Agent having neurotrophic factor-like activity
a neurotrophic factor and agent technology, applied in the field of agents having neurotrophic factorlike activity, can solve the problems of having difficulty in reaching the brain, affecting the ability of neurons to regenerate, and affecting the ability of neurons to be activated, so as to achieve preventive and improve, high safety, and preventive
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
production example 1
Decanoic acid methyl ester, Compound 2
[0067]Decanoic acid was dissolved in thionyl chloride (10 ml) and treated in water bath for 3 hours to distill off excess thionyl chloride. Methanol (30 ml) was added to chloride of decanoic acid and refluxed in water bath for 2 hours. After cooling, the reaction mixture was added to 1N HCl (80 ml) to be acidic and distributed with ethyl acetate (hereinafter expressed as “EtOAc”). The distributed liquid was purified with column chromatography (developing solvent: n-hexane (C6H14)-EtOAc (3:1)) after concentration, and decanoic acid methyl ester shown in the following was isolated.
[0068]Colorless oily substance, C11H22O2 MW 186, EIMS m / z (%): 186 (M+, 7), 143 (21), 101 (10), 87 (55), 74 (100)
production example 2
Decanoic acid ethyl ester, Compound 3
[0069]Decanoic acid was dissolved in thionyl chloride (10 ml) and treated in water bath for 3 hours to distill off excess thionyl chloride. Ethanol (30 ml) was added to chloride of decanoic acid and refluxed in water bath for 2 hours. After cooling, the reaction mixture was added to 1N HCl (80 ml) to be acidic and distributed with EtOAc. The distributed liquid was purified with column chromatography (developing solvent: C6H14-EtOAc (3:1)) after concentration, and decanoic acid ethyl ester shown in the following was isolated.
[0070]Colorless oily substance, C12H24O2 MW 200, EIMS m / z (%): 200 (M+, 10), 155 (26), 101 (44), 88 (100), 73 (19)
production example 3
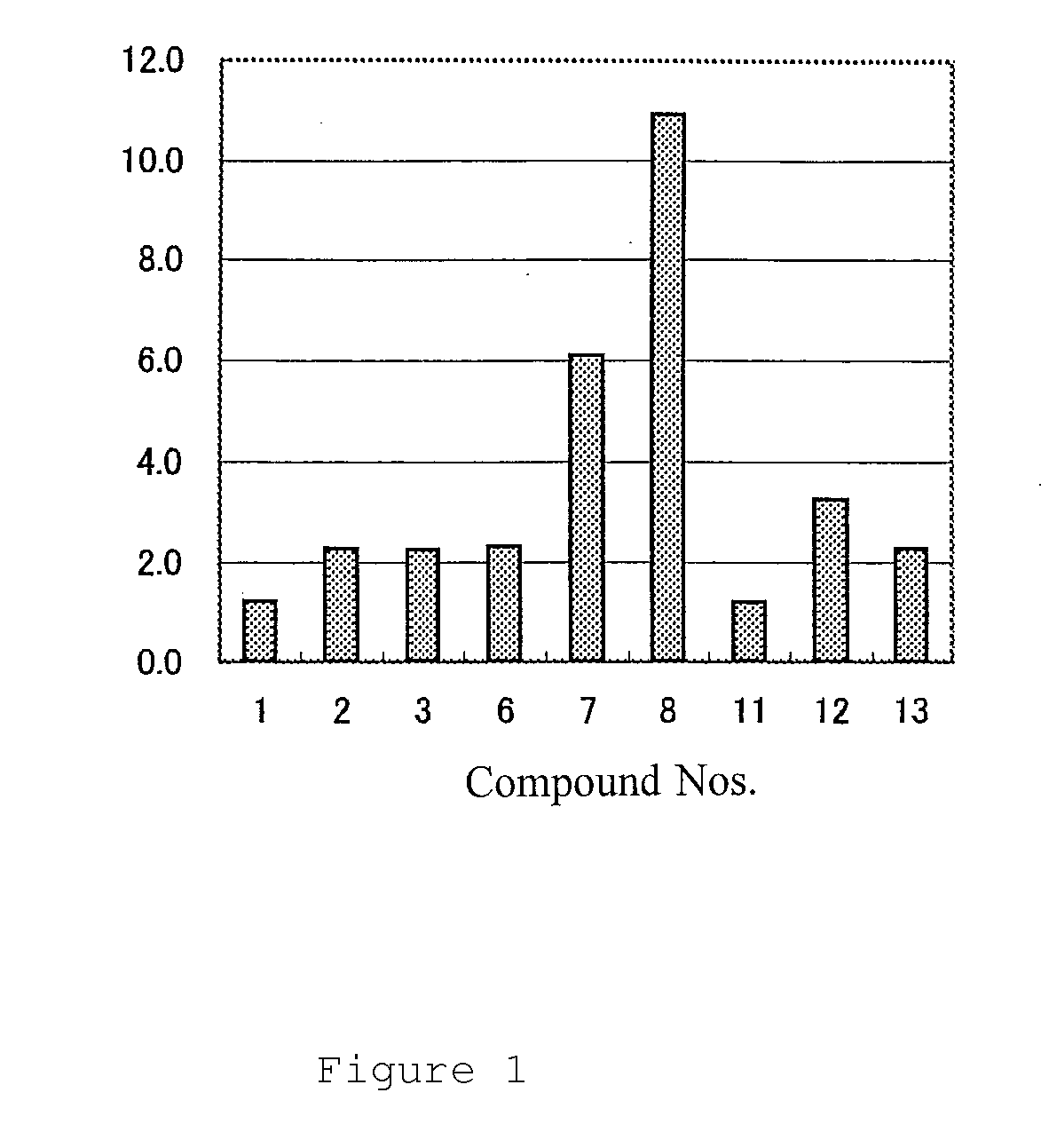
Trans-2-decenoic acid methyl ester, Compound 7
[0071]Trans-2-decenoic acid was dissolved in thionyl chloride (10 ml) and treated in water bath for 3 hours to distill off excess thionyl chloride. Methanol (30 ml) was added to chloride of trans-2-decenic acid and refluxed in water bath for 2 hours. After cooling, the reaction mixture was added to 1N HCl (80 ml) to be acidic and distributed with EtOAc. The distributed liquid was purified with column chromatography (developing solvent: C6H14-EtOAc (3:1)) after concentration, and trans-2-decenoic acid methyl ester shown in the following was isolated.
[0072]Colorless oily substance, C11H20O2 MW 184, EIMS m / z (%): 184 (M+, 5), 167 (6), 153 (43), 123 (16), 113 (41), 87 (90), 69 (39), 43 (100)
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com