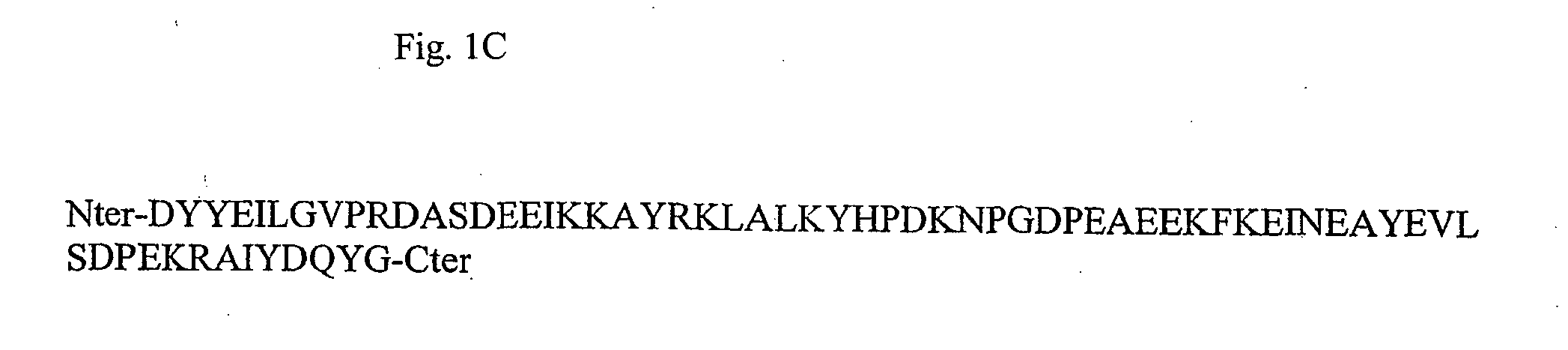Patents
Literature
32 results about "Viral Components" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Virus infection detection and identification method based on metagenomics
ActiveCN105112569AHigh detection sensitivityLow initial sample size requirementMicrobiological testing/measurementViral nucleic acidNucleic acid sequence
The invention provides a virus infection detection and identification technology based on metagenomics. The virus infection detection and identification technology based on the metagenomics comprises the four portions of sample preparation, high-throughput sequencing, bioinformatic analysis and result re-checking. In the sample preparation portion, viral nucleic acid is effectively extracted or enriched from detection samples according to the requirements of the virus infection detection and identification technology based on the metagenomics and characteristics of different types of detection samples, and a nucleic acid library which can be used for a next-generation sequencing instrument is established. In the high-throughput sequencing portion, the nucleic acid library established in the sample preparation step is sequenced so to obtain sufficient high-quality nucleic acid sequence information. In the bioinformatic analysis portion, a large number of high-quality nucleic acid sequences obtained in the high-throughput sequencing step is analyzed to further obtain viral component information prompted by the nucleic acid of the samples. In the result re-checking portion, a bioinformatic analysis result and other information, such as technical contrast, are integrated to perform comprehensive study and judgment, finally alternative infection virus is determined, and other technologies, such as PCR, are utilized to perform re-checking.
Owner:INST OF PATHOGEN BIOLOGY CHINESE ACADEMY OF MEDICAL SCI
Imaging methods and compositions comprising fluorescent dyes associated with viral components for nerve imaging
Disclosed herein are compositions and methods for imaging nerve cells. The composition comprises a fluorescent dye; and a viral component selected from a neurotropic, replication-defective virus, a viral protein of a neurotropic virus, and a capsid of a neurotropic virus. Although the fluorescent dye in itself cannot penetrate nerve cells, the fluorescent dye is bound to the viral component to form a dye / viral component complex that is capable of penetrating nerve cells.
Owner:STRYKER CORP
Imaging methods and compositions comprising fluorescent dyes associated with viral components for nerve imaging
Disclosed herein are compositions and methods for imaging nerve cells. The composition comprises a fluorescent dye; and a viral component selected from a neurotropic, replication-defective virus, a viral protein of a neurotropic virus, and a capsid of a neurotropic virus. Although the fluorescent dye in itself cannot penetrate nerve cells, the fluorescent dye is bound to the viral component to form a dye / viral component complex that is capable of penetrating nerve cells.
Owner:STRYKER EUROPEAN OPERATIONS LIMITED
Imaging methods and compositions comprising fluorescent dyes associated with viral components for nerve imaging
InactiveUS20120078093A1Reduce riskViral/bacteriophage medical ingredientsViruses/bacteriophagesMedicineFluorescence
Owner:NOVADAQ TECH ULC
Therapy of respiratory influenza virus infection using free and liposome-encapsulated ribonucleotides
InactiveUS6544958B2Sugar derivativesMicrobiological testing/measurementRibonucleotide synthesisLiposome
Owner:HER MAJESTY THE QUEEN AS REPRESENTED BY THE MINIST OF NAT DEFENCE OF HER MAJESTYS CANADIAN GOVERNMENT
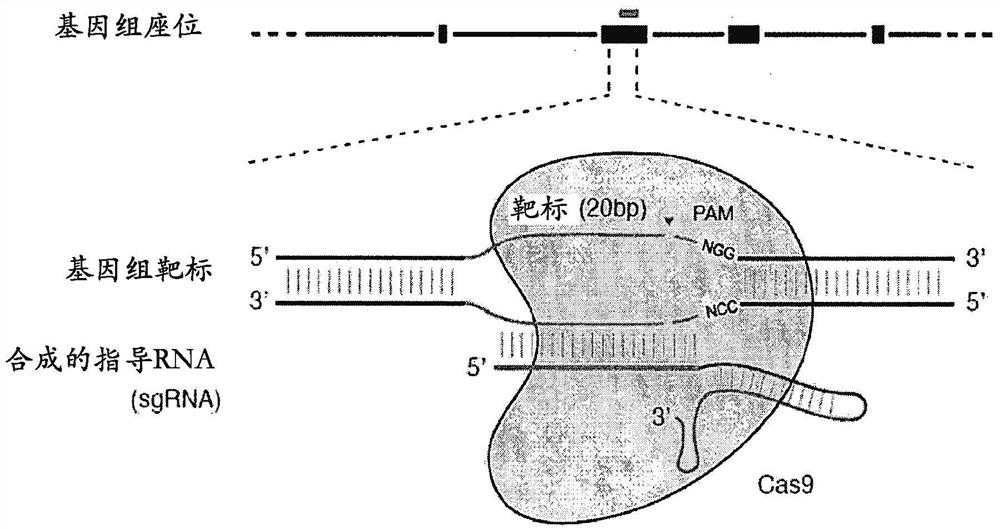
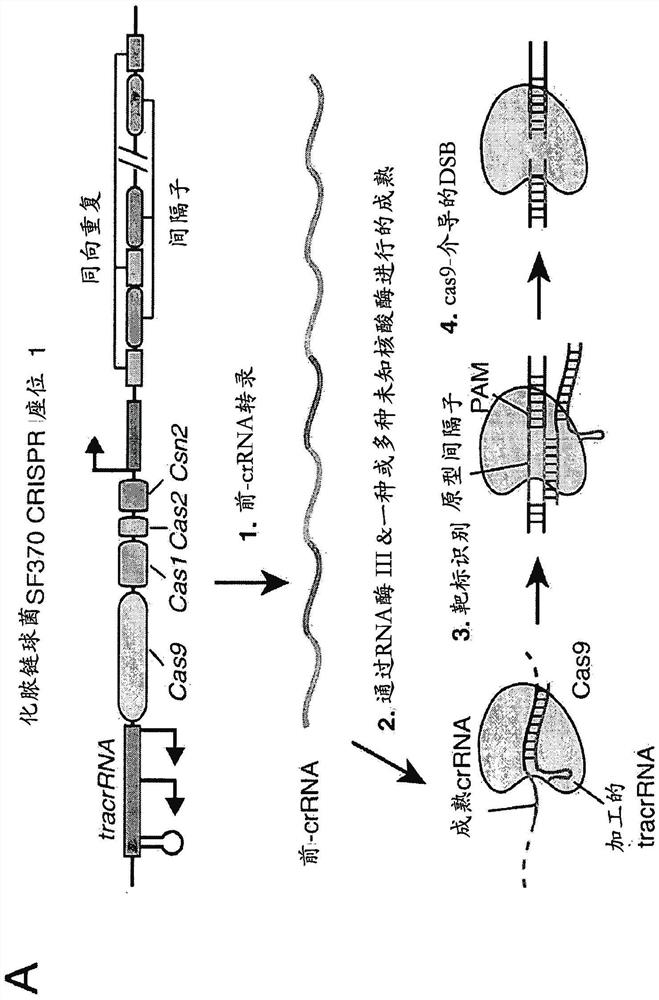
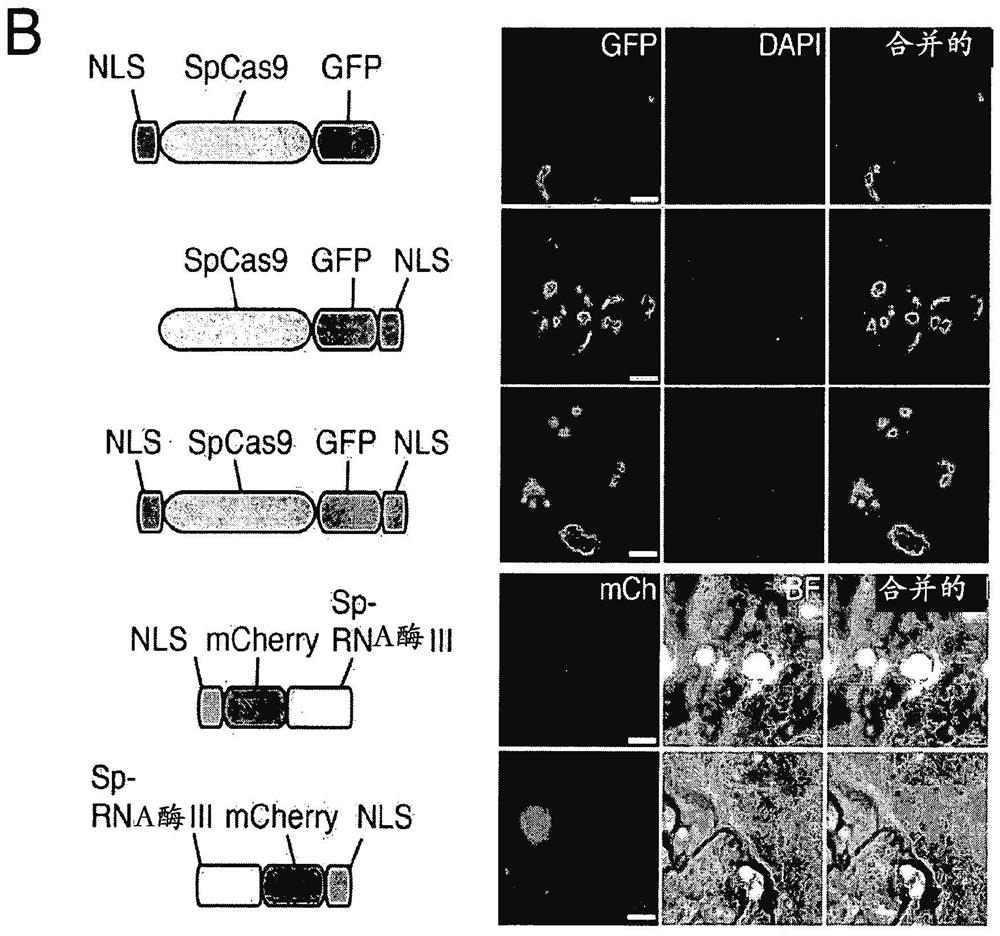
Delivery, use and therapeutic applications of the crispr-cas systems and compositions for targeting disorders and diseases using viral components
ActiveCN105793425AAvoid off-target bindingAvoid side effectsHydrolasesGenetic material ingredientsDiseaseVector system
The invention provides for delivery, engineering and optimization of systems, methods, and compositions for manipulation of sequences and / or activities of target sequences. Provided are delivery systems and tissues or organ which are targeted as sites for delivery. Also provided are vectors and vector systems some of which encode one or more components of a CRISPR complex, as well as methods for the design and use of such vectors. Also provided are methods of directing CRISPR complex formation in eukaryotic cells to ensure enhanced specificity for target recognition and avoidance of toxicity and to edit or modify a target site in a genomic locus of interest to alter or improve the status of a disease or a condition.
Owner:THE BROAD INST INC +2
Transfection, storage and transfer of male germ cells for generation of trangenic species
A composition for in vivo transfection of vertebrate male germ cells comprises a nucleic acid or transgene, and a gene delivery system, and optionally a protective internalizing agent, such as an endosomal lytic agent, a virus or a viral component, which is internalized by cells along with the transgene and which enhances gene transfer through the cytoplasm to the nucleus of the male germ cell. A pharmaceutical preparation and a transfer kit utilize the composition. A method for introducing a polynucleotide into vertebrate male germ cells comprises the administration of the composition to a vertebrate. A method for isolating or selecting transfected cells utilizes a reporter gene, and a method for administering transfected male germ cells utilizes male germ cells which have been transfected in vitro.
Owner:WINSTON ROBERT +1
Systems and Methods for Virus Propagation in Cell Cultures for Vaccine Manufacture
ActiveUS20150064768A1Improve the immunityEliminate wasteSsRNA viruses negative-senseBioreactor/fermenter combinationsInfected cellSystems design
The present invention provides a closed system to propagate virus-infected cells without the effect of shear force, while providing quicker access to nutrients than is available conventionally. This system design allows for a high density of infected cell growth to increase the virus yields and to maintain homogeneity of the contents of the main container. The system further provides a nuclease to degrade the cellular DNA prior for purification of the virus or viral components. As the system is designed for maximum containment at low risk, the live virus can be a hazardous virus such as a Bio-safety Level 3 (BSL 3), BSL 4 or BSL5 virus.
Owner:INVENTPRISE INC
Formulations for therapeutic viruses having enhanced storage stability
Therapeutic viral formulations having enhanced storage stability are described. The formulations comprise a viral vector in addition to one or more of an aqueous cosolvent, a reversible viral-encoded protease inhibitor and a mild reducing agent or other agent that prevents specific degradation of viral components.
Owner:CELLS GENESYS INC
Novel Anti-Viral Method
ActiveUS20120039978A1Reduce infectivitySsRNA viruses negative-sensePowder deliveryLipid formationBiological activation
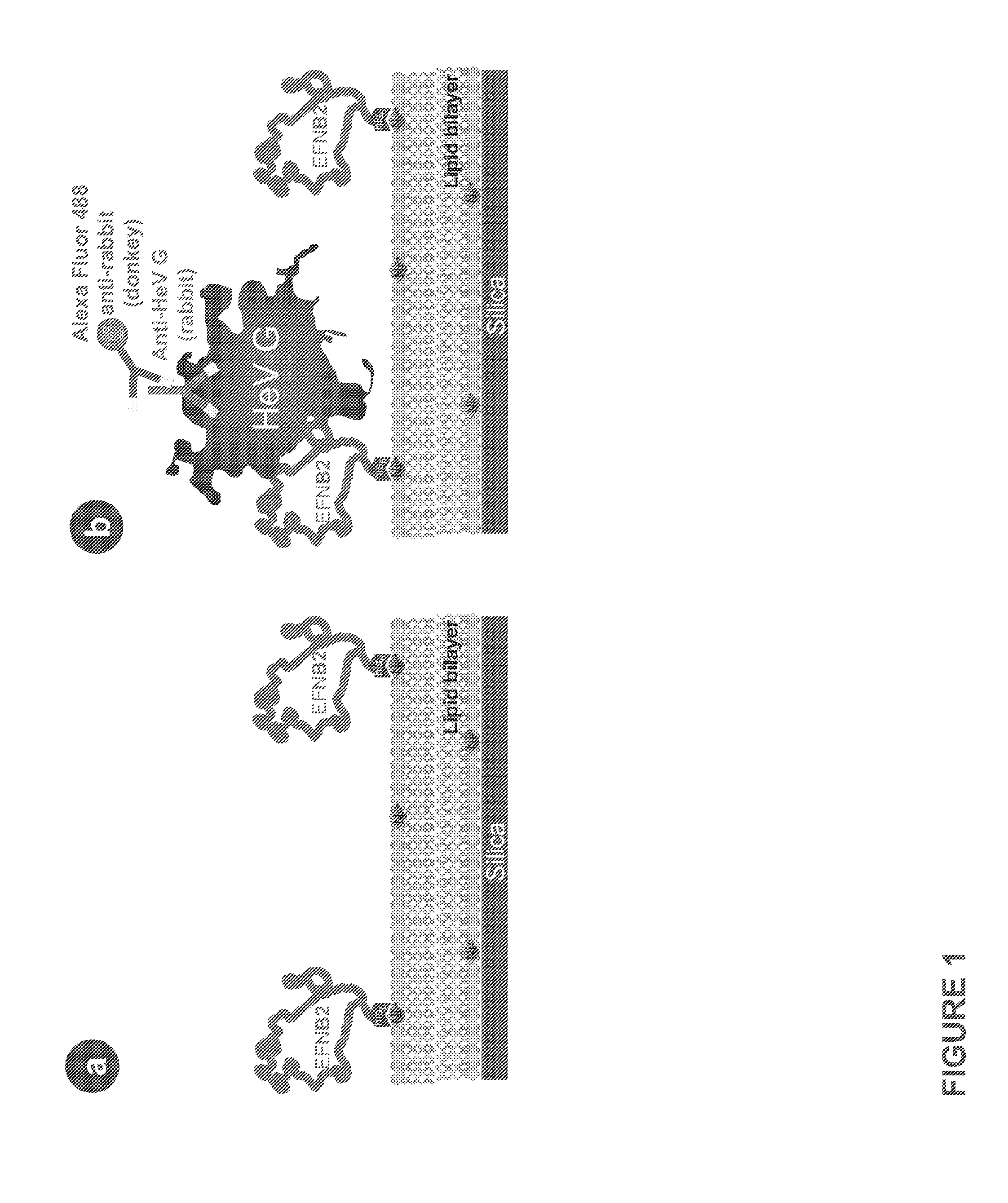
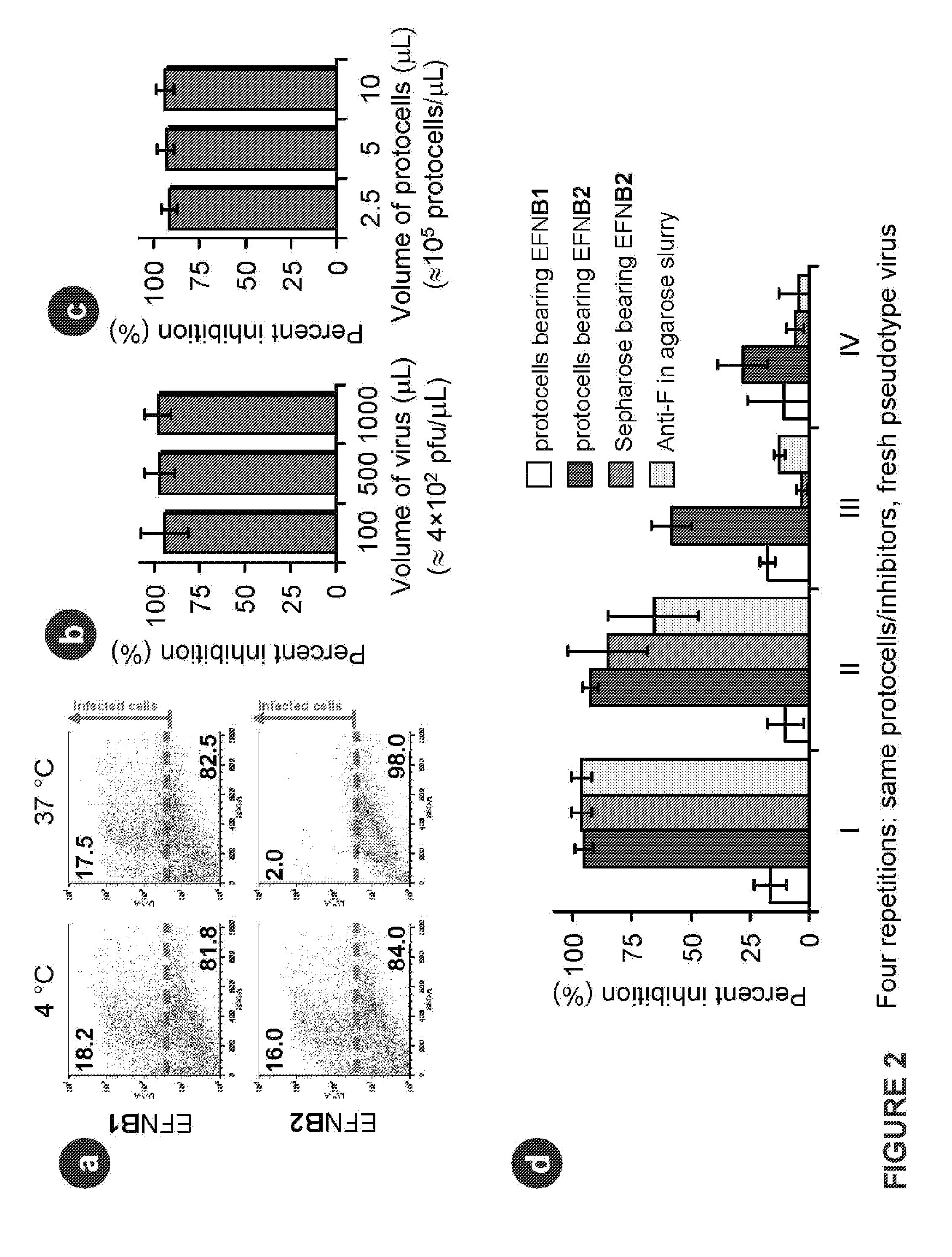
The invention provides a method of reducing viral infectivity in a sample compnsing contacting the sample with a earner matrix wherein lipids are attached to the carrier matrix, and wherein at least one virus-specific agent is attached to the lipids The virus-specific agent is capable of binding to at least one viral component The virus specific agent may be a receptor The earner matrix may be a silica particle, which may be coated with a lipid bilayer The invention further provides a method of inactivating a virus comprising contacting the virus with a earner matrix wherein lipids are attached to the earner matrix, and wherein at least one receptor is attached to the lipids, binding the receptor to at least one viral receptor-binding protein, and activating at least one viral fusion protein, wherein activation inactivates the virus, and releases the virus from the earner matrix
Owner:CORNELL UNIVERSITY +1
Macrocyclic lactone combination compositions, vaccines and methods for producing same
ActiveUS20100266628A1Sufficient amountQuick cureAntibacterial agentsOrganic active ingredientsDiseaseRotavirus RNA
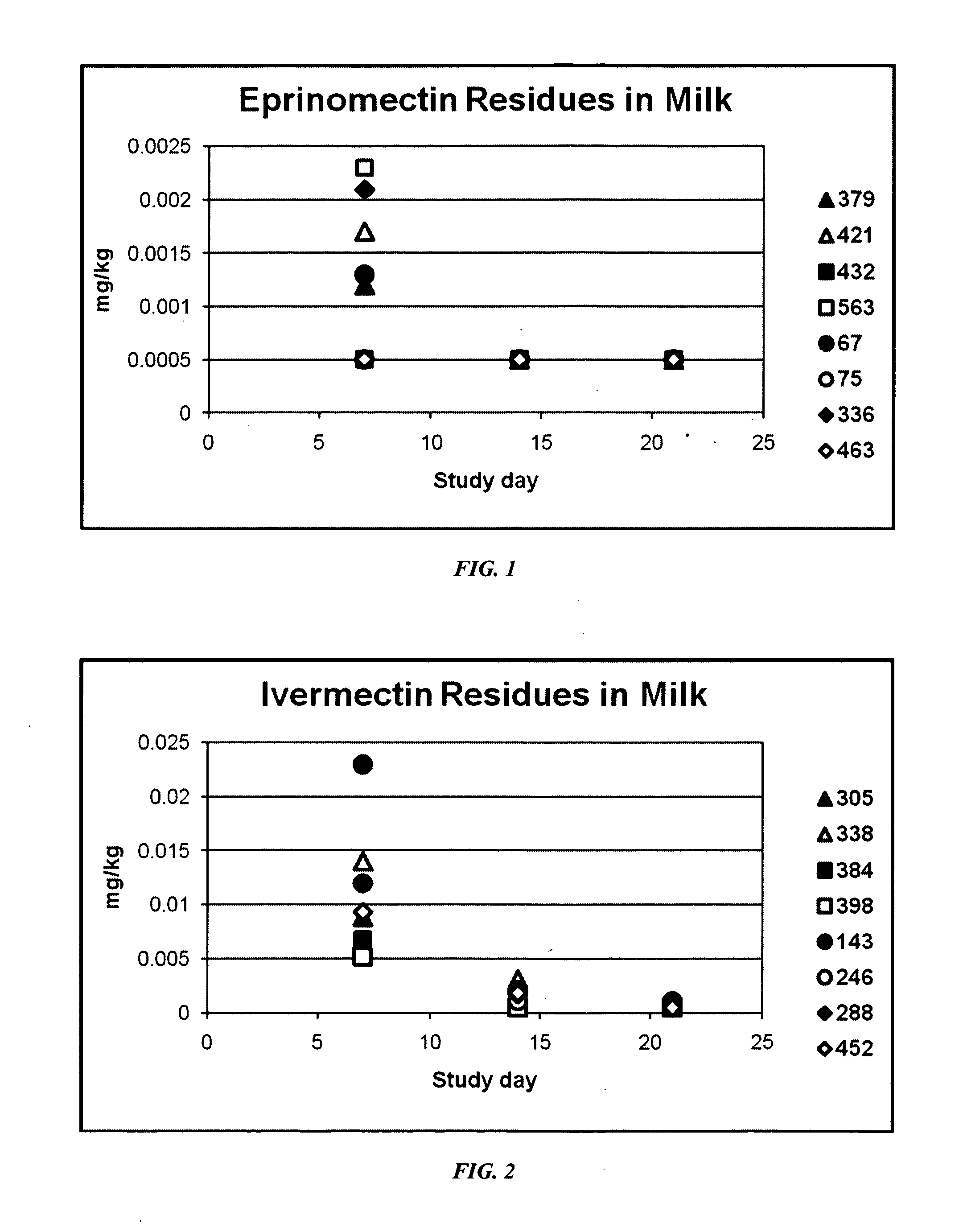
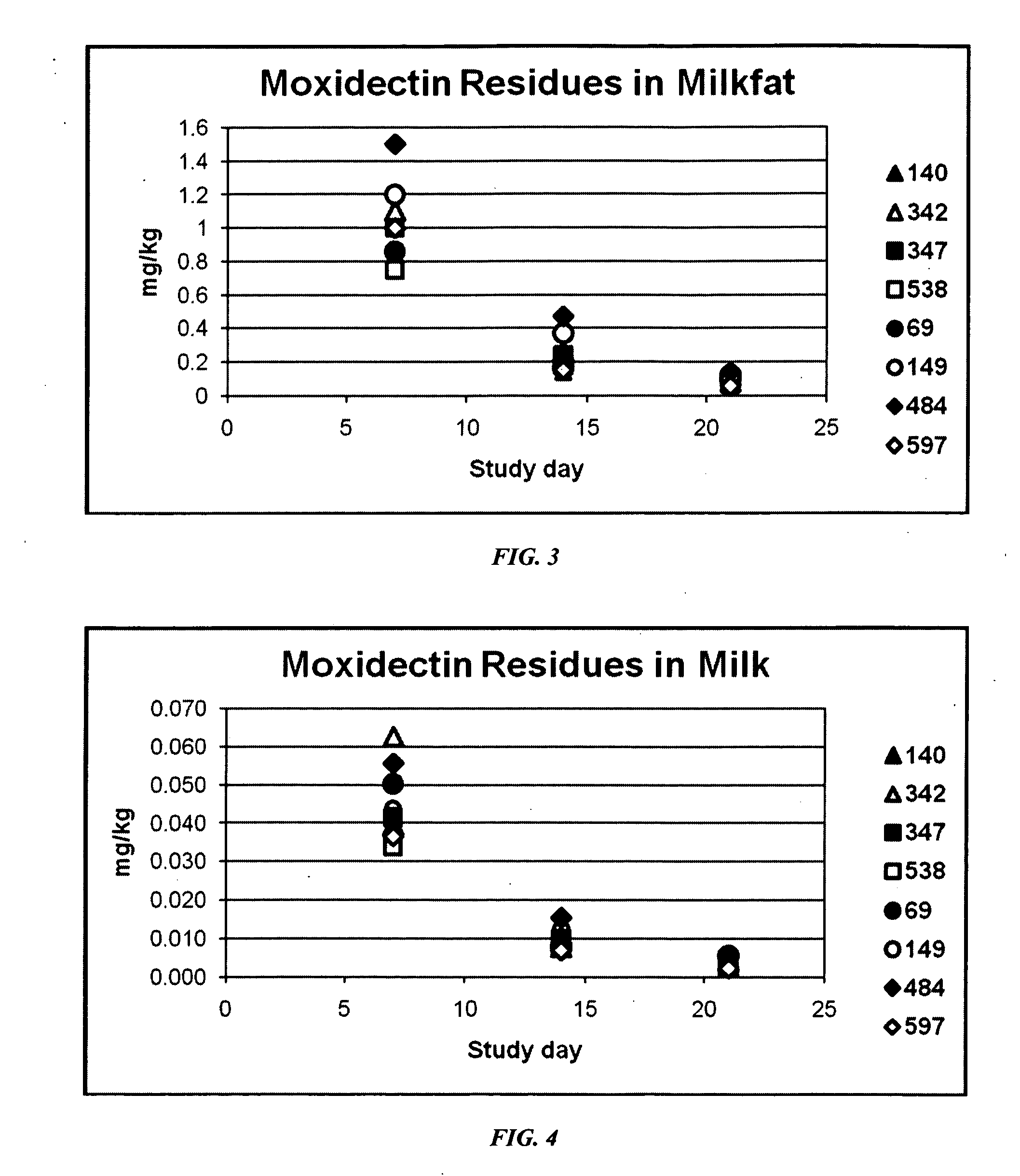
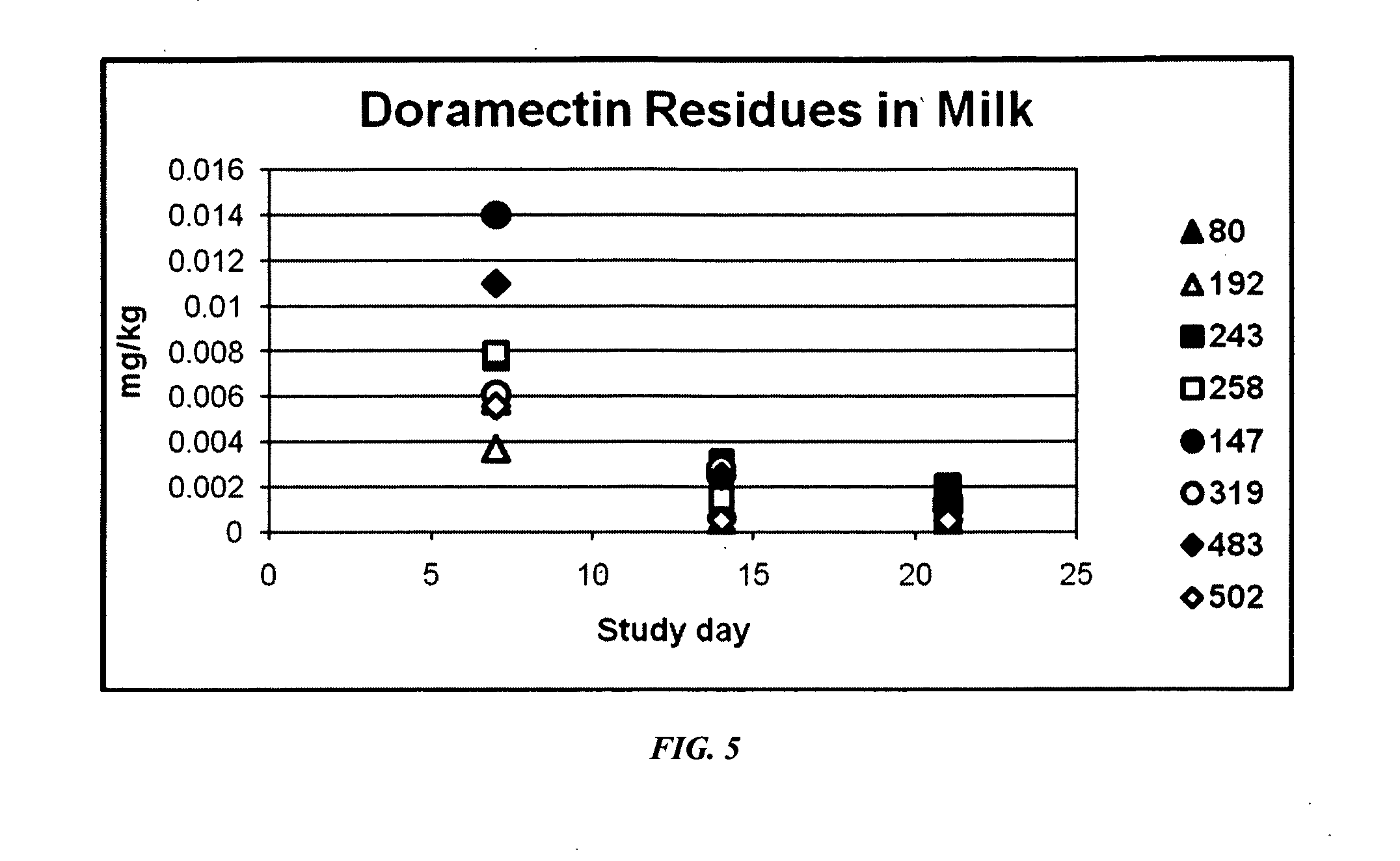
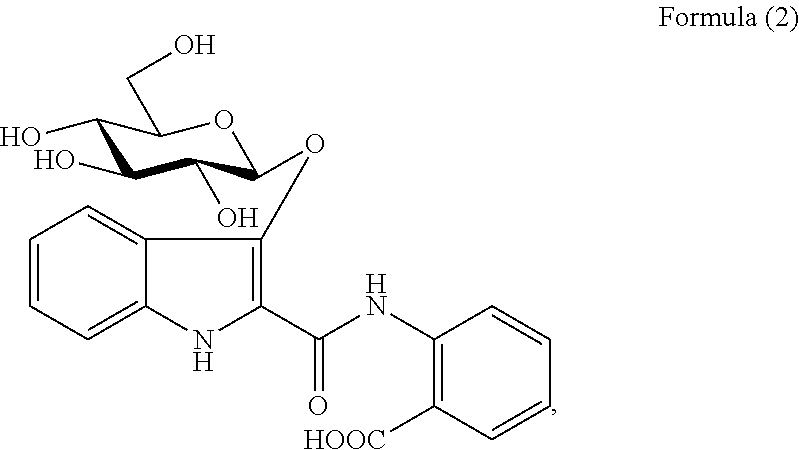
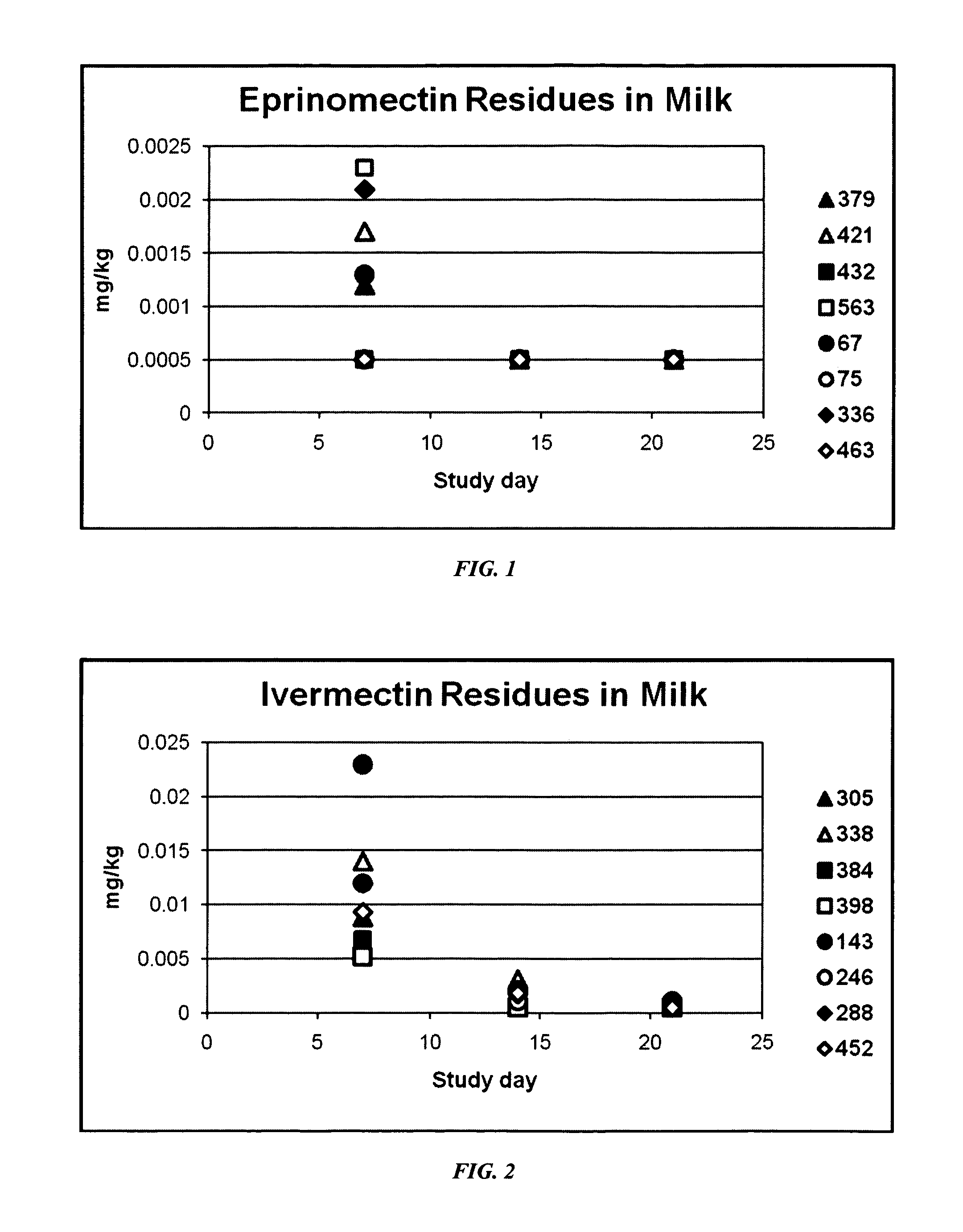
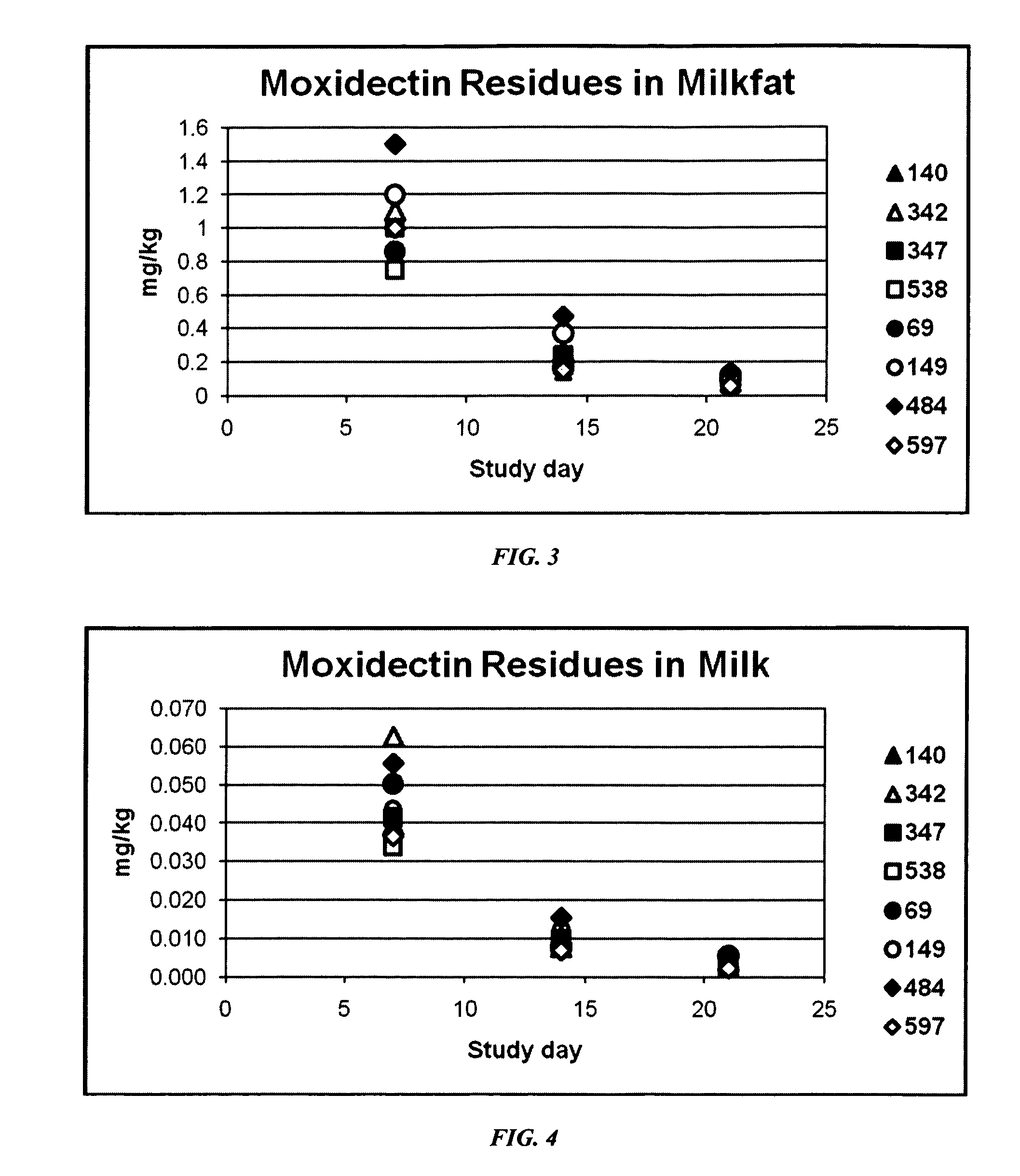
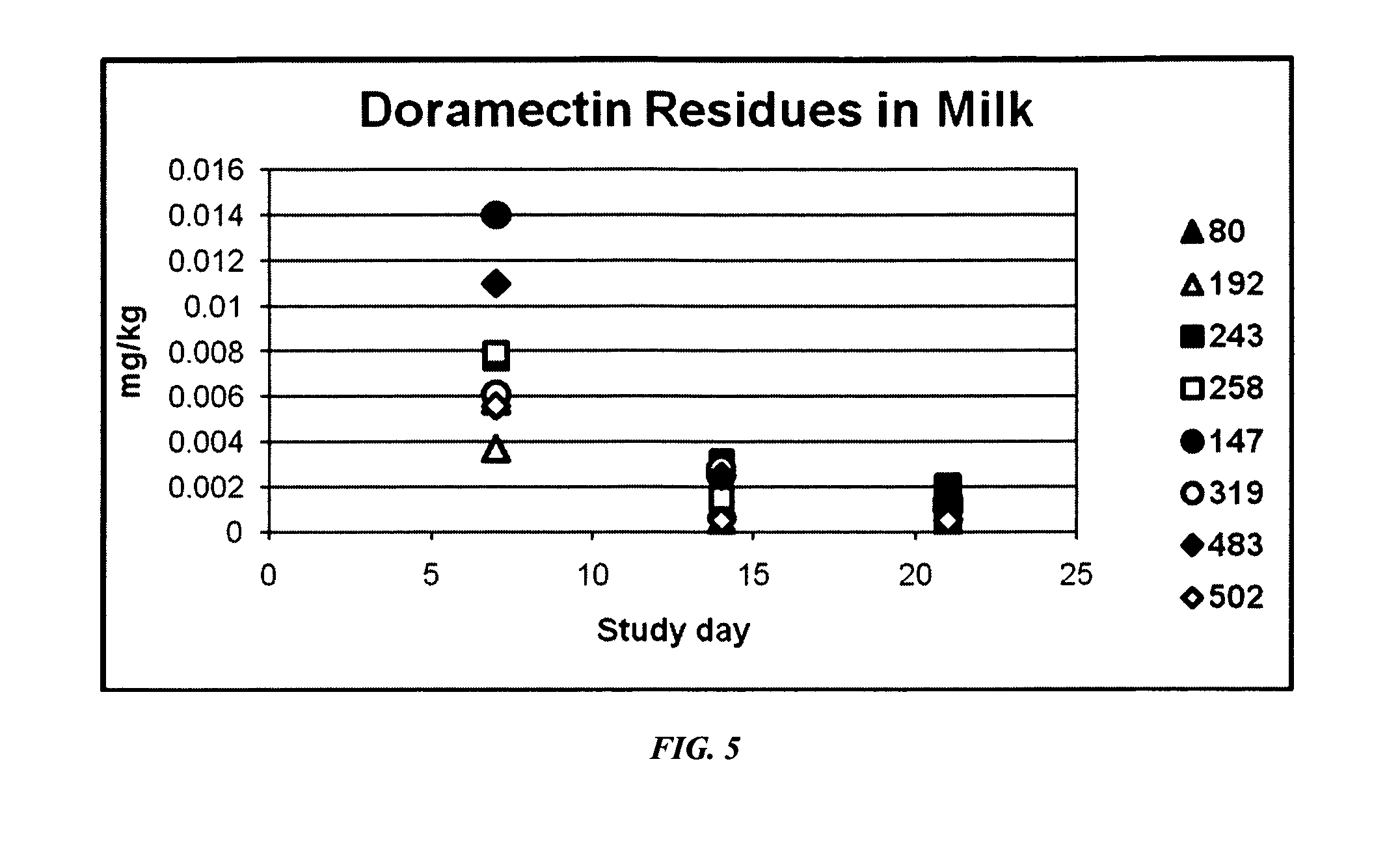
An injectable composition, capable of preventing or controlling parasitic, viral, or bacterial infections or diseases, for example scours, in pregnant cows and viral infections or diseases in neonatal calves by parenterally administering to each cow in a herd of pregnant cows, a dose of a combination composition comprising: (a) at least one inactivated viral component derived from rotavirus and / or coronavirus; (b) a macrocyclic lactone active compound; and (c) a pharmaceutically acceptable parenteral carrier and preservative. The injectable compositions which include eprinomectin result in extremely low milk residues.
Owner:MERIAL INC
Adjuvant compositions
InactiveUS20090047306A1Augmenting alum/MF59-inducedBalanced adjuvant activity profileBacterial antigen ingredientsOrganic chemistryAdjuvantGlycolipid
An adjuvant composition comprises a Th1-activating alkaloid, optionally further comprising an auxiliary adjuvant selected from a type 2 adjuvant (e.g. alum and / or MF59), a type 1 adjuvant and / or a balanced adjuvant. Vaccines comprising the adjuvant composition include nucleic acid(s) which encode one or more antigenic protein(s); protein(s) or peptide(s); glycoprotein(s); polysaccharide(s) (e.g. carbohydrate(s)); fusion protein(s); lipid(s); glycolipid(s); peptide mimic(s) of polysaccharides carbohydrate(s) and a protein(s) in admixture; carbohydrate-protein conjugate(s); cells or extracts thereof; dead or attenuated cells or extracts thereof; tumour cells or extracts thereof; viral particles (e.g. attenuated viral particles or viral components); allergen(s) mixtures thereof.
Owner:SUMMIT
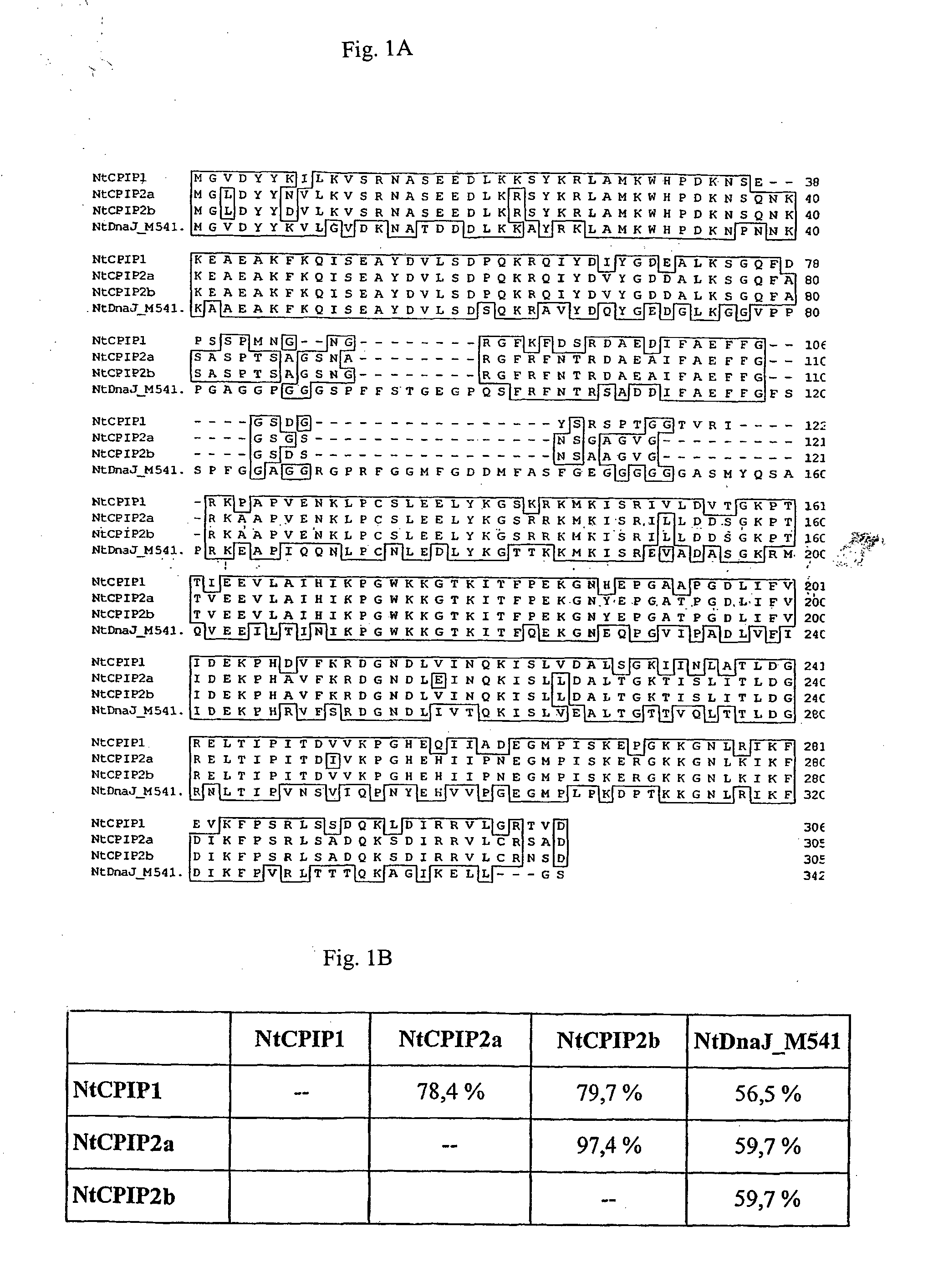
Method for the production of transgenic plants with increased virus resistance by silencing vegetable DnaJ-like proteins
InactiveUS20050251879A1Good antiviral effectEasily put into practiceOther foreign material introduction processesFermentationPlant cellGMO Plants
The invention relates to plants and plant cells which have a transient or permanent virus resistance as a result of modulation of the gene expression and / or binding behavior of vegetable DnaJ-like proteins. The invention also relates to methods for the production of transgenic plants with increased virus resistance, wherein the expression of vegetable DnaJ-like proteins which interact with viral components is substantially prevented by silencing the DnaJ-like proteins. The invention further relates to methods for the production of transgenic plants with increased virus resistance, wherein the interaction of viral components with vegetable DnaJ-like proteins is substantially prevented by expression of dominant-negative mutants of the DnaJ-like proteins, by antibodies against DnaJ-like proteins or by specific inhibitors.
Owner:HOFIUS DANIEL +2
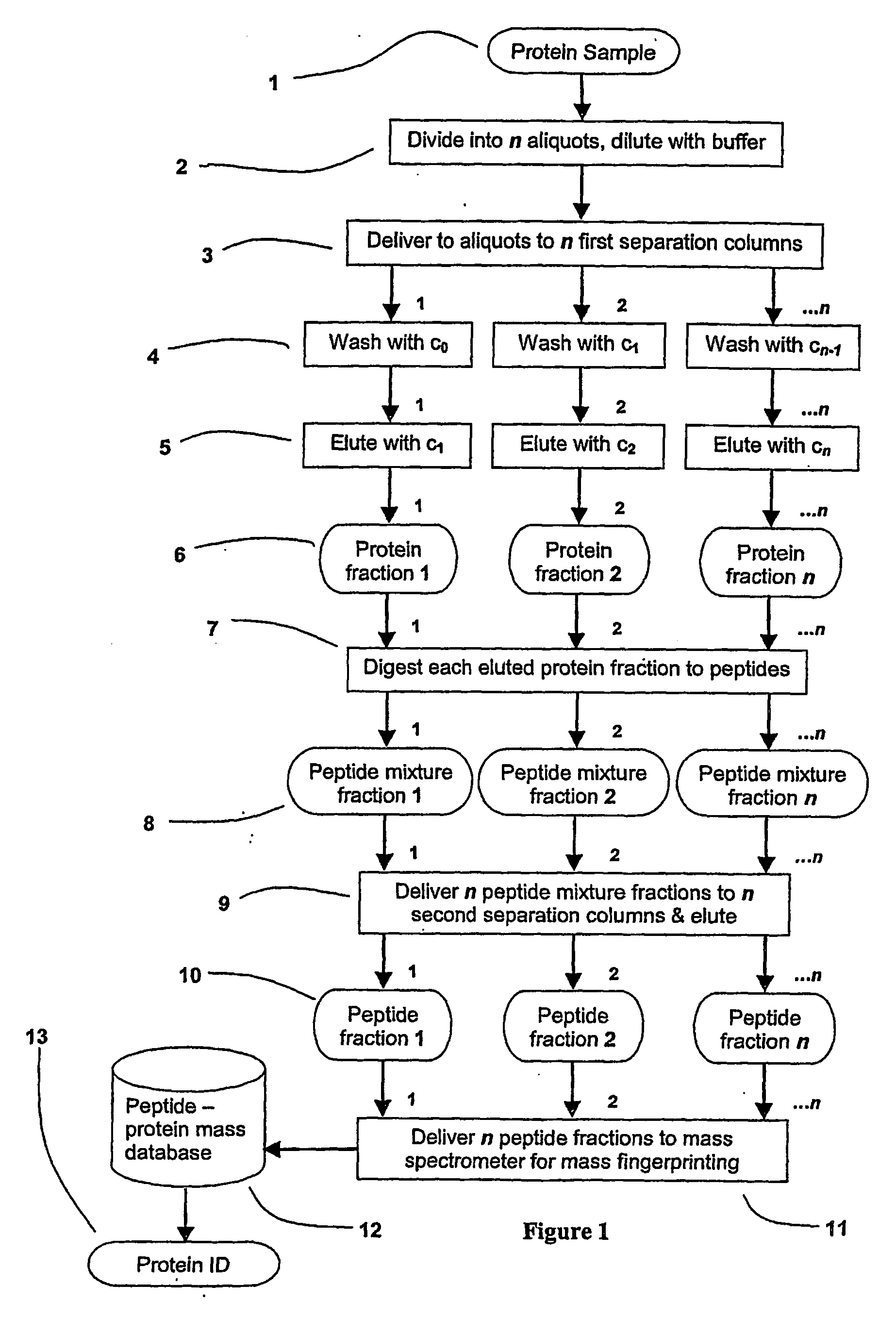
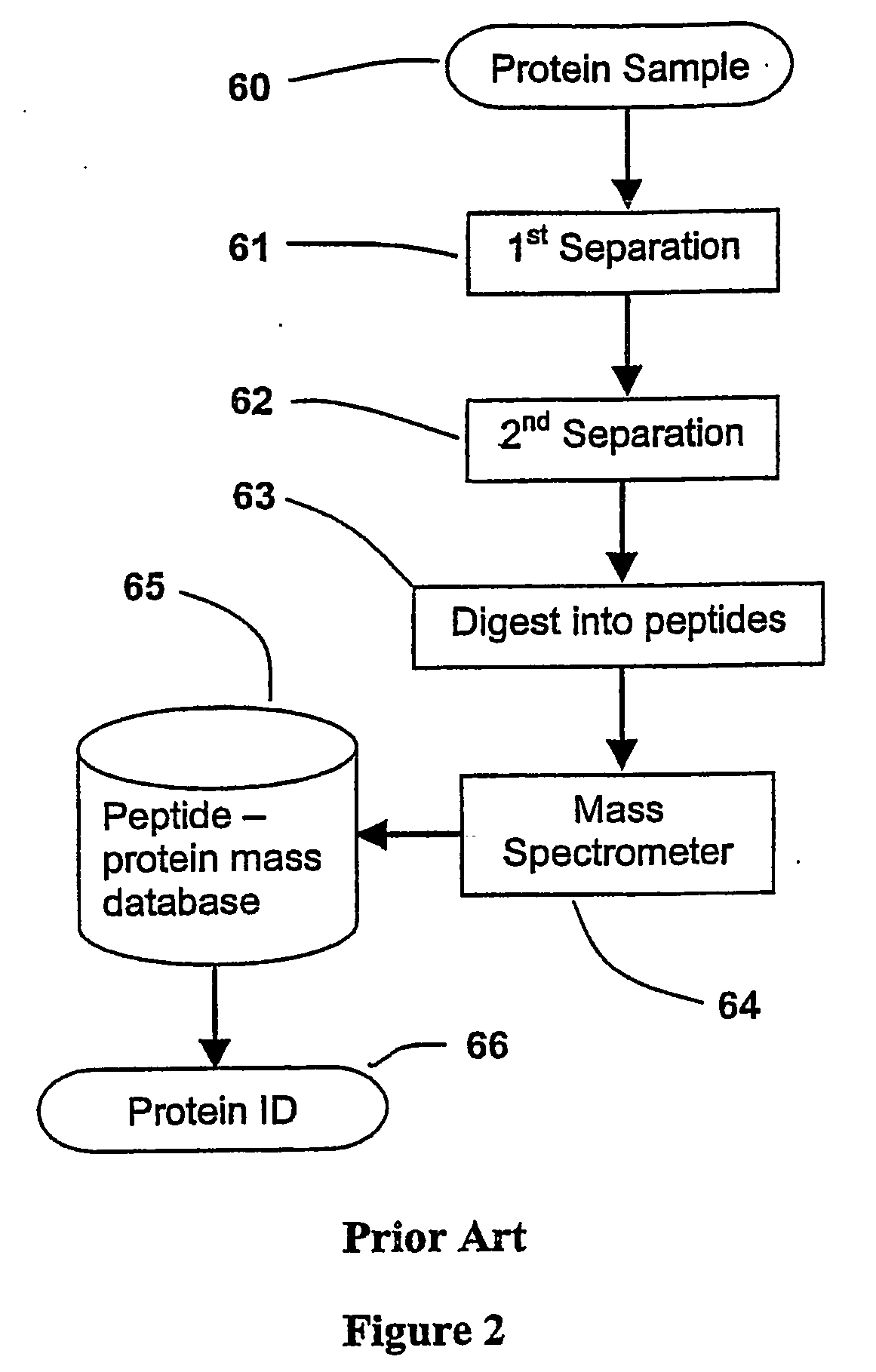
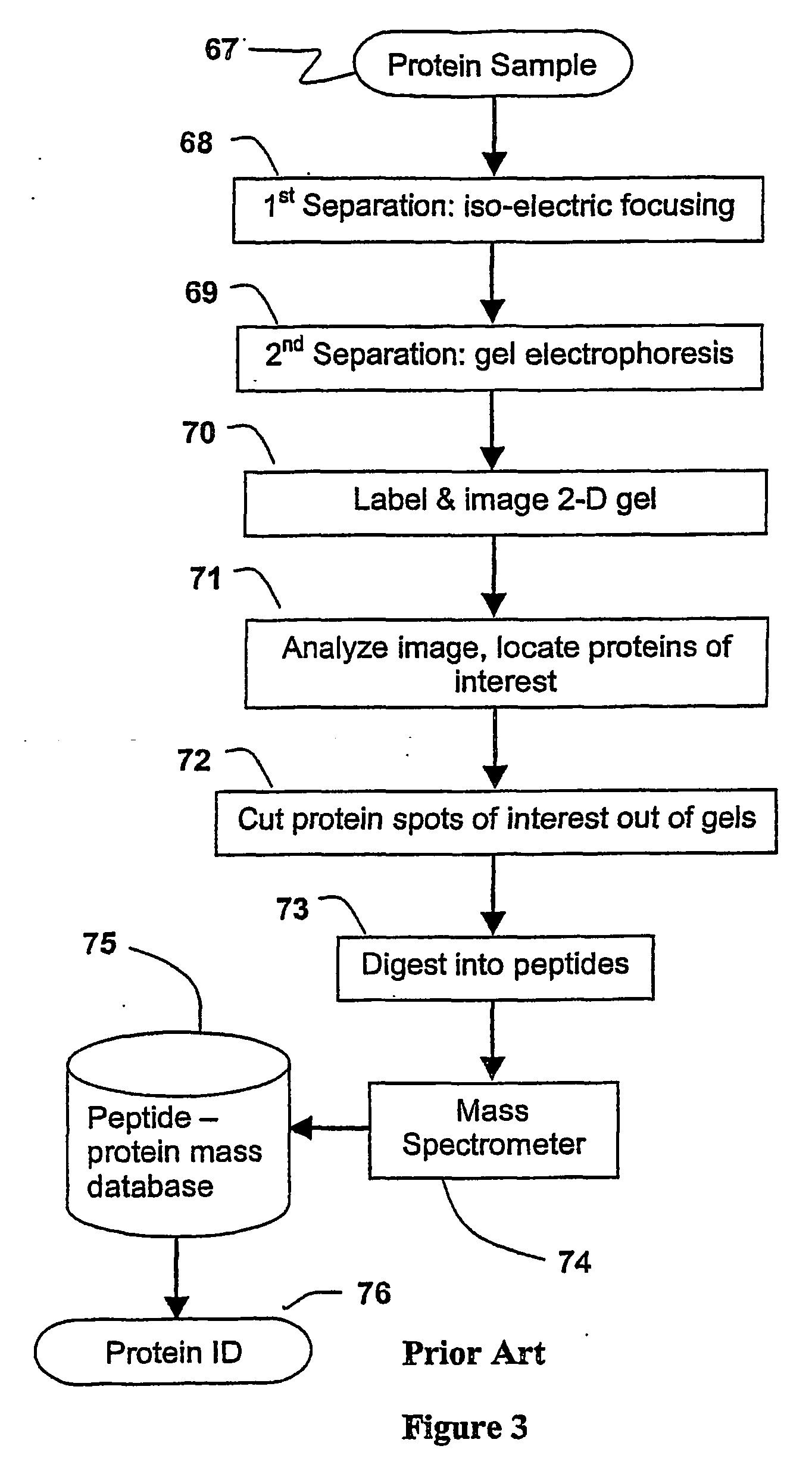
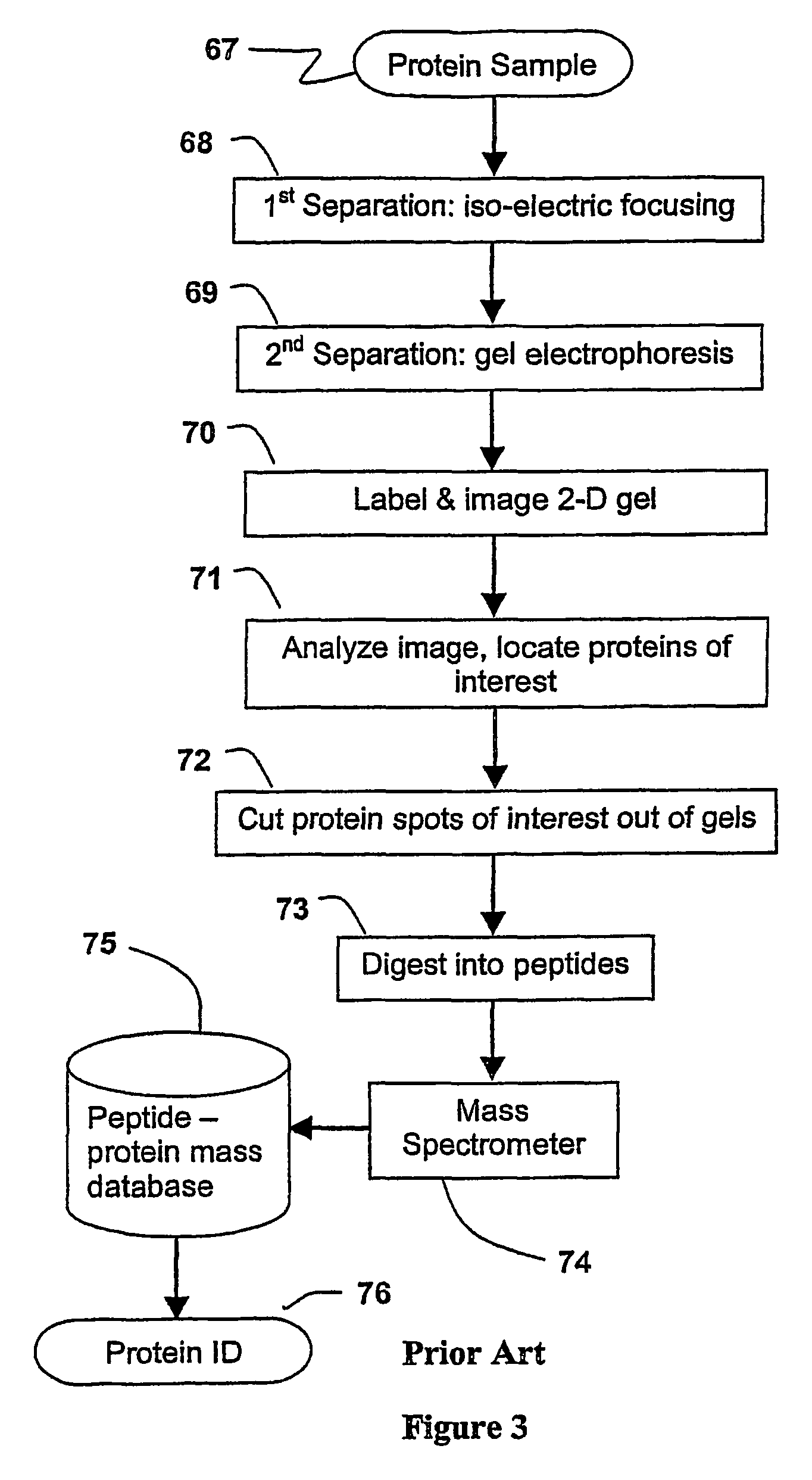
Parallel process for protein or virus separation from a sample
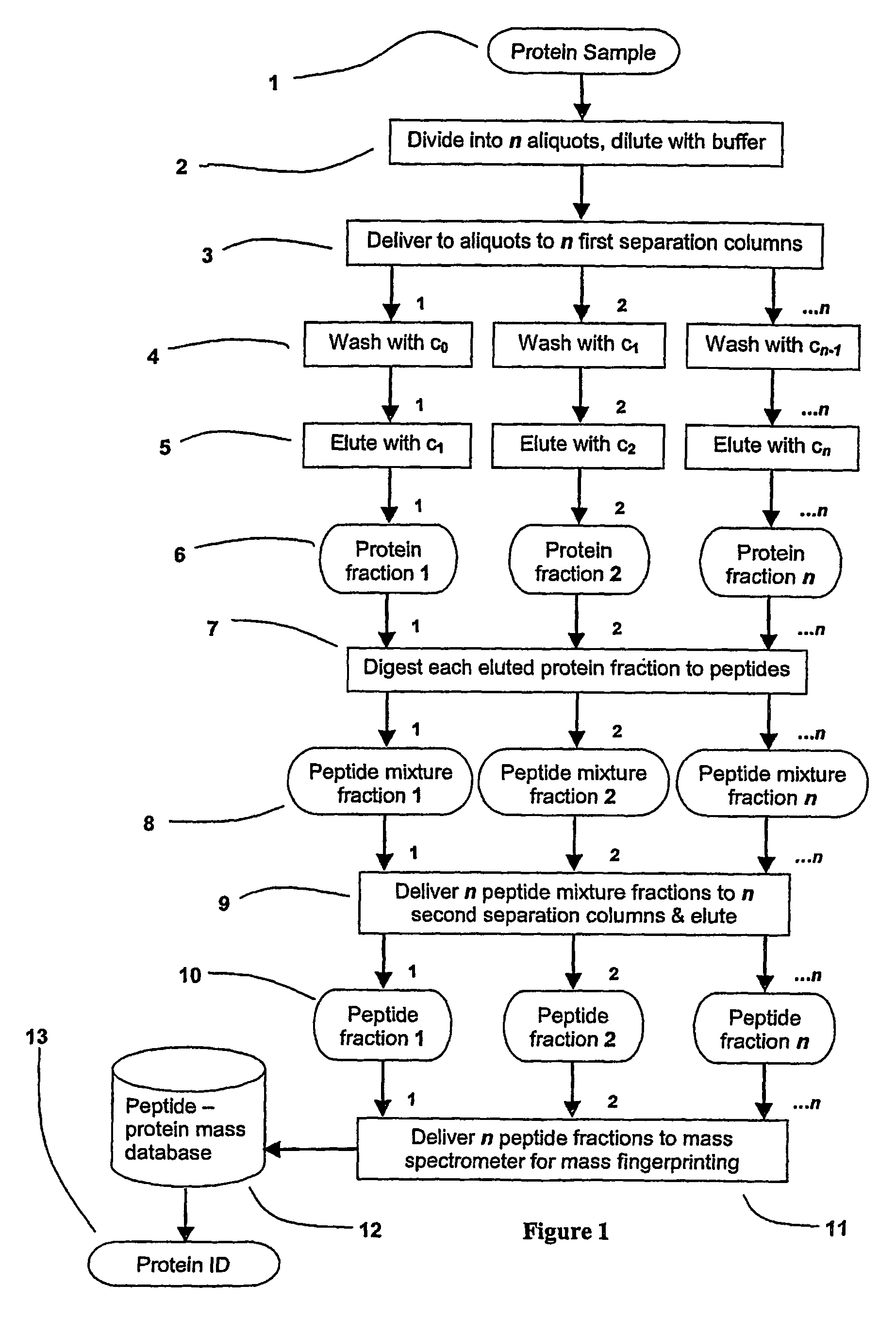
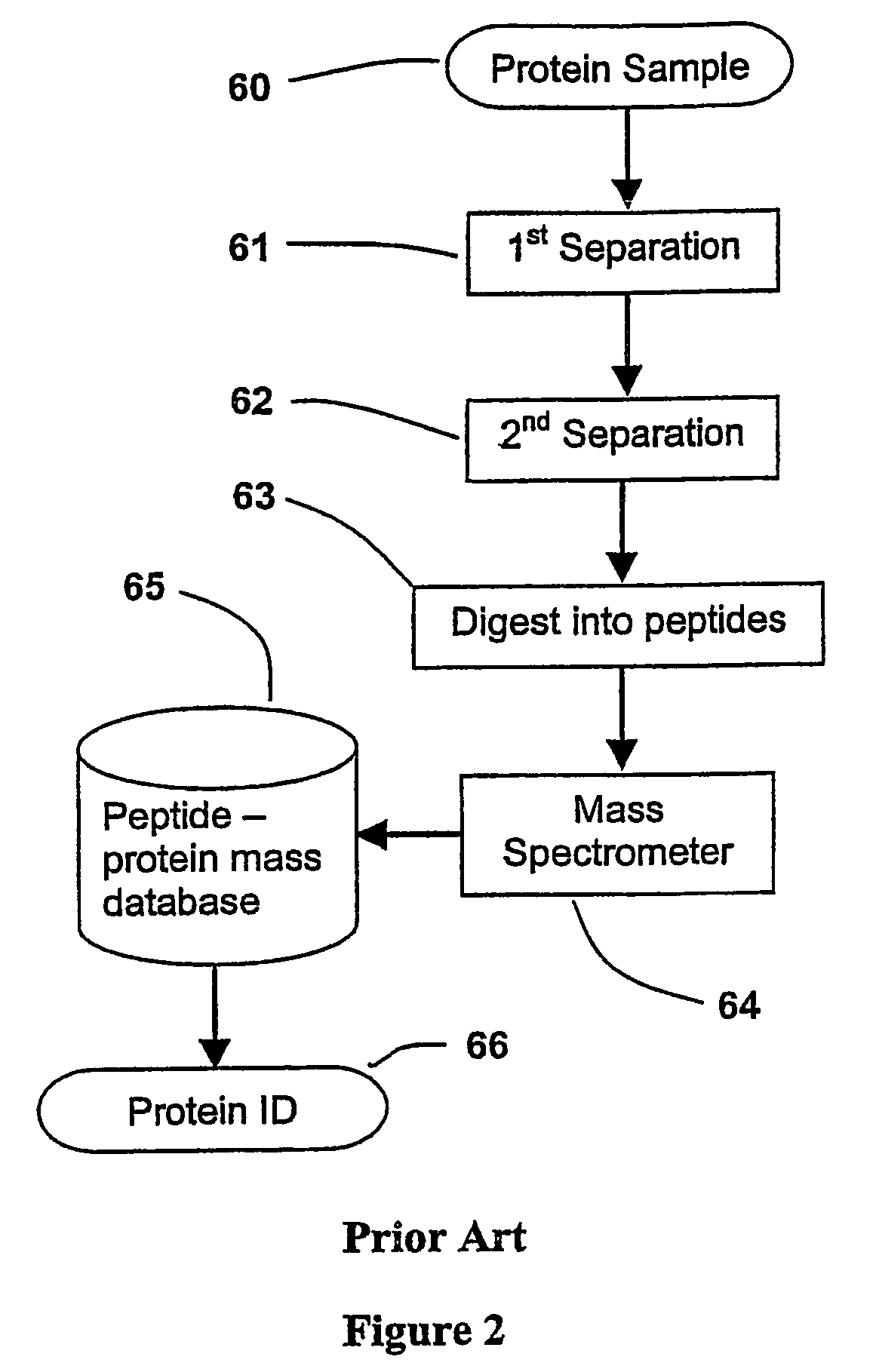
InactiveUS20070020616A1Improve efficiencyImprove automationSamplingComponent separationThroughputAutomation
A sample is divided into a series of aliquots with the aliquots being subjected to at least two successive parallel separation steps in order to resolve protein or viral components thereof. The separation steps are performed not only on a sample but subsamples each containing a prelabeled tag to afford comparisons between subsamples. The parallel separation is amenable to high throughput and automation.
Owner:PERKINELMER HEALTH SCIENCES INC
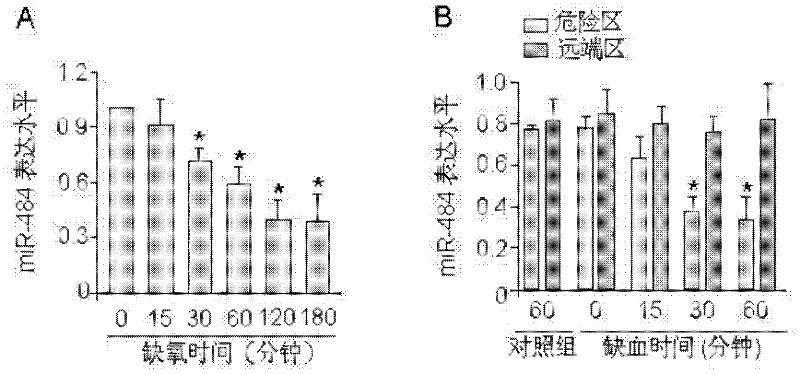
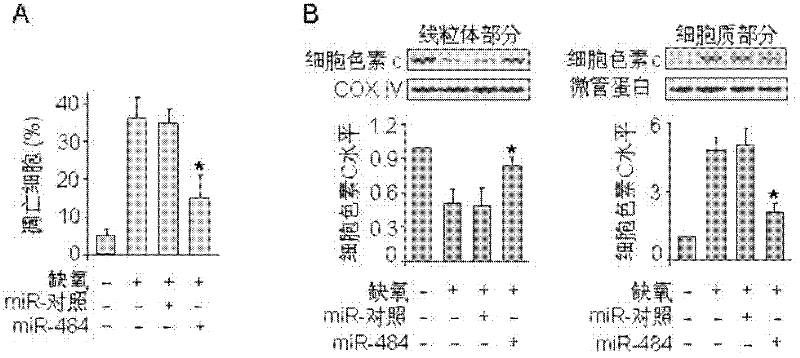
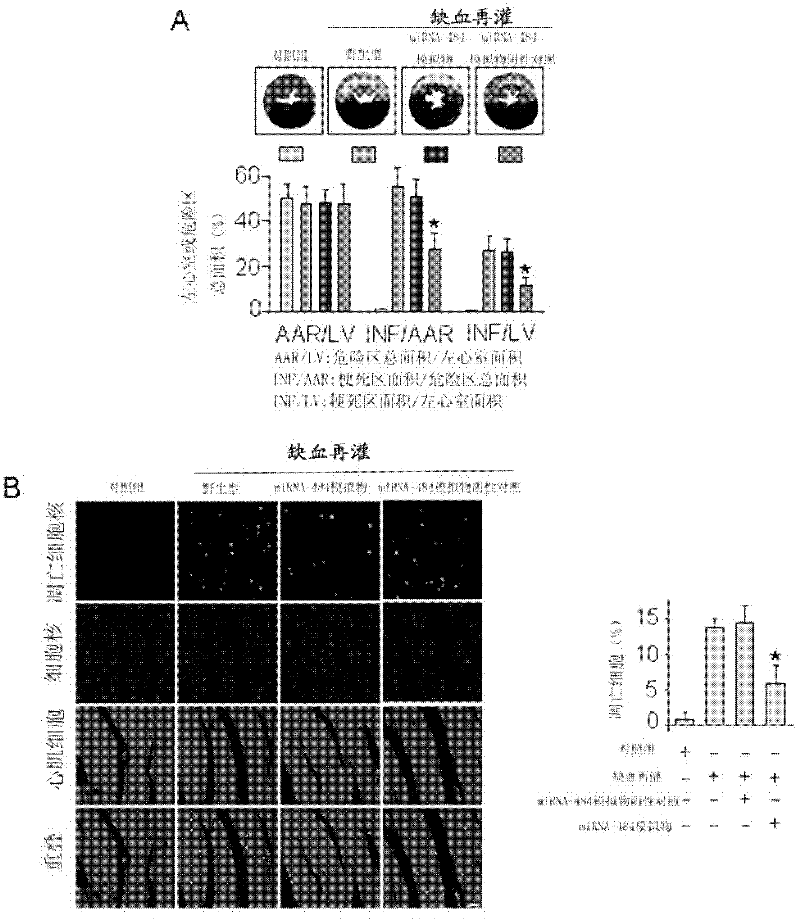
Novel application of miRNA-484 and pharmaceutical composition containing miRNA-484 and its application
InactiveCN102266570AProtectiveReduce myocardial infarct sizeGenetic material ingredientsGene therapyDiseaseApoptosis
The invention provides a new application of miRNA-484, a pharmaceutical composition containing miRNA-484 and the application thereof. The use of the miRNA-484 in the preparation of medicines for preventing or treating heart disease, the pharmaceutical composition, the pharmaceutical composition is composed of an effective dose of miRNA-484 pharmaceutically acceptable carrier, virus or auxiliary material. miRNA-484 has a protective effect on the heart by inhibiting cardiomyocyte apoptosis, and miRNA-484 has potential preventive and therapeutic value for many heart diseases.
Owner:INST OF ZOOLOGY CHINESE ACAD OF SCI
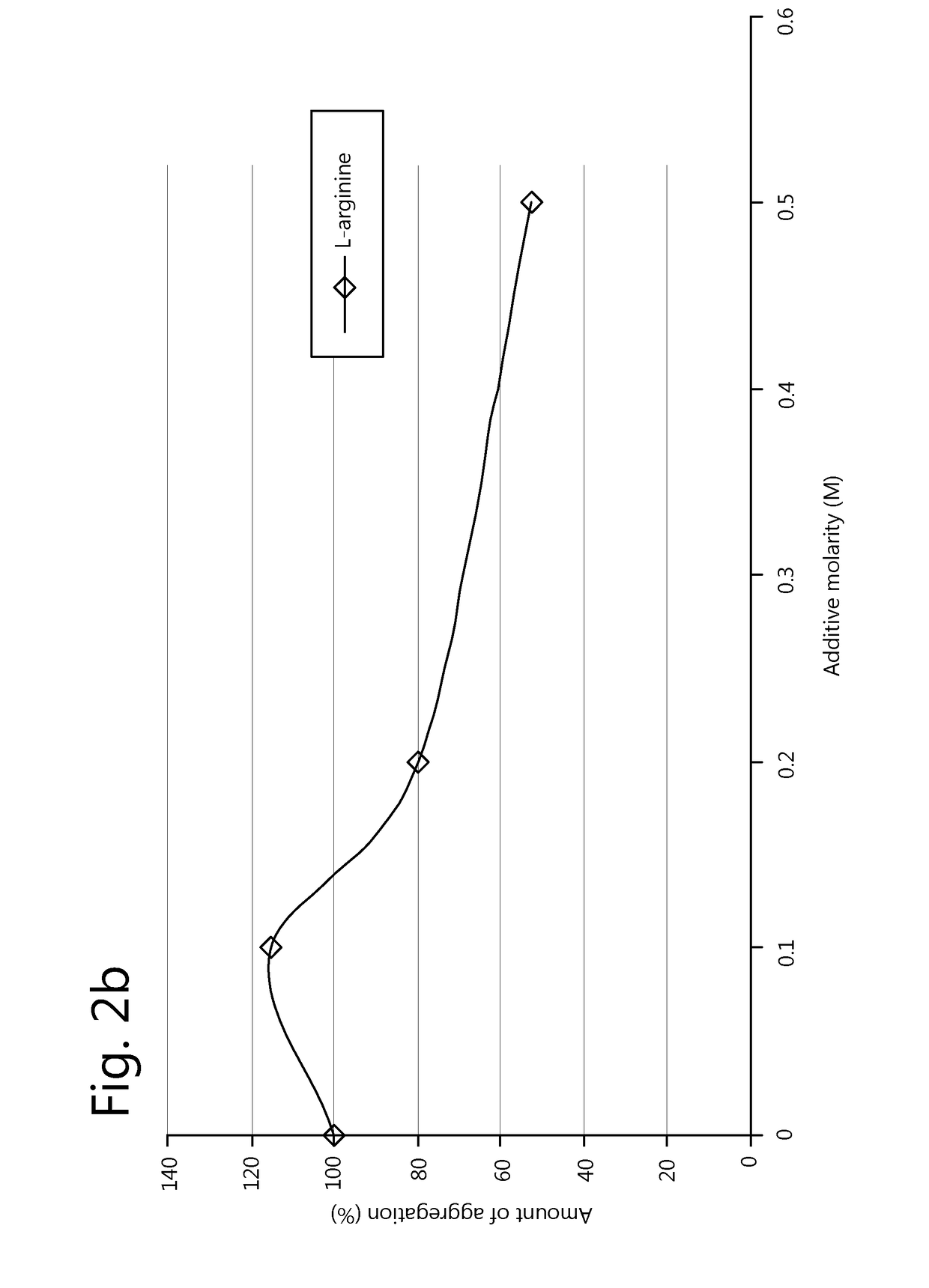
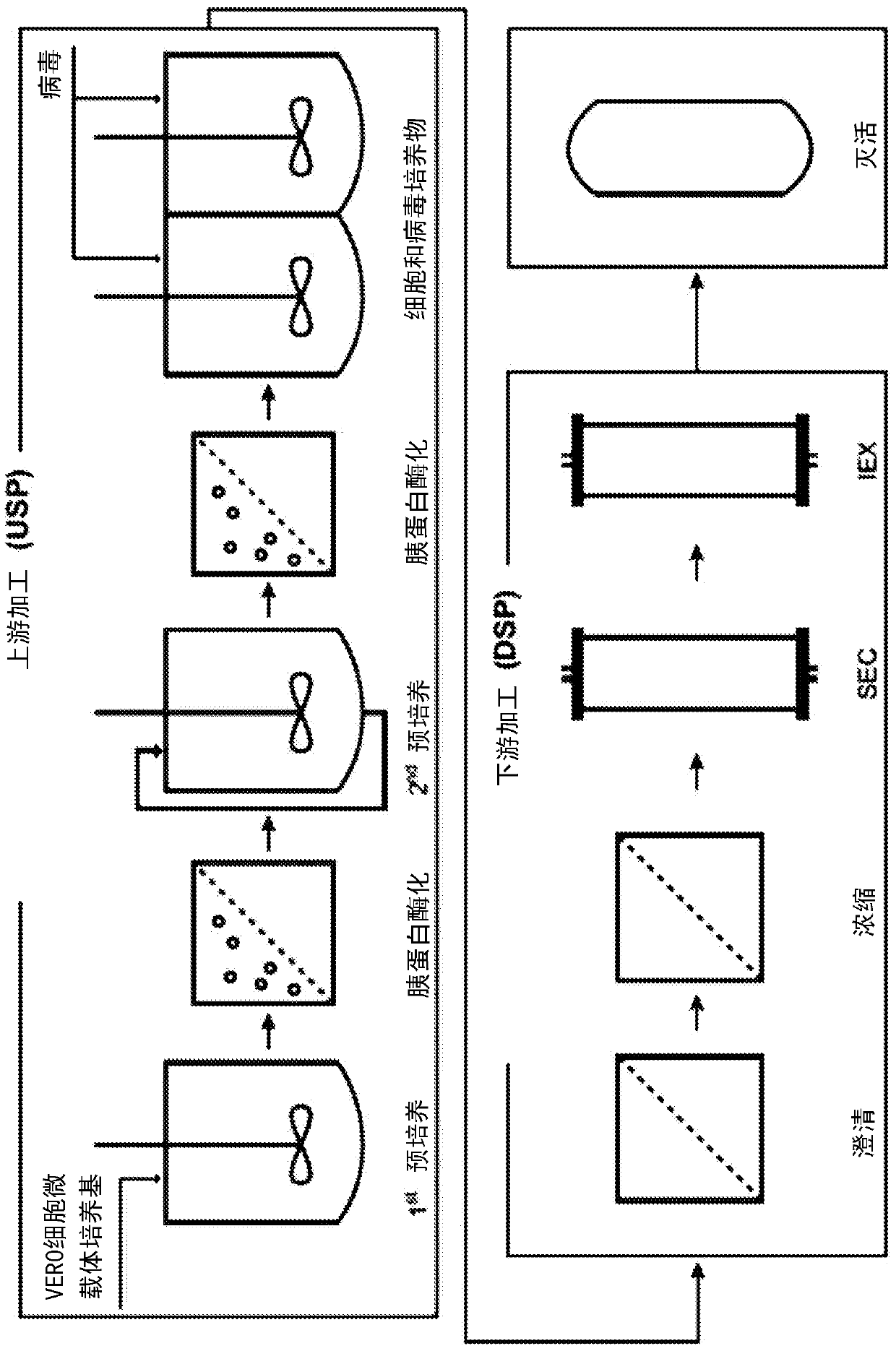
Methods for the prevention of aggregation of viral components
ActiveUS10080793B2Reduce compositionIncrease concentrationSsRNA viruses negative-senseSsRNA viruses positive-senseViral Components
The present invention relates to a method for prevention and / or reduction of aggregation of viral components.
Owner:INTRAVACC BV
Methods for the prevention of aggregation of viral components
The present invention relates to a method for prevention and / or reduction of aggregation of viral components.
Owner:INTRAVACC LLC
Chris-like virus, compositions, vaccines and uses thereof
A novel virus, the Chris-like virus, has been identified and characterised. Sequences derived from a new paramyxovirus, Chris-like virus (CV), are provided. These sequences encode viral proteins with similarity to recognized viral proteins in other known and characterized paramyxoviruses. The CV viral sequences and the polypeptides encoded therein are useful for the detection, immunological protection and therapy of CV. The sequences provided by the invention are also useful for the isolation and characterization of CV, for the propagation of CV or its viral components in cells or tissue culture, for the generation of immunogenic polypeptides and vaccines, and for the screening and identification of antiviral agents for CV.
Owner:PALESE PETER +2
Method of isolating Anti-viral ingredients from baphicacanthus cusia, compositions comprising them and their medical use
ActiveUS20170281702A1Hydroxy compound active ingredientsMagnoliophyta medical ingredientsLignanAlkaloid
The present invention provides a method of isolating at least one ingredient with anti-viral efficacy from Baphicacanthus cusia. The ingredient can be an alkaloid, a triterpenoid, a lignan, a phenylethanoid, a sesquiterpene lactone, or a flavonoid. Two new alkaloids are produced, which have not been previously reported. Moreover, the method isolates 12 compounds which could not or have not been previously isolated. A pharmaceutical composition includes the at least one ingredient and at least one pharmaceutical tolerable excipient. A method of treating a subject suffering from a viral disease includes administering at least one ingredient isolated from Baphicacanthus cusia.
Owner:MACAU UNIV OF SCI & TECH
Macrocyclic lactone combination compositions, vaccines and methods for producing same
An injectable composition, capable of preventing or controlling parasitic, viral, or bacterial infections or diseases, for example scours, in pregnant cows and viral infections or diseases in neonatal calves by parenterally administering to each cow in a herd of pregnant cows, a dose of a combination composition comprising: (a) at least one inactivated viral component derived from rotavirus and / or coronavirus; (b) a macrocyclic lactone active compound; and (c) a pharmaceutically acceptable parenteral carrier and preservative. The injectable compositions which include eprinomectin result in extremely low milk residues.
Owner:MERIAL INC
Regulated nucleic acid expression system
InactiveUS20060121579A1Improve usabilityIncrease productionBiocideBacteriaRegulation of gene expressionCell biology
The present invention provides nucleic acid constructs, expression systems, and methods relating to the regulation of gene expression. The invention may be applied to regulate the expression of any coding sequence of interest, including those coding for viral components necessary for the packaging of viral particles.
Owner:VIRXSYS
Compositions and methods for identifying and differentiating viral components of multivalent shipping fever vaccines
InactiveUS9238843B2Overcome deficienciesHigh sensitivityMicrobiological testing/measurementBovine Viral Diarrhea VirusesOligonucleotide Primer
Disclosed are methods and compositions for identifying viral-specific polynucleotide sequences in a biological sample, and particularly in samples of veterinary origin. Also disclosed are oligonucleotide primer pairs, as well as labeled oligonucleotide detection probes useful in detecting the presence of one or more particular species, strains, types, or subtypes of one or more mammalian pathogens of viral origin, as well as systems, diagnostic kits and articles of manufacture comprising virus-specific primers and labeled detection probes, including those useful in identifying viral components of a multivalent vaccine, and quantitating the potency of particular attenuated, live viruses that comprise a livestock vaccine. In certain embodiments, real-time, quantitative PCR methods have been utilized to identify and distinguish between the three known genetic subtypes of bovine viral diarrhea viruses (BVDV-1a, BVDV-1b, and BVDV-2) in a multivalent bovid shipping fever vaccine.
Owner:ELANCO US INC
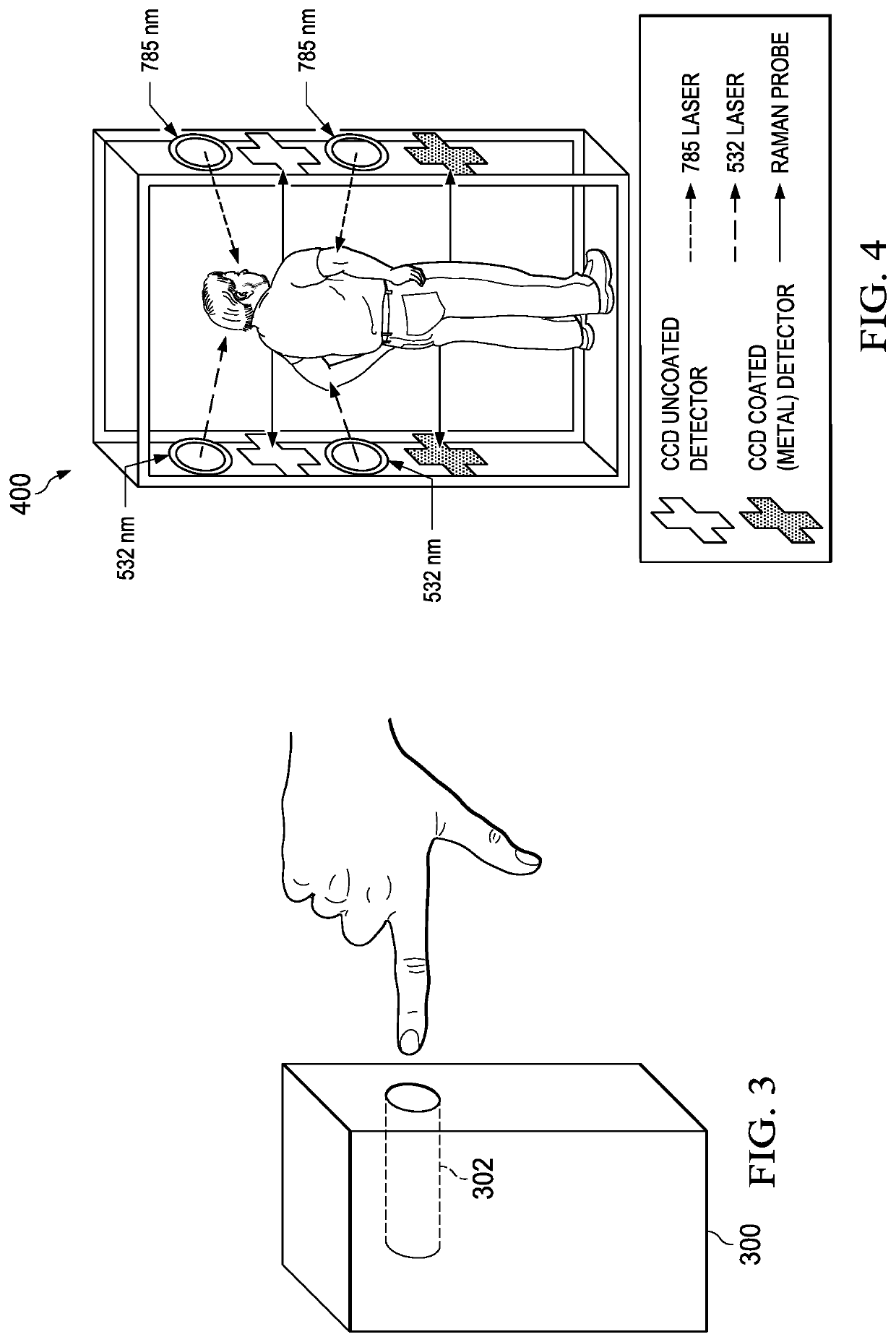
Self-administered, non-invasive, transcutaneous viral detector
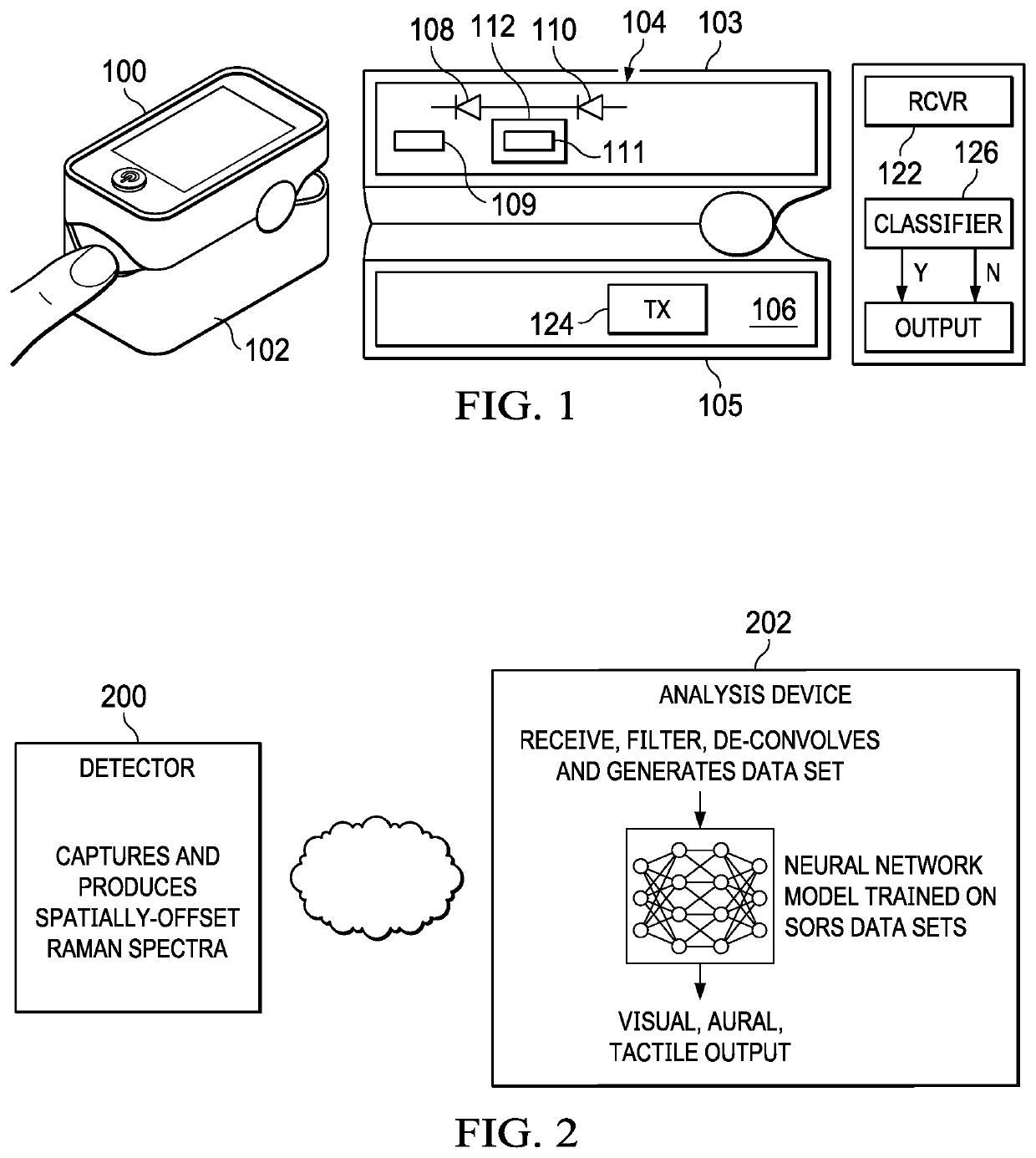
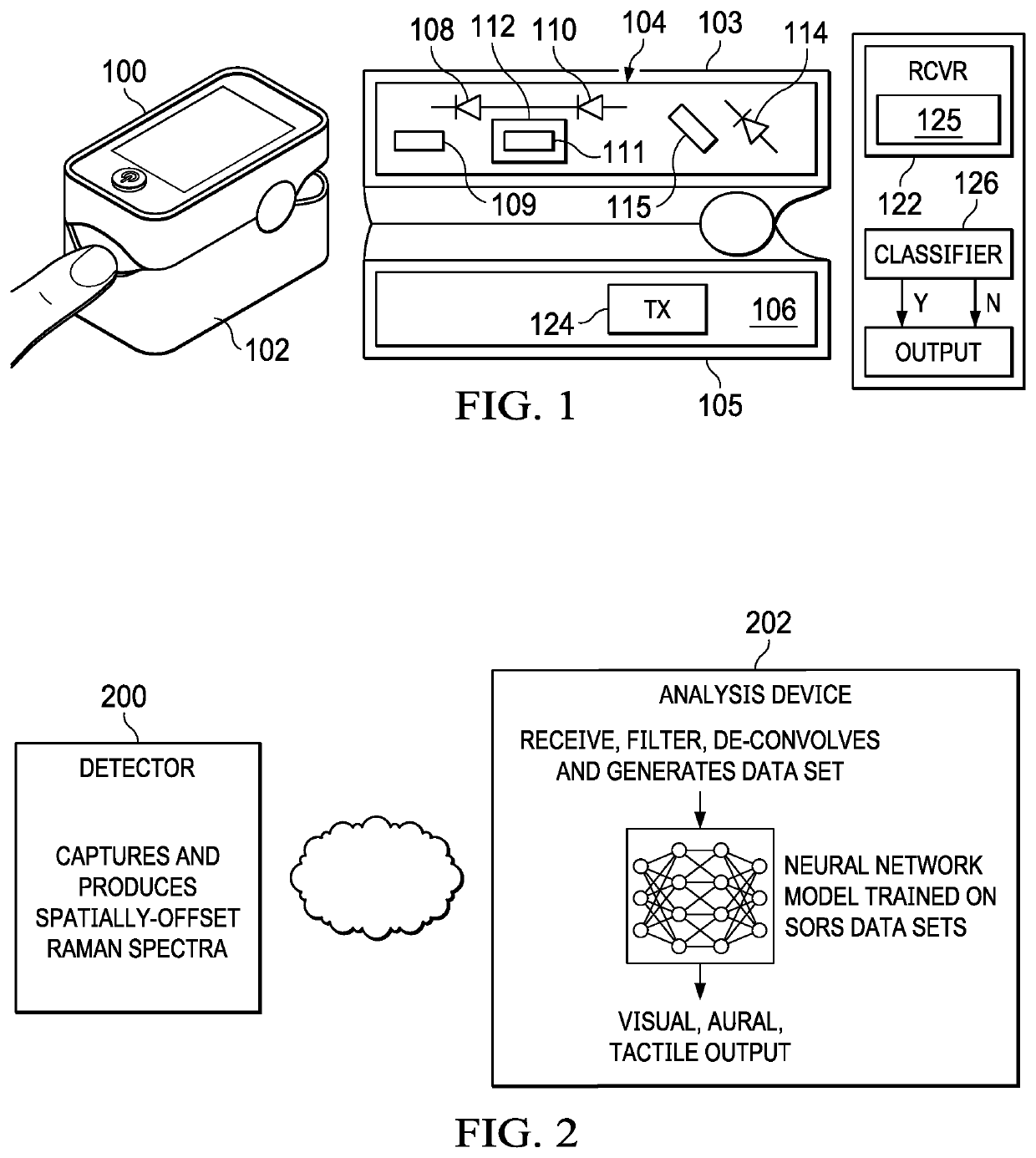
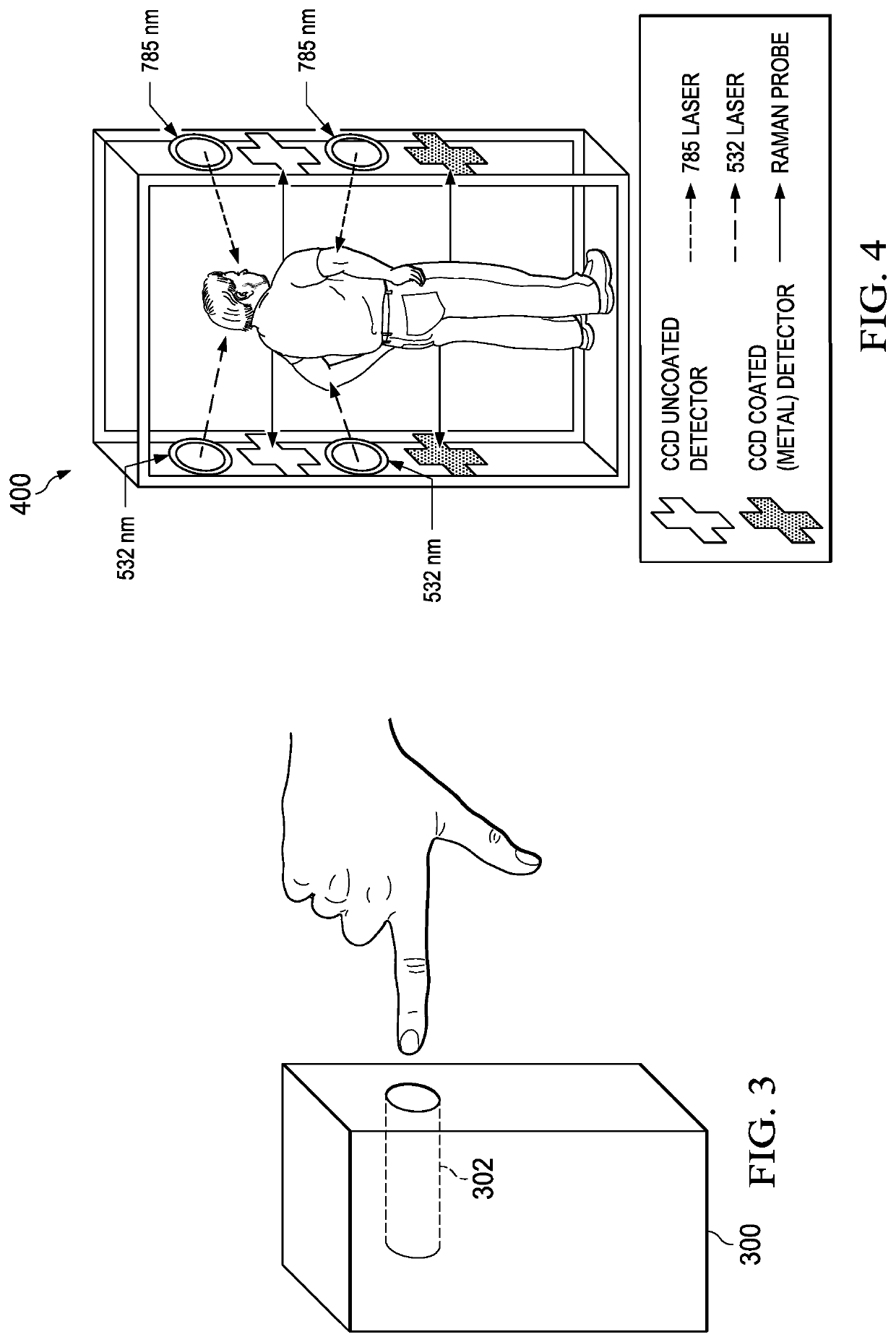
A non-invasive, transcutaneous, real-time viral detection device that is configured for self-administration, e.g., at a user's home. In one embodiment, and after positioning the device relative to the human body part (e.g., the user's finger), light sources in the device are activated (excited), and resulting data captured. In particular, a set of Raman spectra are collected from a configured set of emitters and detectors in the device and delivered to a nearby receiver, preferably wirelessly. The receiver filters and de-convolves the Raman spectra producing a data set representative of the constituent elements in the user's tissue of interest. The data set is applied against a statistical classifier, e.g., a neural network that has been trained to recognize and distinguish the absence or presence of viral components, e.g., C-19, or its associated blood-borne acute phase reactants. The classifier outputs an appropriate indicator, preferably in real-time, providing the user with an immediate indication of whether C-19 (or other virus of interest) is present.
Owner:123IV INC
Parallel process for protein or virus separation from a sample
InactiveUS7652129B2Well representedIncrease automation and efficiencySamplingComponent separationProtein insertionThroughput
A sample is divided into a series of aliquots with the aliquots being subjected to at least two successive parallel separation steps in order to resolve protein or viral components thereof. The separation steps are performed not only on a sample but subsamples each containing a prelabeled tag to afford comparisons between subsamples. The parallel separation is amenable to high throughput and automation.
Owner:PERKINELMER HEALTH SCIENCES INC
Delivery, use and therapeutic applications of CRISPR-Cas systems and compositions targeting disorders and diseases using viral components
ActiveCN105793425BAvoid off-target bindingAvoid side effectsHydrolasesGenetic material ingredientsDiseaseGenome
The present invention provides for the delivery, engineering and optimization of systems, methods, and compositions for manipulating the sequence and / or activity of target sequences. A delivery system and a tissue or organ targeted as a site for delivery is provided. Also provided are vectors and vector systems, some of which encode one or more components of a CRISPR complex, and methods for designing and using such vectors. Also provided are methods of directing the formation of CRISPR complexes in eukaryotic cells to ensure enhanced specificity and avoid toxicity for target recognition, and to edit or modify target sites in genomic loci of interest in order to alter or ameliorate disease or the state of the disease.
Owner:THE BROAD INST INC +2
Vaccines and immunoglobulins targeting African swine fever virus and methods of making and using same
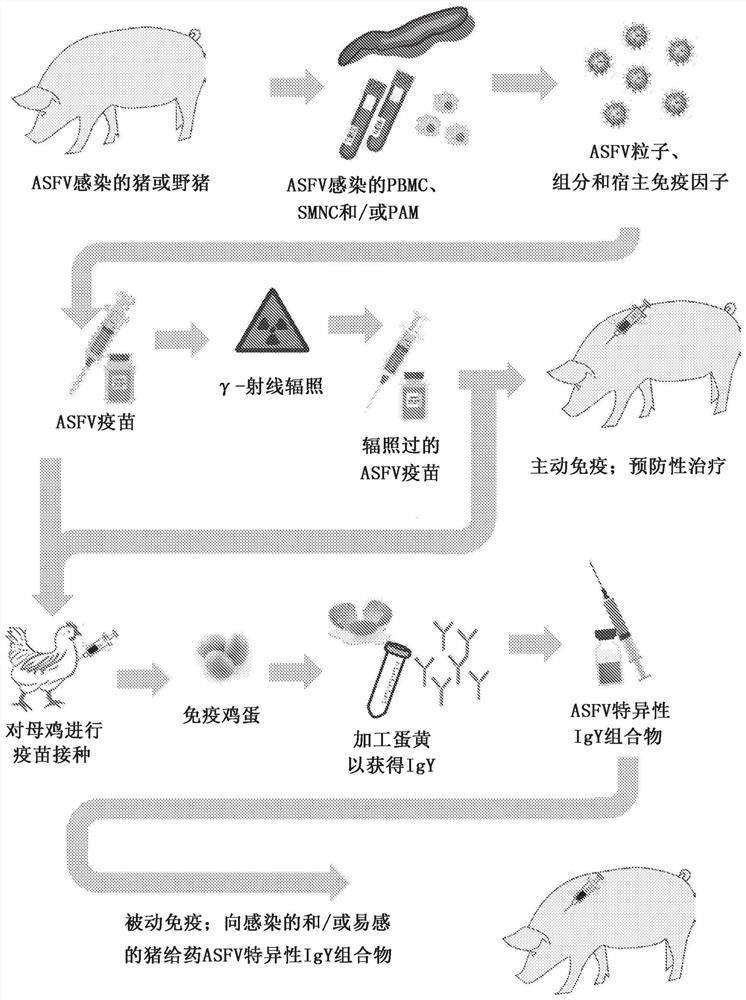
PendingCN114746110AViral antigen ingredientsImmunoglobulins against virusesAfrican swine feverEngineering
The present disclosure provides a method of isolating and preparing live African swine fever (ASF) virus (ASFV) and ASFV vaccines comprising intact ASF virions, viral components, and / or immunosuppressive protein factors. The ASFV vaccine may be used to immunize pigs and wild boars, or may be used to immunize species other than pigs or wild boars, such as poultry, cattle, goats, rabbits, donkeys, or horses, to produce polyclonal immunoglobulins with broad spectrum specificity for ASFV. The ASFV-specific immunoglobulins may then be extracted and purified. The ASFV-specific immunoglobulins may provide acute treatment for ASF-infected pigs or wild boars, or prophylactic treatment for pigs or wild boars at risk of ASF, for example, those may have been exposed to ASFV or ASFV infection.
Owner:IGY免疫技术和生命科学公司
Anti-viral method
ActiveUS9732324B2Reduce infectivitySsRNA viruses negative-senseBiocideLipid formationSilica particle
The invention provides a method of reducing viral infectivity in a sample comprising contacting the sample with a earner matrix wherein lipids are attached to the carrier matrix, and wherein at least one virus-specific agent is attached to the lipids The virus-specific agent is capable of binding to at least one viral component The virus specific agent may be a receptor The earner matrix may be a silica particle, which may be coated with a lipid bilayer The invention further provides a method of inactivating a virus comprising contacting the virus with a earner matrix wherein lipids are attached to the earner matrix, and wherein at least one receptor is attached to the lipids, binding the receptor to at least one viral receptor-binding protein, and activating at least one viral fusion protein, wherein activation inactivates the virus, and releases the virus from the earner matrix.
Owner:CORNELL UNIVERSITY +1
Regulated nucleic acid expression system
InactiveUS8043611B2Improve usabilityHigh potencyBiocideBacteriaRegulation of gene expressionCell biology
The present invention provides nucleic acid constructs, expression systems, and methods relating to the regulation of gene expression. The invention may be applied to regulate the expression of any coding sequence of interest, including those coding for viral components necessary for the packaging of viral particles.
Owner:VIRXSYS
Compositions and methods for identifying and differentiating viral components of multivalent shipping fever vaccines
InactiveUS20130266934A1Facilitate initiationOvercome deficienciesMicrobiological testing/measurementBovine Viral Diarrhea VirusesCattle Diseases
Disclosed are methods and compositions for identifying viral-specific polynucleotide sequences in a biological sample, and particularly in samples of veterinary origin. Also disclosed are oligonucleotide primer pairs, as well as labeled oligonucleotide detection probes useful in detecting the presence of one or more particular species, strains, types, or subtypes of one or more mammalian pathogens of viral origin, as well as systems, diagnostic kits and articles of manufacture comprising virus-specific primers and labeled detection probes, including those useful in identifying viral components of a multivalent vaccine, and quantitating the potency of particular attenuated, live viruses that comprise a livestock vaccine. In certain embodiments, real-time, quantitative PCR methods have been utilized to identify and distinguish between the three known genetic subtypes of bovine viral diarrhea viruses (BVDV-1a, BVDV-1b, and BVDV-2) in a multivalent bovid shipping fever vaccine.
Owner:ELANCO US INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com