Neurotherapeutic Nanoparticle Compositions and Devices
a nanoparticle and nanotechnology, applied in the field of neuroprotection, can solve the problems of no effective cure, no effective cure, and no good cure for neurodegeneration, and achieve the effect of slowing down the progression of neurodegeneration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
1.1 Dopaminergic Neurons Derived from E14 Ventral Mesencephalon (VM) from Rat Foetuses Express the Components of the LIF Receptor Complex
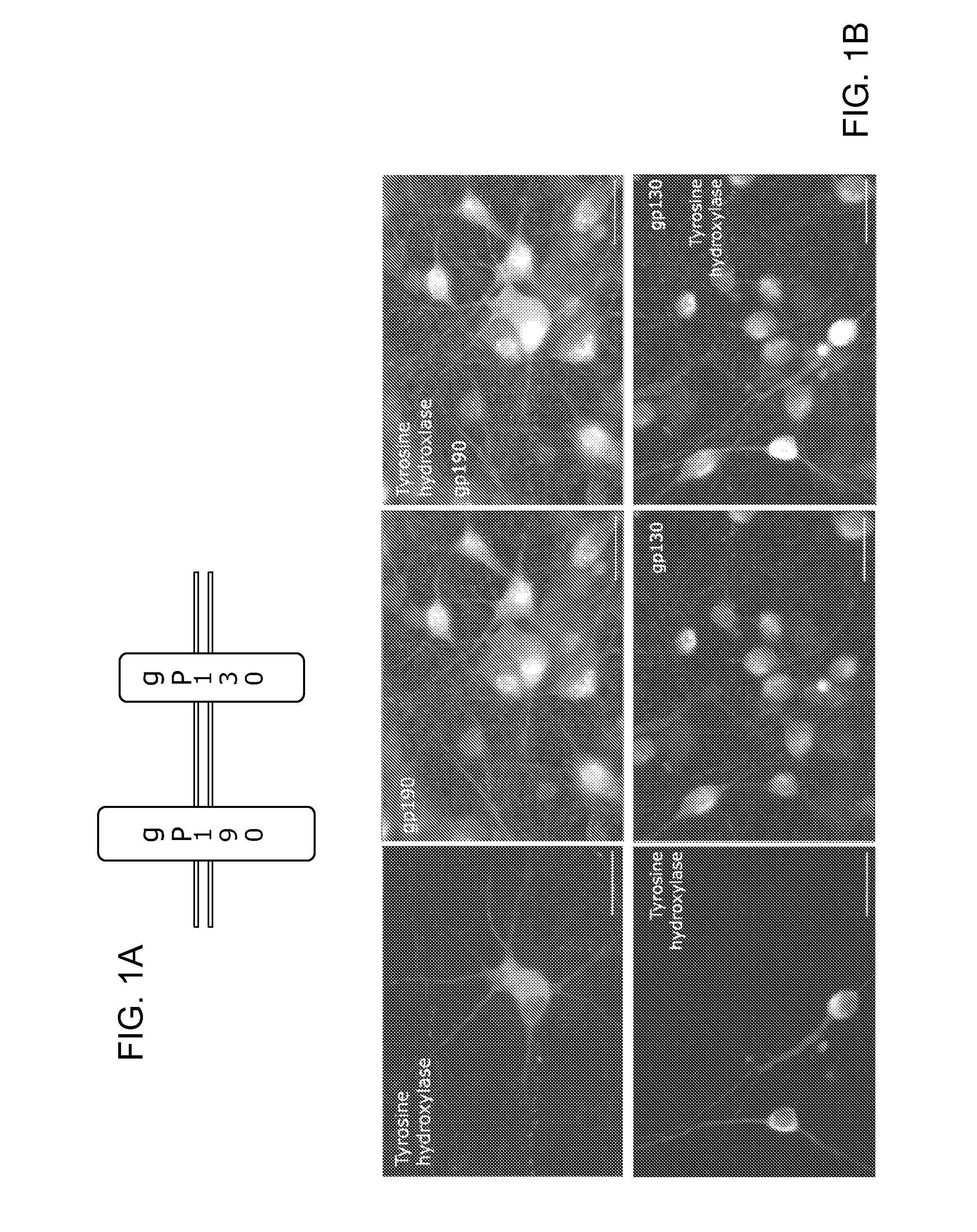
[0117]The expression of gp130 and gp190, the two components of the LIF receptor complex (FIG. 1A), on dopaminergic neurons of embryonic day 14 ('E14′) VM was analysed via immunocytochemistry of E14 VM cultures after 3 days in vitro (DIV) (FIG. 1B). FIG. 1B shows that both components of the LIF receptor complex are expressed by dopaminergic neurons in E14 ventral mesencephalon (VM) cultures. FIG. 1A: The LIF receptor is a heterodimer consisting of two proteins: gp130 and gp190. FIG. 1B: Immunocytochemistry of 5 day old E14 VM cultures with antibodies against tyrosine hydroxylase and gp130 or gp190 demonstrated that dopaminergic neurons express gp130 and gp190. Dopaminergic neurons were demonstrated to express both gp130 and gp190, suggesting a potential for responsiveness to LIF treatment.
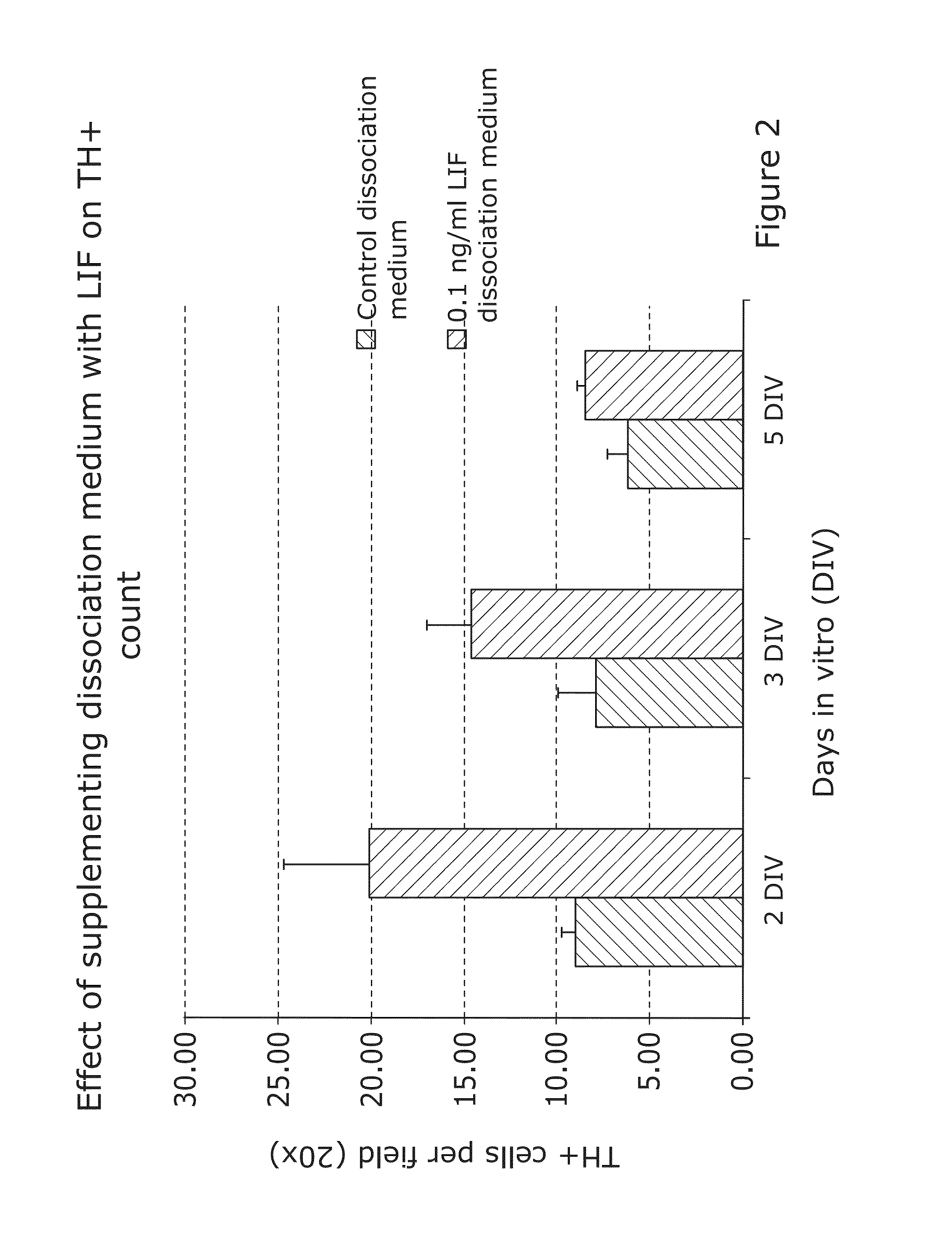
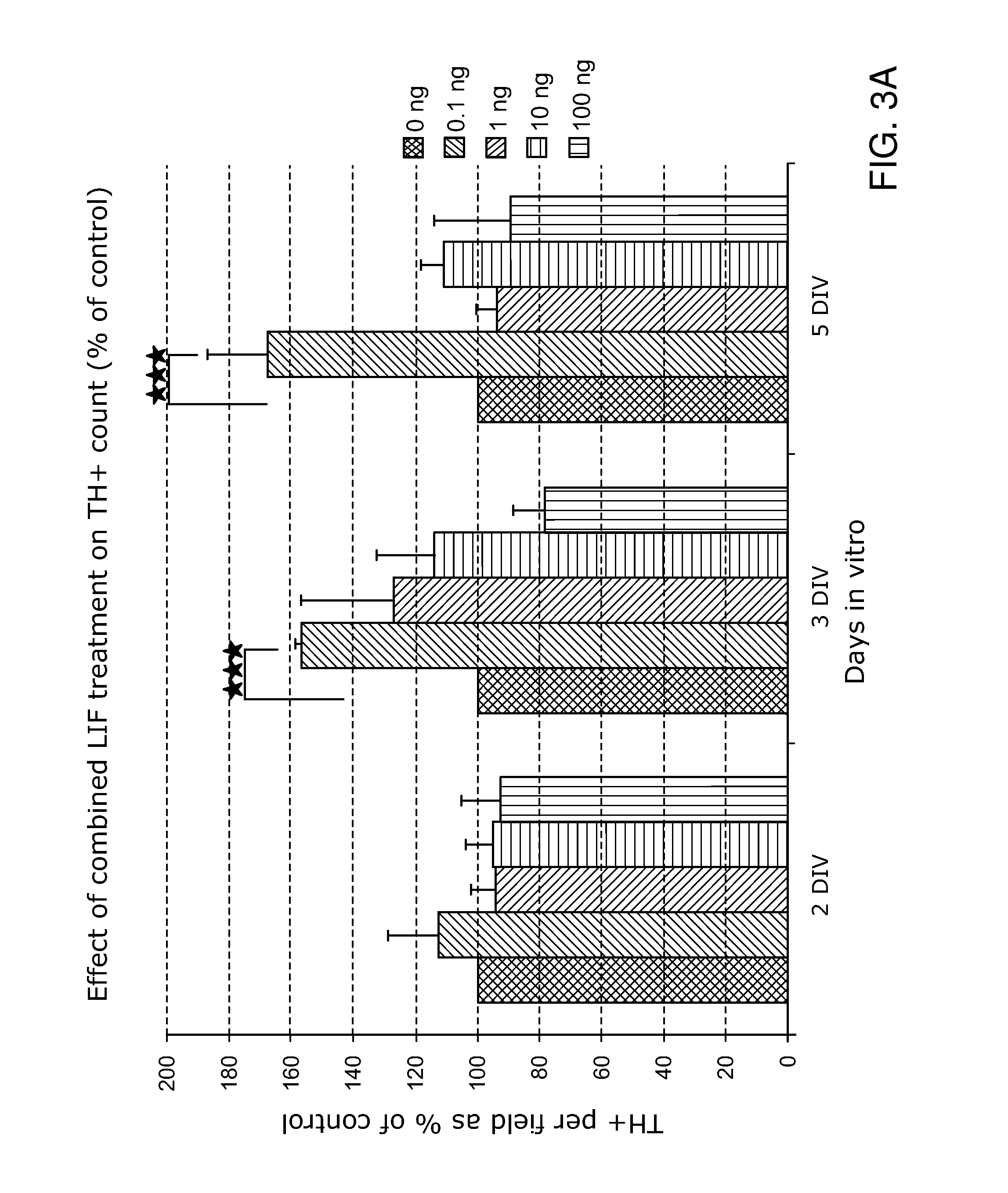
1.2 LIE Treatment during Tissue Dissociation Increases the Su...
example 2
2.1 Human: Monolayer and Neurosphere Cultures—Expansion of Cell Numbers to Provide Sufficient Cells for Testing Therapeutic LIE Nanoparticles
[0130]6-8 week old human foetal midbrain was dissected and cultured as neurospheres in proliferation medium before being sectioned and stained. Upon passage, parallel cultures as monolayer were grown in differentiation medium. Dopaminergic cells are positive for tyrosine hydroxylase (TH). Total cell numbers are stained with nuclear antigen (Hoechst). See FIG. 12, where: Primary=primary cultures; Passage 0=first subculture; Passage 1=second subculture.
[0131]Results show (i) maturation of TH+ neurons within the neurosphere microenvironment; (ii) differentiation of TH+ neurons grown in monolayer. Passage 2 also contains dopaminergic (DA) cells. These amplified cells were used to test LIF-nano effects on DA cell maturation and overall cell survival.
2.2 Human Foetal Ventral Mesencephalon LIF-Nanoparticle Therapy Enhances Human Dopaminergic Neuron De...
example 3
3.1 Treatment of Human Foetal Ventral Mesencephalon (hfVM) with LIF-Nanoparticle Therapy, or XAV939-Nanoparticle Therapy, Enhances Human Dopaminergic Neuron Derivation and Increases hfVM Cell Survival both In Vitro and In Vivo
[0133]To measure the effect of nanotherapy in vivo: hfVM cells were prepared as for the in vitro experiments as outlined in FIG. 17, primary human fetal mesencephalon tissue was stored at 4° C. for upto 4 days in Hibernate E storage medium. The cells were then seeded on coverslips and cultured 4d in differentiation medium after which cells were stained by DAPI to enumerate nuclei and for tyrosine hydroxylase to identify and enumerate differentiated dopaminergic cells. Pooled tissue was then prepared for cell transplantation following the clinical TransEuro Protocol. The protocol summarised in FIG. 18 follows that of the TransEuro clinical trial assessing hfVM cell grafts as cell therapy in patients with Parkinson's disease: http: / / www.transeuro.org.uk. The harv...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com