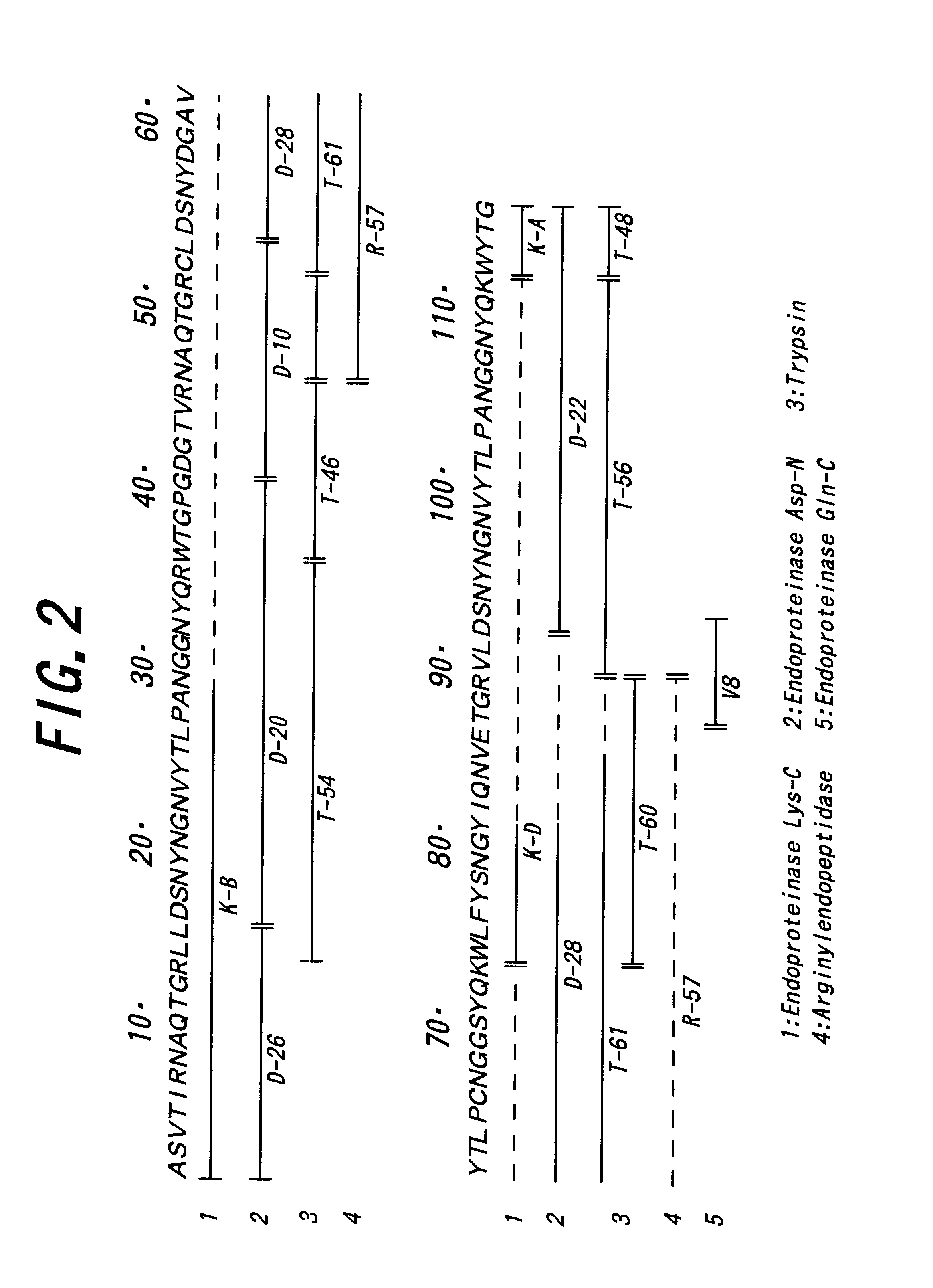
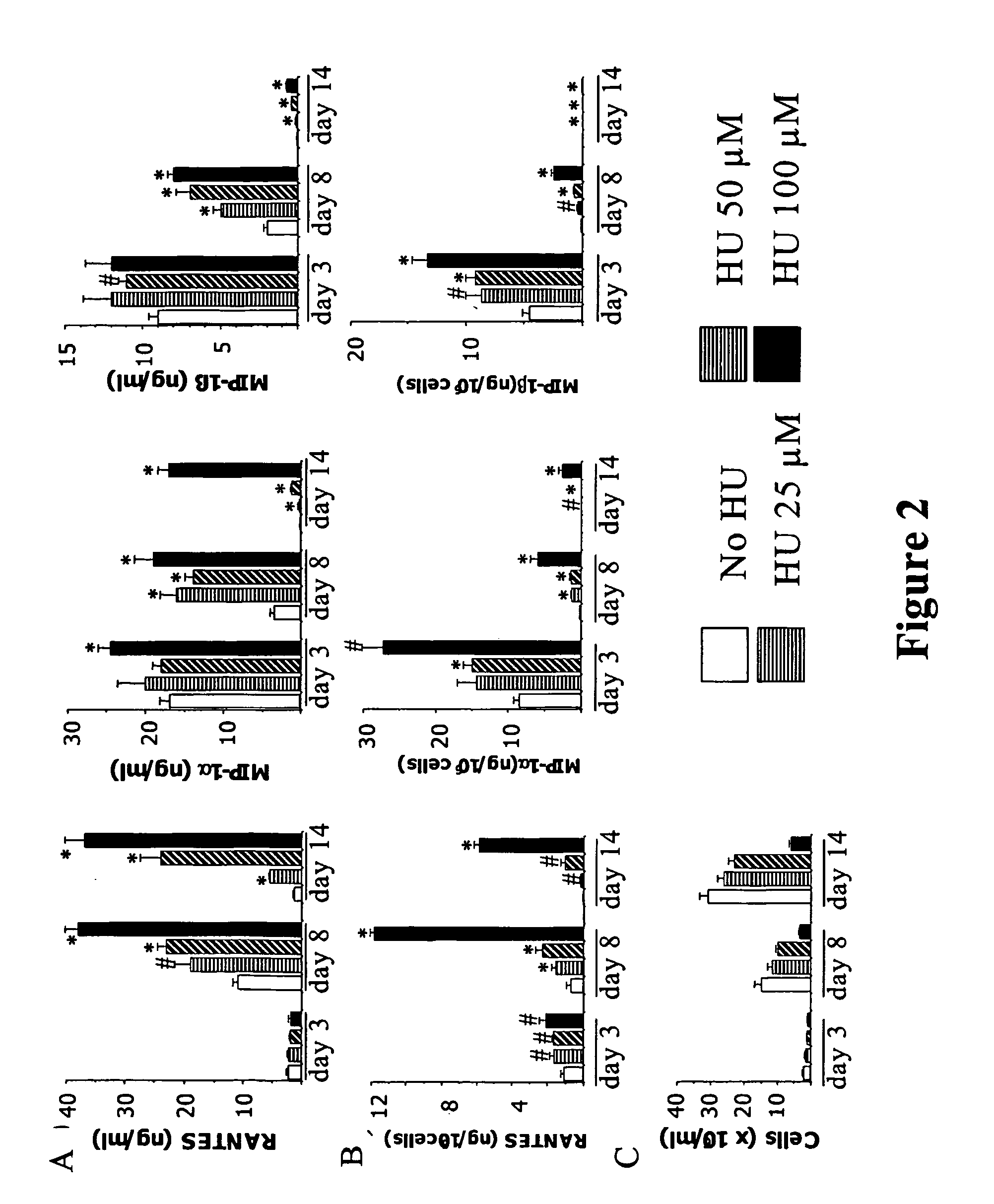
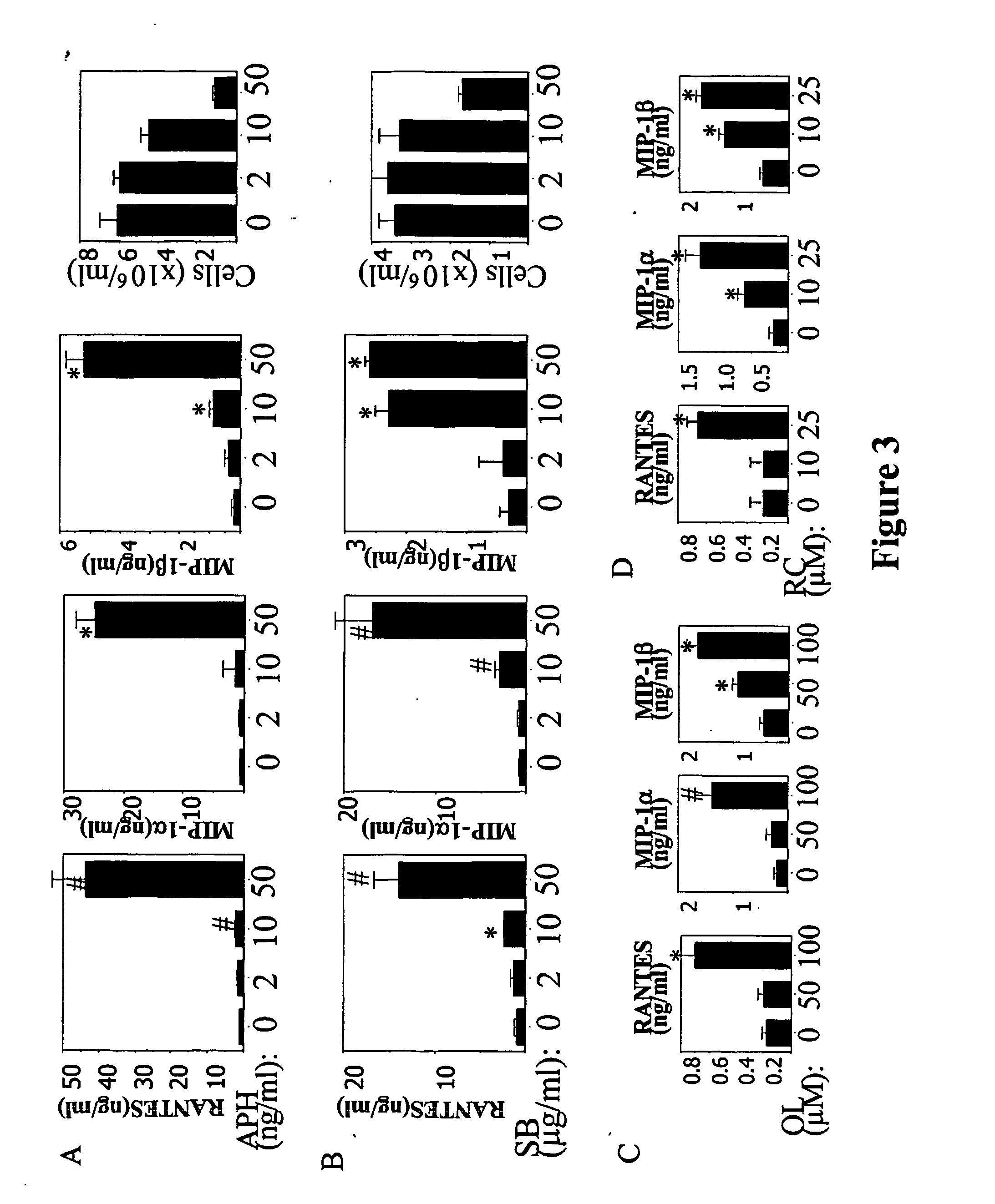
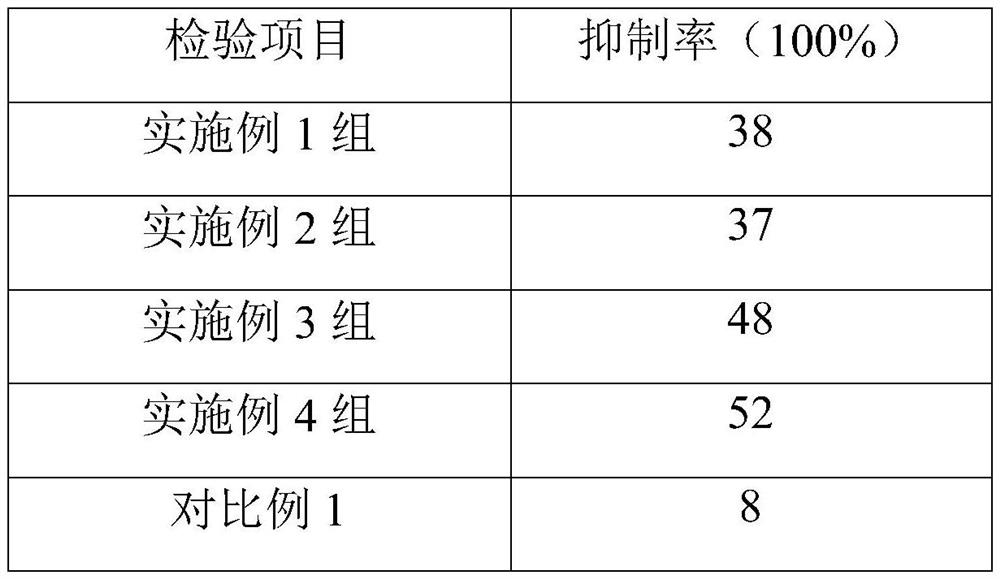
Patents
Literature
37 results about "Viral Receptor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cell surface molecules that are capable of interacting with virus particles, thereby mediating their entry into the cell or otherwise eliciting a cellular response.
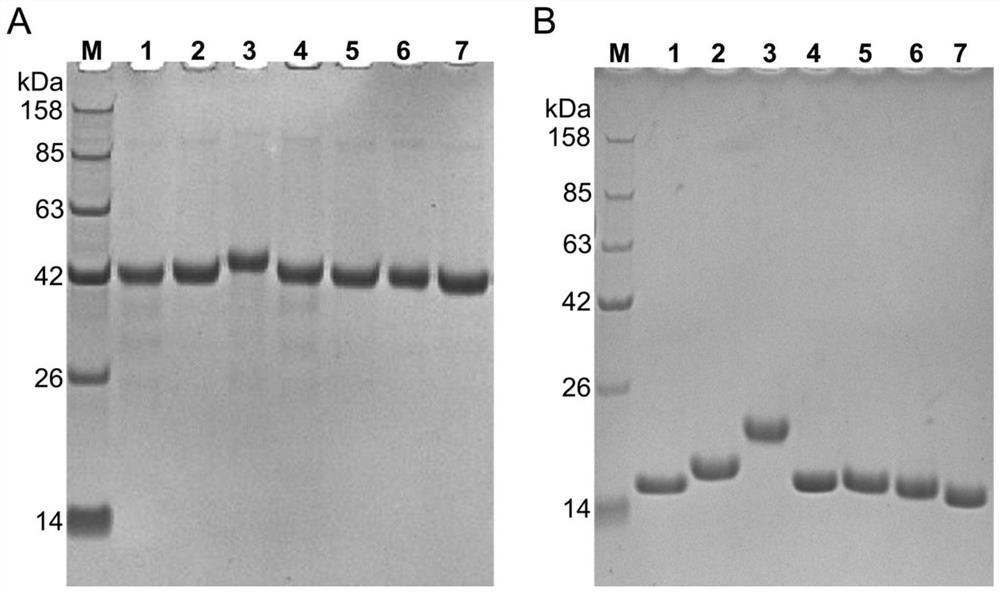
Nanobody against SARS-COV-2 virus S protein RBD structure domain and use thereof
InactiveCN111825762AAvoid infectionStrong specificityImmunoglobulins against virusesAntiviralsViral ReceptorPharmaceutical drug
The invention provides a nanobody against a SARS-COV-2 virus S protein RBD structure domain. The nanobody can recognize the RBD structure domain of SARS-COV-2 virus S protein, and includes one or moreof nanobody1-nanobody19. The nanobody can specifically bind to an S protein RBD region of SARS-COV-2 and block the binding of the virus to a cell receptor ACE2, and EC50 of the binding of the antibody to RBD is 0.4212ng / ml-325.24ng / ml. The nanobody competes with viral receptor ACE2 protein, and the IC50 is 2.433nm-100.02nm. The invention further provides use of the nanobody and recombinant antibody thereof in preparing drugs for inhibiting SARS-COV-2 virus infection, and preparing a SARS-COV-2 virus detection reagent or kit.
Owner:CUSABIO TECH LLC
Alpaca-derived nanobody binding to SARS-CoV-2 RBD
ActiveCN112094342AImprove stabilityHigh expressionHybrid immunoglobulinsImmunoglobulins against virusesViral ReceptorEpitope specificity
The present invention relates to an alpaca-derived nanobody binding to SARS-CoV-2 RBD or an antigen-binding fragment thereof, and particularly relates to an alpaca-derived nanobody capable of bindingto a neocoronavirus (SARS-CoV-2) receptor binding Region (RBD) with high affinity or a double epitope-specific antibody composed thereof or an antigen-binding fragment thereof, which can be used for preventing, treating and / or diagnosing SARS-CoV-2 infection.
Owner:UNIV OF SCI & TECH OF CHINA
Modified erythrocytes and uses thereof
InactiveUS20070082392A1Genetically modified cellsArtificial cell constructsRed cell acanthocytosisHIV receptor
The present invention provides modified erythrocytes which comprise viral receptor proteins capable of mediating entry of respective viruses into the modified erythrocytes. The present invention also provides methods of using the modified erythrocytes for the treatment or prevention of viral infections. In one embodiment, the modified erythrocytes of the present invention comprise CD4 and at least one HIV coreceptor, such as CXCR4 or CCR5. The modified erythrocytes, when administered to an HIV patient, bind to the plasma virus and induce the injection of the HIV ribonucleoprotein complex into the cells. The entrapped viral content is either degraded or deactivated within the erythrocytes, or destroyed by erythrophagocytosis.
Owner:GLASER LAWRENCE F
Vector mediated organelle transfection
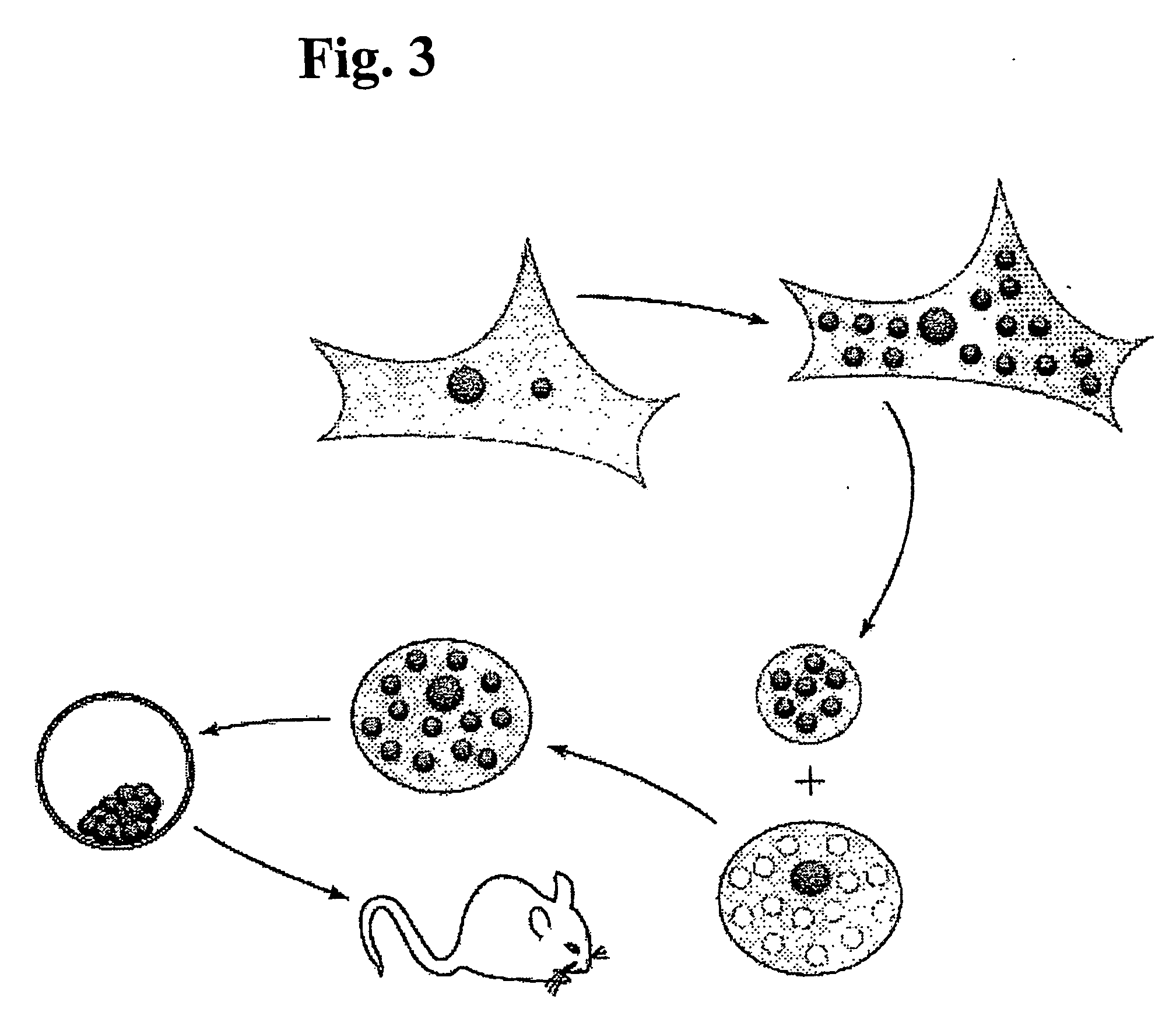
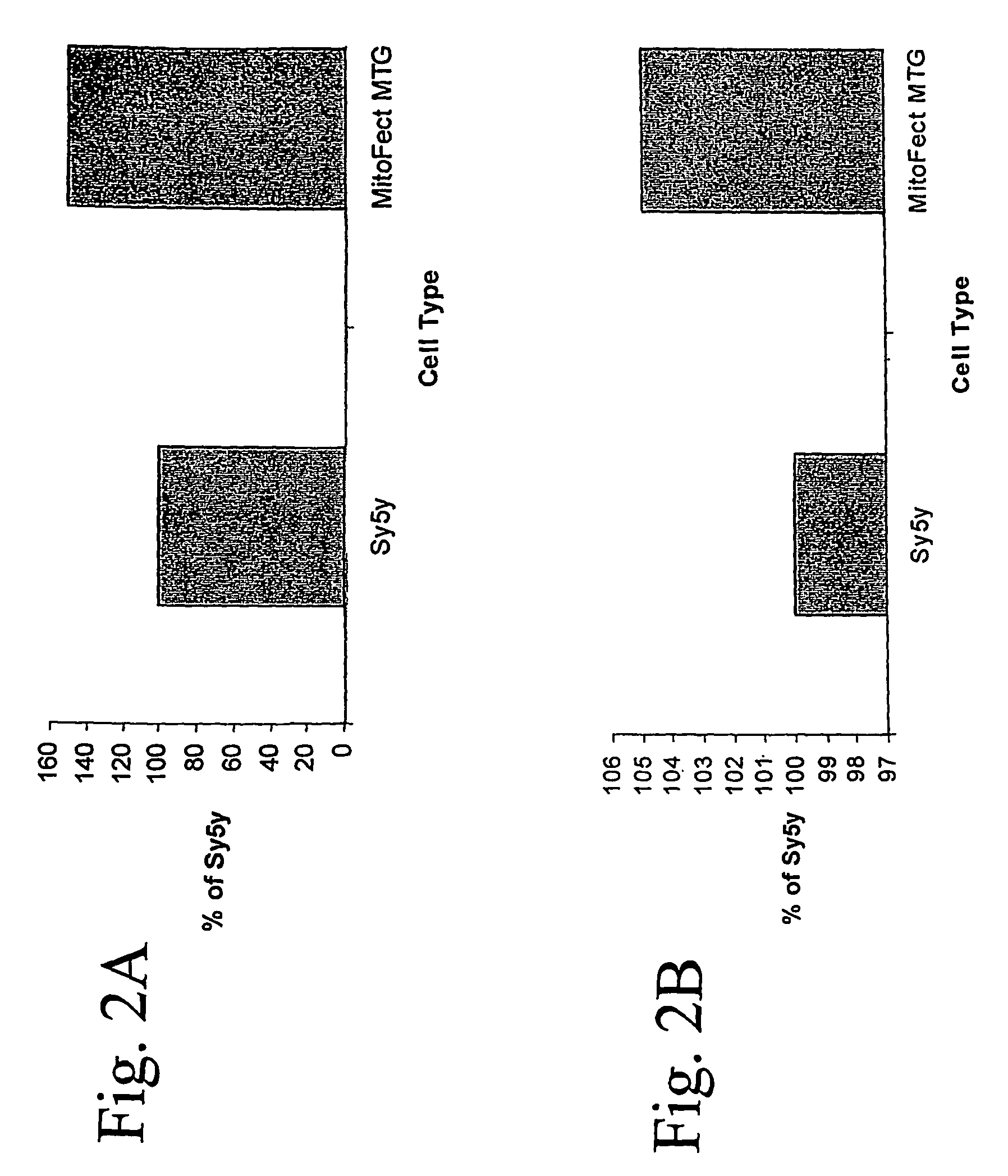
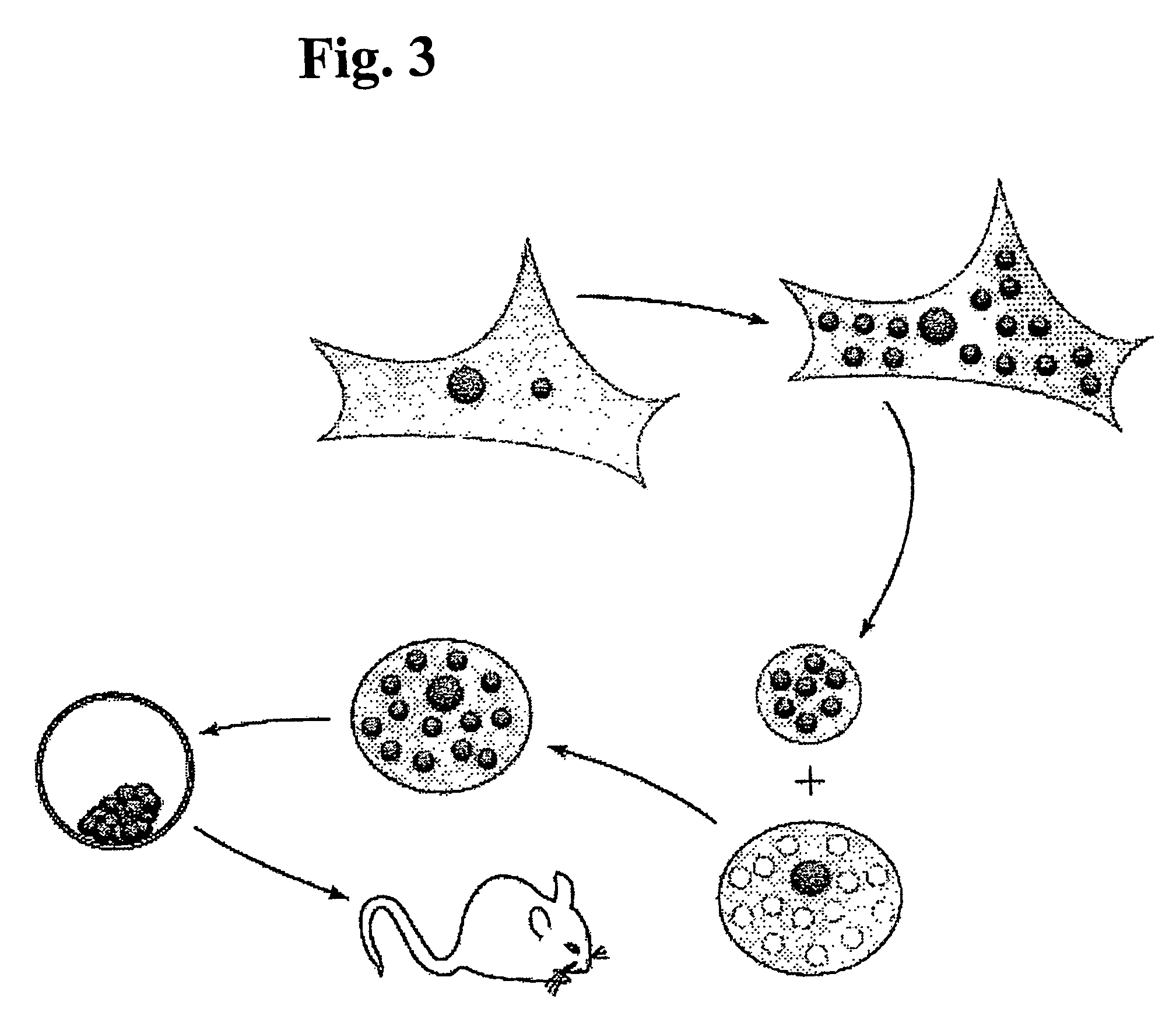
ActiveUS20070011759A1High expressionFunction increaseSugar derivativesPeptide/protein ingredientsEucaryotic cellDNA construct
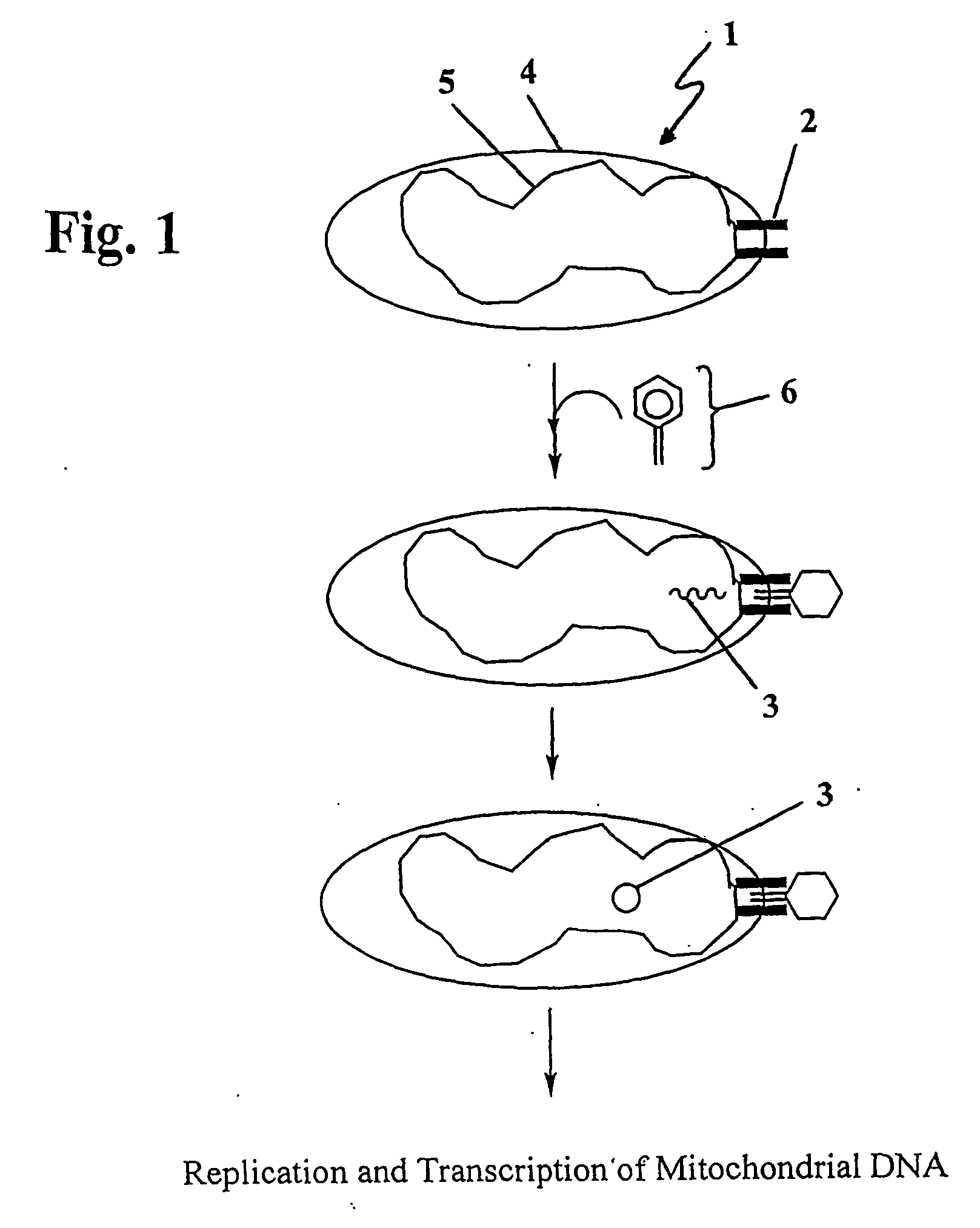
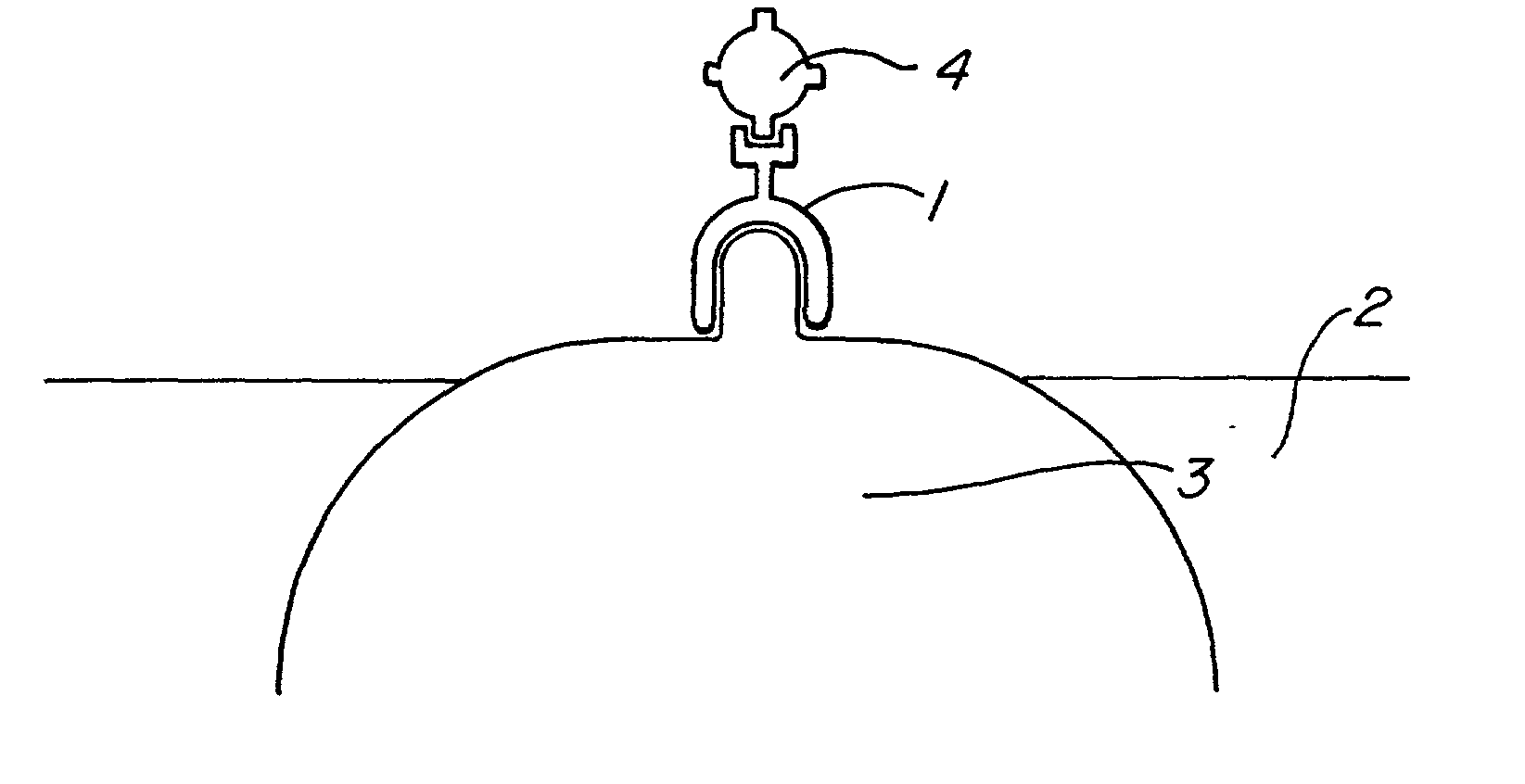
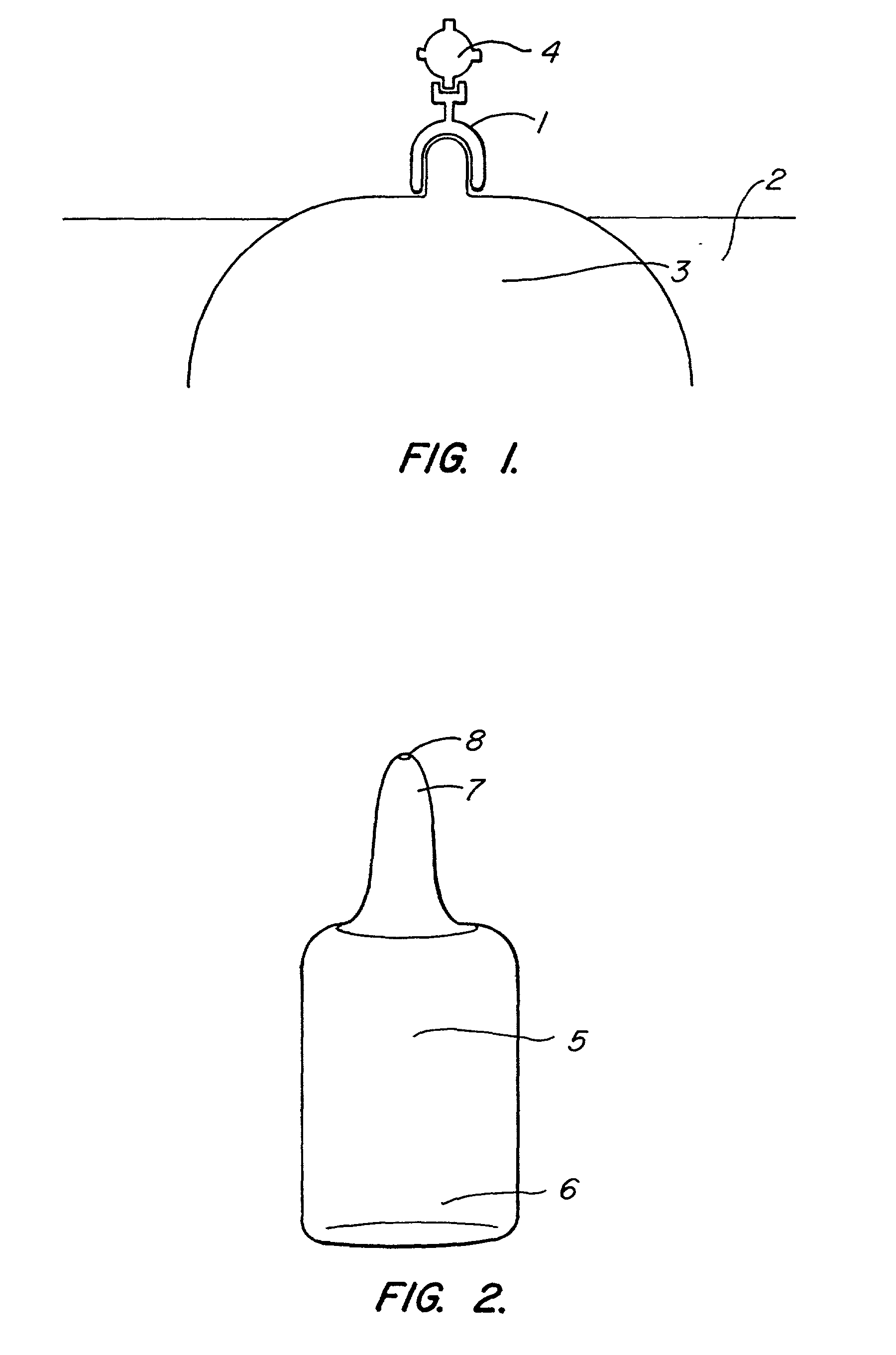
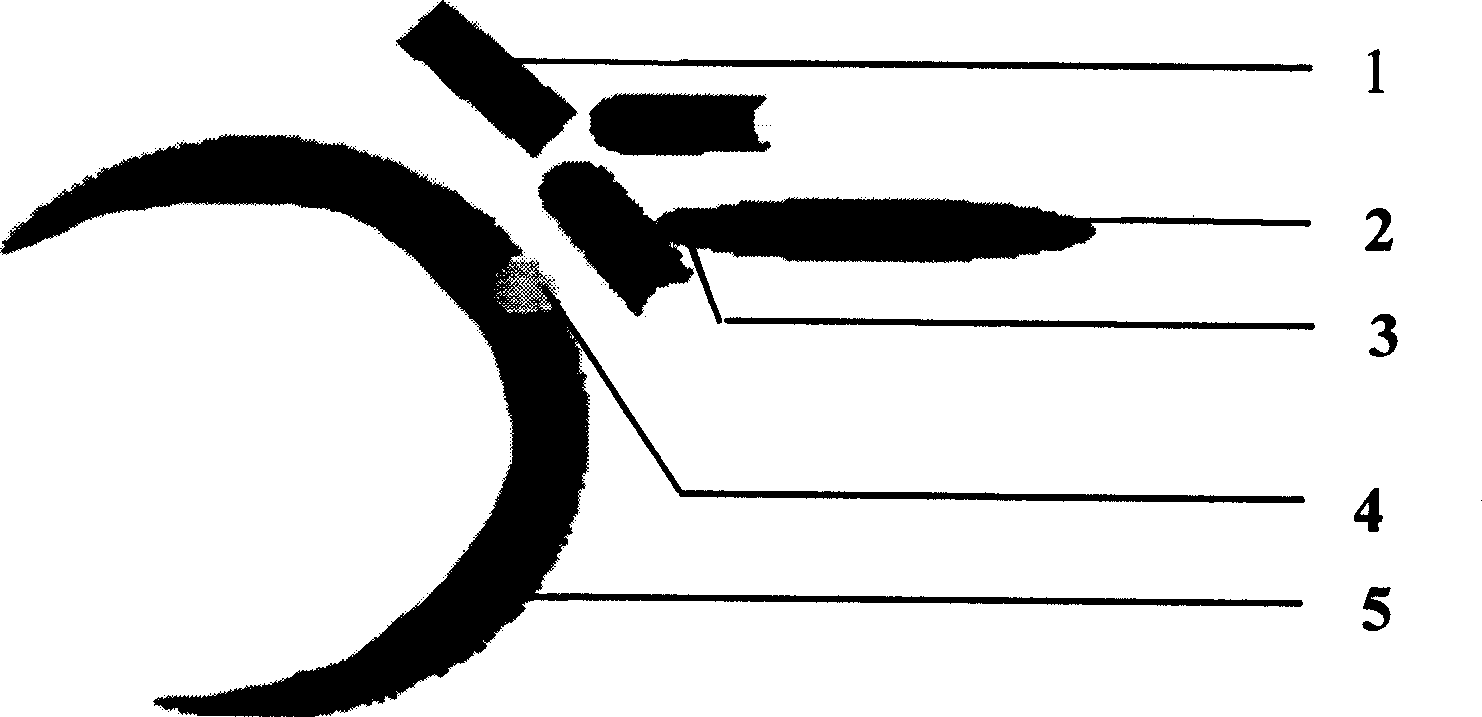
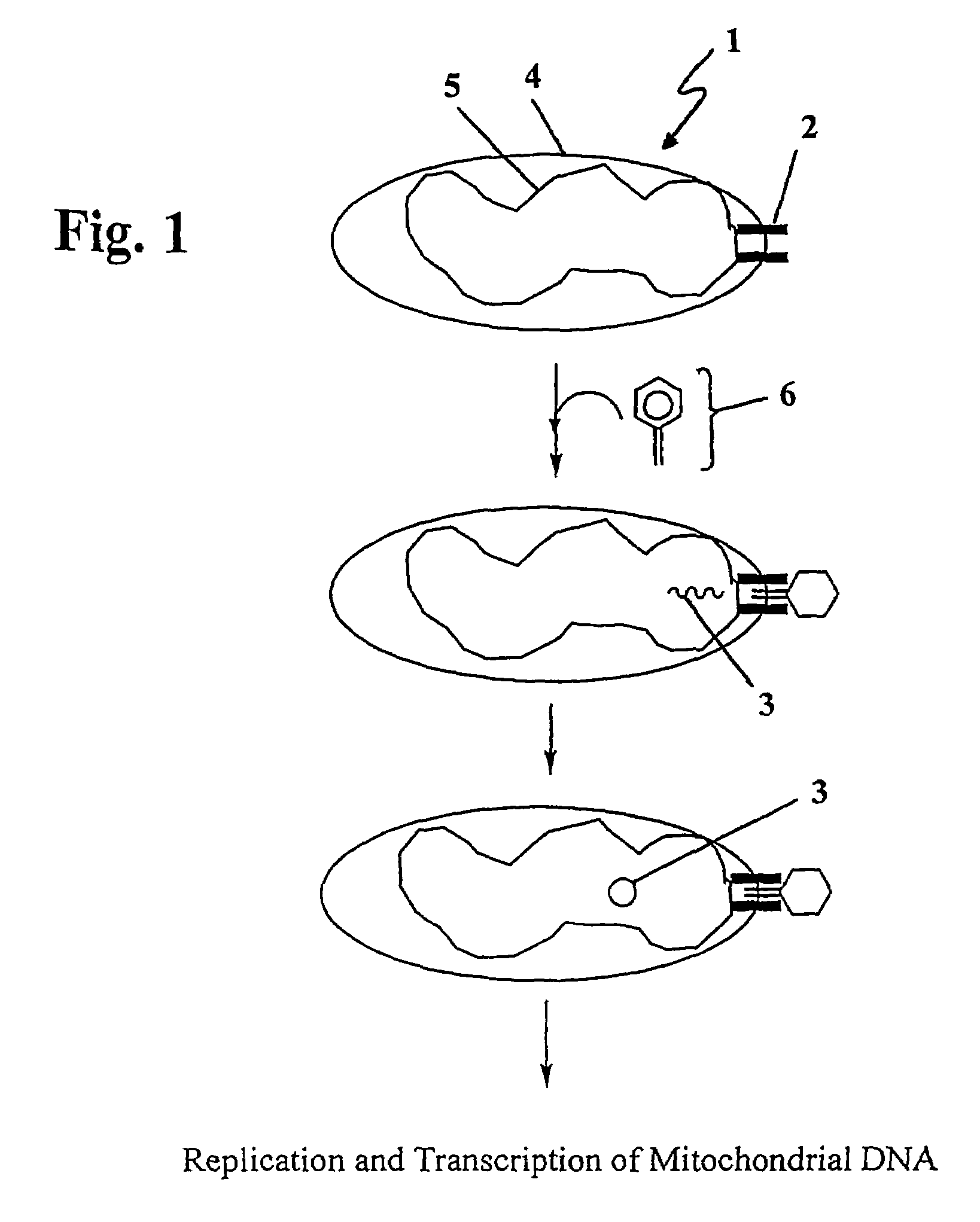
The present invention provides compositions and methods for direct transfection of mitochondria and chloroplast DNA in living cells. More particularly, the present invention is based on the use of viral vectors that specifically bind to receptors uniquely found on the target organelle. In one embodiment, as shown in FIG. 1, a eukaryotic cell containing an organelle (1) that has been modified to express a viral receptor (2) on the organelle's surface is provided. A viral vector (6) comprising a desired recombinant DNA construct (3) is introduced into the cytosol of the cell, wherein the viral vector binds to its receptor and introduces the recombinant DNA into the interior of the organelle.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
Modified erythrocytes and uses thereof
InactiveUS7462485B2Genetically modified cellsArtificial cell constructsViral ReceptorRed cell acanthocytosis
The present invention provides modified erythrocytes which comprise viral receptor proteins capable of mediating entry of respective viruses into the modified erythrocytes. The present invention also provides methods of using the modified erythrocytes for the treatment or prevention of viral infections. In one embodiment, the modified erythrocytes of the present invention comprise CD4 and at least one HIV coreceptor, such as CXCR4 or CCR5. The modified erythrocytes, when administered to an HIV patient, bind to the plasma virus and induce the injection of the HIV ribonucleoprotein complex into the cells. The entrapped viral content is either degraded or deactivated within the erythrocytes, or destroyed by erythrophagocytosis.
Owner:GLASER LAWRENCE F
Method for improving the half-life of soluble viral receptors on mucosal membranes
This invention relates to methods of increasing the half-life of a viral-specific ligand on a mucosal membrane by modifying the viral-specific ligand to bind the bacteria colonized on the mucosal membrane. The invention also provides a chimeric molecule comprising a viral-specific ligand and a bacterial-specific ligand.
Owner:OSEL
Novel Anti-Viral Method
ActiveUS20120039978A1Reduce infectivitySsRNA viruses negative-sensePowder deliveryLipid formationBiological activation
The invention provides a method of reducing viral infectivity in a sample compnsing contacting the sample with a earner matrix wherein lipids are attached to the carrier matrix, and wherein at least one virus-specific agent is attached to the lipids The virus-specific agent is capable of binding to at least one viral component The virus specific agent may be a receptor The earner matrix may be a silica particle, which may be coated with a lipid bilayer The invention further provides a method of inactivating a virus comprising contacting the virus with a earner matrix wherein lipids are attached to the earner matrix, and wherein at least one receptor is attached to the lipids, binding the receptor to at least one viral receptor-binding protein, and activating at least one viral fusion protein, wherein activation inactivates the virus, and releases the virus from the earner matrix
Owner:CORNELL UNIVERSITY +1
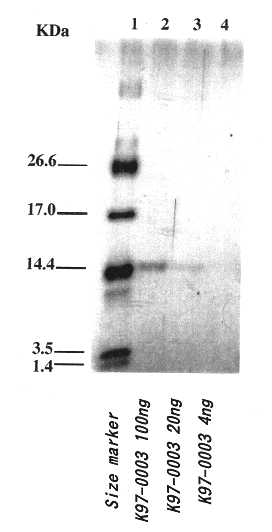
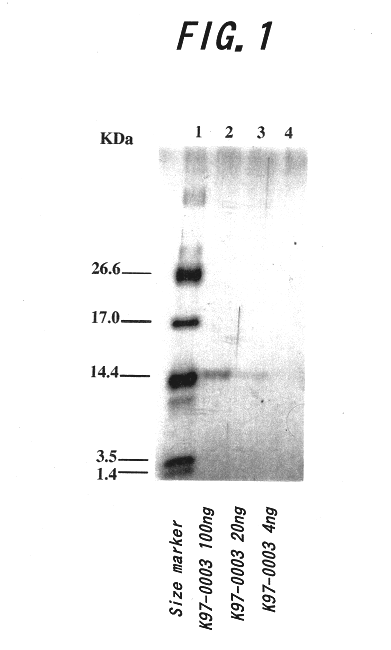
Polypeptide having human HIV inhibitory activity, a gene encoding the polypeptide, a method to produce the polypeptide
InactiveUS6482412B1Bacterial antigen ingredientsPeptide/protein ingredientsViral ReceptorHuman immunodeficiency
A novel compound, which is effective for treatment of AIDS and has inhibitory activity on human immunodeficiency viruses (HIV), was examined. The K97-0003 peptide, which has anti-HIV activity caused by inhibition of syncytium formation by fusion of envelope glycoprotein of HIV and the host cells expressing the receptor to said virus, was provided by the present invention. Furthermore, the base sequence of the gene coding for said polypeptide, and the method for preparing said polypeptide using strain K97-0003 were provided.
Owner:KIIM PHARM LAB +1
Modified and fusion enhanced erythrocytes, cells and uses thereof
InactiveUS20110091973A1Short half-lifeGenetically modified cellsBlood/immune system cellsADAMTS ProteinsChemokine receptor CCR5
Modified fusion enhanced erythrocytes (or other cell types and synthetic cells) including human viral receptor proteins, human viral coreceptor proteins and viral derived proteins capable of mediating entry of respective viruses into the modified erythrocytes, cells or pseudo-cells and the method of using the fusion enhanced modified erythrocytes, cells or pseudo-cells for the treatment or prevention of viral infections. The fusion enhanced modified erythrocytes comprises CD4 and at least one HIV coreceptor, such as CXCR4 or CCR5 and as well, at least one of cholesterol rafts, fusin, actin, a viral derived protein such as fusion peptide derived from HIV GP120 or HIV GP41 or a shorter protein derived from a long viral protein, such as a portion of HIV derived GP120, or HIV GP41 such as the 23 N-terminal peptide of the HIV-1 gp 41 protein (AVGIGALFLGFLGAAGSTMGARS) called FP23 (Fusion Peptide). These viral-fusion enhanced cells may also be electrostatic charge enhanced through further additions named in this invention. The modified erythrocytes, when administered to an HIV patient, bind to the plasma virus and induce the injection of the HIV ribonucleoprotein complex into the cells. The entrapped viral content is sequestered within said cell for at least the period of time that the cell maintains its outer membrane integrity. The virus is thereafter either degraded or deactivated within the erythrocytes, cells or pseudo-cells, or destroyed by erythrophagocytosis.
Owner:GLASER LARRY F
Hog fever virus csfv E2 protein ligand epitope polypeptide and application thereof
ActiveCN102268079AAvoid infectionReduce infection ratePeptide/protein ingredientsMicrobiological testing/measurementClassical swine fever virus CSFVViral Receptor
The invention relates to a (classical swine fever virus) CSFV E2 protein ligand epitope peptide and application thereof. The peptide has an amino acid sequence of VHASDERLGPMPCRPKEIGSSAGPVRKTSCTFNYAKTGKNKYYEPRDSYF and a molecular weight of 5.73kDa. With the PK-15 cell as the target cell, and by means of infection and harvest of the CSFV, TCID50 and the MOI (multiplicity of infection) of the CSFV can be determined. A binding test of a synthetic peptide and the target cell, a virus blocking test and a test for the peptide to block the CSFV from infecting the target cell shows that the screened specific binding target cell can inhibit the virus from infecting the ligand epitope peptide, and ligand epitope of the CSFV is positioned accurately. The peptide SE24 of the invention can inhibit the CSFV from infecting the PK-15 cell, and with the increase of the peptide concentration, the infection rate of the PK-15 cell is reduced. When the peptide concentration is 0.2mmol / L, the CSFV can be completely inhibited from infecting the PK-15 cell. The ligand epitope peptide of the invention provides the theoretical basis for a further study of the virus ligand epitope from the aspect of interactions between virus ligands and virus receptors.
Owner:XINXIANG UNIV
Mutant Paramyxovirus and Method for Production Thereof
ActiveUS20090269850A1Reduce the amount requiredReduce adverse effectsSsRNA viruses negative-senseAntibody mimetics/scaffoldsSurface markerTissue targeting
The present invention provides a modified paramyxovirus containing a reduced amount of receptor-binding protein compared with the wild type; a method of preparing a modified paramyxovirus, comprising the following steps: (1) a step for introducing a nucleic acid that suppresses the expression of a receptor-binding protein of a paramyxovirus into an animal cell, (2) a step for infecting the paramyxovirus to the cell, and (3) a step for isolating paramyxovirus particles replicated in the cell; and a modified paramyxovirus prepared by the method of preparation mentioned above.The present invention also provides a chimera protein wherein a fusion protein of a virus has been joined or bound to a peptide that binds specifically to a cell surface marker; a nucleic acid that encodes the chimera protein; an animal cell capable of expressing the chimera protein on the cell surface thereof; a modified paramyxovirus expressing the chimera protein on the virus particle surface thereof; and a method of preparing a tissue targeting paramyxovirus, comprising: (1) a step for supplying a nucleic acid that encodes a chimera protein wherein a fusion protein of a virus has been joined or bound to a peptide that binds specifically to a cell surface marker of the target cells, (2) a step for introducing the nucleic acid supplied in (1) into an animal cell in an expressible state, and expressing the same, (3) a step for infecting a paramyxovirus to the cell, and (4) a step for isolating paramyxovirus particles replicated in the cell.
Owner:IMMUNOMEDICINE INC
Compositions for inducing increased levels of beta-chemokines and methods of use therefor
InactiveUS20060099170A1Improve the level ofImprove availabilityBiocideAntiviralsViral ReceptorG1/S checkpoint
The present invention relates to compositions and methods for inducing increased levels and availability of β-chemokines by administering to a subject at least one G1 phase arresting compound, wherein the increased levels and availability of β-chemokines block chemokine / viral receptors thereby preventing or treating viral infections.
Owner:UNIV OF MARYLAND BIOTECH INST
Affinity peptide related to coxsackie adenovirus receptor
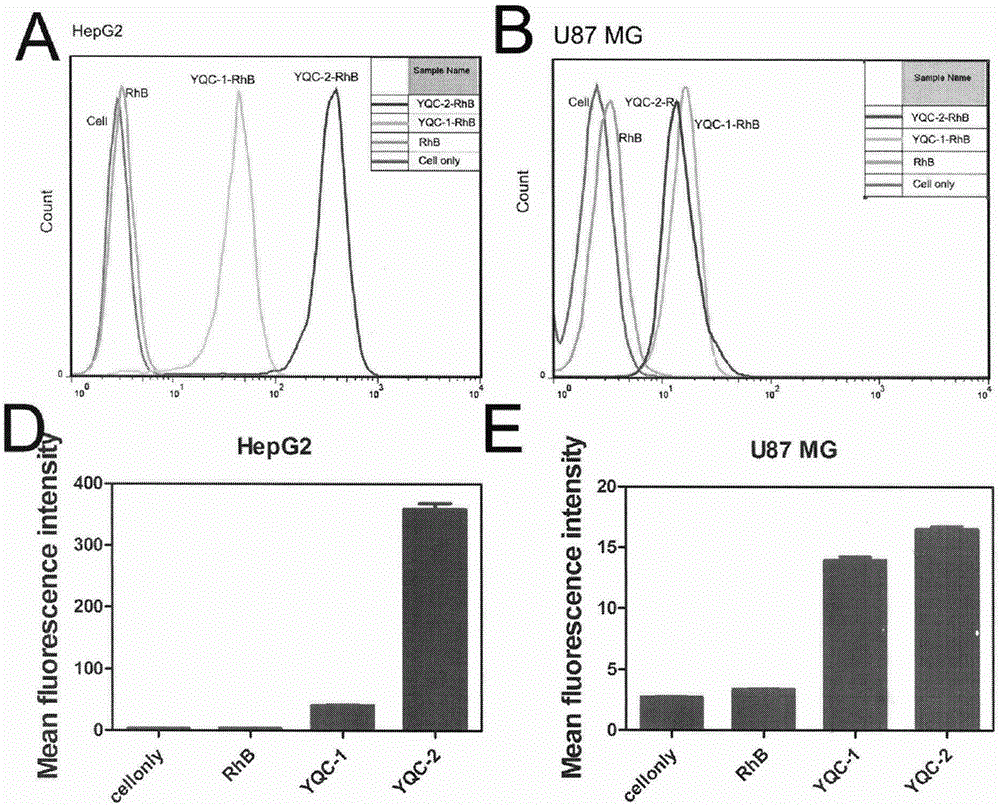
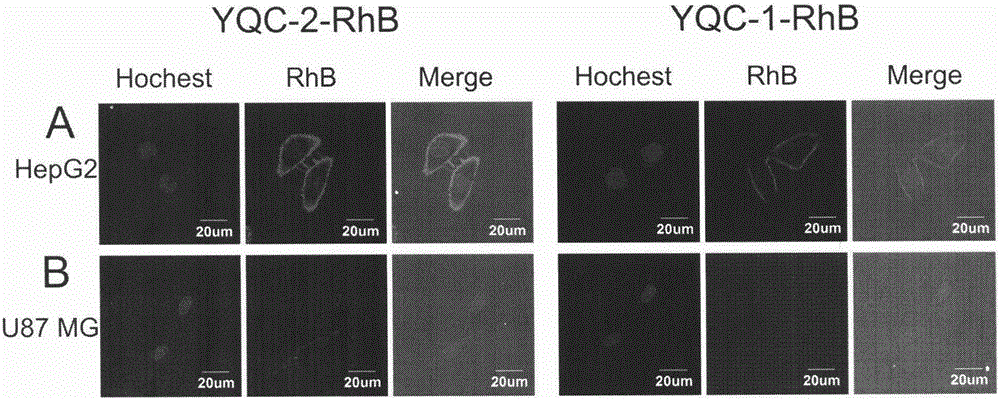
ActiveCN106632613ALow immunogenicityImprove biological activityPeptidesIn-vivo testing preparationsAbnormal tissue growthCoxsackie-Adenovirus Receptor
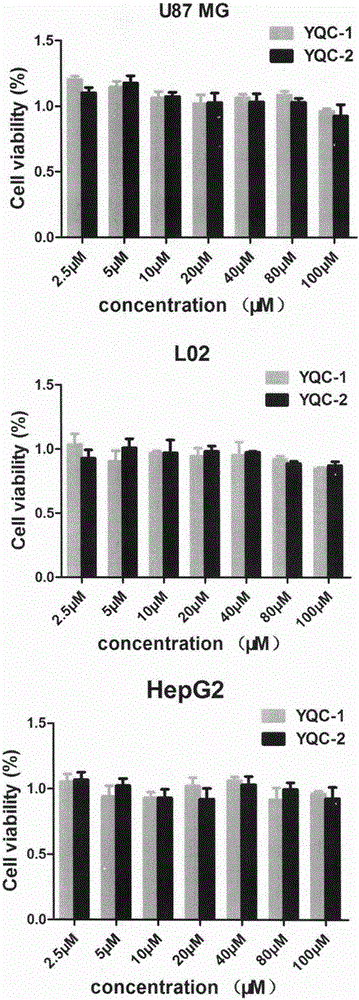
The invention discloses a series of affinity polypeptides YQC-1 and YQC-2 related to a coxsackie adenovirus receptor. The polypeptides possibly realize near-infrared imaging of specific malignant tumors due to the property of specific binding of the coxsackie adenovirus receptor, can be used for preparation of drugs for cell adhesion inhibition, diagnosis or tracing of tumors and targeted chemotherapy and used for pharmaceutical necessities or attachments, so that the aim of nondestructively monitoring early malignant tumors in situ in real time is achieved. The invention relates to the field of drugs related to tumor diagnosis, and in particular to multiple polypeptides, affinity tests of the polypeptides, pharmaceutical compositions taking the polypeptides as active ingredients, and application of the polypeptides in preparation of diagnostic drugs.
Owner:NANJING NUOYUAN MEDICAL DEVICES CO LTD
Methods of blocking asfv infection through interruption of cellular and viral receptor interactions
PendingUS20220241391A1Peptide/protein ingredientsViral antigen ingredientsViral ReceptorTreated animal
A method of preventing and treating viral infections in animals (and preferably ASFV in porcine), by inhibiting viral ligand interactions with critical cellular receptors that are involved either directly (endocytosis and / or macropinocytosis) or indirectly (phagocytosis of RBCs that have been aggregated by viral interactions) with cellular entry in an animal, and preventing and treating the viral infection in the animal. A method of treating a viral infection in an individual with a virus that is both lysogenic and lytic. A composition for treating a viral infection in an individual with a virus that is both lysogenic and lytic. A vaccine for preventing viral infection, including whole and / or partial domains of proteins of both a lysogenic and lytic phase of a virus.
Owner:CHEN DALU +1
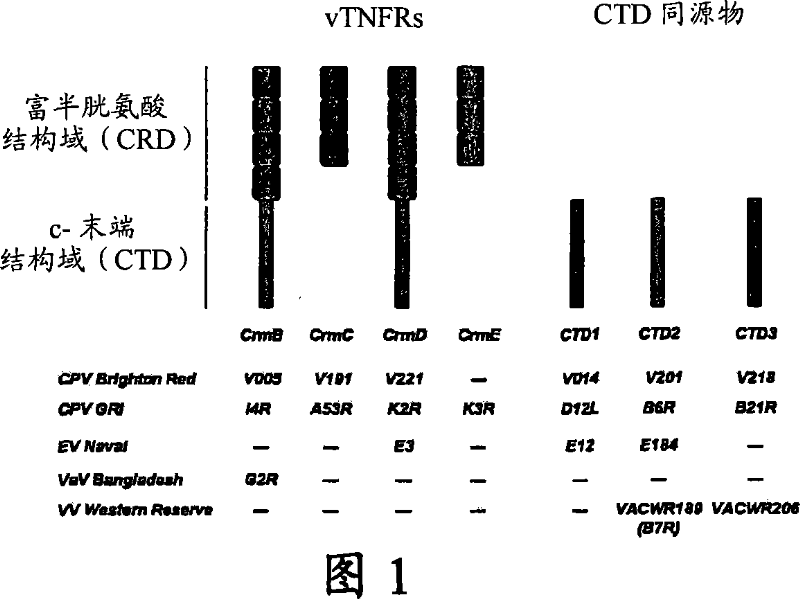
Chemokine binding activity of viral TNF receptors and related proteins
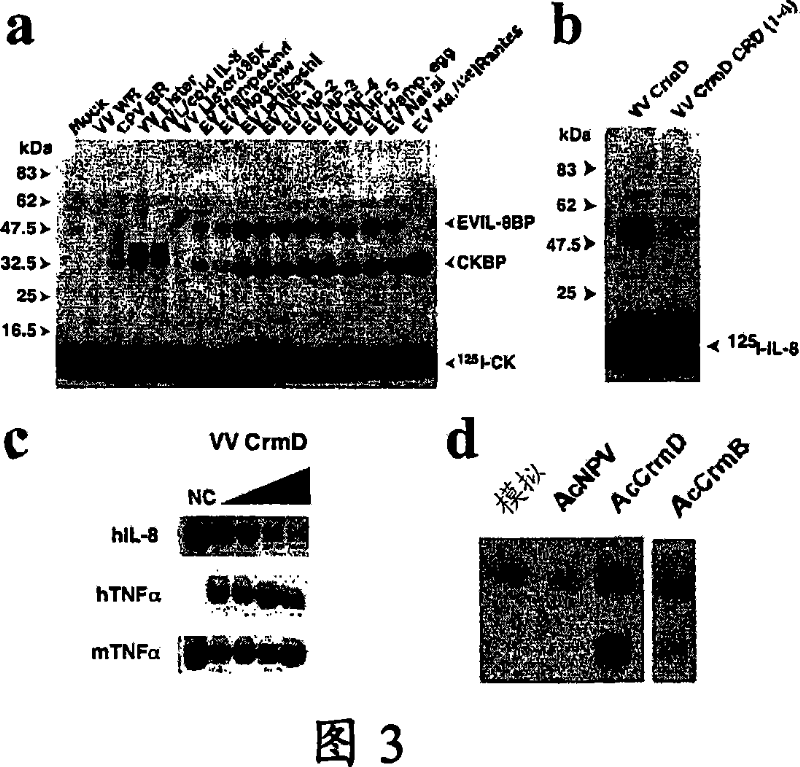
Chemokine binding activity of viral TNF receptors and related proteins. The invention relates to a C-terminal domain (CTD) of viral tumour necrosis factor receptors (vTNFRs) CrmB or CrmD or CTD homologues (CTD1, CTD2 and CTD3) from poxvirus and their functional homologues, including derivatives, and fragments, for use in binding chemokines and their analogues and / or to enhance the immunomodulatory properties of TNFRs or in bloking binding of chemokines to their corresponding cell surface receptors and / or to modulate chemokine biological activity.
Owner:安东尼奥·阿尔卡米佩特霍
Chimeric viral receptor polypeptides, human viral receptor polypeptides and uses thereof
InactiveUS7071301B1Overcome deficienciesPeptide/protein ingredientsAntibody mimetics/scaffoldsViral ReceptorCell specific
Target cell specificity of delivery vectors is provided by incorporation of a target cell specific binding domain by the use of any binding domain, which binds specifically to a binding site on the target cell. The binding site may be endogenous to the target cell, provided by engineering the target cell, or a suitable binding site may be associated with the target cell. Target cell may also be associated with a CVR polypeptide to provide specificity for the delivery vector. The association of the CVR polypeptide confers target cell specificity for a second virus host cell range, which specificity differs from the viral host cell range of the endogeneous target cell or animal host cell viral receptors. The CVR polypeptide may thus comprise a chimeric virus binding site which binds a second virus env binding domain specific for a second virus host cell range, selected from at least one of the group consisting of amphotropic, polytropic, xenotropic, ecotropic and tissue specific.
Owner:NEW YORK UNIV
SARS-CoV-2 receptor bind region glycosylation modification antigen and application thereof
ActiveCN113637055AImprove featuresBoost neutralizing antibody levelsSsRNA viruses positive-senseViral antigen ingredientsAntigen epitopeVariant strain
The invention discloses an SARS-CoV-2 receptor bind region (RBD) glycosylation modification antigen, which is characterized in that the N glycosylation site of the SARS-CoV-2 receptor bind region (RBD) is truncated and connected in series to form a single-chain polymer form, and natural glycosyl shielding on the surface of the RBD is reduced so as to more favorably expose antigen epitopes. Compared with a wild RBD monomer and RBD polymer form, the antigen can excite an organism to generate a higher-level SARS-CoV-2 specific antibody and a neutralizing antibody; an immune effect obtained through one-needle immunization can be equal to the immune effect of two-needle immunization of the wild type RBD monomer; and immune serum has similar neutralizing titer by aiming at an original strain and a variant strain, and shows a good cross protection effect. The invention also provides application of the glycosylation modification antigen in preparation of SARS-CoV-2 treatment and prevention drugs or vaccines.
Owner:ACADEMY OF MILITARY MEDICAL SCI
Neutralization monoclonal antibody of white spot syndrome virus and preparation method
ActiveCN1660909APromote growthUniform sizeImmunoglobulins against virusesImmunoglobulins against cell receptors/antigens/surface-determinantsAntigenPrawn
A neutralizing monoclonal antibody F for white spot virus is secreted by the hybrid tumor cell (CCTCC-C200423). Its preparing process includes such steps as purifying the white spot virus, using it as antigen, creating its monoclonal antibody library by immunological method, incubating the monoclonal antibodies in said library by use of the white spot virus, and screening the neutralizing monoclonal antibody by reacting on the cell membrane of spraw's blood cell. It can be used as the raw material of the passive immune vaccine for prawn.
Owner:OCEAN UNIV OF CHINA
Nanoparticle-rhACE-2 compound for blocking coronavirus from infecting target cells, preparation method and application of nanoparticle-rhACE-2 compound
InactiveCN113599536AHigh mutation rateAvoid infectionPowder deliveryPeptide/protein ingredientsViral ReceptorNanoparticle
The invention discloses a nanoparticle-rhACE-2 compound for blocking coronavirus from infecting target cells, a preparation method and application of the nanoparticle-rhACE-2 compound. The preparation method comprises the following steps: S1, preparing and purifying rhACE-2 protein; S2, labelling the rhACE-2 protein with biotin; and S3, preparing the nanoparticle-rhACE-2 compound: washing nanoparticles with PBS for three times, specifically, washing the nanoparticles with 1ml of PBS for the first time, washing the nanoparticles with PBS with the same amount as a sample for the later two times, adding the soluble rhACE-2 protein in the S2 according to different molar ratios, performing incubating for 30 minutes at 37 DEG C to enable the rhACE-2 to be effectively combined to the surfaces of the nanoparticles, and then performing washing with PBS for three times, wherein the product at the moment is the nanoparticle-rhACE-2 compound. According to the invention, a coronavirus receptor angiotensin converting enzyme 2 is used as an antagonist; and the nanoparticles are used as a carrier to block and neutralize virus particles, so that the infection of viruses to target cells is inhibited.
Owner:南京纳科生物材料有限公司
Vector mediated organelle transfection
InactiveUS7741112B2High expressionFunction increaseSugar derivativesPeptide/protein ingredientsDNA constructViral vector
The present invention provides compositions and methods for direct transfection of mitochondria and chloroplast DNA in living cells. More particularly, the present invention is based on the use of viral vectors that specifically bind to receptors uniquely found on the target organelle. In one embodiment, as shown in FIG. 1, a eukaryotic cell containing an organelle (1) that has been modified to express a viral receptor (2) on the organelle's surface is provided. A viral vector (6) comprising a desired recombinant DNA construct (3) is introduced into the cytosol of the cell, wherein the viral vector binds to its receptor and introduces the recombinant DNA into the interior of the organelle.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
Preparation method of gene editing novel coronavirus vaccine vector
InactiveCN112646823ASolve the permanent preservation problemSolving Recycling ProblemsSsRNA viruses positive-senseVirus peptidesAmniotic fluid cellCord blood stem cell
The invention discloses a preparation method of a gene editing novel coronavirus vaccine vector, which is characterized in that tissue cells such as amniocytes, neonatal cord blood, umbilical cords and placentas, which are originally abandoned and left after prenatal diagnosis, are transformed into a recyclable novel coronavirus vaccine vector which have a stem cell natural treatment function and an infinite passage immortalization function and a novel coronavirus ACE2 receptor missing characteristic by methods of novel coronavirus susceptible receptor ACE2 gene editing, SV40LT and / or hTERT gene transfection; the gene editing novel coronavirus vaccine vector is specially used for preparing a vaccine for individualized treatment of COVID-19, and can be pre-stored in a stem cell library with a temperature of minus 196 DEG C for a long time according to names, ABO blood types or HLA typing; and when the novel coronavirus infectious disease breaks out or a certain individual needs to use the gene editing novel coronavirus vaccine vector, stem cell treatment or vaccine preparation can be carried out after passage and amplification of an autologous or homotype gene editing immortal stem cell line, so as to eliminate and reduce immunological rejection in traditional stem cell treatment and vaccine use.
Owner:翁炳焕
Alpaca-derived nano antibody combined with SARS-CoV-2 RBD
ActiveCN114249820AImprove stabilityHigh expressionAntibody mimetics/scaffoldsBiological material analysisAntigenViral Receptor
The present disclosure relates to an alpaca-derived antibody or an antigen-binding fragment thereof that binds to SARS-CoV-2 RBD, and in particular, to an alpaca-derived nanoantibody or an antigen-binding fragment thereof that can bind to a new coronavirus (SARS-CoV-2) receptor binding region (RBD) with high affinity, which can be used for the prevention, treatment and / or diagnosis of SARS-CoV-2 infection.
Owner:UNIV OF SCI & TECH OF CHINA
A highly tropic bladder cancer-targeted tumor-killing adenovirus
InactiveCN104328140BAddressing CAR Receptor DependenceHigh infection efficiencyFermentationAntineoplastic agentsViral ReceptorUrinary bladder epithelium
The present invention relates to high tropism bladder cancer targeting tumor-killing adenovirus, and discloses modified replication defective adenovirus for treating bladder cancer. According to the present invention, the RGD sequence is integrated into the adenovirus cilium zone to change the adenovirus tropism so as to improve the tropism on the bladder cancer lowly expressing the adenovirus receptor and solve the problem of low infection capability of the adenovirus vector on the bladder cancer lowly expressing the adenovirus receptor; in the tropism-modified adenovirus, the bladder epithelium-specific UPII promoter is adopted to control the expression of the suicide gene TK so as to construct the selectively expressing adenovirus; and the CAR receptor dependence problem of the adenovirus in the prior art and the targeting problem of the adenovirus in the prior art can be solved, and the treatment effect can be increased.
Owner:LANZHOU UNIVERSITY
Alpaca-derived nano antibody combined with SARS-CoV-2 RBD
ActiveCN114249822AImprove stabilityHigh expressionHybrid immunoglobulinsBiological material analysisAntigenViral Receptor
The present disclosure relates to an alpaca-derived antibody or an antigen-binding fragment thereof that binds to SARS-CoV-2 RBD, and in particular, to an alpaca-derived nanoantibody or an antigen-binding fragment thereof that can bind to a new coronavirus (SARS-CoV-2) receptor binding region (RBD) with high affinity, which can be used for the prevention, treatment and / or diagnosis of SARS-CoV-2 infection.
Owner:UNIV OF SCI & TECH OF CHINA
Mask for enhancing prevention of infectious disease transmission and use method
PendingCN113152105ALittle effect on breathingNot cause breathing difficultiesBiochemical treatment with enzymes/microorganismsVegetal fibresCelluloseInfectious Disorder
The invention relates to a mask for enhancing prevention of infectious disease transmission and a use method. The method comprises the following steps of introducing gauze cellulose hydroxyl into an activated cyano group through cyanogen bromide under the reaction conditions of alkalinity, dimethyl sulfoxide and the like, carrying out covalent coupling on the activated cyano group and amino in trypsin, and then preparing immobilized trypsin. According to a solid-phase enzyme technology, the trypsin is fixed to the gauze mask, a dilute alkaline solution is sprayed to gauze through a small spraying bottle, enzyme is in a moist environment, solid-phase pancreatin is in the optimum working pH range, and exhaled air enables the pancreatin to work at a proper temperature. The pancreatin destroys spike protein of protein on the pathogen surface under the proper conditions, so that the spike protein cannot be combined with a virus receptor in the human body. Meanwhile, after a virus protection layer is damaged, nucleic acid of the virus protection layer is easily degraded by nuclease in air or exhaled by people, and then the virus protection layer is used in cooperation with the medical mask, so that the effect of preventing infectious diseases is improved.
Owner:ZHEJIANG UNIV
Alpaca-derived nano antibody combined with SARS-CoV-2 RBD
ActiveCN114249821AImprove stabilityHigh expressionPolypeptide with localisation/targeting motifAntibody mimetics/scaffoldsAntigenViral Receptor
The present disclosure relates to an alpaca-derived antibody or an antigen-binding fragment thereof that binds to SARS-CoV-2 RBD, and in particular, to an alpaca-derived nanoantibody or an antigen-binding fragment thereof that can bind to a new coronavirus (SARS-CoV-2) receptor binding region (RBD) with high affinity, which can be used for the prevention, treatment and / or diagnosis of SARS-CoV-2 infection.
Owner:UNIV OF SCI & TECH OF CHINA
Mask for relieving respiratory tract infectious diseases and using method thereof
PendingCN113142706AMaintain enzyme activityLittle effect on breathingBiocideDisinfectantsCelluloseDisease
The invention relates to a mask for slowing down respiratory tract infectious diseases and a using method thereof, which are characterized in that hydroxyl on cellulose in gauze is introduced into carboxyl under an alkaline condition through a sodium chloroacetate method, and the introduced carboxyl is covalently coupled with amino in trypsin under the action of a water-soluble carbodiimide cross-linking agent to obtain the mask for slowing down the respiratory tract infectious diseases; the trypsin is covalently bound to the gauze to prepare the immobilized trypsin. According to the immobilized enzyme technology, protease is immobilized on gauze to form immobilized enzyme. A solution with the pH of 7.2-8.0 is filled in a small plastic spray bottle to spray steam mist to gauze, so the pancreatin is in a moist environment, the solid-phase pancreatin is in the optimum working pH range, and then the pancreatin is kept at a proper temperature through human respiration. The pancreatin destroys spike protein of protein on a surface of a new crown under proper conditions, so the pancreatin cannot be combined with a virus receptor in a human body, and the prevention and control effect of blocking the new crown virus is improved.
Owner:ZHEJIANG UNIV
Composition for nursing nasal cavity and aerosol and preparation method thereof
PendingCN113679834AAchieve non-flammable environmental characteristicsTo achieve environmental characteristicsOrganic active ingredientsAerosol deliveryBiotechnologyLiving environment
The invention discloses a composition for nursing a nasal cavity. The composition is prepared from the following components in parts by weight: 0.1-0.2 part of alpaca nano antibody, 0.5-2.5 parts of radish root fermentation product extract, 0.5-2.5 parts of bifidus yeast fermentation product lysate and 0.005-0.02 part of vitamin E. The alpaca nano antibody is ingeniously used for binding a virus receptor, meanwhile, leuconostoc in the radish root fermentation product extract is combined, a severe living environment is created for viruses, the viruses are clamped from the source and the living environment, and therefore the prevention purpose is achieved; and the aerosol disclosed by the invention can also be used for preventing the new coronavirus.
Owner:ZHONGSHAN TIANTU FINE CHEM CO LTD
Alpaca-derived Nanobodies Binding to SARS-CoV-2 RBD
ActiveCN112094342BImprove stabilityHigh expressionHybrid immunoglobulinsImmunoglobulins against virusesViral ReceptorEpitope specificity
The present disclosure relates to an alpaca-derived antibody or an antigen-binding fragment thereof that binds to the SARS-CoV-2 RBD, in particular to an alpaca-derived nanoparticle capable of binding to the receptor binding region (RBD) of the new coronavirus (SARS-CoV-2) with high affinity. Antibodies or bi-epitope-specific antibodies or antigen-binding fragments thereof, which can be used for preventing, treating and / or diagnosing SARS-CoV-2 infection.
Owner:UNIV OF SCI & TECH OF CHINA
Alpaca-derived Nanobodies Binding to SARS-CoV-2 RBD
ActiveCN112094343BImprove stabilityHigh expressionHybrid immunoglobulinsImmunoglobulins against virusesViral ReceptorEpitope specificity
The present disclosure relates to an alpaca-derived antibody or an antigen-binding fragment thereof that binds to the SARS-CoV-2 RBD, in particular to an alpaca-derived nanoparticle capable of binding to the receptor binding region (RBD) of the new coronavirus (SARS-CoV-2) with high affinity. Antibodies or bi-epitope-specific antibodies or antigen-binding fragments thereof, which can be used for preventing, treating and / or diagnosing SARS-CoV-2 infection.
Owner:UNIV OF SCI & TECH OF CHINA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com