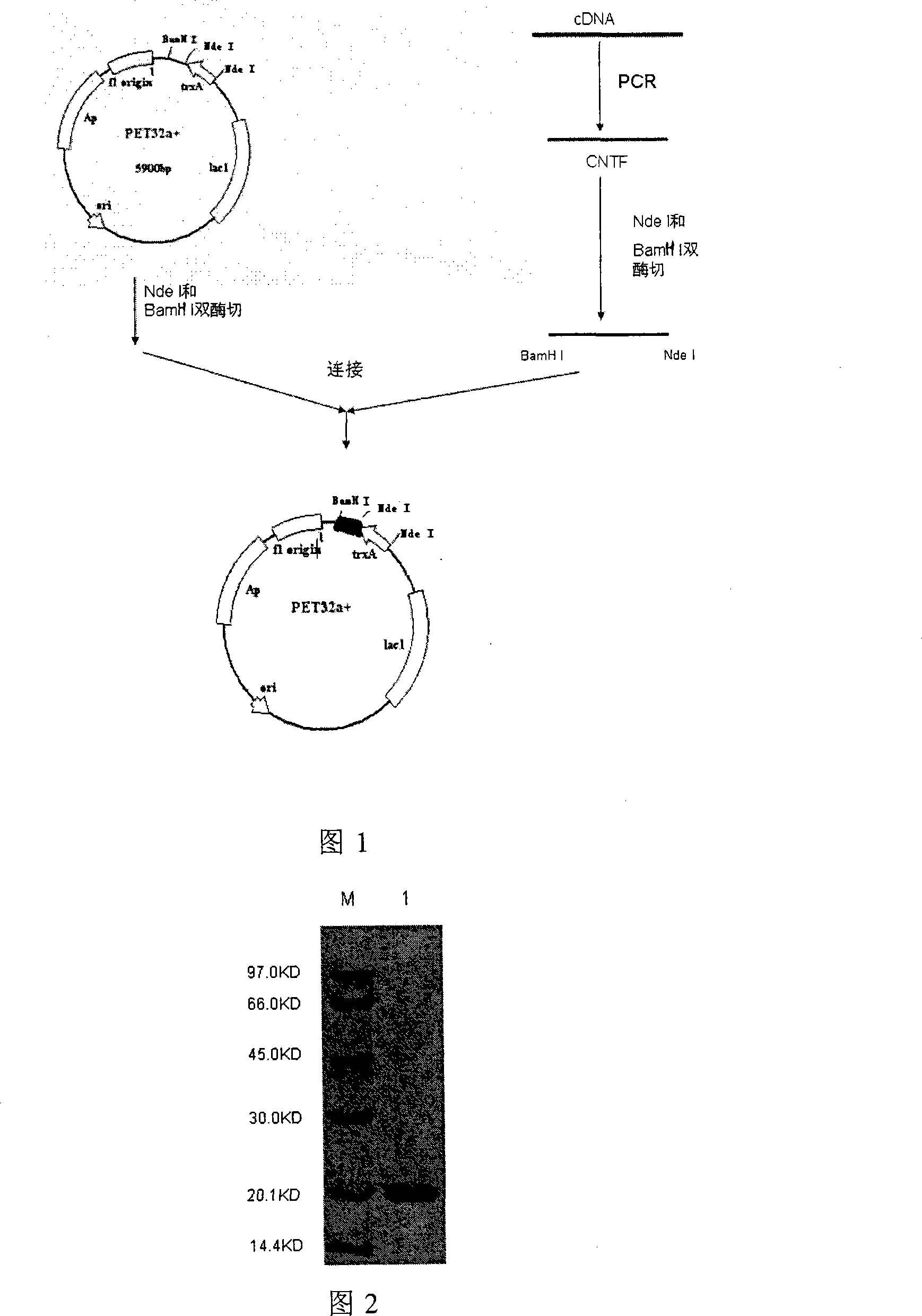
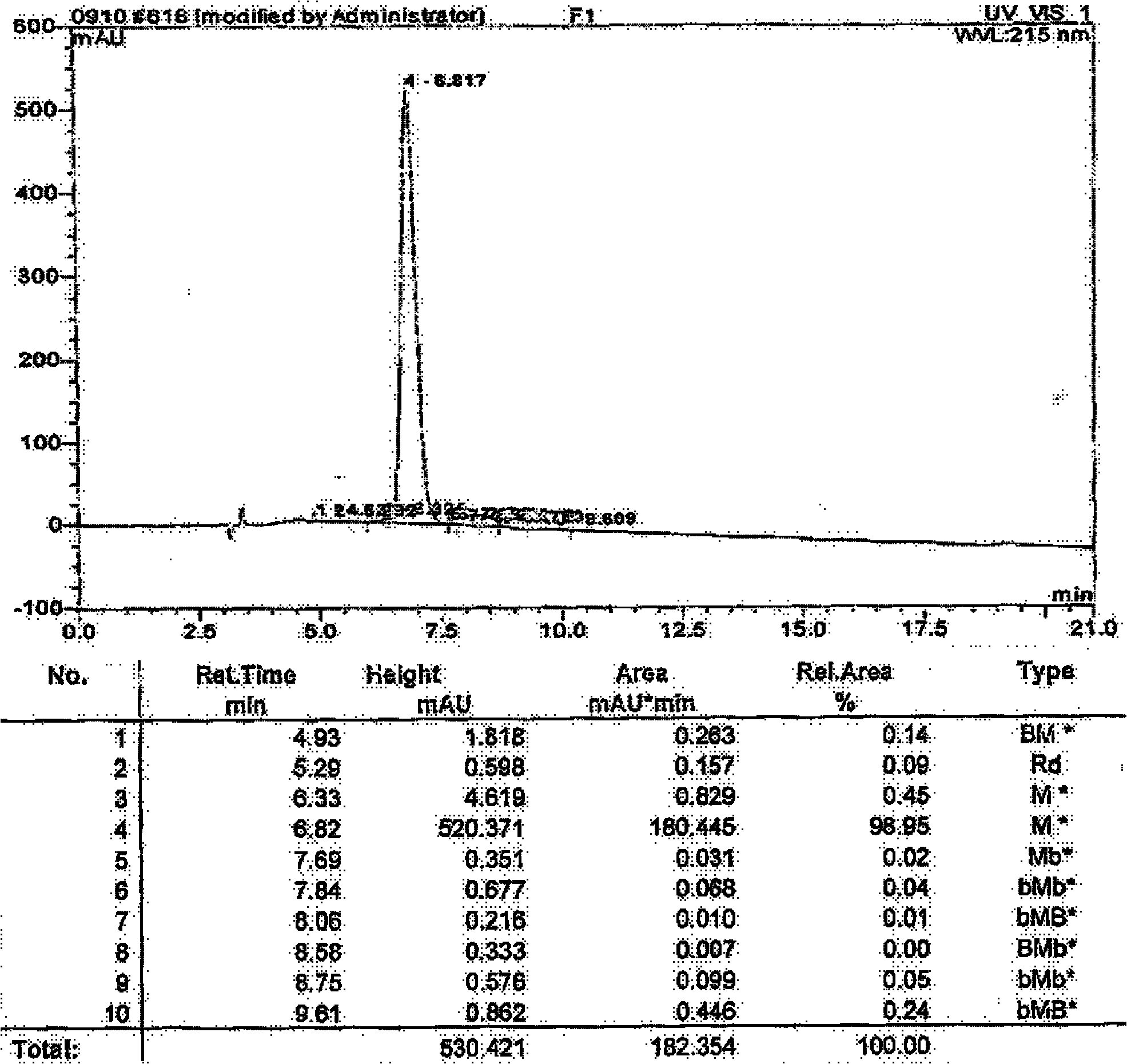
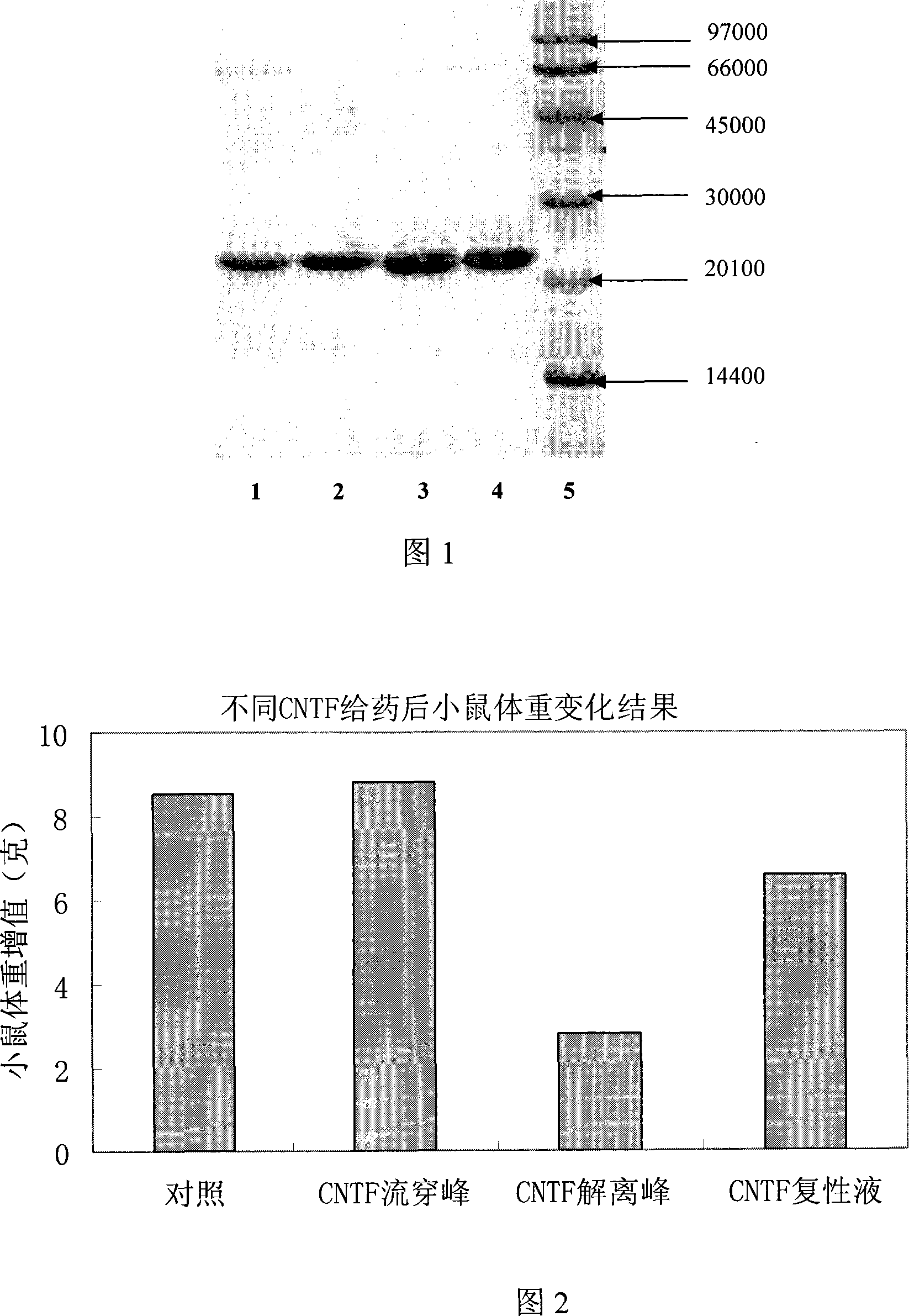
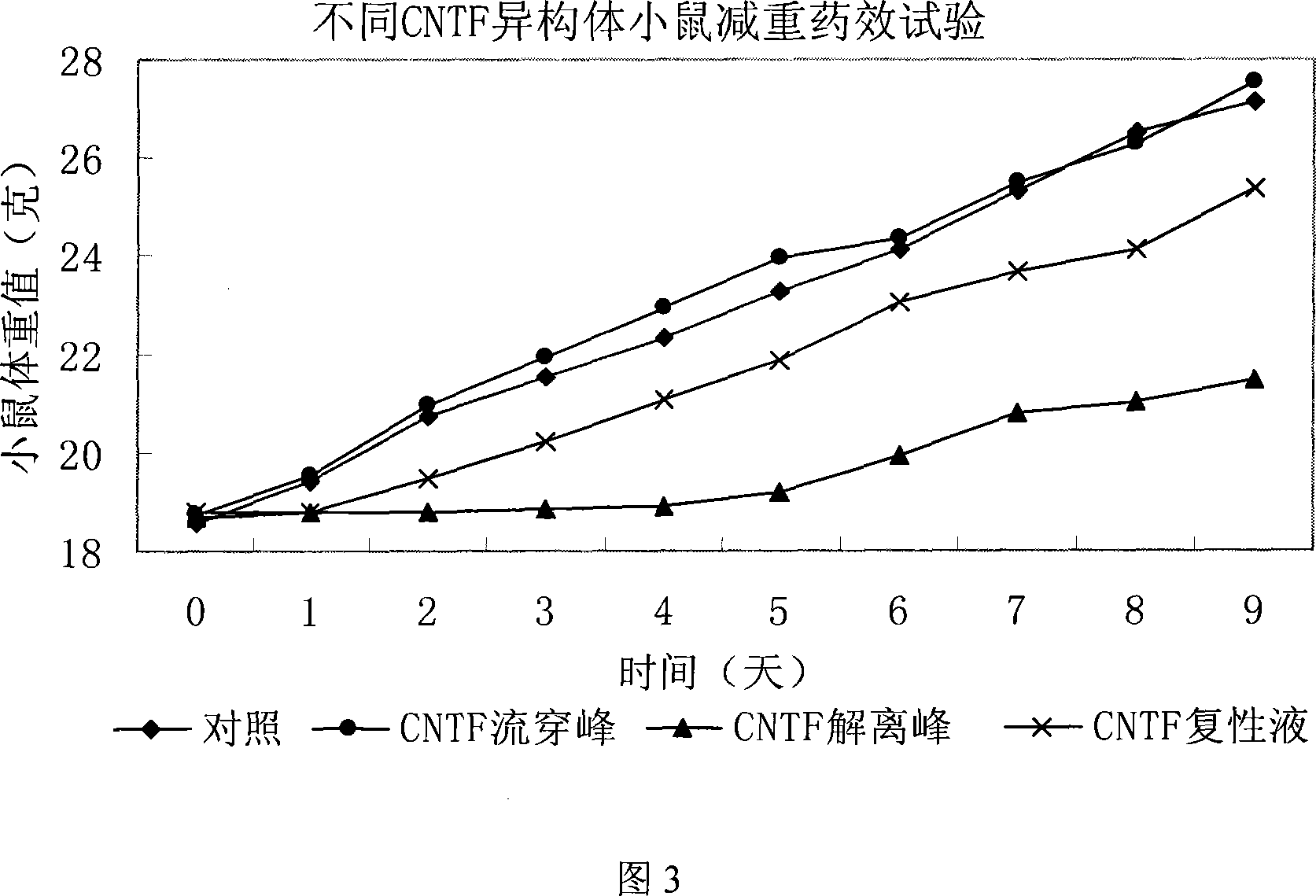
InactiveCN101113174AEfficient separationGood treatment effectPeptide preparation methodsGrowth factors/regulatorsTE bufferInclusion bodies
The invention relates to a method to separate isomeric protein form gene recombinant expression protein. The method of the invention is that: the collected wet bacterium is made into bacterium soliquoid in a cleaning buffer liquid, and shattered and decentered by ultrasonic, and the clear liquid is abandoned, the precipitation is cleaned by TE buffer liquid containing urea and TritonX-100, then protein endosome is acquired, and the endosomes is fully dissolved by a buffer liquid containing guanidine hydrochloride or urea; the dissolving liquid is diluted and renatured by the buffer liquid containing Tris-HCl, arginine and EDTA, then stand for 24-48h, and becomes a CNTF renatured protein liquid; after being ultrafiltered and concentrated, the renatured protein is transferred into the Tris buffer liquid through gel filtration chromatography to collect protein peak; finally the CNTF renatured protein liquid with transferred liquid is sent to Q sephrose-Fast Flow anion exchange column, to remove unrenatured CNTF proteins and other proteins.
Owner:兰州生物制品研究所
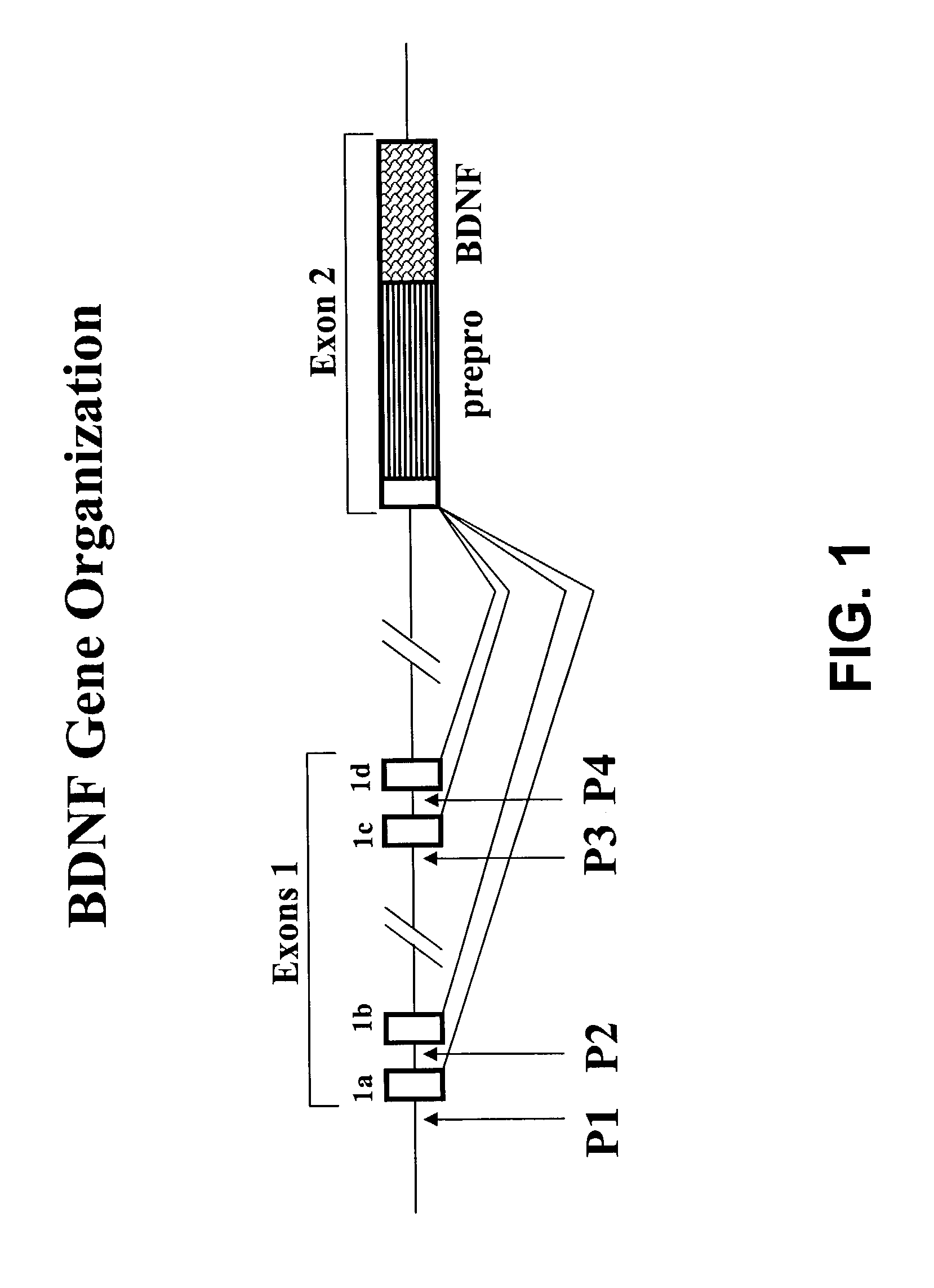
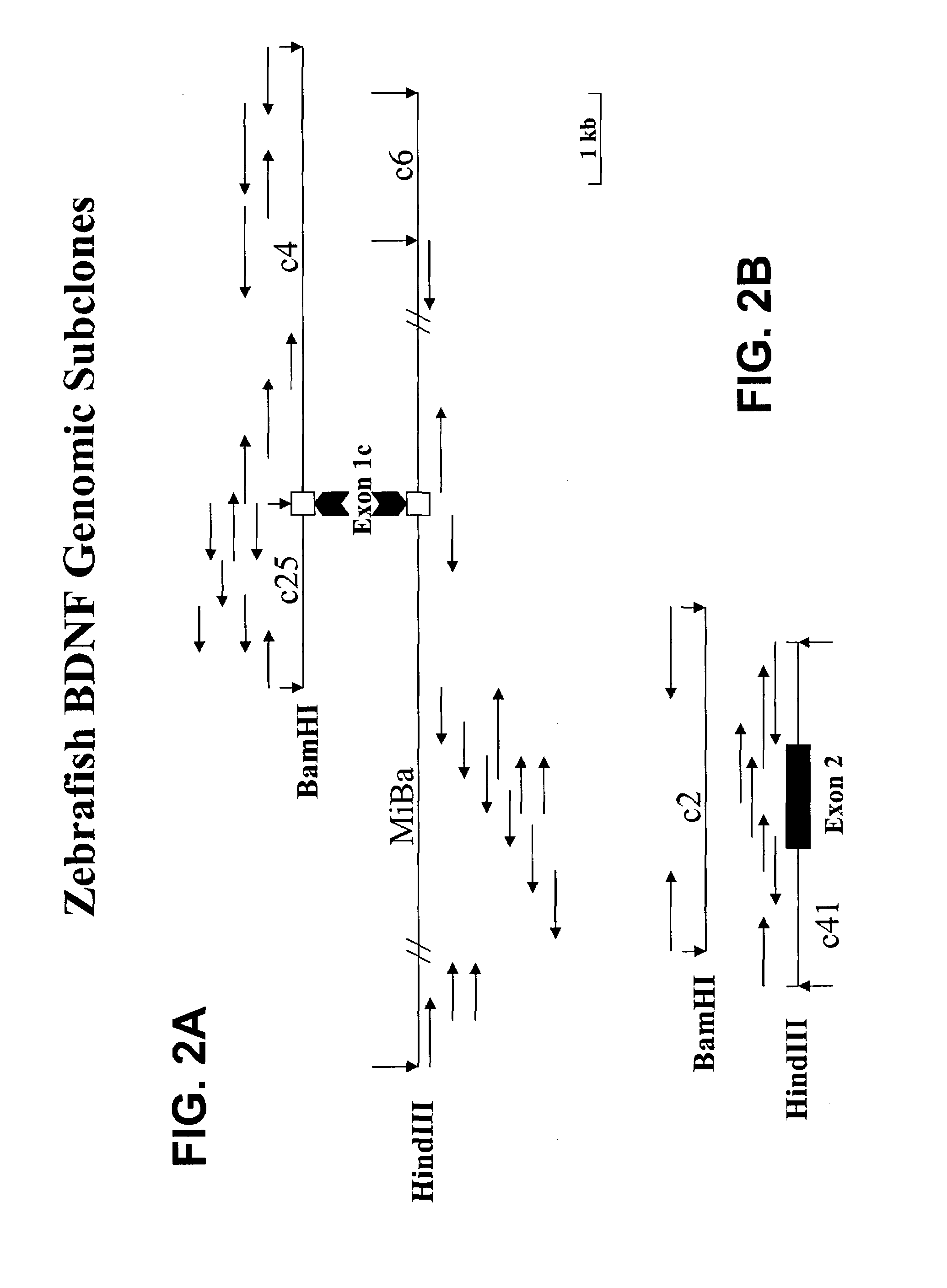
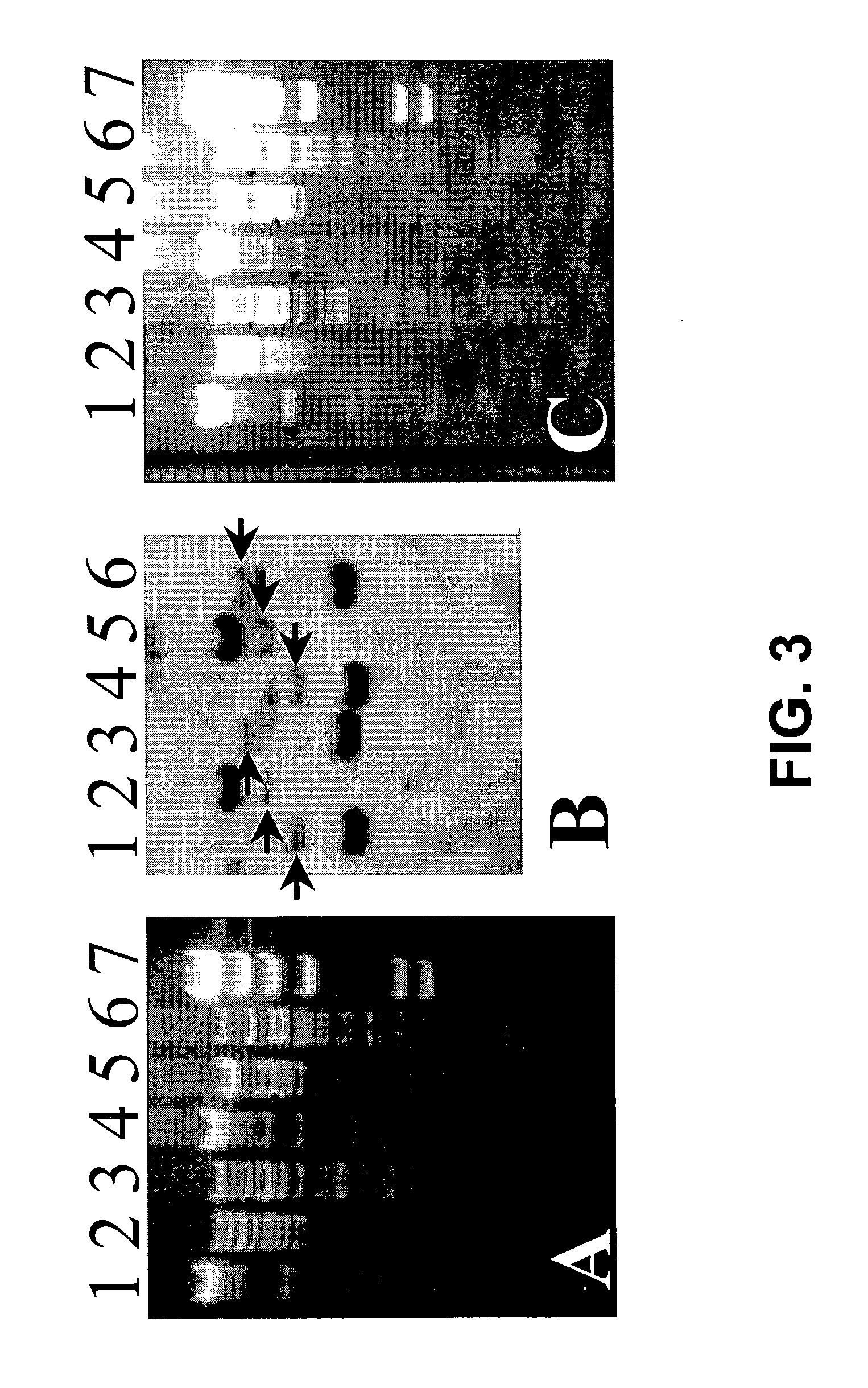
Transgenic screen and method for screening modulators of brain-derived neurotrophic factor (BDNF) production
InactiveUS7491810B2Influence and modulate productionUsed commerciallySugar derivativesMicrobiological testing/measurementFluorescenceEmbryo
A transgenic screen and method for screening biological and chemical test substances or molecules for their ability to influence or modulate the production of BDNF in cells, includes a fusion gene having a zebrafish BDNF gene fragment (promoter) and a fluorescent marker gene inserted downstream of the BDNF gene fragment. When the fusion gene is injected into a zebrafish embryo, the BDNF promoter causes the production of fluorescent protein in various cell types. The embryo is exposed to a test substance for determining the effect thereof on the production of the fluorescent marker protein.
Owner:VETERANS AFFAIRS U S DEPT OF
Methods and compositions for diagnosis and prognosis of renal injury and renal failure
InactiveUS20130316370A1Eliminate needEasy to adaptDisease diagnosisBiological testingWhite blood cellPancreatic hormone
The present invention relates to methods and compositions for monitoring, diagnosis, prognosis, and determination of treatment regimens in subjects suffering from or suspected of having a renal injury. In particular, the invention relates to using assays that detect one or more biomarkers selected from the group consisting of Beta-nerve growth factor, Interleukin-17A, Follitropin subunit beta, Collagenase 3, Follistatin, Vitamin D Binding Protein, Islet amyloid polypeptide, Insulin C-peptide, Complement Factor H, Gastric inhibitory polypeptide, Glucagon-like peptide 1, Glucagon, Involucrin, Type II cytoskeletal Keratin-1 / Keratin-10, Type II cytoskeletal Keratin-6A / 6B / 6C, Osteocalcin, Lipopolysaccharide, Pancreatic prohormone, Peptide YY, Agouti-related protein, Ciliary neurotrophic factor, Appetite-regulating hormone, Transthyretin, Insulin receptor substrate 1, and NF-kappa-B inhibitor alpha as diagnostic and prognostic biomarker assays in renal injuries.
Owner:ASTUTE MEDICAL
T. cruzi-derived neurotrophic agents and methods of use therefor
The invention relates to T. cruzi trans-sialidase (TS) and to the neurotrophic and IL-6 secretion-inducing activities of the protein. TS, neurotrophic variants and / or neurotrophic peptides based upon the sequence of TS can be administered to a mammal to directly or indirectly provide neurotrophic support for neurons. A mammalian neurotrophic factor (e.g., CNTF, LIF) can be co-administered with the TS, neurotrophic variant and / or neurotrophic peptide. TS, IL-6 secretion-inducing variants and / or IL-6 secretion-inducing peptides based upon the sequence of TS can be administered to a mammal to induce the secretion of IL-6. TS, active variants and / or active peptides can be administered to a mammal having an acquired or congenital condition characterized by neuronal degeneration or to a mammal that has experienced trauma to the brain, spinal cord or peripheral nerves. The invention also relates to neurotrophic and IL-6 secretion-inducing variants of TS and to neurotrophic and IL-6 secretion-inducing peptides. The invention also relates to compositions comprising TS, active variants thereof and / or active peptides and a physiologically acceptable carrier.
Owner:TRUSTEES OF TUFTS COLLEGE
Recombinant TAT-MANF (trans-activator of transcription-mesencephalic astrocyte derived neurotrophic factor) fusion protein and application thereof
InactiveCN103254315AImprove protectionSlow down neuronal lesionsNervous disorderPeptide/protein ingredientsCell membraneTrans-Activators
The invention relates to a recombinant TAT-MANF (trans-activator of transcription-mesencephalic astrocyte derived neurotrophic factor) fusion protein and an application thereof. A sequence of the recombinant TAT-MANF fusion protein comprises a TAT sequence positioned at an amino terminal and an MANF sequence positioned at a carboxyl terminal. The recombinant TAT-MANF fusion protein is used for nervous system diseases. The fusion protein can enter cells after striding cell membranes, can pass through a blood brain barrier, has a nerve cell protection effect and can be used for relieving neuron lesions related to Parkinson disease.
Owner:SUZHOU RES INST OF TONGJI UNIV
Agent having neurotrophic factor-like activity
InactiveUS20120264959A1Improve securityPreventive andBiocideNervous disorderEthyl esterCiliary neurotrophic factor
Owner:NIPPON ZOKI PHARM CO LTD
Treatment of brain derived neurotrophic factor (BDNF) related diseases by inhibition of natural antisense transcript to BDNF
ActiveUS9074210B2Modulate its functionCompound screeningSenses disorderDiseaseBrain-derived neurotrophic factor
The present invention relates to antisense oligonucleotides that modulate the expression of and / or function of Brain derived neurotrophic factor (BDNF), in particular, by targeting natural antisense polynucleotides of Brain derived neurotrophic factor (BDNF). The invention also relates to the identification of these antisense oligonucleotides and their use in treating diseases and disorders associated with the expression of BDNF.
Owner:CURNA INC
Pharmacological modulation of positive ampa receptor modulator effects on neurotrophin expression
InactiveUS20090192199A1Good effectImprove survivalBiocideNervous disorderBrain-derived neurotrophic factorFactor ii
Antagonists of group 1 metabotropic glutamate receptors (mGluR) potentiate the effect of positive AMPA receptor modulators on neurotrophin expression, such as brain-derived neurotrophic factor (BDNF). The findings described herein suggest a combinatorial approach for drug therapies, using both positive AMPA receptor modulators and mGluR antagonists. to enhance brain neurotrophism.
Owner:RGT UNIV OF CALIFORNIA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com